Abstract
Enterovirus D68 (EV-D68) is a respiratory viral pathogen that primarily infects children under the age of 8. Although EV-D68 infection typically leads to moderate to severe respiratory illnesses, recent years have seen increasing cases of EV-D68 triggered neurological complications such as the acute flaccid myelitis (AFM). There is currently no vaccine or antiviral available for EV-D68, we therefore aimed to develop potent and specific small molecule antivirals against EV-D68. In this study, we report our discovery of a viral capsid inhibitor R856932 that inhibits multiple contemporary EV-D68 strains with single-digit to submicromolar efficacy. Mechanistic studies have shown that the tetrazole compound R856932 binds to the hydrophobic pocket of viral capsid protein VP1, thereby preventing viral uncoating and releasing of viral genome in the infected cells. The mechanism of action of R856932 was confirmed by time-of-addition, western blot, RT-qPCR, viral heat inactivation, serial viral passage and reverse genetics experiments. A single mutation located at VP1, A129V, confers resistance against R856932. However, recombination virus encoding VP1-A129V appeared to have compromised fitness of replication compared to the wild type EV-D68 virus as shown by the competition growth assay. Overall, the hit compound identified in this study, R856932, represents a promising starting point with a confirmed mechanism of action that can be further developed into EV-D68 antivirals.
Keywords: EV-D68, antiviral, capsid inhibitor, enterovirus, pleconaril
Graphical Abstract
INTRODUCTION
Enterovirus D68 (EV-D68) is a non-enveloped, positive-sense, and single-stranded RNA virus that causes moderate to severe respiratory illnesses in children.1–3 EV-D68 belongs to the enterovirus genus of Piconaviridae family. The enterovirus genus also contains many other important human pathogens, including poliovirus, enterovirus A71 (EV-A71), rhinovirus, and coxsackievirus.2, 4 EV-D68 was previously mis-classified as rhinovirus 87 due to their similar route of infection and clinical manifestations. However, genetic studies have shown that it is more closely related to enterovirus. EV-D68 has two distinct features compared to other viruses in the enterovirus genus:5 1) EV-D68 is cold-adapted and prefers a lower growth temperature (33°C) instead of the regular body temperature (37°C); 2) EV-D68 is acid sensitive,6 which explains why it cannot infect through the gastrointestinal tract as other enteroviruses, possibly due to the low pH in the gut.
EV-D68 was first isolated in California in 1962, and remained a neglected rare disease until 2014.7 EV-D68 came to the public attention when recent outbreaks coincided with neurological complications.1, 4, 8 Several outbreaks occurred in the United States as well as other parts of the world since 2014, and there appears to be a biennial pattern of EV-D68 outbreak since then.9, 10 This virus seems to be evolving over time, leading to more severe pathogenicity.8, 9, 11, 12 For example, infection with contemporary EV-D68 virus was linked to neurological complications, and children infected with EV-D68 had symptoms like muscle weakness, paralysis and even death in the severe cases.13, 14 Recent study has shown that contemporary EV-D68 strains (US/KY/14), but not historical EV-D68 strains (Fermon), were able to infect neuronal cells and cause cytopathic effect.11 In mouse model studies, EV-D68 could be isolated from central nervous system (CNS) tissues including spinal cord, brain, and cerebral fluid.15–17 Although EV-D68 virus was rarely identified in the CNS tissues in human patients, there is nevertheless a strong correlation between EV-D68 infection and neurological complications as shown by a number of studies.8, 18
Despite the severe disease outcome posed by EV-D68, there is currently no antiviral or vaccine available,2 and current treatment is limited to supportive care. Several compounds were reported in the literature that inhibit EV-D68 amplification in cell culture, and many of the compounds tested were either originally developed as rhinovirus antivirals or repurposed from approved drugs.19–21 Unfortunately, many of the reported antivirals against EV-D68 either have moderate potency or concerning side effects.16, 21, 22 Therefore, it is imperative to develop potent and specific EV-D68 antivirals for the prophylaxis and treatment of EV-D68 infection.23, 24
EV-D68 is a non-enveloped virus that is surrounded by viral capsid proteins.25 The virion capsid consists of four proteins: VP1, VP2, VP3, and VP4. A concave pocket is formed in VP1 and it is important for cell receptor binding. The concave pocket is frequently referred as canyon. Small molecules such as pleconaril that are capable of binding to this canyon can stabilize the capsid protein and prevent viral uncoating, leading to inhibition of viral replication.25, 26 As such, the VP1 protein is a validated drug target. In this study, we report our discovery of an EV-D68 specific capsid inhibitor R856932 that was identified through a high-throughput screening. Mechanistic studies have shown that R856932 inhibits the early stage of viral replication by blocking viral attachment to the host cell and protects virion against thermal denaturation. Additional evidences supporting the proposed mechanism of action include the results from serial viral passage and reverse genetics experiments in which a single mutation VP1-A129V located at the drug-binding site (canyon) led to reduced drug sensitivity. Although resistance could be selected against the capsid inhibitor R856932 through serial viral passage experiments, the resistant mutant appears to compromise the fitness of viral replication compared to the wild-type (WT) EV-D68 virus as shown by the competition growth assay. Collectively, the compound R856932 reported herein represents a novel EV-D68 capsid inhibitor, and further development of R856932 might lead to promising EV-D68 antivirals. They can be used either alone or in combination with other direct-acting antivirals to combat EV-D68 infections.
RESULTS AND DISCUSSION
R856932 inhibits various EV-D68 strains, but not EV-A71 in vitro
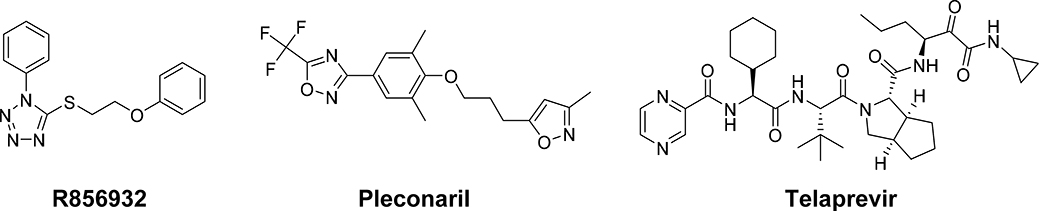
In searching for potent EV-D68 antivirals, we carried out a cell-based cytopathic effect (CPE) screening assay against EV-D68 US/MO/14–18947 strain in rhabdomyosarcoma (RD) cell. US/MO/14–18947 was chosen because of its clinical significance: it is a member of the B1 clade, the predominant circulating EV-D68 clade in 2014 that has been shown to be associated with AFM.13 Compound R856932 (Fig. 1) showed complete protection of RD cells against EV-D68 infection at 15 μM. To confirm the results, R856932 was re-synthesized and titrated to determine the antiviral EC50 value and cellular cytotoxicity CC50 value. It was found that R856932 inhibited EV-D68 US/MO/14–18947 in RD cells with an EC50 value of 0.46 μM and a CC50 value of 32.0 μM (Table 1). The antiviral efficacy of R856932 was further confirmed in four additional contemporary EV-D68 strains with EC50 values ranging from 1.40 to 4.36 μM. Previously reported EV-D68 antivirals pleconaril 25, 27 and telaprevir 23 (Fig. 1) were included as controls and were found to be active against all the EV-D68 strains as well. However, when tested against two EV-A71 strains, compound R856932 was not active, neither were the two control compounds. These results suggest that the antiviral activity of R856932 is specific to EV-D68.
Figure 1.
Chemical structures of R856932, pleconaril, and telaprevir. Pleconaril is a known EV-D68 viral VP1 capsid binding inhibitor,25 and telaprevir is a EV-D68 2A protease inhibitor.23
Table 1.
