Abstract
自驱动微纳米马达(MNMs)是一种能够将周围环境中的化学能或者外部能量转化为机械动能从而产生自主运动的新型人造微纳米机器。基于其自主运动能力,这些微纳米马达能够自主地运动到目标位置,因此在生物医学方面展现出了广阔的应用前景,尤其是在药物递送领域。研究证明微纳米马达能够有效地进行药物的装载并主动地递送至病灶区域,因此与常规纳米药物载体相比,能够更大限度地提高疗效并同时降低毒副作用。本文首先概述了微纳米马达的各种驱动机制,包括基于氧化还原反应的化学驱动和基于光、磁、电、超声等外部能量的外场驱动。随后总结了近十年来基于化学驱动和外场驱动的微纳米马达在药物递送领域的最新研究进展,最后对当前存在的挑战和未来的发展方向进行了展望。
Keywords: 微纳米马达, 药物递送, 化学驱动, 外场驱动
Abstract
As a new type of micro-/nanomachines, self-propelled micro-/nanomotors (MNMs) can convert chemical or external energies from the surrounding environment into mechanical forces to produce autonomous motion. The ability of autonomous movement allows these MNMs to move actively to the targeted locations, and thus confers great potentials on the MNMs for applications in biomedicine, especially in drug delivery. MNMs have been shown to effectively load therapeutic payloads for active delivery to the disease site, which greatly improves the therapeutic efficacy and reduces side effects compared with the traditional nanodrugs. In this review, we provide an overview of different propulsion mechanisms of MNMs, including chemical propulsion based on redox reaction and external field propulsion driven by external energy such as light, magnetic field, electric field and ultrasound, followed by a review of the recent progress in active drug delivery based on MNMs in the past decade. We also discuss the current challenges and future perspectives of the application of the MNMs.
Keywords: micro-/nanomotors, drug delivery, chemically propulsion, external field propulsion
药物在体内通常具有多个部位(包括正常组织)的作用靶点,这使得药物能够同时作用于病灶部位以及正常组织,从而对正常组织造成一定的毒副作用。尤其是化疗药物等细胞毒性药物,这些药物虽然对肿瘤具有较好的疗效,但同时对正常组织特别是增殖旺盛的区域也表现出了很高的细胞毒性。为了降低药物的毒副作用同时提高其疗效,研究者们提出了药物递送系统的概念。其中基于微纳米技术的药物递送系统更是备受科研工作者们的广泛关注[1]。目前,已有诸多基于微纳米技术的药物载体被开发出来,包括脂质体[2]、树状大分子[3]、聚合物[4]、介孔二氧化硅[5]、纳米乳[6]等。但传统的纳米药物载体均是基于被动扩散的纳米粒子,这些纳米粒子自身没有动力来源,只能在体液中进行布朗运动,因此其主动性以及选择性均相对不足。
受自然界中高效生物马达的启发,科研工作者们提出了人造马达的概念。人造马达是一种能够将周围环境中的化学能或外部能量转化为机械动能从而产生自主运动的人造机器。通过自上而下以及自下而上等手段,人造马达已实现从厘米级[7]至微纳米级[8]的突破。经过十几年的发展,人们开发出了基于各种结构的微纳米马达,如微纳米管[9]、微纳米线[10]、微纳米棒[11]、双面神微纳米马达[12]、螺旋型微纳米马达[13]和自组装聚合微纳米马达[14]等。由于其微小的结构以及自主运动的能力,微纳米马达在主动药物递送[15-16]、细胞捕获与分离[17]、分析检测[18]、环境修复[19]等多个方面展现出了广泛的应用前景。
我们首先概述了微纳米马达主要的驱动机理,一种是化学驱动,即利用燃料的氧化还原反应来提供马达运动的驱动力;另一种是外场驱动,通常是利用光场、磁场、超声和电场等外加物理场来实现马达的运动。随后我们按照驱动机理总结了基于化学驱动和外场驱动的微纳米马达在药物递送领域的最新研究进展,最后对微纳米马达当前存在的挑战以及未来的发展方向进行展望。
1. 自驱动微纳米马达的驱动机理
1.1. 化学驱动
化学驱动的微纳米马达一般基于燃料的氧化还原反应来生成不对称的产物分布(包括气体),从而推动其运动。微纳米马达的化学驱动机理主要有四种:气泡驱动[20]、自扩散泳驱动[21]、自电泳驱动[22]、界面张力梯度驱动[23-24]。
气泡驱动是目前微纳米马达中最为常见且发展较为成熟的一种驱动方式。微纳米马达上修饰的催化剂能够催化燃料的氧化还原反应产生气泡,而气泡从马达表面的脱离会对马达产生连续的动量变化,从而使马达朝着远离催化剂的方向运动。Gao等[20]利用模板电沉积法制备了聚苯胺/Pt微管马达。在底物H2O2溶液中,微管内层修饰的金属Pt能催化H2O2的分解,释放O2来实现马达的运动(图 1A)。
1.
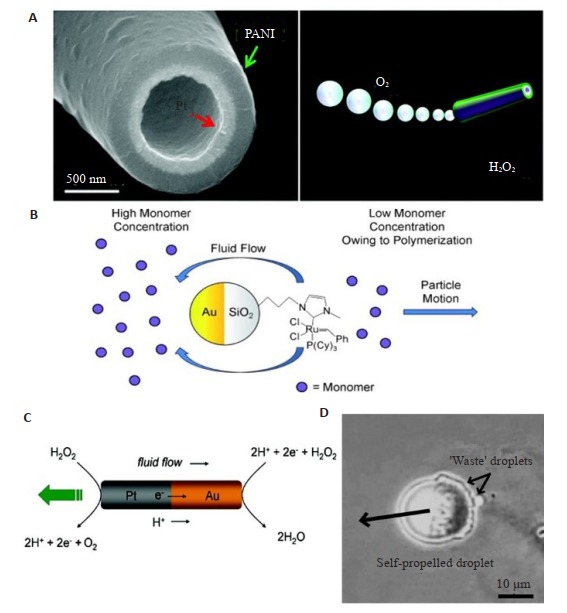
化学驱动的微纳米马达
Representative examples of chemically propelled MNMs. A: MNMs based on bubble propulsion[20]; B: MNMs based on self-diffusiophoresis[21]; C: MNMs based on self-electrophoresis[22]; D: Oil droplet based on interfacial tension gradient mechanism[24].
