Abstract
Colorectal cancer is a worldwide health burden, with high incidence and mortality, especially in the advanced stages of the disease. Preclinical models are very important and valuable to discover and validate early and specific biomarkers as well as new therapeutic targets. In order to accomplish that, the animal models must replicate the clinical evolution of the disease in all of its phases. In this article, we review the existent mouse models, with their strengths and weaknesses in the replication of human cancer disease progression, with major focus on orthotopic models.
Keywords: colorectal cancer, mouse model, orthotopic model
1. INTRODUCTION
Colorectal cancer (CRC) is a major medical concern, being the third most common cancer type and the fourth most common cause of cancer‐related death, accounting for 9% of the total cancer incidence.1 In spite of the progress in clinical and biological knowledge, CRC remains a main public health issue,2 and despite the rapid development of treatments in the last years, the mortality rate related to this type of cancer remains high,3 especially in the advanced stages of disease.4 About half of the patients with CRC will develop liver metastases, and from that subpopulation only 25% are eligible for surgery with curative intent.5
The staging of CRC is the most important prognostic factor for survival, and when patients are in an advanced stage (with the development of metastasis), the prognosis is extremely poor and survival is estimated in months.6 Surgery and chemotherapy have been valuable allies in the treatment of CRC, and are able to treat 75% of patients, but more than 30% of these patients develop new neoplastic lesions, and 10% evolve to second malignancy.7
Nowadays it is clear that CRC is a heterogeneous disease that is caused by changes in different complex pathogenic pathways8 and molecular changes in a multistep carcinogenesis cascade that differ from tumor to tumor and reveal a wide range of clinical behaviors.9 At a molecular level, CRC is the tip of the iceberg of an intricate and ample array of gene alterations, affecting supramolecular processes.2
Therefore, it is understandable that CRC is a composite and multistep process, and when it is put together with recent discoveries in the CRC carcinogenesis, like the influence of the tumor microenvironment in primary and secondary tumor development and possible applications in CRC treatment,10, 11, 12, 13, 14, 15 together with the existence and influence of cancer stem cells in tumor progression, aggressiveness, and resistance to therapeutics,6, 16 new data, possibilities, and major dilemmas in CRC carcinogenesis must be taken into consideration.
So, the expansion of new strategies of screening that allow early and higher rates of CRC detection and development and creation of new preclinical models for the study of the CRC carcinogenesis process with the discovery of sensitive and specific biomarkers, not only for initial detection, but also to identify patients who will have disease recurrence and patients who will probably progress despite adjuvant therapy are critical and essentials steps in managing CRC.17, 18
2. WHY THE ANIMAL MODEL?
Despite differences in animals, their genomes are built on DNA, the chemical basis of life. By comparing the human genome with animal genome, it is possible to better understand the structure and function of human genes, and apply that knowledge to study human diseases in order to develop new strategies and mechanisms to prevent, detect, and treat CRC. In this context, several animals had their genome sequenced, such as the mouse (Mus musculus), the fruit fly (Drosophila melanogaster), and the malaria‐carrying mosquito (Anopheles gambiae), among others.19
Of all those animals, the mouse is the most used animal model in the study of carcinogenesis, with its use as the main model in biomedical research dating back to the beginning of the human civilization, when humans recorded coat‐color mutations for millennia. In 1700, in China and Japan, mice were domesticated as pets, and then imported by Europeans who bred them with their local varieties, creating hybrid progenitors of modern laboratory mice.20 Application of Mendel's law of inheritance to mice in an analogue way to the peas gave birth to new theories of inheritance, and with DNA recombinant technologies/DNA sequence‐based polymorphisms, new models were created.20
Although yeasts and flies reveled themselves as excellent models for studying cell cycle, mice are better models for studying the immune, endocrine, nervous, and other physiological systems, because their genetic and physiology are closer to those of humans. Like humans, mice have the ability to develop several diseases, such as cancer. In recent times, the use of innovative genetic technologies enabled the production of transgenic mice, with gene insertion in the germinal line, and even more advanced changes with “knock out” and “knock in” genes (eg, p53 knockout with disabled TP53 tumor suppressor gene), allied with state of the art reproductive technologies, provided the necessary tools for a deep study of carcinogenesis mechanisms in mice.21
An ideal animal CRC model should allow the development of local tumors, it should replicate all stages of CRC evolution, permit the assessment of the disease progression with radiology and endoscopic methods, (it should allow us to) understand the toxicities related with therapeutic procedures, and be largely adjustable to laboratories without surgical knowledge, enabling its replication.5, 22 However, the development of animal models is an arduous work, depending majorly on the strain of animals and cancer cells that are used.23
Therefore, mice are very valuable in cancer investigation and many transgenic mice were created in order to provide models to study the development and behavior of different types of tumors (gastric, pancreatic, bone, colon, etc). In this article, mouse CRC models will be discussed in detail, with special focus on orthotopic models.
3. WHAT TYPE OF CRC MODELS EXIST?
3.1. Sporadic CRC models/chemical‐induced models
Several models of mouse CRC have been developed along the time. Perhaps the first model developed was one that demonstrated the relation between intestinal tumor genesis and the ingestion of polycyclic aromatic hydrocarbon.24 At that time, the relation between CRC development and the ingestion of some types of food was under keen study, creating sporadic CRC models; more models were developed according to this idea: ingestion of radioactive yttrium25 of 4‐aminodephenyl and 3,2‐dimethyl‐4‐aminodiphenyl26 and 1,2‐dimethylhydrazine azoxymethane.27, 28, 29 In time, other carcinogens that induced colon tumors were discovered, such as heterocyclic and aromatic amines, and alkylnitrosamide compounds. A recent and one of the most used sporadic CRC models promotes CRC by chronic use of dextran sodium sulfate, recreating the conditions of an inflammatory bowel disease.30, 31
These models, despite their utility as pioneers in the first step of tumor carcinogenesis, are extremely limited, because they have low tumor development—only a fraction of the mice that experienced those conditions developed tumors, and when these develop, they reveal a widely variability in location, diffusion, and differentiation.32
The relation between exposure to carcinogens and tumor development is well‐know, and the above described models are still used, but it was necessary to create new and more “profitable” ones.