Antiviral efficacy and cellular cytotoxicity of R856932 against EV-D68 and EV-A71 in RD cells.a
| Viruses | R856932 CC50 = 32.0 ± 3.4 (μM) | Pleconaril CC50 = 14.1 ± 4.1 (μM) | Telaprevir CC50 = 46.2 ± 14.3 (μM) | |||
|---|---|---|---|---|---|---|
| EC50 (μM) | SI | EC50 (μM) | SI | EC50 (μM) | SI | |
| EV-D68 US/MO/14–18947 (clade B1) | 0.46 ± 0.09 | 70 | 0.03 ± 0.01 | 470 | 0.86 ± 0.07 | 54 |
| EV-D68 US/MO/14–18949 (clade B1) | 1.40 ± 0.04 | 22.9 | 0.15 ± 0.01 | 94 | 0.50 ± 0.04 | 92 |
| EV-D68 US/IL/14–18952 (clade B2) | 4.39 ± 0.21 | 7.29 | 0.14 ± 0.03 | 101 | 1.54 ± 0.07 | 30.0 |
| EV-D68 US/IL/14–18956 (clade B2) | 1.77 ± 0.05 | 18.1 | 0.19 ± 0.02 | 74 | 0.44 ± 0.00 | 105 |
| EV-D68 US/KY/14–18953 (clade D) | 3.34 ± 0.31 | 9.58 | 0.09 ± 0.02 | 157 | 0.39 ± 0.05 | 118 |
| EV-A71 Tainan/4643/1998 | >50 | N.A. | >5 | N.A. | >15 | N.A. |
| EV-A71 MP4 | >50 | N.A. | >5 | N.A. | >15 | N.A. |
The EC50 values of different strains of EV-D68 and EV-A71 in RD cells were determined using a cytopathic effect (CPE) assay. The CC50 values were determined by a CPE assay in RD cells after 3 days of drug incubation. EC50 = mean ± standard deviation. SI: Selection Index; N.A.: Not Available; The values are the mean ± standard deviation from three replicates.
R856932 inhibits viral RNA and protein synthesis
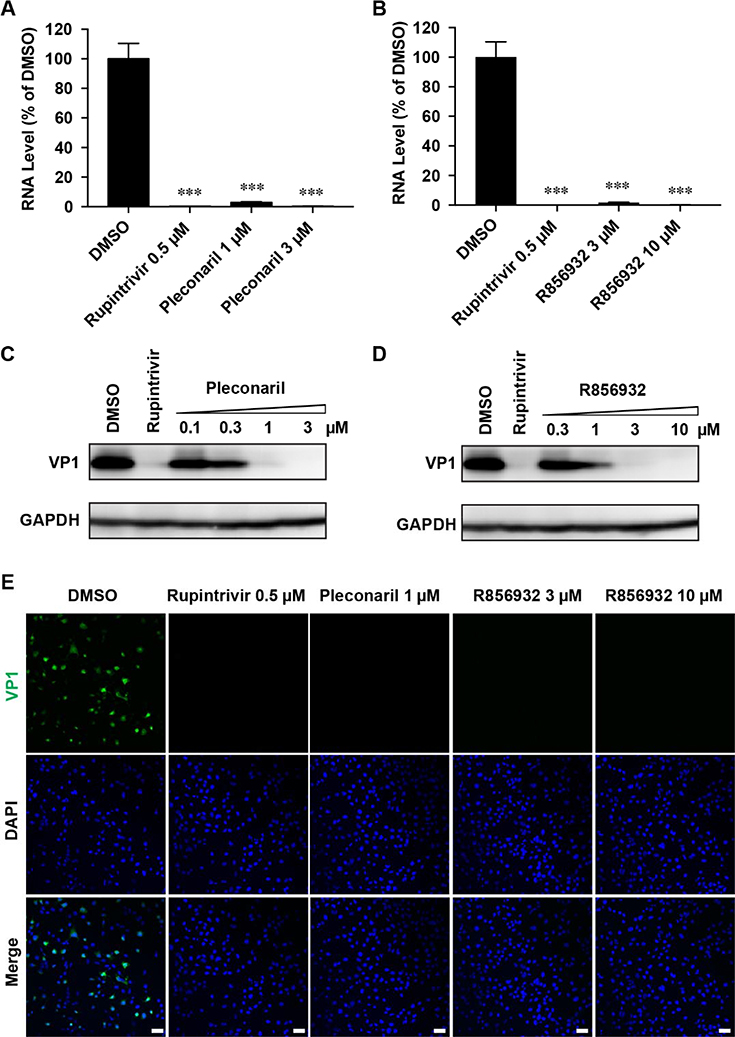
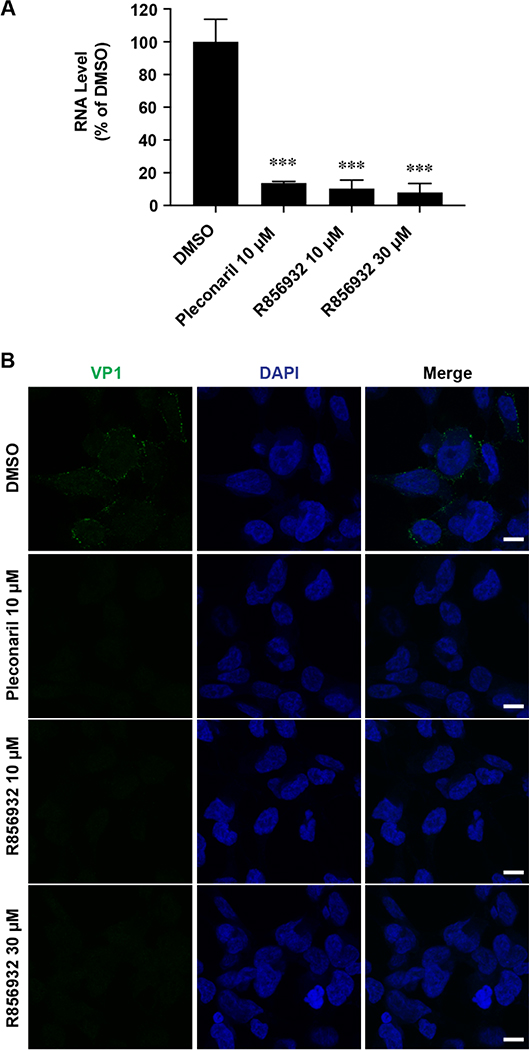
To confirm the antiviral effect of R856932 on EV-D68 replication, we quantified the viral RNA and protein levels with and without R856932 treatment by RT-qPCR, immunofluorescence assay and western blot, respectively. In this experiment, RD cells were infected with EV-D68 US/MO/14–18947 virus at a multiplicity of infection (MOI) of 1 with and without compound treatment (Fig. 2). At 9 hours post-infection (hpi), RD cells were harvested and cellular viral RNA level was quantified by real-time quantitative PCR (RT-qPCR). Compound R856932 greatly reduced viral RNA level, similarly as rupintrivir and pleconaril (Fig. 2A and 2B). Rupintrivir is a known EV-D68 3C protease inhibitor and pleconaril is an EV-D68 virus capsid VP1 binding inhibitor.19, 25, 27 Next, the viral protein expression levels of the capsid protein VP1 were monitored by western blot (Fig. 2C and 2D) and immunofluorescence imaging (Fig. 2E). Compound R856932 significantly reduced the viral VP1 protein level at 9 hpi in a dose-dependent manner (Fig. 2D), similarly to pleconaril treatment (Fig. 2C). Immunofluorescence imaging also showed a similar reduction of VP1 protein fluorescence signal with compound R856932 treatment (Fig. 2E).
Figure 2. Compound R856932 reduced the EV-D68 viral RNA and protein levels.
(A-D) R856932 reduced the EV-D68 viral RNA and protein levels. RD cells were infected with EV-D68 strain US/MO/14–18947 (MOI = 1) with treatment of the indicated compound. At 9 hpi, cells were harvested for viral RNA quantification by RT-qPCR (A and B) or viral protein quantification by western blot (C and D). Asterisks indicate a statistically significant difference in comparison with the DMSO control (one-way analysis of variance analysis by Prism 5, *** P < 0.001). The images were representatives of three independent experiments. (E) R856932 inhibited EV-D68 VP1 synthesis in immunofluorescence assay. RD cells infected with EV-D68 strain US/MO/14–18947 (MOI = 1) were fixed at 9 hpi and stained with anti-VP1 and DAPI for detecting VP1 and nucleus, respectively. Drug concentrations used were shown in the figure.
