Abstract
Background:
Exposure to air pollution in early life has been linked to cognitive deficits and adverse neurodevelopmental effects. However, studies examining associations between air pollutants and Attention-Deficit / Hyperactivity Disorder (ADHD) have had conflicting findings.
Methods:
Individuals born in Denmark 1992-2007 (n=809,654) were followed for the development of ADHD from 1997 to 2013. Data on daily concentrations of nitrogen dioxide (NO2) and fine particulate matter (PM2.5) from air-modeling data at a 1 km x 1 km resolution at residences within the first five years of life, was linked with population-based data from the Danish national registers, including data on clinical diagnoses of ADHD. We estimated incidence rate ratios (IRRs) with 95% confidence intervals (CI) for ADHD, according to increases in exposures, adjusting for age, year, sex, and parental education and income.
Results:
Exposure to NO2 and PM2.5 during early life was associated with a significantly increased risk of ADHD: IRR of 1.38 (Cl: 1.35 to 1.42) per 10 μg/m3 increase in NO2 and an IRR of 1.51 (Cl: 1.40 to 1.62) per 5 μg/m3 increase in PM2.5. In two-pollutant models, the association between NO2 and ADHD did not change (IRR 1.35; 95% CI: 1.31 to 1.39), while the association with PM2.5 was substantially attenuated (IRR 1.07; 95% CI: 0.98 to 1.16), although in stratified models an elevated association with PM2.5 was found in the lowest quintile of NO2 exposure.
Conclusions:
In this large nationwide prospective cohort study, residential air pollution exposure, specifically NO2, during early childhood was associated with the development of ADHD, even when adjusted for parental level of income and education.
Keywords: Ambient air pollution, Attention deficit hyperactivity disorder, Cohort study, Epidemiology, Health
1. Introduction
Today, air pollution is suggested to be one of the largest health threaths on a global scale. It is well documented that long-term exposure to air pollution is associated with adverse health effects (“Ambient (outdoor) air quality and health. Fact sheet no. 313. Updated March 2014. Geneva: World Health Organization,” 2015; Landrigan et al., 2018) and increased mortality (Di et al., 2017) and more recently, exposure to environmental toxins has been proposed as a risk factor for neurodevelopmental problems (Grandjean & Landrigan, 2014). Studies have also found associations between air pollution exposure and cognitive deficits and neurodevelopmental disorders in children (Suades-Gonzalez, Gascon, Guxens, & Sunyer, 2015).
Fetal life and early childhood have been suggested as periods of particular vulnerability, due to extensive cellular differentiation and growth, relevant for brain maturation and neural network development (Block et al., 2012). The underlying biological mechanisms are still not fully understood but animal studies suggest that exposure to air pollution is associated with neuro-inflammatory changes in the central nervous system (Calderon-Garciduenas et al., 2003; Campbell et al., 2005). Mice exposed to airborne particulate matter have higher levels of cytokines and immune-related transcription factors in the brain (Campbell et al., 2005) and exposure to air pollution is associated with chronic brain inflammation in animals (Calderon-Garciduenas et al., 2003). Observational data on humans also suggest adverse associations. An autopsy-study of children and young adults found increased cerebral neuro-inflammation among individuals having resided in high-pollution areas, versus low-pollution areas (Calderon-Garciduenas et al., 2008). Elevated levels of cytokines contribute to widespread neuro-inflammation in the brain, leading to the damage and diffuse loss of neural tissue in various areas of the brain. Affected structures include the prefrontal cortex and olfactory bulb as well as midbrain structures such as hippocampus, structures which are central to development of behavior and cognitive function (Brockmeyer & D’Angiulli, 2016).
Attention-Deficit / Hyperactivity Disorder (ADHD) is a prevalent neurodevelopmental disorder with childhood onset, which for the majority persists into adolescence and adulthood (S. V. Faraone, Biederman, & Mick, 2006). The etiology of ADHD is largely unknown but does include both genetic and environmental risk factors, which may interact to increase susceptibility to develop neurodevelopmental problems (Demontis et al., 2017; Stephen V. Faraone & Larsson, 2018). Several studies have investigated whether exposure to air pollution during early life is associated with ADHD or symptoms of this disorder, but with conflicting results (Abid, Roy, Herbstman, & Ettinger, 2014; Forns et al., 2016; Gong et al., 2014; Min & Min, 2017; Mortamais et al., 2017; Newman et al., 2013; Siddique, Banerjee, Ray, & Lahiri, 2011). Siddique et al. found positive associations between particulate matter (PM10) and ADHD (Siddique et al., 2011), as did Min & Min who also found positive associations between Nitrogen dioxide (NO2) and ADHD (Min & Min, 2017). Newman et al. found that exposure to elemental carbon (EC) during infancy was associated with higher hyperactivity scores in 7-years old children (Newman et al., 2013) and Forns et al. found that exposure to black carbon (BC), EC and NO2 was associated with more behavioral problems (Forns et al., 2016). Two studies did not find evidence of a positive association. Abid et al. found an inverse association between exposure to polycyclic aromatic hydrocarbons (PAHs) measured by urinary metabolite concentrations and ADHD (Abid et al., 2014) and a study by Gong et al. did not find an association between exposure to Nitrogen oxides (NOx) or PM10 and ADHD (Gong et al., 2014). However, Abid et al. used a cross-sectional design and the study by Gong et al. was likely underpowered. In comparison, the studies with positive findings tend to have applied stronger methodologies in their study design and sampling.