In order to overcome the low tumor development rate, a simple strategy was formulated. Instead of exposing the animals to carcinogenic elements, why not expose them directly to cancer cells? Why not study the behavior of cancer cells? And why not expose them to carcinogenic elements with in situ application? This train of thought gave birth to new type of cancer models which require surgical skill and the application of cancer elements (tissue/cells) directly in the animal as well as in vitro studies.
Many models were then created, developed, and improved by the discoveries and comprehension of CRC pathways and progression.
3.2. Cell culture models
One of the main and most used models is the human cancer cell lines model. Cell lines harvested from tumor tissue from patients with CRC are used to model the disease, and there are even cell lines established for this objective. Since the cells have origin in human tissue they provide high fidelity and allow various types of experiences relevant for human disease, and they are not very expensive—a major attractive quality. This model allows to mimic/to replicate tumor cells behavior in culture, providing further analysis of several aspects such as: aggregation, migration, colonies formation, responsiveness to therapeutics, and even to measure the production of intercellular messengers; it is also possible to evaluate parameters like oxidative stress, viability and apoptosis, cell cycle analysis, and determination of surface antigens.33, 34, 35, 36, 37
A major disadvantage of this model is the fact that it does not reproduce the tumor cell/environment interaction. The environment in culture is artificial and does not simulate the host response to tumor presence—immune response and angiogenesis.34 Also, tumor cells are maintained over many passages lacking intra‐tumoral heterogeneity and regularly showing few similarities, physiological and genetic, to the tumor from which they derive.38
Despite this drawback, the cell culture is a widely used model to study human carcinomas and a great complement to other models, but in order for the study to be accurate, the tumor must be preferentially studied in vivo.
3.3. Carcinogens in situ
A way to overcome the limitation of the sporadic cancer models with ingestion of carcinogens was to bypass the digestive tract, eliminating enzymatic alterations, and introduce them directly into the desired place—commonly by intrarectal exposure.38 This model reveals greater efficiency than sporadic CRC models, but the income on tumor formation is still far from the ideal. However, this model type had its efficiency greatly improved with the creation of genetic mouse models, but even so, in some cases, when the metastases are detected, the animals are too ill to expose them to therapeutic options.5
3.4. Peritoneum models
The injection of tumor cells into the peritoneum and peritoneal cavity was probably one of the first ideas in order to promote tumor development in mice and its study. It is still used nowadays leading to the development of tumor nodules on the peritoneum and dissemination through the peritoneal cavity.39
However, its biological behavior does not mimic/replicate the human CRC and other solid tumors, leaving the peritoneum models a tool for evaluating nonsolid tumors like leukemia's and to assess pharmacologic responses,40 since peritoneal metastatic carcinoma has worse prognosis compared with other metastasis and the treatments available did not achieve/have not achieved a good rate of effectiveness yet.41
3.5. Xenografts and orthotopic models
In the last decades, the xenograft model has been adopted as a way to bypass the limitations of the cell culture model. Tumor cell lines or suspensions are injected subcutaneously in mice, that in order to prevent tumor rejection usually are nude (unable to produce T cells) or have immune deficiency34—Figure 1. This type of model was a large success and nowadays it is widely used by researchers around the world to induce CRC in mice for carcinogenesis study and response to therapeutics.42, 43, 44 But like other models, the xenograft model has its limitations, one of them, the low metastatic capacity, reason that motivated some researchers to try new approaches like injection into the spleen, portal vein, and liver.45, 46, 47, 48, 49, 50, 51 The liver injection model does not represent a metastatic model but rather a heterotopic implantation of colon cancer cells and the spleen and portal vein injection models result in highly infiltrative tumors that impair the possibility of radiological characterization and treatment approaches.5
FIGURE 1.