Time-of-addition and viral attachment assays indicate that R856932 blocks viral attachment and entry
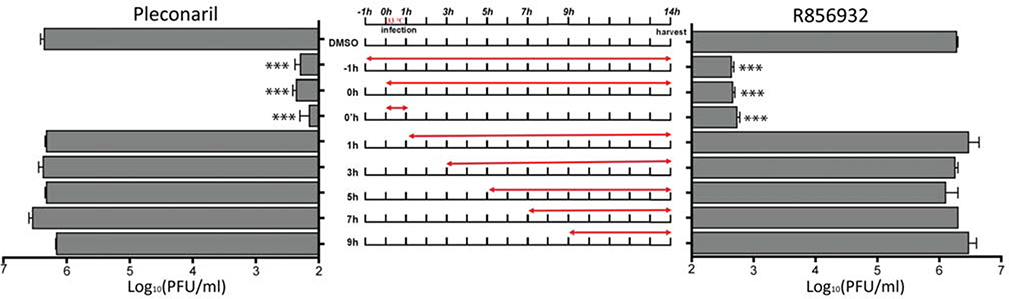
To dissect the antiviral mechanism of R856932, we first checked which stage(s) of the viral replication cycle was affected by R856932 in a drug time-of-addition experiment. In this experiment, RD cells were infected with EV-D68 US/MO/14–18947 with a MOI of 0.01, and R856932 was added at different time points either before, during or after viral infection (Fig. 3). Viruses in the cell supernatant were harvested at 14 hpi and the titer was quantified by plaque assay. The life cycle of EV viruses ranges from 9 to 14 hrs,28 and this experiment mimics one cycle of viral replication. A known viral capsid inhibitor, pleconaril, was included as a control. As shown in Fig. 3, when compound R856932 was applied 1 hpi or afterwards, its antiviral efficacy was completely abolished. In contrast, the antiviral efficacy of R856932 remained when it was added either before (−1h–14h) or during infection (0h–14h). Significantly, the antiviral efficacy of R856932 during the 0h–1h treatment alone was similar to that of −1h–14h and 0h–14h treatments, suggesting R856932 exerted its antiviral activity solely at the viral attachment or entry process. The time-of-addition experiment results of R856932 matched with that of pleconaril, suggesting these two compounds might have a similar mechanism of action.
Figure 3. Time-of-addition experiments by quantify progeny virus yield in cell culture supernatant.
RD cells were infected with EV-D68 US/MO/14–18947 at MOI = 0.01 at 0h time-point. At 1 hpi, inoculant virus was removed and washed with PBS buffer, progeny viruses in cell culture supernatant were harvested at 14 hpi and were quantified with plaque assay. Red arrow represents the period of time that drug (compound R856932 or pleconaril) was present in the cell culture. The concentrations used for R856932 and pleconaril were 25 μM and 12.5 μM, respectively. Asterisks indicate a statistically significant difference in comparison with the DMSO control (one-way analysis of variance analysis by Prism 5, *** P < 0.001). The value is the mean of two independent experiments ± S.D.
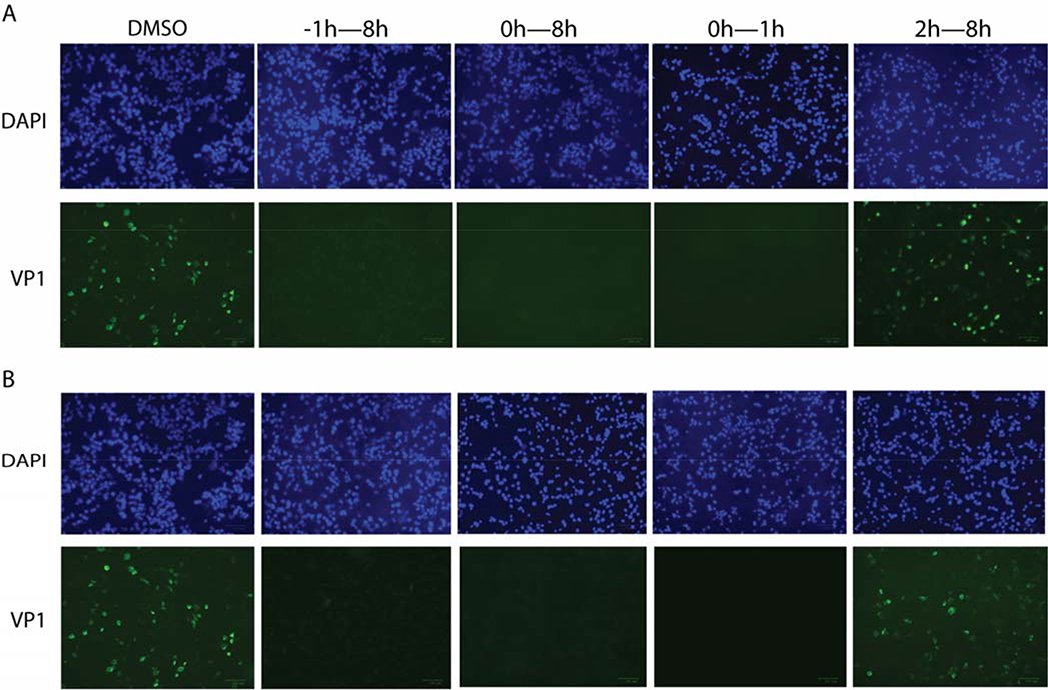
The time-of-addition experiments described above quantify the progeny viruses in the cell supernatant. To independently confirm the results, we performed another set of time-of-addition experiments by quantifying the intracellular viral protein level using immunofluorescence imaging (Fig. 4). In this experiment, RD cells were infected with EV-D68 US/MO/14–18947 at MOI of 1, and compound R856932 was added at different time points either before, during, or after viral infection. Cells were fixed at 8 hpi and viral VP1 protein was stained with anti-VP1 antibody. As shown in Fig. 4, R856932 treatment at −1h–8h, 0h–8h, and 0h–1h time periods all led to complete inhibition of viral protein synthesis, while treatment at 2h–8h time period had almost no effect on viral protein synthesis (Fig. 4B). The immunofluorescence pattern of R856932 treatment (Fig. 4B) was once again similar to that of pleconaril (Fig. 4A). Taken together, these two sets of time-of-addition experiments suggest that R856932 inhibits the early stage of viral replication during either the attachment or entry step.
Figure 4: Time-of-addition experiments by immunostaining VP1 protein inside the host cells.
RD cells were infected with EV-D68 US/MO/14–18947 at MOI = 1 at 0 h time-point, and at 1 hpi, inoculant virus was removed and washed with PBS buffer. A) Pleconaril or B) Compound R856932 was applied at −1, 0 or 2 hpi to 8 hpi; for the 0h–1h condition, Pleconaril or compound R856932 was only present during the infection stage for one hour, then pleconaril or compound R856932 was washed away and fresh media was applied. The concentrations used for R856932 and pleconaril were 50 μM and 12.5 μM, respectively. Cells were fixed at 8 hpi and viruses were detected via staining VP1 protein with anti-VP1. The images were representatives of three independent experiments.
Next, to determine whether R856932 blocks viral attachment or entry, we performed viral attachment assay. In this experiment, RD cells were cooled to 4 °C and mixed with EV-D68 US/MO/14–18947 at MOI of 30 at 4 °C for 2 h, then unbound viruses were washed away, and the attached viruses on cell surface were quantitated by real-time PCR (Fig. 5A) or immunostaining (Fig. 5B). The purpose of keeping the cells at 4 °C for the attachment assay was to prevent viral entry or internalization. The viral RNA levels with R856932 and pleconaril treatments were less than 10% of the DMSO control (Fig. 5A). Similarly, viruses were detected at the surface of RD cells that were treated with DMSO control, but not with R856932 and pleconaril treatments (Fig. 5B).
Figure 5. R856932 reduced the EV-D68 viral attachment to host cells (A and B).
Pre-cooled RD cells were infected with EV-D68 strain US/MO/14–18947 (MOI of 30) and were incubated at 4 °C for 2 h. After removing unbound viruses by washing with ice-cold PBS, the attached viruses on cell surface were quantitated by real-time PCR (A) or visualized by immunostaining (B). Asterisks indicate a statistically significant difference in comparison with the DMSO control (one-way analysis of variance analysis by Prism 5, *** P < 0.001). The value is the mean of three independent experiments ± S.D. The images were representatives of three independent experiments. Drug concentrations used were shown in the figure.
