Abstract
Given the potential adverse health effects related to toxic trace metal exposure and insufficient or excessive levels of essential trace metals in pregnant women and their fetuses, the present study characterizes biomarkers of metal and metalloid exposure at repeated time points during pregnancy among women in Puerto Rico. We recruited 1,040 pregnant women from prenatal clinics and collected urine, blood, and questionnaire data on demographics, product use, food consumption, and water usage at up to three visits. All samples were analyzed for 16 metal(loid)s: arsenic (As), barium (Ba), beryllium (Be), cadmium (Cd), cobalt (Co), chromium (Cr), cesium (Cs), copper (Cu), mercury (Hg), manganese (Mn), nickel (Ni), lead (Pb), titanium (Ti), uranium (U), vanadium (V), and zinc (Zn). Urine samples were additionally analyzed for molybdenum (Mo), platinum (Pt), antimony (Sb), tin (Sn), and tungsten (W). Mean concentrations of most metal(loid)s were higher among participants compared to the general US female population. We found weak to moderate correlations for inter-matrix comparisons, and moderate to strong correlations between several metal(loid)s measured within each biological matrix. Blood concentrations of Cu, Zn, Mn, Hg, and Pb, and urinary concentrations of As, Ni, and Co, were shown to reflect reliable biomarkers of exposure. For other metals, repeated samples are recommended for exposure assessment in epidemiology studies. Predictors of metal(loid) biomarkers included fish and rice consumption (urinary As), fish and canned food (blood Hg), drinking public water (blood Pb), smoking (blood Cd), and iron/folic acid supplement use (urinary Cs, Mo, and Sb). Characterization of metal(loid) biomarker variation over time and between matrices, and identification of important exposure sources, may inform future epidemiology studies and exposure reduction strategies.
Keywords: Exposure assessment, Pregnancy, Metals, Blood, Urine, Biomarkers
INTRODUCTION
Metals and metalloids occur naturally in the environment and enter the human body through ingestion of food, water, and supplements, and the use of metal-containing products via inhalation, dermal absorption, and incidental ingestion (Borowska and Brzoska 2015; Gorman Ng et al. 2017; Martin and Griswold 2009; Rehman et al. 2018; Singh et al. 2011). In the United States, reports from the National Health and Nutrition Examination Survey (NHANES) show that children and adults have detectable concentrations of a range of metal(loid)s in their bodies (Centers for Disease Control and Prevention (CDC) 2019), including pregnant women and their fetuses because of trans-placental metal(loid) transfer (Caserta et al. 2013; Chen et al. 2014; Punshon et al. 2016). Some of these metals are essential for human health and required for fetal growth (Horning et al. 2015; Nordberg et al. 2001), such as cobalt (Co), copper (Cu), iron (Fe), magnesium (Mg), manganese (Mn), molybdenum (Mo), nickel (Ni), selenium (Se) and zinc (Zn). Excess or insufficient levels of these metals may pose risks to pregnancy (Nordberg et al. 2001; O'Neal and Zheng 2015). Other metal(loid)s do not play an essential physiologic role and can be toxic if present even at low concentrations (Jaishankar et al. 2014; Jan et al. 2015; Singh et al. 2011); some, including lead (Pb) and mercury (Hg), are reproductive toxicants and neurotoxicants, while others, such as cadmium (Cd) and arsenic (As), are known human carcinogens. Several metal(loid)s (Pb, Hg, Cd, As, Mn, Zn) are also suspected endocrine disruptors (Bloom et al. 2010; De Coster and van Larebeke 2012; Diamanti-Kandarakis et al. 2009; Mendiola et al. 2011).
Puerto Rico has a long-standing history of contamination with environmental chemicals, with 200+ hazardous waste sites and 16 active Superfund sites (the hazardous waste lands identified by the EPA as a site for cleanup because it poses a risk to human health and/or the environment) (Padilla et al. 2011). Many contaminated sites are above unlined landfills that overlie Karst aquifers, creating pathways for contamination of groundwater and potential drinking water sources. Therefore, the risk of human exposure to metal(loid) contamination is high. However, little is known regarding the extent and specific sources of human metal(loid) exposure on the island. This is the first study to examine distributions, time trends, and predictors of urinary and blood metal(loid) biomarkers measured at multiple times during pregnancy among women living in Northern Puerto Rico. Characterizing relationships of metal(loid) biomarkers over time and between matrices, and identifying important exposure sources to metal(loid)s, may inform risk evaluations in epidemiology and targeted approaches to reduce metal(loid) exposure.
METHODS
2.1. Study Population
This study was conducted among pregnant women participating in the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) project (Ashrap et al. 2018; Cantonwine et al. 2014; Meeker et al. 2013; Watkins et al. 2015), an ongoing prospective birth cohort in the Northern Karst Region of Puerto Rico that began in 2010. PROTECT aims to explore environmental toxicants and other factors contributing to preterm birth risk and other adverse birth outcomes in Puerto Rico.
Study participants were recruited at approximately 14 ± 2 weeks of gestation at seven prenatal clinics and hospitals throughout Northern Puerto Rico and followed until delivery. The present analysis reflects 1,040 women recruited into the study thus far at 18 to 40 years of age. Details on the recruitment and inclusion criteria have been described previously (Cantonwine et al. 2014; Meeker et al. 2013). Spot urine samples were collected from women at three separate study visits (18 ± 2 weeks, 22 ± 2 weeks, and 26 ± 2 weeks of gestation) and blood samples were collected during the first and third visits. During the initial visit, questionnaires collecting demographic information were administered to participants. Information on housing characteristics, employment status, and family situation were collected during a second, in-home visit using a nurse-administered questionnaire. Household product, personal care product use, and water source and usage information were collected at each visit.
The research protocol was approved by the Ethics and Research Committees of the University of Puerto Rico and participating clinics, the University of Michigan, and Northeastern University. The study was described in detail to all participants, and informed consent was obtained prior to study enrollment.
2.2. Measurement of Metal(loid)s
Spot urine was collected in sterile polypropylene cups and aliquoted within one hour after collection, while blood samples were collected in metal-free whole blood tubes. All samples were frozen and stored at −80°C and shipped on dry ice to NSF International (Ann Arbor, MI, USA) for analysis. Concentrations of 16 metals and metalloids (As) were measured in both urine and blood: As, barium (Ba), beryllium (Be), Cd, Co, chromium (Cr), cesium (Cs), Cu, Hg, Mn, Ni, Pb, titanium (Ti), uranium (U), vanadium (V), and Zn; an additional 5 metals and metalloids (antimony) were measured in urine only: Mo, platinum (Pt), antimony (Sb), tin (Sn), and tungsten (W). Metal(loid) concentrations were measured using inductively coupled plasma mass spectrometry (ICPMS) as described previously (Kim et al. 2018). Considering that biological samples have high levels of carbon and chloride in the matrix, the laboratory selected the appropriate isotopes for the requested elements to best avoid interferences where possible. The ICPMS was calibrated with a blank and a minimum of 4 standards for each element of interest. An R2 value of >0.995 was the minimum criteria for an acceptable calibration curve. The calibration curves were verified by initial checks at three calibration points within the curve. Continuing calibration checks and blanks after every 10 samples were also utilized throughout the analytical run to ensure the ICPMS system was maintaining acceptable performance. Urinary specific gravity (SG) was measured at the University of Puerto Rico Medical Sciences Campus using a hand-held digital refractometer (Atago Co., Ltd., Tokyo, Japan) as an indicator of urine dilution.
2.3. Questionnaire
The product use questionnaire was adapted from questionnaires used in other studies of adults to capture information on potential exposure sources with which the pregnant women may have been in contact (Cantonwine et al. 2014; Meeker et al. 2013). At each visit, the questionnaire was administered by a study nurse to collect data on product and water use. The household/personal care product use section contained yes/no questions about the use of different products in the 48-h period preceding biological sample collection: bar soap, cologne/perfume, colored cosmetics, conditioner, deodorant, fingernail polish, hair cream, hairspray/ hair gel, laundry products, liquid soap, lotion, mouthwash, other hair products, shampoo, and shaving cream. In the water use section, participants were asked about the type of water utilized for drinking and cooking (municipal water, private well water, bottled/delivered water) as well as water storage behaviors (use of water cistern, filtration). In the second visit, participants also completed a food frequency questionnaire on the consumption of milk, cheese, fish, rice, yogurt, and other foods (never, <1 per month, 1 per month, 2–3 per month, 1 per week, 2 per week, 3–4 per week, 5-6 per week, 1 per day and 2 or more per day) as well as yes/no questions regarding supplement use (iron, folic acid, multivitamin, etc.).
2.4. Statistical Methods
To account for urinary dilution, metal(loid) concentrations in urine were corrected for SG using the equation: Pc = P[(SGp – 1)/(SGi – 1)]; where Pc is the SG corrected biomarker concentration (ng/mL), P is the measured biomarker concentration, SGp is the median urinary specific gravity in this population (1.019), and SGi is the individual’s urinary specific gravity. Biomarker concentrations below the limit of detection (LOD) were replaced by LOD/√2. For statistical analysis, we included metal(loid)s with at least 50% of samples having concentrations above the LOD (Carmichael et al. 2010; Curwin et al. 2005; Dorgan et al. 1999).
Descriptive statistics and comparison to NHANES
Descriptive statistics [geometric means (GM), geometric standard deviation (GSD), select percentiles] of urine and blood concentrations were calculated to describe distributions of metal(loid) concentrations among study participants and for comparison with previous reports. Using GM and selected percentiles, we compared concentrations measured in the present study with those measured in NHANES (2009-2010, 2011-2012, 2013-2014, 2015-2016), including women aged between 18 and 40 years (N for urine=1604, N for blood=3585).
