Abstract
Background
The greater cane rat (GCR), reputed to be African's second largest rodent, is a precocial hystricomorph with an uncommon phenotype and life history. Scientific and socio‐economic interests in the GCR have led to heightened research efforts targeted towards a better understanding of its biology and exploration of its economic and translational usefulness.
Methods
Records of all online scientific publications on the GCR from Google, Google Scholar, PubMed, science.gov, Ebscohost and Worldwide science, with the exception of research theses, proceedings, unpublished projects and abstracts, were collated and analyzed using descriptive statistics.
Results
A total of 146 published scholarly articles spanning about six decades were retrieved, with 98% of the GCR publications originating from African countries. Nigeria boasts the highest number of publications (58.22%) followed by Ghana (21.23%) and South Africa (5.48%) while Senegal contributed the least (0.69%). Publications were sorted into ten field categories. The field with the highest number of articles (41.78%) was animal breeding and management recording, closely followed by anatomy (37.67%). Lesser contributions were made by parasitology (5.48%), biochemistry/hematology (4.8%), pharmacology/toxicology (4.11%), pathology (2.06%), and surgery/anesthesia and physiology (1.37% apiece). The fields with fewest contributions were microbiology and developmental biology (0.69% each).
Conclusion
This study chronicles the spectrum of knowledge available on the GCR, highlighting the knowledge gap that still exists in various fields in order to provide advocacy for new frontiers in research efforts on this rodent. We suggest the need for a clearly defined and well integrated national/regional policy aimed at establishing Africa's foremost micro‐livestock rodent, the greater cane rat, on the world's scientific radar.
Keywords: African rodent, grasscutter, greater cane rat, micro‐livestock, review, wildlife
1. INTRODUCTION
African cane rats belong to the family Thryonomyidae, which is represented by a single genus, Thryonomys. Nearly all the earliest fossil thryonomyids are African, suggesting that the family originated there in the early Miocene. The group diversified to a maximum of six genera during the middle Miocene,1 and by the Pliocene only the genus Thryonomys remained.2, 3 Misonne4 and Woods5 have provided short reviews of extant species of thryonomyids, concluding that only two species exist at present. These are the greater cane rat (GCR) (Thryonomys swinderianus, Temminck 1827) and the lesser cane rat, (Thryonomys gregorianus, Thomas 1894).6 Although, other species such as T logani 7 and T arkelli 8 were initially described, they are no longer recognized as taxons distinct from T swinderianus.9
The GCR is commonly referred to in Nigeria as “oya” in the Yoruba parlance, “nchi” by the Igbos, “Gegbi” by the Hausas, “agouti” by French‐speaking Africans, “grasscutter” in West Anglophone Africa, and “hedgehog” in Central Africa.10 They are predominantly found in the humid and sub‐humid regions of Africa, from the far west (Senegal) through the grasslands of East Africa to Southern Africa, extending to the eastern Cape.11 The presence and predominance of dense thick cane‐like grasses such as elephant grass (Pennisectum purpureum) and guinea grass (Panicum maximum) influence the geographical distribution of these rodents. As obligate herbivores, they feed primarily on reeds, roots, shoots and stems of grasses using their broad and sharp incisors to cut easily through tough plant materials. However, they can be major pests in sugar cane, wheat and maize fields.12, 13
The skin of GCR has very coarse hairs speckled with yellow or grey, giving it a bristly appearance.14, 15 With a broad head, round muzzle, small round ears and a short tail, these hystricomorphic rodents are heavily built, reaching up to about 9 kg.16, 17 Unsurprisingly therefore, they are reputed to be the fourth largest extant rodent18, 19 and the second largest African rodent after the African porcupine, Hystrix africaeaustralis.20, 21, 22, 23 Their large carcass yield and the high nutritional value of their meat make them a premium alternative source of protein, especially in sub‐Saharan Africa, where they command high prices leading to huge economic returns.24, 25, 26, 27 Furthermore, their meat is widely accepted, with few to no cultural and religious prohibitions, thus making them Africa's foremost micro‐livestock.6, 28, 29
This precocial African rodent has an uncommon phenotype and life history, with an unusually long gestational period of 150 days.30 Reproduction occurs all year round and litter sizes vary from 2 to 4.6, 31 Pups are born with eyes wide open with thick fur on their skin and become accomplished runners shortly after birth.6
Recently, scientific and socio‐economic interest in the GCR has led to increased research efforts to better understand of its biology and explore of its economic and translational benefits. The aim of this paper therefore is to chronicle the spectrum of knowledge available on the GCR over a period of six decades, highlighting the knowledge gap that still exists in order to provide advocacy for new frontiers in research efforts on this rodent.