自扩散泳通常是由于催化产物在微纳米马达周围不对称地分布,而具有一定浓度梯度的产物的扩散使得环境中流体发生扰动,从而推动马达的运动。Pavlick等[21]制备了一种Grubbs催化剂修饰的Au-SiO2双面神粒子。Grubbs催化剂能够催化降冰片烯单体的开环易位聚合。当马达置于含降冰片烯单体的溶液中,不对称修饰的催化剂消耗了SiO2一侧的单体,与Au侧的单体形成了一定的浓度梯度,从而在粒子上产生渗透力。这种力使得净流体从单体浓度较低的一侧(催化侧)流向单体浓度较高的一侧(非催化侧),导致粒子朝着与流体流动相反的方向运动(图 1B)。
自电泳是由于马达表面离子型产物的不对称分布而在马达周围形成一个局部电场所引起的电泳效应。Paxton等[8]发现Pt-Au纳米棒在2~3%的H2O2溶液中能够自主地运动。经过深入地研究,他们发现自电泳是驱动此马达的主要机理[22]。在Pt-Au纳米马达中,阳极(Pt端)失去电子发生H2O2的氧化反应,随后电子从阳极端迁移到阴极(Au端)发生H2O2的还原反应,由此形成一个原电池结构。由于阳极端生成H+,阴极端消耗H+,因此H+在Pt端形成高浓度区,在Au端形成低浓度区。质子的不对称分布导致电场从Pt端指向Au端,带负电的纳米棒由此朝Pt端方向进行自电泳驱动(图 1C)。
界面张力梯度是指在催化点附近产生的具有浓度梯度的产物能够降低溶液与微纳米马达之间的界面张力,即在溶液与马达之间产生界面张力梯度,从而在马朗格尼效应作用下使微纳米马达朝着催化剂的方向运动[23]。这一驱动原理不仅仅局限于微纳米马达,对一些宏观的微粒也同样有效。Toyota等[24]报道了一个能够通过催化表面活性剂的分解进而实现自主运动的油滴。当将油滴置于水相时,存在于油滴中的催化剂不断分解油滴表面的表面活性剂,从而在油滴周围产生界面张力梯度来推动油滴的运动(图 1D)。
1.2. 外场驱动
除了利用化学反应来提供微纳米马达自主运动的动力以外,还可以通过施加外场来实现其驱动[25]。较为常用的外场包括光场[26-27]、电场[28-29]、磁场[30-31]和超声波[32-33]等。
光驱动的微纳米马达主要通过在马达上引入光活性材料如光催化、光致变色和光热材料等。在光照下,这些光活性材料能够吸收光能,进而引发光催化反应、光异构化或光热转化等,从而产生不对称场来推动马达的自主运动。Wang等[26]制备了一种核壳型硅纳米线,如图 2A所示。他们通过在p型Si纳米线(红色)表面形成一层n+-Si壳(绿色),其中p-Si只暴露在纳米线的一端,然后将Pt纳米粒作为电催化剂沉积在表面。在可见/近红外光下,硅纳米线的p-Si和n+-Si表面在H2O2溶液中发生氧化还原反应,分别产生H+和OH-离子。不平衡的离子分布所产生的电场反过来推动带负电荷的硅纳米线的运动。
2.
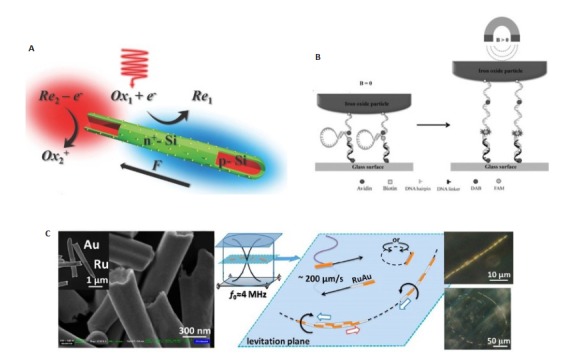
外场驱动的微纳米马达
Representative examples of external field-propelled MNMs. A: Light-propelled MNMs[26]; B: Magnetically propelled MNMs[30]; C: Ultrasonically propelled MNMs[32].
除了光场外,外部电场也可以用于驱动微纳米马达的运动。在微纳米马达中引入导电材料,通过电场来调节马达的表面电荷或界面上的电化学反应,可以相应地调控微纳米马达的运动行为。Kim等[28]报道了一个利用电镊驱动金属纳米线的纳米驱动系统。在电镊的作用下,纳米线马达能通过直流电场产生的电泳力进行移动,并通过交流电场产生的介电泳转矩进行排列。该纳米马达驱动系统以纳米线为转子,阵列化的纳米磁铁为轴承自下而上组装而成。在交变电场的作用下,Au/Ni/Au纳米线能有效极化,从而实现高速旋转(超过18 000 r/min)。
在微纳米马达上添加某种磁性物质,马达也可以在外加磁场的作用下进行运动,并且通过施加或去除外加磁场即可实现马达的启停控制。Bamrungsap等[30]将磁性粒子结合在DNA分子探针的5'端,利用偶联在磁性粒子上的DNA发夹成功地构建了磁性驱动的DNA纳米马达(图 2B)。施加的外部磁场能够吸引磁性粒子,从而触发DNA发夹的开启。当去除外部磁场后,DNA分子探针重组为发夹结构。
此外,超声波也是微纳米马达外场驱动中常用的能量源。Wang等[32]制备了具有凹端(Au)及凸端(Ru)的金属纳米棒。在超声下,金属纳米棒的凹端可以聚集所应用的声能,而凸端可以快速消散能量。因此,纳米棒的不对称结构导致了声压分布的不均匀,作用在凹端的声压相对更强,从而推动纳米棒沿Ru末端向前运动(图 2C)。
2. 自驱动微纳米马达在主动药物递送中的应用
与常规微纳米药物载体相比,自驱动微纳米马达由于其具有自主运动能力,在穿透组织和细胞屏障时具有更高的效率,因此被广泛的应用于主动药物递送。大量的研究表明自驱动微纳米马达在主动药物递送中的有效性[15-16],它们不仅可以递送传统化学药物,还可以递送核酸(siRNA、DNA)[34]、酶[35]、蛋白质[36]和抗体[37]等生物大分子药物。在这一节中,我们将基于马达的驱动机理分别介绍近年来自驱动微纳米马达主动药物递送的研究进展。
2.1. 化学驱动的微纳米马达在药物递送中的应用
鉴于化学驱动的微纳米马达具有相对较强的动力,近年来涌现出了很多微纳米马达用于药物递送的研究报道。Kagan等[38]利用磁性金属Ni制备得到了Ni/(Au50/Ag50)/Ni/Pt纳米线马达,第一次报道了微纳米马达用于输送和释放药物。这些纳米线能通过磁性相互作用拾取、运输、释放含磁性的载药PLGA微粒和载药脂质体(图 3A)。然而,这种药物递送模式首先依赖于额外的载药微纳米粒。其次,粒子和马达之间相对较弱的作用力也可能导致载药粒子在到达目标位置之前就从马达系统上脱离。为此,科研工作者们又展开了能够直接包载药物的微纳米马达的研究。Wu等[39]通过层层自组装制备了Pt纳米粒不对称修饰的聚苯乙烯磺酸/聚丙烯胺盐酸盐空心胶囊纳米马达(图 3B)。当加入乙醇时,溶液的极性发生变化,这导致马达的胶囊壁保持开放的状态。因此,可以通过溶剂极性来控制胶囊壁的开放与闭合,从而实现对模型药物的封装与释放。
3.