Correlations between and within blood and urine concentrations
Spearman correlation coefficients and p values were calculated between blood and urine concentrations for 10 metal(loid)s (As, Cd, Co, Cs, Cu, Hg, Mn, Ni, Pb, and Zn) that were measured in both matrices and detected in >50% of samples; correlations were calculated using all samples that have measurements in both matrices. The ratio of urine concentration to blood concentration was constructed for each metal(loid) to further evaluate the relationship between the two biomarkers. Spearman rank correlations and p values were also calculated to assess relationships between different metal(loid)s within the same matrix; two sets of correlations were calculated using samples collected at each visit and using GM of metal(loid) concentrations over study visits.
Change in biomarkers across pregnancy (ICCs) and over time
To test for significant changes in biomarker concentrations across pregnancy (i.e., time points in gestation), linear mixed models (LMM) were used to account for repeated measurements from individuals. We also assessed the proportion of variance attributed to between-person variability across the three time points in pregnancy, using intra-class correlation coefficients (ICCs) and their 95% confidence intervals (Hankinson et al. 1995). Ranging between 0 (no reproducibility) and 1 (perfect reproducibility), ICCs reflect a poor degree of reliability when below 0.40, a moderate to good reliability when between 0.40 and 0.75, and an excellent reliability when above 0.75 (Rosner 2015). Next, to examine the changes in urinary and blood metal(loid) concentrations over time (2011-2017), tests of linear trends across study period were conducted by modeling the GM for each individual’s repeated measurements, including the year of visit as a continuous variable, and assessing statistical significance using the Wald test.
Predictor selection
Two approaches were taken to identify potential predictors of metal(loid) concentrations in urine and blood. Covariates (predictors) of interest (n=61) included demographic characteristics, 48-h recall of product use, dietary supplement intake, food consumption, and water use and sources. In the first approach, we regressed each covariate of interest against each measured biomarker, using linear mixed effects models (LMMs) with random intercepts. LMM accounts for the intra-individual correlation and variation of repeated measures over time and lead to smaller and more precise standard errors around means. With LMMs, we assessed log- transformed metal(loid) concentrations individually as continuous dependent variables; for urinary metal(loid)s, log-transformed concentrations were further corrected for SG. Potential predictors were modeled individually as independent variables. With the purpose of determining a subset of important predictor variables for each metal(loid), in the second approach, we fit multivariable LMMs with LASSO (least absolute shrinkage and selection operator) regularization (LMMLasso). LASSO regularization shrinks estimated regression coefficients corresponding to “weakly associated” covariates to zero, thereby embedding variable selection into the estimation procedure (Groll and Tutz 2014). An optimal choice of the coefficient for the LASSO regularization (λ), corresponding with the lowest Bayesian Information Criterion (BIC), maximizes the probability of selecting the best model. In our analysis, for each metal, all predictor variables were entered in the LMMLasso models at the same time. The λ was identified using the R package glmmLasso version 1.3.3.
Furthermore, we analyzed associations between log-transformed metal(loid)s concentrations and food frequency questionnaire information collected at the second visit, using linear regression. To use the same/close time period for biomarkers and supplement use to assess these relationships, urine metal(loid) concentrations measured at the second visit and blood metal(loid) concentrations measured at the third visit were used, as blood samples were not collected during the second visit. Data were analyzed using R version 3.2.2 and SAS 9.4 (SAS Institute Inc., Cary, NC)
RESULTS
Demographics
A total of 1,285 urine samples and 1,183 blood samples from 1,040 women with measured metal(loid) concentrations in either blood and/or urine samples were included in this analysis. Among those 1040 women, 660 and 824 women provided urine and blood samples, respectively. Demographic characteristics of those women were described previously (Aker et al. 2019; Ashrap et al. 2018) and are summarized in SI Table S1. Most women in our study had private insurance, had an education above high school, were employed, and were married or in a domestic partnership. Nearly half of them had household incomes below $30,000/year. More than 80% of women never smoked while less than 2% smoked during pregnancy and 6% reported second-hand smoking exposure (>1 hour per day). Nearly all women reported no consumption of alcohol within the last few months. Demographic characteristics do not differ between women who provided urine samples (660 women) and blood samples (842 women).
GM and percentiles
Descriptive statistics (GM, GSD, select percentiles) are presented in Table 1. Nearly all of the samples had detectable concentrations for most of the metals (98-100% > LOD), while a majority had detectable Cd (74.5% > LOD), Pb (72.1% > LOD), and Sb (90% > LOD) in urine and half had detected As (49% > LOD) and Cd (61% > LOD) in blood. 14 urinary metal(loid)s (As, Ba, Cd, Co, Cs, Cu, Hg, Mn, Mo, Ni, Pb, Sb, Sn, and Zn) and 10 blood metal(loid)s (As, Cd, Co, Cs, Cu, Hg, Mn, Ni, Pb, and Zn) with at least 50% of samples having concentrations higher than LOD levels were included in the statistical analysis.
Table 1.
Urinary and blood concentration of metal(loid)s (ng/ml) in 1,040 pregnant women from Puerto Rico in 2011–2017
| Metal (loid) |
Specimen | N (Sample) |
LO D |
% > LOD |
GM | GSD | 25% | 50% | 75% | 95% | rd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Urinea | 1285 | 0.3 | 100 | 10.9 | 2.5 | 6.1 | 10.8 | 19.0 | 46.4 | 0.27** |
| Bloodb | 1183 | 0.3 | 48.9 | 0.34 | 1.8 | 0.21 | 0.21 | 0.48 | 0.99 | ||
| Ba | Urinea | 1285 | 0.1 | 99.3 | 2.5 | 2.9 | 1.3 | 2.5 | 5.0 | 12.9 | |
| Bloodb | |||||||||||
| Cd | Urinea | 1285 | 0.06 | 74.5 | 0.12 | 2.3 | 0.06 | 0.12 | 0.20 | 0.58 | 0.25** |
| Bloodb | 1183 | 0.1 | 60.9 | 0.12 | 1.7 | 0.07 | 0.12 | 0.16 | 0.27 | ||
| Co | Urinea | 1285 | 0.05 | 100 | 1.0 | 1.9 | 0.70 | 1.0 | 1.5 | 2.8 | 0.51** |
| Bloodb | 1183 | 0.2 | 98.2 | 0.34 | 1.4 | 0.28 | 0.34 | 0.41 | 0.57 | ||
| Cs | Urinea | 1285 | 0.01 | 100 | 4.9 | 1.7 | 3.7 | 5.3 | 7.1 | 10.7 | 0.43** |
| Bloodb | 1183 | 0.04 | 99.9 | 1.1 | 1.4 | 0.94 | 1.2 | 1.4 | 1.9 | ||
| Cu | Urinea | 1285 | 2.5 | 99.3 | 14.0 | 1.8 | 10.0 | 14.2 | 19.5 | 34.5 | 0.21** |
| Bloodb | 1183 | 9 | 99.9 | 1552 | 1.3 | 1393 | 1562 | 1740 | 2096 | ||
| Hg | Urinea | 1285 | 0.05 | 98.6 | 0.60 | 2.9 | 0.30 | 0.59 | 1.2 | 3.6 | 0.33** |
| Bloodb | 1183 | 0.2 | 99.9 | 1.2 | 1.7 | 0.85 | 1.2 | 1.7 | 3.0 | ||
| Mn | Urinea | 1285 | 0.08 | 100 | 1.2 | 1.6 | 0.95 | 1.2 | 1.6 | 2.3 | 0.01 |
| Bloodb | 1183 | 2 | 99.9 | 11.3 | 1.4 | 9.0 | 11.3 | 14.0 | 19.4 | ||
| Mo | Urinea | 1285 | 0.3 | 100 | 58.9 | 2.0 | 38.9 | 62.9 | 92.2 | 166 | |
| Bloodb | |||||||||||
| Ni | Urinea | 1285 | 0.8 | 98.9 | 5.4 | 2.0 | 3.5 | 5.5 | 8.5 | 15.5 | 0.06 |
| Bloodb | 1183 | 0.5 | 96.4 | 1.0 | 1.6 | 0.81 | 1.0 | 1.3 | 2.2 | ||
| Pb | Urinea | 1285 | 0.1 | 72.1 | 0.25 | 2.7 | <LOD | 0.27 | 0.51 | 1.2 | 0.17** |
| Bloodb | 1183 | 0.02 | 99.9 | 3.3 | 1.6 | 2.5 | 3.3 | 4.3 | 6.4 | ||
| Sb | Urinea | 1285 | 0.04 | 90 | 0.09 | 1.9 | 0.06 | 0.08 | 0.12 | 0.22 | |
| Bloodb | |||||||||||
| Sn | Urinea | 1285 | 0.1 | 100 | 2.1 | 3.0 | 1.0 | 1.9 | 4.0 | 14.0 | |
| Bloodb | |||||||||||
| Zn | Urinea | 1285 | 2 | 100 | 266 | 2.5 | 155 | 300 | 498 | 947 | 0.07 |
| Bloodb | 1183 | 24 | 99.9 | 4682 | 1.3 | 4248 | 4752 | 5252 | 6055 |
Includes uncorrected urinary metal concentrations for up to 3 repeated samples per woman (n = 1285 samples)
Includes blood metal concentrations for up to 2 repeated samples per woman (n = 1183 samples)
LOD, limit of detection; GM, geometric mean; GSD, geometric standard deviation
Spearman correlation coefficient calculated for blood and urine metal concentrations
P value for the Spearman test <0.01.
