Abstract
Objective
We investigated how pancreatic cancer developed resistance to FAK inhibition over time.
Design
Pancreatic ductal adenocarcinoma (PDAC) tumors from KPC mice (p48-CRE; LSL-KRasG12D/wt; p53flox/wt) treated with FAK inhibitor were analyzed for the activation of a compensatory survival pathway in resistant tumors. We identified pathways involved in the regulation of STAT3 signaling upon FAK inhibition by gene set enrichment analysis (GSEA), and verified these outcomes by RNA interference studies. We also tested combinatorial approaches targeting FAK and STAT3 in syngeneic transplantable mouse models of PDAC and KPC mice.
Results
In KPC mice, the expression levels of pSTAT3 were increased in PDAC cells as they progressed on FAK inhibitor therapy. This progression corresponded to decreased collagen density, lowered numbers of SMA+ fibroblasts, and downregulation of the TGFβ/SMAD signaling pathway in FAKi-treated PDAC tumors. Furthermore, TGFβ production by fibroblasts in vitro drives repression of STAT3 signaling and enhanced responsiveness to FAK inhibitor therapy. Knockdown of SMAD3 in pancreatic cancer cells abolished the inhibitory effects of TGFβ on pSTAT3. We further found that tumor-intrinsic STAT3 regulates the durability of the anti-proliferative activity of FAK inhibitor, and combinatorial targeting of FAK and JAK/STAT3 act synergistically to suppress pancreatic cancer progression in mouse models.
Conclusion
Stromal depletion by FAK inhibitor therapy leads to eventual treatment resistance, through the activation of STAT3 signaling. These data suggest that, similar to tumor-targeted therapies, resistance mechanisms to therapies targeting stromal desmoplasia may be critical to treatment durability.
INTRODUCTION
The prognosis for pancreatic cancer (PC) patients is dismal, with the 5-year survival rate less than 9%. This poor survival rate is driven by the high propensity of this disease to metastasize, and the lack of therapeutic efficacy from cytotoxic, targeted, and immune-based therapeutics. One proposed mechanism of resistance to therapy has been the uniquely desmoplastic tumor microenvironment (TME) of pancreatic ductal adenocarcinoma (PDAC). Included in this TME, high stromal density including excessive collagen deposition and activated fibroblasts are thought to provide a barrier to the delivery of cytotoxic and targeted agents and effector T cells, and likely improve PDAC cell survival even when these agents are delivered into the tumor1,2. Disruptors of stromal density are actively being investigated in multiple clinical trials. However, the limitations of and/or mechanisms of resistance to such approaches are only now becoming apparent.
Dysregulation of signal transducer and activator of transcription 3 (STAT3) occurs often in many human solid tumors3. The Janus kinases (JAK) play the most relevant biological role in linking STAT3 to the activity of cytokines. JAK/STAT3 signaling mediates multiple aspects of cytokine signaling in cancer cells, chiefly accelerating proliferation, increasing resistance to apoptosis, and promoting angiogenesis and metastatic potential4,5. The intrinsic activation of STAT3 in tumor cells is frequently observed in human solid malignancies, and is mainly caused by an oversupply of cytokines and growth factors present in the tumor microenvironment6,7. Recent studies have shown that excessive STAT3 activity in tumor cells provides a common mechanism by which the cells acquire resistance to targeted treatment8,9.
Focal adhesion kinases (FAKs) are non-receptor tyrosine kinases, which include FAK1 and PYK2/FAK2. Several studies have demonstrated that elevated FAK1 expression enhances tumor malignancy and correlates with poor prognosis10. Previous studies by our lab and others, have shown that pharmacologic targeting of FAK in pancreatic cancer models results in decreased stromal density and thus increases the responsiveness of the tumor to chemotherapy and immunotherapy, while simultaneously suppressing tumor progression11,12. In these studies, we observed that in response to FAK inhibition, most tumors exhibited a period of disease stabilization followed by resistant growth11. In the present study, we investigated how stromal-depletion leads to altered susceptibility to FAK inhibitor growth suppression through STAT3 activation.
MATERIALS AND METHODS
Pancreatic cancer tissue microarray cohort and analysis
Tissue microarray (TMA) studies were conducted on surgically resected PDAC specimens from patients diagnosed in the Department of Pathology at Washington University. To assemble TMAs, clearly defined areas of tumor tissue were demarcated, and two biopsies (1.0-mm diameter) were taken from each donor block. Four-micrometer paraffin sections were used for immunohistochemical (IHC) analyses. All human tissue studies were approved by the Washington University School of Medicine Ethics Committee. Fully automated image acquisition was performed using a Zeiss Axio Scan Z1 Slide Scanner system with a 10× objective (Carl Zeiss) to capture whole-slide digital images.
Genetic mouse model of PDAC
KPC (p48-CRE; LSL-KRas/KrasG12D/wt; p53flox/wt), KPPC, (p48-CRE; LSL-KrasG12D/wt; p53flox/flox) mice were generated in-house, and C57BL/6 breeders were obtained from the Jackson Laboratory. KPC and KPPC mice were backcrossed to C57BL/6 over six generations and validated as C57BL/6 congenic through SNP scanning. Mice were maintained within the Washington University Laboratory for Animal Care barrier facility, and all studies involving animals were approved by the Washington University School of Medicine Institutional Animal Studies Committee.
Orthotopic model and preclinical animal cohorts
Syngeneic orthotopic PDAC tumors were established by surgical implantation, as previously described11. Approximately 200,000 cells in 50 µL Matrigel (BD-Biosciences) were injected into the pancreas of each mouse. Cohorts of mice were randomized into different treatment groups upon gross palpation of tumor in the pancreas. In the transplantable model, 200,000 cells in 50 µL Matrigel (BD-Biosciences) were injected into each mouse’s back/flank. Cohorts of mice were randomized into different treatment groups by tumor volume (calculated as length*(width2)/2). Preclinical studies were conducted with 10~15-week-old female mice. Tumor burden was measured by establishing the gross wet weight of the pancreas and comparing it to that of five parallel mice sacrificed at the beginning of treatment. Mice were maintained within the Washington University Laboratory for Animal Care barrier facility. All studies involving animals were approved by the Washington University School of Medicine Institutional Animal Studies Committee.
Inhibitors and neutralizing antibodies
FAK inhibitor (FAKi) was provided by Verastem Inc. VS-4718 is a selective bispecific inhibitor for FAK and Pyk2 kinases, with a biochemical half-maximal inhibitory concentration (IC50) of 1.5 nM. VS-4718 was administered at 50 mg/kg by oral gavage b.i.d. STAT3 inhibitor (Stattic) and JAK1/2 inhibitor (ruxolitinib) were purchased from MedChem Express. Stattic was administered at 25 mg/kg by intraperitoneal injection once a day. Ruxolitinib was administered at 100 mg/kg by oral gavage b.i.d. For the survival study, KPC or KPPC mice were started on FAKi treatment at 3.5 or 1.5 months old, respectively. Mice were considered to have reached the survival endpoint when the pancreatic tumor reached 1.8 cm in diameter or the mice lost >20% of body weight, whichever occurred earlier. For T cell depletion, CD4- and CD8- neutralizing IgG antibodies (anti-mCD4 clone GK1.5, anti-mCD8 clone 2.43, BioXCell) were administered via i.p. injection every 4–5 days, with the first injection containing 500 µg before tumor implantation and subsequent injections containing 250 µg. For TGF-β1 neutralization, TGF-β1 antibody (clone 1D11.16.8, BioXCell) was given by intraperitoneal (i.p.) injection every other day for 14 days at 200 µg. In vitro, KP cells cultured alone, or co-cultured with pancreatic fibroblasts, were treated with 4 µg/mL TGF-β1 antibody (clone 1D11.16.8, BioXCell).
