Abstract
MRI已经成为临床疾病诊断的重要手段之一,而40%~50%的临床MRI检查需要使用造影剂来提高MRI的灵敏度,因此,MRI造影剂也成为重要的临床诊断药物。本文主要介绍了MRI造影剂及其分类,市售MRI造影剂的优缺点,并对极小磁性氧化铁纳米粒子、点式核壳型铁钆复合纳米粒子和极小氧化钆纳米粒子进行了评述,这三者因其r1值很高且r2/r1比值很低,T1-加权MRI成像的效果很好,有望转化为临床医用T1-加权MRI造影剂。本文对将MRI造影剂与各种肿瘤治疗策略相结合的诊断治疗一体化体系进行了评述,如MRI指导的铁凋亡治疗、MRI指导的放射增敏治疗和MRI指导的光热治疗,它们实现了对肿瘤的高效治疗和实时监控,有望成为可行的癌症治疗策略。最后对MRI基多功能诊疗制剂这一领域的未来可能研究方向进行了展望。
Keywords: 磁共振成像造影剂, 极小磁性氧化铁纳米粒子, 点式核壳型铁钆复合纳米粒子, 极小氧化钆纳米粒子, 肿瘤诊断与治疗
Abstract
Magnetic resonance imaging (MRI) is an important imaging modality for clinical disease diagnosis, and nearly 50% of clinical MRI examinations require contrast agents to enhance the diagnostic sensitivity. This review provides a summary of the major MRI contrast agents and their classification, and the advantages and limits of the commercially available MRI contrast agents, and elaborates on the exceedingly small magnetic iron oxide nanoparticles (ES-MIONs), dotted core-shell iron and gadolinium hybrid nanoparticles (FeGd-HN) and exceedingly small gadolinium oxide nanoparticles (ES-GON). These nanoparticles can greatly improve the efficiency of T1-weighted MRI due to their high r1 value and low r2/r1 ratio, and are expected to be translated into clinical contrast agents for T1-weighted MRI. The authors also review the diagnostic and therapeutic integration system that combines MRI contrast agents with various tumor therapies, such as MRI-guided ferroptosis therapy, radiosensitization therapy, and photothermal therapy, which allow efficient treatment as well as real-time monitoring of tumors and serve as potential cancer therapy strategies. The possible future research directions in the field of MRI-based multifunctional diagnostic and therapeutic formulations are also discussed.
Keywords: magnetic resonance imaging contrast agents, exceedingly small magnetic iron oxide nanoparticles, dotted coreshell iron and gadolinium hybrid nanoparticles, exceedingly small gadolinium oxide nanoparticles, tumor theranostics
自首次发表使用梯度磁场产生磁共振图像以来[1],MRI因其较强的软组织分辨能力而广泛应用于临床,尤其在肿瘤早期诊断和肿瘤术前分期等方面尤为重要[2]。通过使用造影剂,可以提高MRI的灵敏度,有文献报道40%~50%的临床MRI检查使用了造影剂[3]。目前,临床上应用的MRI造影剂主要有两种,即阳性造影剂(或T1-加权MRI造影剂)和阴性造影剂(或T2-加权MRI造影剂)。T1-加权MRI造影剂是通过缩短质子的纵向弛豫时间(T1)使T1-加权像上的信号强度增高,从而产生明亮的图像。T1造影剂因其产生亮信号,故适用范围广。临床上使用最广泛的MRI造影剂是钆螯合物基T1造影剂,被美国食品和药物监督管理局(FDA)和欧洲药品管理局批准应用于临床的造影剂包括:Magnevist(Gd-DTPA)(1988年被FDA批准进入临床的第1个MRI造影剂);Gadovist(Gd-DO3A-Butriol);Eovist(Gd-EOB-DTPA);ProHance(Gd-DO3A-HP);Multihance(Gd-BOPTA);OptiMARK(Gd-DTPABMEA);Dotarem(Gd-DTOA);Omniscan(Gd-DTPABMA);Vasovist(Gd-diphenyl-cyclohexy-lphosphodiester-DTPA)[4]。然而,市售钆螯合物基T1造影剂具有如下问题:(1)具有一定的肾毒性,可造成部分患者的肾源性系统性纤维化[5-6],有研究报道了肾源性系统纤维化可能与钆螯合物有关[7],FDA因其肾毒性而对其临床应用提出了警告[8];(2)可在脑部沉积,FDA于2017年5月22日确认了钆螯合物基造影剂的脑部沉积,将继续评估钆螯合物基T1造影剂的安全性[9];(3)钆螯合物基T1造影剂均为小分子,对任何组织和细胞均不存在靶向性;(4)纵向磁豫率(r1)较低,仅仅约为4 mM-1 s-1,从而导致MRI成像效果不佳。
T2-加权MRI造影剂是通过缩短质子的横向弛豫时间(T2)使T2或T2*-加权像上的信号强度下降,从而产生暗图像[4]。T2造影剂的优点包括:(1)静脉给药后,它们可以被肝脏、脾脏、淋巴和骨髓的网状内皮系统吞噬,具有一定的靶向性[10-13];(2)安全性高,它们可以在溶酶体中被降解,降解的铁最终从体内清除或重新存储在体内进入正常铁代谢途径[14-15]。尽管T2造影剂有着明显的优势,然而其临床应用却很少,多数原本已经上市的T2造影剂已经退出市场[16],主要原因在于T2造影剂存在如下问题:(1)T2信号为暗信号,不利于临床观察对比,而且易与其他致病原因造成的暗信号混淆,如出血、钙化、金属沉积等[15, 17];(2)T2造影剂的高磁矩会引起磁敏感性伪影,破坏病灶周围背景导致图像模糊,造成MRI图像局部失真[17-18];(3)T2造影剂粒径较大(60~180 nm),在体内的降解和清除速度较慢,可能会导致长期毒性[15];(4)T2-加权MRI的处理时间比T1-加权MRI长得多,不利于临床应用。
为了克服铁基T2-加权MRI造影剂的缺点,有研究首次探索了作为潜在T1造影剂的超小磁性氧化铁纳米粒子(ES-MIONs),研究发现粒径小于5 nm的ES-MIONs具有较小的纵向磁化强度,而Mz与横向弛豫率r2成正比,故ES-MIONs的纵向弛豫率r1值为2~50 mM-1 s-1,而r2/r1比值则降低至小于5,这大大提高了T1成像效率(r1值越大且r2/r1比值越小,T1成像效果越好)[19-20]。与钆基T1造影剂相比,铁基T1造影剂有明显的优势:铁的安全性高,且可作为注射型补铁剂,在体内的循环时间长。所以铁基T1造影剂的深入研究越来越受研究者青睐[21-22],与之相反,钆基T1造影剂可能面临淘汰(图 1)[23]。
1.
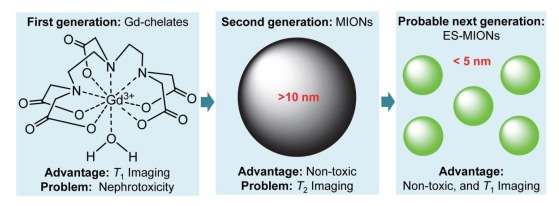
MRI造影剂的主要问题和优势[23]
Main problems and advantages of MRI contrast agents[23].
1. ES-MIONs
近年来出现的ES-MIONs可作为T1造影剂,有望克服钆螯合物基T1造影剂和铁基T2造影剂的缺点。迄今为止,可以用热分解法[24]、溶剂热法[25]、多元醇法[26]、还原沉淀法[27-28]、共沉淀法[29-30]合成粒径均小于5 nm的ESMIONs。然而,ES-MIONs的粒径与r1值和r2/r1比值之间的关系未知,故ES-MIONs作为T1-加权MRI造影剂的最佳粒径仍然未知。阐明这一关系的关键是合成不同粒径的ES-MIONs(小于5 nm)。有研究报道了尺寸可控的锰掺杂磁性氧化铁纳米粒子的合成,然而,他们合成的纳米粒子的粒径都大于5 nm(5、7、9、12 nm)[31]。有研究合成了粒径分别为3.2、4.8和7.5 nm的磁性氧化铁纳米粒子[32]。另有研究合成了不同粒径的磁性氧化铁纳米粒子,但在5 nm以下也只有两种尺寸(2.2、3 nm)[33],不足以阐明ES-MIONs粒径与r1值和r2/r1比值之间的关系。
为此,Shen等[34]发展了一种以聚丙烯酸(PAA)为稳定剂的共沉淀法,成功合成了7种粒径均在5 nm以下的ES-MIONs(分别为1.9、2.6、3.3、3.6、4.2、4.8和4.9 nm),并在MRI扫描仪(7T)上测量了7种不同粒径ES-MIONs的r1和r2值,结果表明当ES-MIONs的粒径从1.9 nm增大到3.6 nm时,r1值逐渐增大,r2/r1比值逐渐减小;当ES-MIONs的粒径从3.6 nm增大到4.9 nm,r1值逐渐降低,r2/r1比值逐渐增大(图 2)[34]。由于r1值高和r2/r1比值低都有利于T1成像[35-37],因此,ES-MIONs作为T1-加权MRI造影剂的最佳粒径是3.6 nm。该研究对合成的ES-MIONs进行了表征,结果表明所合成的ES-MIONs具有良好的水相分散性,满足生物医学应用的要求[34]。
2.
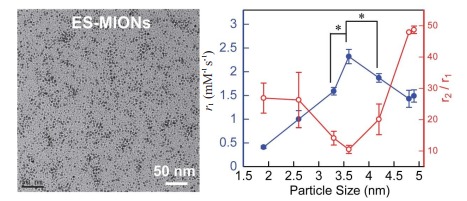
ES-MIONs的TEM图像及r1值或r2/r1比值与粒径(< 5 nm)的关系[34]
TEM images of ES-MIONs, and the relationships of r1 value or r2/r1 ratio with particle size (< 5 nm) (Mean±SD, n=3). *P < 0.02[34].
阐明了ES-MIONs作为T1-加权MRI造影剂的最佳粒径(3.6 nm,ES-MION3)后,为了解决正常细胞对纳米颗粒的非特异性摄取问题,Shen等[34]还基于ES-MION3(3.6 nm)构建了一种肿瘤精准靶向的T1-加权MRI造影剂和化疗药物递送系统,即先将靶向配体RGD二聚体(RGD2)通过酰胺键偶联至ES-MION3表面,再把亲水性聚合物甲氧基聚乙二醇(mPEG)通过不耐酸的β-巯基丙酸酯交联剂接枝到偶联有RGD2的ES-MION3上,最后将抗癌化疗药物阿霉素(DOX)通过氢键、离子键和(或)配位键装载至ES-MION3,最终构建了复合纳米粒子DOX@ES-MION3@RGD2@mPEG(图 3)[34]。当复合纳米粒子DOX@ES-MION3@RGD2@mPEG进入体内血循环时(pH=7.4),RGD2(小分子)隐藏于亲水性mPEG(高分子)之中,阻止了正常细胞表面非特异性受体对RGD2的识别与结合,从而抑制了正常细胞对纳米粒子DOX@ES-MION3@RGD2@mPEG的非特异性摄取。但是,弱酸性肿瘤微环境能破坏不耐酸的β-巯基丙酸酯交联剂,使其连接的mPEG脱落,从而暴露被mPEG隐藏的RGD2,使之被肿瘤细胞表面过量表达的αvβ3受体识别与结合,从而使肿瘤细胞通过受体介导的细胞內吞特异性摄取DOX@ES-MION3@RGD2复合纳米粒子[38]。DOX@ES-MION3@RGD2复合纳米粒子进入肿瘤细胞形成内涵体,随着内涵体酸性增强(DOX的氨基和ES-MION3表面羧基都能结合H+),渗透压增大,致使内涵体溶胀甚至破裂,从而释放ES-MION3和化疗药物DOX,从而达到肿瘤诊断与治疗的目的。
3.
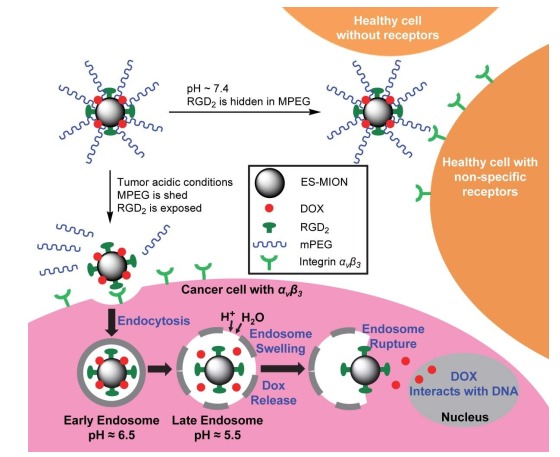
DOX@ES-MION@RGD2@mPEG复合纳米粒子的设计及降低正常细胞非特异性摄取纳米颗粒的原理[34]
Design of DOX@ES-MION@RGD2@mPEG composite nanoparticles and the mechanism to reduce nonspecific uptake of nanoparticles by normal cells[34].
Shen等[34]通过FITC-PEG-OH荧光光谱测试了聚合物的脱落,通过激光扫描共聚焦显微镜(LSCM)和流式细胞仪分别定性和定量测试了肿瘤细胞对纳米粒子的摄取,研究结果表明RGD2可在血循环中隐藏而在弱酸性肿瘤微环境中暴露,以此减少正常细胞对复合纳米粒子的非特异性摄取,并提高表达αvβ3的肿瘤细胞对复合纳米粒子的特异性摄取。U87 MG(表达αvβ3)荷瘤小鼠实验结果表明,DOX@ES-MION3@RGD2@mPEG3复合纳米粒子大大提高了MRI成像的对比度,并具有良好的肿瘤治疗效果[34]。
2. 点式核壳型铁钆复合纳米粒子
近几十年来,钆螯合物作为T1-加权MRI造影剂在临床上被广泛使用,但是由于其肾毒性,FDA已经对其使用发出了警告[8]。为了通过降低使用剂量而降低肾毒性风险,需要改善钆基T1造影剂的磁豫率(即提高r1值,降低r2/r1比值),以提高T1成像效果。在过去的十几年中,研究者们致力于设计各种钆基T1造影剂,以提高其r1值和降低r2/r1比值。有学者研制了r1值为20.5 mM-1 s-1且r2/r1比值为7.1(B0=3.0 T)的Gd2O3嵌入式氧化铁纳米板(GdIOPs)[39]。有研究设计了以MnFe2O4(15 nm)为核,SiO2(4,8,12,16,20 nm)为隔离层,Gd2O(CO3)2(1.5 nm)为外壳的核壳型纳米粒子[即MnFe2O4@SiO2@Gd2O(CO3)2],其r1值高达33.1 mM-1 s-1,但相应的r2/r1比值较高,达到了8.3(B0=4.7 T)[40]。有研究发展了一种r1值为45.24 mM-1 s-1且r2/r1比值为4.1(B0=1.5 T)的核壳式Fe3O4@Gd2O3纳米立方体[41]。有研究合成了r1值为4.2 mM-1 s-1且r2/r1比值为4.1(B0=3.0 T)的Fe3O4@SiO2核壳式纳米粒子,并在其表面偶联了Gd-DTPA和RGD肽(精氨酸-甘氨酸-天冬氨酸)[42]。有研究用热分解法合成了r1值为7.87 mM-1 s-1且r2/r1比值为23.2(B0=9.4 T)的Gd掺杂的氧化铁纳米粒子[43]。Jung等[44]用Gd(Ⅲ)- DOTA-1, 2-二硬脂酰基-Sn-甘油-3-磷酸乙醇胺脂制备了Gd(Ⅲ)-DOTA修饰的声敏脂质体,得到的钆基磁脂质体r1值为6.6~7.8 mM-1 s-1(B0=4.7 T),而r2/r1比值未提供。
即使考虑到B0的影响,上述设计的钆基T1造影剂有相对较高的r1值,但其相应的r2/r1比值并不低。为此,Shen等[45]以PAA为稳定剂,采用共沉淀法合成了ESMIONs种子(图 4A);然后加入Gd3+,通过与PAA的羧基形成离子键而吸附到ES-MIONs表面,并在ESMIONs表面上原位合成了氧化钆纳米粒子,从而得到了铁钆复合纳米粒子(FeGd-HN)。该研究发现在不同钆浓度(CGd)下合成的FeGd-HN具有3种不同结构(图 4B)。在中等CGd(即,Gd/Fe摩尔比=0.49)条件下,得到的点式核壳型FeGd-HN具有超高r1值(73.5 mM-1 s-1,B0= 1.5 T)和较低r2/r1比值(1.95,B0=1.5 T)。然而,在较低CGd或较高CGd下得到的Gd嵌入型FeGd-HN或全核壳型FeGd-HN,其r1值相对较低,且r2/r1比值较高(图 5)。为了实现肿瘤的主动靶向,该研究将靶向配体RGD2偶联至点式核壳型FeGd-HN(FeGd-HN3)表面,构建了FeGd-HN3-RGD2复合纳米粒子(图 4C)[45]。
4.