NO2 and PM are among the air pollutants with the strongest evidence of adverse health consequences. To clarify a potential role for these air pollutants in a country with low to moderate air pollutant levels, we examined whether exposure to higher concentrations of NO2 and PM2.5 during early life, was associated with the development of ADHD, examining a large Danish nationwide cohort. Although the prenatal period is considered to represent the most vulnerable period for brain development, prenatal exposure to air pollution would be through the mother’s exposure (both at home and at work). In most previous studies, concerning both the prenatal and postnatal period, exposure-data on air pollution have been limited by once-annual measurements, measurements over a short period or have relied on extrapolated data from monitoring stations purposely placed in highly polluted areas. This offers little evidence of which exposure window reflects the most vulnerable period. In this study, we investigated exposure to air pollution in the postnatal period from age 0 to 5, since we had continuous information about exposure to daily concentrations of pollutants, based on the children’s complete history of residential addresses within the first five years of life.
2. Methods
2.1. Study population and data sources
We used the Danish Civil Registration System (CRS) to identify the study population (Figure S1 in the Supplementary Appendix). The CRS was established in Denmark in 1968 and includes all people living in Denmark in 1968 and onwards. Since then, all live-born children and new residents in Denmark have been assigned a personal identification number (enabling accurate linkages), which is stored in the CRS with information on sex, date and place of birth, current and former residential addresses, vital status (date of death, if relevant) and the personal identification number of the persons’ parents.
Our study population included all singletons born in Denmark between January 1, 1992 and December 31, 2007, who were alive and resident in Denmark on their 5th birthday, whose parents were both born in Denmark, and whose mothers were residents in Denmark at time of conception, defined as nine months before giving birth.
2.2. Assessment of ADHD and other mental disorders
Information about clinical diagnoses of ADHD among cohort members and any mental disorders in their parents was obtained from the Danish Psychiatric Central Research Register (DPCR) and the Danish National Patient Register (DNPR). The DPCR contains information about all inpatient admissions to Danish psychiatric facilities since 1969, and from 1995 also information on all contacts to outpatient psychiatric departments and visits to psychiatric emergency care units. The DNPR has information about all inpatient admissions to public hospitals in Denmark since 1977 and outpatient visits from 1995 and onwards. Diagnosis of ADHD was based on the International Classification of Diseases, 10th revision, Diagnostic Criteria for Research (ICD-10-DCR) (codes F90x or F98.8), as assessed by a child and adolescent psychiatrist. The ICD-10-DCR was adopted for use in Denmark starting 1994 and the validity of the diagnosis of ADHD in the DPCR is considered high (Mohr-Jensen, Vinkel Koch, Briciet Lauritsen, & Steinhausen, 2016). Date of diagnosis of ADHD was defined as the first day with the diagnosis (inpatient or outpatient contact) and individuals with a date of first diagnosis of ADHD before their fifth birthday were excluded from the cohort. The incidence of a diagnosis of ADHD before five years of age is very low and negligible before two years of age (Dalsgaard et al., 2019). So, although data on ADHD was only available from 1994, we included also children born in 1992 and 1993, thereby possibly missing very early incident diagnoses of ADHD (before age 2 and 1, respectively), unless they had a second hosapital contact under the same diagnosis before age 5 (but after 1994). Parents were classified as having a mental disorder, if they had a hospital contact with a psychiatric diagnosis (ICD-10 codes F00-F99, ICD-8 codes: 290-315) before the child was born. Date of diagnosis of mental disorders was defined as the first day with the diagnosis (inpatient or outpatient contact). We obtained information about birthweight, gestational age and five-minute Apgar score from the Danish Medical Birth Register. Statistics Denmark provided information about highest completed parental education at the time of delivery of the child (The Education register) and parental income at the year of delivery (The Income register), for all cohort members.
2.3. Air pollution exposure assessment
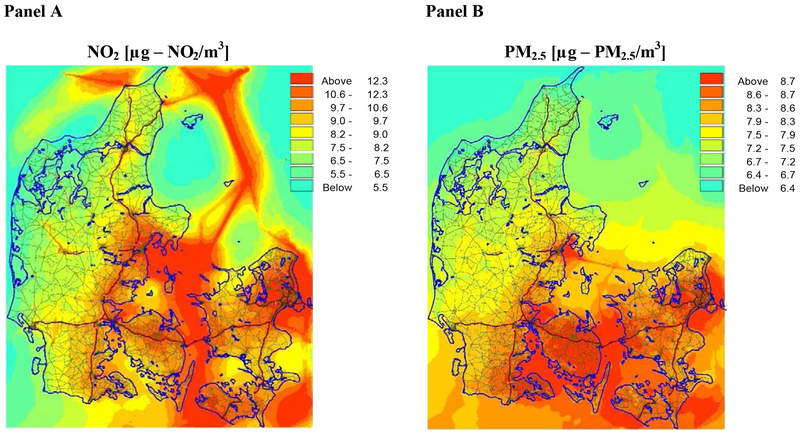
We used data with daily concentrations of NO2 and PM2.5 to estimate exposure to ambient air pollution (Figure 1). Air pollution concentrations all over Denmark are simulated using the high resolution THOR modeling system which covers the years 1979-2015. The THOR model system consist of a coupling of several air pollution models, covering different areas and with different resolution. The Danish Eulerian Hemispheric Model (DEHM) covers the Northern Hemisphere and includes three nested domains over Europe (50 km x 50 km), Northern Europe (16.7 km x 16.7 km) and Denmark (5.6 km x 5.6 km) for higher resolution over the area of interest. DEHM is coupled to the Urban Background Model (UBM) which covers Denmark with a high resolution of 1 km x 1 km and uses background concentrations from the DEHM model. Inputs to THOR include data on air pollutant measurements, layout of roads and traffic counts, fleet-specific emission concentrations, weather patterns, long-range transport, and secondary pollutants formation. For this study, we have used detailed emission inventories with a resolution of 1 km x 1 km (J. Brandt, Christensen, Frohn, & Berkowicz, 2003; Jørgen Brandt et al., 2001; J. Brandt et al., 2012). Analyses of the THOR model system show that the performance of the models does not vary significantly over years. The overall long-term trends of the main air pollutants (NO2, NOx, PM2.5 and PM10) using surface observations from eight Danish monitoring stations, from the 1990ties and onwards, are captured well by the model. These trends are in general driven by similar trends in Danish and international emissions (Im et al., 2018).