Cell line, constructs, and siRNAs
KP2 cells were derived from a KPC tumor obtained in house. Kras-INK (KI) cells were obtained from Dr. Douglas Hanahan’s laboratory. Panc-1 and Capan-1 cells were obtained from Dr. Kian H. Lim’s laboratory. Primary pancreatic fibroblasts were isolated from the normal pancreas from 8-week old female C57BL/6 mice. Briefly, the normal pancreas was dissected by manual mincing using a scalpel, followed by enzymatic digestion with 3.0 mg/ml collagenase A (Roche) and DNase I (Sigma) dissolved in Dulbecco’s Modified Eagle’s Medium (DMEM); (GIBCO) for 40 min at 37°C with constant stirring. Digestion mixtures were quenched by adding DMEM containing 10% fetal bovine serum (FBS) and filtered through 40-µm nylon strainers (Fisher Scientific). Single-cell suspensions were cultured in DMEM with 10% FBS for several passages to enrich mesenchyme-like fibroblasts. All experiments were performed with low-passage (passage 4–6) fibroblasts. Multiple batches of fibroblasts were used. All cell lines tested negative for MAP and mycoplasma.
To generate tumor cell lines stably expressing shSTAT3, KP2 cells were transduced with lentivirus particles carrying shRNA for 48 hours, following standard transduction protocols. Subsequently, cells were cultured in regular DMEM+F12 medium (Gibco) containing 7 µg/mL puromycin (Sigma-Aldrich) for 2 weeks. Surviving cells were tested for knockdown efficiency by immunoblotting.
The siRNAs targeting mouse STAT3, SMAD3, and human SMAD3 were purchased from Integrated DNA Technologies (IDT). Sequences are listed below: siSTAT3#1 5’-CACAUGGGCUAAAUUCUGCAAAGAA-3’; siSTAT3#2 5’-UUCUUUGCAGAAUUUAGCCCAUGUGAU-3’; siSMAD3 5’-CAAGUUGCAUCAAUGAAUUCACCTA-3’ and Human siSMAD3 5’-AGUCAGUUGCAUUCAUUAAAUCAAC-3’. Short hairpin RNA (shRNA) constructs targeting mouse STAT3 were purchased from the Genome Center at Washington University. The targeting sequence is listed in Supplementary Table 1.
Synergism analysis
On day 0, 1,000 KP2, Panc-1, and Canpan-1 cells were seeded in triplicates on 96-well plates pre-coated with collagen, and the cells were treated with eight fixed-ratio concentrations (all in µM) of VS-4718 to ruxolitinib (1:10, 0.5:5, 0.25:2.5, 0.125:1.25, 0.0625:0.625; 0.03125:0.3125; 0.015625:0.15625; 0.0078125:0.078125) and of VS-4718 to Stattic (1:0.5, 0.5:0.25, 0.25:0.125, 0.125:0.0625, 0.0625:0.03125, 0.03125:0.015625; 0.015625:0.0078125, 0.0078125:0.00390625). On day 3, old medium was replaced with fresh drug-containing medium. On day 5, cells were processed for the Alamar blue assay. Briefly, for each 100 µL cell culture medium, 25 µL 5× Alamar Blue reagent (Sigma-Aldrich) was added and the culture was shaken at room temperature (RT) for about 10 minutes, then incubated at 37°C for 1–2 hours. Fluorescence was read at 540 nm and 590 nm using a molecular device plate reader. All wells were normalized with the DMSO-treated group. Combination indices were calculated using Compusyn software (ComboSyn, Inc.).
RNA isolation and PCR
Total RNA was extracted from tissue or cells, using an E.Z.N.A.® Total RNA Kit (OMEGA). cDNAs were synthesized using qScript cDNA SuperMix (QuantaBio). Quantitative real-time PCR Taqman primer probe sets (Applied Biosystems) were used, and the relative gene expression was determined on an ABI 7900HT quantitative PCR machine (ABI Biosystems) using Taqman Gene Expression Master Mix (Applied Biosystems). The comparative threshold cycle method was used to calculate fold changes in gene expression, which were normalized to the expression of HPRT, GAPDH, and/or TBP as reference genes.
Immunofluorescence
Five-micrometer-thick fresh cryosections were air-dried and fixed in 4% PFA (Ted Pella) for 15 min before being washed three times with PBS. Tissues were permeabilized by incubating the slides in 0.5% Triton X-100 in PBS for 15 min at RT, and peroxidase-quenched by incubating in 1% hydrogen peroxide (Invitrogen) for 10 min at RT. After blocking for 1 hour at RT in blocking buffer (5% goat serum, 2.5% BSA in 1× PBS), slides were incubated overnight in a humidified chamber at 4°C with the anti-mouse antibodies listed in Supplementary Table 2. Following PBST (1× PBS with 0.05% Tween-20) washes, slides were incubated with Alexa Fluor 594- or Alexa Fluor 647-conjugated goat anti-mouse/rabbit secondary antibody (Invitrogen). For immunofluorescence staining by Tyramide Signal Amplification (TSA), slides were additionally blocked using an Avidin/Biotin Blocking Kit (Vector Labs) after using blocking buffer. After primary incubation and washes, streptavidin-HRP conjugate (PerkinElmer) was added and incubated for 30 min at RT. After three washes in PBST, the slides were incubated with TSA-Biotin (PerkinElmer) for 8 min at RT. After PBST washes, slides were incubated with Streptavidin-Alexa Fluor 594 (Life Technologies) for 30 min. Slides were subsequently washed and mounted using Vectashield containing DAPI (Vector Labs). For cell immunofluorescence staining, 5,000 normal pancreatic fibroblasts were seeded into an 8-well chamber slide (Lab-Tek II; Thermo Fisher Scientific) and cultured overnight. Slides were then processed as described previously for tissue immunofluorescence staining.
RNA in situ hybridization and Immunohistochemistry (IHC)
Fresh FFPE slides of KPC tumors were subjected to automated RNA in situ hybridization (ISH) using ACD RNAscope 2.5 LS reagents on the Leica Bond III RXm autostainer. TGF-β1 transcript (Mm- TGF-β1 probe Cat#407758) was visualized using DAB immunohistochemistry. Stained slides were scanned on the Zeiss Axio Scan Z1 Slide Scanner (Carl Zeiss Microscopy) at a magnification of 20× and analyzed on Indica Labs HALO platform. Tissues were fixed in formalin and embedded in paraffin according to standard protocols. Immunostaining was performed on the Leica Bond III RXm autostainer using Leica Bond ancillary reagents and Refine Polymer DAB detection system. Primary antibodies are listed in Supplementary Table 2. For TGF-β1 ISH plus aSMA IHC staining, ACD RNAscope 2.5 LS reagents without proteinase were used. Immunostaining was performed according to ISH and IHC protocols.
Microarray and data analysis
Microarray analysis was performed by the Genome Technology Access Center (GTAC) at Washington University, and the data generated has been deposited in NCBI’s Gene Expression Omnibus with accession number GSE75233. Gene Set Enrichment Analysis (GSEA) using KEGG_TGF_BETA_SIGNALING_PATHWAY-databases was performed as previously described13.