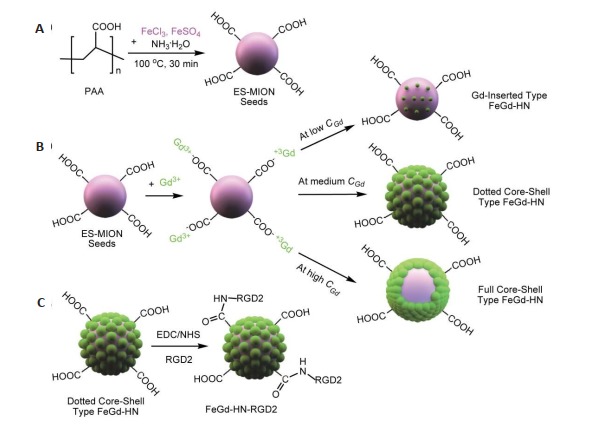
FeGd-HN-RGD2复合纳米粒子合成步骤示意图[45]
Schematic illustration of the procedures for synthesis of FeGd-HN-RGD2 composite nanoparticles[45]. A: The seeds of ES-MIONs were synthesized using PAA as a stabilizer; B: Gd3+ions are adsorbed on the surfaces of ES-MION seeds via formation of ionic bonds between them and the -COOH groups from PAA, and then GdON are synthesized in situ on the surfaces of ES-MION seeds to form three different structures at different CGd; C: The targeted ligand RGD2 is conjugated onto the surface of the dotted core-shell type FeGd-HN to construct FeGd-HN-RGD2 nanoparticles.
5.
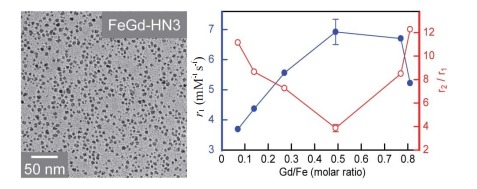
FeGd-HN3的TEM图像及r1值和r2/r1比值与Gd/Fe摩尔比的关系(B0=7.0 T)[45]
An transmission electron microscopic image of FeGd-HN3 and the relationships of r1 value or r2/r1 ratio with Gd/Fe molar ratio (Mean±SD, n=3)[45].
Shen等[45]对合成的FeGd-HN3和FeGd-HN3- RGD2进行了表征,结果显示合成了稳定性、分散性良好的点式核壳型铁钆复合纳米粒子。根据Gd浓度计算和根据Fe和Gd浓度之和计算,分别在1.5 T临床MRI扫描系统和7.0 TMRI扫描系统上测量了FeGd-HN3和FeGd-HN3-RGD2的r1值和r2值(表 1)。根据Fe和Gd浓度之和计算时,在7.0 T MRI扫描系统上测得FeGd-HN3和FeGd-HN3-RGD2的r1值为6.92和7.07 mM-1 s-1,与报道的GdIOPs(6.8±1.3 mM-1 s-1)[39]相当,远高于ESMIONs(2.40 mM-1 s-1)[34]和Magnevist(3.54 mM-1 s-1);r2/r1比值分别为6.86和6.80,远低于GdIOPs(23.4)[39],但高于Magnevist(1.47)。根据Gd浓度计算时,在1.5 T MRI扫描系统上测得FeGd-HN3和FeGd-HN3-RGD2的r1值均大于70.0 mM-1 s-1,远高于文献报道的T1造影剂[39-44]和市售T1造影剂(如Magnevist),其r2/r1比值均小于2.0,与Magnevist相当,低于大多数文献报道的T1造影剂[39-44]。FeGd-HN3和FeGd-HN3-RGD2因其超高r1值和较低r2/r1比值,T1-加权MRI成像的效果很好,有望转化为临床医用T1-加权MRI造影剂。
1.
| Sample Nomenclature | H0(T)a |
r1(mM-1s-1) (Fe+Gd)b |
r2(mM-1s-1) (Fe+Gd)b |
r2/r1b |
r1(mM-1s-11) (Gd)c |
r2(mM-1s-1) (Gd)c |
r2/r1b |
| FeGd-HN3 | 7.0 | 6.92±0.42 | 47.4±2.2 | 6.86±0.31 | 21.0±1.3 | 143.9±2.9 | 6.86±0.31 |
| 1.5 | 25.1±0.9 | 48.9±0.9 | 1.95±0.07 | 73.5±2.6 | 143.0±2.7 | 1.95±0.07 | |
| FeGd-HN3-RGD2 | 7.0 | 7.07 | 48.1 | 6.80 | 20.7 | 140.5 | 6.80 |
| 1.5 | 24.0 | 47.6 | 1.98 | 70.0 | 139.2 | 1.98 | |
| Magnevist® | 7.0 | 3.54 | 5.22 | 1.47 | 3.54 | 5.22 | 1.47 |
| 1.5 | 4.25±0.07 | 4.51±0.02 | 1.06±0.02 | 4.25±0.07 | 4.51±0.02 | 1.06±0.02 |
Shen等[45]通过3(- 4, 5-二甲基噻唑-2)-2, 5-二苯基四氮唑溴盐(MTT)测定法,用U-87 MG细胞(整合素αvβ3阳性)和MCF-7细胞(整合素αvβ3阴性)评估了FeGd-HN3- RGD2纳米粒子的细胞毒性,并用正常健康裸鼠、U-87 MG荷瘤裸鼠和静脉注射FeGd-HN3-RGD2后的U-87 MG荷瘤裸鼠的主要器官进行组织学分析,研究结果表明FeGd-HN3-RGD2具有良好的生物相容性。
使用LSCM和流式细胞仪研究了U-87 MG细胞和MCF-7细胞对FeGd-HN3-RGD2和FeGd-HN3的摄取,结果显示U-87 MG细胞摄取了大量的R6G-FeGdHN3-RGD2纳米粒子,而MCF-7细胞摄取的R6GFeGd-HN3-RGD2较少。此外,U-87 MG细胞和MCF-7细胞摄取的R6G-FeGd-HN3也很少。以上结果表明,靶向配体RGD2能通过受体介导的细胞內吞帮助整合素αvβ3阳性细胞摄取纳米颗粒[45]。
T1加权MRI成像的体内研究表明,FeGd-HN3-RGD2的MR成像效果要比Magnevist好,静脉注射FeGdHN3-RGD2后肿瘤最大ΔSNR高达(477±44)%,明显高于Magnevist(75±11)%,且远高于文献报道值[34, 39, 46-47]。
通常情况下,由于肝脾很容易通过网状内皮系统的巨噬细胞吞噬纳米粒子,文献报道的大多数纳米粒子在肝、脾部位的富集量明显高于肿瘤部位[48-50]。该研究中,MRI和电感耦合等离子体(ICP)结果进一步证实,由于RGD2的靶向性和8.5 nm的流体动力学粒径,FeGdHN3-RGD2在肿瘤部位的富集量很大,甚至高于在肝脏和脾脏的富集[45]。该结果表面,FeGd-HN3-RGD2实现了对整合素αvβ3阳性肿瘤组织的主动靶向。
3. 极小氧化钆纳米粒子
尽管上述点式核壳型铁钆复合纳米粒子FeGdHN3具有超高r1值(73.5 mM-1 s-1,B0=1.5 T),但r2/r1比值(1.95,B0=1.5 T)不算太低,仍有下调空间。为保持超高r1值的同时降低r2/r1比值,Shen等还发展了一种湿化学合成法,以PAA为稳定剂合成极小氧化钆纳米颗粒(ES-GON-PAA),作为T1-加权MRI造影剂[51]。
该研究以不同浓度的Gd(NO3)3(1000~62.5 mmol/L,其他反应条件一致)为原料合成了ES-GON1-5-PAA(图 6A),对合成的ES-GON1-5-PAA进行了表征[51],结果显示成功合成了分散性良好的ES-GON1-5-PAA。通过7.0 T MRI扫描仪系统测得ES-GON1-5-PAA、Gd(NO3)3、Dotarem、Gadavist和Magnevist的r1值和r2/r1比值(表 2),结果发现,随着PAA/Gd摩尔比的增加,r1值增加而r2/r1比值减小(图 6B)。因此,与ES-GON1- 4-PAA相比,ES-GON5-PAA是最佳的T1-加权MRI造影剂。此外,ES-GON5-PAA的r1值(21.3 mM-1 s-1)远高于Gd(NO3)3(10.4 mM-1 s-1)、Dotarem(3.68 mM-1 s-1)、Gadavist(4.30 mM-1 s-1)和Magnevist(3.54 mM-1 s-1)。通过1.5 T临床MRI扫描仪系统测得ES-GON1-5-PAA和Magnevist的r1值和r2/r1比值(表 2),结果发现,ESGON5-PAA同时具有超高r1值(70.2±1.8 mM-1 s-1)和极低r2/r1比值(1.02±0.03)。ES-GON5-PAA水溶液的T1-加权MRI图像进一步证实了ES-GON5-PAA具有很强的MRI成像效果[51]。
6.
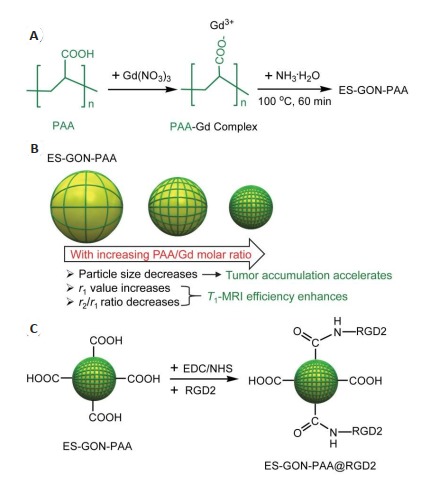
ES-GON5-PAA@RGD2复合纳米粒子合成步骤示意图[51]
Schematic illustration of the synthesis of ES-GON5- PAA@RGD2 composite nanoparticles[51]. A: Scheme for synthesis of the PAA-stabilized exceedingly small gadolinium oxide nanoparticles (ES-GON-PAA); B: With increasing PAA/Gd molar ratio, (1) the particle size of ES-GON-PAA decreases resulting in accelerated tumor accumulation, (2) r1 value increases and r2/r1 ratio decreases leading to enhanced T1- weighted MRI efficiency; C: RGD2-conjugated ES-GON-PAA (ES-GON-PAA@RGD2).
2.
| Sample Nomenclature | H0 (T)a | r1(mM-1s-1) | r2(mM-1s-1) | r2/r1b |
| Magnevist® | 7.0 | 3.54 | 5.22 | 1.47 |
| 1.5 | 4.20±0.16 | 4.52±0.12 | 1.08±0.06 | |
| ES-GON1-PAA | 7.0 | 10.4 | 59.0 | 5.69 |
| 1.5 | 49.3±2.4 | 64.6±2.0 | 1.32±0.10 | |
| ES-GON2-PAA | 7.0 | 13.0 | 61.1 | 4.71 |
| 1.5 | 54.2±3.0 | 65.8±1.3 | 1.22±0.08 | |
| ES-GON3-PAA | 7.0 | 17.2 | 64.2 | 3.74 |
| 1.5 | 60.7±1.9 | 62.4±2.8 | 1.03±0.02 | |
| ES-GON4-PAA | 7.0 | 19.5 | 55.6 | 2.85 |
| 1.5 | 65.7±2.6 | 68.2±2.4 | 1.04±0.08 | |
| ES-GON5-PAA | 7.0 | 21.3±0.9 | 57.8±2.1 | 2.71±0.14 |
| 1.5 | 70.2±1.8 | 71.7±2.0 | 1.02±0.03 | |
| ES-GON5-PAA@RGD2 | 7.0 | 19.9±0.8 | 54.8±2.7 | 2.75±0.23 |
| 1.5 | 68.7±2.3 | 70.5±1.6 | 1.03±0.03 |
为实现肿瘤的主动靶向,该研究将靶向配体RGD2偶联至ES-GON-PAA表面,构建了ES-GON5-PAA @RGD2复合纳米粒子(图 6C),所得ES-GON5-PAA @RGD2具有很好的稳定性和水相分散性[51]。如表 2所示,ES-GON5-PAA@RGD2也具有超高r1值和极低r2/r1比值,其r1值(68.7±2.3 mM-1 s-1,1.5 T)远高于临床钆螯合物(≈4 mM-1 s-1)[52-53]和文献报道的GONs(4.4~ 47.2 mM-1 s-1)[54-59],其r2/r1比值(1.03±0.03)也低于临床钆螯合物(≈1.1)和文献报道的GONs(1.1~6.8)[58-59]。MTT测定结果和组织学分析表明,ES-GON5- PAA@RGD2具有良好的生物相容性。T1-加权MRI成像的体内研究表明,ES-GON5-PAA@ RGD2的MRI成像效果很好,静脉注射ES-GON5-PAA @ RGD2后2 h,肿瘤的ΔSNR最大值达(372±56)%,明显高于钆螯合物(< 80%)[34, 39, 45-47]和ES-GON5-PAA(225±29)%。ICP结果进一步证实,ES-GON5-PAA@RGD2具有良好的肿瘤靶向性,其肿瘤富集量明显高于肝脏和脾脏[51]。
4. MRI指导的铁凋亡治疗
癌症已在全球范围内成为威胁人类生命的重要杀手,根据国家癌症中心的最新数据显示,我国癌症发病率与死亡率逐年上升,每天超过1万余人,每分钟约7人被确诊为癌症[60],恶性肿瘤的根治成为亟待解决的重要难题。目前,治疗恶性肿瘤的方法有:手术治疗[61]、化学治疗[62-65]、放射治疗[66-67]、光热疗法[68-71]、光动力疗法[72-74]、肿瘤免疫治疗[75-77]、基因治疗[78-80]、高强度聚焦超声治疗[8-82]、磁热疗[83-85]、硼中子捕获治疗[86-87]和重离子治疗[88]。这些方法有其有效性,但与此同时又存在一定局限性。具体地,手术治疗能治愈大多早期肿瘤,但无法用于多数中晚期肿瘤,肿瘤组织难以通过手术完全切除,有些部位手术难度很大;化学治疗和放射治疗都能杀伤肿瘤细胞,对癌症产生一定的控制效果,但同时也会杀伤正常组织和细胞,导致严重的毒副作用;光热疗法和光动力疗法疗法原理主要是利用特定波长的光激活光热剂或光敏剂产生热或活性氧(ROS),从而杀死肿瘤细胞,具有损伤小、对人体副作用小的优点,但光的穿透能力有限,故光热疗法和光动力疗法都难以用于深部肿瘤的治疗;肿瘤免疫治疗副作用小、反应快,但治疗费用昂贵、治疗效果依赖于宿主的免疫系统,而且可能会造成自身免疫攻击过度;基因治疗的生物安全性令人担忧;高强度聚焦超声治疗受超声穿透深度的限制,对深层肿瘤的治疗效果不佳;磁热疗存在磁性纳米粒子肿瘤低积聚的问题[89];硼中子捕获治疗和重离子治疗的设备非常昂贵,成本极高。因此,为了克服现有治疗方式的弊端,需要发展新的治疗方式来补充现有治疗方案。
细胞死亡是维持组织机能和形态所必须的,在预防过量增生性质的疾病(如肿瘤)中有着非常重要的作用。细胞死亡的方式包括:程序性细胞死亡(凋亡和自噬)、坏死和一种新型的细胞死亡方式即铁凋亡[90-91]。铁凋亡是一种铁依赖性的以细胞内ROS堆积为特征的非细胞凋亡形式的细胞死亡形式。铁凋亡这一概念是Dixon等[92]在研究Erastin杀死含有致癌基因RAS突变的肿瘤细胞的作用机制时发现并提出的,随后铁凋亡引起了越来越多研究者的关注。目前,铁凋亡的一些调控机制和细胞信号通路已经被证实,铁凋亡的机制是通过Fenton反应产生和积累ROS,从而杀死肿瘤细胞[93-95]。在铁凋亡治疗(FT)被正式命名前,已有一些研究利用铁基纳米材料基于铁凋亡机理来治疗癌症[96-99]。已报道的FT机理是利用铁基纳米材料通过Fenton反应产生ROS。据报道,在弱酸性肿瘤微环境中更容易释放铁的非晶态铁纳米颗粒是最有效的铁基纳米材料,但即使是非晶态铁纳米颗粒,FT疗效仍然不高,在荷瘤小鼠中诱导FT所需的铁剂量很高(75 mg/kg)[99]。
因此,有研究设计了一种偶联有乳铁蛋白(LF)和RGD2、并装载有顺铂的Fe3O4/Gd2O3复合纳米粒子(FeGd-HN@Pt@LF/RGD2),实现了在癌细胞内同时提高Fenton反应中所有反应物(Fe2+、Fe3+和H2O2)的局部浓度,从而大大提高了肿瘤的FT疗效[100]。首先,以PAA为稳定剂,采用两步共沉淀法合成了FeGd-HN[101-102]。然后,通过顺铂与PAA的羧基反应制得装载有顺铂的FeGd-HN(FeGd-HN@Pt)。血脑屏障(BBB)表面存在大量LF受体,LF能介导纳米粒子的跨BBB转运[103],脑肿瘤表达有整合素αvβ3,RGD2能实现对脑肿瘤的主动靶向,故研究将LF和RDG2偶联至FeGd-HN@Pt表面,制得FeGd-HN@Pt@LF/RGD2复合纳米粒子(图 7A),从而实现了MRI指导的原位脑肿瘤的FT,具体原理如下:FeGd-HN@Pt@LF/RGD2纳米粒子通过LF受体介导跨BBB运转,进入大脑,与脑肿瘤细胞表面的RGD2受体(即整合素αvβ3)结合,通过细胞内吞作用进入肿瘤细胞形成内涵体,随着内涵体酸性增强,渗透压增大,以致溶胀、破裂,并释放Fe2+、Fe3+和顺铂。Fe2+和Fe3+为Fenton反应3种反应物中的2种,可以直接加速Fenton反应。此外,释放的顺铂还能激活NADPH氧化酶,将NADPH转化为NADP+,释放电子,产生O2· -,形成H2O2[104-106],而H2O2也是Fenton反应的一种底物。因此,FeGd-HN@Pt@LF/RGD2纳米粒子能提高肿瘤细胞内参与Fenton反应的所有反应物的浓度,加速Fenton反应,从而大大提高了肿瘤细胞的FT疗效(图 7B)[100]。
7.