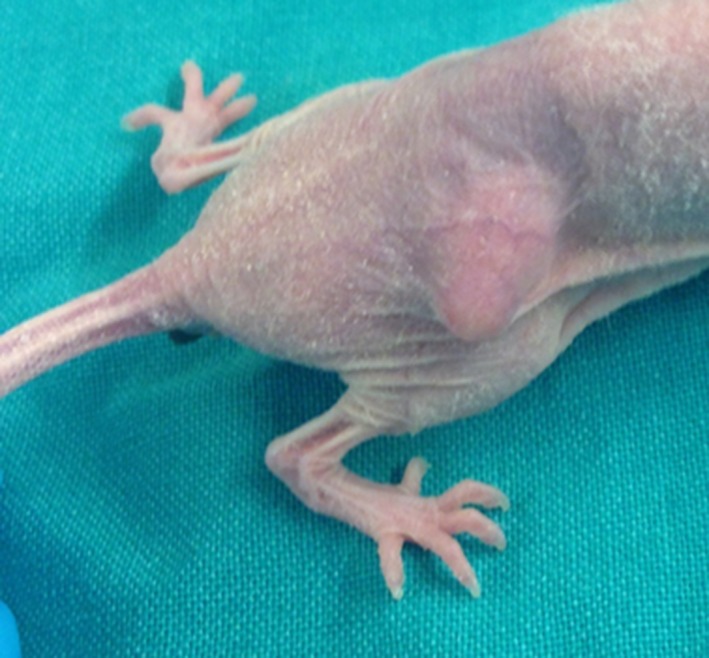
A mice exhibiting a subcutaneous heterotopic tumor—in this case, the cells were inoculated at the right side of dorsum
Other limitation of subcutaneous xenografts is the lack of reproduction of the tumor/microenvironment interaction—a well‐recognized element of predisposal to tumor indolent/aggressive behavior and distant metastases.11, 15, 52, 53, 54 The xenograft model allows the detection of cancer stem cells,3, 55, 56 but lacks the direct relation with local invasion and metastases. Despite its limitations, it is still amply used nowadays, especially in therapeutic studies.57
A way to overcome the lacunae in the previously described model was the development of a model where tumor cells are injected directly in the anatomical position of interest—thus giving birth to the orthotopic model, also in nude mice or with immune deficiency.34 Orthotopic models enhance the possibility of distant metastatic spread in a superior manner when compared with the subcutaneous models.58
In 1987 was created an orthotopic model of CRC in mice with injection of tumor cells in the ceacum, which enabled the study of local tumor invasion as well as metastatic dissemination—it was a more patient‐like animal tumor model.59 The success of this model was very high, turning it into a valuable asset in the study of the CRC, and amply used, with even some adaptations as injection of tumor cells in the rectum,60, 61, 62, 63, 64 and even exploring microvascular patterns of the colon concerning the differences between the mesenteric and antimesenteric side.32
The model was refined, with artificial selection of more aggressive CRC cells and the use of genetic engineering in order to create mice that were adequate to the studies.65 Finally, in 2009, with the improvement of surgical approaches and techniques, it was possible to create an orthotopic model recurring to a cecostomy surgical skill. This kind of model represented a major income/breakthrough due to the possibility of more sensitive tumor monitoring, real‐time visualization, and repeated tumor sampling66—Figure 2.
FIGURE 2.
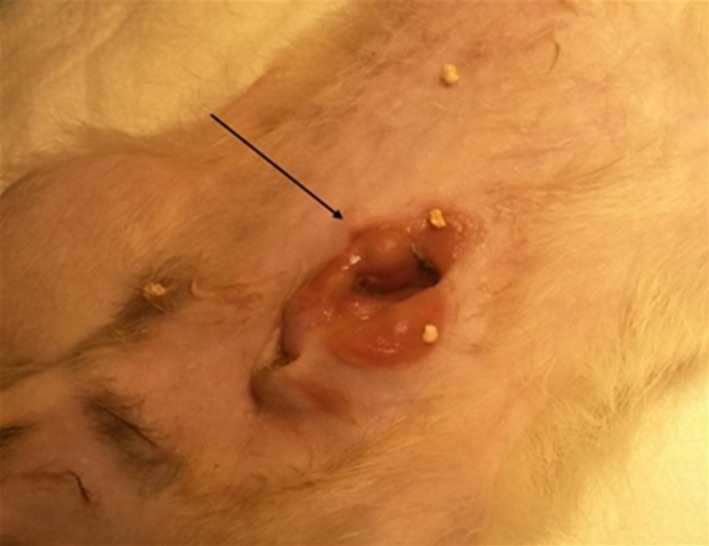
Colostomy with a nodular and submucosal lesion (black arrow), which on histological examination revealed an adenocarcinoma
It must be referred, though, that the use of surgical skills (ostomy creations) for cancer studying had already been tried in 1994 with a double colostomy in the transverse colon and application of the chemical‐induced model principles.67
It seemed that the optimal mouse model for the study of CRC had been found. However, at the light of recent genomic studies of the colon and differences between proximal, transverse, distal, sigmoid, and rectosigmoid components,68, 69, 70, 71, 72 conjugated with the already known embryologic, anatomic, and physiological differences,73, 74, 75, 76 the high percentage of tumor in the left side of the colon77, 78, 79, 80 and with the knowledge that only orthotopic models for the right colon were described, it is easily perceived the lack of a “left side orthotopic tumor” model and its detailed study.