Overall, the results from the two sets of time-of-addition experiments as well as the viral attachment assay collectively suggest that R856932 inhibits viral attachment to the host cell, and the mechanism of action of R856932 is similar to that of pleconaril, a known viral capsid VP1 inhibitor.
Compound R856932 protects EV-D68 virus from heat inactivation
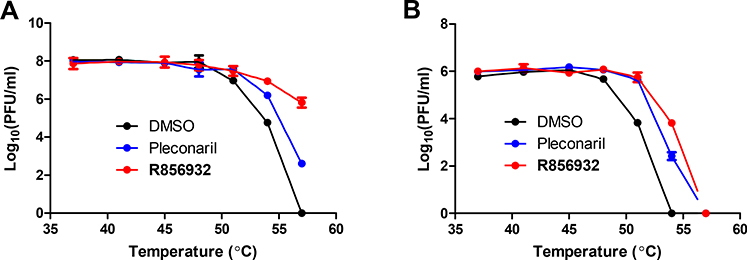
It has been shown before that viral capsid inhibitors can protect virus from heat inactivation.29 To determine whether R856932 directly binds to the EV-D68 capsid proteins, we performed thermal protection assay. In this assay, EV-D68 US/MO/14–18947 virus was heated for 2 minutes at temperatures ranging 37 to 57 °C in the absence or presence of 50 μM compound R856932 or 12.5 μM of pleconaril (Fig. 6A). At temperatures of 54 °C or above, compound R856932 significantly reduced the virus inactivation by heat, and pleconaril displayed similar pattern, though less effective (Fig. 6A). Next, the thermal protection assay was repeated with another EV-D68 strain US/MO/14–18949. Although US/MO/14–18949 was more sensitive to heat inactivation than US/MO/14–18947 strain, the overall protection pattern was very similar to that of US/MO/14–18947 strain with compound treatment (Fig. 6B). These data indicate that compound R856932 directly binds to the virus capsid protein(s) and stabilize viral capsid from heat inactivation.
Figure 6. Thermo-protection assay showing that R856932 prevented EV-D68 US/MO/14–18947 (A) and US/MO/14–18949 viruses (B) from heat inactivation.
Protective effects of pleconaril or compound R856932 on heat inactivation of EV-D68 US/MO/14–18947 (A) and US/MO/14–18949 viruses (B). 12.5 μM Pleconaril or 50 μM compound R856932 or DMSO was incubated with viruses (2×10^6 pfu/ml) for 30 minutes. The virus/compound mixture was then heated for 2 minutes at temperature ranging from 37 to 57 °C, followed by a rapid cool down to 4 °C. The resulting infectious virus was quantified via plaque assay using RD cells. The values are the mean ± standard deviation from three replicates.
Selection of EV-D68 mutants that are resistant to compound R856932 via serial viral passage experiment
Based the above results, it appears that compound R856932 directly interacts with EV-D68 virus capsid proteins. In order to determine which viral capsid protein(s) compound R856932 interacts with, we carried out a serial passage experiment in the presence increasing concentration of compound R856932 with an attempt to select drug-resistant mutants.23 Since the purpose was to select resistant mutants, we used high drug selection pressure (8.7×EC50 to 34.8×EC50) to speed up this process (Table 2). 23, 30 At passage 3 (P3), the EC50 value increased about 80-fold in the presence of R856932 selection pressure, while for the DMSO control, the EC50 value remained the same (Table 2), suggesting the observed resistance was not due to cell culture passage adaption.
Table 2:
Serial viral passage experiment of R856932 against EV-D68 US/MO/14–18947.a
| Passage # | DMSO | R856932 | ||
|---|---|---|---|---|
| Selection pressure (μM) | EC50 (μM) | Selection Pressure (μM) | EC50 (μM) | |
| 0 | 0 | 0.46 ± 0.09 | 0 | 0.46 ± 0.09 |
| 1 | 0 | N.D. | 4 | N.D. |
| 2 | 0 | N.D. | 8 | N.D. |
| 3 | 0 | 0.77 ± 0.44 | 16 | 35.60 ± 5.10 |
US/MO/14–18947 (MOI = 0.1) were treated with DMSO or compound R856932 at the indicated concentration (μM); EC50 (μM) = mean ± standard deviation; The values are the mean ± standard deviation from three replicates. N.D.: Not determined.
We then sequenced the whole viral genome of both DMSO and R856932 P3 viruses by Sanger sequencing. No mutation was identified in DMSO P3 virus; however, a few mutations were identified in the R856932 P3 virus: A45V and A129V in VP1 protein, T139A in VP2 protein, and I44V in non-structural 3A protein.
VP1-A129V and VP2-T139A mutants confer R856932 resistance
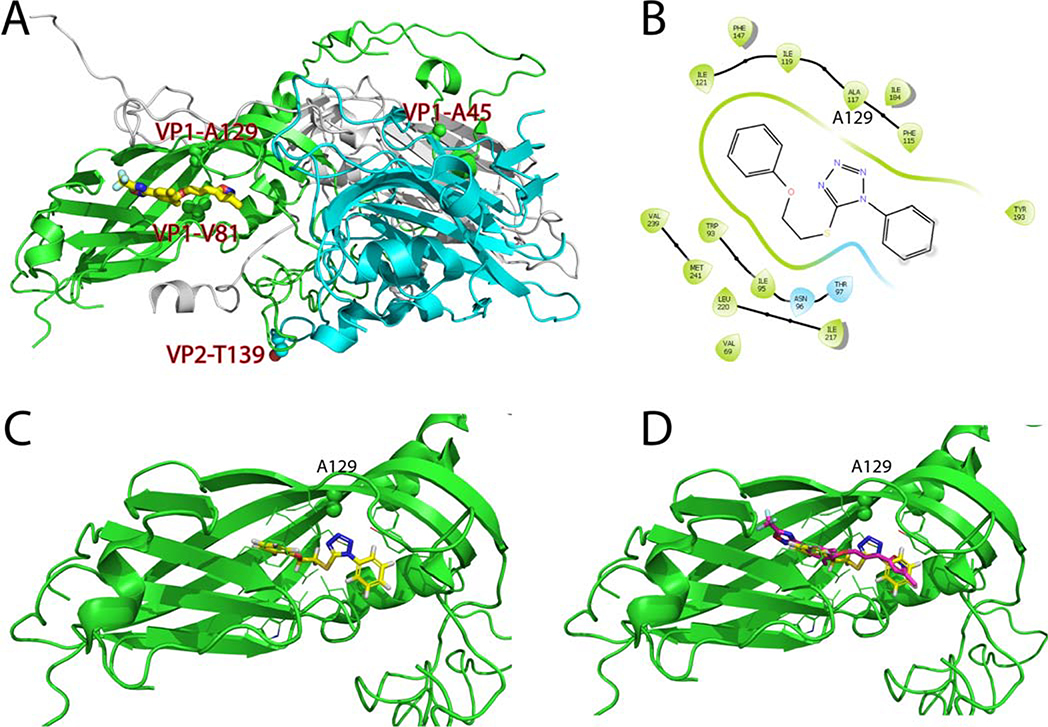
Based on the results from time-of-addition, viral attachment, and thermo-protection assay results, we hypothesized that compound R856932 acts on the EV-D68 capsid proteins. Therefore, we focused our efforts on examining the mutations in viral capsid proteins: VP1-A45V, VP1-A129V, and VP2-T139A. To rationalize the selected resistant mutants, we examined the X-ray crystal structure of the EV-D68 capsid proteins in complex with pleconaril (PDB: 4WM7) (Fig. 7A).25 Residues VP1-A45 (numbered A33 in the crystal structure) was located at the surface of VP1, and there was no drug-binding site close to this region, therefore the VP1-A45V mutant was probably unrelated to R856932 resistance. In contrast, residue VP1-A129 was located right above the pleconaril drug-binding site in the canyon region, and it was close to the pleconaril resistant mutant VP1-V81A (numbered V69 in the crystal structure). It was reported that VP1-V81A confers resistance against pleconaril.19 Residue VP2-T139 was located at a flexible loop that was near the entrance of the pleconaril drug-binding site, and it might affect drug entrance in the canyon region. We therefore hypothesized that R856932 might have an overlapping drug-binding site as pleconaril and mutants VP1-A129V and VP2-T139A might lead to R856932 resistance. To test this hypothesis, we first performed molecular docking of R856932 in the pleconaril drug-binding site using Schrödinger Glide. Pleconril was set as the centroid of the grid box and the docking was performed using the Glide standard precision. In the docking model, R856932 fitted snuggly in the pleconaril drug-binding site and was surrounded by hydrophobic residues V69, W93, I95, T97, F115, I119, F147, I184, Y193, I217, L220, V239, and M241 (numbering in the X-ray crystal structure) (Fig. 7B & 7C). It was noted that the tetrazole from R856932 was 3.4 Å apart from the resistant mutant residue VP1-A129 (corresponds to A117 in the crystal structure). Mutation of VP1-A129 to V129 was therefore likely to create a steric clash with R856932, leading to drug resistance. In addition, in the overlay structure of R856932 with pleconaril (Fig. 7D), the 1-phenyl ring from R856932 overlapped with the isoxazole ring from pleconaril, and the distal phenyl ring from R856932 overlapped with the central benzene ring from pleconaril. Overall, molecular docking suggests that R856932 might bind to the same canyon site as pleconaril and the selected resistant mutant VP1-A129V might lead to R856932 resistance. In addition, based on the docking model (Fig. 7D), it is reasonable to speculate that replacing the 1, 5-tetrazole linker in R856932 with either the 2,5-disubstituted tetrazole linker or the 1,4-disubstituted 1H-1,2,3-triazole linker would lead to analogs with increased antiviral potency since it will extend the molecule to occupy the trifluoromethyl oxadiazole pocket.