Figure 1. Average NO2 and PM2.5 concentrations in Denmark in 2012.
The figure shows the geographical distribution of the mean concentrations of NO2 (Panel A) and PM2.5 (Panel B), across Denmark in the year 2012. For NO2, higher concentrations are especially found along major roads and ship lanes. For PM2.5, there is a north-south gradient, with higher concentrations found in southern parts of Denmark, in part due to long-range transport from neighboring countries.
From the CRS we had a complete history of cohort member’s current and former residential addresses as well as dates of changes in addresses within the first five years of life. To obtain exact geographical coordinates we linked each residential address with information from the Danish Register on Official Standard Addresses and Coordinates. The residential address information was linked with longitudinal information on daily concentrations of NO2 and PM2.5 by geographical coordinates, and for each individual, we estimated the average exposure to these pollutants between birth and the fifth birthday, accounting for residential changes in Denmark. If an individual had a residence in another country during the first five years, it was not possible to assign exposure status for this period residing outside of Denmark. Before running the analyses, it was decided that only individuals, resident in Denmark >80% of the days between birth and their fifth birthday, whose mothers were resident in Denmark for >80% of the days between conception and birth and who had a residence in Denmark at the beginning of follow-up, would be included in the study.
2.4. Statistical analyses
Individuals in the study population (n=809,654) were followed from their fifth birthday until ADHD diagnosis, death, emigration from Denmark or end of study (July first , 2013) (whichever came first). Incidence rate ratios (IRRs) for ADHD were estimated by Poisson regression in SAS (version 9·4. SAS Institute Inc.; 2013)and P-values and 95% confidence intervals (CIs) were based on likelihood ratio tests (Clayton & Hills, 1993). The adjusted-score test (N.E. Breslow, 1996) suggested that the regression models were not subject to over-dispersion. The rationale for defining the exposure window as the first five years of life was to have equal time of exposure for all individuals in the study population, ensure temporality (i.e., that the exposure preceed the outcome) and to follow-up all cohort members from the same age. This also explains the rationale for excluding individuals diagnosed with ADHD before five years of age (otherwise associations could be the result of reversed causation).
The effect of NO2 and PM2.5 on ADHD was estimated using exposure levels as five-level categorical and as continuous variables per 10 μg/m3 increase in NO2 and per 5 μg/m3 increase in PM2.5 (because a 10 μg/m3 increase in PM2.5 was out of scale). We adjusted for age, calendar year, child sex, and parental socioeconomic status (SES; mother’s and father’s level of education and income in year of child’s birth). In all analyses, we treated age and calendar period as time-dependent variables and other variables as time-independent.
In sensitivity analyses, we included additional covariates: Parental socioeconomic status at the child’s fifth birthday, parental history of psychiatric illness before child’s birth and obstetrical factors (birthweight, five-minute Apgar score and gestational age). To investigate if prenatal exposure made an additional contribution to the effect of the childhood exposure, we expanded the exposure window to also include the prenatal period. Furthermore, we did a sensitivity analysis including only individuals, for whom we had full information on exposure throughout their first five years of life. As both the incidence of ADHD and the concentration of air pollutants vary temporally and geographically across Denmark, we performed robustness-checks, stratifying first by year of birth, second by the five geographical regions in Denmark, and third by degree of urbanization at birth (Section 3 and 4 in the Supplementary Appendix).
We also conducted two-pollutant analyses in which the risk of ADHD associated with exposure to NO2 was adjusted for the exposure to PM2.5 and vice-versa, to investigate potential mutual confounding. Finally, we did a test for interaction between NO2 and PM2.5, (Section 6 in the Supplementary Appendix).
2.5. Ethics
The Danish Protection Agency and the Danish Health and Medicines Authority approved this study.
3. Results
3.1. Study population
Among the included 809,654 children born in Denmark, a total of 19,045 (2.4%) children developed ADHD during the 6,969,066 person-years of risk from 1997 to 2013 (Table 1). Individuals in the study population were followed for up to 16 years (mean=8.6 years; SD 4.6 years). In 4,272 children and adolescents (0.5%), follow-up was ended before the end of the study because they emigrated from Denmark (0.4%) or were lost to follow-up or died (0.1%). Mean age at first ADHD diagnosis was 10.6 years (SD 3.96).