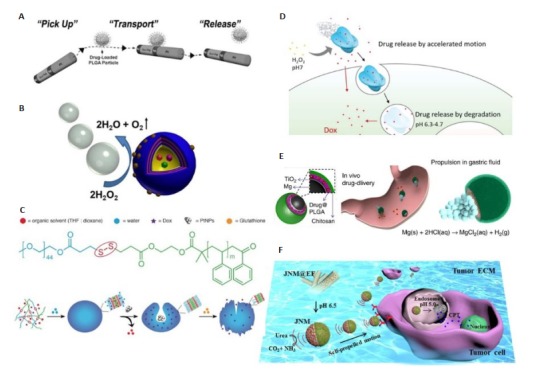
化学驱动的微纳米马达用于主动的药物递送
Representative examples of active drug delivery by chemically propelled MNMs. A: The drug-loaded PLGA particles dynamic pick-up, transport and release by a Ni/(Au50/Ag50)/Ni/Pt nanomotor[38]; B: Janus capsule motors for drug delivery[39]; C: Selfassembly and GSH-triggered disassembly of a redox-sensitive nanomotor[40]; D: DOX delivery by cat-β@SAFs micromotors[41]; E: Mg-based micromotors for drug delivery[46]; F: Urease-powered Janus nanomotors for drug delivery[12].
为了进一步提高微纳米马达的药物递送效率,对马达运动的控制十分重要。通常在微纳米马达中引入磁性物质,利用外加磁场对磁性物质的作用可以实现对马达运动方向的控制[9]。此外,局部的化学刺激也能诱导马达的运动。Peng等[14]报道了一种具有趋化行为的超分子碗形囊泡纳米马达。其中,催化剂Pt纳米粒被包载在碗形囊泡的空腔中,模型药物DOX被包载在内腔中。他们发现这些马达能够感知底物H2O2的浓度梯度,具有朝着H2O2浓度较高区域运动的能力。当在马达系统中引入氧化还原响应的二硫键时,细胞内高浓度的谷胱甘肽可以引起马达结构中的二硫键断裂,从而诱导纳米马达的解组装,进而实现可控的药物释放(图 3C)[40]。
除了运动方向的控制外,对微纳米马达运动速度的调控也同样重要。Gao等[41]开发了一种基于pH特异性响应来调节运动速度的微米马达。他们将琥珀酰化的β-乳球蛋白和H2O2酶通过超组装方式嵌入多孔框架材料制成微米马达。这些微米马达可以通过pH的调节来实现可逆的速率调控。在中性pH条件下,底物H2O2能够进入马达,从而激活马达的自主运动。然而在弱酸性条件下,琥珀酰化的β-乳球蛋白会发生可逆的凝胶化,因此阻止了底物的进入。在H2O2的存在下,包载DOX的微米马达在中性环境中能加速运动。当进入肿瘤细胞后,肿瘤细胞的酸性环境使马达降解,从而实现药物释放(图 3D)。
前文报道的化学驱动的微纳米马达大多基于H2O2的催化反应,然而H2O2的毒性极大地限制了微纳米马达的生物医学应用[42]。于是,科研工作者们开始寻找各种替代燃料来驱动微纳米马达。其中葡糖糖[43]、尿素[43-44]、水[45]等体内现有的天然物质受到了科研工作者们的广泛关注。de Avila等[46]制备了一种基于Mg的微米马达,其表面涂覆了含有药物克拉霉素(CLR)的PLGA层和壳聚糖层,这种马达能在小鼠模型中以胃酸为燃料进行运动并治疗胃炎(图 3E)。微米马达中的Mg能与胃液中的H+反应生成氢气气泡推动马达,同时使胃液pH提高。马达中的Mg核心溶解,导致这些微米马达降解进而实现药物释放。同时,带正电荷的壳聚糖外涂层可使马达粘附于胃壁,促进PLGA中的药物有效地进行局部释放。此外,Chen等[12]制备了一种脲酶驱动的双面神纳米马达用于药物递送。包载抗癌药物喜树碱(CPT)的马达在生理尿素浓度下朝向肿瘤细胞自主运动。进入肿瘤细胞后,肿瘤细胞内的微酸性环境使得马达结构中的羟磷灰石降解,从而释放药物CPT(图 3F)。
2.2. 外场驱动的微纳米马达在药物递送中的应用
外场驱动的微纳米马达一般不需要燃料的催化反应来推动马达的运动,因此不存在燃料耗竭的问题。与化学驱动的微纳米马达相比,具有长期的可持续性,同时因其良好的生物相容性,更适合应用于主动的药物递送。
临床中磁共振成像(MRI)的广泛应用说明了磁场于人体的安全性,因此磁驱动的微纳米马达展现出了其潜在的临床应用前景。2012年,Gao等[47]首次报道了利用磁驱动纳米马达来实现定向的药物递送。在外加磁场作用下,这些磁性纳米马达可以捕获并运输包载有DOX的磁性粒子,并将这些粒子靶向运送到预定的HeLa细胞。随后Qiu等[48]受细菌运动的启发,开发了一种在低强度旋转磁场作用下可进行精确三维运动的人造磁性螺旋微米马达。他们用含有pDNA的脂质复合物对这些马达进行修饰。在低强度旋转磁场的作用下,这些微米马达将pDNA靶向输运到了人胚胎肾细胞(HEK 293)内,成功转染后表达编码蛋白用于基因治疗(图 4A)。此外,Chen等[10]制备了一种FeGa@P(VDFTrFE)核壳型磁电纳米线马达。他们在纳米线表面修饰聚多巴胺,通过聚多巴胺中的氨基、羟基、羰基与抗癌药物紫杉醇之间的相互作用,实现了紫杉醇的装载。当载药纳米线暴露在交变磁场时,磁电效应引起极化状态的短暂变化破坏了药物分子与聚多巴胺的相互作用,从而释放出紫杉醇(图 4B)。最近,Wang等[49]成功地开发了具有pH响应特性的磁驱动螺旋微米马达。微米马达表面修饰的沸石咪唑骨架8(ZIF-8)锌基有机框架具有pH响应特性,在弱酸条件下能被降解,从而释放药物。该团队将罗丹明B作为模型药物包载到微米马达上,随后在pH 7.4和pH 6.0的条件下与乳腺癌细胞孵育(图 4C)。鉴于肿瘤微环境的弱酸性,这些具有pH响应特性的磁驱动螺旋微米马达能高效地递送抗癌药物。
4.