Bearing this in mind, in 2012, an orthotopic model for distal colon carcinoma was created, able to develop a distal colon cancer in vivo, that on a histological level induced tumors remarkably similar with human colon cancer. It resorted on the implantation of CRC cells in the submucosa of the distal colon of animals previously submitted to a descending colostomy with mucosal‐cutaneous fistula of the sigmoid colon, avoiding a fatal colon stenosis. However, it did not record the existence of metastatic disease.81 This model was further refined in 2016, with the use of different CRC cellular lines (different colonic origins—ascending, descending, rectosigmoid) in a murine model, which led to the development of distinct morphophysiological characteristics of the primary tumor with neural invasion and cancer stem cells identification, also similar to those observed in human disease; it is a simple and reproducible model of distal colon cancer, that enables the study of genetic and molecular pathways of CRC, their interaction with the microenvironment, and the study of the metastatic process.82
Some groups bypassed surgical skills and perform injections of colon cancer cells in the rectum and used mechanical means, like metallic stents, to overcome obstruction, with metastatic development; however, the results did not have statistical significance.83 The orthotopic model is a very interesting approach to CRC studies, since on a local level, it is very similar to what happens on human tissue, replicating human disease with high reliability; the need of surgical skills may be a lesser drawback, but many research on the literature resorts to this method.84, 85, 86
Still, the injection method has its limitations, with tumor developing rates that can go from 60% to 70%, which can be explained by incorrect parietal injection of tumor cells, low viability of tumor cells, and host reaction to the cells.32 Also, the metastases may not reach the metastatic site of interest, and when they do, sometimes they take a huge amount of time.87
In the last years, there has been application of different techniques to orthotopic models, to improve the tumor development success rate, such as electrocoagulation, with apparent maximization of tumor development, both locally and distant.88 Another approach was the development of patients' derived xenografts (PDX), which consist in the graft of tumor from human patients into an immune‐deficient animal, replicating in this manner the human scenario.38 Several approaches were made with PDX subcutaneous and orthotopic engraftments, reporting development of secondary disease in the orthotopic location, with nuclear medicine techniques and histological confirmation.89, 90, 91 The PDX models allow a strong preservation of the tumoral and stromal architecture, with a high degree of fidelity to the donor tumor—microscopic, genetic, and functional.38, 90
Despite all the favorable characteristics, the model presents some major limitations: samples are usually taken from patients with highly advanced tumors92 and from patients who had already undergone cycles of chemotherapy90; there is also concern about the amount of viable tumor being engrafted and intra‐tumoral heterogeneity93 and also the various strains of mouse and grafting techniques used.90 Recently, a new drawback has been raised with the demonstration that the human stroma is usually replaced by the murine stroma, regardless of the maintenance of histological characteristics of the tumor,94, 95 and it occurs very rapidly in CRC PDX.96
A similar approach has been performed, but using syngraft/isograft models, which consist of grafting tumor fragments or cells derived from 1 mouse into a genetically similar inbred and immune competent mouse.97 This method would allow bypassing the two major limitations of the xenograft models—species mismatch and the stroma issue, however the model is not human and it is highly time consuming and laborious.98
3.6. Genetic engineered mouse models
The development of gene targeting provided the possibility of genetic models of CRC, with many advantages because of the availability of genetic information and easy gene manipulation,34 especially in those relevant for the carcinogenic process.58
The evolution of genetic engineering and genetic manipulation techniques enabled the creation of models capable of replicating genetic abnormalities that cause CRC, such as: hereditary nonpolyposis CRC,99, 100, 101 familial adenomatous polyposis102, 103 and improvement of the APC heterozygous model101 and the modifier of Min model.104, 105
For most tumor types, the genetic engineered mouse models (GEMM) can be used to study the human disease, and even to develop therapeutics; however, the models only possess evidence in the early stages of disease, having little evidence in the advanced stages.106 GEMM models do not replicate the native process of metastasis, showing lower grade of dissemination and thus, needing more time to metastasize, and when metastases occur, they have a high grade of variability and are less reproducible,107 and mostly depend on concomitant Kras and Pr3 mutations.108 Besides, some GEMM of CRC (with Apc mutations) usually develops small bowel and not colon tumors, and tumor burden usually diminishes the lifespan of the animal limiting malignant progression with the majority of them not reporting secondary disease.22, 98
Additional limitations to the GEMM models include more heterogeneity in human tumors, which may be explained by a more varied diet and different microbiome in humans and consequently more exposition to toxins,109 different time of exposition to toxins (chronic exposition in humans and more time limited in mouse), lack of population genetic heterogeneity in mouse due to inbred ,and the fact that in GEMM tumor arise by the same genetic mutation which limits the number of genetic tumor pathways.98
Genetic engineered mouse models are particularly effective for the study of initial phases of disease but have not replaced the xenograft models as research tools for treatment methodologies in metastatic disease.58
The addition of new methodologies such as CRISPR‐Cas9 technology has provided plasticity to genomic editing and is appointed as a very successfully tool to achieve metastatic disease, especially when associated with CRC organoids;108 however, it is not a tool available in the majority of the laboratories.
4. CONCLUSION/FINAL CONSIDERATIONS
For the last years, many types of mouse models have been developed in order to study CRC. In the age of the genetic information and genetic manipulation techniques, it would seem that the GEMM models would be the most suitable for the task, but they have major limitations in the study of the later stages of the disease and in the metastatic process.
Of all the available models, the orthotopic model seems to have the “leading position” in cancer study because it allows the study of the tumoral microenvironment in vivo and the metastatic process. Because of the different characteristics of the colon segments, the creation of a model to study the carcinogenesis of the left colon is of major importance and presents a solution to the technical limitations of the other CRC models.
Each animal model has its pros and cons—Table 1, and in some cases, association of more than one form in CRC induction is necessary.110 The model should be chosen accordingly to the study expectations and aim, maximizing its potential.111
TABLE 1.
Comparison between the different mouse models, with advantages and drawbacks
| Animal model | Advantages | Drawbacks |
|---|---|---|
| Sporadic/chemical induced |
|
|
| Carcinogens in situ |
|
|
| Peritoneum models |
|
|
| Subcutaneous xenografts |
|
|
| Orthotopic xenografts |
|
|
| Syngenic |
|
|
| Genetic engineered mouse models |
|
|
Translational investigation for CRC is increasing, due to the high demand of proper models to study the complexity of in vivo biological behaviors. The application of the “left colon model” and its deep study will definitely bring new considerations about the carcinogenesis of rectosigmoid tumors, helping to unveil the pathways of metastization of CRC, the main cause of death in humans with the disease.