2. METHODS
This review of published literature on the greater cane rat (GCR) was conducted at the Federal University of Agriculture Abeokuta (FUNAAB). It was predicated on a database search using the search engines Google, Google Scholar, PubMed, science.gov, Ebscohost and Worldwide science, in no particular order, to access online journals. The keywords used were “greater cane rat”, “GCR”, “grasscutter”, “African greater cane rat”. All publications up until July 2019 were collated and analyzed. Theses, proceedings, unpublished projects and abstracts were excluded from this search. All pooled publications were broadly classified based on the following field categories: anatomy, animal breeding and management, biochemistry/hematology, surgery/anesthesia, microbiology, parasitology, pathology, pharmacology/toxicology, physiology, and developmental biology. The field of anatomy was further subdivided into various body organ systems, namely: circulatory, digestive, endocrine, immune and lymphatics, integumentary, muscular, nervous, respiratory, skeletal, and urinary systems. In addition, publications were grouped into originating countries based on the institutional affiliations of lead authors. Publication rate per decade was defined as the total number of publications within a decade divided by the time period under consideration (10 years), while publication differentials was calculated as the differences in publication rates between successive decades. All data generated were presented in pictorial (bar and pie) charts and linear graphs using Microsoft Excel (version 2016).
3. RESULTS
A total of one hundred and forty‐six (146) published scholarly articles were found using a combination of selected search engines. The earliest publication on the GCR dates back to 1969 (Figure 1), thus indicating an average publication rate of 2.4 articles per year on this unique African rodent over a 60‐year period. About one‐third of the total publications on this rodent were written in the first four decades (1969‐2009), as seen in the publication rate per decade and publication differentials between successive decades (Figures 1 and 2). This was followed by a sharp publication spurt in the last decade (2009‐2019) with almost two‐thirds of the research articles documented within this time frame (Figure 3). With the exclusion of three articles whose authorships were from France, all other publications on the GCR came from African countries (about 98%). Of the total publication, Nigeria boasts the highest number of publications on the GCR (58.22%) followed by Ghana (21.23%) and South Africa (5.48%), while Senegal has provided the smallest contribution, with only one publication (0.69%) (Figures 4 and 5).
Figure 1.

Distribution pattern of all published articles on the GCR in the last six decades
Figure 2.

Publication rate per decade and publication differentials of GCR articles within the last six decades
Figure 3.

Pie chart of the publication distribution on the GCR in successive decades from 1960 to 2019
Figure 4.
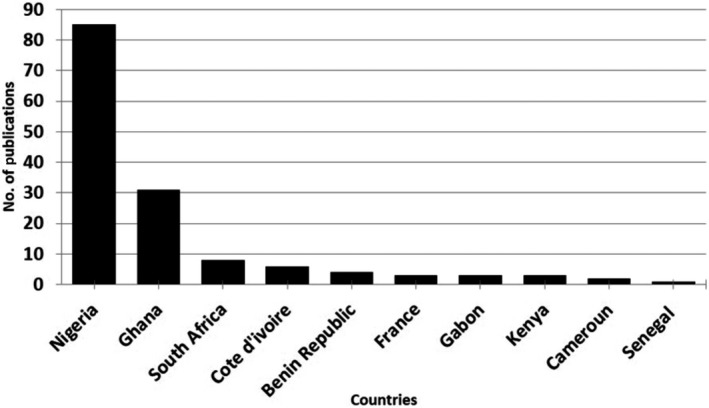
Bar chart showing number of published articles on the GCR based on originating countries
Figure 5.