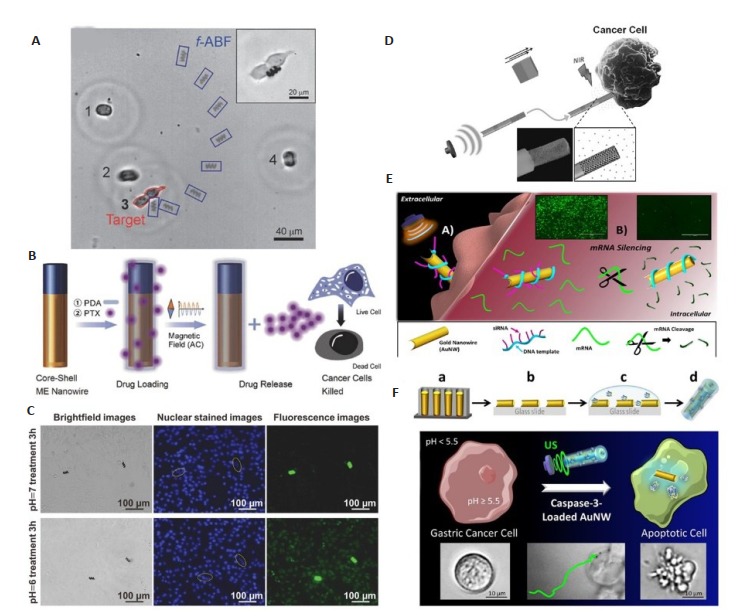
外场驱动的微纳米马达用于主动的药物递送
Representative examples of active drug delivery by external field-propelled MNMs. A: Magnetic helical microswimmers functionalized with lipoplexes for targeted gene delivery[48]; B: Schematic representation of a PDA treated FeGa@P(VDF-TrFE) core-shell nanowire for drug delivery[10]; C: Magnetic helical microswimmers functionalized with ZIF- 8 for in vitro drug delivery[49]; D: Ultrasound-propelled drug-loaded nanoporous Au nanomotors for drug delivery[50]; E: Ultrasonically propelled nanomotors for intracellular siRNA delivery[34]; F: Ultrasound-propelled high-pH-responsive polymer coated gold nanowire for CASP-3 delivery[35].
超声在医学上也具有广泛的应用。基于超声的安全性,使用超声来驱动生物介质中的微纳米马达也同样具有很大的应用前景。Garcia-Gradilla等[50]制备了一种超声驱动的多孔金纳米线马达(Au/Ni/Au/Pau,图 4D)。基于其多孔结构,这些金纳米线具有更大的表面积和更高的载药量,因此药物DOX可以通过静电相互作用实现有效的负载。在超声驱动及磁场导向下,马达能够朝着目标癌细胞运动。当到达目标靶点后通过近红外光辐射破坏DOX与阴离子聚合物间的静电相互作用实现药物释放。除了递送小分子药物外,微纳米马达也能用于核酸和蛋白质等大分子药物的递送。de Avila等[34]制备了超声驱动的环形扩增DNA链修饰的金纳米线用于siRNA治疗。如图 4E所示,在超声驱动下,这些金纳米线能够进入靶细胞并在细胞内快速运动,从而大幅提高siRNA的基因沉默效率。与现有的基因沉默方法相比,这些金纳米线的基因沉默率高达94%。此外,de Avila等[35]报道了一种具有pH响应性的纳米马达用于剪切酶的递送。他们用包载了可致细胞凋亡的caspase-3(CASP-3)的pH敏感聚合物对超声驱动的金纳米线进行修饰。如图 4F所示,在胃液低pH环境下,修饰在金纳米线上的聚合物涂层将保护CASP-3在到达细胞内环境之前不被释放及失活。当进入pH值较高的靶细胞内时,聚合物涂层溶解,使活性CASP-3释放,从而引起细胞凋亡。
3. 结论和展望
微纳米马达是近二十年来发展起来的新型人造微纳米机器人,广泛地应用于药物递送、细胞捕获与分离、分析检测、环境修复等研究领域。在本文第二部分,我们详细阐述了人造微纳米马达化学驱动(气泡驱动、自电泳、自扩散泳、界面张力梯度等)和外场驱动(光、磁、电、超声等)的主要机制。在第三部分中,我们总结了近十年来微纳米马达在主动药物递送领域的研究进展。在这十年里,微纳米马达从只能利用磁性相互作用将磁性药物载体吸附到马达上进行药物递送,到实现载药微纳米马达的直接构建;从使用具有细胞毒性的H2O2燃料驱动马达,到实现外场驱动以及开发出具有生物相容性的替代燃料;从运动无方向性和无速度可控性,到已经可以实现运动速度、运动方向及药物释放速率的控制。一项项的突破都向我们展示了微纳米马达在药物递送领域具有巨大的应用前景。然而尽管取得了一定的进展,但微纳米马达要想真正应用于人体,还面临着巨大的挑战:(1)生物体内环境复杂,如何保证微纳米马达的功能在人体复杂的生物环境中不受影响;(2)目前多数微纳米马达的结构都是基于金属材料,对人体具有一定的伤害且不易降解,如何开发出具有生物相容且生物可降解的微纳米马达;(3)目前大多数的研究仍局限于对单个马达的控制,如何实现对多个微纳米马达或马达集群的控制使其协同运动至疾病部位并发挥疗效;(4)由于生物介质的黏度相对较大且含有较多的电解质,微纳米马达在生物介质中的运动速度会严重减弱,因此需要具有更高效的马达或马达以新型的运动机制来实现在体内更加有效地输送。尽管微纳米马达仍处于起步阶段,但是随着多学科的交叉合作,该领域正在高速地发展。随着科研人员的不懈努力,我们坚信高效、智能和生物兼容的微纳米马达将为药物递送等生物医学领域带来革命性的进展。
Biographies
刘美焕,在读硕士研究生,E-mail: 1033293607@qq.com
涂彬彬,住院医师,E-mail: bbtu_1985@126.com。刘美焕、涂彬彬共同为第一作者
Funding Statement
国家自然科学基金(31800835);广东省自然科学基金(2018A030313521);广东省青年珠江学者配套项目
Supported by National Natural Science Foundation of China (31800835)
Contributor Information
刘 美焕 (Meihuan LIU), Email: 1033293607@qq.com.