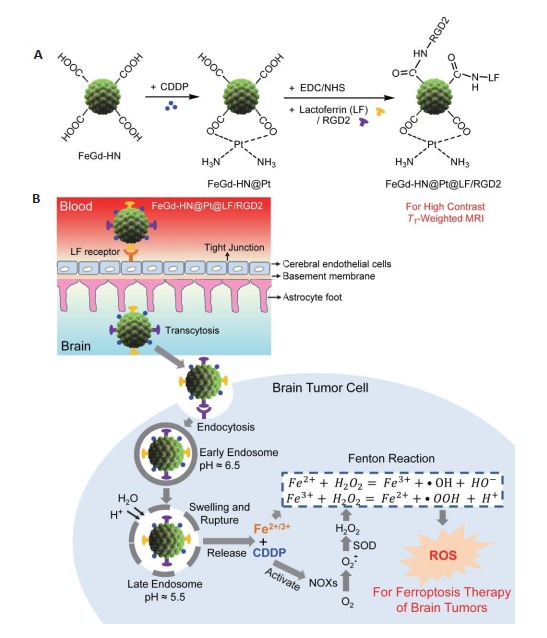
FeGd-HN@Pt@LF/RGD2的合成(A), MRI指导下原位脑肿瘤的铁凋亡治疗机制(B)[100]
Synthesis of FeGd-HN@PT@LF/RGD2 (A) and the mechanism illustration of MRI-guided ferroptosis therapy for orthotopic brain tumors(B)[100].
该研究对合成的FeGd-HN@Pt2@LF/RGD2和FeGd-HN@Pt2进行了表征,结果表明成功合成了分散性良好的纳米粒子[100]。通过1.5 T临床MRI扫描仪系统和7.0 T MRI扫描仪系统测得FeGd-HN@Pt2@LF/RGD2和FeGd-HN@Pt2的r1值和r2值(表 3),结果显示,FeGdHN@Pt@LF/RGD2具有较高的r1值(56.57 mM-1 s-1)和较低的r2/r1比值(1.25,B0=1.5 T)。体外和皮下肿瘤的T1-加权MRI结果进一步表明,FeGdHN@Pt@LF/RGD2的MRI成像效果好,是一种优异的T1-加权MRI造影剂[100]。
3.
| Sample Nomenclature | H0 (T)a | r1(mM-1s-1) (Gd)b | r2(mM-1s-1) (Fe)b | r2/r1 |
| FeGd-HN@Pt2 | 7.0 | 15.83 | 52.32 | 3.30 |
| 1.5 | 67.75 | 72.01 | 1.06 | |
| FeGd-HN@Pt2@LF/RGD2 | 7.0 | 12.70 | 50.72 | 3.99 |
| 1.5 | 56.57 | 70.56 | 1.25 |
该研究通过2, 7-二氯二醋酸荧光素结合LSCM和流式细胞仪,测试了细胞内ROS的产生和FT疗效,结果表明FeGd-HN@Pt2@LF/RGD2可以通过Fenton反应产生ROS,而铁螯合剂甲磺酸去铁胺(DFO)或活性氧清除剂N-乙酰-L-半胱氨酸(NAC)会抑制ROS产生[100]。用MTT法进一步直接评估了MCF-7和U-87 MG细胞的FT疗效,实验结果显示,FeGd-HN@Pt2@LF/RGD2对U-87 MG细胞有高度毒性,且高于游离顺铂,具有较高的FT效果。FeGdHN@Pt2@LF/RGD2与DFO或NAC共同孵育后,细胞毒性降低,表明FeGdHN@Pt2@LF/RGD2的细胞毒作用可被DFO或NAC阻断。上述结果也证明了FeGdHN@Pt2@LF/RGD2的FT机理是通过依赖于铁的Fenton反应产生ROS。体内外跨BBB运转的研究结果表明,LF可用于介导FeGdHN@Pt2@LF/RGD2纳米粒子的BBB转运。进一步地,该研究采用生物发光成像和弥散加权磁共振成像,评价了FeGdHN@Pt2@LF/RGD2纳米粒子对原位脑肿瘤模型的FT疗效[100]。与对照组相比,FeGd-HN@Pt@LF/RGD2成功抑制了小鼠原位脑瘤的生长,延长了小鼠的生存时间(图 8),表明可加速Fenton反应和可穿过BBB的FeGdHN@Pt2@LF/RGD2纳米粒子对原位脑瘤具有很高的FT疗效。免疫组化结果显示FeGd-HN@Pt@LF/RGD2纳米粒子具有良好的生物相容性。此外,纳米粒子固有的MRI能力可以用来指导和监测肿瘤对FT的反应(即自我MRI监测),以便在耐药效果产生初期及时更改治疗方案。总之,FeGd-HN@Pt2@LF/RGD2因其FT疗效高,有望成为可行的癌症治疗策略[100]。
8.
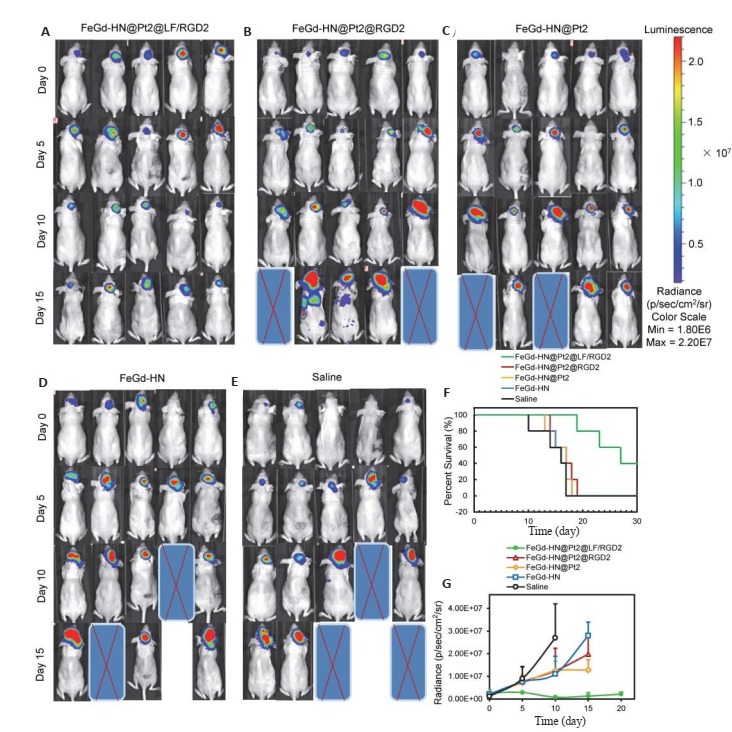
不同治疗组原位脑肿瘤模型小鼠的生物发光图像(A-E);不同治疗组原位脑瘤模型小鼠的存活率(F);不同治疗组原位脑瘤模型小鼠的生长曲线(G)[100]
(A-E) Bioluminescence images of mice bearing orthotopic brain tumors in different treatment groups; (F) Percent survival of the mice bearing orthotopic brain tumors after different treatments; (G) Growth curves of the orthotopic brain tumors in different treatment groups (Mean±SE, n=5)[100].
5. MRI指导的放射增敏治疗
胶质母细胞瘤(GBM)是成人中最常见的原发性中枢神经系统的恶性肿瘤[107],是胶质瘤中恶性程度最高的一种类型,属WHO分级中高级别(Ⅳ级)的胶质瘤,易侵犯周围的正常组织,预后不良[108]。近几十年来,GBM发病率不断增加,据美国肿瘤协会调查统计,每年约89 000位新确诊为原发性脑肿瘤的患者中就有32.8%被诊断为GBM[109]。然而,由于GBM的高度侵袭性,现有临床治疗手段的治疗效果并不理想,GBM患者的中位生存时间仅有15月左右,5年生存率低于5%[108, 110-111]。
替莫唑胺是具有抗肿瘤活性的烷化剂,为口服制剂,口服后可迅速完全吸收,可通过BBB,生物利用率高,是抗GBM的标准一线药物。然而超过45%的GBM可能对替莫唑胺有耐药性,GBM对替莫唑胺的耐药性是化疗失败的主要原因[112-113]。虽然也有其他尚处于临床试验阶段的药物用于GBM的治疗,但由于大多不能通过BBB,药物很难进入中枢神经系统发挥作用。因此,为了促进GBM治疗药物向中枢神经系统转运,许多研究者致力于BBB可转运纳米材料的研究,包括受体介导的BBB跨越、细胞介导的BBB跨越、穿梭肽介导的BBB跨越和细胞穿透肽介导的BBB跨越。例如,有研究通过聚乙二醇-嵌段-聚([N-(3-甲基丙烯酰胺基丙基)胍-co-4(-4, 4, 5, 5-四甲基-1, 3, 2-二氧杂硼烷-2-基)苄基-丙烯酸酯)]/Ang-聚乙二醇-嵌段-聚[N-(3-甲基丙烯酰胺丙基)胍]与siRNA的络合作用,构建了以AngPEG-2肽(37.8 nm)功能化的用于靶向GBM治疗的ROS响应型siRNA纳米药物(3I-NM@siRNA)[114]。有研究设计了环肽iRGD偶联固体脂质纳米粒子,并携带针对表皮生长因子受体和PD-L1的小干扰RNA(24.1 nm),用于联合靶向和免疫治疗GBM[111]。有文献报道了一种偶联有载脂蛋白E肽的聚合物泡囊(> 40 nm),可介导高效GBM治疗[115]。有研究设计了巨噬细胞质膜修饰的、二硬脂酰磷脂酰乙醇胺-聚乙二醇包覆近红外Ib荧光染料(IR-792)的纳米粒子(MDINPs)(99±6 nm),用于靶向GBM成像和光热治疗[116]。
尽管粒径较小的纳米材料有更高的BBB通透性,上述设计的可跨越BBB纳米材料的粒径都大于24 nm[111, 114-116]。为此,研究开发了一种新型的小于14 nm的可跨越BBB纳米材料[117],用于高对比度MRI和原位GBM的放射增敏治疗。具体地,在水相中合成PAA稳定的、还原性牛血清白蛋白(rBSA)修饰的极小氧化钆纳米粒子(ES-GON-rBSA)。由于钆原子序数高(Z= 64),钆基纳米粒子具有作为放射敏化剂的潜力,可以提高放射治疗的效果[118-120]。以不同rBSA加入量合成了ES-GON-rBSA1-4纳米粒子,在7.0 T MRI系统上测量了ES-GON-rBSA1-4的r1值和r2值,ES-GON-rBSA3因其相对较高的r1值、较低的r2/r1比值和较高的rBSA含量而被认为是最佳选择。为了实现对GBM的靶向和促进跨BBB转运,通过在ES-GON-rBSA3上偶联RGD2和LF,得到了复合纳米粒子ES-GON-rBSA3-LF-RGD2(图 9)。
9.
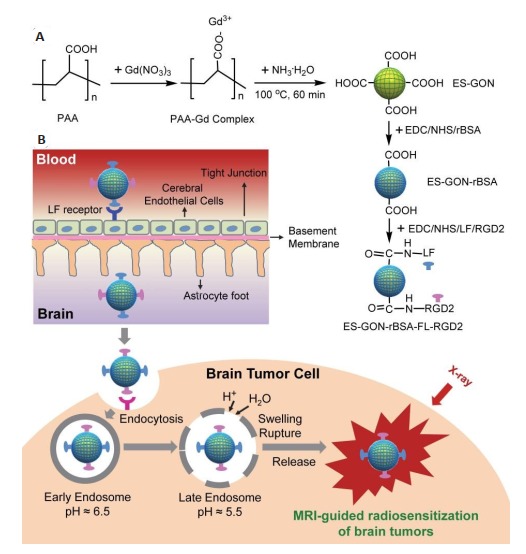
ES-GON-rBSA-FL-RGD2的合成(A);MRI指导的脑肿瘤放射增敏治疗的机制(B)[117]
Synthesis of ES-GON-RBSA-FL-RGD2(A) and mechanism of MRI-guided radiosensitization therapy for brain tumors (B)[117].
分别在1.5 T临床MRI扫描仪系统和7.0 T MRI扫描仪系统上测量了ES-GON-rBSA3和ES-GONrBSA3-LF-RGD2的r1值和r2值(表 4)。ES-GONrBSA3-LF-RGD2的r1值为60.8 mM-1 s-1,远高于临床Gd螯合物(≈4 mM-1 s-1)[52-53]和已报道的GONs(4.4~ 47.2 mM-1 s-1)[54-59]。ES-GON-rBSA3-LF-RGD2的r2/r1比值为1.1,与临床Gd螯合物(≈1.1)[52-53]相当,低于已报道的GONs(1.1~6.8)[54-59]。较高的r1值和较低的r2/r1比值表明ES-GON-rBSA3-LF-RGD2具有高对比度MRI成像效果,可作为T1-加权MRI造影剂,用于肿瘤治疗的评价和监测[117]。
4.