Table 1:
Distribution of 19,045 cases of ADHD and 6,969,066 person-years at risk in the overall cohort
| Number of cases with ADHD | Number of person-years at risk | Rate per 10,000 person-years | |
|---|---|---|---|
| Year of birth | |||
| 1992-1996 | 8,062 | 3,744,066 | 21.5 |
| 1997-2001 | 7,109 | 2,230,553 | 31.9 |
| 2002-2007 | 3,874 | 994,445 | 38.9 |
| Gender | |||
| Male | 13,983 | 3,554,061 | 39.3 |
| Female | 5,062 | 3,415,004 | 14.8 |
| Region of residence at birth | |||
| North Denmark | 1,530 | 790,878 | 19.3 |
| Central Denmark | 4,888 | 1,688,392 | 28.9 |
| South Denmark | 3,804 | 1,588,730 | 23.9 |
| Capital Region | 5,855 | 1,912,955 | 30.6 |
| Zealand | 2,968 | 988,109 | 30.0 |
| Degree of urbanization at birth Ɨ | |||
| Capital | 2,180 | 791,297 | 27.6 |
| Capital suburb | 2,717 | 860,128 | 31.6 |
| Municipalities with a town with >100,000 inhabitants | 1,827 | 834,035 | 21.9 |
| Municipalities with a town with 10,000 – 100,000 inhabitants | 5,691 | 1,985,318 | 28.7 |
| Other municipalities (largest town < 10,000 inhabitants) | 6,619 | 2,496,712 | 26.5 |
| Mean NO2 exposure first five years of life (μg/m3) | |||
| 5th quintile (21.28 – 57.62 μg/m3) | 4,991 | 1,800,266 | 27.7 |
| 4th quintile (17.22 – 21.27 μg/m3) | 3,866 | 1,365,063 | 28.3 |
| 3rd quintile (14.07 – 17.21 μg/m3) | 3,873 | 1,455,080 | 26.6 |
| 2nd quintile (11.41 – 14.06 μg/m3) | 3,691 | 1,318,829 | 27.9 |
| 1st quintile (4.39 -11.4 μg/m3) | 2,624 | 1,029,826 | 25.5 |
| Mean PM2.5 exposure first five years of life (μg/m3) | |||
| 5th quintile (14.62 – 26.36 μg/m3) | 4,834 | 2,264,760 | 21.3 |
| 4th quintile (13.59 – 14.61 μg/m3) | 4,694 | 1,691,056 | 27.8 |
| 3rd quintile (12.77 – 13.58 μg/m3) | 4,553 | 1,432,455 | 31.8 |
| 2nd quintile (11.76 – 12.76 μg/m3) | 3,374 | 1,036,992 | 32.5 |
| 1st quintile (8.13 – 11.75 μg/m3) | 1,590 | 543,801 | 29.2 |
| Mother’s level of educationƗǂ | |||
| Primary school | 7,436 | 1,608,469 | 46.2 |
| Short education | 8,749 | 3,550,742 | 24.6 |
| Medium long education | 2,324 | 1,406,226 | 16.5 |
| Long education | 410 | 381,711 | 10.7 |
| Father’s level of educationƗǂ | |||
| Primary school | 7,146 | 1,557,206 | 45.9 |
| Short education | 9,701 | 3,962,861 | 24.5 |
| Medium long education | 1,194 | 815,192 | 14.6 |
| Long education | 630 | 565,688 | 11.1 |
| Mother’s level of incomeƗ§ | |||
| Below the 20th percentile | 505 | 130,633 | 38.7 |
| 20th to the 40th percentile | 2,537 | 566,222 | 44.8 |
| 40th to the 60th percentile | 9,075 | 2,681,180 | 33.8 |
| 60th to the 80th percentile | 5,673 | 2,731,616 | 20.8 |
| Above the 80th percentile | 1,255 | 859,384 | 19.6 |
| Father’s level of incomeƗ§ | |||
| Below the 20th percentile | 334 | 71,861 | 46.5 |
| 20th to the 40th percentile | 1,413 | 273,549 | 51.7 |
| 40th to the 60th percentile | 3,040 | 694,136 | 43.8 |
| 60th to the 80th percentile | 7,096 | 2,282,666 | 31.1 |
| Above the 80th percentile | 7,153 | 3,644,837 | 19.6 |
| Birthweight (g) | |||
| < 2500 | 1,182 | 274,791 | 43.0 |
| 2500-3999 | 14,257 | 5,281,812 | 27.0 |
| ≥4000 | 3,606 | 274,791 | 43.0 |
| Gestational age (weeks) | |||
| < 37 | 1,459 | 366,928 | 39.8 |
| 37 - 41 | 16,081 | 5,995,285 | 26.8 |
| ≥ 42 | 1,505 | 606,852 | 24.8 |
| Apgar score (5 min) | |||
| 1-9 | 1,891 | 570,287 | 33.2 |
| 10 | 17,154 | 6,398,778 | 26.8 |
| Maternal history of psychiatric disorder | |||
| Yes | 1,227 | 188,813 | 65.0 |
| No | 17,818 | 6,780,252 | 26.3 |
| Paternal history of psychiatric disorder | |||
| Yes | 943 | 145,094 | 65.0 |
| No | 18,102 | 6,823,972 | 26.5 |
Cohort consisted of 809 654 children born 1992-2007.
May not sum to 19,045 cases and 6,969,066 person- years at risk because of missing information.
Highest finished education measured at the end of the year of the child’s birth. Education is defined as primary school, short education (e.g. high school, vocational training) medium-long education (e.g. bachelor’s degree from college or teaching training) or higher education (e.g. college degree or PhD degree).
Level of income at the year of the child’s birth.
3.2. Air pollution and ADHD in one-pollutant models
The average NO2 and PM2.5 daily concentrations (from birth to the fifth birthday) ranged from 4.4 to 57.6 μg/m3 and from 8.1 to 26.4 μg/m3, respectively. NO2 and PM2.5 were moderately correlated (Pearson 0.55) and had small overlaps in spatial distributions (Figure 1).
Children exposed to the highest quintile of NO2 during childhood had a 1.70-fold (95% CI: 1.61 to 1.78) increased risk of ADHD, compared to children exposed to the lowest quintile of NO2, in analyses adjusted for age, calendar year, child sex, and parental socioeconomic status at the year of birth. Similarly, children exposed to the highest quintile of PM2.5 during childhood had a 1.63-fold (95% CI: 1.52 to 1.76) increased risk of ADHD, compared to children exposed to the lowest quintile of PM2.5. In analyses of the air pollutants as continuous variables, the adjusted IRR for ADHD increased 1.38 (95% Cl: 1.35 to 1.42) fold per 10 μg/m3 increase in NO2 and 1.51 (95% Cl: 1.40 to 1.62) fold per 5 μg/m3 increase in PM2.5 (Table 2). A sensitivity analysis, in which only individuals, for whom we had 100% exposure information during their first five years of life, were included in the cohort, showed similar results (data not shown).