涂 彬彬 (Binbin TU), Email: bbtu_1985@126.com.
涂 盈锋 (Yingfeng TU), Email: tuyingfeng1@smu.edu.cn.
References
- 1.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_30de8739e895a294e0cae1625a79f32c. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery [J].ACS Nano, 2009, 3(1): 16-20.] [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–60. doi: 10.1038/nrd1632. [Torchilin VP. Recent advances with liposomes as pharmaceutical carriers[J]. Nat Rev Drug Discov, 2005, 4(2): 145-60.] [DOI] [PubMed] [Google Scholar]
- 3.Gillies ER, Frechet JMJ. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10(1):35–43. doi: 10.1016/S1359-6446(04)03276-3. [Gillies ER, Frechet JMJ. Dendrimers and dendritic polymers in drug delivery[J]. Drug Discov Today, 2005, 10(1): 35-43.] [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Feijen J. Polymersomes for drug delivery: design, formation and characterization. J Controlled Release. 2012;161(2):473–83. doi: 10.1016/j.jconrel.2011.10.005. [Lee JS, Feijen J. Polymersomes for drug delivery: design, formation and characterization[J]. J Controlled Release, 2012, 161(2): 473-83.] [DOI] [PubMed] [Google Scholar]
- 5.Slowing Ⅱ, Vivero-Escoto JL, Wu CW, et al. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60(11):1278–88. doi: 10.1016/j.addr.2008.03.012. [Slowing Ⅱ, Vivero-Escoto JL, Wu CW, et al. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers[J].Adv Drug Deliv Rev, 2008, 60(11): 1278-88.] [DOI] [PubMed] [Google Scholar]
- 6.Choudhury H, Gorain B, Pandey M, et al. Recent update on nanoemulgel as topical drug delivery system. J Pharm Sci. 2017;106(7):1736–51. doi: 10.1016/j.xphs.2017.03.042. [Choudhury H, Gorain B, Pandey M, et al. Recent update on nanoemulgel as topical drug delivery system[J]. J Pharm Sci, 2017, 106(7): 1736-51.] [DOI] [PubMed] [Google Scholar]
- 7.Ismagilov RF, Schwartz A, Bowden N, et al. Autonomous movement and self-assembly. Angew Chem Int Ed. 2002;41(4):652–4. doi: 10.1002/1521-3773(20020215)41:4<652::AID-ANIE652>3.0.CO;2-U. [Ismagilov RF, Schwartz A, Bowden N, et al. Autonomous movement and self-assembly[J].Angew Chem Int Ed, 2002, 41(4): 652-4.] [DOI] [Google Scholar]
- 8.Paxton WF, Kistler KC, Olmeda CC, et al. Catalytic nanomotors: autonomous movement of striped nanorods. J Am Chem Soc. 2004;126(41):13424–31. doi: 10.1021/ja047697z. [Paxton WF, Kistler KC, Olmeda CC, et al. Catalytic nanomotors: autonomous movement of striped nanorods[J]. J Am Chem Soc, 2004, 126(41): 13424-31.] [DOI] [PubMed] [Google Scholar]
- 9.Wu ZG, Wu YJ, He WP, et al. Self-propelled polymer-based multilayer nanorockets for transportation and drug release. Angew Chem Int Ed Engl. 2013;52(27):7000–3. doi: 10.1002/anie.201301643. [Wu ZG, Wu YJ, He WP, et al. Self-propelled polymer-based multilayer nanorockets for transportation and drug release[J]. Angew Chem Int Ed Engl, 2013, 52(27): 7000-3.] [DOI] [PubMed] [Google Scholar]
- 10.Chen XZ, Hoop M, Shamsudhin N, et al. Hybrid magnetoelectric nanowires for nanorobotic applications: fabrication, magnetoelectric coupling, and magnetically assisted in vitro targeted drug delivery. Adv Mater. 2017;29(8):1605458. doi: 10.1002/adma.201605458. [Chen XZ, Hoop M, Shamsudhin N, et al. Hybrid magnetoelectric nanowires for nanorobotic applications: fabrication, magnetoelectric coupling, and magnetically assisted in vitro targeted drug delivery[J].Adv Mater, 2017, 29(8): 1605458.] [DOI] [PubMed] [Google Scholar]
- 11.Ma HR, Tang K, Luo W, et al. Photonic nanorods with magnetic responsiveness regulated by lattice defects. Nanoscale. 2017;9(9):3105–13. doi: 10.1039/C6NR10022H. [Ma HR, Tang K, Luo W, et al. Photonic nanorods with magnetic responsiveness regulated by lattice defects[J]. Nanoscale, 2017, 9 (9): 3105-13.] [DOI] [PubMed] [Google Scholar]
- 12.Chen ZJ, Xia T, Zhang ZL, et al. Enzyme-powered Janus nanomotors launched from intratumoral depots to address drug delivery barriers. http://cn.bing.com/academic/profile?id=ea235432725281cb05194d48cf71660d&encoded=0&v=paper_preview&mkt=zh-cn. Chem Eng J. 2019;375(2019):122109. [Chen ZJ, Xia T, Zhang ZL, et al. Enzyme-powered Janus nanomotors launched from intratumoral depots to address drug delivery barriers [J]. Chem Eng J, 2019, 375(2019): 122109.] [Google Scholar]
- 13.Qiu FM, Mhanna R, Zhang L, et al. Artificial bacterial flagella functionalized with temperature-sensitive liposomes for controlled release. http://cn.bing.com/academic/profile?id=ae6a33af2a134bc097b19881ea99bdc0&encoded=0&v=paper_preview&mkt=zh-cn. SensActuators B. 2014;196:676–81. [Qiu FM, Mhanna R, Zhang L, et al. Artificial bacterial flagella functionalized with temperature-sensitive liposomes for controlled release[J]. SensActuators B, 2014, 196: 676-81.] [Google Scholar]