| Sample Nomenclature | H0 (T)a | r1(mM-1s-1) | r2(mM-1s-1) | r2/r1b |
| Magnevist® | 7.0 | 3.6 | 5.4 | 1.5 |
| 1.5 | 4.3 | 4.6 | 1.1 | |
| ES-GON-rBSA3 | 7.0 | 18.6 | 64.0 | 3.4 |
| 1.5 | 64.5 | 76.6 | 1.2 | |
| ES-GON-rBSA3-LF-RGD2 | 7.0 | 15.1 | 59.0 | 3.9 |
| 1.5 | 60.8 | 67.3 | 1.1 |
TEM结果显示,ES-GON-rBSA3和ES-GON-rBSA3-LF-RGD2的粒径小于5 nm、分散性良好。DLS测得的ES-GON-rBSA3-LF-RGD2的水力学直径为13.4 nm,小粒径和LF受体均可促进跨BBB转运[100, 121-123]。其他表征结果显示,ES-GON-rBSA3-LF-RGD2的稳定性好,血液循环时间长。另外,LSCM和流式细胞仪的研究结果表明,由于RGD2的主动靶向作用,整合素αvβ3阳性表达的U-87 MG细胞大量摄取了R6G-ES-GON-rBSA3-LF-RGD2,但极少摄取R6G-ES-GON-rBSA3。整合素αvβ3阴性表达的MCF-7细胞几乎不摄取R6G-ESGON-rBSA3-LF-RGD2和R6G-ES-GON-rBSA3。这些结果表明,ES-GON-rBSA3-LF-RGD2可以通过RGD2受体介导的细胞内吞作用被脑瘤细胞摄取。ESGON-rBSA3-LF-RGD2的体内和体外BBB转运性能的研究结果表明,LF可介导ES-GON-rBSA3-LFRGD2纳米粒子的跨BBB转运[117]。
小鼠的T1加权MRI成像结果表明,尾静脉注射ESGON-rBSA3-LF-RGD2后12 h,肿瘤部位的的ΔSNR高达(423±42)%,明显高于临床Gd螯合物(< 80%)[34, 39, 46-47],而且肿瘤的信号强于肝脏,这归因于ES-GON-rBSA3- LF-RGD2的小粒径和RGD2主动靶向,使ES-GONrBSA3-LF-RGD2在肿瘤中聚集度较高。ICP测量的体内生物分布实验进一步证实了ES-GON-rBSA3-LFRGD2在肿瘤中的高聚集度。MTT和体内毒性研究结果表明,ES-GON-rBSA3-LF-RGD2具有良好的生物相容性。细胞、皮下GBM模型和原位GBM模型的放射增敏治疗结果表明,ES-GON-rBSA3-LF-RGD2可作为一种有效的放射增敏剂,能增强脑肿瘤的放射治疗效果,在MRI指导的脑肿瘤放射增敏治疗方面有很好的应用前景[117]。
6. MRI指导的光热治疗
光热治疗是利用特定波长的光激活光热转换剂,将光能转换成热能,产生热量,从而杀死肿瘤细胞,具有损伤小、对人体副作用小的优点,但由于光的穿透深度有限,限制了光热治疗在深部肿瘤中的应用[68-71]。其次,光热转换剂在肿瘤部位的积累效率也限制了光热治疗的临床应用[124-125]。因此,为了达到更好的肿瘤治疗效果,越来越多的研究致力于克服光热治疗的这些缺陷。与传统的小分子药物相比,纳米材料因其小尺寸效应、表面效应、体积效应、量子效应和肿瘤的高通透性和滞留效应,在肿瘤部位的聚集度较高,从而在肿瘤的诊疗中显示出了巨大的潜力[126-129]。目前,越来越多的光热纳米材料被发现并用于光热治疗,包括:贵金属纳米材料[130-132]、半导体纳米材料[133-134]、碳基纳米材料[135-136]、导电聚合物纳米材料[137-138]。然而,这并不能满足日益增长的对先进功能纳米材料的需求,需要通过不同合成方法(如:湿化学合成、自组装等)不断提高纳米材料的物理性能,从而得到更多层次化和刺激响应性的纳米结构[139-144]。一般来说,由于肿瘤的高通透性和滞留效应,纳米材料能在肿瘤部位积累,但是,纳米材料在到达肿瘤部位之前,会受到体内生物屏障的阻碍,使其在肿瘤部位的积累效率受到限制。纳米粒子克服体内生物屏障的能力与其自身的物理属性(如尺寸和刺激响应性等)密切相关[145-149]。因此,如何开发具有更佳物理性能的肿瘤诊疗用纳米粒子成为亟待解决的问题。
在各种光热纳米材料中,金纳米颗粒具有独特的光学及光热性质。光学特性主要表现在金纳米颗粒具有较强的表面等离子体共振效应,表面等离子体共振效应可以提高金纳米颗粒的光吸收和光散射能力,从而增加肿瘤部位对纳米粒子的吸收量[150]。金纳米材料的光热效应是通过表面等离子体共振效应将光能转换成热能,从而使局部温度升高。不同大小及形态的金纳米粒子(如金纳米棒、纳米笼、纳米壳等)均具有光热效应,在生物医学领域(如光声成像和光热治疗等)方面表现出了巨大的应用前景[151]。光热治疗效果与金纳米材料的光热效率和通过各种成像技术确定的最佳治疗时间窗等密切相关[152-153]。ES-MIONs前面已有介绍,是一种潜在的可以替换Gd螯合物的T1-加权MRI造影剂[34]。
有研究用已报道过的银(Ag)-模版介导法制备了Au纳米环,再以Au纳米环为模版通过种子生长法合成了Au纳米花环(AuNW)[154],AuNW表面的突起结构和内部的孔洞可以增加纳米粒子自身的等离子体耦合,相对于Au纳米环,光热转换效率得到了大大的提高[151, 155]。为了构建磁性AuNW,研究用二氧化硅包裹AnNW,并利用层层自组装方法依次组装了含有二硫键的带正电的高分子材料和带负电的ES-MIONs,最后用PEG进行修饰,从而合成了谷胱甘肽(GSH)响应型磁性AuNW(图 10A)。在组装体中,ES-MIONs作为T1-加权MRI造影剂的能力被抑制,而在肿瘤微环境中,高表达的GSH可以切断高分子的二硫键,将ES-MIONs从组装体释放出来,重新开启其T1成像功能[156-161],使磁性AuNW作为T1-加权MRI造影剂和光热剂用于肿瘤的诊疗(图 10B)[162]。
10.
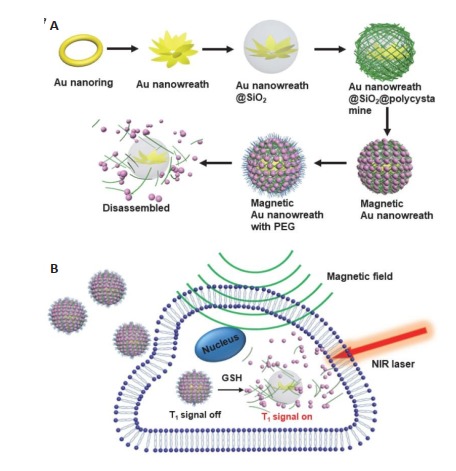
磁性AuNW的合成原理图(A), 磁性AuNW作为GSH响应型T1-加权MRI造影剂和光热剂的应用(B)[162]
Schematic illustration of the synthesis of magnetic AuNW (A) and the applications of magnetic AuNW as GSH-responsive T1- weighted MRI contrast agents and photothermal agents (B)[162].
磁性AuNW纳米粒子的表征结果显示,其粒径为100.7 nm,相对于粒径为3.6 nm的单分散ES-MIONs[34],粒径较大的磁性AuNW不易从肾代谢,从而增加了ESMIONs在肿瘤中的富集。另外,磁性AuNW具有极佳的光热效应。MTT实验结果表明,磁性AuNW的细胞毒性相对较低,但在激光照射下,磁性AuNW的光热效应可以有效地杀死癌细胞[162]。
经7.0 T MRI扫描仪测量,磁性AuNW的r1值和r2值分别为1.1 mM-1 s-1和198.6 mM-1 s-1。当组装体中的二硫键断裂,ES-MIONs从磁性AuNW中释放出来后,r1值增加到了3.2 mM-1 s-1,r2值降低到了33.9 mM-1 s-1,这表明磁性AuNW具有GSH响应性的T1信号。进一步地,研究用U87 MG荷瘤裸鼠模型评估了磁性AuNW的体内T1-加权MRI成像能力,实验结果显示,与单个ES-MIONs相比,磁性AuNWs的肿瘤富集得到了显著提高,磁性AuNW的T1图像比ES-MIONs明亮。另外,注射磁性AuNWs 24 h后,T1成像信号增强了2.5倍,表明注射后24 h是进行光热疗法的最佳时间点。磁性AuNW的体内光声成像研究显示,肿瘤的光声成像信号在注射后24 h达到了最高,增强了5倍(图 11A,B),进一步证实了注射后24 h是进行光热治疗的最佳时间点。磁性AuNW的体内光热治疗研究表明,磁性AuNW能通过光热治疗有效消除模型小鼠的肿瘤(图 11C~E)[162]。因此,磁性AuNW可作为一种有效的光热转换剂,在MRI指导的光热治疗方面有较好的发展前景。
11.
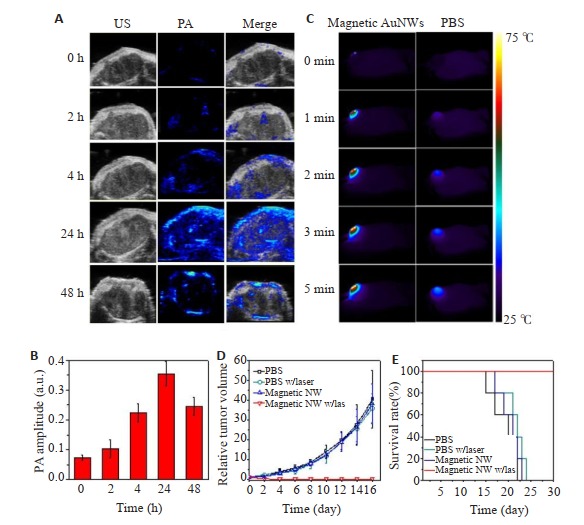
磁性AuNW的体内光声成像与光热治疗[162]
In vivo photoacoustic imaging and photothermal therapy with magnetic AuNWs[162].
7. 总结与展望
近几十年来,钆螯合物作为T1-加权MRI造影剂占据了MRI造影剂的大部分市场。然而,钆螯合物的肾毒性、体内残留(特别是脑残留)、非特异性和低r1值限制了其进一步应用。新型纳米MRI造影剂(如:超小磁性氧化铁纳米粒子、点式核壳型铁钆复合纳米粒子和极小氧化钆纳米粒子),具有良好的生物相容性、较高的肿瘤富集度以及显著的MRI成像效果,有望实现临床转化。这些新型纳米MRI造影剂仍处于实验室研究阶段,其进一步的临床研究结果令人期待。
另外,将MRI与各种治疗方式相结合的诊疗一体化研究发展迅速,在疾病诊疗尤其是肿瘤诊疗方面,结合了诊断与治疗功能的诊疗一体化制剂,可实现对肿瘤的高效治疗和实时监控,如:MRI指导的铁凋亡治疗、MRI指导的放射增敏治疗和MRI指导的光热治疗等,有望成为可行的癌症治疗策略。在MRI基多功能诊疗制剂领域,可通过控制纳米粒子的尺寸并进行不同方式的表面修饰,制备基于MRI的多功能诊疗制剂,更好地应用于肿瘤的靶向成像和治疗,可为肿瘤的临床诊断、治疗及疗效评估提供新型有效的方法。
Biography
周惠敏,硕士研究生,E-mail: zhouhuimin21910426@163.com
Funding Statement
国家自然科学基金(51761145021);浙江省自然科学基金杰出青年科学基金(LR19E030001);国家重点研发计划(2016YFC1400605,2018YFD0800302)
Supported by National Natural Science Foundation of China (51761145021)
Contributor Information
周 惠敏 (Huimin ZHOU), Email: zhouhuimin21910426@163.com.
邱 小忠 (Xiaozhong QIU), Email: qqiuxzh@163.com.
沈 折玉 (Zheyu SHEN), Email: sz@smu.edu.cn.
References
- 1.Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature. 1973;242(5394):190–1. doi: 10.1038/242190a0. [Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance[J]. Nature, 1973, 242(5394): 190-1.] [DOI] [PubMed] [Google Scholar]
- 2.Denis HC. Paramagnetic contrast media for magnetic resonance imaging of the mediastinum and lungs. J Thorac Imaging. 1985;1(1):74–8. doi: 10.1097/00005382-198512000-00010. [Denis HC. Paramagnetic contrast media for magnetic resonance imaging of the mediastinum and lungs[J]. J Thorac Imaging, 1985, 1 (1): 74-8.] [DOI] [PubMed] [Google Scholar]
- 3.Shellock FG, Kanal E. Safety of magnetic resonance imaging contrast agents. J Magn Reson Imaging. 1999;10(3):477–84. doi: 10.1002/(SICI)1522-2586(199909)10:3<477::AID-JMRI33>3.0.CO;2-E. [Shellock FG, Kanal E. Safety of magnetic resonance imaging contrast agents[J]. J Magn Reson Imaging, 1999, 10(3): 477-84.] [DOI] [PubMed] [Google Scholar]
- 4.李 坤成. 磁共振成像对比剂的临床应用. 北京: 人民卫生出版社; 2016. pp. 20–4. [李坤成.磁共振成像对比剂的临床应用[M].北京:人民卫生出版社, 2016: 20-4.] [Google Scholar]
- 5.Zou ZT, Lin M. Nephrogenic systemic fibrosis: review of 408 biopsy-confirmed cases. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_6c75c58348afe6db03ee89ca67aacb91. Cardiovasc Imaging. 2011;56(1):65–73. doi: 10.4103/0019-5154.77556. [Zou ZT, Lin M. Nephrogenic systemic fibrosis: review of 408 biopsy-confirmed cases[J]. Cardiovasc Imaging, 2011, 56(1): 65- 73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peak AS, Sheller A. Risk factors for developing GadoliniumInduced nephrogenic systemic fibrosis. Ann Pharmacother. 2007;41(9):1481–5. doi: 10.1345/aph.1K295. [Peak AS, Sheller A. Risk factors for developing GadoliniumInduced nephrogenic systemic fibrosis[J]. Ann Pharmacother, 2007, 41(9): 1481-5.] [DOI] [PubMed] [Google Scholar]
- 7.Grobner T. Gadolinium-a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis. Nephrol Dial Transplant. 2006;21(4):1104–8. doi: 10.1093/ndt/gfk062. [Grobner T. Gadolinium-a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis[J]. Nephrol Dial Transplant, 2006, 21(4): 1104-8.] [DOI] [PubMed] [Google Scholar]
- 8.Mendichovszky IA, Stephen DM, Simcock CM, et al. Gadolinium and nephrogenic systemic fibrosis: time to tighten practice. Pediatr Radiol. 2008;38(5):489–96. doi: 10.1007/s00247-007-0633-8. [Mendichovszky IA, Stephen DM, Simcock CM, et al. Gadolinium and nephrogenic systemic fibrosis: time to tighten practice[J]. Pediatr Radiol, 2008, 38(5): 489-96.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulani V, Calamante F, Shellock FG, et al. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16(7):564–70. doi: 10.1016/S1474-4422(17)30158-8. [Gulani V, Calamante F, Shellock FG, et al. Gadolinium deposition in the brain: summary of evidence and recommendations[J]. Lancet Neurol, 2017, 16(7): 564-70.] [DOI] [PubMed] [Google Scholar]
- 10.Wisner ER, Amparo EG, Vera DR, et al. Arabinogalactan-coated superparamagnetic Iron oxide: effect of particle size in liver MRI. J ComputAssist Tomogr. 1995;19(2):211–5. doi: 10.1097/00004728-199503000-00008. [Wisner ER, Amparo EG, Vera DR, et al. Arabinogalactan-coated superparamagnetic Iron oxide: effect of particle size in liver MRI [J]. J ComputAssist Tomogr, 1995, 19(2): 211-5.] [DOI] [PubMed] [Google Scholar]
- 11.Chouly C, Pouliquen D, Lucet I, et al. Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface Nature on biodistribution. J Microencapsul. 1996;13(3):245–55. doi: 10.3109/02652049609026013. [Chouly C, Pouliquen D, Lucet I, et al. Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface Nature on biodistribution[J]. J Microencapsul, 1996, 13(3): 245-55.] [DOI] [PubMed] [Google Scholar]
- 12.Réty F, Clément O, Siauve N, et al. Mr lymphography using Iron oxide nanoparticles in rats: pharmacokinetics in the lymphatic system after intravenous injection. J Magn Reson Imaging. 2000;12(5):734–9. doi: 10.1002/1522-2586(200011)12:5<734::AID-JMRI10>3.0.CO;2-R. [Réty F, Clément O, Siauve N, et al. Mr lymphography using Iron oxide nanoparticles in rats: pharmacokinetics in the lymphatic system after intravenous injection[J]. J Magn Reson Imaging, 2000, 12(5): 734-9.] [DOI] [PubMed] [Google Scholar]
- 13.Allkemper T, Bremer C, Matuszewski L, et al. Contrast-enhanced blood-pool Mr angiography with optimized Iron oxides: effect of size and dose on vascular contrast enhancement in rabbits. Radiology. 2002;223(2):432–8. doi: 10.1148/radiol.2232010241. [Allkemper T, Bremer C, Matuszewski L, et al. Contrast-enhanced blood-pool Mr angiography with optimized Iron oxides: effect of size and dose on vascular contrast enhancement in rabbits[J]. Radiology, 2002, 223(2): 432-8.] [DOI] [PubMed] [Google Scholar]
- 14.Briley-Saebo K, Bjrnerud A, Grant D, et al. Hepatic cellular distribution and degradation of Iron oxide nanoparticles following single intravenous injection in rats: implications for magnetic resonance imaging. Cell Tissue Res. 2004;316(3):315–23. doi: 10.1007/s00441-004-0884-8. [Briley-Saebo K, Bjrnerud A, Grant D, et al. Hepatic cellular distribution and degradation of Iron oxide nanoparticles following single intravenous injection in rats: implications for magnetic resonance imaging[J]. Cell Tissue Res, 2004, 316(3): 315-23.] [DOI] [PubMed] [Google Scholar]
- 15.Luo G, Ronnie HF, Michael JS, et al. In vivo clearance and toxicity of monodisperse Iron oxide nanocrystals. ACS Nano. 2012;6(6):4947–54. doi: 10.1021/nn300456z. [Luo G, Ronnie HF, Michael JS, et al. In vivo clearance and toxicity of monodisperse Iron oxide nanocrystals[J]. ACS Nano, 2012, 6(6): 4947-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi-Xiang JW. Current status of superparamagnetic Iron oxide contrast agents for liver magnetic resonance imaging. World J Gastroenterol. 2015;21(47):13400–9. doi: 10.3748/wjg.v21.i47.13400. [Yi-Xiang JW. Current status of superparamagnetic Iron oxide contrast agents for liver magnetic resonance imaging[J]. World J Gastroenterol, 2015, 21(47): 13400-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyon BN, Song IC, Hyeon T. Inorganic nanoparticles for MRI contrast agents. Adv Mater. 2009;21(21):2133–48. doi: 10.1002/adma.200802366. [Hyon BN, Song IC, Hyeon T. Inorganic nanoparticles for MRI contrast agents[J].Adv Mater, 2009, 21(21): 2133-48.] [DOI] [Google Scholar]
- 18.Bulte JM, Kraitchman DL. Iron oxide Mr contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17(7):484–99. doi: 10.1002/nbm.924. [Bulte JM, Kraitchman DL. Iron oxide Mr contrast agents for molecular and cellular imaging[J]. NMR Biomed, 2004, 17(7): 484- 99.] [DOI] [PubMed] [Google Scholar]
- 19.Pilgrimm H. Superparamagnetic particles with increased r1 relaxivity, process for producing said particles and use thereof: United States, 6638494 B1[P]. 2000.