Figure 7. Molecular docking of R856932 in EV-D68 viral capsid protein VP1.
(A) X-ray crystal structure of EV-D68 (Fermon CA62–1) capsid proteins in complex with pleconaril (PDB: 4WM7).25 VP1, VP2 and VP3 were colored as green, cyan, and grey, respectively. R856932-selected resistant mutants VP1-A129V, VP1-A45V, and VP2-T139A, as well as the pleconaril-resistant mutant VP1-V81A, were labeled and the side chains were shown as spheres. (B) Ligand interaction diagram of R856932 in the pleconaril binding site in VP1. The figure was generated in Schrödinger Glide. Note that A117 in the crystal structure corresponds to A129 in the conventional numbering. (C) Docking model of R856932 in VP1. VP1-A129 side chain was shown as spheres. (D) Overlay of R856932 and pleconaril in the canyon region of VP1. Docking was performed using Schrödinger Glide standard precision.
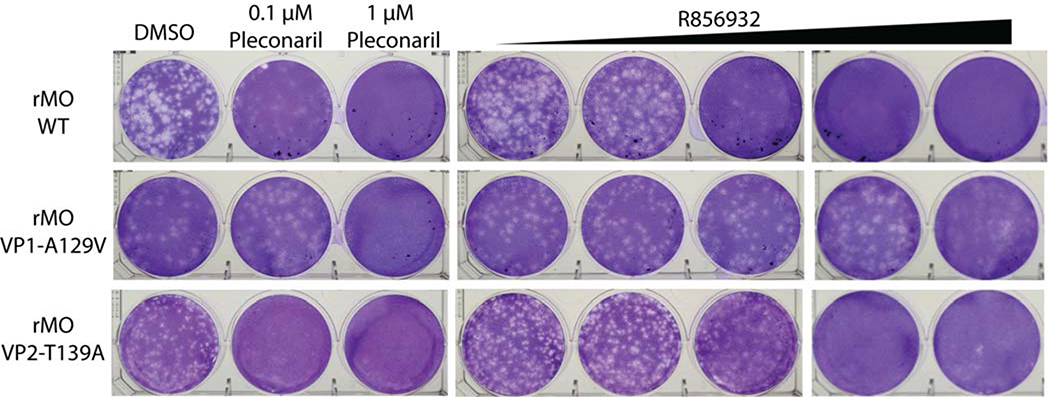
To experimentally validate the relevance of VP1-A129V and VP2-T139A mutations in R856932 resistance, we used reverse genetics approach to generate recombinant EV-D68 viruses that encode either the VP1-A129V mutant or the VP2-T139A mutant and compared their drug sensitivity with the WT EV-D68 virus. Specifically, we introduced VP1-A129V or VP2-T139A into EV-D68 US/MO/14–18947 genome individually via a reverse genetics approach using a pHH21 vector.23 We named the resulting virus rMO VP1-A129V and rMO VP2-T139A, respectively. The drug sensitivity of these recombinant viruses against R856932 was evaluated with a plaque reduction assay (PRA), and recombinant rMO WT virus was included as a control (Fig. 8). The introduction of VP1-A129V mutation alone increased EC50 value of compound R856932 from 0.39 μM to 16.00 μM; while the VP2-T139A mutation increased EC50 value from 0.39 μM to 1.15 μM (Table 3). The plaque assay results were also independently confirmed by the cytopathic effect assay (CPE). As shown in Table 3, CPE assay also showed that the antiviral efficacy of compound R856932 was greatly reduced against rMO VP1-A129V virus, and slightly reduced against rMO VP2-T139A virus. Overall, both plaque reduction assay and cytopathic effect assay indicate that VP1-A129V is the major mutation that confers R856932 resistance, while VP2-T139A had a partial role in R856932 resistance. Taken together, the molecular docking and reverse genetics collectively suggested that R856932 had an overlapping drug-binding site with pleconaril in the canyon region of VP1, and resistant mutants VP1-A129V and VP2-T139A both conferred drug resistance against R856932.
Figure 8. Representative plaque reduction assay with compound R856932 against recombinant rMO WT, rMO VP1-A129V and rMO VP2-T139A viruses.
Approximately 100 pfu/well of recombinant rMO WT, rMO VP1-A129V or rMO VP2-T139A viruses were applied to RD cell monolayers. 0.1 to 10 μM compound R856932, or 0.1 or 1.0 μM pleconaril was present in the 1.2% avicel overlay. RD cells were stained with crystal violet 3 days after infection. The images were representatives of three independent experiments.
Table 3:
Drug sensitivity against recombinant EV-D68 US/MO/14–18947 viruses
| R856932 | Pleconaril | |||
|---|---|---|---|---|
| CPE EC50 (μM) | PRA EC50 (μM) | CPE EC50 (μM) | PRA EC50 (μM) | |
| rMO WT | 0.86 ± 0.14 | 0.39 ± 0.03 | 0.07 ± 0.02 | 0.03 ± 0.01 |
| rMO VP1-A129V | 16.6 ± 1.2 | 16.0 ± 7.4 | 0.44 ± 0.04 | 0.23 ± 0.04 |
| rMO VP2-T139A | 1.46 ± 0.39 | 1.15 ± 0.14 | 0.13 ± 0.05 | 0.13 ± 0.02 |
CPE: cytopathic effect assay; PRA: Plaque reduction assay; EC50 (μM) = mean ± standard deviation. The values are the mean ± standard deviation from three replicates.
Recombinant rMO VP1-A129V virus had compromised fitness of replication compared to rMO WT virus
We compared the fitness of replication of recombinant rMO VP1-A129V virus with the rMO WT virus by a co-cultivation competition assay in RD cells.30 We started the co-cultivation by mixing the rMO VP1-A129V virus with the rMO WT virus in a 100 to 1 ratio, and applied the virus mixture to infect the RD cells. Every 2 days after infection, culture media supernatant was harvested and viruses were quantified with plaque assay, and 28,000 PFU progeny viruses were used for the next round of infection. The VP1 coding sequences of the resulting viruses of each passage were determined by reverse transcription-PCR, and followed by Sanger sequencing.