Oliveira RC, Abrantes AM, Tralhão JG, Botelho MF. The role of mouse models in colorectal cancer research—The need and the importance of the orthotopic models. Animal Model Exp Med. 2020;3:1–8. 10.1002/ame2.12102
REFERENCES
- 1. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grizzi F, Di Caro G, Marchesi F, Laghi L. Prognostic Value of Innate and Adaptive Immunity in Cancers In: Cancer Immunology: A Translational Medicine Context. Berlin, Heidelberg: Springer; 2015:275‐284. [Google Scholar]
- 3. Chen S, Song X, Chen Z, et al. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta‐analysis. PLoS One. 2013;8(2):e56380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skirrow C, Cross JH, Harrison S, et al. Temporal lobe surgery in childhood and neuroanatomical predictors of long‐term declarative memory outcome. Brain. 2015;138(1):80‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. White SB, Procissi D, Chen J, et al. Characterization of CC‐531 as a rat model of colorectal liver metastases. PLoS One. 2016;11(5):e0155334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langan RC, Mullinax JE, Raiji MT, et al. Colorectal cancer biomarkers and the potential role of cancer stem cells. J Cancer. 2013;4(3):241‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roy S, Majumdar AP. Cancer stem cells in colorectal cancer: genetic and epigenetic changes. J Stem Cell Res Ther. 2012;Suppl 7(6):10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schweiger MR, Hussong M, Röhr C, Lehrach H. Genomics and epigenomics of colorectal cancer. Wiley Interdiscip Rev Syst Biol Med. 2013;5(2):205‐219. [DOI] [PubMed] [Google Scholar]
- 9. Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu H, Sun L, Guo C, et al. Tumor cell‐microenvironment interaction models coupled with clinical validation reveal CCL2 and SNCG as two predictors of colorectal cancer hepatic metastasis. Clin Cancer Res. 2009;15(17):5485‐5493. [DOI] [PubMed] [Google Scholar]
- 11. Luca AC, Mersch S, Deenen R, et al. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One. 2013;8(3):e59689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang H, Verovski VN, Leonard W, et al. Hepatocytes determine the hypoxic microenvironment and radiosensitivity of colorectal cancer cells through production of nitric oxide that targets mitochondrial respiration. Int J Radiat Oncol Biol Phys. 2013;85(3):820‐827. [DOI] [PubMed] [Google Scholar]
- 13. Belluco C, Mammano E, Petricoin E, et al. Kinase substrate protein microarray analysis of human colon cancer and hepatic metastasis. Clin Chim Acta. 2005;357(2):180‐183. [DOI] [PubMed] [Google Scholar]
- 14. Achyut BR, Yang L. Transforming growth factor‐β in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141(4):1167‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu Y, Brodt P, Sun H, et al. Insulin‐like growth factor‐I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010;70(1):57‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li L, Cole J, Margolin DA. Cancer stem cell and stromal microenvironment. Ochsner J. 2013;13(1):109‐118. [PMC free article] [PubMed] [Google Scholar]
- 17. Carrato A. Adjuvant treatment of colorectal cancer. Gastrointest Cancer Res. 2008;2(4 Suppl):S42‐S46. [PMC free article] [PubMed] [Google Scholar]
- 18. Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134(5):1296‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spencer G. Background on Comparative Genomic Analysis—National Human Genome Research Institute (NHGRI). National Human Genome Research Institute. 2012. https://www.genome.gov/10005835/ Accessed December 01, 2019. [Google Scholar]
- 20. Background on the History of the Mouse – National Human Genome Research Institute (NHGRI). https://www.genome.gov/10005832/background-on-the-history-of-the-mouse/ Accessed October 28, 2018. [Google Scholar]
- 21. Spencer G. Background on Mouse as a Model Organism – National Human Genome Research Institute (NHGRI). National Human Genome Research Institute. 2002. https://www.genome.gov/10005834/background-on-mouse-as-a-model-organism/ Accessed December 01, 2019. [Google Scholar]
- 22. O'Rourke KP, Loizou E, Livshits G, et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat Biotechnol. 2017;35(6):577‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fidler IJ, Kripke ML. The challenge of targeting metastasis. Cancer Metastasis Rev. 2015;34(4):635‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lorenz E, Stewart HL. Intestinal carcinoma and other lesions in mice following oral administration of 1,2,5,6‐dibenzanthracene and 20‐methylcholanthrene. J Natl Cancer Inst. 1940;1(1):17‐40. [Google Scholar]
- 25. Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30(2):183‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walpole AL, Williams MHC, Roberts DC. The carcinogenic action of 4‐aminodiphenyl and 3:2′‐dimethyl‐4‐amino‐diphenyl. Br J Ind Med. 1952;9(4):255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laqueur GL, Mickelsen O, Whiting MG, Kurland LT. Carcinogenic properties of nuts from Cycas circinalis l. Indigenous to guam. J Natl Cancer Inst. 1963;31:919‐951. [PubMed] [Google Scholar]
- 28. Laqueur GL. Carcinogenic effects of cycad meal and cycasin, methylazoxymethanol glycoside, in rats and effects of cycasin in germfree rats. Fed Proc. 1964;23:1386‐1388. [PubMed] [Google Scholar]
- 29. Druckrey H, Preussmann R, Matzkies F, Ivankovic S. Selective production of intestinal cancer in rats by 1,2‐dimethylhydrazine. Naturwissenschaften. 1967;54(11):285‐286. [DOI] [PubMed] [Google Scholar]