RESULTS
STAT3 signaling is hyperactivated in FAKi non-responsive tumors
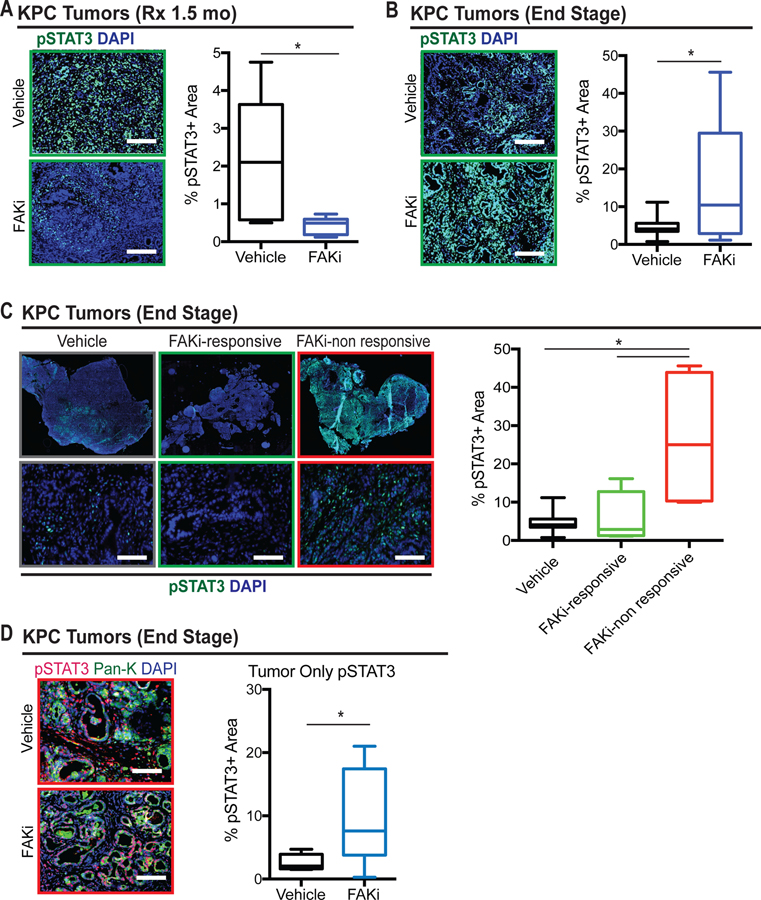
In our previous studies, we showed that both genetic and pharmacological targeting of FAK suppresses tumor progression in pancreatic cancer models11. We also observed that inhibition of FAK caused a period of stable disease followed by rapid progression, during which FAKi-treated tumors grew at the same rate as vehicle-treated tumors (Supplementary Figure 1A). Previously, reduced levels of phosphorylated STAT3 observed after short term FAKi-treatment were considered a biomarker of FAK inhibition, and this paralleled decreased proliferation in tumor and stromal cells. To determine if loss of disease control during FAK inhibition was associated with altered STAT3 signaling, we analyzed the expression levels of phosphorylated-STAT3 (pSTAT3) in PDAC tissues from either KPC mice treated for 1.5 months (wherein tumor progression is controlled in most animals) or from end-stage animals being treated for 3–5 months (where FAK inhibition had eventually failed in most animals). The total levels of pSTAT3 were significantly decreased in mice treated for 1.5 months with FAKi, compared to those of vehicle-treated mice (Figure 1A). In contrast, we observed a dramatic increase in pSTAT3 expression in end-stage FAKi-treated tumors (Figure 1B), indicating that STAT3 signaling becomes hyperactivated after prolonged inhibition of FAK signaling. Although many KPC mice in the study showed prolonged stable disease following treatment with FAK inhibitors, a subset of mice exhibited rapid progression11. To explore if STAT3 signaling was altered in those mice that showed limited de novo response to FAK inhibition, we analyzed PDAC tissues for pSTAT3 levels of end-stage KPC mice stratified by duration of response to FAKi, and found that there was a significant increase in the level of pSTAT3 in tumors with limited response to FAKi (Figure 1C and Supplementary Figure 1B). Moreover, the survival of FAKi-treated mice was inversely correlated with the overall pSTAT3 expression in the tumor, suggesting that pSTAT3 might be a predictive biomarker for FAK inhibition (Supplementary Figure 1C).
Figure 1. Elevated expression of pSTAT3 in end-stage FAKi-treated tumors.
(A) Representative immunofluorescence staining for pSTAT3 in PDAC tissue from 1.5-month vehicle-and FAKi-treated KPC mice. Scale bar, 100 µm. Right, the percentage of pSTAT3+ area for each treatment group (n = 5–6 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(B) Representative immunofluorescence staining for pSTAT3 in PDAC tissue from end-stage vehicle-and FAKi-treated KPC mice. Scale bar, 100 µm. Right, the percentage of pSTAT3+ area for each treatment group (n = 8–11 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(C) Representative whole-tissue and 10× inset of immunofluorescence staining for pSTAT3 in PDAC tissue from end-stage vehicle- and FAKi-treated (responsive and non-responsive) KPC mice. Scale bar, 100 µm. Right, the percentage of pSTAT3+ area for each treatment group (n = 5–8 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(D) Representative immunofluorescence staining for pSTAT3 and tumor marker pan-keratin (Pan-K) in tumor from end-stage vehicle- and FAKi-treated KPC mice. Scale bar, 50 µm. Right, the percentage of pSTAT3+ area in tumor from each treatment group (n = 7 mice per group). Error bars represent mean s.e.m. *P < 0.05 by Student’s t-test.
To explore which compartments in the tumor microenvironment (TME) express high levels of pSTAT3, we differentiated pSTAT3 levels in both tumor and stromal cells of non-responsive KPC tumors. We found that pSTAT3 was highly expressed in both tumor and stromal cells (Figure 1D and Supplementary Figure 1G). To confirm our findings from genetic mouse models of PDAC, we further analyzed pSTAT3 in tumors from both KP and KI transplantable models14. Consistent with the data in KPC mice, we found that there was a significant increase in the expression of the pSTAT3 protein in KI and KP2 tumors that outgrew FAK inhibition (Supplementary Figure 1D, 1E, and 1F). Taken together, these data suggest that STAT3 signaling is activated, with a corresponding loss of disease control, during FAK inhibitor therapy.
Tumor cell-intrinsic STAT3 regulates the durability of the FAK inhibitor response.