Table 2.
One pollutant models, estimating adjusted incidence rate ratios (IRRs)€ for ADHD by cumulative exposure to NO2 and PM2.5, within the first five years of life
| Exposure to NO2 | IRR (95% CI) | Exposure toPM2.5 | IRR (95% CI) |
|---|---|---|---|
| 1st quintile (4·39 -11·4 μg/m3) | 1·00 (ref) | 1st quintile (8·13 – 11·75 μg/m3) | 1·00 (ref) |
| 2nd quintile (11·41 – 14·06 μg/m3) | 1·22 (1·16, 1·28) | 2nd quintile (11·76 – 12·76 μg/m3) | 1·30 (1·22, 1·39) |
| 3rd quintile (14·07 – 17·21 μg/m3) | 1·30 (1·24, 1·37) | 3rd quintile (12·77 – 13·58 μg/m3) | 1·52 (1·42, 1·62) |
| 4th quintile (17·22 – 21·27 μg/m3) | 1·47 (1·40, 1·55) | 4th quintile (13·59 – 14·61 μg/m3) | 1·53 (1·43, 1·64) |
| 5th quintile (21·28 – 57·62 μg/m3) | 1·70 (1·61, 1·78) | 5th quintile (14·62 – 26·36 μg/m3) | 1·63 (1·52, 1·76) |
| Trend effect of NO2 | Trend effect of PM2.5 | ||
| IRR (per 10 μg/m3 increase) | 1·38 (1·35, 1·42) | IRR (per 5 μg/m3 increase) | 1·51 (1·40, 1·62) |
Adjusted for age, calendar year, sex, and mother’s and father’s level of education and income
The associations between NO2 and PM2.5 and ADHD remained unchanged after adjustments for obstetric factors and parental psychiatric disorders (Table 3). Effects of NO2 and PM2.5 on the risk of ADHD were consistent within each of the five geographically distinct Danish regions (Table 4) and when stratified by year of birth, the effect was consistent across birth years (Table S1) and within each level of urbanization (Table S2). A sensitivity analysis, in which we adjusted for parental socioeconomic status at the child’s fifth birthday showed similar results (data not shown) as when we adjusted for parental socioeconomic status at the year of birth.
Table 3:
One-pollutant models estimating incidence rate ratios (IRRs) for ADHD by cumulative exposure to NO2 and PM2.5 within the first five years of life, after adjustment for different confounders
| IRR (95% CI), per 10 μg/m3 increase in NO2 | IRR (95% CI), per 5 μg/m3 increase in PM2.5 | |
|---|---|---|
| Base adjustment€ | 1·38 (1·35, 1·42) | 1·51 (1·41, 1·62) |
| Base adjustment€ + parental psychiatric history | 1·37 (1·33, 1·41) | 1·48 (1·37, 1·59) |
| Base adjustment€ + obstetrical factorsǂ | 1·38 (1·34, 1·42) | 1·50 (1·4, 1·61) |
Adjusted for age, calendar year, sex, and mother’s and father’s level of education and income
Weight at birth, 5-minute Apgar score and gestational age
Table 4:
One-pollutant models, estimating adjusted incidence rate ratios (IRRs)€ for ADHD by an averaged exposure to NO2 and PM2.5 within the first five years of life, stratified by region of residence, at time of birth
| Region in Denmark | Number of cases with ADHD | Number of persons-years at risk | IRR (95% CI), per 10 μg/m3 increase in NO2 | IRR (95% CI), per 5 μg/m3 increase in PM2.5 |
|---|---|---|---|---|
| North Denmark | 1,530 | 45,176,80 | 1·29 (1·14, 1·47) | 1·71 (1·30, 2·26) |
| Central Denmark | 4,888 | 98,388,98 | 1·27 (1·19, 1·36) | 2·02 (1·74, 2·34) |
| South Denmark | 3,804 | 90,881,90 | 1·14 (1·04, 1·24) | 1·66 (1·45, 1·91) |
| Capital region# | 5,855 | 113,365,81 | 1·14 (1·06, 1·22) | 1·56 (1·36, 1·80) |
| Zealand | 2,968 | 57,641,91 | 1·43 (1·34, 1·54) | 1·25 (1·08, 1·47) |
Adjusted for age, calendar year, sex, and mother’s and father’s level of education and income
Excluding the island of Bornholm from the Capital Region
All presented IRRs were significantly increased (p < 0·00001)
When investigating whether prenatal air pollutant exposure provided additional contributions beyond childhood exposure, we found that prenatal exposure to NO2 (P=0.07064) or PM2.5 (P=0.79286) did not contribute further to the prediction of ADHD beyond the effect of exposure to the two pollutants during the first five years of life.
3.3. Air pollution and ADHD in two-pollutant models
In two-pollutant models, the positive trend association for every 10 μg/m3 increase in NO2 and risk of ADHD remained unchanged (IRR 1.35; 95% CI: 1.31 to 1.39), while the trend association for every 5 μg/m3 increase in PM2.5 was substantially attenuated (IRR 1.07; 95% CI: 0.98 to 1.16). We found significant interactions between NO2 and PM2.5 with ADHD (P=<0.00001). In analyses of the effect of PM2.5 (per 5 μg/m3 increase) stratified by quintiles of NO2, we found a positive association with PM2.5 only at the lowest concentrations of NO2 (1st quintile), while the increased risk of ADHD associated with exposure to increased levels of NO2 was found across all concentrations of PM2.5 exposures (Table 5 and tables S3–S5).