Pie chart showing percentage contribution of GCR publications from originating countries
The field of physiology was the first to be explored in this rodent, with a report by Ewer12 on the form and function of the GCR describing its structural and behavioural primary feeding adaptations. No other significant contribution was made in this field until 43 years later when Yapi et al32 provided more insight into the physiology of this rodent, highlighting the caecum as an important site for fermentation in digestion.
The field with the highest number of articles was animal breeding and management; 61 articles were allocated to this field accounting for 41.78% of the total publications on the GCR (Figures 6 and 7), the earliest being on food preference and carcass composition of the GCR.24 Subsequently, several authors elucidated breeding characteristics and traits in the GCR. van der Merwe33 described the GCR as an aseasonal breeder with a very low fecundity rate, producing a maximum of two litters per annum. This low fecundity is attributable to the long gestation period of about 150 days. Other works reported in the literature include: characterization of the estrus cycle,34 determination of the ovulatory mechanisms,35 detection of mating and pregnancy,36 efficacy of vaginal mucous plug formation in pregnancy detection,37 and efficacy of sex determination using ano‐genital distance,38 amongst others. More recently, advanced techniques such as the use of mitochondrial D‐loop and double‐digest restriction‐site associated DNA (RAD) sequencing markers (single nucleotide polymorphisms, SNPs) have been deployed to improve understanding of genetic diversity in the GCR and for genetic management of production.39, 40
Figure 6.

Bar chart showing publication distributions on the GCR based on field categories within the last six decades
Figure 7.

Pie chart showing percentage contributions of different research fields on the GCR over a 60‐year period
Second to animal breeding and management in terms of percentage of publications is the field of anatomy, with 37.67% (55 articles) of the total publications pooled allocated to this field (Figures 6 and 7). This field was further classified based on body organ‐systems. The sub‐field with the greatest number of publications was reproductive anatomy (36.4%), followed by neuroanatomy (23.7%), and the digestive (16.4%), skeletal (10.9%) and the integumentary and urinary systems (3.6% apiece). Fewest publications were allocated to the fields of muscular, immune and lymphatics and endocrine systems, each comprising 1.8% of the total (Figure 8). No article on the cardiovascular and respiratory systems of the GCR was found in this search.
Figure 8.

Bar chart showing distribution of publications on veterinary anatomy of the GCR based on body organ‐systems within the last six decade
Eight publications, representing 5.48% of the total number of articles found on the GCR, were allocated to the field of parasitology (Figures 6 and 7). In a survey of ecto‐ and endo‐parasites carried out in Ghana by Yeboah and Simpson,41 6 species of helminths were identified. They include 2 cestodes (Furhmanella transvaalensis and Railettina mahonae) and 4 nematodes (Longistriata spira, Trachypharynx natalensis, Paralibyostrongylus vondwei and Trichuris paravispicularis) were reported. However, in a similar study carried out in Nigeria 14 nematodes, 5 trematodes, 4 cestodes and 1 acanthocephala were reported in wild GCRs42 while 7 nematodes, 2 trematodes and 2 cestodes were identified in domesticated rats.43 In Gabon, Jori et al44 identified Paralibyostrongylus hebreniticus and Taenia species at post mortem in captive cane rats. Natural occurrence of hemoparasites such as Trypansoma spp., Plasmodium spp. and Babesia spp. in wild and captive‐reared GCRs have also been reported.42 To date, only 7 species of ticks have been found on the body of wild GCRs and none has been identified on their captive‐bred counterparts. These ticks include: Ixodes aulacodi, Ixodes sp Rhipicephalus simpsoni, Rhipicephalus (Boophilus) microplus, Amblyomma compressum, Haemaphysalis parmata, and Haemaphysalis leachi.41, 45
Only 2.06% of the total publications on the GCR were allocated to the field of pathology (Figures 6 and 7). Stressful conditions as a result of transportation, poor handling, traumatic injuries, respiratory diseases, especially pneumonia, and septicaemia have been identified as the leading causes of mortality in the GCR.46, 47, 48 In most instances, Klebsiella pneumonia, Staphylococcus aureus, Streptococcus D haemolyticum and Candida albicans were isolated from postmortem lung samples of this rodent.44 Neoplasia, although rare, has been reported by Jori and Cooper.49 The least number of publications were allocated to the fields of microbiology and developmental biology, each comprising 0.69% of the total (Figures 6 and 7).