The comparisons with distributions among women 18 to 40 years old from NHANES 2009-10, 2011-12, 2013-14 and 2015-16 were included in SI Table S2 and S3. In the NHANES cohort, some metals (Cu, Ni, and Zn) were not measured in urine samples and only Cd, Hg, Mn, and Pb were measured in blood samples. When comparing uncorrected urinary metal(loid) distributions with women of childbearing age enrolled in NHANES, women in our study had higher GM concentrations of all urinary metal(loid)s except for Cd, which were lower among PROTECT women, and Pb, which were similar in the two cohorts. Median concentrations of As, Ba, Co, Hg, Mo, and Sb were 2-fold greater among women in this study compared to NHANES. PROTECT women had a median concentration of Mn and Sn that were 13 and 5 times greater than NHANES, respectively. For blood samples, PROTECT women had higher concentrations of Hg and Mn compared to NHANES while NHANES women had Cd and Pb concentrations (GM) that were twice as high as PROTECT women. Among women of childbearing age enrolled in NHANES, a small portion was pregnant (85 and 185 women in the urine and blood analysis, respectively) and the metal concentrations measured among these pregnant women were similar to the levels measured among other women included in our NHANES comparison.
Correlations between and within blood and urine concentrations
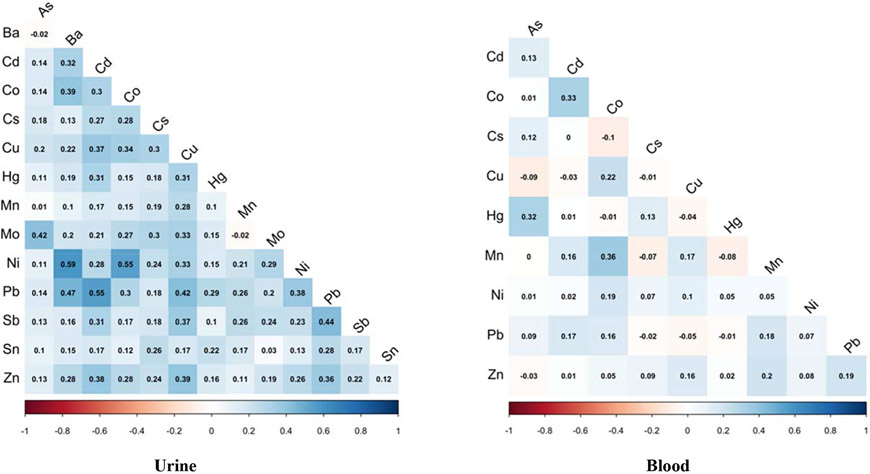
Spearman correlations between metal(loid)s within the same matrix did not differ when we calculated using GM of metal(loid) concentrations over study visit or using samples collected at each visit. Therefore, we presented the correlations between GM concentrations in Figure 1. When looking across metal(loid)s measured in urine, there were some moderate to strong correlations [r=0.47 (Pb and Ba), 0.55 (Cd and Pb), 0.55 (Ni and Co), 0.59 (Ni and Ba)]. There were also weak to moderate (r = 0.30 to 0.45) but statistically significant (p< 0.05) correlations between several metal(loid)s. The correlations between metal(loid)s in blood were generally weaker compared to urinary metal(loid)s with only a few pairs being moderately correlated (Mn and Co, r=0.36; Cd and Co, r=0.33; As and Hg, r=0.32).
Figure 1.
Heat map of pairwise correlations between urine and blood GM concentrations among pregnant women in the PROTECT studyab.
a The correlation heat map was created using natural log-transformed urinary or blood metal(loid) concentrations;
b All urinary concentrations were SG-corrected.
Spearman correlation coefficients for the same metal(loid)s across urine and blood matrices are presented in the last column of Table 1. Most of the metal concentration in two matrices were significantly correlated, with Co (r=0.51) and Cs (r=0.43) having the highest coefficient followed by Hg (r=0.33) and As (r=0.27). Mn, Ni, Zn concentrations measured in urine and blood were not correlated.
Ratio
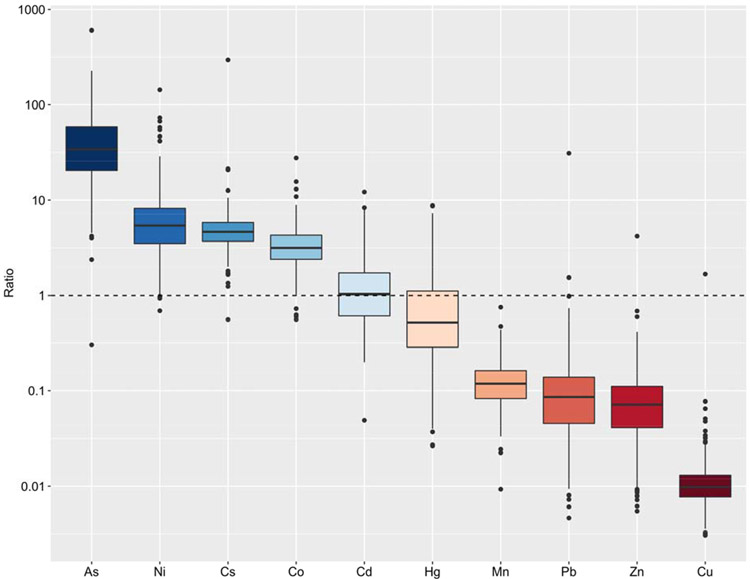
Distribution of urine/blood ratios for 10 metals are presented in Figure 2. GM and median of urine/blood ratios were <1 for Cu, Zn, Pb, Mn, and Hg, indicating generally higher concentration measured in blood vs urine. Inversely, GM and median of urine/blood ratios were >1 for As, Ni, Cs, and Co, indicating higher concentrations measured in urine vs blood. Cd concentrations were similar in two matrices (median urine/blood ratio of 1).
Figure 2.
Ratio of metal(loid) concentrations in urine and blood samples (n=509)a.
a All urinary concentrations were SG-corrected.
Change in biomarkers across pregnancy (ICCs) and over time
SI Figure S1 and S2 show comparisons of urinary and blood concentration distributions for each biomarker between study visits. SG-corrected urinary concentrations of metal(loid)s were not significantly different between the three visits except for Co, Cs, Cu, Mo, and Zn (p<0.05 for all). First visit concentrations were higher compared to later visits for Cs, Mo, and Zn, while Co and Cu were higher at the third visit. Blood concentrations of Cs were higher at the first visit, while blood concentrations of Cd, Co, Cu, Mn, and Zn were lower, compared to the third visit.
ICCs for urine and blood metal(loid) concentrations and the urine/blood ratio are presented in Table 2. Metals with a urine/blood ratio <1 (Cu, Zn, Pb, Mn, Hg) presented good to excellent reliability in blood with ICCs ranging from 0.54-0.78. Among the four metals with only urine measurements available, Sn had moderate reproducibility (ICC=0.55), whereas Mo, Sb, and Ba had weak reproducibility (ranging from 0.15 to 0.19). Reproducibility varied widely for the urine/blood ratio for each metal(loid), with ICCs ranging from 0.07 to 0.48.
Table 2.
Intraclass correlation coefficients (ICCs) and 95% confidence for natural log-transformed urinary and blood concentrations of biomarkers and ratio of urine and blood concentrations.
| Urineab | Bloodc | Urine/Blood Ratiode |
|
|---|---|---|---|
| biomarker | ICC (95% CI) | ICC (95% CI) | ICC (95% CI) |
| As | 0.21 (0.15,0.29) | 0.25 (0.17,0.36) | 0.20 (0.08,0.42) |
| Ba | 0.19 (0.13,0.28) | - | - |
| Cd | 0.18 (0.12,0.26) | 0.48 (0.41,0.56) | 0.18 (0.07,0.39) |
| Co | 0.27 (0.21,0.36) | 0.16 (0.07,0.3) | 0.07 (0.00,0.53) |
| Cs | 0.31 (0.25,0.38) | 0.77 (0.72,0.8) | 0.40 (0.25,0.56) |
| Cu | 0.21 (0.15,0.3) | 0.68 (0.62,0.74) | 0.22 (0.06,0.56) |
| Hg | 0.51 (0.46,0.57) | 0.62 (0.56,0.68) | 0.43 (0.29,0.59) |
| Mn | 0.13 (0.07,0.21) | 0.54 (0.44,0.6) | 0.31 (0.15,0.53) |
| Mo | 0.15 (0.09,0.23) | - | - |
| Ni | 0.13 (0.07,0.23) | 0.13 (0.05,0.27) | 0.22 (0.08,0.48) |
| Pb | 0.08 (0.03,0.2) | 0.78 (0.73,0.81) | 0.22 (0.07,0.48) |
| Sb | 0.17 (0.11,0.26) | - | - |
| Sn | 0.55 (0.49,0.61) | - | - |
| Zn | 0.39 (0.33,0.46) | 0.75 (0.7,0.79) | 0.48 (0.35,0.62) |
Among 660 women who had urine samples available, 184 had data from all three visits, 257 had data from two visits, and 219 had data from one visit
specific gravity corrected concentration
Among 842 women who had blood samples available, 341 had data from both visits, and 501 had data from one visit
Among 403 women who had both urine and blood samples available, 106 had data from both visits, and 297 had data from one visit
specific gravity corrected urinary concentration was used to calculate the ratio.