- 14.Peng F, Tu YF, van Hest JC, et al. Self-guided supramolecular cargoloaded nanomotors with chemotactic behavior towards cells. Angew Chem Int Ed Engl. 2015;54(40):11662–5. doi: 10.1002/anie.201504186. [Peng F, Tu YF, van Hest JC, et al. Self-guided supramolecular cargoloaded nanomotors with chemotactic behavior towards cells[J]. Angew Chem Int Ed Engl, 2015, 54(40): 11662-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo M, Feng YZ, Wang TW, et al. Micro-/Nanorobots at work in active drug delivery. Adv Funct Mater. 2018;28(25):1706100. doi: 10.1002/adfm.201706100. [Luo M, Feng YZ, Wang TW, et al. Micro-/Nanorobots at work in active drug delivery[J].Adv Funct Mater, 2018, 28(25): 1706100.] [DOI] [Google Scholar]
- 16.Wang SH, Liu K, Wang F, et al. The application of micro- and nanomotors in classified drug delivery. Chem Asian J. 2019;14(14):2336–47. doi: 10.1002/asia.201900274. [Wang SH, Liu K, Wang F, et al. The application of micro- and nanomotors in classified drug delivery[J]. Chem Asian J, 2019, 14 (14): 2336-47.] [DOI] [PubMed] [Google Scholar]
- 17.Balasubramanian S, Kagan D, Hu CM, et al. Micromachine-enabled capture and isolation of cancer cells in complex media. Angew Chem Int Ed Engl. 2011;50(18):4161–4. doi: 10.1002/anie.201100115. [Balasubramanian S, Kagan D, Hu CM, et al. Micromachine-enabled capture and isolation of cancer cells in complex media[J]. Angew Chem Int Ed Engl, 2011, 50(18): 4161-4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maria-Hormigos R, Jurado-Sanchez B, Escarpa A. Labs-on-a-chip meet self-propelled micromotors. Lab Chip. 2016;16(13):2397–407. doi: 10.1039/C6LC00467A. [Maria-Hormigos R, Jurado-Sanchez B, Escarpa A. Labs-on-a-chip meet self-propelled micromotors[J]. Lab Chip, 2016, 16(13): 2397- 407.] [DOI] [PubMed] [Google Scholar]
- 19.Zarei M, Zarei M. Self-propelled micro/nanomotors for sensing and environmental remediation. Small. 2018;14(30):1800912. doi: 10.1002/smll.201800912. [Zarei M, Zarei M. Self-propelled micro/nanomotors for sensing and environmental remediation[J]. Small, 2018, 14(30): 1800912.] [DOI] [PubMed] [Google Scholar]
- 20.Gao W, Sattayasamitsathit S, Orozco J, et al. Highly efficient catalytic microengines: template electrosynthesis of polyaniline/platinum microtubes. JAm Chem Soc. 2011;133(31):11862–4. doi: 10.1021/ja203773g. [Gao W, Sattayasamitsathit S, Orozco J, et al. Highly efficient catalytic microengines: template electrosynthesis of polyaniline/platinum microtubes[J]. JAm Chem Soc, 2011, 133(31): 11862-4.] [DOI] [PubMed] [Google Scholar]
- 21.Pavlick RA, Sengupta S, McFadden T, et al. A polymerizationpowered motor. Angew Chem Int Ed Engl. 2011;50(40):9374–7. doi: 10.1002/anie.201103565. [Pavlick RA, Sengupta S, McFadden T, et al. A polymerizationpowered motor[J].Angew Chem Int Ed Engl, 2011, 50(40): 9374-7.] [DOI] [PubMed] [Google Scholar]
- 22.Paxton WF, Baker PT, Kline TR, et al. Catalytically induced electrokinetics for motors and micropumps. J Am Chem Soc. 2006;128(46):14881–8. doi: 10.1021/ja0643164. [Paxton WF, Baker PT, Kline TR, et al. Catalytically induced electrokinetics for motors and micropumps[J]. J Am Chem Soc, 2006, 128(46): 14881-8.] [DOI] [PubMed] [Google Scholar]
- 23.Zhao GJ, Pumera M. Macroscopic self-propelled objects. Chem Asian J. 2012;7(9):1994–2002. doi: 10.1002/asia.201200206. [Zhao GJ, Pumera M. Macroscopic self-propelled objects[J]. Chem Asian J, 2012, 7(9): 1994-2002.] [DOI] [PubMed] [Google Scholar]
- 24.Toyota T, Maru N, Hanczyc MM, et al. Self-propelled oil droplets consuming "fuel" surfactant. J Am Chem Soc. 2009;131(14):5012–3. doi: 10.1021/ja806689p. [Toyota T, Maru N, Hanczyc MM, et al. Self-propelled oil droplets consuming "fuel" surfactant[J]. J Am Chem Soc, 2009, 131(14): 5012-3.] [DOI] [PubMed] [Google Scholar]
- 25.Xu TL, Gao W, Xu LP, et al. Fuel-free synthetic micro-/nanomachines. Adv Mater. 2017;29(9):1603250. doi: 10.1002/adma.201603250. [Xu TL, Gao W, Xu LP, et al. Fuel-free synthetic micro-/nanomachines [J].Adv Mater, 2017, 29(9): 1603250.] [DOI] [PubMed] [Google Scholar]
- 26.Wang JZ, Xiong Z, Zhan XJ, et al. A silicon nanowire as a spectrally tunable light-driven nanomotor. Adv Mater. 2017;29(30):1701451. doi: 10.1002/adma.201701451. [Wang JZ, Xiong Z, Zhan XJ, et al. A silicon nanowire as a spectrally tunable light-driven nanomotor[J]. Adv Mater, 2017, 29(30): 1701451.] [DOI] [PubMed] [Google Scholar]