Recently, developmental milestones across the entire period of gestation in GCR using external morphogenetic features were reported by Mustapha et al.31 The authors noted that the GCR had a relatively longer period of embryogenesis and a consequently shorter period of fetogenesis compared to other precocial mammals like the guinea pig, sheep and pig. They also reported evidence‐based findings that prenatal development in the GCR might be associated with a reproductive delay.31
4. DISCUSSION
One hundred and forty‐six (146) scientific publications on the GCR were found from an online search covering a period of six decades. Considering the exclusion criteria deployed in this study, this publication pool, while extensive, is not exhaustive. The increasing rate of publications on the GCR, particularly the spurt in the last ten years (2009‐2019), attests to a recent rise in research activity on this rodent. This is traceable to the current drive by African scientists to identify indigenous animals suitable for domestication and use as spontaneous research models, especially within the African context.19, 31, 50, 51 The publication spurt also highlights an increase in awareness and exploitation of this rodent for economic purposes – the GCR is regarded as providing premium, choice meat, particularly in West Africa, where they command high prices leading to huge economic returns.28, 29 It is therefore unsurprising that the fields of animal breeding and management, and anatomy were most highly represented among all publications on the GCR. The lower percentages recorded for other fields such as parasitology, physiology and developmental biology, amongst others, reflect a huge dearth of information on the biology of this rodent in health and disease. For instance, while pneumonia has been reported as one of the major causes of mortalities in the GCR,44, 48 it remains to be ascertained whether there are morpho‐physiological configurations and/or predispositions that make the GCR susceptible to this condition.
All publications excluding three from France originated from African countries. This comes as no surprise as the GCR is predominantly found in Africa.11 Specifically, the GCR is found in the humid and sub‐humid areas of Africa, south of the Sahara, inhabiting virtually all countries of west, east and southern Africa.52 The three publications recorded from outside Africa suggest a collaboration between the French speaking African countries (in this case Gabon and Cote d’Ivoire) and France. Indeed, Adu et al11 noted that the distribution of this rodent is largely influenced by the availability of adequate and/or favored grass species. Interestingly, all publications on the GCR emanating from Africa were from countries found within the geographical zones naturally inhabited by this rodent. Nigeria had the highest number of research publications on the GCR, followed by Ghana. This might reflect the level of farming of the GCR in these countries, with its attending socioeconomic impact, as most of the rats used for research were obtained from established commercial farms.
Several repetitions in some of the scholarly works on the GCR were noted. These repetitions were published without proper acknowledgement or recognition of previously published works. For instance, the hematological and plasma biochemical parameters of young and adult GCRs reared in captivity and in the wild were reported by Ogunsanmi et al,53 Opara and Fagbemi42 and Byanet et al54 A similar study by Soro et al55 was published without fully citing the works of the previous reports mentioned above, suggesting that the authors did not conduct an in‐depth literature review on the subject matter. Olude et al56 have also linked repetition of work on the African giant rat to lack of a unified national/regional research focus and continuity in the affected fields, poor research funding in sub‐Saharan Africa, and inadequate specialized, advanced equipment for research and the accompanying technical know‐how in most local institutions.