Distributions of urinary and blood biomarker concentrations stratified by year are shown in SI Figure S4 and S5. Results from linear trend tests indicated that the distributions of some biomarkers changed slightly over the course of our study period. For example, median levels of urine Ba, Cd, Cr, Cs, and blood Cs increased by 20-50% (P for trend<0.05) when comparing earlier and later years in the study period; while urinary Mn, Pb, Sb, Sn, and blood Ni and Pb were characterized by smaller, 20-30% decreases (P for trend<0.05).
Predictor selection
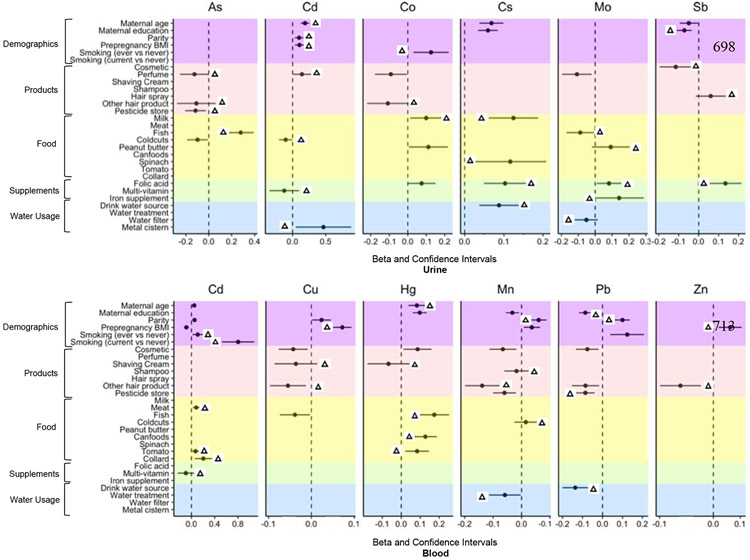
Variable selection analysis revealed several important predictors of urine and blood metal(loid) levels. Considering the concentrations of metal(loid)s measured in two matrices and reproducibility of different metal(loid)s in our analysis, we presented results for urinary concentrations of As, Co, Cs, Mo, and Sb (urine/blood ratio>1) and blood concentrations of Cu, Hg, Mn, Pb, and Zn (urine/blood ratio <1). Results from both urinary and blood concentrations of Cd were included as the average urine/blood ratio was 1. No significant predictors were found for either blood or urine Ba, Ni, Sn (data not shown). Here we describe predictors identified by both univariable LMMs and multivariable LMMLasso, while Figure 3 shows all the variables selected through either approach. The two statistical approaches gave very similar effect estimates, therefore, Figure 3 presents effect estimates (β) and confidence intervals (CIs) obtained from the univariable LMMs. GM of urinary and blood metal(loid) concentrations in relation to different categories of demographic variables, self-reported product use, dietary supplement intake, food consumption, and water use are also shown in SI Table S4 and S5.
Figure 3.
Beta and confidence intervals extracted from individual linear mixed models for metal(loid) concentrations and potential predictorsab.
Δ Variables also selected as predictors of metal(loid) exposure from multivariable LMMLasso models;
a In this figure, covariates that were not associated with any metal(loid) concentrations in the univariable and multivariable analysis were not included in the y-axis;
b Drinking water source: bottle water (1) vs AAA public water (0).
Urine
As Consuming fish 48 h prior to sample collection had the strongest relationship to urinary As concentration, while “other hair product” use, perfume use, and pesticide storage were negatively associated with As.
Cd We found strong positive associations between using a metal cistern to store water and urine Cd concentration, there was a 0.04 ng/ml difference on Cd concentration between women reporting the use of metal cistern and those who used plastic cistern or did not use cistern. Weak but significant positive associations were identified between urinary Cd concentration with age, parity, pre-pregnancy BMI, and use of perfume.
Co Smoking and consuming milk was associated with significantly higher urinary Co; self-reported use of other hair product was negatively associated with Co.
Cs Consumption of milk, spinach, folic acid supplement and drinking bottled water (vs public water) were positive predictors of higher Cs levels in urine.
Mo We found positive associations between self-reported folic acid, iron supplement, and peanut butter consumption and urine Mo concentration, while fish consumption and drinking filtered water were negatively associated with Mo concentration.
Sb Use of hair spray and consumption of folic acid were associated with higher Sb levels, while education and use of cosmetics were associated with lower Sb levels.
Blood
Cd For Cd, smoking (ever, current vs never) was significantly associated with blood levels among pregnant women in the study, and the GM concentration difference between current smoker vs never smoker (0.13 ng/mL) was stronger than ever smoker vs never smoker (0.02 ng/mL). Cd concentrations were higher for women who consumed meat, tomatoes, or collards, and lower for women who consumed multi-vitamins, compared to women who did not consume these items.
Cu Self-reported use of shaving cream and other hair product were important predictors of lower Cu levels; There was a trend for increasing concentration of Cu with increasing pre-pregnancy categories of BMI.
Hg Consuming fish, canned foods (e.g. canned tuna) and tomatoes were the strongest predictors of blood Hg levels. Hg concentrations were also higher among women with >12 years of education.
Mn Mn concentrations were associated with parity, where concentrations among women who had one or more children were significantly higher compared to women who had not yet had children. Blood Mn concentrations were lower among women who reported using shampoo and other hair products. Water treatment was also negatively associated with Mn concentration.
Pb Using bottled water as main drinking source was identified as the most significant predictor of lower Pb levels- participants who reported using bottled water as their main drinking source had significantly lower concentrations of Pb (0.30 μg/dL) compared to participants who drink public supply water (0.36 μg/dL). There were decreasing Pb concentrations associated with higher education levels.
Zn Pre-pregnancy BMI and using other hair products were negatively associated with blood Zn concentration.
Findings from the food frequency analysis
Our analysis of food frequency questionnaire information and metal(loid) concentrations found a trend for increasing concentrations of urinary As with increasing rice consumption frequency (p<0.05) (SI Table S6). The geometric mean concentration of As was 2 fold higher among women who consumed rice once per day or more compared to women who consumed rice 2-3 times per month or less. Fish consumption frequency was negatively associated with urinary Cd and Pb concentrations (SI Table S6). A similar trend was also observed for yogurt consumption frequency and urinary Sb concentration. In line with the results from the main predictor analysis above, there were positive linear trend relationships between meat consumption frequency and blood Cd, and fish consumption frequency and blood As and Hg levels (SI Table S7). Blood Cs levels also increased with increased fish consumption.
DISCUSSION
Our study provided much needed information on exposures to metal(loid)s among pregnant women in Northern Puerto Rico. We quantified levels of toxic and essential metal(loid)s in maternal urine and blood, characterized variability of levels across pregnancy, and correlation between different metal(loid)s and matrices to better inform the use of metal(loid) biomarkers in epidemiology studies. We also identified important predictors of each metal(loid) in blood and urine which may suggest possible strategies and considerations for reducing exposure.
Comparison with other studies
Table 3 provides an overview of reported metal(loid) concentrations in other studies of pregnant women. Urinary and blood concentrations of some essential metals such as Co, Cu, and Zn were within the range of what was reported in previous studies (Callan et al. 2013; Fort et al. 2014; Hansen et al. 2011; Lewis et al. 2018; Rudge et al. 2009; Shirai et al. 2010). The concentrations of Cs in urine and blood were lower in this study compared with other studies of pregnant women in Australia and Spain (Fort et al. 2014; Hinwood et al. 2015). Urinary Mn concentrations (GM=1.2 ng/mL) in this study exceeded those seen in Australia (Callan et al. 2013) and Mexico (Lewis et al. 2018), while blood Mn concentrations (GM=11.3 ng/mL) were comparable with those detected in other studies where the GM or median concentrations ranged from 6.5 to 16.1 ng/mL (Callan et al. 2013; Fort et al. 2014; Hansen et al. 2011; Nakayama et al. 2019).
Table 3.
Urinary and blood metal(loid) concentrations among pregnant women in PROTECT and previous studiesa.