- 27.Wu ZG, Si TY, Gao W, et al. Superfast near-infrared light-driven polymer multilayer rockets. Small. 2016;12(5):577–82. doi: 10.1002/smll.201502605. [Wu ZG, Si TY, Gao W, et al. Superfast near-infrared light-driven polymer multilayer rockets[J]. Small, 2016, 12(5): 577-82.] [DOI] [PubMed] [Google Scholar]
- 28.Kim K, Xu XB, Guo JH, et al. Ultrahigh-speed rotating nanoelectromechanical system devices assembled from nanoscale building blocks. http://cn.bing.com/academic/profile?id=d5fb05efc75675608887a218128816e4&encoded=0&v=paper_preview&mkt=zh-cn. Nat Commun. 2014;5(5):3632. doi: 10.1038/ncomms4632. [Kim K, Xu XB, Guo JH, et al. Ultrahigh-speed rotating nanoelectromechanical system devices assembled from nanoscale building blocks[J]. Nat Commun, 2014, 5(5): 3632.] [DOI] [PubMed] [Google Scholar]
- 29.Rahman MM, Chowdhury MM, Alam MK. Rotating-electric-fieldinduced carbon-nanotube-based nanomotor in water: a molecular dynamics study. Small. 2017;13(19):1603978. doi: 10.1002/smll.201603978. [Rahman MM, Chowdhury MM, Alam MK. Rotating-electric-fieldinduced carbon-nanotube-based nanomotor in water: a molecular dynamics study[J]. Small, 2017, 13(19): 1603978.] [DOI] [PubMed] [Google Scholar]
- 30.Bamrungsap S, Phillips JA, Xiong XL, et al. Magnetically driven single DNA nanomotor. Small. 2011;7(5):601–5. doi: 10.1002/smll.201001559. [Bamrungsap S, Phillips JA, Xiong XL, et al. Magnetically driven single DNA nanomotor[J]. Small, 2011, 7(5): 601-5.] [DOI] [PubMed] [Google Scholar]
- 31.Peng F, Tu YF, Men YJ, et al. Supramolecular adaptive nanomotors with magnetotaxis behavior. Adv Mater. 2017;29(6):1604996. doi: 10.1002/adma.201604996. [Peng F, Tu YF, Men YJ, et al. Supramolecular adaptive nanomotors with magnetotaxis behavior[J].Adv Mater, 2017, 29(6): 1604996.] [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Castro LA, Hoyos M, et al. Autonomous motion of metallic microrods propelled by ultrasound. ACS Nano. 2012;6(7):6122–32. doi: 10.1021/nn301312z. [Wang W, Castro LA, Hoyos M, et al. Autonomous motion of metallic microrods propelled by ultrasound[J]. ACS Nano, 2012, 6(7): 6122- 32.] [DOI] [PubMed] [Google Scholar]
- 33.Kagan D, Benchimol MJ, Claussen JC, et al. Acoustic droplet vaporization and propulsion of perfluorocarbon-loaded microbullets for targeted tissue penetration and deformation. Angew Chem Int Ed Engl. 2012;51(30):7519–22. doi: 10.1002/anie.201201902. [Kagan D, Benchimol MJ, Claussen JC, et al. Acoustic droplet vaporization and propulsion of perfluorocarbon-loaded microbullets for targeted tissue penetration and deformation[J]. Angew Chem Int Ed Engl, 2012, 51(30): 7519-22.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Avila BE, Angell C, Soto F, et al. Acoustically propelled nanomotors for intracellular siRNA delivery. ACS Nano. 2016;10(5):4997–5005. doi: 10.1021/acsnano.6b01415. [de Avila BE, Angell C, Soto F, et al. Acoustically propelled nanomotors for intracellular siRNA delivery[J]. ACS Nano, 2016, 10 (5): 4997-5005.] [DOI] [PubMed] [Google Scholar]
- 35.de Avila BE, Ramirez-Herrera DE, Campuzano S, et al. Nanomotorenabled pH-responsive intracellular delivery of caspase-3: toward rapid cell apoptosis. ACS Nano. 2017;11(6):5367–74. doi: 10.1021/acsnano.7b01926. [de Avila BE, Ramirez-Herrera DE, Campuzano S, et al. Nanomotorenabled pH-responsive intracellular delivery of caspase-3: toward rapid cell apoptosis[J].ACS Nano, 2017, 11(6): 5367-74.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan DL, Yin ZZ, Cheong R, et al. Subcellular-resolution delivery of a cytokine through precisely manipulated nanowires. Nat Nanotechnol. 2010;5(7):545–51. doi: 10.1038/nnano.2010.104. [Fan DL, Yin ZZ, Cheong R, et al. Subcellular-resolution delivery of a cytokine through precisely manipulated nanowires[J]. Nat Nanotechnol, 2010, 5(7): 545-51.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vilela D, Orozco J, Cheng G, et al. Multiplexed immunoassay based on micromotors and microscale tags. Lab Chip. 2014;14(18):3505. doi: 10.1039/C4LC00596A. [Vilela D, Orozco J, Cheng G, et al. Multiplexed immunoassay based on micromotors and microscale tags[J]. Lab Chip, 2014, 14(18): 3505.] [DOI] [PubMed] [Google Scholar]
- 38.Kagan D, Laocharoensuk R, Zimmerman M, et al. Rapid delivery of drug carriers propelled and navigated by catalytic nanoshuttles. Small. 2010;6(23):2741–7. doi: 10.1002/smll.201001257. [Kagan D, Laocharoensuk R, Zimmerman M, et al. Rapid delivery of drug carriers propelled and navigated by catalytic nanoshuttles[J]. Small, 2010, 6(23): 2741-7.] [DOI] [PubMed] [Google Scholar]