Evidenced‐based findings suggesting a reproductive delay in the GCR were recently reported by Mustapha et al31 However, the exact type(s) of delay exhibited by this rodent remain to be fully ascertained. More studies are therefore needed in order to investigate, characterize and confirm this. It will also be interesting to know whether this delay is specific to the GCR or rather a phenomenon of precocial hystricomorph rodents.
This study has highlighted the spectrum of research opportunities yet to be explored in various fields of study that will contribute to a better understanding and further domestication of this rodent. It also underscores the need for a clearly defined and well integrated national push to establish Africa's foremost mini‐livestock rodent on the world's scientific radar. This can be achieved by concerted and purposeful scientific investigation targeted towards exploring its suitability as a spontaneous indigenous research animal model while also leveraging its huge economic potential, especially in sub‐Saharan Africa.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
OAM, AMO and JOO conceived and designed the study. OAM, EET and OSE collected and analyzed the data. OAM and EET wrote the original draft; AMO, AKA and JOO revised the manuscript. All authors critically read and contributed to the manuscript and approved the final manuscript for submission.
Mustapha OA, Teriba EE, Ezekiel OS, Olude AM, Akinloye AK, Olopade JO. A study of scientific publications on the greater cane rat (Thryonomys swinderianus, Temminck 1827). Animal Model Exp Med. 2020;3:40–46. 10.1002/ame2.12103
REFERENCES
- 1. McKenna MC, Bell SK. Classification of Mammals Above the Species Level. New York, NY: Columbia University Press; 1997. [Google Scholar]
- 2. Wesselman HB, Black MT, Asnake M. Small mammals In: Haile‐Selassie Y, Wolde Gabriel G, eds. Ardipithecus Kadabba: Late Miocene Evidence from the Middle Awash, Ethiopia. Berkeley, CA: University of California Press; 2009:105‐133. [Google Scholar]
- 3. Denys C. Rodents In: Harrison T, ed. Paleontology and Geology of Laetoli: Human Evolution in Context. New York, NY: Springer; 2011:15‐53. [Google Scholar]
- 4. Misonne X. The Mammals of Africa: An Identification Manual. Washington, DC: Smithsonian Institution Press; 1971:23. [Google Scholar]
- 5. Woods CA. Suborder Hystricognathi In: Wilson DE, Reeder DM, eds. Mammal Species of the World: A Taxonomic and Geographic Reference. 2nd ed Washington: Smithsonian Institution Press; 1993:771‐806. [Google Scholar]
- 6. Okorafor KA, Okete JA, Andem AB, Eleng IE. Assessment of grasscutters’ (Thryonomys swinderianus) sellers and hunters conservation knowledge, rate of hunting and methods of hunting in Oyo State, Nigeria. Eur J Zool Res. 2012;1:86‐92. [Google Scholar]
- 7. Romer AS, Nesbitt PH. LXXIX—an extinct cane‐rat (Thryonomys logani, sp. n.) from the Central Sahara. Ann Mag Nat Hist. 1930;6:687‐690. [Google Scholar]
- 8. Bate DMA. An extinct reed‐rat (Thryonomys arkelli) from the Sudan. Ann Mag Nat Hist. 1947;14:65‐71. [Google Scholar]
- 9. van der Merwe M. Discriminating between Thryonomys swinderianus and Thryonomys gregorianus . Afr Zool. 2007;42:165‐171. [Google Scholar]
