Several studies have documented that topical antibiotics do not reduce the risk of surgical site infection following uncomplicated clean cutaneous surgery compared to petrolatum.1,2 Although evidence-based recommendations from the Centers for Disease Control and Prevention recommend avoiding topical antibiotic use, nearly half of dermatology wound care handouts advise using topical antibiotics after such procedures.3,4 However, there is a lack of information regarding actual clinician prescribing practices for topical antibiotics following these procedures and how this has changed over time.
Using the National Ambulatory Medical Care Survey (NAMCS), we investigated the frequency of topical antibiotic use associated with biopsies and excisions between 2006 and 2015. Each encounter that was coded as including a biopsy or excision was evaluated for prescribing of topical antibiotics (i.e. mupirocin, gentamicin, neomycin, bacitracin, polymyxin, clindamycin, erythromycin). Using logistic regression, we evaluated the frequency of topical antibiotic use following clean biopsies and excisions, stratified by specialty (dermatologists versus non-dermatologists). To improve accuracy and better characterize temporal trends in antibiotic use due to limited number of observations available in NAMCS, the study period was divided into 5 two-year periods, as has been recommended elsewhere.4
In 2014–2015, among patients seen by dermatologists, there were an estimated 503,227 (10.2% of visits) and 268,264 (5.7% of visits) topical antibiotic prescriptions each year associated with biopsies and excisions, respectively. Among patients seen by non-dermatologists in 2014–2015, there were an estimated 210,536 (1.9% of visits) and 401,684 (5.3% of visits) topical antibiotic prescriptions each year associated with biopsies and excisions, respectively.