| Urinary Metal(loid)s Summary | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Country/Region | Year | nb | As | Ba | Cd | Co | Cs | Cu | Hg | Mn | Mo | Ni | Pb | Sb | Sn | Zn | Corr ectionc |
GMd /Median |
Unit |
| Present study | Puerto Rico | 2011-2017 | 1285 | 10.9 | 2.5 | 0.12 | 1.0 | 4.9 | 14.0 | 0.60 | 1.2 | 58.9 | 5.4 | 0.25 | 0.09 | 2.1 | 266 | SG | GM | ng/mL |
| Kalloo et al, 2018 | Ohio, US | 2003-2006 | 389 | 5.3 | - | 0.20 | - | - | - | 0.60 | - | - | - | 0.70 | - | - | - | - | GM | ng/mL |
| Lewis et al 2018 | Mexico | 1997-2004 | 212 | 13.8 | 4.0 | 0.18 | 1.2 | - | - | - | 0.82 | 17.3 | 9.5 | 2.9 | - | - | 288 | - | GM | ng/mL |
| Callan et al. 2013 | Australia | 2008-2011 | 157 | 13.2 | - | - | 1.2 | - | 10.4 | - | 0.53 | - | 2.3 | - | - | - | 396 | Crt | Median | μg/g |
| Hinwood et al, 2013 | Australia | 2008-2011 | 157 | - | - | 0.78 | - | - | - | <0.40 | - | - | - | 0.70 | - | - | - | Crt | Median | μg/g |
| Hinwood et al, 2015 | Australia | 2008-2011 | 157 | - | - | - | - | 8.3 | - | - | - | - | - | - | - | - | - | Crt | Median | μg/g |
| Birgisdottir et al, 2013 | Norway | 2003 | 184 | 79.6 | - | 0.16 | - | - | - | 1.2 | - | - | - | - | - | - | - | Crt | Median | μg/g |
| Fort et al, 2014 | Spain (1st trime) | 2004-2006 | 489 | 32.0 | - | 0.61 | 0.45 | 8.0 | 12.0 | - | - | - | 3.9 | 3.8 | 0.36 | - | 256 | Crt | Median | μg/g |
| Spain (3rd trime) | 2004-2006 | 489 | 35.0 | - | 0.54 | 1.3 | 6.8 | 15.0 | - | - | - | 3.9 | 3.9 | 0.28 | - | 290 | Crt | Median | μg/g | |
| Shirai et al, 2010 | Japan | 2007-2008 | 78 | 76.9 | - | 0.77 | - | - | 12.8 | - | - | 79.0 | - | 0.48 | - | 0.23 | 393 | Crt | GM | μg/g |
| Blood Metal(loid)s Summary | ||||||||||||||||||||
| Reference | Country/Region | Year | nb | As | Ba | Cd | Co | Cs | Cu | Hg | Mn | Mo | Ni | Pbf | Sb | Sn | Zn | Corr ectionc |
GMd /Median |
Unit |
| Present study | Puerto Rico | 2011-2017 | 1183 | 0.34 | 0.12 | 0.34 | 1.1 | 1552 | 1.2 | 11.3 | 1.0 | 0.33 | 4682 | - | GM | ng/mL | ||||
| Kalloo et al, 2018 | Ohio, US | 2003-2006 | 389 | - | - | - | - | - | - | - | 0.70 | - | - | GM | ng/mL | |||||
| Callan et al. 2013 | Australia | 2008-2011 | 172 | 1.3 | - | 0.28 | - | 1252 | - | 6.5 | <2.0 | - | 2330 | - | Median | ng/mL | ||||
| Hinwood et al, 2013 | Australia | 2008-2011 | 172 | - | 0.38 | - | - | - | 0.46 | - | - | 0.37 | - | - | Median | ng/mL | ||||
| Hinwood et al, 2015 | Australia | 2008-2011 | 172 | - | - | - | 1.9 | - | - | - | - | - | - | - | Median | ng/mL | ||||
| Birgisdottir et al 2013 | Norway | 2003 | 184 | 5.9 | 0.45 | - | - | - | 4.0 | - | - | 2.5 | - | - | Median | ng/mL | ||||
| Hansen et al, 2011 | Norway | 2007-2009 | 211 | 1.4 | 0.15 | 0.10 | - | 1650 | 1.2 | 10.7 | - | 0.75 | 5110 | - | GM | ng/mL | ||||
| Mathee et al, 2014 | South Africa | 2010 | 307 | 8.0 | 0.20 | - | - | - | 0.60 | - | - | 1.4 | - | - | Median | ng/mL | ||||
| Rudge et al. 2009 | South Africa | nrg | 62 | 0.57 | 0.15 | 0.60 | - | 1730 | 0.65 | 16.8 | - | 2.3 | 6290 | - | Median | ng/mL | ||||
| Nakayama et al, 2019 | Japan | 2011-2014 | 1799 | - | 0.71 | - | - | - | 3.8 | 16.1 | - | 0.64 | - | - | GM | ng/mL | ||||
To allow for comparison on same scale, the urine concentrations were converted to ng/mL for unadjusted urine, μg/g for creatinine adjusted urine, and blood concentrations were converted to ng/mL
Sample Size
- No correction applied, SG corrected for specific gravity Crt corrected for creatinine
GM geometric mean
trim trimester
the unit for blood Pb concentration is μg/dL
nr not reported.
Ba was only measured in urine and concentrations (GM=2.5 ng/mL) were lower in this study compared with Mexican pregnant women (GM=4.0 ng/mL) (Lewis et al. 2018).The levels of Mo and Ni present in the urine samples from Puerto Rican pregnant women were similar to the levels reported in other studies (Callan et al. 2013; Fort et al. 2014; Lewis et al. 2018; Shirai et al. 2010). Studies of Sb and Sn among pregnant women have been much more limited in number compared with other essential metals. The concentrations of urine Sb in our study, GM=0.1 ng/mL, were lower than the levels reported among Spanish pregnant women (Fort et al. 2014). Sn levels measured in urine (GM=2.1 ng/mL) were one order of magnitude higher than the Japan study (Shirai et al. 2010), where the GM was 0.2 ng/mL; however, this comparison needs to be interpreted cautiously given that Sn was only detected among 53% of the samples in the Japan study (Shirai et al. 2010).
The urinary As concentration reported in our study was comparable to other studies of pregnant women while blood As concentrations were lower. The discrepancy between two matrices may be attributable to the fact that As in blood is more susceptible to variation as the half-life of inorganic As in blood is a few hours compared with a few days in urine (Hall et al. 2006). Our study found that the GM blood Hg value among Puerto Rican pregnant women was 1.2 ng/mL with 3 participants having levels exceeding 5.8 μg/L, U.S. EPA’s current reference dose for blood mercury (USEPA 2007).
Pregnant women in this study had lower urine and blood concentrations of Cd and Pb, compared to previous studies mentioned above. This is particularly significant where blood Pb concentrations among this population, with GM of 0.33 μg/dL, is the lowest when compared to women in NHANES (GM=0.64 μg/dL), and pregnant women in Australia (median= 0.37 μg/dL) (Hinwood et al. 2013), Japan (GM=0.64 μg/dL) (Nakayama et al. 2019), Ohio, US (GM=0.7 μg/dL) (Kalloo et al. 2018), Norway (two studies: median=2.5 μg/dL and GM=0.75 μg/dL) (Birgisdottir et al. 2013; Hansen et al. 2011), and South Africa (two studies: median=1.4 μg/dL and median= 2.3 μg/dL) (Mathee et al. 2014; Rudge et al. 2009). In epidemiological studies, higher Pb exposure may mask the effects of other exposures (Sanders et al. 2015), therefore, having lower concentrations of Pb, this population may provide an opportunity to study the health effects of other metal(loid)s/exposures independent of Pb.
None of the blood samples in our study had Pb concentrations that exceeded the level of concern set by CDC, a blood level of 5 μg/dL for pregnant women (Ettinger and Wengrovitz 2010). However, concerns have been raised that even at low levels, prenatal Pb exposure may pose a toxic effect on fetal development (Anderson et al. 2016; Mushak et al. 1989; Polanska et al. 2018; Silver et al. 2016; Takser et al. 2005; CM Taylor et al. 2017; Wu et al. 2017).
These differences in metal(loid) concentrations among pregnant women could be mainly due to population differences, including different geographical and demographic environment, life style and dietary behaviors. The impact of demographic, dietary, and product use patterns during pregnancy on the variation of levels for metal(loid)s will be further discussed in this paper.
Variability of metal(loid) exposures
Variability across study visits
Limited studies have measured and/or compared metal(loid) concentrations at different times during pregnancy and mainly compared just a few metal(loid)s measured in blood or serum. As mentioned above, urinary concentrations of Co, Cs, Cu, Mo, and Zn among pregnant women in our study were statistically different between three visits. These different trends in concentration may due to an actual increase/decrease of metal(loid) concentrations in the body influenced by the change in fetal demand and maternal nutrient supply (King 2000). Metabolic changes during pregnancy, such as the change in glomerular filtration rate (Cheung and Lafayette 2013; Weaver et al. 2014) and plasma volume expansion (Hytten and Paintin 1963) may also result in different filtration of metal(loid)s from blood into urine throughout pregnancy.
Our study reported a significant increase in blood Cd, Co, Cu, Mn, and Zn as gestation progresses. Similar increasing trends have been observed in previous studies considering concentrations of Co, Cu, and Mn in blood or serum (Arbuckle et al. 2016; Izquierdo Alvarez et al. 2007; Khoushabi et al. 2016; Li et al. 2019; Liu et al. 2010; Spencer 1999; Tabrizi and Pakdel 2014). The increasing levels of these metal(loid)s during pregnancy may be attributed to the increased intake and/or release of essential nutrients (Khayat et al. 2017; Marangoni et al. 2016). For Cs, lower concentrations in the blood were observed during the third visit which may be explained by increasing plasma volume during pregnancy (Hytten and Paintin 1963). However, we would expect to see similar trends for all metals if the difference is due to metabolic changes during pregnancy.
We also found that urine/blood ratio remained constant for most of the metal over the course of pregnancy, except for Cd and Mn where the ratio was higher at the first visit and for Cu which had a higher ratio at the third visit (SI Figure S3). These trends may reflect the absolute concentration changes of the metals in either matrix (the results are consistent with the single matrix results described above) and/or the different adjustments of toxicokinetics (distribution, excretion) of those metals throughout pregnancy.
Correlation between metal(loid)s within urine and blood
Moderate to strong correlations were observed between urinary Pb and Ba (r=0.47) and Ni and Ba (r=0.59) (Figure 1). Lewis et al also reported a strong correlation between urinary Pb and Ba (r=0.57) among Mexican pregnant women (Lewis et al. 2018). There were also a few blood metal(loid)s pairs that were moderately correlated (Mn and Co, r=0.36; As and Hg, r=0.32; Cd and Co, r=0.33) in our study (Figure 1). Similar correlations between maternal blood Mn and Co, and As and Hg were reported among Norwegian pregnant women (Hansen et al. 2011). The correlation between As and Hg reflects the common source of exposures, seafood, which is consistent with results from our predictor analysis, whereas the pattern of correlations we observed between Pb and Ba, Ni and Ba, and Mn and Co concentrations could be due to combined use in products, demographic factors, and personal behaviors.