- 20.Schnorr J, Susanne W, Abramjuk C, et al. Comparison of the iron oxide-based blood-pool contrast medium VSOP-C184 with gadopentetate dimeglumine for first-pass magnetic resonance angiography of the aorta and renal arteries in pigs. Invest Radiol. 2004;39(9):546–53. doi: 10.1097/01.rli.0000133944.30119.cc. [Schnorr J, Susanne W, Abramjuk C, et al. Comparison of the iron oxide-based blood-pool contrast medium VSOP-C184 with gadopentetate dimeglumine for first-pass magnetic resonance angiography of the aorta and renal arteries in pigs[J]. Invest Radiol, 2004, 39(9): 546-53.] [DOI] [PubMed] [Google Scholar]
- 21.Iqbal MZ, Ma X, Chen T, et al. Silica coated super-paramagnetic iron oxide nanoparticles (SPIONPs): a new type contrast agent of T1 magnetic resonance imaging (MRI) J Mater Chem B. 2015;3:5172–81. doi: 10.1039/C5TB00300H. [Iqbal MZ, Ma X, Chen T, et al. Silica coated super-paramagnetic iron oxide nanoparticles (SPIONPs): a new type contrast agent of T1 magnetic resonance imaging (MRI)[J]. J Mater Chem B, 2015, 3: 5172-81.] [DOI] [PubMed] [Google Scholar]
- 22.Zeng LY, Ren WZ, Zheng JJ, et al. Ultrasmall water-soluble metaliron oxide nanoparticles as T1-weighted contrast agents for magnetic resonance imaging. Phys Chem Chem Phys. 2012;14(8):2631–42. doi: 10.1039/c2cp23196d. [Zeng LY, Ren WZ, Zheng JJ, et al. Ultrasmall water-soluble metaliron oxide nanoparticles as T1-weighted contrast agents for magnetic resonance imaging[J]. Phys Chem Chem Phys, 2012, 14 (8): 2631-42.] [DOI] [PubMed] [Google Scholar]
- 23.Shen ZY, Wu AG, Chen XY. Iron oxide nanoparticle based contrast agents for magnetic resonance imaging. Mol Pharm. 2017;14(5):1352–64. doi: 10.1021/acs.molpharmaceut.6b00839. [Shen ZY, Wu AG, Chen XY. Iron oxide nanoparticle based contrast agents for magnetic resonance imaging[J]. Mol Pharm, 2017, 14(5): 1352-64.] [DOI] [PubMed] [Google Scholar]
- 24.He W, Oliver TB, Michael GK, et al. Exceedingly small Iron oxide nanoparticles as positive MRI contrast agents. Proceed National Academy Sci. 2017;114(9):2325–30. doi: 10.1073/pnas.1620145114. [He W, Oliver TB, Michael GK, et al. Exceedingly small Iron oxide nanoparticles as positive MRI contrast agents[J]. Proceed National Academy Sci, 2017, 114(9): 2325-30.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu L, Jia Y, Yu Y, et al. RGD-functionalized ultrasmall Iron oxide nanoparticles for targeted T1-weighted Mr imaging of gliomas. Nanoscale. 2015;7(34):14538–46. doi: 10.1039/C5NR04003E. [Yu L, Jia Y, Yu Y, et al. RGD-functionalized ultrasmall Iron oxide nanoparticles for targeted T1-weighted Mr imaging of gliomas[J]. Nanoscale, 2015, 7(34): 14538-46.] [DOI] [PubMed] [Google Scholar]
- 26.Park JY, Patel D, Lee GH, et al. Highly water-dispersible PEG surface modified ultra small superparamagnetic iron oxide nanoparticles useful for target-specific biomedical applications. Nanotechnology. 2008;19(36):365603–9. doi: 10.1088/0957-4484/19/36/365603. [Park JY, Patel D, Lee GH, et al. Highly water-dispersible PEG surface modified ultra small superparamagnetic iron oxide nanoparticles useful for target-specific biomedical applications[J]. Nanotechnology, 2008, 19(36): 365603-9.] [DOI] [PubMed] [Google Scholar]
- 27.Yung KP, Chien L, Hsieh C, et al. Antiferromagnetic Iron nanocolloids: a new generation in vivo T1 MRI contrast agent. J Am Chem Soc. 2013;135(49):18621–8. doi: 10.1021/ja409490q. [Yung KP, Chien L, Hsieh C, et al. Antiferromagnetic Iron nanocolloids: a new generation in vivo T1 MRI contrast agent[J]. J Am Chem Soc, 2013, 135(49): 18621-8.] [DOI] [PubMed] [Google Scholar]
- 28.Chien L, Yung KP, Chou SW, et al. One-step, room-temperature synthesis of glutathione-capped iron-oxide nanoparticles and their application in in vivo T1-weighted magnetic resonance imaging. Small. 2014;10(19):3962–9. doi: 10.1002/smll.201303868. [Chien L, Yung KP, Chou SW, et al. One-step, room-temperature synthesis of glutathione-capped iron-oxide nanoparticles and their application in in vivo T1-weighted magnetic resonance imaging[J]. Small, 2014, 10(19): 3962-9.] [DOI] [PubMed] [Google Scholar]
- 29.Zhen L, Pei WY, Qiao S, et al. Ultrasmall water-soluble and biocompatible magnetic iron oxide nanoparticles as positive and negative dual contrast agents. Adv Funct Mater. 2012;22(11):2387–93. doi: 10.1002/adfm.201103123. [Zhen L, Pei WY, Qiao S, et al. Ultrasmall water-soluble and biocompatible magnetic iron oxide nanoparticles as positive and negative dual contrast agents[J]. Adv Funct Mater, 2012, 22(11): 2387-93.] [DOI] [Google Scholar]
- 30.Zhen L, Bien T, Mathieu A, et al. Direct coprecipitation route to monodisperse dual-functionalized magnetic iron oxide nanocrystals without size selection. Small. 2008;4(2):231–9. doi: 10.1002/smll.200700575. [Zhen L, Bien T, Mathieu A, et al. Direct coprecipitation route to monodisperse dual-functionalized magnetic iron oxide nanocrystals without size selection[J]. Small, 2008, 4(2): 231-9.] [DOI] [PubMed] [Google Scholar]
- 31.Huang GM, Hui L, Chen JH, et al. Tunable T1 and T2 contrast abilities of manganese-engineered Iron oxide nanoparticles through size control. Nanoscale. 2014;6(17):10404–15. doi: 10.1039/C4NR02680B. [Huang GM, Hui L, Chen JH, et al. Tunable T1 and T2 contrast abilities of manganese-engineered Iron oxide nanoparticles through size control[J]. Nanoscale, 2014, 6(17): 10404-15.] [DOI] [PubMed] [Google Scholar]
- 32.Pavel K, He JB, Vijay TJ, et al. Superparamagnetic iron oxide nanoparticles with variable size and an Iron oxidation state as prospective imaging agents. Langmuir. 2013;29(2):710–6. doi: 10.1021/la3037007. [Pavel K, He JB, Vijay TJ, et al. Superparamagnetic iron oxide nanoparticles with variable size and an Iron oxidation state as prospective imaging agents[J]. Langmuir, 2013, 29(2): 710-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byung HK, Nohyun L, Hyoungsu K, et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolutionT1magnetic resonance imaging contrast agents. J Am Chem Soc. 2011;133(32):12624–31. doi: 10.1021/ja203340u. [Byung HK, Nohyun L, Hyoungsu K, et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolutionT1magnetic resonance imaging contrast agents[J]. J Am Chem Soc, 2011, 133(32): 12624-31.] [DOI] [PubMed] [Google Scholar]
- 34.Shen ZY, Chen TX, Ma XH, et al. Multifunctional theranostic nanoparticles based on exceedingly small magnetic Iron oxide nanoparticles for T1-Weighted magnetic resonance imaging and chemotherapy. ACS Nano. 2017;11(11):10992–1004. doi: 10.1021/acsnano.7b04924. [Shen ZY, Chen TX, Ma XH, et al. Multifunctional theranostic nanoparticles based on exceedingly small magnetic Iron oxide nanoparticles for T1-Weighted magnetic resonance imaging and chemotherapy[J].ACS Nano, 2017, 11(11): 10992-1004.] [DOI] [PubMed] [Google Scholar]
- 35.Charalambos K, Shaffer TM, Ogirala A, et al. Environmentresponsive nanophores for therapy and treatment monitoring via molecular MRI quenching. http://cancurecancer.org/wp-content/uploads/2015/02/nanophores.pdf. Nat Commun. 2014;5(1):3384–9. doi: 10.1038/ncomms4384. [Charalambos K, Shaffer TM, Ogirala A, et al. Environmentresponsive nanophores for therapy and treatment monitoring via molecular MRI quenching[J]. Nat Commun, 2014, 5(1): 3384-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikhaylov G, Mikac U, Magaeva AA, et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat Nanotechnol. 2011;6(9):594–602. doi: 10.1038/nnano.2011.112. [Mikhaylov G, Mikac U, Magaeva AA, et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment[J]. Nat Nanotechnol, 2011, 6(9): 594-602.] [DOI] [PubMed] [Google Scholar]
- 37.Alexander AK, Xu HY, Stephen RA, et al. Paramagnetic fluorinated nanoemulsions for sensitive cellular fluorine-19 magnetic resonance imaging. Nat Mater. 2016;15(6):662–8. doi: 10.1038/nmat4585. [Alexander AK, Xu HY, Stephen RA, et al. Paramagnetic fluorinated nanoemulsions for sensitive cellular fluorine-19 magnetic resonance imaging[J]. Nat Mater, 2016, 15(6): 662-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen ZY, Hao W, Yang S, et al. A novel Trojan-horse targeting strategy to reduce the non-specific uptake of nanocarriers by noncancerous cells. https://www.sciencedirect.com/science/article/pii/S0142961215006808. Biomaterials. 2015;70(3):1–11. doi: 10.1016/j.biomaterials.2015.08.022. [Shen ZY, Hao W, Yang S, et al. A novel Trojan-horse targeting strategy to reduce the non-specific uptake of nanocarriers by noncancerous cells[J]. Biomaterials, 2015, 70(3): 1-11.] [DOI] [PubMed] [Google Scholar]
- 39.Zhou ZJ, Wu CQ, Liu HY, et al. Surface and interfacial engineering of Iron oxide nanoplates for highly efficient magnetic resonance angiography. ACS Nano. 2015;9(3):3012–22. doi: 10.1021/nn507193f. [Zhou ZJ, Wu CQ, Liu HY, et al. Surface and interfacial engineering of Iron oxide nanoplates for highly efficient magnetic resonance angiography[J].ACS Nano, 2015, 9(3): 3012-22.] [DOI] [PubMed] [Google Scholar]
- 40.Jin-sil C, Jae-Hyun L, Tae-Hyun S, et al. Self-confirming "AND" logic nanoparticles for fault-free MRI. J Am Chem Soc. 2010;132(32):11015–7. doi: 10.1021/ja104503g. [Jin-sil C, Jae-Hyun L, Tae-Hyun S, et al. Self-confirming "AND" logic nanoparticles for fault-free MRI[J]. J Am Chem Soc, 2010, 132(32): 11015-7.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F, Zhi D, Luo Y, et al. Core/shell Fe3O4/Gd2O3 nanocubes as T1-T2 dual modal MRI contrast agents. Nanoscale. 2016;8(25):12826–33. doi: 10.1039/C6NR02620F. [Li F, Zhi D, Luo Y, et al. Core/shell Fe3O4/Gd2O3 nanocubes as T1-T2 dual modal MRI contrast agents[J]. Nanoscale, 2016, 8(25): 12826- 33.] [DOI] [PubMed] [Google Scholar]
- 42.Hong Y, Zhuang YM, Yun S, et al. Targeted dual-contrast T1- and T2- weighted magnetic resonance imaging of tumors using multifunctional gadolinium-labeled superparamagnetic Iron oxide nanoparticles. Biomaterials. 2011;32(20):4584–93. doi: 10.1016/j.biomaterials.2011.03.018. [Hong Y, Zhuang YM, Yun S, et al. Targeted dual-contrast T1- and T2- weighted magnetic resonance imaging of tumors using multifunctional gadolinium-labeled superparamagnetic Iron oxide nanoparticles [J]. Biomaterials, 2011, 32(20): 4584-93.] [DOI] [PubMed] [Google Scholar]
- 43.Zhang GL, Du RH, Zhang L, et al. Gadolinium-doped Iron oxide nanoprobe as multifunctional bioimaging agent and drug delivery system. Adv Funct Mater. 2015;25(38):6101–11. doi: 10.1002/adfm.201502868. [Zhang GL, Du RH, Zhang L, et al. Gadolinium-doped Iron oxide nanoprobe as multifunctional bioimaging agent and drug delivery system[J].Adv Funct Mater, 2015, 25(38): 6101-11.] [DOI] [Google Scholar]
- 44.Jung S, Na K, Seul AL, et al. Gd(Ⅲ)-DOTA-modified sonosensitive liposomes for ultrasound-triggered release and Mr imaging. Nanoscale Res Lett. 2012;7(1):462–73. doi: 10.1186/1556-276X-7-462. [Jung S, Na K, Seul AL, et al. Gd(Ⅲ)-DOTA-modified sonosensitive liposomes for ultrasound-triggered release and Mr imaging[J]. Nanoscale Res Lett, 2012, 7(1): 462-73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen ZY, Song JB, Zhou ZJ, et al. Dotted core-shell nanoparticles for T1-weighted MRI of tumors. Adv Mater. 2018;30(33):1803163–8. doi: 10.1002/adma.201803163. [Shen ZY, Song JB, Zhou ZJ, et al. Dotted core-shell nanoparticles for T1-weighted MRI of tumors[J]. Adv Mater, 2018, 30(33): 1803163-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huan Z, Li L, Xiao LL, et al. Ultrasmall ferrite nanoparticles synthesized via dynamic simultaneous thermal decomposition for high-performance and multifunctional T1 magnetic resonance imaging contrast agent. ACS Nano. 2017;11(4):3614–31. doi: 10.1021/acsnano.6b07684. [Huan Z, Li L, Xiao LL, et al. Ultrasmall ferrite nanoparticles synthesized via dynamic simultaneous thermal decomposition for high-performance and multifunctional T1 magnetic resonance imaging contrast agent[J].ACS Nano, 2017, 11(4): 3614-31.] [DOI] [PubMed] [Google Scholar]
- 47.Zhou ZJ, Wang LR, Chi XQ, et al. Engineered iron-oxide-based nanoparticles as enhanced T1 contrast agents for efficient tumor imaging. ACS Nano. 2013;7(4):3287–96. doi: 10.1021/nn305991e. [Zhou ZJ, Wang LR, Chi XQ, et al. Engineered iron-oxide-based nanoparticles as enhanced T1 contrast agents for efficient tumor imaging[J].ACS Nano, 2013, 7(4): 3287-96.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhuang L, Cai WB, He LA, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2(1):47–52. doi: 10.1038/nnano.2006.170. [Zhuang L, Cai WB, He LA, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice[J]. Nat Nanotechnol, 2007, 2(1): 47-52.] [DOI] [PubMed] [Google Scholar]
- 49.Maldiney T, Bessière A, Seguin J, et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat Mater. 2014;13(4):418–26. doi: 10.1038/nmat3908. [Maldiney T, Bessière A, Seguin J, et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells[J]. Nat Mater, 2014, 13(4): 418-26.] [DOI] [PubMed] [Google Scholar]
- 50.Du BJ, Jiang XY, Das A, et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat Nanotechnol. 2017;12(11):1096–102. doi: 10.1038/nnano.2017.170. [Du BJ, Jiang XY, Das A, et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime[J]. Nat Nanotechnol, 2017, 12(11): 1096-102.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen Z, Fan W, Yang Z, et al. Exceedingly small gadolinium oxide nanoparticles with remarkable relaxivities for magnetic resonance imaging of tumors. Small. 2019;15(41):1903422–8. doi: 10.1002/smll.201903422. [Shen Z, Fan W, Yang Z, et al. Exceedingly small gadolinium oxide nanoparticles with remarkable relaxivities for magnetic resonance imaging of tumors[J]. Small, 2019, 15(41): 1903422-8.] [DOI] [PubMed] [Google Scholar]
- 52.Ananta JS, Godin B, Sethi R, et al. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrast. Nat Nanotechnol. 2010;5(11):815–21. doi: 10.1038/nnano.2010.203. [Ananta JS, Godin B, Sethi R, et al. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrast[J]. Nat Nanotechnol, 2010, 5(11): 815-21.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caravan P, Ellison JJ, Mcmurry TJ, et al. Gadolinium(Ⅲ) chelates as MRI contrast agents: structure, dynamics, and applications. https://www.ncbi.nlm.nih.gov/pubmed/11749483. Chem Rev. 1999;58(9):2293–352. doi: 10.1021/cr980440x. [Caravan P, Ellison JJ, Mcmurry TJ, et al. Gadolinium(Ⅲ) chelates as MRI contrast agents: structure, dynamics, and applications[J]. Chem Rev, 1999, 58(9): 2293-352.] [DOI] [PubMed] [Google Scholar]