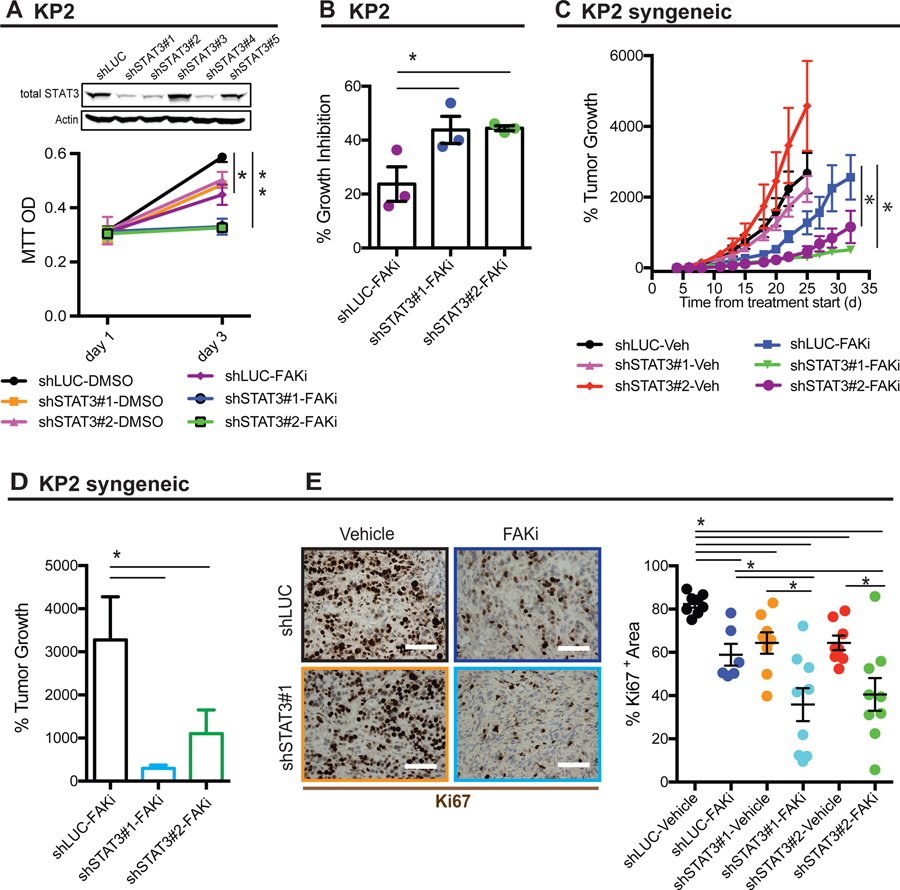
Base on the dramatic increase in pSTAT3 expression in end-stage FAKi-treated tumors, we hypothesized that intrinsic STAT3 expression in tumor cells might be critical in their response to FAK inhibitor. To test this, we knocked down STAT3 in in the KPC-derived PDAC cell line KP2, and found that although STAT3 depletion did not change cell proliferation on its own, loss of STAT3 did dramatically increased the sensitivity of cells to FAK inhibitor (Figure 2A and 2B). In vivo, we also found that STAT3-deficient PDAC cells had prolonged responsiveness to FAKi and greater tumor repression compared to STAT3-proficient tumors (Figure 2C and 2D). Concomitant with this increased susceptibility to FAKi treatment, proliferation as marked by Ki67 was markedly reduced in FAKi-treated STAT3-deficient tumors (Figure 2E). To access the impact of STAT3 expression in pancreatic fibroblasts on FAK-responsiveness, we co-cultured KP cells with either control (siNC) or STAT3-depleted (siSTAT3) fibroblasts in the presence of FAKi. We found that loss of pSTAT3 in fibroblasts did not affect the inhibitory effects of FAKi on KP cells (Supplementary Figure 2A and 2B). Taken together, these data suggest that STAT3 in fibroblasts may not be the critical driver of FAK inhibitor resistance, and support the conclusion that tumor cell-intrinsic STAT3 regulates the durability of the FAK inhibitor response.
Figure 2. Tumor-intrinsic STAT3 contributes to FAKi response.
(A) Representative immunoblot for total STAT3 and β-actin (loading control) in KP cells stably expressing control shRNA (shLUC) or shSTAT3 constructs (shSTAT3#1, 2, 3, 4, and 5). Bottom, MTT proliferation assay using KP cells stably expressing shLUC or shSTAT3 constructs (shSTAT3#1 and 2) treated with DMSO or 1 µM FAKi. Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(B) Histogram showing the percentage of growth inhibition in KP cells stably expressing shLUC or shSTAT3 constructs (shSTAT3#1 and 2) treated with DMSO or 1 µM FAKi. Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(C) Syngeneic tumor growth of KP cells stably expressing shLUC or shSTAT3 in mice treated with vehicle or 50 mg/kg FAKi. All animal experiments included 8–9 mice per group unless otherwise specified. Error bars represent mean ± s.e.m. *P < 0.05; by one-way ANOVA with Dunnett’s multiple-comparison test.
(D) Histogram showing the percentage of tumor growth KP cells stably expressing shLUC or shSTAT3 in mice treated with vehicle or 50 mg/kg FAKi. Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(E) Representative immunohistochemistry staining for pSTAT3 in PDAC tissues from C57BL/6 mice subcutaneously transplanted with 200,000 KP cells stably expressing shLUC or shSTAT3 constructs (shSTAT3#1 and 2) and then treated with FAKi or vehicle. Scale bar, 100 µm. Right, the percentage of pSTAT3+ area for each treatment group (n = 6–9 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test
FAKi downregulates TGF-β/SMAD3 signaling via reducing fibroblasts and collagen
To determine if STAT3 signaling was directly upregulated by FAK inhibition, we stimulated KP (Supplementary Figure 3A) and KI cells (Supplementary Figure 3B) with FAKi, and found that there was no change in pSTAT3 levels among different groups. Moreover, silencing of both FAK1 and PYK2 in KP cells did not modulate pSTAT3 expression, suggesting that activation of STAT3 signaling was not due to the loss of FAK1/PYK2 in tumor cells (Supplementary Figure 3C). Additionally, long-term selection for FAKi-resistant cell lines did not result in elevated pSTAT3 signaling (Supplementary Figure 3D). These data suggest that exposure to FAK inhibition alone does not alter STAT3 signaling, and that FAKi-induced changes in the TME might be at involved.
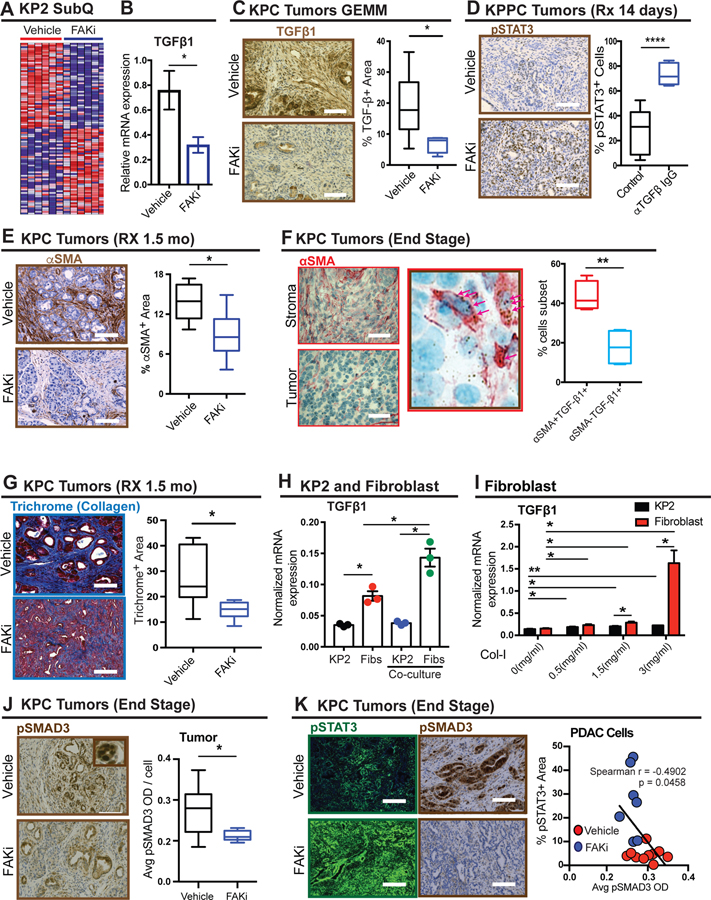
To further explore how FAK inhibition might change the TME, we profiled gene expression in PDAC tissue in vehicle- or FAKi-treated mice. Gene set enrichment analysis (GSEA) revealed enrichment of genes involved in the TGF-β signaling pathway in downregulated gene sets (Figure 3A, Supplementary Figure 3E, and Tables 3–6). To better understand the mechanisms leading to downregulation of the TGF-β pathway, we evaluated several TGF-β receptor ligands and pathway signaling component genes. Among TGF beta receptor ligands, the mRNA of TGF-β1 was expressed at 10- to 100-fold higher than that of TGF-β2 and 3, or BMP-5, 6 and 7 (Supplementary Figure 3G). Additionally, TGF-β1 was significantly downregulated in FAKi-treated tumors (Figure 3B), but the majority of other signaling components were not (Supplementary Figure 3I). Consistent with these results, the protein levels of TGF-β1 were also suppressed by FAKi (Figure 3C). To validate whether TGF-β1 was involved in repressing pSTAT3 hyperactivation, we treated KPPC mice with a TGF-β-neutralizing antibody and observed that pSTAT3 was upregulated in PDAC tissue from TGF-β-neutralized mice (Figure 3D and Supplementary Figure 3F). These data suggest that prolonged FAK inhibition leads to downregulation of TGF-β in the TME and thus potentiates STAT3 signaling.