- 30. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694‐702. [DOI] [PubMed] [Google Scholar]
- 31. Okayasu I, Yamada M, Mikami T, Yoshida T, Kanno J, Ohkusa T. Dysplasia and carcinoma development in a repeated dextran sulfate sodium‐induced colitis model. J Gastroenterol Hepatol. 2002;17(10):1078‐1083. [DOI] [PubMed] [Google Scholar]
- 32. Boni L, Benevento A, Dionigi G, Rovera F, Diurni M, Dionigi R. Injection of colorectal cancer cells in mesenteric and antimesenteric sides of the colon results in different patterns of metastatic diffusion: An experimental study in rats. World J Surg Oncol. 2005;3:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nautiyal J, Banerjee S, Kanwar SS, et al. Curcumin enhances dasatinib‐induced inhibition of growth and transformation of colon cancer cells. Int J Cancer. 2011;128(4):951‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Young M, Ordonez L, Clarke AR. What are the best routes to effectively model human colorectal cancer? Mol Oncol. 2013;7(2):178‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kannen V, Hintzsche H, Zanette DL, et al. Antiproliferative effects of fluoxetine on colon cancer cells and in a colonic carcinogen mouse model. PLoS One. 2012;7(11):e50043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bradshaw‐Pierce EL, Pitts TM, Tan A‐C, et al. Tumor P‐glycoprotein correlates with efficacy of PF‐3758309 in in vitro and in vivo models of colorectal cancer. Front Pharmacol. 2013;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McKinley ET, Smith RA, Zhao P, et al. 3′‐deoxy‐3′‐18F‐fluorothymidine PET predicts response to V600EBRAF‐targeted therapy in preclinical models of colorectal cancer. J Nucl Med. 2013;54(3):424‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown KM, Xue A, Mittal A, Samra JS, Smith R, Hugh TJ. Patient‐derived xenograft models of colorectal cancer in preclinical research: a systematic review. Oncotarget. 2016;7(40):66212‐66225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schoffelen R, van der Graaf WTA, Sharkey RM, et al. Quantitative immuno‐SPECT monitoring of pretargeted radioimmunotherapy with a bispecific antibody in an intraperitoneal nude mouse model of human colon cancer. J Nucl Med. 2012;53(12):1926‐1932. [DOI] [PubMed] [Google Scholar]
- 40. Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170(3):793‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qiu C, Li Y, Liang X, et al. A study of peritoneal metastatic xenograft model of colorectal cancer in the treatment of hyperthermic intraperitoneal chemotherapy with Raltitrexed. Biomed Pharmacother. 2017;92:149‐156. [DOI] [PubMed] [Google Scholar]
- 42. de la Cueva A, Ramírez de Molina A, Álvarez‐Ayerza N, et al. Combined 5‐FU and ChoKα inhibitors as a new alternative therapy of colorectal cancer: evidence in human tumor‐derived cell lines and mouse xenografts. PLoS One. 2013;8(6):e64961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abuqayyas L, Balthasar JP. Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer. J Pharmacokinet Pharmacodyn. 2012;39(6):683‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bradshaw‐Pierce EL, Pitts TM, Kulikowski G, et al. Utilization of quantitative in vivo pharmacology approaches to assess combination effects of everolimus and irinotecan in mouse xenograft models of colorectal cancer. PLoS One. 2013;8(3):e58089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim C, Broqueres‐You D, Brouland J‐P, et al. Hepatic ischemia‐reperfusion increases circulating bone marrow‐derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J Surg Res. 2013;184(2):888‐897. [DOI] [PubMed] [Google Scholar]
- 46. Shen F, Li JL, Cai WS, et al. Interleukin‐12 prevents colorectal cancer liver metastases in mice. Onco Targets Ther. 2013;6:523‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang X, Zou Y, Lian L, et al. Changes of T cells and cytokines TGF‐β1 and IL‐10 in mice during liver metastasis of colon carcinoma: implications for liver anti‐tumor immunity. J Gastrointest Surg. 2013;17(7):1283‐1291. [DOI] [PubMed] [Google Scholar]
- 48. Young GC, Wise BS, Ayvazian SG. A tagging study on tailor (Pomatomus saltatrix) in Western Australian waters: their movement, exploitation, growth and mortality. Mar Freshw Res. 1999;50(7):633‐642. [Google Scholar]
- 49. Thalheimer A, Otto C, Bueter M, et al. Tumor cell dissemination in a human colon cancer animal model: orthotopic implantation or intraportal injection? Eur Surg Res. 2009;42(3):195‐200. [DOI] [PubMed] [Google Scholar]
- 50. Frampas E, Maurel C, Thedrez P, Remaud‐Le Saëc P, Faivre‐Chauvet A, Barbet J. The intraportal injection model for liver metastasis. Nucl Med Commun. 2011;32(2):147‐154. [DOI] [PubMed] [Google Scholar]
- 51. Schuh JC. Trials, tribulations, and trends in tumor modeling in mice. Toxicol Pathol. 2004;32(Suppl 1):53‐66. [DOI] [PubMed] [Google Scholar]
- 52. Jobin C. Colorectal cancer: CRC—all about microbial products and barrier function? Nat Rev Gastroenterol Hepatol. 2012;9(12):694‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kruse J, Von Bernstorff W, Evert K, et al. Macrophages promote tumour growth and liver metastasis in an orthotopic syngeneic mouse model of colon cancer. Int J Colorectal Dis. 2013;28(10):1337‐1349. [DOI] [PubMed] [Google Scholar]