- 10. Ibitoye O, Kolejo O, Akinyemi G. Burgeoning and domestication of grasscutter (Thryonomys swinderianus) in a post‐Ebola era: a reassessment of its prospects and challenges in Nigeria. World Sci News. 2019;130:216‐237. [Google Scholar]
- 11. Adu EK, Asafu‐Adjaye A, Hagan BA, Nyameasem JK. The grasscutter: an untapped resource of Africa’s grasslands. Livest Res Rural Dev. 2017;29. [Google Scholar]
- 12. Ewer RF. Form and function in the grass cutter, thryonomys swinderlanus temm. (rodentia , thryonomidae). Ghana J Sci. 1969;9:131‐141. [Google Scholar]
- 13. van Zyl A, Delport JH. Digestibility of nutrients and aspects of the digestive physiology of the greater cane rat, Thryonomys swinderianus in two seasons. Afr Zool. 2010;45:254‐264. [Google Scholar]
- 14. Aluko FA, Salako AE, Ngere LO, Eniolorunda OO. A review of the habitat, feeds and feeding, behavior and economic importance of Grasscutter farming. Am J Res Commun. 2015;3:96‐107. [Google Scholar]
- 15. Akpan MO, Samuel OM, Emikpe BO. Regional skin histomorphology in adult greater cane rats (Thryonomys swinderianus): a pilot study. Int J Vet Sci Med. 2018;6:219‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Henry AJ. Reproductive performance of grasscutter does at first parity and growth performance of their F1 generation. Asian J Anim Sci. 2011;5:289‐295. [Google Scholar]
- 17. Igado OO, Adebayo AO, Oriji CC, Oke BO. Cranio‐facial and ocular morphometrics of the male greater cane rat (Thryonomys swinderianus). Nig Vet J. 2016;37:54‐63. [Google Scholar]
- 18. Eben AB. Grasscutter: Importance, Habitat, Characteristics, Feed and Feeding, Breeding and Diseases. Kumasi, Ghana: Centre for Biodiversity Utilization and Development (CBUD); 2004:1‐6. [Google Scholar]
- 19. Ibe CS, Salami SO, Wanmi N. Brain size of the African grasscutter (Thryonomys swinderianus, Temminck, 1827) at defined postnatal periods. Folia Vet. 2017;61:5‐11. [Google Scholar]
- 20. Skinner JD, Chimimba CT. The Mammals of the Southern African Subregion. 3rd ed Cambridge, UK: Cambridge University Press; 2005:93‐96. [Google Scholar]
- 21. Nzalak JO, Byanet O, Salami SO, et al. Comparative morphometric studies of the cerebellum and forebrain of the African giant rat (AGR) (Crycetomys gambianus‐Waterhouse) and that of grasscutter (Thryonomys swinderianus). J Anim Vet Adv. 2008;7:1090‐1092. [Google Scholar]
- 22. Ajayi I, Shawulu JC, Daniel NW. Organ body weight relationship of some organs in the male African Grasscutter (Thryonomys swinderianus). J Adv Vet Res. 2012;2:86‐90. [Google Scholar]
- 23. Mustapha OA, Aderounmu OA, Olude MA, et al. Anatomical studies on the spinal cord of the GCR (Thryonomys swinderianus, Temminck) 1: gross morphometry. Niger Vet J. 2015;36:1192‐1202. [Google Scholar]
- 24. Ajayi SS, Tewe OO. Food preferences and carcass composition of the grasscutter (Thryonomys swinderianus) in captivity. Afr J Ecol. 1980;18:133‐140. [Google Scholar]
- 25. van der Merwe M. Tooth succession in the greater cane rat Thryonomys swinderianus (Temminck, 1827). J Zool. 2000;251:541‐545. [Google Scholar]
- 26. Opara MN. Grasscutter: the haematology and major parasites. Res J Parasitol. 2010;5:214‐223. [Google Scholar]
- 27. Akinola L, Etela I, Emiero S. Grasscutter production in West Africa: prospects, challenges and role in disease transmission. Am J Exp Agr. 2015;6:196‐207. [Google Scholar]