During the study period, the odds of receiving a topical antibiotic after a biopsy initially fell amongst dermatologists, with a nadir in 2010–2011 (OR 0.20, CI 0.06–0.63), before increasing back to baseline rates in subsequent years (Figure 1). Among non-dermatologists, the odds of receiving a topical antibiotic post-biopsy remained largely unchanged, with the exception of 2012–2013 (OR = 3.98, CI 1.07–14.82).
Figure 1.
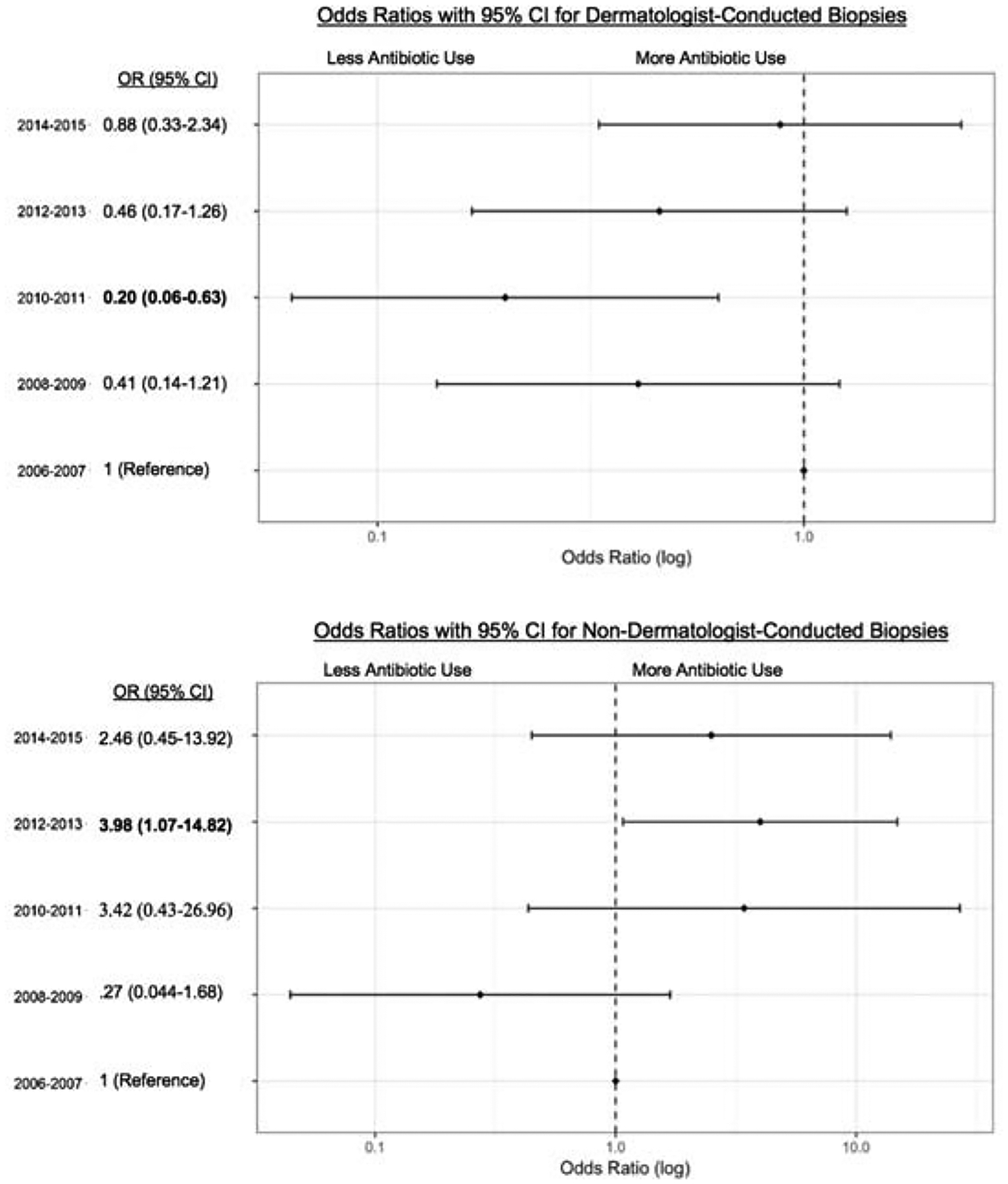
Odds of a receiving a topical antibiotic following encounters involving a biopsy by dermatologists (top) and non-dermatologists (bottom). 2006–2007 is the reference year.
With respect to excisions, a similar initial decrease and subsequent increase in prescribing was noted among dermatologists, although these changes did not reach statistical significance (Figure 2). Among non-dermatologists, the odds of receiving a topical antibiotic after an encounter including an excision significantly increased throughout the study period, peaking in 2014–2015 (OR 5.16, CI 1.77–14.99).
Figure 2.
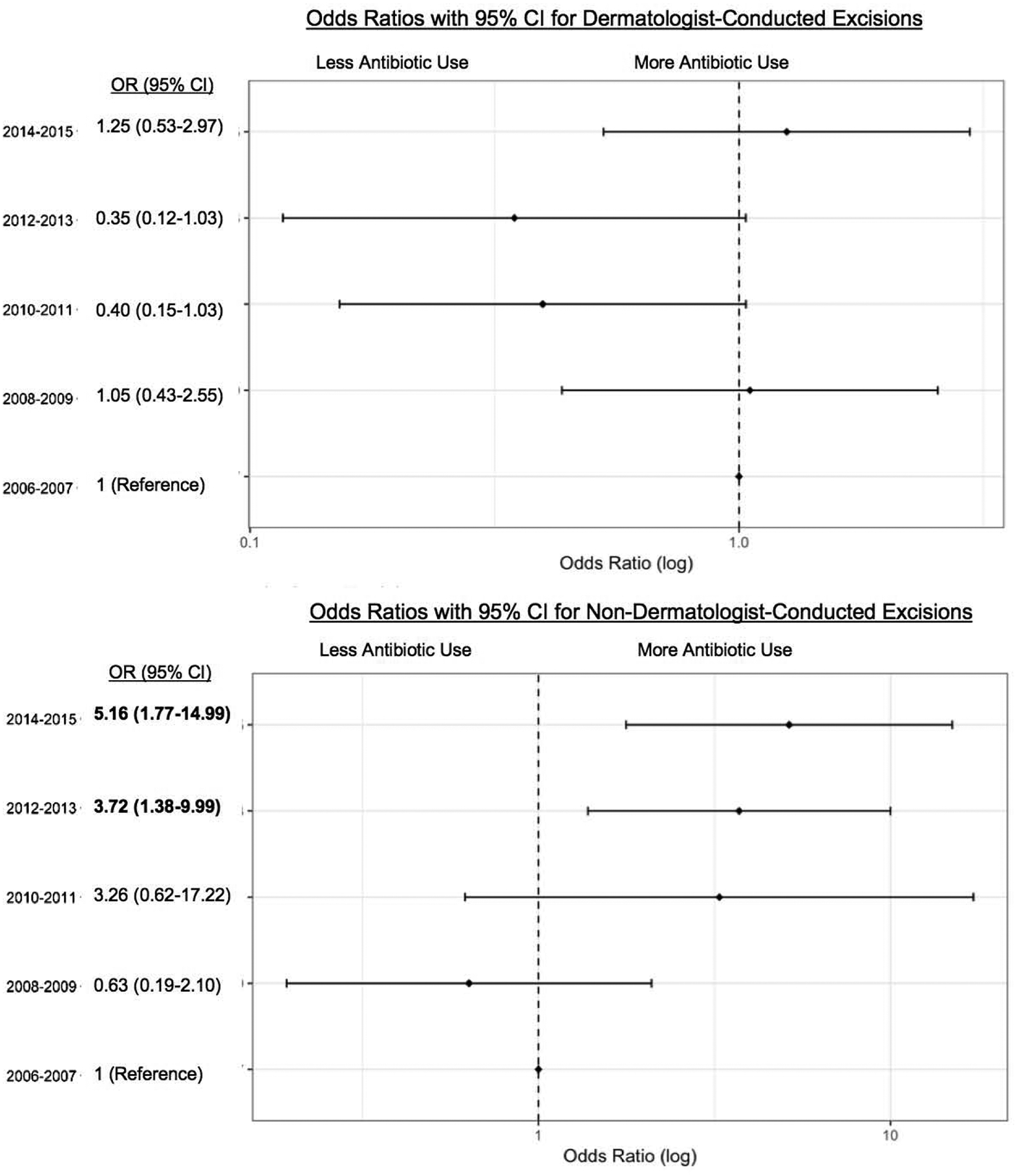
Odds of a receiving a topical antibiotic following encounters involving an excision by dermatologists (top) and non-dermatologists (bottom). 2006–2007 is the reference year.
This work builds on a prior study investigating the use of topical antibiotics following clean dermatologic procedures between 1993–2007, which reported antibiotic use in 5.0% of these procedures.5 We identified substantially higher rates of antibiotic use following biopsies and excisions, particularly when conducted by dermatologists. Despite high-quality evidence from randomized controlled trials suggesting multiple advantages of using petrolatum over topical antibiotics after clean cutaneous surgery,2 physicians continue to prescribe topical antibiotics after procedures, with over 750,000 prescriptions annually by dermatologists