- 54. Xu Q, Guo L, Gu X, et al. Prevention of colorectal cancer liver metastasis by exploiting liver immunity via chitosan‐TPP/nanoparticles formulated with IL‐12. Biomaterials. 2012;33(15):3909‐3918. [DOI] [PubMed] [Google Scholar]
- 55. Gao W, Chen LU, Ma Z, et al. Isolation and phenotypic characterization of colorectal cancer stem cells with organ‐specific metastatic potential. Gastroenterology. 2013;145(3):636‐646.e5. [DOI] [PubMed] [Google Scholar]
- 56. Gemei M, Mirabelli P, Di Noto R, et al. CD66c is a novel marker for colorectal cancer stem cell isolation, and its silencing halts tumor growth in vivo. Cancer. 2013;119(4):729‐738. [DOI] [PubMed] [Google Scholar]
- 57. Mittal VK, Singh Bhullar J, Kumar J. Animal models of human colorectal cancer: current status, uses and limitations. World J Gastroenterol. 2015;21(41):11854‐11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Francia G, Cruz‐Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11(2):135‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guerreiro ADS, Rola RC, Rovani MT, Costa SRD, Sandrini JZ. Antifouling biocides: impairment of bivalve immune system by chlorothalonil. Aquat Toxicol. 2017;189:194‐199. [DOI] [PubMed] [Google Scholar]
- 60. Metildi CA, Kaushal S, Snyder CS, Hoffman RM, Bouvet M. Fluorescence‐guided surgery of human colon cancer increases complete resection resulting in cures in an orthotopic nude mouse model. J Surg Res. 2013;179(1):87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cohen G, Lecht S, Arien‐Zakay H, et al. Bio‐imaging of colorectal cancer models using near infrared labeled epidermal growth factor. PLoS One. 2012;7(11):e48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Devaud C, Rousseau B, Netzer S, et al. Anti‐metastatic potential of human Vδ1+ γδ T cells in an orthotopic mouse xenograft model of colon carcinoma. Cancer Immunol Immunother. 2013;62(7):1199‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen Q, Chen L, Zhao R, Yang XD, Imran K, Xing CG. Microarray analyses reveal liver metastasis‐related genes in metastatic colorectal cancer cell model. J Cancer Res Clin Oncol. 2013;139(7):1169‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chowdhury S, Ongchin M, Wan G, Sharratt E, Brattain MG, Rajput A. Restoration of PTEN activity decreases metastases in an orthotopic model of colon cancer. J Surg Res. 2013;184(2):755‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Washington MK, Powell AE, Sullivan R, et al. Pathology of rodent models of intestinal cancer: progress report and recommendations. Gastroenterology. 2013;144(4):705‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jin H, Yang Z, Wang J, Zhang S, Sun Y, Ding Y. A superficial colon tumor model involving subcutaneous colon translocation and orthotopic transplantation of green fluorescent protein‐expressing human colon tumor. Tumor Biol. 2011;32(2):391‐397. [DOI] [PubMed] [Google Scholar]
- 67. Zhang J, Lam LKT. Colonoscopic colostomy model in rats for colon tumorigenesis studies. Carcinogenesis. 1994;15(8):1571‐1576. [DOI] [PubMed] [Google Scholar]
- 68. Birkenkamp‐Demtroder K, Olesen SH, Sørensen FB, et al. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005;54(3):374‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Glebov OK, Rodriguez LM, Nakahara K, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12(8):755‐762. [PubMed] [Google Scholar]
- 70. Frattini M, Balestra D, Suardi S, et al. Different genetic features associated with colon and rectal carcinogenesis. Clin Cancer Res. 2004;10(12):4015–4021. [DOI] [PubMed] [Google Scholar]
- 71. Minoo P, Zlobec I, Peterson M, Terracciano L, Lugli A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int J Oncol. 2010;37(3):707‐718. [DOI] [PubMed] [Google Scholar]
- 72. Slattery ML, Curtin K, Wolff RK, et al. A comparison of colon and rectal somatic DNA alterations. Dis Colon Rectum. 2009;52(7):1304‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ferretti G, Felici A, Cognetti F, Mandala M. Is there a right‐sided shift for colorectal cancer in women compared with men? Cancer Epidemiol Biomarkers Prev. 2006;15(5):1054. [DOI] [PubMed] [Google Scholar]
- 74. Gervaz P, Bucher P, Morel P. Two colons‐two cancers: paradigm shift and clinical implications. J Surg Oncol. 2004;88(4):261‐266. [DOI] [PubMed] [Google Scholar]
- 75. Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10(3):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403‐408. [DOI] [PubMed] [Google Scholar]
- 77. Milne D. Right or left, right or wrong? debate whirls over colorectal cancer distribution. J Natl Cancer Inst. 1994;86(19):1442‐1443. [DOI] [PubMed] [Google Scholar]
- 78. Pocard M, Salmon RJ, Muleris M, et al. Two colons–two cancers? Proximal or distal adenocarcinoma: arguments for a different carcinogenesis. Bull Cancer. 1995;82(1):10‐21. [PubMed] [Google Scholar]
- 79. Distler P, Holt PR. Are right‐ and left‐sided colon neoplasms distinct tumors? Dig Dis. 1997;15(4‐5):302‐311. [DOI] [PubMed] [Google Scholar]
- 80. Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right‐ versus left‐sided colon cancers? Ann Surg Oncol. 2008;15(9):2388‐2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Priolli DG, Abrantes AM, Neves S, Batista JN, Cardinalli IA, Botelho MF. Novo modelo de câncer de cólon distal em camundongos atímicos. Acta Cir Bras. 2012;27(6):355‐360. [DOI] [PubMed] [Google Scholar]
- 82. Caetano‐Oliveira R, Gomes MA, Abrantes AM, et al. Revisiting colorectal cancer animal model—an improved metastatic model for distal rectosigmoid colon carcinoma. Pathophysiology. 2018;25(2):89‐99. [DOI] [PubMed] [Google Scholar]