Table 5:
Two-pollutant models, estimating the trend effect of NO2 (per 10 μg/m3 increase), across quintiles of PM2.5, and the trend effect of PM2.5 (per 5 μg/m3 increase), across quintiles of NO2, on the risk of ADHD. Adjusted incidence rate ratios (IRRs)€ for ADHD by cumulative exposure to NO2 of PM2.5 within the first five years of life.
| Effect of NO2 on the risk of ADHD, across quintiles of PM2.5 | Effect of PM2.5 on the risk of ADHD, across quintiles of NO2 | ||
|---|---|---|---|
| Quintiles of PM2.5 | IRR (95% CI) | Quintiles of NO2 | IRR (95% CI) |
| 5th quintile (14·62 – 26·36 μg/m3) | 1·33 (1·26, 1·40) | 5th quintile (21·28 – 57·62 μg/m3) | 1·00 (0·88, 1·14) |
| 4th quintile (13·59 – 14·61 μg/m3) | 1·37 (1·29, 1·46) | 4th quintile (17·22 – 21·27 μg/m3) | 0·92 (0·81, 1·05) |
| 3rd quintile (12·77 – 13·58 μg/m3) | 1·40 (1·31, 1·49) | 3rd quintile (14·07 – 17·21 μg/m3) | 1·02 (0·89, 1·16) |
| 2nd quintile (11·76 – 12·76 μg/m3) | 1·31 (1·20, 1·43) | 2nd quintile (11·41 – 14·06 μg/m3) | 1·03 (0·89, 1·18) |
| 1st quintile (8·13 – 11·75 μg/m3) | 1·39 (1·20, 1·60) | 1st quintile (4·39 -11·4 μg/m3) | 1·43ǂ (1·21, 1·70) |
Adjusted for age, calendar year, sex, and mother’s and father’s level of education and income
When NO2 was divided into deciles, the effect of PM2.5 was primarily found in the 1st decile of NO2 (IRR 1·66; 95% CI: 1·28 to 2·15)
4. Discussion
This nationwide prospective cohort study of 809,654 Danish children showed that children exposed in the first five years of life to higher concentrations of NO2 and PM2.5, considered separately, had a significantly increased risk of developing ADHD. These associations could not be explained by SES, obstetrical factors or parental psychiatric history and the associations were robust across birth cohorts, urbanicity levels and all Danish geographical regions. The effect of NO2 remained robust after accounting for PM2.5, whereas the overall effect of PM2.5 was substantially attenuated after accounting for NO2. The effect of increasing PM2.5 levels on the risk for ADHD was only significant within the lowest quintile of NO2 exposure.
Studies from Asian countries with annual levels of NO2 and PM substantially higher than those recommended in the United States (annual mean standards: 99.64 μg/m3 for NO2 and 12 μg/m3 for PM2.5) (EPA, 2018) and the European Union (annual mean standards: 40 μg/m3 for NO2 and 25 μg/m3 for PM2.5) (“European Union,” 2008) have found that postnatal exposure to very high levels of air pollution was associated with an increased risk of ADHD (Min & Min, 2017; Siddique et al., 2011; Wang et al., 2009). If these previously identified associations represent a true causal effect, discerning such associations in a less polluted European country with lesser exposure contrasts requires a large sample and a substantial period of follow-up.
We studied a large nationwide population-based cohort that included all children born in Denmark between 1992 and 2007 and followed up for up to 16 years. Another strength of the study is that our study used information on daily estimates of exposure over the first five years of life within a 1 km x 1 km area in which the child’s residence was located, including all residential changes during this period. Residential areas also correlate with socioeconomic status, which could confound the estimated effects. To minimize this, we included detailed data on parental level of education and income, measured the year the child was born and at the child’s fifth birthday. Also, in this study, we relied on clinical diagnoses of ADHD from the registers instead of dimensional measurements of ADHD symptoms using questionnaires or computer-tests at a single time point. A diagnosis of ADHD from the registers is assessed by a child and adolescent psychiatrist and is considered of high validity (Mohr-Jensen et al., 2016).
Worldwide, the population prevalence of ADHD in children and adolescents is 5% (Polanczyk, de Lima, Horta, Biederman, & Rohde, 2007) and in this study the prevalence of ADHD was only 2.4%. Our study participants may have more severe ADHD and our findings may not be representative of children with mild to moderate ADHD. Moreover, although the incidence of a diagnosis of ADHD increased and concentrations of NO2 and PM2.5 decreased over the study period, the estimated association may still in part be causally linked. Studies have estimated that increased incidence of ADHD does not reflect a true increase in the prevalence of ADHD at population-level, but rather reflects changes in diagnostic practices over time, including lower thresholds for referral and more resources becoming available for diagnosing true cases in later years. To address these temporal changes in incidence of ADHD not only did we control for calendar year in our main analyses, we also performed a sensitivity analysis stratified on birth year and found comparable estimates of the association in all strata. In addition, a potential limitation of any study of air pollution is related to geographical differences in both the occurrence of ADHD and level of pollution. Denmark consists of five regions, which are also the administrative centers of public health care, and large variations in incidence rates of ADHD exists, despite that all individuals have free access to public health care in Denmark (Madsen, Ersbøll, Olsen, Parner, & Obel, 2015). Some of the variation in incidence could be explained by unequal proximity to services or different diagnostic practices in the five regions. However, when stratified on the five geographically distinct regions, we found that the effects of NO2 and PM2.5 on the risk of ADHD were consistent within each of the five Danish regions. Another study limitation is that we did not have data on addresses of mothers’ workplace and hence, for our main analyses we did not include the pregnancy period in the exposure window, as maternal exposure to air pollution using only the residential addresses would be insufficient. However, we performed a sensitivity analysis using an expanded exposure window to cover the time from conception until the fifth birthday, resulting in estimates of the association being virtually unchanged. Children (also) do not stay at home the first five years of life, but spend time in daycare, which is also a limitation in our study. However, this likely biases our estimates to a lesser degree, since exposure levels are likely to be similar because most children in Denmark attend daycare in the same area, as their residential address (affairs, 2015). Finally, it is possible that the association between air pollution and ADHD in our study could partly be explained by residual confounding by unmeasured factors, such as noise pollution, indoor air pollution, and other outdoor air pollutants. Both NO2 and PM2.5 arise in large part from vehicle traffic and other combustion sources. These sources emit a wide variety of air pollutants in addition to NO2 and PM2.5, which may all be linked together and we cannot exclude the possibility, that the etiologic agent responsible for the observed association, is one or more correlated air pollutants, not included in this study.