Variability across matrices
Urine and blood are commonly used to measure metal(loid)s in humans (Keil et al. 2011; Sani and Abdullahi 2017; Wang et al. 2014). For most metal(loid)s examined in our study, weak to moderate correlations were observed between concentrations measured in both matrices. Most studies use a single human specimen (blood or urine) to determine exposure to various metal(loid)s. However, each metal(loid) exhibits unique physiochemical properties and toxicokinetics, such as half-life, storage, or elimination rate from the body. As such, the preference for either blood or urine concentration as a better indicator for exposure to a given metal(loid) must be coordinated with the predicted toxicokinetics of the metal(loid) involved, the time between exposure and specimen collection, and the goals for a particular study (e.g. health outcome). For example, since As is excreted relatively rapidly via urine, urinary concentration of As is used as an indicator of recent exposure (Buchet et al. 1981; Kubota et al. 2002). In contrast, blood is the preferred specimen for Pb because Pb has a long biological half-life, resulting in less variability of blood concentrations over time (Barbosa et al. 2005). Blood is also the preferred specimen to identify exposure to methyl-mercury, the most toxic form of Hg, whereas urine excretion represents inorganic Hg exposure (Centers for Disease Control and Prevention (CDC) 2014; Göthe et al. 1985; Yoshida 1985). For Cd, both urine and blood are useful for detecting exposures, as blood Cd primarily reflects recent exposure and urine Cd represents long-term exposure (Centers for Disease Control and Prevention (CDC) 2014; Sanders et al. 2015).
Repeated measures of metal(loid) concentrations in both blood and urine samples enabled us to characterize metal(loid) exposures in different biological matrices, their interrelation, and variability during pregnancy, and select a better exposure indicator with higher reproducibility and abundance for each metal for application in epidemiology studies of pregnancy outcomes. Distributions of the ratio of urine/blood for non-essential metal(loid)s and ICCs for two matrices are consistent with previous knowledge; 1) the absolute concentrations of Pb and Hg were generally higher in blood than in urine (urine/ blood ratios<1 for most samples) and blood samples had good to excellent reproducibility (ICC for Pb=0.78, ICC for Hg=0.62); 2) concentrations of As were higher in urine (urine/blood ratio >1 for most samples); 3) concentrations of Cd were similar in both matrices (mean urine/blood ratio = 1). The concepts presented here for these non-essential metals can be applied to other metals with similar ratio and reproducibility. It is evident from Figure 2 that, metals with mean urine/blood ratio <1 (Cu, Zn, Mn, Hg, Pb) presented good to excellent reliability for blood measurements with ICC ranging from 0.54-0.78, this is consistent with studies indicating that blood Mn and Zn concentrations serve as a reasonable indicator of exposure (Agency for Toxic Substances and Disease Registry (ATSDR) 2005; Myers et al. 2003; Roels et al. 1992). The findings also indicate that repeated measurement of essential and non-essential metal(loid)s during pregnancy was necessary, particularly for most urinary biomarkers.
Predictors
Our predictor analysis revealed that some demographics, dietary factors, product use/water use behaviors can affect the distribution of various metal(loid)s.
Demographics factors and food/ supplement consumption
Smoking was the most significant predictor of blood Cd. We also found that the consumption of several food items (meat, tomato, collard) were additional predictors of Cd exposure. These results were somewhat expected given that diet and smoking are known sources of human Cd exposure (Agency for Toxic Substances and Disease Registry (ATSDR) 2012). In this population, we identified the consumption of fish as a significant predictor of As levels; rice consumption frequency was also positively associated with As levels. These findings are consistent with studies reporting increased exposure and possible health hazards associated with consuming As contaminated rice (Das et al. 2004; Donohue and Abernathy 1999; Hassan et al. 2017; Mahaffey et al. 1975; V Taylor et al. 2017). The forms of As found in rice are mostly inorganic and far more toxic than the organic form found in the environment and food like fish (Valko et al. 2005). Fish was also one of the main predictors of blood Hg levels along with canned food and tomatoes. Fish and canned food (especially canned tuna) are food groups known to be potentially high in Hg (Hughner et al. 2008; Mahaffey et al. 2008; Shim et al. 2004). However, our finding on tomato consumption and blood Hg are contrary to what was reported in previous studies where the consumption of tomato products and tropical fruits were associated with lower blood Hg (Gagné et al. 2013; Passos et al. 2003; Passos et al. 2007). The reported use of supplements during pregnancy, including folic acid and iron supplements, were significant and positive predictors of urinary Cs, Mo, and Sb concentrations. Cs and Mo are often in multivitamin and multi-mineral dietary supplements (Blotsky and Figueroa 2014; Paradissis et al. 1999). It is also plausible that other specific supplementation that wasn’t included in our questionnaires may contain those essential metal(loid)s and women in our study may be consuming those supplementations along with folic acid and iron supplements. Prenatal multivitamin use significantly decreased both blood and urine levels of Cd among this population, and this observation is supported by findings on the protective effect of vitamin E on heavy metal(loid)s absorption among animals (Osfor et al. 2010; Sears 2013).
Personal care product use
For blood concentrations of metal(loid)s, self-reported use of shaving cream and/or shampoo and/or other hair products were important predictors of lower Cu, Hg, Mn, and Zn levels. This inverse association may due to a higher frequency of washing behaviors (showering, face washing) which could help remove metals from the skin and reduce continued exposure.
Water usage
While Pb concentrations in the study population were relatively low overall, we found that those whose drink AAA public water have higher levels of blood Pb compared to those who mostly drink from bottled water. According to a report published in 2017 by the Natural Resources Defense Council, drinking water violations in Puerto Rico had the highest rate among all the U.S. jurisdictions with the presence of Pb and other pollutants in the water coming out of the taps during 2005-2015 (Natural Resources Defense Council 2017). Water treatment was inversely associated with blood Mn levels (among the questionnaire answers from women in our study, most treatments are referring to filtration). A study that assessed heavy metal(loid) concentrations in urban rivers of Puerto Rico found that Mn was the only metal found to exceed maximum contaminant levels established by the EPA for drinking water (US EPA: 5 μg/l) (Ortiz-Colon et al. 2016). It is plausible that treatment of drinking water in homes may help reduce the levels of Mn in the water, therefore reducing exposure. Participants in our study who reported using metal cisterns to store water had elevated levels of urinary Cd. Various studies have found significantly higher levels of Cd in collected tank water and suggested that the main source of Cd in the tank water may be the corrosion of rooftop material since Cd is a common impurity in the Zn coating (Chubaka et al. 2018; Magyar et al. 2008; Van Metre and Mahler 2003). These findings suggest that proper and careful attention should be given to modifying household environments and water treatment behaviors when developing metal(loid) exposure remediation strategies.
Strengths and Limitation
To our knowledge, this is the first study to assess exposure to multiple metal(loid)s among pregnant women in Puerto Rico. PROTECT, a large prospective longitudinal cohort study in Puerto Rico, provides a unique opportunity to characterize metal(loid) exposure in this population. The study design allows for repeated collection of biological samples and questionnaire data to account for the varying levels of exposures during pregnancy, and LMM incorporated this full richness and structure of the data across pregnancy (Meeker et al. 2013). We measured a large panel of metal(loid)s in two biological matrices, urine and blood, which helps to inform future epidemiological analyses because different matrices may be more appropriate for assessing exposure to different metal(loid)s (Fort et al. 2014). The study does have several limitations. We did not collect detailed information regarding the amount of personal product use, and the collection of maternal supplement use is not detailed as to specific ingredients and amount ingested. This may have caused non-differential misclassification and attenuated our results toward the null in the linear mixed models. Though our findings are possibly generalizable to the general pregnant population in Puerto Rico, they may not be generalizable to other pregnant women populations, considering that race/ethnicity, personal care product use, dietary patterns, and toxicokinetics may be quite different compared to pregnant women in Puerto Rico.
Conclusion
In conclusion, we reported metal(loid)s exposure levels for 14 toxic and essential trace metal(loid)s in urine and blood samples from 1,040 pregnant women in Northern Puerto Rico. Exposure to many toxic and essential metal(loid)s are high among these women compared to women of reproductive age from the general US population. Blood concentrations of Cu, Zn, Mn, Hg, and Pb, and urinary concentrations of As, Ni, and Co, were shown to reflect reliable biomarkers of exposure. For other metal(loid)s, repeated samples are recommended for exposure assessment in epidemiology studies. We further examined a variety of predictors of prenatal metal(loid) exposure and found significant associations between potential predictors and biomarkers, including fish and rice consumption (urinary As), fish and canned food (blood Hg), drinking public water (blood Pb), smoking (blood Cd), and iron/folic acid supplement use (urinary Cs, Mo, and Sb). Improved understanding of biomarkers, sources, and pathways of metal(loid)s exposure can inform strategies to reduce exposure among Puerto Rico’s residents.
Supplementary Material
Highlights.
First study to assess exposure to multiple metal(loid)s among pregnant women in Puerto Rico
Concentration of most metals among pregnant women were higher than women of same age in NHANES
Reliable biomarkers of exposures identified by examining biomarkers over time and between matrices.
Exposure predictors: fish, rice-As, fish-Hg, public water-Pb, smoking-Cd, supplement-Cs, Mo, and Sb.
Acknowledgements
We would like to extend our gratitude to all PROTECT study participants and their families. The authors also thank the nurses and research staff who participated in cohort recruitment and follow up, as well as the Federally Qualified Health Centers (FQHC) in Puerto Rico that facilitated participant recruitment, including Morovis Community Health Center, Prymed in Ciales, Camuy Health Services, Inc. and the_Delta OBGyn Group in Manati, as well as the Manati Medical Center and the Metro Pavia Hospital in Arecibo.