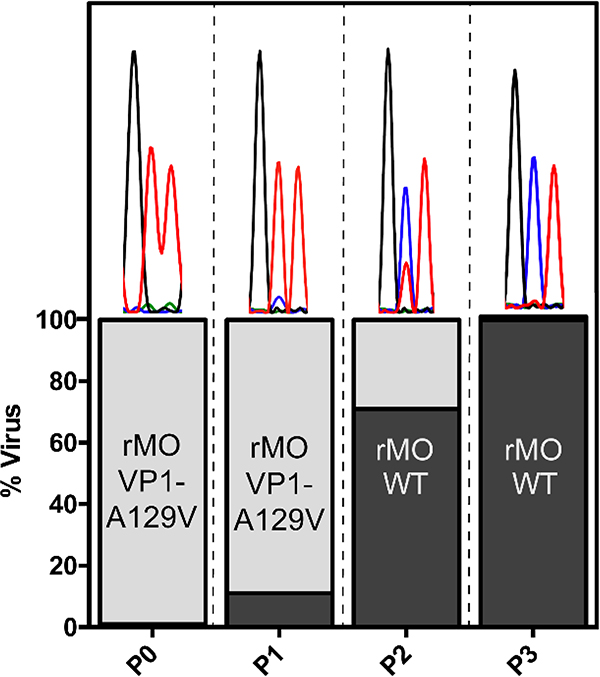
After passage P3, no VP1-A129V mutation could be detected in the virus mixture (Fig. 9), suggesting that rMO VP1-A129V was overtaken by rMO WT. Comparing to the VP1 sequences in P1 and P2, it was found that mutant rMO VP1-A129V virus was gradually replaced by rMO WT virus from P1 to P3. These results indicate that recombinant rMO VP1-A129V had compromised fitness of replication than rMO WT virus in cell culture.
Figure 9. Competition growth assay to assess the replication fitness of rMO VP1-A129V virus.
280 PFU rMO WT and 28,000 PFU rMO VP-A129V viruses (ratio = 1/100) were used to infect RD cells in a T25 flask. Every 2 days after infection, culture media supernatant was harvested and viruses were quantified with plaque assay, and 28,000 PFU progeny viruses were used for the next round of infection. The VP1 coding sequences were determined in each round of passage. The percentage of each viruses was estimated by measuring the height of the nucleotide sequence electropherogram peak as shown in the bar graph.
CONCLUSION
Recent years of upswing of EV-D68 virus infections and its association with polio-like neurological disorder such as acute flaccid myelitis (AFM), coupled with the lack of effective vaccine and antivirals,14, 15, 31 prompted us carry out a high-throughput screening assay to identify novel EV-D68 antivirals. Compound R856932 was identified to have potent antiviral efficacy in this screening. Further testing showed that compound R856932 was effective against all the contemporary EV-D68 strains tested, but not the EV-A71 strains (Table 1).
To elucidate the mechanism of action by compound R856932, we performed drug time-of-addition, viral attachment, thermal protection, and drug-resistance selection assays. The time-of-addition and viral attachment assays showed that compound R856932 inhibited viral attachment (Figs. 2–5). Moreover, compound R856932 displayed very similar patterns as the known capsid-binder pleconaril in all of the assays performed. Furthermore, the thermal protection assay revealed that compound R856932 could protect EV-D68 viruses from heat inactivation (Fig. 6). In the independent serial viral passage experiments, the drug-resistant mutant viruses were obtained and the mutations were located in the capsid proteins VP1 and VP2, namely VP1-A129V and VP2-T139A. Molecular docking and reverse genetics suggested that the binding site of R856932 overlapped with pleconaril (Figs. 7–8), and VP1-A129V had a direct effect on R856932 binding, while VP2-T139A might have an indirect effect by affecting the drug entry into the binding site.
Development of capsid inhibitors targeting the hydrophobic pocket in the canyon region is a promising strategy in combating picornavirus infection. Pirodavir, pleconaril and vapendavir successfully advanced to clinical trials, though none of them was approved.2, 26 Pleconaril is one of the best studied capsid-binding inhibitor against picornaviruses. It has broad-spectrum antiviral activity against several human enteroviruses and rhinovirses. It should be noted that pleconaril does not inhibit enterovirus A71, as we confirmed in our study (Table 1). Pleconaril originally was developed to treat common cold caused by human rhinoviruses, and made all the way to clinical trial Phase III.32 However FDA rejected pleconaril as a drug to treat rhinoviruses caused common cold, citing pleconaril’s concerning side effect and inducing drug resistant mutations.33 In the clinical trial pleconaril caused some women to bleed between menstrual periods and reduced the effectiveness of oral contraceptives.33 Although compound R856932 had overlapping drug-binding site as pleconaril, compound R856932 possesses a different scaffold from pleconaril and other known capsid inhibitors, such as pirodavir and vapendavir. Therefore, compound R856932 possibly represents a new starting point to the development of effective capsid-binding inhibitor with high efficacy against all temporary EV-D68 strains, favorable drug-like properties, and limited side effects. Future SAR campaigns will help optimize the potency and pharmacological properties of this class of capsid-binding antivirals. Such study is ongoing and will be reported when available.
Another concern with viral capsid inhibitors is the drug resistance. Although resistance could be raised for R856932 in the in vitro cell culture with a high drug selection pressure, it should be noted that the resistant mutant virus encoding VP1-A129V had a compromised fitness of replication compared to the WT EV-D68 virus. Moreover, EV-D68 is an acute infection and treatment might not need to last more than a couple of weeks, so the chances of resistance remain low. In addition, it is expected that combining capsid inhibitors with other EV-D68 antivirals, such as the protease inhibitors,23 might further decrease the pace of resistance evolution.
MATERIAL AND METHODS
Chemicals, cells and virus
R856932 was purchased from Sigma-Aldrich (Cat # R856932); Pleconril was purchased from Sigma-Aldrich (Cat # SML0307); Telaprevir was purchased from Achemblock (Cat #F-4593).
Human rhabdomyosarcoma (RD; ATCC CCL-136) was maintained at 37°C in a 5% CO2 atmosphere and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin antibiotics. The following reagents were obtained through BEI Resources, NIAID, NIH: Human Enterovirus D68, US/MO/14–18949, NR-49130; Enterovirus D68, US/MO/14–18947, NR-49129; Enterovirus D68, US/IL/14–18952, NR-49131; Enterovirus D68, US/KY/14–18953, NR-49132; Enterovirus D68, US/IL/14–18956, NR-49133; Human Enterovirus 71 (HEV-71), Tainan/4643/1998, NR-471; and Human Enterovirus 71 (HEV-71), MP4, NR-472. All viruses were amplified in RD cells prior to infection assays.
Cytopathic effect assay (CPE) and plaque reduction assay (PRA)
RD cells for antiviral assays were seeded and grown overnight at 37°C in a 5% CO2 atmosphere to ~90% confluence on the next day. For EV-D68 virus infections, cells were washed with PBS saline containing magnesium and calcium and infected with virus diluted in DMEM with 2% FBS and 30 mM MgCl2. Viruses were incubated for at least 1 h at 33°C in a 5% CO2 atmosphere, followed by addition of compound as well as 1% penicillin-streptomycin. The CC50 was measured similarly but in the absence of viral infection. For cytopathic effect (CPE) assays, cells were stained with 66 μg/ml neutral red for 2 h, and neutral red uptake was measured at an absorbance at 540 nm using a Multiskan FC microplate photometer (Thermo Fisher Scientific). The EC50 and CC50 values were calculated from best-fit dose-response curves using GraphPad Prism software. For plaque reduction assays, a 1.2% Avicel microcrystalline cellulose (FMC BioPolymer, Philadelphia, PA) overlay in DMEM media supplemented with 2% FBS and 30 mM MgCl2 was used, and the cells were stained after 3 days at 33 °C as previously described.23, 27 For EV-A71 virus infection, the procedures are identical as EV-D68 virus, except that 30 mM MgCl2 was omitted in all the media and viruses were infected and incubated at 37°C instead of 33 °C.
High-throughput screening (HTS)
High-throughput screening was performed in 96-well plate using the CPE assay as described above. The library was purchased from Sigma (MyriaScreen, product number T990000) which contains 10,000 drug-like compounds. All compounds were initially screened at 15 μM, and hits showing more than 50% inhibition were repurchased from Sigma and tested in the serial titration experiment to determine the EC50 values. The calculated Z-factor was 0.68, indicating this was an excellent assay for HTS.
Western blot
RD cells infected with EV-D68 strain US/MO/14–18947 (MOI = 1) were treated with indicated compound for 9 hours. Then total proteins were extracted using RAPI lysis buffer (50 mM Tris [pH 8.0], 1% NP-40, 0.1% SDS, 150 mM NaCl, 0.5% sodium deoxycholate, 5 mM EDTA, 10 mM NaF, 10 mM NaPPi, 2 mM phenylmethylsulfonyl, 1 mM PMSF). Equal amounts of total proteins were separated by electrophoresis and transferred to PVDF membrane. Target protein was detected using protein-specific antibody [anti-VP1 antibody: GTX132313 (GeneTex) or anti-GAPDH antibody: MAB374 (EMD Millipore); 1:3,000 dilution] and corresponding Horseradish peroxidase (HRP)-conjugated secondary antibody (catalog number 32430 or 656120; Thermo Fisher Scientific; 1:3,000 dilutions) and Supersignal West Femto substrate (Thermo Fisher Scientific).