- 28. Jori F, Edderai D, Houben P. Potential of rodents for minilivestock in Africa In: Paoletti MG, ed. Ecological Implications of Mini‐livestock; Rodents, Frogs, Snails and Insects for Sustainable Development. Enfield, USA: Science Publications; 2005:25‐47. [Google Scholar]
- 29. Ibe CS. Structural and Immunohistochemical Studies of Prenatal and Postnatal Brain Development in the African Grasscutter (Thryonomys swinderianus ‐ Temminck, 1827), Dissertation. Zaria (Nigeria): Ahmadu Bello University; 2016. [Google Scholar]
- 30. Krüger J. A Study of the Anatomy and Physiology of Sleep in African Rodents with Unusual Phenotypes and Life Histories, Dissertation. Johannesburg (South Africa): University of Witwatersrand, Johannesburg, South Africa; 2015. [Google Scholar]
- 31. Mustapha OA, Olude MA, Ezekiel S, Seeger J, Fietz SA, Olopade JO. Developmental horizons in the pre‐natal development of the Greater cane rat (Thryonomys swinderianus). Anat Histol Embryol. 2019;48:486‐497. [DOI] [PubMed] [Google Scholar]
- 32. Yapi YM, Gidenne T, Farizon Y, Segura M, Zongo D, Enjalbert F. Post‐weaning changes in the digestive physiology and caecal fermentative activity in the Greater Cane Rat (Thryonomys swinderianus). Afr Zool. 2012;47:311‐320. [Google Scholar]
- 33. van der Merwe M. Breeding season and breeding potential of the greater cane rat (Thryonomys swinderianus) in captivity in South Africa. South Afr J Zool. 1999;34:69‐73. [Google Scholar]
- 34. Nyameasem JK, Adu EK, Amoah KO, Hagan BA. The effect of male proximity on vaginal patency, estrous cycle length and feed intake of female grasscutters. Trop Anim Health Prod. 2016;48:445‐449. [DOI] [PubMed] [Google Scholar]
- 35. Addo P, Dodoo A, Adjei S, Awumbila B, Awotwi E. Determination of the ovulatory mechanism of the grasscutter (Thryonomys swinderianus). Anim Reprod Sci. 2002;71:125‐137. [DOI] [PubMed] [Google Scholar]
- 36. Addo PG, Awumbila B, Awotwi E, Ankrah NA. Comparative characterization of the oestrous cycles of the grasscutter (Thryonomys swinderianus) and the guinea pig (Cavia porcellus) by the hystricomorph vaginal membrane perforation phenomenon. Livest Res Rural Dev. 2007;19. [Google Scholar]
- 37. Adu EK, Yeboah S. The efficacy of the vaginal plug formation after mating for pregnancy diagnosis and embryonic resorption in utero in the greater cane rat (Thryonomys swinderianus, Temminck). Trop Anim Health Pro. 2000;32:1‐10. [DOI] [PubMed] [Google Scholar]
- 38. Adu EK, Wallace PA, Ocloo TO. Efficacy of sex determination in the greater cane rat (Thryonomys swinderianus, Temminck). Trop Anim Health Pro. 2002;34:27‐33. [DOI] [PubMed] [Google Scholar]
- 39. Adenyo C, Hayano A, Kayang BB, Owusu E, Inoue‐Murayama M. Mitochondrial D‐loop diversity of grasscutter (Thryonomys swinderianus Rodentia: Hystricomorpha) in Ghana. Open J Anim Sci. 2013;3:145‐153. [Google Scholar]
- 40. Adenyo C, Ogden R, Kayang B, Onuma M, Nakajima N, Inoue‐Murayama M. Genome‐wide DNA markers to support genetic management for domestication and commercial production in a large rodent, the Ghanaian grasscutter (Thryonomys swinderianus). Anim Genet. 2017;48:113‐115. [DOI] [PubMed] [Google Scholar]
- 41. Yeboah S, Simpson PK. A preliminary survey of the ecto and endoparasites of the grasscutter (Thryonomys swinderianus Temminck): case study in Ekumfi, Central Region of Ghana. J Ghana Sci Assoc. 2001;3:30‐36. [Google Scholar]