Figure 3. FAKi triggers pSTAT3 via inhibiting fibroblast- mediated TGF-β1/ SMAD3 signaling.
(A) Heatmap of top 50 downregulated and upregulated genes by FAK inhibition in KP transplantable tumors following treatment with vehicle or FAKi (n = six mice per group).
(B) mRNA expression analysis of TGF-β1 from a gene array of KP2 transplantable tumors following treatment with vehicle or FAKi (n = six or seven mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test
(C) Representative immunohistochemistry staining for TGF-β1 in PDAC tissue from 1.5-month vehicle-and FAKi-treated KPC mice. Scale bar, 100 µm. Right, the percentage of TGF-β+ area for each treatment group (n = 6–7 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(D) Representative immunohistochemistry staining for pSTAT3 in PDAC from KPPC mice treated with vehicle and TGF-β neutralizing antibody. Scale bar, 100 µm. Right, the percentage of pSTAT3+ cells for each treatment group (n = 5–8 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(E) Representative immunohistochemistry staining for αSMA in PDAC tissue from 1.5-month vehicle-and FAKi-treated KPC mice. Scale bar, 100 µm. Right, the percentage of αSMA+ area for each treatment group (n = 6–7 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(F) Representative immunohistochemistry staining for αSMA combined with TGF-β1 RNA in situ hybridization in PDAC tissue from end-stage KPC mice. Scale bar, 25 µm. Arrows indicate TGF-β1 mRNA ISH. Right, the percentage of αSMA+ TGF-β+ cells, and αSMA− TGF-β+ cells (n = 4). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(G) Representative immunohistochemistry staining for Trichrome in PDAC tissue from 1.5-month vehicle- and FAKi-treated KPC mice. Scale bar, 100 µm. Right, the percentage of Trichrome+ area in tumor from each treatment group (n = 7 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(H) mRNA level of TGF-β1 in KP cells and fibroblasts cultured alone or co-cultured with each other (n =3). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(I) mRNA level of TGF-β1 in fibroblasts and KP cells cultured on different density of collagen (n =3). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(J) Representative immunohistochemistry staining for pSMAD3 in tumor from end-stage vehicle- and FAKi-treated KPC mice. Scale bar, 100 µm. Right, The mean pSMAD3+ area in tumor from end-stage vehicle- and FAKi-treated KPC mice. Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(K) Representative immunofluorescence staining for pSTAT3 and immunohistochemistry staining for pSMAD3 in PDAC tissue from end-stage vehicle- and FAKi-treated KPC mice. Scale bar, 100 µm. Right, scatter plot showing Spearman’s correlation between the percentage of pSTAT3+ area and percentage of pSMAD3+ area in PDAC tissue from end-stage vehicle- and FAKi-treated KPC mice.
Because FAK inhibitor treatment leads to both stromal depletion and STAT3 activation, we hypothesized the two might be linked through TGF-β1. We first verified, in concordance with our published data, that FAK inhibition led to reduced collagen density and SMA+ fibroblast presence in the PDAC TME at time points corresponding to reduced TGF-β1 expression (Figure 3E and G). Next, we evaluated the relative expression of TGF-β1 in PDAC cells and SMA+ fibroblasts in PDAC tissue using RNA in situ hybridization (ISH) and found that the TGF-β1 transcript was expressed at higher levels in SMA+ fibroblasts (Figure 3F). Consistent with this result, in vitro studies demonstrated that the mRNA levels of TGF-β1 in pancreatic fibroblasts were higher than those in corresponding PDAC cells, and increased significantly when co-cultured with PDAC cells (Figure 3H). Additionally, we observed that collagen density, which is reduced in vivo by FAK inhibition (Figure 3G), significantly increased TGF-β1 expression in fibroblasts compared to their PDAC cell counterparts (Figure 3I). Notably, FAK inhibition did not alter TGF-β1 mRNA expression in either fibroblasts or PDAC cells (Supplementary Figure 3J). Taken together, these results suggest that prolonged FAK inhibition reduces TGF-β1 in the PDAC TME by reducing αSMA+ fibroblast number and collagen density.
In conjunction with TGF-β1 loss and STAT3 activation, we also observed decreased pSMAD3 levels in PDAC cells, but not stromal cells, in end-stage FAKi-treated tumors (Figure 3J and Supplementary Figure 3H). Additionally, the expression levels of pSTAT3 were inversely correlated with the expression of pSMAD3, but not of pSMAD2 (Figure 3K, Supplementary Figure 4A–D), suggesting SMAD3 may be a critical mediator of STAT3 activation in PDAC cells.
High levels of pFAK1 and pSMAD3 predict unfavorable prognoses in pancreatic cancer patients
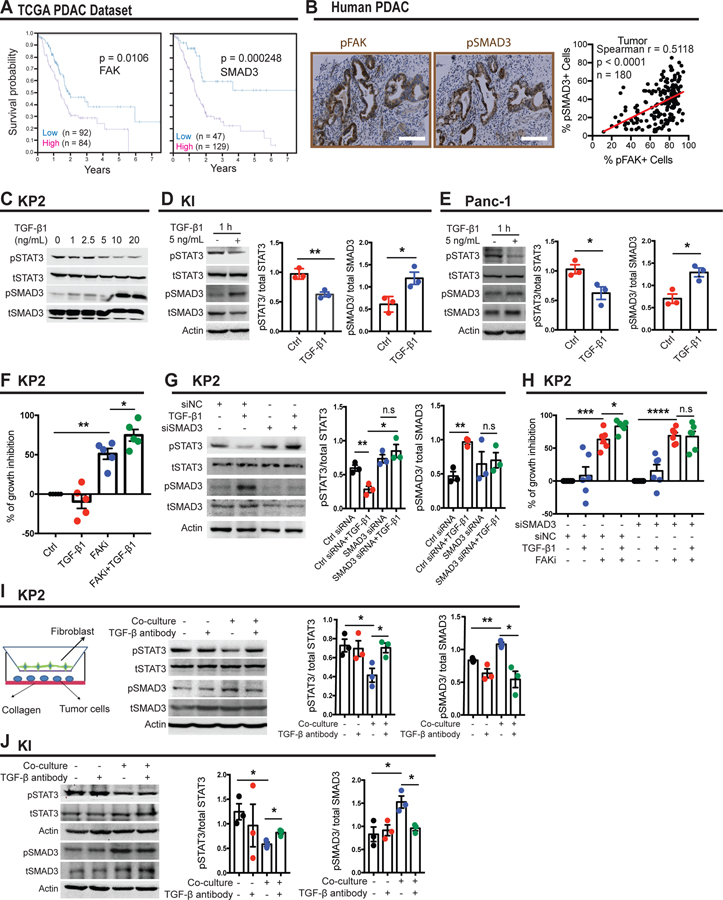
Next, we investigated the preponderance and impact of FAK and SMAD3 pathway upregulation in human PDAC patients. The Cancer Genome Atlas (TCGA) database revealed that elevated expression of pFAK or pSMAD3 in human PDAC tumors independently defined a subset of patients with worse prognosis (Figure 4A). To confirm these findings, we stained pancreatic cancer tissue microarrays (TMAs) for pFAK or pSMAD3 by immunohistochemistry. Consistent with our findings in KPC mice, pSMAD3 is expressed in both the tumor and stroma, whereas pFAK is mainly upregulated in ductal tumor cells, and a statistically significant positive correlation was observed between pFAK and pSMAD3 (Figure 4B, Supplemental Figure 4E, 5A and B), indicating FAK and SMAD3 might be key mediators of clinical outcomes in pancreatic cancer patients.
Figure 4. pSTAT3 is suppressed by TGF-β1/ SMAD3 pathway.