- 83. Malgras B, Brullé L, Lo Dico R, et al. Insertion of a stent in obstructive colon cancer can induce a metastatic process in an experimental murine model. Ann Surg Oncol. 2015;22(Suppl 3):S1475‐S1480. [DOI] [PubMed] [Google Scholar]
- 84. Hite N, Klinger A, Hellmers L, et al. An optimal orthotopic mouse model for human colorectal cancer primary tumor growth and spontaneous metastasis. Dis Colon Rectum. 2018;61(6):698‐705. [DOI] [PubMed] [Google Scholar]
- 85. Liao HW, Hung MC. Intracaecal orthotopic colorectal cancer xenograft mouse model. Bio Protoc. 2017;7(11):e2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tseng W, Leong X, Engleman E. Orthotopic mouse model of colorectal cancer. J Vis Exp. 2007;10:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ellis L, Lehet K, Ku SY, Azabdaftari G, Pili R. Generation of a syngeneic orthotopic transplant model of prostate cancer metastasis. Oncoscience. 2014;1(10):609‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bhullar JS, Subhas G, Silberberg B, et al. A novel nonoperative orthotopic colorectal cancer murine model using electrocoagulation. J Am Coll Surg. 2011;213(1):54‐60. [DOI] [PubMed] [Google Scholar]
- 89. Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. 2015;163(1):39‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Puig I, Chicote I, Tenbaum SP, et al. A personalized preclinical model to evaluate the metastatic potential of patient‐derived colon cancer initiating cells. Clin Cancer Res. 2013;19(24):6787‐6801. [DOI] [PubMed] [Google Scholar]
- 91. Hoffman RM. Patient‐derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 2015;15(8):451‐452. [DOI] [PubMed] [Google Scholar]
- 92. Dangles‐Marie V, Pocard M, Richon S, et al. Establishment of human colon cancer cell lines from fresh tumors versus xenografts: comparison of success rate and cell line features. Cancer Res. 2007;67(1):398‐407. [DOI] [PubMed] [Google Scholar]
- 93. Hidalgo M, Amant F, Biankin AV, et al. Patient‐derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Isella C, Terrasi A, Bellomo SE, et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet. 2015;47(4):312‐319. [DOI] [PubMed] [Google Scholar]
- 95. Rosfjord E, Lucas J, Li G, Gerber HP. Advances in patient‐derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 2014;91(2):135‐143. [DOI] [PubMed] [Google Scholar]
- 96. Julien S, Merino‐Trigo A, Lacroix L, et al. Characterization of a large panel of patient‐derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18(19):5314‐5328. [DOI] [PubMed] [Google Scholar]
- 97. Stenholm S, Tiainen K, Rantanen T, et al. Long‐term determinants of muscle strength decline: prospective evidence from the 22‐year Mini‐Finland follow‐up survey. J Am Geriatr Soc. 2012;60(1):77‐85. [DOI] [PubMed] [Google Scholar]
- 98. Mcintyre RE, Buczacki SJA, Arends MJ, Adams DJ. Mouse models of colorectal cancer as preclinical models. BioEssays. 2015;37(8):909‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kuraguchi M, Yang K, Wong E, et al. The distinct spectra of tumor‐associated Apc mutations in mismatch repair‐deficient Apc1638N mice define the roles of MSH3 and MSH6 in DNA repair and intestinal tumorigenesis. Cancer Res. 2001;61(21):7934‐7942. [PubMed] [Google Scholar]
- 100. Casorelli I, Pannellini T, De Luca G, et al. The Mutyh base excision repair gene influences the inflammatory response in a mouse model of ulcerative colitis. PLoS One. 2010;5(8):e12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Orner GA, Dashwood WM, Blum CA, Díaz GD, Li Q, Dashwood RH. Suppression of tumorigenesis in the Apcmin mouse: down‐regulation of β‐catenin signaling by a combination of tea plus sulindac. Carcinogenesis. 2003;24(2):263‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cooper K, Squires H, Carroll C, et al. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2010;14(32):1‐205. [DOI] [PubMed] [Google Scholar]
- 103. Albuquerque C, Breukel C, van der Luijt R, et al. The “just‐right” signaling model: APC somatic mutations are selected based on a specific level of activation of the beta‐catenin signaling cascade. Hum Mol Genet. 2002;11(13):1549‐1560. [DOI] [PubMed] [Google Scholar]
- 104. Petrova TV, Nykänen A, Norrmén C, et al. Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell. 2008;13(5):407‐419. [DOI] [PubMed] [Google Scholar]
- 105. Dopeso H, Mateo‐Lozano S, Mazzolini R, et al. The receptor tyrosine kinase EPHB4 has tumor suppressor activities in intestinal tumorigenesis. Cancer Res. 2009;69(18):7430‐7438. [DOI] [PubMed] [Google Scholar]
- 106. Heijstek MW, Kranenburg O, Borel Rinkes IHM. Mouse models of colorectal cancer and liver metastases. Dig Surg. 2005;22(1‐2):16‐25. [DOI] [PubMed] [Google Scholar]
- 107. Taketo MM, Edelmann W. Mouse models of colon cancer. Gastroenterology. 2009;136(3):780‐798. [DOI] [PubMed] [Google Scholar]
- 108. Romano G, Chagani S, Kwong LN. The path to metastatic mouse models of colorectal cancer. Oncogene. 2018;37(19):2481‐2489. [DOI] [PubMed] [Google Scholar]
- 109. Nguyen TLA, Vieira‐Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. De‐souza ASC, Costa‐casagrande TA. Animal models for colorectal cancer. Arq Bras Cir Dig. 2018;31(2):e1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Evans JP, Sutton PA, Winiarski BK, et al. From mice to men: murine models of colorectal cancer for use in translational research. Crit Rev Oncol Hematol. 2016;98:94‐105. [DOI] [PubMed] [Google Scholar]