Funding
This study was supported by the Superfund Research Program of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH; grant number P42ES017198). Additional support was provided from NIEHS grant number P30ES017885 and the NIH Environmental influences on Child Health Outcomes (ECHO) program grant number UH3OD023251. ECHO is a nationwide research program supported by the NIH, Office of the Director to enhance child health.
Footnotes
Conflict of Interest
The authors declare that they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Agency for Toxic Substances and Disease Registry (ATSDR). 2005. Toxicological profile for zinc. [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). 2012. Toxicological profile for cadmium. [PubMed]
- Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Cordero JF, et al. 2019. The associations between prenatal exposure to triclocarban, phenols and parabens with gestational age and birth weight in northern puerto rico. Environ Res 169:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DW, Mettil W, Schneider JS. 2016. Effects of low level lead exposure on associative learning and memory in the rat: Influences of sex and developmental timing of exposure. Toxicol Lett 246:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TE, Liang CL, Morisset AS, Fisher M, Weiler H, Cirtiu CM, et al. 2016. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The mirec study. Chemosphere 163:270–282. [DOI] [PubMed] [Google Scholar]
- Ashrap P, Watkins DJ, Calafat AM, Ye X, Rosario Z, Brown P, et al. 2018. Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in northern puerto rico: Predictors and trends. Environ Int 121:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa F Jr., Tanus-Santos JE, Gerlach RF, Parsons PJ. 2005. A critical review of biomarkers used for monitoring human exposure to lead: Advantages, limitations, and future needs. Environ Health Perspect 113:1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgisdottir BE, Knutsen HK, Haugen M, Gjelstad IM, Jenssen MT, Ellingsen DG, et al. 2013. Essential and toxic element concentrations in blood and urine and their associations with diet: Results from a norwegian population study including high-consumers of seafood and game. Sci Total Environ 463–464:836–844. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Parsons PJ, Steuerwald AJ, Schisterman EF, Browne RW, Kim K, et al. 2010. Toxic trace metals and human oocytes during in vitro fertilization (ivf). Reprod Toxicol 29:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blotsky RD, Figueroa R. 2014. Mineral, nutritional, cosmetic, pharmaceutical, and agricultural compositions and methods for producing the same.Google Patents. [Google Scholar]
- Borowska S, Brzoska MM. 2015. Metals in cosmetics: Implications for human health. J Appl Toxicol 35:551–572. [DOI] [PubMed] [Google Scholar]
- Buchet JP, Lauwerys R, Roels H. 1981. Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. Int Arch Occup Environ Health 48:71–79. [DOI] [PubMed] [Google Scholar]
- Callan AC, Hinwood AL, Ramalingam M, Boyce M, Heyworth J, McCafferty P, et al. 2013. Maternal exposure to metals--concentrations and predictors of exposure. Environ Res 126:111–117. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. 2014. Urinary phthalate metabolite concentrations among pregnant women in northern puerto rico: Distribution, temporal variability, and predictors. Environ Int 62:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Herring AH, Sjodin A, Jones R, Needham L, Ma C, et al. 2010. Hypospadias and halogenated organic pollutant levels in maternal mid-pregnancy serum samples. Chemosphere 80:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta D, Graziano A, Lo Monte G, Bordi G, Moscarini M. 2013. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur Rev Med Pharmacol Sci 17:2198–2206. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). 2014. Laboratory procedure manual (cadmium, lead, manganese, mercury, and selenium).
- Centers for Disease Control and Prevention (CDC). 2019. Fourth national report on human exposure to environmental chemicals updated tables.
- Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, et al. 2014. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol 24:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KL, Lafayette RA. 2013. Renal physiology of pregnancy. Adv Chronic Kidney Dis 20:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubaka CE, Whiley H, Edwards JW, Ross KE. 2018. Lead, zinc, copper, and cadmium content of water from south australian rainwater tanks. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin BD, Hein MJ, Sanderson WT, Nishioka MG, Reynolds SJ, Ward EM, et al. 2005. Pesticide contamination inside farm and nonfarm homes. J Occup Environ Hyg 2:357–367. [DOI] [PubMed] [Google Scholar]
- Das H, Mitra AK, Sengupta P, Hossain A, Islam F, Rabbani G. 2004. Arsenic concentrations in rice, vegetables, and fish in bangladesh: A preliminary study. Environment international 30:383–387. [DOI] [PubMed] [Google Scholar]
- De Coster S, van Larebeke N. 2012. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J Environ Public Health 2012:713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. 2009. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr Rev 30:293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue JM, Abernathy CO. 1999. Exposure to inorganic arsenic from fish and shellfish In: Arsenic exposure and health effects iii:Elsevier, 89–98. [Google Scholar]
- Dorgan JF, Brock JW, Rothman N, Needham LL, Miller R, Stephenson HE Jr., et al. 1999. Serum organochlorine pesticides and pcbs and breast cancer risk: Results from a prospective analysis (USA). Cancer Causes Control 10:1–11. [DOI] [PubMed] [Google Scholar]
- Ettinger AS, Wengrovitz AM. 2010. Guidelines for the identification and management of lead exposure in pregnant and lactating women. [Google Scholar]
- Fort M, Cosin-Tomas M, Grimalt JO, Querol X, Casas M, Sunyer J. 2014. Assessment of exposure to trace metals in a cohort of pregnant women from an urban center by urine analysis in the first and third trimesters of pregnancy. Environ Sci Pollut Res Int 21:9234–9241. [DOI] [PubMed] [Google Scholar]
- Gagné D, Lauzière J, Blanchet R, Vézina C, Vaissière É, Ayotte P, et al. 2013. Consumption of tomato products is associated with lower blood mercury levels in inuit preschool children. Food and chemical toxicology 51:404–410. [DOI] [PubMed] [Google Scholar]
- Gorman Ng M, MacCalman L, Semple S, van Tongeren M. 2017. Field measurements of inadvertent ingestion exposure to metals. Ann Work Expo Health 61:1097–1107. [DOI] [PubMed] [Google Scholar]
- Göthe C-J, Langworth S, Carleson R, Tufvesson B. 1985. Biological monitoring of exposure to metallic mercury. Journal of Toxicology: Clinical Toxicology 23:381–389. [DOI] [PubMed] [Google Scholar]
- Groll A, Tutz G. 2014. Variable selection for generalized linear mixed models by l 1-penalized estimation. Statistics and Computing 24:137–154. [Google Scholar]
- Hall M, Chen Y, Ahsan H, Slavkovich V, van Geen A, Parvez F, et al. 2006. Blood arsenic as a biomarker of arsenic exposure: Results from a prospective study. Toxicology 225:225–233. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. 1995. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol Biomarkers Prev 4:649–654. [PubMed] [Google Scholar]
- Hansen S, Nieboer E, Sandanger TM, Wilsgaard T, Thomassen Y, Veyhe AS, et al. 2011. Changes in maternal blood concentrations of selected essential and toxic elements during and after pregnancy. J Environ Monit 13:2143–2152. [DOI] [PubMed] [Google Scholar]
- Hassan FI, Niaz K, Khan F, Maqbool F, Abdollahi M. 2017. The relation between rice consumption, arsenic contamination, and prevalence of diabetes in south asia. EXCLI J 16:1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood AL, Callan AC, Ramalingam M, Boyce M, Heyworth J, McCafferty P, et al. 2013. Cadmium, lead and mercury exposure in non smoking pregnant women. Environ Res 126:118–124. [DOI] [PubMed] [Google Scholar]
- Hinwood AL, Stasinska A, Callan AC, Heyworth J, Ramalingam M, Boyce M, et al. 2015. Maternal exposure to alkali, alkali earth, transition and other metals: Concentrations and predictors of exposure. Environ Pollut 204:256–263. [DOI] [PubMed] [Google Scholar]
- Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. 2015. Manganese is essential for neuronal health. Annu Rev Nutr 35:71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughner ReS, Maher JK, Childs NM. 2008. Review of food policy and consumer issues of mercury in fish. Journal of the American College of Nutrition 27:185–194. [DOI] [PubMed] [Google Scholar]
- Hytten FE, Paintin DB. 1963. Increase in plasma volume during normal pregnancy. J Obstet Gynaecol Br Emp 70:402–407. [DOI] [PubMed] [Google Scholar]
- Izquierdo Alvarez S, Castanon SG, Ruata ML, Aragues EF, Terraz PB, Irazabal YG, et al. 2007. Updating of normal levels of copper, zinc and selenium in serum of pregnant women. J Trace Elem Med Biol 21 Suppl 1:49–52. [DOI] [PubMed] [Google Scholar]
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. 2014. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QM. 2015. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16:29592–29630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalloo G, Wellenius GA, McCandless L, Calafat AM, Sjodin A, Karagas M, et al. 2018. Profiles and predictors of environmental chemical mixture exposure among pregnant women: The health outcomes and measures of the environment study. Environ Sci Technol 52:10104–10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil DE, Berger-Ritchie J, McMillin GA. 2011. Testing for toxic elements: A focus on arsenic, cadmium, lead, and mercury. Laboratory Medicine 42:735–742. [Google Scholar]
- Khayat S, Fanaei H, Ghanbarzehi A. 2017. Minerals in pregnancy and lactation: A review article. J Clin Diagn Res 11:QE01–QE05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoushabi F, Shadan MR, Miri A, Sharifi-Rad J. 2016. Determination of maternal serum zinc, iron, calcium and magnesium during pregnancy in pregnant women and umbilical cord blood and their association with outcome of pregnancy. Mater Sociomed 28:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Meeker JD, Carroll R, Zhao S, Mourgas MJ, Richards MJ, et al. 2018. Urinary trace metals individually and in mixtures in association with preterm birth. Environ Int 121:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JC. 2000. Physiology of pregnancy and nutrient metabolism. The American journal of clinical nutrition 71:1218S–1225S. [DOI] [PubMed] [Google Scholar]