(A) Kaplan-Meier survival curves for FAK and SMAD3 mRNA expression in the TCGA patient dataset for pancreatic adenocarcinoma (PAAD).
(B) Representative immunohistochemistry staining for pFAK and pSMAD3 in human pancreatic cancer tissue microarray. Scale bar, 100 µm. Right, scatter plot showing Spearman’s correlation between the percentage of pFAK+ cells and percentage of pSMAD3+ cells in human pancreatic cancer tissue samples.
(C) Representative western blot for pSTAT3, total STAT3, pSMAD3, total SMAD3 in KP cells treated with the denoted dosages of TGF-β1 for 1 hour.
(D) Representative immunoblot for pSTAT3, total STAT3, pSMAD3, total SMAD3 and β-actin (loading control) in KI cells treated with 5 ng/mL TGF-β for 1 hour.
(E) Representative immunoblot for pSTAT3, total STAT3, pSMAD3, total SMAD3 and β-actin (loading control) in Panc-1 cells treated with 5 ng/mL TGF-β for 1 hour.
(F) Histogram showing the percentage of growth inhibition in KP cells. Cells were grown on 7.5 mg/mL basement membrane extract and treated with 5ng/mL TGF-β1 in the presence or absence of 1 µM FAKi for 4 days. Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(G) Representative immunoblot for pSTAT3, total STAT3, pSMAD3, total SMAD3 and β-actin (loading control) in KP cells transiently transfected with control siRNA (siNC) or siRNA targeting SMAD3 in the presence or absence of 5 ng/mL TGF-β for 1 hour.
(H) Histogram showing the percentage of growth inhibition in KP cells. Cells were grown on 7.5 mg/mL basement membrane extract and transiently transfected with control siRNA (siNC) or siRNA targeting SMAD3. Then cells were treated with 5 ng/mL TGF-β1 in the presence or absence of 1 µM FAKi for 4 days. Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(I) Representative immunoblot for pSTAT3, total STAT3, pSMAD3, total SMAD3 and β-actin (loading control) in KP cells cultured alone or co-cultured with fibroblasts in the presence or absence of 4 µg/mL TGF- β neutralizing antibody for 48 hours.
(J) Representative immunoblot for pSTAT3, total STAT3, pSMAD3, total SMAD3 and β-actin (loading control) in KI cells cultured alone or co-cultured with fibroblasts in the presence or absence of 4 µg/mL TGF- β neutralizing antibody for 48 hours.
TGF-β1/ SMAD3 signaling represses activation of STAT3 signaling
Next, we sought to test whether SMAD3 activation suppresses STAT3 signaling in pancreatic cancer cells. To accomplish this, we treated KP2 cells growing on collagen-I with TGF-β1. Intriguingly, TGF-β1 preferentially activated pSMAD3 and suppressed pSTAT3 in a time- and dose-dependent manner (Figure 4C and Supplementary Figure 5C). This signaling regulation was further confirmed in KI (Figure 4D), Panc-1 (Figure 4E) and Capan-1 cells (Supplementary Figure 5D). To explore whether TGF-β1 inhibited pSTAT3 through SMAD3, we knocked down SMAD3 in KP2 and Panc-1 cells and observed that TGF-β1 no longer inhibited pSTAT3 expression (Figure 4G and Supplementary Figure 5G). Additionally, we observed that TGF-β1 increased the growth inhibition sensitivity of KP2 cells to FAK inhibitor, in a SMAD3-dependent manner (Figure 4F, H). These data suggest a critical role for the TGF-β/SMAD3 signaling in FAKi response through STAT3. We also observed that after 1 hour of TGF-β1 exposure, pJAK1 is downregulated in a SMAD3-dependent manner (Supplementary Figure 5G), suggesting direct signaling inhibition. Exploring this further, we found that when Ruxolinitib, a JAK1/2 inhibitor, was given to Panc-1 cells, TGF-β1 could not further inhibit pSTAT3 expression (Supplementary Figure 5H). As demonstrated in Supplementary Figure 5E and F, we concluded that the non-canonic TGF-β signaling pathway was not required in FAKi resistance. Based on our in vivo data, which implicate SMA+ fibroblasts in the production of TGF-β1, we sought to determine whether fibroblast-secreted TGF-β1 regulated pSMAD3/pSTAT3 signaling in vitro. As seen in Figure 4I and J, neutralizing TGF-β in the media of tumor cells had no impact on either pSMAD3 or pSTAT3. In contrast, co-culture with fibroblasts increased pSMAD3 and decreased pSTAT3 levels in both KP2 and KI cells, and TGF-β neutralization abolished these effects. These data suggest that fibroblast- secreted TGF-β1 suppresses pSTAT3 signaling in PDAC cells and thus mediates FAKi sensitivity.
Pharmacological inhibition of JAK/STAT signaling improves response to FAK inhibition
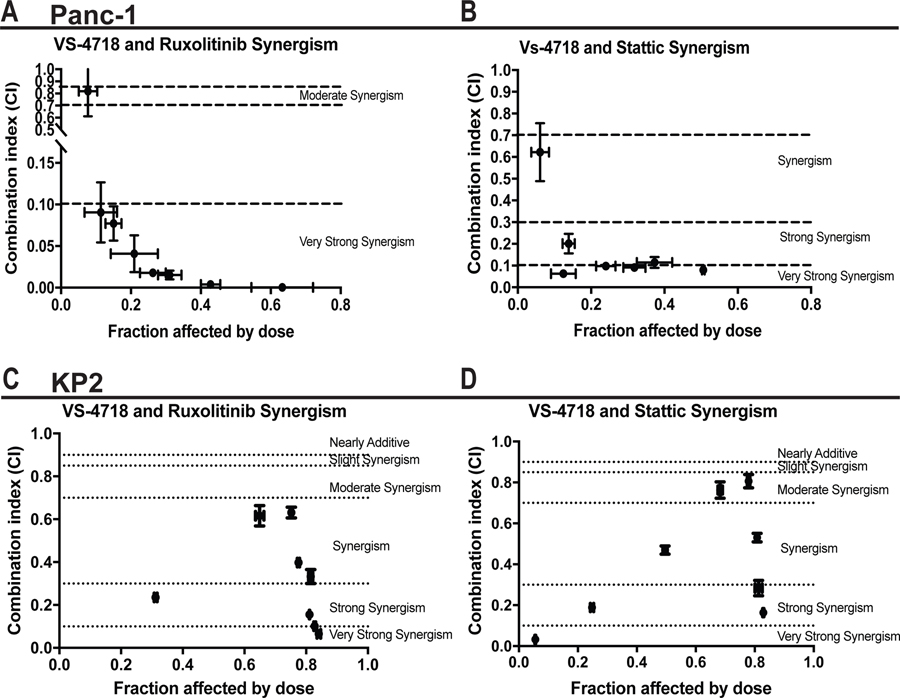
Given the fact that genetic knockdown of STAT3 improves response to FAK inhibition, we sought to test the ability of pharmacologic inhibition of STAT3 to improve FAK inhibitor efficacy both in vitro and in vivo. To target STAT3 signaling, we used ruxolitinib (JAK1/2 inhibitor) and Stattic (STAT3 inhibitor). We first treated human cell lines (Panc-1, Capan-1) or murine PDAC lines (KP2) with control, ruxolitinib, or Stattic in the presence or absence of FAKi. We found that single-agent ruxolitinib or Stattic had little impact on cell proliferation, whereas FAKi significantly inhibited cell proliferation (Supplementary Figure 6A and B). By contrast, the combination of FAK inhibitor with ruxolitinib or Stattic suppressed cell proliferation synergistically in vitro (Figure 5A–D and Supplementary Figure 6C–D).
Figure 5. Synergism of FAK inhibitor and Ruxolitinib/Stattic.