Air pollutants are connected by rather complex atmospheric chemistry and the effects ascribed to one pollutant, may be influenced by the underlying toxicity of several air pollutants in the mixture. The two pollutants examined in this study are linked in that NO2 may serve as a precursor of PM2.5. Few other studies have investigated this potential co-pollutant confounding between PM2.5 and NO2. A European study of associations with mortality found that the association with PM was substantially attenuated, when adjusting for NO2 (Katsouyanni et al., 2001). A US study did not find any evidence of such confounding by NO2 (Samet, Dominici, Zeger, Schwartz, & Dockery, 2000) While our results point to NO2 as a stronger risk factor for ADHD compared to PM2.5, this pattern may not be generalizable to other locations such as the US, where the sources of PM2.5 and NO2 differ (e.g. different degrees of diesel fuel use in Europe and the US). PM2.5 is simply defined by size of particles and includes a mixture of particles with different chemistry arising from different sources. When PM2.5 is present and concentration of NO2 is very low, this likely indicates low traffic-related emissions and thus may indicate a different predominant source for PM2.5. Our finding of a robust association between PM2.5 and ADHD only when NO2 was low, is consistent with a hypothesis that different types or sources of PM2.5 may have different biological effects.
5. Conclusions
This nationwide study suggests that early life exposure to NO2 and PM2.5 may be associated with an increased risk of developing ADHD, even in a country with a low general level of air pollution. If causal, then lowering the maximum permissible value of these pollutants could potentially have important preventive impacts on the risk of ADHD as well as improvements in the public health in general. Given the complex relationship between air pollutants, it is difficult to separate out the effect of each single pollutant within the complex air pollutant mixture. Future studies should examine the role of several air pollutants in the association with ADHD, as well as the interplay between them.
Supplementary Material
Acknowledgments
Funding
The project was funded by grants from Aarhus University Research Foundation (AUFF-E-2015-FLS-8-61), Research training supplement from the Graduate School of Health Sciences at Aarhus University and The Lundbeck Foundation (iPSYCH grant no R102-A9118 and R155-2014-1724). Data management was supported by Center for Integrated Register-based Research at Aarhus University (CIRRAU). Dr’s. J. Brandt, C. B. Pedersen and C. Geels are supported by NordForsk, under the Nordic Programme on Health and Welfare (NordicWelfAir grant no 75007). C.B. Pedersen is supported by the Novo Nordisk Foundation (Big Data Centre for Environment and Health, grant no NNF17OC0027864). Dr.’s A. Kalkbrenner, D. Schendel, J. Brandt, C. B. Pedersen, M. Thygesen and S. Dalsgaard are supported by the National Institutes of Health (R01ES026993). Dr. Dalsgaard is additionally supported by the Novo Nordisk Foundation (grant no 22018), and the European Commission (Horizon 2020, grant no 667302).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abid Z, Roy A, Herbstman JB, & Ettinger AS (2014). Urinary polycyclic aromatic hydrocarbon metabolites and attention/deficit hyperactivity disorder, learning disability, and special education in U.S. children aged 6 to 15. J Environ Public Health, 2014, 628508. doi: 10.1155/2014/628508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- affairs, M. o. S. (2015). Legal guidelines - Daycare [in Danish]. (j.nr. 2015-868).
- Ambient (outdoor) air quality and health. Fact sheet no. 313. Updated March 2014. Geneva: World Health Organization; (2015). [Google Scholar]
- Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, … Wright RJ (2012). The outdoor air pollution and brain health workshop. Neurotoxicology, 33(5), 972–984. doi: 10.1016/j.neuro.2012.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Christensen JH, Frohn LM, & Berkowicz R (2003). Air pollution forecasting from regional to urban street scale—implementation and validation for two cities in Denmark. Physics and Chemistry of the Earth, Parts A/B/C, 28(8), 335–344. doi: 10.1016/S1474-7065(03)00054-8 [DOI] [Google Scholar]
- Brandt J, Christensen JH, Frohn LM, Palmgren F, Berkowicz R, & Zlatev Z (2001). Operational air pollution forecasts from European to local scale. Atmospheric Environment, 35, S91–S98. doi: 10.1016/S1352-2310(00)00415-5 [DOI] [Google Scholar]
- Brandt J, Silver JD, Frohn LM, Geels C, Gross A, Hansen AB, … Christensen JH (2012). An integrated model study for Europe and North America using the Danish Eulerian Hemispheric Model with focus on intercontinental transport of air pollution. Atmospheric Environment, 2012 v.53, pp. 156–176. doi: 10.1016/j.atmosenv.2012.01.011 [DOI] [Google Scholar]
- Brockmeyer S, & D’Angiulli A (2016). How air pollution alters brain development: the role of neuroinflammation. Translational Neuroscience, 7(1), 24–30. doi: 10.1515/tnsci-2016-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Maronpot RR, Torres-Jardon R, Henriquez-Roldan C, Schoonhoven R, Acuna-Ayala H, … Swenberg JA (2003). DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol, 31(5), 524–538. doi: 10.1080/01926230390226645 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, … Reed W (2008). Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol, 36(2), 289–310. doi: 10.1177/0192623307313011 [DOI] [PubMed] [Google Scholar]
- Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, … Kleinman M (2005). Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology, 26(1), 133–140. doi: 10.1016/j.neuro.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, … Neale BM (2017). Discovery Of The First Genome-Wide Significant Risk Loci For ADHD. bioRxiv. doi: 10.1101/145581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, … Schwartz JD (2017). Air Pollution and Mortality in the Medicare Population. N Engl J Med, 376(26), 2513–2522. doi: 10.1056/NEJMoa1702747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. (2018). Environmental Protection Agency. https://www.epa.gov/pm-pollution/2012-national-ambient-air-quality-standards-naaqs-particulate-matter-pm.