RNA extraction and RT-qPCR
RD cells infected with EV-D68 strain US/MO/14–18947 (MOI = 1) were treated with indicated compound for 9 hours. Then total RNA was extracted using TRIzol reagents (Thermo Fisher Scientific). After digestion of genomic DNA with RQ1 RNase-free DNase (Promega), 1.5 μg of total RNA was used to synthesize the first strand of cDNA of viral RNA and host mRNA using SuperScript III reverse transcriptase (Thermo Fisher Scientific) and oligo(dT)18. Target gene was amplified on a QuantStudio 5 Real- Time PCR System (Thermo Fisher Scientific) using FastStart Universal SYBR Green Master (Rox) (Roche) and virus-specific primers: D68-F (5′-CGCTGAACTTGGCGTGGTCC-3′) and D68- R (5′-GGCTGCCCTGCTAAGAAAATTCTCC-3′). GAPDH was amplified to serve as a control using GAPDH-specific primers (GAPDH-F: 5′-ACACCCACTCCTCCACCTTTG-3′ and GAPDH- R: 5′-CACCACCCTGTTGCTGTAGCC-3′). The amplification conditions were: 95 °C for 10 min; 40 cycles of 15 s at 95 °C and 60 s at 60 °C. The melting curve analysis was performed to verify the specificity of each amplification. Details regarding the PCR amplification method and sequencing primers are available upon request.
Immunostaining
RD cells were infected with EV-D68 strain US/MO/14–18947 (MOI = 1). At 9 hpi, cells were fixed with 4% formaldehyde for 10 min, followed by permeabilization with 0.2% Triton X-100 for another 10 min. After blocking with 10% bovine serum, cells were stained with rabbit anti-VP1 antibody and followed by staining with anti-rabbit immunoglobulin secondary antibody conjugated to Alexa Fluor 488 (Thermo Fisher Scientific). The nuclei were stained with 300 nM DAPI (Thermo Fisher Scientific) after secondary antibody incubation. Fluorescent images were acquired using a Leica SP5-II spectral confocal microscope (Leica).34–36
Virus attachment assay
RD cells were pre-cooled at 4 °C for 30 min, followed by infection with EV-D68 strain US/MO/14–18947 (MOI of 30). After 2 h incubation at 4 °C, unbound viruses were removed by two times of washing with ice-cold PBS. The attached viruses on cell surface were harvested for quantitated by real-time PCR or fixed for visualized by immunostaining.
Time-of-addition assay
Approximately 90% confluent RD cells were treated with compound R856932 and pleconaril at 1 h before infection (−1 hpi), at the time of infection (0 hpi), or at 1–7 hpi with EV-D68 US/MO/14–18947. For quantification the progeny virus in the supernatant via plaque assay, MOI of 0.01 was used to infect the RD cells, the supernatant was harvested at 14 hpi, and the progeny viruses were quantified via plaque assay. For quantification the progeny virus inside the RD cells via immunostaining, MOI of 1 was used to infect the RD cell, and the cells were fixed at 8 hpi, and immunostaining was carried as described in immunostaining section.
Thermo-protection assay
12.5 μM Pleconaril or 50 μM compound R856932 or same volume DMSO was incubated with viruses at 30 °C for 30 min. then the viruses were heated for 2 min temperature ranging from 37 to 57 °C, followed a rapid cool down to 4 °C on PCR machine. The resulting live virus was quantified via regular plaque assay on RD cells.
Serial passage experiment and viral genome sequencing
Serial passage experiment and viral genome sequencing were performed using an approach similar to that described previously.30, 36, 37 Briefly, serial passages were carried out in the presence of increasing concentration of compound R856932 or DMSO as shown in Table 2. The virus was harvested after approximately 3 days after infection with EV-D68 US/MO/14–18947 at MOI=0.1 when significant cytopathic effect was observed, and the viral titers were determined by a plaque assay. The passage P3 virus drug sensitivity was determined via CPE assay, and their genome sequences were determined by first purifying viral RNA using a QIAamp viral RNA minikit (Qiagen, Hilden, Germany), followed by reverse transcription using a SuperScript III first-strand reverse transcriptase (Invitrogen) with an oligo(dT) primer and PCR amplification. The whole viral genome was sequenced via 14 sequencing reactions by Eton Bioscience, Inc. Details regarding the PCR amplification method and the sequencing primers are available upon request.
Reverse genetics of EV-D68 virus
A plasmid-based reverse genetic system for EV-D68 US/MO/14–18947 was generated in a pHH21 vector, based on the study by Pan et al.23, 38 The mutations were introduced via site-directed Mutagenesis with Agilent Technologies QuikChange II XL kit according to manufacturer protocol.
Virus growth competition assay30
To compare the relative viral fitness of rMO VP1-A129V with rMO WT virus, rMO WT virus was mixted with rMO VP1-A129V virus at a 1:100 ratio (280 PFU rMO WT and 28,000 PFU rMO A129V). Every 2 days after infection, culture media supernatant was harvested and viruses were quantified with plaque assay, and 28,000 PFU progeny viruses were used to next round of infection. The VP1 coding sequences of the resulting viruses of each passage were determined by reverse transcription-PCR, and followed by sequencing reaction as described in the serial passage experiment and virus genome sequencing section. The percentage of each viruses was estimated by measuring the height of the nucleotide sequence electropherogram peak.30, 39
Molecular Modeling
Molecular docking was performed using Schrödinger Glide standard precision. The X-ray crystal structure of EV-D68 capsid proteins in complex with pleconaril was used as the template (PDB: 4WM7).25 The centroid of the grid box was set as the pleconaril. Figures were generated in Pymol.
Acknowledgements
This research is supported by the NIH grants (AI119187, AI144887, and AI147325), the Young Investigator Award grant from the Arizona Biomedical Research Centre to J.W.