(A-D) Median effect analysis showing the interaction between FAKi and Ruxolitinib or Stattic in Panc-1 (A-B) or KP2 (C-D), analyzed using Compusyn software.
Data represent the combined mean ± s.e.m of three independent experiments each performed in duplicate. Horizontal dotted lines indicate the boundaries for each interaction classification.
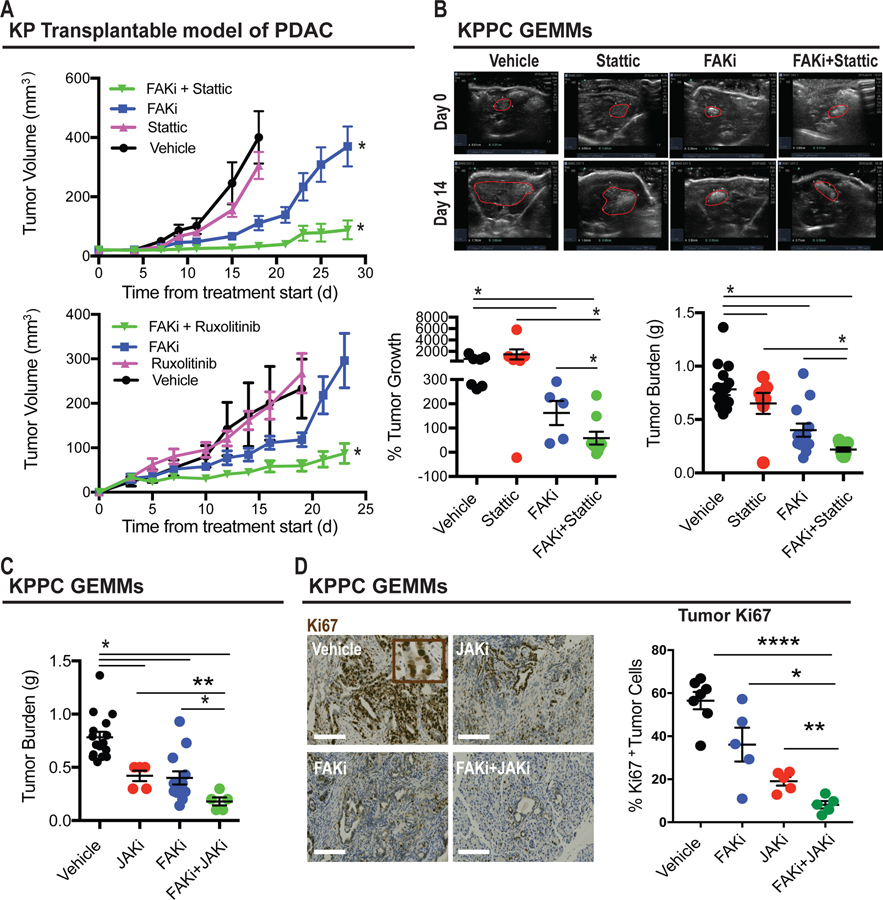
We next tested the ability of FAKi and either JAK or STAT3 inhibitors to restrain tumor growth in vivo using syngeneic transplantable tumors and the genetic KPPC PDAC model. We found that single-agent Stattic or ruxolitinib did not markedly alter tumor progression in either model, but the combination of these agents synergized to enhance the ability of FAKi to restrain tumor progression in both model systems (Figure 6A–C). To better understand how the combination of FAKi and Stattic regulated cell fate in vivo, we evaluated cell proliferation and apoptosis in tumors from mice treated with vehicle, ruxolitinib, FAKi, or the combination of ruxolitinib and FAKi. We found that ruxolitinib combined with FAKi dramatically suppressed cell proliferation when compared to FAKi alone (Figure 6D). Neither FAKi nor the combination of FAKi and JAKi induced apoptotic cells (Supplementary Figure 7A). To investigate the impact of FAK plus JAK inhibition on the TME, we evaluated collagen density and T cell infiltration and found that in contrast to PDAC cell proliferation, there were no synergistic effects of adding JAK inhibitors to FAK blockade (Supplementary Figure 7B and C). Further, depletion of CD4+ or CD8+ T cells did not impact the efficiency of the combination (Supplementary Figure 7D). Taken together, these findings suggest that pharmacologic inhibition of JAK/STAT3 signaling sustains PDAC responsiveness to FAK inhibitor via suppression of tumor cell proliferation.
Figure 6. Pharmacological inhibition of STAT3 improves tumor responsive to FAKi.
(A) Tumor growth curve of KP tumor-bearing mice treated with vehicle, Stattic, FAKi, or Stattic + FAKi (upper panel). Lower panel, tumor growth curve of KP tumor-bearing mice treated with vehicle, Ruxolitinib, FAKi, or Ruxolitinib + FAKi. All graphs depict mean ± s.e.m. * indicates P < 0.05 by one-way ANOVA with Tukey’s method for multiple comparisons.
(B) Representative ultrasound images of tumors for KPPC mice treated with vehicle, Stattic, FAKi, or FAKi + Stattic, with tumor outlined in red (upper panel). Lower panel, scatter dot plots showing the percentage of tumor outgrowth and tumor weight (in grams) for each treatment group (n = 5–16 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
(C) Scatter dot plots showing tumor weight (in grams, right) for KPPC mice treated with vehicle, Ruxolitinib, FAKi, or FAKi + Ruxolitinib (n = 5–16 mice per group). All graphs depict mean ± s.e.m. * indicates P < 0.05 by unpaired two-sided Student’s t-tests.
(D) Representative immunohistochemistry staining for Ki67 in tumor from KPPC mice treated with vehicle, Ruxolitinib, FAKi, or FAKi + Ruxolitinib. Scale bar, 100 µm. Scatter dot plots (right) showing the number of Ki67+ tumor cells for each treatment group (n = 5–7 mice per group). Error bars represent mean ± s.e.m. *P < 0.05 by Student’s t-test.
DISCUSSION
In this study, we demonstrate that prolonged FAK inhibition leads to STAT3 hyper-activation and resistance to FAK-targeted therapy. Ironically, this resistance is incurred by the stromal depleting activity of FAK inhibitors, which lead to loss of stromal TGF-β1-mediated antagonism of STAT3 activation. We find this is an intriguing concept, whereby a targeted agent causes alterations in stromal desmoplasia, leading to eventual therapeutic resistance. Nonetheless, in these studies we found that this could be overcome by dual inhibition of FAK and JAK/STAT3 signaling to induce more durable suppression of PDAC progression.
FAK signaling has been heavily studied in the context of cancer cell migration, proliferation, and survival10, suggesting that FAK might be a potential therapeutic target. We recently found that FAK functions as a central driver of the fibrotic and immunosuppressive microenvironment that protects tumors from immune surveillance and drives resistance to immunotherapy11. Although single-agent-FAK inhibition caused dramatic survival extension in genetic mouse models of PDAC, we observed that some tumors progressed rapidly upon FAK inhibition, indicating that these FAKi-treated tumors acquired resistance. Here, we have discovered that FAK inhibition results in activation of STAT3 signaling through downregulation of the TGF-β pathway. We have shown that TGF-β1 originates from the stroma, and we have demonstrated that FAK inhibition can reduce TGF-β1 expression at the mRNA level. Furthermore, knockdown of SMAD3 in tumor cells abolished the inhibitory effects of TGF-β1 on pSTAT3 expression levels. We also found that pSMAD3 is positively correlated with pFAK in human PDAC patients, suggesting that the TGF-β pathway might be a new target in PDAC. Interestingly, we found that SMAD2 was upregulated in tumor cells upon FAK inhibition (Supplementary Figure 4C), suggesting that SMAD2 activation might induce STAT3 activation. Consistent with our findings, previous studies reported that phosphorylation status determines the opposing functions of SMAD2/SMAD3 as STAT3 cofactors in TH17 differentiation15. The mechanism by which SMAD2 and SMAD3 might function in opposite ways during PDAC progression needs to be further explored. The interplay between TGF-β signaling and STAT3-dependent signaling exists in various physiological and pathophysiological contexts, such as tumor development16, as well as murine embryonic stem cell self-renewal and differentiation17. Further study is needed to better understand how this direct interplay between Stat3 and Smad3 impacts these biological functions.