alone. In addition, given that data in NAMCS may not capture over-the-counter antibiotic use or samples given in the office, it is likely that total topical antibiotic use frequency is higher than our estimates. Future studies are needed to understand the factors driving this persistent prescribing and to identify how to optimize topical antibiotic use to improve patient outcomes and prevent resistance in the community.
Acknowledgements:
Funding/Support: Dr. Barbieri is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number T32-AR-007465 and receives partial salary support through a Pfizer Fellowship grant to the Trustees of the University of Pennsylvania.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Smack DP. Infection and Allergy Incidence in Ambulatory Surgery Patients Using White Petrolatum vs Bacitracin Ointment: A Randomized Controlled Trial. JAMA. 1996;276(12): 972. [PubMed] [Google Scholar]
- 2.Saco M, Howe N, Nathoo R, Cherpelis B. Topical antibiotic prophylaxis for prevention of surgical wound infections from dermatologic procedures: a systematic review and meta-analysis. Journal of Dermatological Treatment. 2014;26(2): 151–158. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen JK, Huang A, Siegel DM, Jagdeo J. Variability in Wound Care Recommendations Following Dermatologic Procedures: Dermatologic Surgery. March 2019:1. [DOI] [PubMed] [Google Scholar]
- 4.Ahn CS, Allen M-M, Davis SA, Huang KE, Fleischer AB, Feldman SR. The National Ambulatory Medical Care Survey: A resource for understanding the outpatient dermatology treatment. Journal of Dermatological Treatment. 2013;25(6):453–458. [DOI] [PubMed] [Google Scholar]
- 5.Levender Michelle M., et al. “Use of topical antibiotics as prophylaxis in clean dermatologic procedures.” Journal of the American Academy of Dermatology 66.3 (2012): 445–451. [DOI] [PubMed] [Google Scholar]