- European Union. (2008). http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0050&from=DA.
- Faraone SV, Biederman J, & Mick E (2006). The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med, 36(2), 159–165. doi: 10.1017/s003329170500471x [DOI] [PubMed] [Google Scholar]
- Faraone SV, & Larsson H (2018). Genetics of attention deficit hyperactivity disorder. Molecular Psychiatry. doi: 10.1038/s41380-018-0070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Dadvand P, Foraster M, Alvarez-Pedrerol M, Rivas I, Lopez-Vicente M, … Sunyer J (2016). Traffic-Related Air Pollution, Noise at School, and Behavioral Problems in Barcelona Schoolchildren: A Cross-Sectional Study. Environ Health Perspect, 124(4), 529–535. doi: 10.1289/ehp.1409449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T, Almqvist C, Bolte S, Lichtenstein P, Anckarsater H, Lind T, … Pershagen G (2014). Exposure to air pollution from traffic and neurodevelopmental disorders in Swedish twins. Twin Res Hum Genet, 17(6), 553–562. doi: 10.1017/thg.2014.58 [DOI] [PubMed] [Google Scholar]
- Grandjean P, & Landrigan PJ (2014). Neurobehavioural effects of developmental toxicity. Lancet Neurol, 13(3), 330–338. doi: 10.1016/s1474-4422(13)70278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im U, Christensen J, Ketzel M, Ellermann T, Geels C, Hansen K, … Brandt J (2016). Air pollutant trends over Denmark over the last 36 years as simulated by the integrated THOR model system. In Air Pollution Modelling and its Application XXV Springer, Springer Proceedings in Complexity, pp. 49–54. [Google Scholar]
- Im U, Christensen JH, Ketzel M, Ellermann T, Geels C, Hansen KM, … Brandt J (2018). Air Pollutant Trends over Denmark over the Last 37 Years as Simulated by the Integrated Model System THOR In Mensink C & Kallos G (Eds.), Springer Proceedings in Complexity (pp. 49–54). Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Katsouyanni K, Touloumi G, Samoli E, Gryparis A, Le Tertre A, Monopolis Y, … Schwartz J (2001). Confounding and Effect Modification in the Short-Term Effects of Ambient Particles on Total Mortality: Results from 29 European Cities within the APHEA2 Project. Epidemiology, 12(5), 521–531. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, … Zhong M (2018). The Lancet Commission on pollution and health. Lancet, 391(10119), 462–512. doi: 10.1016/s0140-6736(17)32345-0 [DOI] [PubMed] [Google Scholar]
- Madsen KB, Ersbøll AK, Olsen J, Parner E, & Obel C (2015). Geographic analysis of the variation in the incidence of ADHD in a country with free access to healthcare: a Danish cohort study. International Journal of Health Geographics, 14, 24. doi: 10.1186/s12942-015-0018-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JY, & Min KB (2017). Exposure to ambient PM10 and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environment International, 99, 221–227. doi: 10.1016/j.envint.2016.11.022 [DOI] [PubMed] [Google Scholar]
- Mohr-Jensen C, Vinkel Koch S, Briciet Lauritsen M, & Steinhausen HC (2016). The validity and reliability of the diagnosis of hyperkinetic disorders in the Danish Psychiatric Central Research Registry. Eur Psychiatry, 35, 16–24. doi: 10.1016/j.eurpsy.2016.01.2427 [DOI] [PubMed] [Google Scholar]
- Mortamais M, Pujol J, van Drooge BL, Macia D, Martinez-Vilavella G, Reynes C, … Sunyer J (2017). Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environment International, 105, 12–19. doi: 10.1016/j.envint.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Newman NC, Ryan P, Lemasters G, Levin L, Bernstein D, Hershey GK, … Dietrich KN (2013). Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect, 121(6), 731–736. doi: 10.1289/ehp.1205555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, & Rohde LA (2007). The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry, 164(6), 942–948. doi: 10.1176/ajp.2007.164.6.942 [DOI] [PubMed] [Google Scholar]
- Samet JM, Dominici F, Zeger SL, Schwartz J, & Dockery DW (2000). The National Morbidity, Mortality, and Air Pollution Study. Part I: Methods and methodologic issues. Res Rep Health Eff Inst(94 Pt 1), 5–14; discussion 75-84. [PubMed] [Google Scholar]
- Siddique S, Banerjee M, Ray MR, & Lahiri T (2011). Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur J Pediatr, 170(7), 923–929. doi: 10.1007/s00431-010-1379-0 [DOI] [PubMed] [Google Scholar]
- Suades-Gonzalez E, Gascon M, Guxens M, & Sunyer J (2015). Air Pollution and Neuropsychological Development: A Review of the Latest Evidence. Endocrinology, 156(10), 3473–3482. doi: 10.1210/en.2015-1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang J, Zeng X, Zeng Y, Wang S, & Chen S (2009). Association of traffic-related air pollution with children’s neurobehavioral functions in Quanzhou, China. Environ Health Perspect, 117(10), 1612–1618. doi: 10.1289/ehp.0800023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.