- 39.Wu YJ, Wu ZG, Lin XK, et al. Autonomous movement of controllable assembled Janus capsule motors. ACS Nano. 2012;6(12):10910–6. doi: 10.1021/nn304335x. [Wu YJ, Wu ZG, Lin XK, et al. Autonomous movement of controllable assembled Janus capsule motors[J]. ACS Nano, 2012, 6 (12): 10910-6.] [DOI] [PubMed] [Google Scholar]
- 40.Tu YF, Peng F, White PB, et al. Redox-sensitive stomatocyte nanomotors: destruction and drug release in the presence of glutathione. Angew Chem Int Ed Engl. 2017;56(26):7620–4. doi: 10.1002/anie.201703276. [Tu YF, Peng F, White PB, et al. Redox-sensitive stomatocyte nanomotors: destruction and drug release in the presence of glutathione[J].Angew Chem Int Ed Engl, 2017, 56(26): 7620-4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao S, Hou JW, Zeng J, et al. Superassembled biocatalytic porous framework micromotors with reversible and sensitive pH-speed regulation at ultralow physiological H2O2 concentration. Adv Funct Mater. 2019;29(18):1808900. doi: 10.1002/adfm.201808900. [Gao S, Hou JW, Zeng J, et al. Superassembled biocatalytic porous framework micromotors with reversible and sensitive pH-speed regulation at ultralow physiological H2O2 concentration[J]. Adv Funct Mater, 2019, 29(18): 1808900.] [DOI] [Google Scholar]
- 42.Symons MC, Rusakiewicz S, Rees RC, et al. Hydrogen peroxide: a potent cytotoxic agent effective in causing cellular damage and used in the possible treatment for certain tumours. Med Hypotheses. 2001;57(1):56–8. doi: 10.1054/mehy.2000.1406. [Symons MC, Rusakiewicz S, Rees RC, et al. Hydrogen peroxide: a potent cytotoxic agent effective in causing cellular damage and used in the possible treatment for certain tumours[J]. Med Hypotheses, 2001, 57(1): 56-8.] [DOI] [PubMed] [Google Scholar]
- 43.Ma X, Jannasch A, Albrecht UR, et al. Correction to enzymepowered hollow mesoporous Janus nanomotors. Nano Lett. 2015;15(11):7779. doi: 10.1021/acs.nanolett.5b04318. [Ma X, Jannasch A, Albrecht UR, et al. Correction to enzymepowered hollow mesoporous Janus nanomotors[J]. Nano Lett, 2015, 15(11): 7779.] [DOI] [PubMed] [Google Scholar]
- 44.Ma X, Wang X, Hahn K, et al. Motion control of urea-powered biocompatible hollow microcapsules. ACS Nano. 2016;10(3):3597–605. doi: 10.1021/acsnano.5b08067. [Ma X, Wang X, Hahn K, et al. Motion control of urea-powered biocompatible hollow microcapsules[J]. ACS Nano, 2016, 10(3): 3597-605.] [DOI] [PubMed] [Google Scholar]
- 45.Gao W, Pei A, Wang J. Water-driven micromotors. ACS Nano. 2012;6(9):8432–8. doi: 10.1021/nn303309z. [Gao W, Pei A, Wang J. Water-driven micromotors[J]. ACS Nano, 2012, 6(9): 8432-8.] [DOI] [PubMed] [Google Scholar]
- 46.de Avila BE, Angsantikul P, Li JX, et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. http://cn.bing.com/academic/profile?id=333d751766043f490ac08892e295c53a&encoded=0&v=paper_preview&mkt=zh-cn. Nat Commun. 2017;8(1):272. doi: 10.1038/s41467-017-00309-w. [de Avila BE, Angsantikul P, Li JX, et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection[J]. Nat Commun, 2017, 8(1): 272.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao W, Kagan D, Pak OS, et al. Cargo-towing fuel-free magnetic nanoswimmers for targeted drug delivery. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8812d46829b4146913255ff2a903c7dd. Small. 2012;8(3):460–7. doi: 10.1002/smll.201101909. [Gao W, Kagan D, Pak OS, et al. Cargo-towing fuel-free magnetic nanoswimmers for targeted drug delivery[J]. Small, 2012, 8(3): 460-7.] [DOI] [PubMed] [Google Scholar]
- 48.Qiu FM, Fujita S, Mhanna R, et al. Magnetic helical microswimmers functionalized with lipoplexes for targeted gene delivery. Adv Funct Mater. 2015;25(11):1666–71. doi: 10.1002/adfm.201403891. [Qiu FM, Fujita S, Mhanna R, et al. Magnetic helical microswimmers functionalized with lipoplexes for targeted gene delivery[J]. Adv Funct Mater, 2015, 25(11): 1666-71.] [DOI] [Google Scholar]
- 49.Wang XP, Chen XZ, Alcantara CCJ, et al. MOFBOTS: Metal-organicframework-based biomedical microrobots. Adv Mater. 2019;31(27):1901592. doi: 10.1002/adma.201901592. [Wang XP, Chen XZ, Alcantara CCJ, et al. MOFBOTS: Metal-organicframework-based biomedical microrobots[J]. Adv Mater, 2019, 31 (27): 1901592.] [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Gradilla V, Sattayasamitsathit S, Soto F, et al. Ultrasoundpropelled nanoporous gold wire for efficient drug loading and release. https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.201401013. Small. 2014;10(20):4154–9. doi: 10.1002/smll.201401013. [Garcia-Gradilla V, Sattayasamitsathit S, Soto F, et al. Ultrasoundpropelled nanoporous gold wire for efficient drug loading and release [J]. Small, 2014, 10(20): 4154-9.] [DOI] [PubMed] [Google Scholar]