- 54.Bridot JL, Faure AC, Laurent S, et al. Hybrid gadolinium oxide nanoparticles: multimodal contrast agents for in vivo imaging. J Am Chem Soc. 2007;129(16):5076–84. doi: 10.1021/ja068356j. [Bridot JL, Faure AC, Laurent S, et al. Hybrid gadolinium oxide nanoparticles: multimodal contrast agents for in vivo imaging[J]. J Am Chem Soc, 2007, 129(16): 5076-84.] [DOI] [PubMed] [Google Scholar]
- 55.Park JY, Baek MJ, Choi ES, et al. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images. ACS Nano. 2009;3(11):3663–9. doi: 10.1021/nn900761s. [Park JY, Baek MJ, Choi ES, et al. Paramagnetic ultrasmall gadolinium oxide nanoparticles as advanced T1 MRI contrast agent: account for large longitudinal relaxivity, optimal particle diameter, and in vivo T1 MR images[J]. ACS Nano, 2009, 3(11): 3663-9.] [DOI] [PubMed] [Google Scholar]
- 56.Faucher L, Tremblay M, Lagueux J, et al. Rapid synthesis of PEGylated ultrasmall gadolinium oxide nanoparticles for cell labeling and tracking with MRI. ACS Appl Mater Interfaces. 2012;4(9):4506–15. doi: 10.1021/am3006466. [Faucher L, Tremblay M, Lagueux J, et al. Rapid synthesis of PEGylated ultrasmall gadolinium oxide nanoparticles for cell labeling and tracking with MRI[J]. ACS Appl Mater Interfaces, 2012, 4(9): 4506-15.] [DOI] [PubMed] [Google Scholar]
- 57.Zhou Z, Yang T, Wang J, et al. Size-tunable GdO@albumin nanoparticles conjugating chlorin e6 for magnetic resonance imaging-guided photo-induced therapy. Theranostics. 2017;7(3):764–73. doi: 10.7150/thno.15757. [Zhou Z, Yang T, Wang J, et al. Size-tunable GdO@albumin nanoparticles conjugating chlorin e6 for magnetic resonance imaging-guided photo-induced therapy[J]. Theranostics, 2017, 7 (3): 764-73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho M, Sethi R, Narayanan JS, et al. Gadolinium oxide nanoplates with high longitudinal relaxivity for magnetic resonance imaging. Nanoscale. 2014;6(22):13637–45. doi: 10.1039/C4NR03505D. [Cho M, Sethi R, Narayanan JS, et al. Gadolinium oxide nanoplates with high longitudinal relaxivity for magnetic resonance imaging [J]. Nanoscale, 2014, 6(22): 13637-45.] [DOI] [PubMed] [Google Scholar]
- 59.Luo D, Cui S, Liu Y, et al. Biocompatibility of magnetic resonance imaging nanoprobes improved by transformable gadolinium oxide nanocoils. JAm Chem Soc. 2018;140(43):14211–6. doi: 10.1021/jacs.8b08118. [Luo D, Cui S, Liu Y, et al. Biocompatibility of magnetic resonance imaging nanoprobes improved by transformable gadolinium oxide nanocoils[J]. JAm Chem Soc, 2018, 140(43): 14211-6.] [DOI] [PubMed] [Google Scholar]
- 60.Wanqing C, Kexin S, Rongshou Z, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30(1):1–12. doi: 10.21147/j.issn.1000-9604.2018.01.01. [Wanqing C, Kexin S, Rongshou Z, et al. Cancer incidence and mortality in China, 2014[J]. Chin J Cancer Res, 2018, 30(1): 1-12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin OA, Anderson RL, Narayan K, et al. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat Rev Clin Oncol. 2017;14(1):32–44. doi: 10.1038/nrclinonc.2016.128. [Martin OA, Anderson RL, Narayan K, et al. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? [J]. Nat Rev Clin Oncol, 2017, 14(1): 32-44.] [DOI] [PubMed] [Google Scholar]
- 62.Stewart MP, Sharei A, Ding X, et al. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538(7624):183–92. doi: 10.1038/nature19764. [Stewart MP, Sharei A, Ding X, et al. In vitro and ex vivo strategies for intracellular delivery[J]. Nature, 2016, 538(7624): 183-92.] [DOI] [PubMed] [Google Scholar]
- 63.Liao L, Liu J, Dreaden EC, et al. A convergent synthetic platform for single-nanoparticle combination cancer therapy: ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin. JAm Chem Soc. 2014;136(16):5896–9. doi: 10.1021/ja502011g. [Liao L, Liu J, Dreaden EC, et al. A convergent synthetic platform for single-nanoparticle combination cancer therapy: ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin[J]. JAm Chem Soc, 2014, 136(16): 5896-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim EJ, Bhuniya S, Lee H, et al. An activatable prodrug for the treatment of metastatic tumors. J Am Chem Soc. 2014;136(39):13888–94. doi: 10.1021/ja5077684. [Kim EJ, Bhuniya S, Lee H, et al. An activatable prodrug for the treatment of metastatic tumors[J]. J Am Chem Soc, 2014, 136(39): 13888-94.] [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Liu D, Shahbazi MA, et al. Fabrication of a multifunctional nano-in-micro drug delivery platform by micro_x0002_fluidic templated encapsulation of porous silicon in polymer matrix. http://www.ncbi.nlm.nih.gov/pubmed/24737409. Adv Mater. 2014;26(10):4497–503. doi: 10.1002/adma.201400953. [Zhang H, Liu D, Shahbazi MA, et al. Fabrication of a multifunctional nano-in-micro drug delivery platform by micro_x0002_fluidic templated encapsulation of porous silicon in polymer matrix[J]. Adv Mater, 2014, 26(10): 4497-503.] [DOI] [PubMed] [Google Scholar]
- 66.Yang YS, Carney RP, Stellacci F, et al. Enhancing radiotherapy by lipid nanocapsule-mediated delivery of amphi_x0002_philic gold nanoparticles to intracellular membranes. ACS Nano. 2014;8(9):8992–9002. doi: 10.1021/nn502146r. [Yang YS, Carney RP, Stellacci F, et al. Enhancing radiotherapy by lipid nanocapsule-mediated delivery of amphi_x0002_philic gold nanoparticles to intracellular membranes[J]. ACS Nano, 2014, 8(9): 8992-9002.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang XD, Chen J, Min Y, et al. Metabolizable Bi2Se3 nanoplates: biodistribution, toxicity, and uses for cancer radiation therapy and imaging. http://d.old.wanfangdata.com.cn/OAPaper/oai_arXiv.org_1312.1773. Adv Funct Mater. 2014;24(13):1718–29. [Zhang XD, Chen J, Min Y, et al. Metabolizable Bi2Se3 nanoplates: biodistribution, toxicity, and uses for cancer radiation therapy and imaging[J].Adv Funct Mater, 2014, 24(13): 1718-29.] [Google Scholar]
- 68.Cheng L, Wang C, Feng L, et al. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114(21):10869–939. doi: 10.1021/cr400532z. [Cheng L, Wang C, Feng L, et al. Functional nanomaterials for phototherapies of cancer[J]. Chem Rev, 2014, 114(21): 10869-939.] [DOI] [PubMed] [Google Scholar]
- 69.Wang Z, Huang P, Jacobson O, et al. Biomineralization-inspired synthesis of copper sulfide-ferritin nanocages as cancer theranostics. ACS Nano. 2016;10(3):3453–60. doi: 10.1021/acsnano.5b07521. [Wang Z, Huang P, Jacobson O, et al. Biomineralization-inspired synthesis of copper sulfide-ferritin nanocages as cancer theranostics [J].ACS Nano, 2016, 10(3): 3453-60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin J, Wang M, Hu H, et al. Multimodal-imaging-guided cancer phototherapy by versatile biomimetic theranostics with UV and gamma-Irradiation protection. Adv Mater. 2016;28(17):3273–9. doi: 10.1002/adma.201505700. [Lin J, Wang M, Hu H, et al. Multimodal-imaging-guided cancer phototherapy by versatile biomimetic theranostics with UV and gamma-Irradiation protection[J].Adv Mater, 2016, 28(17): 3273-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang W, Guo W, Le W, et al. Albumin-bioinspired Gd:CuS nanotheranostic agent for in vivo photoacoustic/magnetic resonance imaging-guided tumor-targeted photothermal therapy. ACS Nano. 2016;10(11):10245–57. doi: 10.1021/acsnano.6b05760. [Yang W, Guo W, Le W, et al. Albumin-bioinspired Gd:CuS nanotheranostic agent for in vivo photoacoustic/magnetic resonance imaging-guided tumor-targeted photothermal therapy[J]. ACS Nano, 2016, 10(11): 10245-57.] [DOI] [PubMed] [Google Scholar]
- 72.Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CACancer J Clin. 2011;61(4):250–81. doi: 10.3322/caac.20114. [Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update[J]. CACancer J Clin, 2011, 61(4): 250-81.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60(15):1627–37. doi: 10.1016/j.addr.2008.08.003. [Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm[J]. Adv Drug Deliv Rev, 2008, 60 (15): 1627-37.] [DOI] [PubMed] [Google Scholar]
- 74.Ge J, Lan M, Zhou B, et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ec54a84232cfd566370fcba9e99dc721. Nat Commun. 2014;5(12):4596–602. doi: 10.1038/ncomms5596. [Ge J, Lan M, Zhou B, et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation[J]. Nat Commun, 2014, 5(12): 4596-602.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C, Sun W, Wright G, et al. Inflammation-triggered cancer immunotherapy by programmed delivery of CpG and anti-PD1 antibody. Adv Mater. 2016;28(40):8912–20. doi: 10.1002/adma.201506312. [Wang C, Sun W, Wright G, et al. Inflammation-triggered cancer immunotherapy by programmed delivery of CpG and anti-PD1 antibody[J].Adv Mater, 2016, 28(40): 8912-20.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao H, Woods EC, Vukojicic P, et al. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci USA. 2016;113(37):10304–9. doi: 10.1073/pnas.1608069113. [Xiao H, Woods EC, Vukojicic P, et al. Precision glycocalyx editing as a strategy for cancer immunotherapy[J]. Proc Natl Acad Sci USA, 2016, 113(37): 10304-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oberli MA, Reichmuth AM, Dorkin JR, et al. Lipid nanoparticle assisted mRNA delivery for Potent cancer immunotherapy. Nano Lett. 2017;17:1326–35. doi: 10.1021/acs.nanolett.6b03329. [Oberli MA, Reichmuth AM, Dorkin JR, et al. Lipid nanoparticle assisted mRNA delivery for Potent cancer immunotherapy[J]. Nano Lett, 2017, 17: 1326-35.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spring BQ, Sears RB, Zheng LZ, et al. Photoactivable multiinhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways. https://www.ncbi.nlm.nih.gov/pubmed/26780659. Nat Nanotechnol. 2016;11(2):378–87. doi: 10.1038/nnano.2015.311. [Spring BQ, Sears RB, Zheng LZ, et al. Photoactivable multiinhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways[J]. Nat Nanotechnol, 2016, 11(2): 378-87.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Leonard M, Shu Y, et al. Overcoming tamoxifen resistance of human breast cancer by targeted gene silencing using multifunctional pRNA nanoparticles. ACS Nano. 2017;11(1):335–46. doi: 10.1021/acsnano.6b05910. [Zhang Y, Leonard M, Shu Y, et al. Overcoming tamoxifen resistance of human breast cancer by targeted gene silencing using multifunctional pRNA nanoparticles[J]. ACS Nano, 2017, 11(1): 335-46.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen J, Gao P, Yuan S, et al. Oncolytic adenovirus complexes coated with lipids and calcium phosphate for cancer gene therapy. ACS Nano. 2016;10(12):11548–60. doi: 10.1021/acsnano.6b06182. [Chen J, Gao P, Yuan S, et al. Oncolytic adenovirus complexes coated with lipids and calcium phosphate for cancer gene therapy[J]. ACS Nano, 2016, 10(12): 11548-60.] [DOI] [PubMed] [Google Scholar]
- 81.Wang X, Chen H, Chen Y, et al. Perfluorohexane-encapsulated mesoporous silica nanocapsules as enhancement agents for highly efficient high intensity focused ultrasound. Adv Mater. 2012;24(6):785–91. doi: 10.1002/adma.201104033. [Wang X, Chen H, Chen Y, et al. Perfluorohexane-encapsulated mesoporous silica nanocapsules as enhancement agents for highly efficient high intensity focused ultrasound[J]. Adv Mater, 2012, 24 (6): 785-91.] [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Chen H, Sun Y, et al. Multifunctional mesoporous composite nanocapsules for highly efficient MRI-guided high-intensity focused ultrasound cancer surgery. Angew Chem Int Ed Engl. 2011;50(52):12505–9. doi: 10.1002/anie.201106180. [Chen Y, Chen H, Sun Y, et al. Multifunctional mesoporous composite nanocapsules for highly efficient MRI-guided high-intensity focused ultrasound cancer surgery[J]. Angew Chem Int Ed Engl, 2011, 50(52): 12505-9.] [DOI] [PubMed] [Google Scholar]
- 83.Shi D, Cho HS, Chen Y, et al. Fluorescent polystyrene-Fe3O4 composite nanospheres for in vivo imaging and hyperthermia. http://www.researchgate.net/publication/229914772_Fluorescent_PolystyreneFe3O4_Composite_Nanospheres_for_In_Vivo_Imaging_and_Hyperthermia. Adv Mater. 2009;21(15):2170–3. [Shi D, Cho HS, Chen Y, et al. Fluorescent polystyrene-Fe3O4 composite nanospheres for in vivo imaging and hyperthermia[J]. Adv Mater, 2009, 21(15): 2170-3.] [Google Scholar]
- 84.Hayashi K, Nakamura M, Sakamoto W, et al. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics. 2013;3(6):366–76. doi: 10.7150/thno.5860. [Hayashi K, Nakamura M, Sakamoto W, et al. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment[J]. Theranostics, 2013, 3(6): 366-76.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoo D, Jeong H, Noh SH, et al. Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia. Angew Chem Int Ed Engl. 2013;52(49):13047–51. doi: 10.1002/anie.201306557. [Yoo D, Jeong H, Noh SH, et al. Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia [J].Angew Chem Int Ed Engl, 2013, 52(49): 13047-51.] [DOI] [PubMed] [Google Scholar]
- 86.Kuthala N, Vankayala R, Li YN, et al. Engineering novel targeted boron-10-enriched theranostic nanomedicine to combat against murine brain tumors via MR imaging-guided boron neutron capture therapy. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f63bc184846d2b5efb70c30b358d7922. Adv Mater. 2017;29(31):2117–26. doi: 10.1002/adma.201700850. [Kuthala N, Vankayala R, Li YN, et al. Engineering novel targeted boron-10-enriched theranostic nanomedicine to combat against murine brain tumors via MR imaging-guided boron neutron capture therapy[J].Adv Mater, 2017, 29(31): 2117-26.] [DOI] [PubMed] [Google Scholar]
- 87.Li L, Li J, Shi Y, et al. On-demand biodegradable boron nitride nanoparticles for treating triple negative breast cancer with boron neutron capture therapy. ACS Nano. 2019;13(12):13843–52. doi: 10.1021/acsnano.9b04303. [Li L, Li J, Shi Y, et al. On-demand biodegradable boron nitride nanoparticles for treating triple negative breast cancer with boron neutron capture therapy[J].ACS Nano, 2019, 13(12): 13843-52.] [DOI] [PubMed] [Google Scholar]
- 88.Pompos A, Durante M, Choy H. Heavy ions in cancer therapy. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_71ff1fd9b23d4c7c9b5cfd9e6fb6c11d. JAMAOncol. 2016;2(12):1539–40. doi: 10.1001/jamaoncol.2016.2646. [Pompos A, Durante M, Choy H. Heavy ions in cancer therapy[J]. JAMAOncol, 2016, 2(12): 1539-40.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan W, Yung B, Huang P, et al. Nanotechnology for multimodal synergistic cancer therapy. Chem Rev. 2017;117(22):13566–638. doi: 10.1021/acs.chemrev.7b00258. [Fan W, Yung B, Huang P, et al. Nanotechnology for multimodal synergistic cancer therapy[J]. Chem Rev, 2017, 117(22): 13566-638.] [DOI] [PubMed] [Google Scholar]