- Kubota R, Kunito T, Tanabe S. 2002. Chemical speciation of arsenic in the livers of higher trophic marine animals. Mar Pollut Bull 45:218–223. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Basu N, Gauthier AM, Cantoral A, Mercado-Garcia A, et al. 2018. Urinary metal concentrations among mothers and children in a mexico city birth cohort study. Int J Hyg Environ Health 221:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZJ, Liang CM, Xia X, Huang K, Yan SQ, Tao RW, et al. 2019. Association between maternal and umbilical cord serum cobalt concentration during pregnancy and the risk of preterm birth: The ma'anshan birth cohort (mabc) study. Chemosphere 218:487–492. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang H, Shi H, Shen C, Zhou W, Dai Q, et al. 2010. Blood copper, zinc, calcium, and magnesium levels during different duration of pregnancy in chinese. Biol Trace Elem Res 135:31–37. [DOI] [PubMed] [Google Scholar]
- Magyar MI, Mitchell V, Ladson A, Diaper C. 2008. Lead and other heavy metals: Common contaminants of rainwater tanks in melbourne. Proceedings of Water Down Under 2008:409. [Google Scholar]
- Mahaffey K, Corneliussen P, Jelinek C, Fiorino J. 1975. Heavy metal exposure from foods. Environmental health perspectives 12:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Jeffries RA. 2008. Adult women’s blood mercury concentrations vary regionally in the united states: Association with patterns of fish consumption (nhanes 1999–2004). Environmental health perspectives 117:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni F, Cetin I, Verduci E, Canzone G, Giovannini M, Scollo P, et al. 2016. Maternal diet and nutrient requirements in pregnancy and breastfeeding. An italian consensus document. Nutrients 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Griswold W. 2009. Human health effects of heavy metals. Environmental Science and Technology briefs for citizens 15:1–6. [Google Scholar]
- Mathee A, Naicker N, Kootbodien T, Mahuma T, Nkomo P, Naik I, et al. 2014. A crosssectional analytical study of geophagia practices and blood metal concentrations in pregnant women in johannesburg, south africa. S Afr Med J 104:568–573. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. 2013. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in puerto rico. Environ Sci Technol 47:3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Moreno JM, Roca M, Vergara-Juarez N, Martinez-Garcia MJ, Garcia-Sanchez A, et al. 2011. Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: A pilot study. Environ Health 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushak P, Davis JM, Crocetti AF, Grant LD. 1989. Prenatal and postnatal effects of low-level lead exposure: Integrated summary of a report to the u.S. Congress on childhood lead poisoning. Environ Res 50:11–36. [DOI] [PubMed] [Google Scholar]
- Myers JE, Thompson ML, Naik I, Theodorou P, Esswein E, Tassell H, et al. 2003. The utility of biological monitoring for manganese in ferroalloy smelter workers in south africa. Neurotoxicology 24:875–883. [DOI] [PubMed] [Google Scholar]
- Nakayama SF, Iwai-Shimada M, Oguri T, Isobe T, Takeuchi A, Kobayashi Y, et al. 2019. Blood mercury, lead, cadmium, manganese and selenium levels in pregnant women and their determinants: The japan environment and children's study (jecs). J Expo Sci Environ Epidemiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natural Resources Defense Council. 2017. Threats on tap: Drinking water violations in puerto rico.
- Nordberg G, Sandström B, Becking G, Goyer R. 2001. Essentiality and toxicity of trace elements: Principles and methods for assessment of risk from human exposure to essential trace elements. The Journal of Trace Elements in Experimental Medicine: The Official Publication of the International Society for Trace Element Research in Humans 14:261–273. [Google Scholar]
- O'Neal SL, Zheng W. 2015. Manganese toxicity upon overexposure: A decade in review. Curr Environ Health Rep 2:315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Colon AI, Pinero-Santiago LE, Rivera NM, Sosa MA. 2016. Assessment of concentrations of heavy metals and phthalates in two urban rivers of the northeast of puerto rico. J Environ Anal Toxicol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osfor M, Ibrahim H, Mohamed Y, Ahmed S, Abd El Azeem A, Hegazy A. 2010. Effect of alpha lipoic acid and vitamin e on heavy metals intoxication in male albino rats. J Am Sci 6:56–63. [Google Scholar]
- Padilla I, Irizarry C, Steele K. 2011. Historical contamination of groundwater resources in the north coast karst aquifers of puerto rico. Rev Dimens 3:7–12. [PMC free article] [PubMed] [Google Scholar]
- Paradissis GN, Levinson RS, Heeter G, Cuca RC, Vanek PP. 1999. Multi-vitamin and mineral supplements for women.Google Patents. [Google Scholar]
- Passos CJ, Mergler D, Gaspar E, Morais S, Lucotte M, Larribe F, et al. 2003. Eating tropical fruit reduces mercury exposure from fish consumption in the brazilian amazon. Environmental Research 93:123–130. [DOI] [PubMed] [Google Scholar]
- Passos CJS, Mergler D, Fillion M, Lemire M, Mertens F, Guimarães JRD, et al. 2007. Epidemiologic confirmation that fruit consumption influences mercury exposure in riparian communities in the brazilian amazon. Environmental Research 105:183–193. [DOI] [PubMed] [Google Scholar]
- Polanska K, Hanke W, Pawlas N, Wesolowska E, Jankowska A, Jagodic M, et al. 2018. Sex- dependent impact of low-level lead exposure during prenatal period on child psychomotor functions. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punshon T, Li Z, Marsit CJ, Jackson BP, Baker ER, Karagas MR. 2016. Placental metal concentrations in relation to maternal and infant toenails in a u.S. Cohort. Environ Sci Technol 50:1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K, Fatima F, Waheed I, Akash MSH. 2018. Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119:157–184. [DOI] [PubMed] [Google Scholar]
- Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR. 1992. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br J Ind Med 49:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner B 2015. Fundamentals of biostatistics:Nelson Education. [Google Scholar]
- Rudge CV, Rollin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JO. 2009. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of south african delivering women. J Environ Monit 11:1322–1330. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Claus Henn B, Wright RO. 2015. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: A review of recent literature. Curr Environ Health Rep 2:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani A, Abdullahi IL. 2017. Evaluation of some heavy metals concentration in body fluids of metal workers in kano metropolis, nigeria. Toxicol Rep 4:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears ME. 2013. Chelation: Harnessing and enhancing heavy metal detoxification—a review. The Scientific World Journal 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S, Dorworth L, Lasrado J, Santerre C. 2004. Mercury and fatty acids in canned tuna, salmon, and mackerel. Journal of Food Science 69:C681–C684. [Google Scholar]
- Shirai S, Suzuki Y, Yoshinaga J, Mizumoto Y. 2010. Maternal exposure to low-level heavy metals during pregnancy and birth size. J Environ Sci Health A Tox Hazard Subst Environ Eng 45:1468–1474. [DOI] [PubMed] [Google Scholar]
- Silver MK, Li X, Liu Y, Li M, Mai X, Kaciroti N, et al. 2016. Low-level prenatal lead exposure and infant sensory function. Environ Health 15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Gautam N, Mishra A, Gupta R. 2011. Heavy metals and living systems: An overview. Indian J Pharmacol 43:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A 1999. Whole blood manganese levels in pregnancy and the neonate. Nutrition 15:731–734. [DOI] [PubMed] [Google Scholar]
- Tabrizi FM, Pakdel FG. 2014. Serum level of some minerals during three trimesters of pregnancy in iranian women and their newborns: A longitudinal study. Indian J Clin Biochem 29:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takser L, Mergler D, Lafond J. 2005. Very low level environmental exposure to lead and prolactin levels during pregnancy. Neurotoxicol Teratol 27:505–508. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Kordas K, Golding J, Emond AM. 2017. Effects of low-level prenatal lead exposure on child iq at 4 and 8 years in a uk birth cohort study. Neurotoxicology 62:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V, Goodale B, Raab A, Schwerdtle T, Reimer K, Conklin S, et al. 2017. Human exposure to organic arsenic species from seafood. Sci Total Environ 580:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. 2007. Organic mercury teach chemical summary.
- Valko M, Morris H, Cronin MT. 2005. Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208. [DOI] [PubMed] [Google Scholar]
- Van Metre PC, Mahler BJ. 2003. The contribution of particles washed from rooftops to contaminant loading to urban streams. Chemosphere 52:1727–1741. [DOI] [PubMed] [Google Scholar]
- Wang RY, Caldwell KL, Jones RL. 2014. Analytical considerations in the clinical laboratory assessment of metals. J Med Toxicol 10:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Ferguson KK, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD. 2015. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in puerto rico. Int J Hyg Environ Health 218:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Vargas GG, Silbergeld EK, Rothenberg SJ, Fadrowski JJ, Rubio-Andrade M, et al. 2014. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (egfr) in adolescents. Environ Res 132:226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Hivert MF, Cardenas A, Zhong J, Rifas-Shiman SL, Agha G, et al. 2017. Exposure to low levels of lead in utero and umbilical cord blood DNA methylation in project viva: An epigenome-wide association study. Environ Health Perspect 125:087019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M 1985. Relation of mercury exposure to elemental mercury levels in the urine and blood. Scandinavian journal of work, environment & health:33–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.