- 42. Opara MN, Fagbemi BO. Hematological and plasma biochemistry of the adult wild african grasscutter (Thryonomys swinderianus). Ann N Y Acad Sci. 2008;1149:394‐397. [DOI] [PubMed] [Google Scholar]
- 43. Opara MN, Fagbemi BO. Patho‐physiological effects of experimental Trypanosoma congolense and Trypanosoma vivax infections in the grasscutter (Thryonomys swinderianus, Temminck). Nat Sci. 2010;8:88‐101. [Google Scholar]
- 44. Jori F, Cooper JE, Casal J. On post mortem findings in captive cane rats (Thryonomys swinderianus) in Gabon. Vet Rec. 2001;148:624‐628. [DOI] [PubMed] [Google Scholar]
- 45. Faustin ZBZ, Alassane T, Clarisse OK, Yahaya K, Fantodji A. Prevalence of ticks infesting grasscutters (Thryonomys swinderianus Temminck, 1827) in the south of Côte d’Ivoire. J App Bio. 2015;87:8085‐8093. [Google Scholar]
- 46. Ikpeze OO, Ebenebe CI. Productive performance of the grasscutter (Rodentia: Thryonomyidae) reared under three different housing systems. Bio‐Research. 2004;2:19‐21. [Google Scholar]
- 47. Adu EK, Aning KG, Wallace PA, Ocloo TO. Reproduction and mortality in a colony of captive greater cane rats, Thryonomys swinderianus, Temminck. Trop Anim Health Prod. 2000;32:11‐17. [DOI] [PubMed] [Google Scholar]
- 48. Jarikre TA, Awe O, Bakare AA, Emikpe BO. Examination of the Lung and Liver for Pathological Changes in Hunted Grasscutters (Thryonomys swinderianus) in Southwest Nigeria. Nig Vet J. 2018;39:263‐268. [Google Scholar]
- 49. Jori F, Cooper JE. Spontaneous neoplasms in captive African cane rats (Thryonomys swinderianus). Vet Pathol. 2001;38:556‐558. [DOI] [PubMed] [Google Scholar]
- 50. Asibey EOA, Addo PG. The Grasscutter: a promising animal for meat production In: Turnham D, ed. African Perspective: Practices and Policies Supporting Sustainable Development. Zimbabwe: Weaver press; 2000:46. [Google Scholar]
- 51. Mustapha OA, Taiwo S, Olude A, et al. Anatomical studies on the spinal cord of the Greater Cane Rat (Thryonomys swinderianus, Temminck) II: histomorphology and spinal tracings. Nig Vet J. 2017;38:129‐139. [Google Scholar]
- 52. National Research Council . Grasscutter In: Vietmeyer ND, Ruskin FR, eds. Micro‐livestock: Little‐Known small Animals with a promising Economic Future. Washington, DC: National Academy Press; 1991;147‐155, 233–240. [Google Scholar]
- 53. Ogunsanmi AO, Ozegbe PC, Ogunjobi O, Taiwo V, Adu JO. Haematological, plasma biochemistry and whole blood minerals of the captive adult African grasscutter (Thryonomys swinderianus). Trop Vet. 2002;20:27‐35. [Google Scholar]
- 54. Byanet O, Adamu S, Salami SO, Obadiah H. Haematological and plasma biochemical parameters of the young grasscutter (Thyronomys swinderianus) reared in northern Nigeria. J Cell Anim Biol. 2008;2:177‐181. [Google Scholar]
- 55. Soro D, Karamoko Y, Kimse M, Fantodji A. Study of basic haematological parameters: indicators of the general state and immune competence in the male grasscutter (Thryonomys swinderianus, Temminck 1827) bred in captivity in Côte d'Ivoire. J Anim Plant Sci. 2014;22:3379‐3387. [Google Scholar]
- 56. Olude MA, Ogunbunmi TK, Olopade JO. A review of the published anatomical research on the African Giant Rat (Cricetomys gambianus Waterhouse). Bull Anim Health Prod Afr. 2013;61:617‐628. [Google Scholar]