- 90.Fuchs YSH. Programmed cell death in animal development and disease. Cell. 2011;147(4):742–58. doi: 10.1016/j.cell.2011.10.033. [Fuchs YSH. Programmed cell death in animal development and disease[J]. Cell, 2011, 147(4): 742-58.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thompson BC. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456–67. doi: 10.1126/science.7878464. [Thompson BC. Apoptosis in the pathogenesis and treatment of disease[J]. Science, 1995, 267(5203): 1456-67.] [DOI] [PubMed] [Google Scholar]
- 92.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an irondependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. doi: 10.1016/j.cell.2012.03.042. [Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an irondependent form of nonapoptotic cell death[J]. Cell, 2012, 149(5): 1060-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3669455. Nat Chem Biol. 2014;10(1):9–17. doi: 10.1038/nchembio.1416. [Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death[J]. Nat Chem Biol, 2014, 10(1):9-17.] [DOI] [PubMed] [Google Scholar]
- 94.Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression[J]. Nature, 2015, 520(7545): 57-62.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herde K, Krysko DV. Ferroptosis: oxidized PEs trigger death. Nat Chem Biol. 2017;13(1):4–5. doi: 10.1038/nchembio.2261. [Herde K, Krysko DV. Ferroptosis: oxidized PEs trigger death[J]. Nat Chem Biol, 2017, 13(1): 4-5.] [DOI] [PubMed] [Google Scholar]
- 96.Zanganeh S, Hutter G, Spitler R, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–94. doi: 10.1038/nnano.2016.168. [Zanganeh S, Hutter G, Spitler R, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues[J]. Nat Nanotechnol, 2016, 11(11): 986-94.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Z, Song J, Tian R, et al. Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy. Angew Chem Int Ed Engl. 2017;56(23):6492–6. doi: 10.1002/anie.201701181. [Zhou Z, Song J, Tian R, et al. Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy[J]. Angew Chem Int Ed Engl, 2017, 56(23): 6492-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li WP, Su CH, Chang YC, et al. Ultrasound-induced reactive oxygen species mediated therapy and imaging using a fenton reaction activable polymersome. ACS Nano. 2016;10(2):2017–27. doi: 10.1021/acsnano.5b06175. [Li WP, Su CH, Chang YC, et al. Ultrasound-induced reactive oxygen species mediated therapy and imaging using a fenton reaction activable polymersome[J]. ACS Nano, 2016, 10(2): 2017- 27.] [DOI] [PubMed] [Google Scholar]
- 99.Zhang C, Bu W, Ni D, et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew Chem Int Ed Engl. 2016;55(6):2101–6. doi: 10.1002/anie.201510031. [Zhang C, Bu W, Ni D, et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction [J].Angew Chem Int Ed Engl, 2016, 55(6): 2101-6.] [DOI] [PubMed] [Google Scholar]
- 100.Shen Z, Liu T, Li Y, et al. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano. 2018;12(11):11355–65. doi: 10.1021/acsnano.8b06201. [Shen Z, Liu T, Li Y, et al. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors[J]. ACS Nano, 2018, 12(11): 11355-65.] [DOI] [PubMed] [Google Scholar]
- 101.Maleki A, Aghaei M. Sonochemical rate enhanced by a new nanomagnetic embedded core/shell nanoparticles and catalytic performance in the multicomponent synthesis of pyridoimidazoisoquinolines. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ba4fd6b08c698bfac9180d6f87fe802f. Ultrason Sonochem. 2017;38(2):115–9. doi: 10.1016/j.ultsonch.2017.03.014. [Maleki A, Aghaei M. Sonochemical rate enhanced by a new nanomagnetic embedded core/shell nanoparticles and catalytic performance in the multicomponent synthesis of pyridoimidazoisoquinolines[J]. Ultrason Sonochem, 2017, 38(2): 115-9.] [DOI] [PubMed] [Google Scholar]
- 102.Maleki A, Rahimi J, Demchuk OM, et al. Green in water sonochemical synthesis of tetrazolopyrimidine derivatives by a novel core-shell magnetic nanostructure catalyst. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f4a8b086df3d0acdab2afa663e527f6e. Ultrason Sonochem. 2018;43(10):262–71. doi: 10.1016/j.ultsonch.2017.12.047. [Maleki A, Rahimi J, Demchuk OM, et al. Green in water sonochemical synthesis of tetrazolopyrimidine derivatives by a novel core-shell magnetic nanostructure catalyst[J]. Ultrason Sonochem, 2018, 43(10): 262-71.] [DOI] [PubMed] [Google Scholar]
- 103.Qiao R, Jia Q, Huwel S, et al. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano. 2012;6(4):3304–10. doi: 10.1021/nn300240p. [Qiao R, Jia Q, Huwel S, et al. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier[J]. ACS Nano, 2012, 6(4): 3304-10.] [DOI] [PubMed] [Google Scholar]
- 104.Ma PA, Xiao HH, Li CX, et al. Inorganic nanocarriers for platinum drug delivery. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=f79ba99a85c65f4161f5f2b64c7e0983. Mater Today. 2015;18(12):554–64. [Ma PA, Xiao HH, Li CX, et al. Inorganic nanocarriers for platinum drug delivery[J]. Mater Today, 2015, 18(12): 554-64.] [Google Scholar]
- 105.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–84. doi: 10.1038/nrc2167. [Kelland L. The resurgence of platinum-based cancer chemotherapy [J]. Nat Rev Cancer, 2007, 7(8): 573-84.] [DOI] [PubMed] [Google Scholar]
- 106.Johnstone TC, Suntharalingam K, Lippard SJ. The next generation of platinum drugs: targeted Pt(Ⅱ) agents, nanoparticle delivery, and Pt(Ⅳ) prodrugs. Chem Rev. 2016;116(5):3436–86. doi: 10.1021/acs.chemrev.5b00597. [Johnstone TC, Suntharalingam K, Lippard SJ. The next generation of platinum drugs: targeted Pt(Ⅱ) agents, nanoparticle delivery, and Pt(Ⅳ) prodrugs[J]. Chem Rev, 2016, 116(5): 3436-86.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(Suppl 4):v1–13. doi: 10.1093/neuonc/nou223. [Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011[J]. Neuro Oncol, 2014, 16 (Suppl 4): v1- 13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22. doi: 10.1056/NEJMoa1308345. [Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma[J]. N Engl J Med, 2014, 370(8): 709-22.] [DOI] [PubMed] [Google Scholar]
- 109.Kohler BA, Ward E, Mccarthy B J, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–36. doi: 10.1093/jnci/djr077. [Kohler BA, Ward E, Mccarthy B J, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system[J]. J Natl Cancer Inst, 2011, 103(9): 714-36.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang W, Fan W, Lau J, et al. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem Soc Rev. 2019;48(11):2967–3014. doi: 10.1039/C8CS00805A. [Tang W, Fan W, Lau J, et al. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics[J]. Chem Soc Rev, 2019, 48(11): 2967-3014.] [DOI] [PubMed] [Google Scholar]
- 111.Erel-Akbaba G, Carvalho LA, Tian T, et al. Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy. ACS Nano. 2019;13(4):4028–40. doi: 10.1021/acsnano.8b08177. [Erel-Akbaba G, Carvalho LA, Tian T, et al. Radiation-induced targeted nanoparticle-based gene delivery for brain tumor therapy [J].ACS Nano, 2019, 13(4): 4028-40.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lundy DJ, Lee KJ, Peng IC, et al. Inducing a transient increase in blood-brain barrier permeability for improved liposomal drug therapy of glioblastoma multiforme. ACS Nano. 2019;13(1):97–113. doi: 10.1021/acsnano.8b03785. [Lundy DJ, Lee KJ, Peng IC, et al. Inducing a transient increase in blood-brain barrier permeability for improved liposomal drug therapy of glioblastoma multiforme[J]. ACS Nano, 2019, 13(1): 97- 113.] [DOI] [PubMed] [Google Scholar]
- 113.Taphoorn MJB, Henriksson R, Bottomley A. Health-related quality of life in a randomized phase Ⅲ study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. https://www.ncbi.nlm.nih.gov/pubmed/26014298/ J Clin Oncol. 2015;33(11):2166–75. doi: 10.1200/JCO.2014.60.3217. [Taphoorn MJB, Henriksson R, Bottomley A. Health-related quality of life in a randomized phase Ⅲ study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma [J]. J Clin Oncol, 2015, 33(11): 2166-75.] [DOI] [PubMed] [Google Scholar]
- 114.Zheng M, Liu Y, Wang Y, et al. ROS-responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv Mater. 2019;31(37):e1903277–89. doi: 10.1002/adma.201903277. [Zheng M, Liu Y, Wang Y, et al. ROS-responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy[J]. Adv Mater, 2019, 31 (37): e1903277-89.] [DOI] [PubMed] [Google Scholar]
- 115.Jiang Y, Zhang J, Meng F, et al. Apolipoprotein E peptide-directed chimeric polymersomes mediate an ultrahigh-efficiency targeted protein therapy for glioblastoma. ACS Nano. 2018;12(11):11070–9. doi: 10.1021/acsnano.8b05265. [Jiang Y, Zhang J, Meng F, et al. Apolipoprotein E peptide-directed chimeric polymersomes mediate an ultrahigh-efficiency targeted protein therapy for glioblastoma[J]. ACS Nano, 2018, 12(11): 11070-9.] [DOI] [PubMed] [Google Scholar]
- 116.Lai J, Deng G, Sun Z, et al. Scaffolds biomimicking macrophages for a glioblastoma NIR-Ib imaging guided photothermal therapeutic strategy by crossing blood-brain barrier. http://cn.bing.com/academic/profile?id=042efe6b6c23deabcf9b56e912375130&encoded=0&v=paper_preview&mkt=zh-cn. Biomater. 2019;211(3):48–56. doi: 10.1016/j.biomaterials.2019.04.026. [Lai J, Deng G, Sun Z, et al. Scaffolds biomimicking macrophages for a glioblastoma NIR-Ib imaging guided photothermal therapeutic strategy by crossing blood-brain barrier[J]. Biomater, 2019, 211(3): 48-56.] [DOI] [PubMed] [Google Scholar]
- 117.Shen Z, Liu T, Yang Z, et al. Small-sized gadolinium oxide based nanoparticles for high-efficiency theranostics of orthotopic glioblastoma. http://cn.bing.com/academic/profile?id=c79e1b3cf1affbc1912a1d4325266af3&encoded=0&v=paper_preview&mkt=zh-cn. Biomaterials. 2020;235(17):119783–92. doi: 10.1016/j.biomaterials.2020.119783. [Shen Z, Liu T, Yang Z, et al. Small-sized gadolinium oxide based nanoparticles for high-efficiency theranostics of orthotopic glioblastoma[J]. Biomaterials, 2020, 235(17): 119783-92.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luchette M, Korideck H, Makrigiorgos M, et al. Radiation dose enhancement of gadolinium-based AGuIX nanoparticles on HeLa cells. Nanomedicine. 2014;10(8):1751–5. doi: 10.1016/j.nano.2014.06.004. [Luchette M, Korideck H, Makrigiorgos M, et al. Radiation dose enhancement of gadolinium-based AGuIX nanoparticles on HeLa cells[J]. Nanomedicine, 2014, 10(8): 1751-5.] [DOI] [PubMed] [Google Scholar]
- 119.Song G, Chao Y, Chen Y. All-in-One theranostic nanoplatform based on hollow taOx for chelator-free labeling imaging, drug delivery, and synergistically enhanced radiotherapy. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b3e817e8e712de5c255beacd35c675c4. Adv Funct Mater. 2016;26(12):8243–54. [Song G, Chao Y, Chen Y. All-in-One theranostic nanoplatform based on hollow taOx for chelator-free labeling imaging, drug delivery, and synergistically enhanced radiotherapy[J]. Adv Funct Mater, 2016, 26(12): 8243-54.] [Google Scholar]
- 120.Porcel E, Tillement O, Lux F. Gadolinium-based nanoparticles to improve the hadron therapy performances. http://www.ncbi.nlm.nih.gov/pubmed/24846523. Nanomedicine. 2014;10(9):1601–8. doi: 10.1016/j.nano.2014.05.005. [Porcel E, Tillement O, Lux F. Gadolinium-based nanoparticles to improve the hadron therapy performances[J]. Nanomedicine, 2014, 10(9): 1601-8.] [DOI] [PubMed] [Google Scholar]
- 121.Yu Y, Jiang X, Gong S, et al. The proton permeability of selfassembled polymersomes and their neuroprotection by enhancing a neuroprotective peptide across the blood-brain barrier after modification with lactoferrin. Nanoscale. 2014;6(6):3250–8. doi: 10.1039/C3NR05196J. [Yu Y, Jiang X, Gong S, et al. The proton permeability of selfassembled polymersomes and their neuroprotection by enhancing a neuroprotective peptide across the blood-brain barrier after modification with lactoferrin[J]. Nanoscale, 2014, 6(6): 3250-8.] [DOI] [PubMed] [Google Scholar]
- 122.Zhao X, Ting SM, Liu CH, et al. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. http://cn.bing.com/academic/profile?id=46b59fdbc534e25302bcf4e454557d2d&encoded=0&v=paper_preview&mkt=zh-cn. Nat Commun. 2017;8(1):602–9. doi: 10.1038/s41467-017-00770-7. [Zhao X, Ting SM, Liu CH, et al. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage[J]. Nat Commun, 2017, 8(1): 602-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agrawal M, Ajazuddin, Tripathi DK, et al. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer's disease. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=885d2870b02a60e337bbeccf75e28d96. J Control Release. 2017;260(5):61–77. doi: 10.1016/j.jconrel.2017.05.019. [Agrawal M, Ajazuddin, Tripathi DK, et al. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer's disease[J]. J Control Release, 2017, 260(5): 61-77.] [DOI] [PubMed] [Google Scholar]
- 124.Chen H, Zhang W, Zhu G, et al. Rethinking cancer nanotheranostics. http://cn.bing.com/academic/profile?id=2359f6b391277603ff63071b6e1d464a&encoded=0&v=paper_preview&mkt=zh-cn. Nat Rev Mater. 2017;15(2):17024–35. doi: 10.1038/natrevmats.2017.24. [Chen H, Zhang W, Zhu G, et al. Rethinking cancer nanotheranostics [J]. Nat Rev Mater, 2017, 15(2): 17024-35.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51. doi: 10.1038/nbt.3330. [Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery[J]. Nat Biotechnol, 2015, 33(9): 941-51.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matsumoto Y, Nichols JW, Toh K, et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat Nanotechnol. 2016;11(6):533–8. doi: 10.1038/nnano.2015.342. [Matsumoto Y, Nichols JW, Toh K, et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery[J]. Nat Nanotechnol, 2016, 11(6): 533-8.] [DOI] [PubMed] [Google Scholar]
- 127.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. http://d.old.wanfangdata.com.cn/NSTLQK/10.1007-s11051-011-0462-4/ Cancer Res. 1986;46(12):6387–92. [Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs[J]. Cancer Res, 1986, 46(12): 6387-92.] [PubMed] [Google Scholar]
- 128.Setyawati MI, Tay CY, Chia SL, et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8067843cefe65f071104d1e0d3da5871. Nat Commun. 2013;4(5):1673–82. doi: 10.1038/ncomms2655. [Setyawati MI, Tay CY, Chia SL, et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin[J]. Nat Commun, 2013, 4 (5): 1673-82.] [DOI] [PubMed] [Google Scholar]