Drug resistance is a common issue when targeted therapy is deployed in both preclinical areas and in clinical settings18. When advanced cancers are treated with targeted agents acting on an oncogenic driver, resistance emerges almost invariably, either through acquired resistance or intrinsic resistance19,20. Acquired drug resistance is often the result of clonal selection of a pre-existing population of cancer cells that circumvents the targeted agent, often through an additional genetic alteration or bypass of cell signaling. Constitutive activation of STAT3 has frequently been observed in a variety of tumors, including melanoma, pancreatic cancer, lung cancer, colorectal cancer, breast cancer, brain cancer, prostate cancer, glioma, lymphoma, and leukemia3. Increasing evidence has implicated STAT3 in resistance to conventional chemotherapy and immunotherapy21. In the present study, we have uncovered a tumor-intrinsic escape mechanism in response to FAK inhibition. We provide new evidence that STAT3 activation in tumor cells is critical for driving the FAK inhibitor response.
STAT3 is largely believed to be a key oncogene, and intensive efforts have been devoted to developing STAT3 inhibitors. However, no inhibitor that directly targets STAT3 has yet been approved by the FDA for clinical use. As proof of concept, in this study, we used Stattic and ruxolitinib to target different levels of the STAT3 signaling cascade. Surprisingly, both Stattic and ruxolitinib showed a strong synergism with FAKi in vitro and in vivo. Stattic is a nonpeptidic small molecule that selectively inhibits the STAT3 SH2 domain regardless of STAT3 activation state in vitro. Stattic selectively inhibits the activation, dimerization, and nuclear translocation of STAT3, and has been shown to increase the apoptosis of STAT3-addicted breast cancer cell lines22. In our hands, Stattic has shown high potency and efficacy in cell lines and tumors in vivo. However, we have observed toxicity of Stattic in mice (body weight loss, data not shown). By contrast, mice tolerated either single-agent ruxolitinib or a combination of ruxolitinib and FAKi quite well. Our observations raise concerns about the use of Stattic in a clinical setting. There remains an unmet need to develop selective and safe STAT3 inhibitors.
Taken together, these findings provide evidence that feedback activation of STAT3 signaling in response to decreased stromal desmoplasia contributes to single-agent FAKi therapy resistance. Moreover, these data suggest there might be additional mechanisms by which stromal depleting agents can lead to unforeseen resistance to either targeted or cytotoxic agents, which warrant further exploration.
Supplementary Material
The significance of this study.
What is already known on this subject?
The FAK, STAT3, and TGFb/SMAD pathways are all hyperactivated in human PDAC, and these data suggest they undergo reciprocal regulation in PDAC cells.
Targeting of FAK signaling suppresses PDAC progression and enhances overall survival in animal models, but tumors will eventually develop therapeutic resistance.
FAK inhibitor clinical trials are ongoing in human PDAC patients and the development of resistance is expected.
Signal transducer and activator of transcription 3 (STAT3) signaling is linked to treatment resistance in several tumor contexts.
What are the new findings?
Reprogramming of the PDAC tumor microenvironment (TME) by FAK inhibition induces a feedback loop whereby decreased stromal TGFb enhances PDAC cell proliferation.
STAT3 signaling is hyperactivated following prolonged FAK inhibitor treatment and enhances FAK-independent PDAC growth.
TGFβ/SMAD3 signaling suppresses STAT3 activation in PDAC cells, but is diminished as FAK inhibitor treatment decreases stromal desmoplasia.
Combined inhibition of JAK/STAT and FAK signaling shows durable efficacy in PDAC animal models.
How might it impact clinical practice in the near future?
There are two ongoing clinical trials in pancreatic cancer that combine FAK inhibitors with either chemotherapy or combined chemo- and immunotherapy. Thus, understanding how FAK inhibitor reprogramming of the PDAC TME might alter tumor-intrinsic resistance signaling is important.
Combinatorial targeting of JAK/STAT and FAK signaling is tractable for translation into near-term clinical testing.
Acknowledgments:
The authors would like to thank Dr. James Fitzpatrick from the Washington University Center for Cellular Imaging (WUCCI). The authors would like to thank Dr. Greg Williams from the Department of Surgery, Washington University School of Medicine for assistance with TMA analysis. S.H. is supported by a fellowship award from the National Cancer Institute (F99 CA223043).
Funding: This work was supported by funding awarded to D.G.D. from the National Cancer Institute (P50 CA196510, R01 CA177670, R01 CA203890).
Footnotes
Competing interests: None declared.
Ethics approval: The protocol was approved by the Ethics Committee of Washington University School of Medicine.
References
- 1.Feig C et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 110, 20212–20217, doi: 10.1073/pnas.1320318110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozdemir BC et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734, doi: 10.1016/j.ccr.2014.04.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Zaid Siddiquee K & Turkson J STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res 18, 254–267, doi: 10.1038/cr.2008.18 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H, Pardoll D & Jove R STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9, 798–809, doi:nrc2734 [pii] 10.1038/nrc2734 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H, Kortylewski M & Pardoll D Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7, 41–51, doi:nri1995 [pii] 10.1038/nri1995 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Buchert M, Burns CJ & Ernst M Targeting JAK kinase in solid tumors: emerging opportunities and challenges. Oncogene 35, 939–951, doi: 10.1038/onc.2015.150 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Siveen KS et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim Biophys Acta 1845, 136–154, doi: 10.1016/j.bbcan.2013.12.005 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ et al. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell 26, 207–221, doi: 10.1016/j.ccr.2014.05.019 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Huynh J, Etemadi N, Hollande F, Ernst M & Buchert M The JAK/STAT3 axis: A comprehensive drug target for solid malignancies. Semin Cancer Biol 45, 13–22, doi: 10.1016/j.semcancer.2017.06.001 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Sulzmaier FJ, Jean C & Schlaepfer DD FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 14, 598–610, doi: 10.1038/nrc3792 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 22, 851–860, doi: 10.1038/nm.4123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laklai H et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med, doi: 10.1038/nm.4082 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian A et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550, doi: 10.1073/pnas.0506580102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchem JB et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 73, 1128–1141, doi: 10.1158/0008-5472.CAN-12-2731 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon JH et al. Phosphorylation status determines the opposing functions of Smad2/Smad3 as STAT3 cofactors in TH17 differentiation. Nat Commun 6, 7600, doi: 10.1038/ncomms8600 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G et al. STAT3 selectively interacts with Smad3 to antagonize TGF-beta signalling. Oncogene 35, 4422, doi: 10.1038/onc.2016.145 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Ying QL, Nichols J, Chambers I & Smith A BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281–292 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Holohan C, Van Schaeybroeck S, Longley DB & Johnston PG Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 13, 714–726, doi: 10.1038/nrc3599 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Misale S et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486, 532–536, doi: 10.1038/nature11156 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misale S et al. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med 6, 224ra226, doi: 10.1126/scitranslmed.3007947 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Zhao C et al. Feedback Activation of STAT3 as a Cancer Drug-Resistance Mechanism. Trends Pharmacol Sci 37, 47–61, doi: 10.1016/j.tips.2015.10.001 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Schust J, Sperl B, Hollis A, Mayer TU & Berg T Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol 13, 1235–1242, doi: 10.1016/j.chembiol.2006.09.018 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.