REFERENCES
- 1.Cassidy H; Poelman R; Knoester M; Van Leer-Buter CC; Niesters HGM Enterovirus D68-The New Polio? Front Microbiol 2018, 9, 2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggen J; Thibaut HJ; Strating JRPM; van Kuppeveld FJM The life cycle of non-polio enteroviruses and how to target it. Nat Rev Microbiol 2018, 16, 368–381. [DOI] [PubMed] [Google Scholar]
- 3.Sun J; Hu XY; Yu XF Current Understanding of Human Enterovirus D68. Viruses 2019, 11, E490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lugo D; Krogstad P Enteroviruses in the early 21st century: new manifestations and challenges. Curr Opin Pediatr 2016, 28, 107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Royston L; Essaidi-Laziosi M; Perez-Rodriguez FJ; Piuz I; Geiser J; Krause KH; Huang S; Constant S; Kaiser L; Garcin D; Tapparel C Viral chimeras decrypt the role of enterovirus capsid proteins in viral tropism, acid sensitivity and optimal growth temperature. PLoS Pathog 2018, 14, e1006962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y; Sheng J; van Vliet ALW; Buda G; van Kuppeveld FJM; Rossmann MG Molecular basis for the acid-initiated uncoating of human enterovirus D68. Proc Natl Acad Sci U S A 2018, 115, E12209–E12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oermann CM; Schuster JE; Conners GP; Newland JG; Selvarangan R; Jackson MA Enterovirus d68. A focused review and clinical highlights from the 2014 U.S. Outbreak. Ann Am Thorac Soc 2015, 12, 775–781. [DOI] [PubMed] [Google Scholar]
- 8.Dyda A; Stelzer-Braid S; Adam D; Chughtai AA; MacIntyre CR The association between acute flaccid myelitis (AFM) and Enterovirus D68 (EV-D68) - what is the evidence for causation? Eurosurveillance 2018, 23, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morens DM; Folkers GK; Fauci AS Acute Flaccid Myelitis: Something Old and Something New. Mbio 2019, 10, e00521–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messacar K; Schreiner TL; Van Haren K; Yang M; Glaser CA; Tyler KL; Dominguez SR Acute flaccid myelitis: A clinical review of US cases 2012–2015. Ann Neurol 2016, 80, 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DM; Hixon AM; Oldfield LM; Zhang Y; Novotny M; Wang W; Das SR; Shabman RS; Tyler KL; Scheuermann RH Contemporary Circulating Enterovirus D68 Strains Have Acquired the Capacity for Viral Entry and Replication in Human Neuronal Cells. mBio 2018, 9, e01954–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Midgley CM; Watson JT; Nix WA; Curns AT; Rogers SL; Brown BA; Conover C; Dominguez SR; Feikin DR; Gray S; Hassan F; Hoferka S; Jackson MA; Johnson D; Leshem E; Miller L; Nichols JB; Nyquist AC; Obringer E; Patel A; Patel M; Rha B; Schneider E; Schuster JE; Selvarangan R; Seward JF; Turabelidze G; Oberste MS; Pallansch MA; Gerber SI; Group E-DW Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir Med 2015, 3, 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greninger AL; Naccache SN; Messacar K; Clayton A; Yu G; Somasekar S; Federman S; Stryke D; Anderson C; Yagi S; Messenger S; Wadford D; Xia D; Watt JP; Van Haren K; Dominguez SR; Glaser C; Aldrovandi G; Chiu CY A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012–14): a retrospective cohort study. Lancet Infect Dis 2015, 15, 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christy A; Messacar K Acute Flaccid Myelitis Associated With Enterovirus D68: A Review. J Child Neurol 2019, 34, 511–516. [DOI] [PubMed] [Google Scholar]
- 15.Hixon AM; Yu G; Leser JS; Yagi S; Clarke P; Chiu CY; Tyler KL A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog 2017, 13, e1006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurst BL; Evans WJ; Smee DF; Van Wettere A; Tarbet EB Evaluation of antiviral therapies in respiratory and neurological disease models of Enterovirus D68 infection in mice. Virology 2019, 526, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrey JD; Wang H; Hurst BL; Zukor K; Siddharthan V; Van Wettere AJ; Sinex DG; Tarbet EB Causation of Acute Flaccid Paralysis by Myelitis and Myositis in Enterovirus-D68 Infected Mice Deficient in Interferon/Receptor Deficient Mice. Viruses 2018, 10, E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowers JR; Valentine M; Harrison V; Fofanov VY; Gillece J; Delisle J; Patton B; Schupp J; Sheridan K; Lemmer D; Ostdiek S; Bains HK; Heim J; Sylvester T; Prasai S; Kretschmer M; Fowle N; Komatsu K; Brady S; Robinson S; Fitzpatrick K; Ostovar GA; Alsop E; Hutchins E; Jensen K; Keim P; Engelthaler DM Genomic Analyses of Acute Flaccid Myelitis Cases among a Cluster in Arizona Provide Further Evidence of Enterovirus D68 Role. MBio 2019, 10, e02262–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L; Meijer A; Froeyen M; Zhang LL; Thibaut HJ; Baggen J; George S; Vernachio J; Kuppeveld FJM; Leyssen P; Hilgenfeld R; Neyts J; Delang L Antiviral Activity of Broad-Spectrum and Enterovirus-Specific Inhibitors against Clinical Isolates of Enterovirus D68. Antimicrob Agents Chemother 2015, 59, 7782–7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhoden E; Zhang M; Nix WA; Oberste MS In Vitro Efficacy of Antiviral Compounds against Enterovirus D68. Antimicrob Agents Chemother 2015, 59, 7779–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smee DF; Evans WJ; Nicolaou KC; Tarbet EB; Day CW Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antiviral Res 2016, 131, 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messacar K; Sillau S; Hopkins SE; Otten C; Wilson-Murphy M; Wong B; Santoro JD; Treister A; Bains HK; Torres A; Zabrocki L; Glanternik JR; Hurst AL; Martin JA; Schreiner T; Makhani N; DeBiasi RL; Kruer MC; Tremoulet AH; Van Haren K; Desai J; Benson LA; Gorman MP; Abzug MJ; Tyler KL; Dominguez SR Safety, tolerability, and efficacy of fluoxetine as an antiviral for acute flaccid myelitis. Neurology 2019, 92, e2118–e2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musharrafieh R; Ma C; Zhang J; Hu Y; Diesing JM; Marty MT; Wang J Validating Enterovirus D68–2A(pro) as an Antiviral Drug Target and the Discovery of Telaprevir as a Potent D68–2A(pro) Inhibitor. J Virol 2019, 93, e02221–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musharrafieh R; Zhang JT; Tuohy P; Kitamura N; Bellampalli SS; Hu YM; Khanna R; Wang J Discovery of Quinoline Analogues as Potent Antivirals against Enterovirus D68 (EV-D68). J Med Chem 2019, 62, 4074–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y; Sheng J; Fokine A; Meng G; Shin WH; Long F; Kuhn RJ; Kihara D; Rossmann MG Structure and inhibition of EV-D68, a virus that causes respiratory illness in children. Science 2015, 347, 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egorova A; Ekins S; Schmidtke M; Makarov V Back to the future: Advances in development of broad-spectrum capsid-binding inhibitors of enteroviruses. Eur J Med Chem 2019, 178, 606–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smee DF; Evans WJ; Nicolaou KC; Tarbet EB; Day CW Susceptibilities of enterovirus D68, enterovirus 71, and rhinovirus 87 strains to various antiviral compounds. Antiviral Res 2016, 131, 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J; He YQ; Yi LN; Zan H; Kung HF; He ML Viral kinetics of Enterovirus 71 in human abdomyosarcoma cells. World J Gastroenterol 2011, 17, 4135–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacroix C; Laconi S; Angius F; Coluccia A; Silvestri R; Pompei R; Neyts J; Leyssen P In vitro characterisation of a pleconaril/pirodavir-like compound with potent activity against rhinoviruses. Virol J 2015, 12, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musharrafieh R; Ma CL; Wang J Profiling the in vitro drug-resistance mechanism of influenza A viruses towards the AM2-S31N proton channel blockers. Antiviral Res 2018, 153, 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyda A; Stelzer-Braid S; Adam D; Chughtai AA; MacIntyre CR The association between acute flaccid myelitis (AFM) and Enterovirus D68 (EV-D68) - what is the evidence for causation? Euro Surveill 2018, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willyard C An unknown enemy: Drugs sought against EV-68 as paralysis link is explored. Nat Med 2015, 21, 419–421. [DOI] [PubMed] [Google Scholar]
- 33.Senior K FDA panel rejects common cold treatment. Lancet Infect Dis 2002, 2, 264. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y; Zhang J; Musharrafieh R; Hau R; Ma C; Wang J Chemical Genomics Approach Leads to the Identification of Hesperadin, an Aurora B Kinase Inhibitor, as a Broad-Spectrum Influenza Antiviral. Int J Mol Sci 2017, 18, E1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y; Zhang J; Musharrafieh RG; Ma C; Hau R; Wang J Discovery of dapivirine, a nonnucleoside HIV-1 reverse transcriptase inhibitor, as a broad-spectrum antiviral against both influenza A and B viruses. Antiviral Res 2017, 145, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J; Hu Y; Foley C; Wang Y; Musharrafieh R; Xu S; Zhang Y; Ma C; Hulme C; Wang J Exploring Ugi-Azide Four-Component Reaction Products for Broad-Spectrum Influenza Antivirals with a High Genetic Barrier to Drug Resistance. Sci Rep 2018, 8, 4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma C; Zhang J; Wang J Pharmacological Characterization of the Spectrum of Antiviral Activity and Genetic Barrier to Drug Resistance of M2-S31N Channel Blockers. Mol Pharmacol 2016, 90, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan ML; Gao S; Zhou ZW; Zhang KK; Liu SH; Wang ZY; Wang T A reverse genetics system for enterovirus D68 using human RNA polymerase I. Virus Genes 2018, 54, 484–492. [DOI] [PubMed] [Google Scholar]
- 39.Takeda M; Pekosz A; Shuck K; Pinto LH; Lamb RA Influenza A virus M-2 ion channel activity is essential for efficient replication in tissue culture. J Virol 2002, 76, 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]