- 129.Tavares AJ, Poon W, Zhang YN, et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc Natl Acad Sci USA. 2017;114(51):10871–80. doi: 10.1073/pnas.1713390114. [Tavares AJ, Poon W, Zhang YN, et al. Effect of removing Kupffer cells on nanoparticle tumor delivery[J]. Proc Natl Acad Sci USA, 2017, 114(51): 10871-80.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Black KC, Luehmann H, et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano. 2013;7(3):2068–77. doi: 10.1021/nn304332s. [Wang Y, Black KC, Luehmann H, et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment[J].ACS Nano, 2013, 7(3): 2068-77.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang X, El-Sayed IH, Qian W, et al. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. JAm Chem Soc. 2006;128(6):2115–20. doi: 10.1021/ja057254a. [Huang X, El-Sayed IH, Qian W, et al. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods[J]. JAm Chem Soc, 2006, 128(6): 2115-20.] [DOI] [PubMed] [Google Scholar]
- 132.Manikandan M, Hasan N, Wu HF. Platinum nanoparticles for the photothermal treatment of Neuro 2A cancer cells. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c27c8f1f0a0928bb3810418789913c4d. Biomater. 2013;34(16):5833–42. doi: 10.1016/j.biomaterials.2013.03.077. [Manikandan M, Hasan N, Wu HF. Platinum nanoparticles for the photothermal treatment of Neuro 2A cancer cells[J]. Biomater, 2013, 34(16): 5833-42.] [DOI] [PubMed] [Google Scholar]
- 133.Huang Y, Lai Y, Shi S, et al. Copper sulfide nanoparticles with phospholipid-PEG coating for in vivo near-infrared photothermal cancer therapy. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d6f00820edcc3778d15dacb7f8feb18d. ChemAsian J. 2015;10(2):370–6. doi: 10.1002/asia.201403133. [Huang Y, Lai Y, Shi S, et al. Copper sulfide nanoparticles with phospholipid-PEG coating for in vivo near-infrared photothermal cancer therapy[J]. ChemAsian J, 2015, 10(2): 370-6.] [DOI] [PubMed] [Google Scholar]
- 134.Li Y, Lu W, Huang Q, et al. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine (Lond) 2010;5(8):1161–71. doi: 10.2217/nnm.10.85. [Li Y, Lu W, Huang Q, et al. Copper sulfide nanoparticles for photothermal ablation of tumor cells[J]. Nanomedicine (Lond), 2010, 5(8): 1161-71.] [DOI] [PubMed] [Google Scholar]
- 135.Moon HK, Lee SH, Choi HC. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano. 2009;3(11):3707–13. doi: 10.1021/nn900904h. [Moon HK, Lee SH, Choi HC. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes[J]. ACS Nano, 2009, 3(11): 3707-13.] [DOI] [PubMed] [Google Scholar]
- 136.Neves LF, Krais JJ, Van Rite BD, et al. Targeting single-walled carbon nanotubes for the treatment of breast cancer using photothermal therapy. Nanotechnol. 2013;24(37):375104–15. doi: 10.1088/0957-4484/24/37/375104. [Neves LF, Krais JJ, Van Rite BD, et al. Targeting single-walled carbon nanotubes for the treatment of breast cancer using photothermal therapy[J]. Nanotechnol, 2013, 24(37): 375104-15.] [DOI] [PubMed] [Google Scholar]
- 137.Hessel CM, Pattani VP, Rasch M. Copper selenide nanocrystals for photothermal therapy. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=07d6a19fc7942a857416d66d635ae96e. Nano Lett. 2011;11(9):2560–6. doi: 10.1021/nl201400z. [Hessel CM, Pattani VP, Rasch M. Copper selenide nanocrystals for photothermal therapy[J]. Nano Lett, 2011, 11(9): 2560-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou J, Lu Z, Zhu X. NIR photothermal therapy using polyaniline nanoparticles. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0231843759/ Biomater. 2013;34(12):9584–92. doi: 10.1016/j.biomaterials.2013.08.075. [Zhou J, Lu Z, Zhu X. NIR photothermal therapy using polyaniline nanoparticles[J]. Biomater, 2013, 34(12): 9584-92.] [DOI] [PubMed] [Google Scholar]
- 139.Zhao T, Wang P, Li Q, et al. Near-infrared triggered decomposition of nanocapsules with high tumor accumulation and stimuli responsive fast elimination. Angew Chem Int Ed Engl. 2018;57(10):2611–5. doi: 10.1002/anie.201711354. [Zhao T, Wang P, Li Q, et al. Near-infrared triggered decomposition of nanocapsules with high tumor accumulation and stimuli responsive fast elimination[J]. Angew Chem Int Ed Engl, 2018, 57 (10): 2611-5.] [DOI] [PubMed] [Google Scholar]
- 140.Sanchez-Iglesias A, Claes N, Solis DM, et al. Reversible clustering of gold nanoparticles under confinement. Angew Chem Int Ed Engl. 2018;57(12):3183–6. doi: 10.1002/anie.201800736. [Sanchez-Iglesias A, Claes N, Solis DM, et al. Reversible clustering of gold nanoparticles under confinement[J]. Angew Chem Int Ed Engl, 2018, 57(12): 3183-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang Y, Lu Y, Abbaraju PL, et al. Multi-shelled dendritic mesoporous organosilica hollow spheres: roles of composition and architecture in cancer immunotherapy. http://cn.bing.com/academic/profile?id=9bcaac98fa8ce1b3b2077f51681592ed&encoded=0&v=paper_preview&mkt=zh-cn. Angew Chem. 2017;129(21):8566–70. doi: 10.1002/anie.201701550. [Yang Y, Lu Y, Abbaraju PL, et al. Multi-shelled dendritic mesoporous organosilica hollow spheres: roles of composition and architecture in cancer immunotherapy[J]. Angew Chem, 2017, 129 (21): 8566-70.] [DOI] [PubMed] [Google Scholar]
- 142.Gonzalez-Rubio G, Diaz-Nunez P, Rivera A, et al. Femtosecond laser reshaping yields gold nanorods with ultranarrow surface plasmon resonances. Science. 2017;358(6363):640–4. doi: 10.1126/science.aan8478. [Gonzalez-Rubio G, Diaz-Nunez P, Rivera A, et al. Femtosecond laser reshaping yields gold nanorods with ultranarrow surface plasmon resonances[J]. Science, 2017, 358(6363): 640-4.] [DOI] [PubMed] [Google Scholar]
- 143.Zhou J, Jiang Y, Hou S, et al. Compact plasmonic blackbody for cancer theranosis in the near-infrared Ⅱ window. ACS Nano. 2018;12(3):2643–51. doi: 10.1021/acsnano.7b08725. [Zhou J, Jiang Y, Hou S, et al. Compact plasmonic blackbody for cancer theranosis in the near-infrared Ⅱ window[J]. ACS Nano, 2018, 12(3): 2643-51.] [DOI] [PubMed] [Google Scholar]
- 144.Gilroy KD, Peng HC, Yang X, et al. Symmetry breaking during nanocrystal growth. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0228166129/ Chem Commun. 2017;53(11):4530–41. doi: 10.1039/c7cc01121k. [Gilroy KD, Peng HC, Yang X, et al. Symmetry breaking during nanocrystal growth[J]. Chem Commun, 2017, 53(11): 4530-41.] [DOI] [PubMed] [Google Scholar]
- 145.Tang L, Yang X, Yin Q, et al. Investigating the optimal size of anticancer nano_x0002_medicine. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_PM25316794. Proc Natl Acad Sci USA. 2014;111(29):15344–9. doi: 10.1073/pnas.1411499111. [Tang L, Yang X, Yin Q, et al. Investigating the optimal size of anticancer nano_x0002_medicine[J]. Proc Natl Acad Sci USA, 2014, 111(29): 15344-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sykes EA, Chen J, Zheng G, et al. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano. 2014;8(6):5696–706. doi: 10.1021/nn500299p. [Sykes EA, Chen J, Zheng G, et al. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency [J].ACS Nano, 2014, 8 (6): 5696-706.] [DOI] [PubMed] [Google Scholar]
- 147.Du B, Jiang X, Das A, et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4bf142a3a869dbbde33964eb54f1924d. Nat Nanotechnol. 2017;12(8):1096–105. doi: 10.1038/nnano.2017.170. [Du B, Jiang X, Das A, et al. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime[J]. Nat Nanotechnol, 2017, 12(8): 1096-105.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu Y, Wang Z, Liu Y, et al. Suppressing nanoparticle_ x0002_mononuclear phagocyte system interactions of twodimensional gold nanorings for improved tumor accumulation and photothermal ablation of tumors. ACS Nano. 2017;11(12):10539–48. doi: 10.1021/acsnano.7b05908. [Liu Y, Wang Z, Liu Y, et al. Suppressing nanoparticle_ x0002_mononuclear phagocyte system interactions of twodimensional gold nanorings for improved tumor accumulation and photothermal ablation of tumors[J]. ACS Nano, 2017, 11(12): 10539-48.] [DOI] [PubMed] [Google Scholar]
- 149.Zhen X, Zhang C, Xie C, et al. Intraparticle energy level alignment of semiconducting polymer nanoparticles to amplify chemiluminescence for ultrasensitive in vivo imaging of reactive oxygen species. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=33683d5d88d9f94079b7e9d4803f1149. ACS Nano. 2016;10(4):6400–9. doi: 10.1021/acsnano.6b02908. [Zhen X, Zhang C, Xie C, et al. Intraparticle energy level alignment of semiconducting polymer nanoparticles to amplify chemiluminescence for ultrasensitive in vivo imaging of reactive oxygen species[J].ACS Nano, 2016, 10(4): 6400-9.] [DOI] [PubMed] [Google Scholar]
- 150.Stiles PL, Dieringer JA, Shah NC. Surface-enhanced raman spectroscopy. http://d.old.wanfangdata.com.cn/Periodical/gpxygpfx201112023. Annu RevAnal Chem. 2008;22(1):601–26. doi: 10.1146/annurev.anchem.1.031207.112814. [Stiles PL, Dieringer JA, Shah NC. Surface-enhanced raman spectroscopy[J].Annu RevAnal Chem, 2008, 22(1): 601-26.] [DOI] [PubMed] [Google Scholar]
- 151.Abadeer NS, Murphy CJ. Recent progress in cancer thermal therapy using gold nanoparticles. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=97aadb0e80d31759222318ad0223c39d. J Phys Chem C. 2016;120(13):4691–716. [Abadeer NS, Murphy CJ. Recent progress in cancer thermal therapy using gold nanoparticles[J]. J Phys Chem C, 2016, 120(13): 4691- 716.] [Google Scholar]
- 152.Wang S, Lin J, Wang T, et al. Recent advances in photoacoustic imaging for deep-tissue biomedical applications. Theranostics. 2016;6(13):2394–413. doi: 10.7150/thno.16715. [Wang S, Lin J, Wang T, et al. Recent advances in photoacoustic imaging for deep-tissue biomedical applications[J]. Theranostics, 2016, 6(13): 2394-413.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhen X, Xie C, Pu K. Temperature-correlated afterglow of a semiconducting polymer nanococktail for imaging-guided photo_x0002_thermal therapy. Angew Chem. 2018;130(18):4002–6. doi: 10.1002/anie.201712550. [Zhen X, Xie C, Pu K. Temperature-correlated afterglow of a semiconducting polymer nanococktail for imaging-guided photo_x0002_thermal therapy[J]. Angew Chem, 2018, 130(18): 4002-6.] [DOI] [PubMed] [Google Scholar]
- 154.Liu Y, Wang Z, Liu Y, et al. Suppressing nanoparticle-mononuclear phagocyte system interactions of two-dimensional gold nanorings for improved tumor accumulation and photothermal ablation of tumors. ACS Nano. 2017;11(10):10539–48. doi: 10.1021/acsnano.7b05908. [Liu Y, Wang Z, Liu Y, et al. Suppressing nanoparticle-mononuclear phagocyte system interactions of two-dimensional gold nanorings for improved tumor accumulation and photothermal ablation of tumors[J].ACS Nano, 2017, 11(10): 10539-48.] [DOI] [PubMed] [Google Scholar]
- 155.Melancon MP, Zhou M, Li C. Cancer theranostics with nearinfrared light-activatable multimodal nanoparticles. https://www.ncbi.nlm.nih.gov/pubmed/21848277. Acc Chem Res. 2011;44(8):947–56. doi: 10.1021/ar200022e. [Melancon MP, Zhou M, Li C. Cancer theranostics with nearinfrared light-activatable multimodal nanoparticles[J]. Acc Chem Res, 2011, 44(8): 947-56.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kuppusamy P, Li H, Ilangovan G, et al. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. http://cn.bing.com/academic/profile?id=60dd72fbfc45150fdbc61c2036936085&encoded=0&v=paper_preview&mkt=zh-cn. Cancer Res. 2002;62(1):307–12. [Kuppusamy P, Li H, Ilangovan G, et al. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels [J]. Cancer Res, 2002, 62(1): 307-12.] [PubMed] [Google Scholar]
- 157.Gao Z, Hou Y, Zeng J, et al. Tumor microenvironment-triggered aggregation of antiphagocytosis 99mTc-labeled Fe3O4 nanoprobes for enhanced tumor imaging in vivo. https://www.researchgate.net/publication/316089825_Tumor_Microenvironment-Triggered_Aggregation_of_Antiphagocytosis_99m_Tc-Labeled_Fe3_O4_Nanoprobes_for_Enhanced_Tumor_Imaging_In_Vivo. Adv Mater. 2017;29(12):1701095–106. doi: 10.1002/adma.201701095. [Gao Z, Hou Y, Zeng J, et al. Tumor microenvironment-triggered aggregation of antiphagocytosis 99mTc-labeled Fe3O4 nanoprobes for enhanced tumor imaging in vivo[J]. Adv Mater, 2017, 29(12): 1701095-106.] [DOI] [PubMed] [Google Scholar]
- 158.Zhao Z, Fan H, Zhou G, et al. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheetaptamer nanoprobe. JAm Chem Soc. 2014;136(32):11220–3. doi: 10.1021/ja5029364. [Zhao Z, Fan H, Zhou G, et al. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheetaptamer nanoprobe[J]. JAm Chem Soc, 2014, 136(32): 11220-3.] [DOI] [PubMed] [Google Scholar]
- 159.Jiang Y, Li J, Zhen X, et al. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: a comparative study. Adv Mater. 2018;30(14):e1705980. doi: 10.1002/adma.201705980. [Jiang Y, Li J, Zhen X, et al. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: a comparative study[J]. Adv Mater, 2018, 30 (14): e1705980-9.] [DOI] [PubMed] [Google Scholar]
- 160.Li J, Xie C, Huang J, et al. Semiconducting polymer nanoenzymes with photothermic activity for enhanced cancer therapy. Angew Chem Int Ed Engl. 2018;57(15):3995–8. doi: 10.1002/anie.201800511. [Li J, Xie C, Huang J, et al. Semiconducting polymer nanoenzymes with photothermic activity for enhanced cancer therapy[J]. Angew Chem Int Ed Engl, 2018, 57(15): 3995-8.] [DOI] [PubMed] [Google Scholar]
- 161.Lyu Y, Zeng J, Jiang Y, et al. Enhancing both biodegradability and efficacy of semiconducting polymer nanoparticles for photoacoustic imaging and photothermal therapy. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=57d7a73f4843cc9f080666fd6404cddf. ACS Nano. 2018;12(11):1801–10. doi: 10.1021/acsnano.7b08616. [Lyu Y, Zeng J, Jiang Y, et al. Enhancing both biodegradability and efficacy of semiconducting polymer nanoparticles for photoacoustic imaging and photothermal therapy[J]. ACS Nano, 2018, 12 (11): 1801-10.] [DOI] [PubMed] [Google Scholar]
- 162.Liu Y, Yang Z, Huang X, et al. Glutathione-responsive selfassembled magnetic gold nanowreath for enhanced tumor imaging and imaging-guided photothermal therapy. ACS Nano. 2018;12(8):8129–37. doi: 10.1021/acsnano.8b02980. [Liu Y, Yang Z, Huang X, et al. Glutathione-responsive selfassembled magnetic gold nanowreath for enhanced tumor imaging and imaging-guided photothermal therapy[J]. ACS Nano, 2018, 12 (8): 8129-37.] [DOI] [PubMed] [Google Scholar]


