Abstract
Erythrodermic psoriasis (EP) is an extreme and often refractory variant of psoriasis with high morbidity and increased mortality, and is frequently classified as dermatological emergency. The pathophysiology of EP is largely unknown but is thought to differ from that of plaque psoriasis. Treatment of EP is challenging, and usually based on clinical experience and patient comorbidities, due to its low incidence and limited clinical evidence. Conventional treatments, such as topical glucocorticoid therapy, cyclosporin, acitretin, and methotrexate have some but limited efficacy in EP, and treatment discontinuation may result in flares. Newer biological drugs, including anti-TNF, anti-IL-17, and anti-IL-12/23 agents have shown promise in therapeutic management of EP, but most of the available evidence is currently based on small case series and reports. Few studies have compared available treatment options for EP, and further clinical studies are necessary to provide clinical data and optimal treatment guidelines for EP patients. Here, we provide a comprehensive review of the background of EP, assess the available clinical data on the efficacy of targeted therapies, and aim to provide a foundation for clinical decision making for this rare form of psoriasis.
1. Introduction
Erythrodermic psoriasis (EP) is a rare and severe subset of psoriasis characterized by prominent erythema affecting at least 80–90% of the body surface (1) (Figure 1), and is classically accompanied by fever, chills, headache, and general discomfort (2). Due to severe and extensive skin barrier defect, EP patients can present with systemic symptoms such as dehydration, fatigue, staphylococcal infection, insomnia, weight changes, cachexia, and electrolyte abnormalities (3). The onset can be explosive, or can be more gradual, triggered by various factors, including emotional stress, sunburns, infections, or drugs (4–6). A frequent cause of EP is the sudden withdrawal of plaque psoriasis treatment, including steroids, cyclosporin, and methotrexate (7). It has also recently been described after discontinuation of anti-IL-17A treatments (8). It has been shown that serum interferon-γ (IFN-γ) level correlates positively with disease severity (as measured by psoriasis area and severity index (PASI)), and that this level is higher in the EP patients (9). In addition, vascular changes and expression of intercellular adhesion molecules are much higher in EP skin lesions than those in plaque psoriasis (10). However, the use of these observations as a clinical biomarker for EP has not been attempted, and to date, there are no specific biomarkers to predict or monitor the development of EP.
Figure 1. The clinical features of erythrodermic psoriasis (EP).
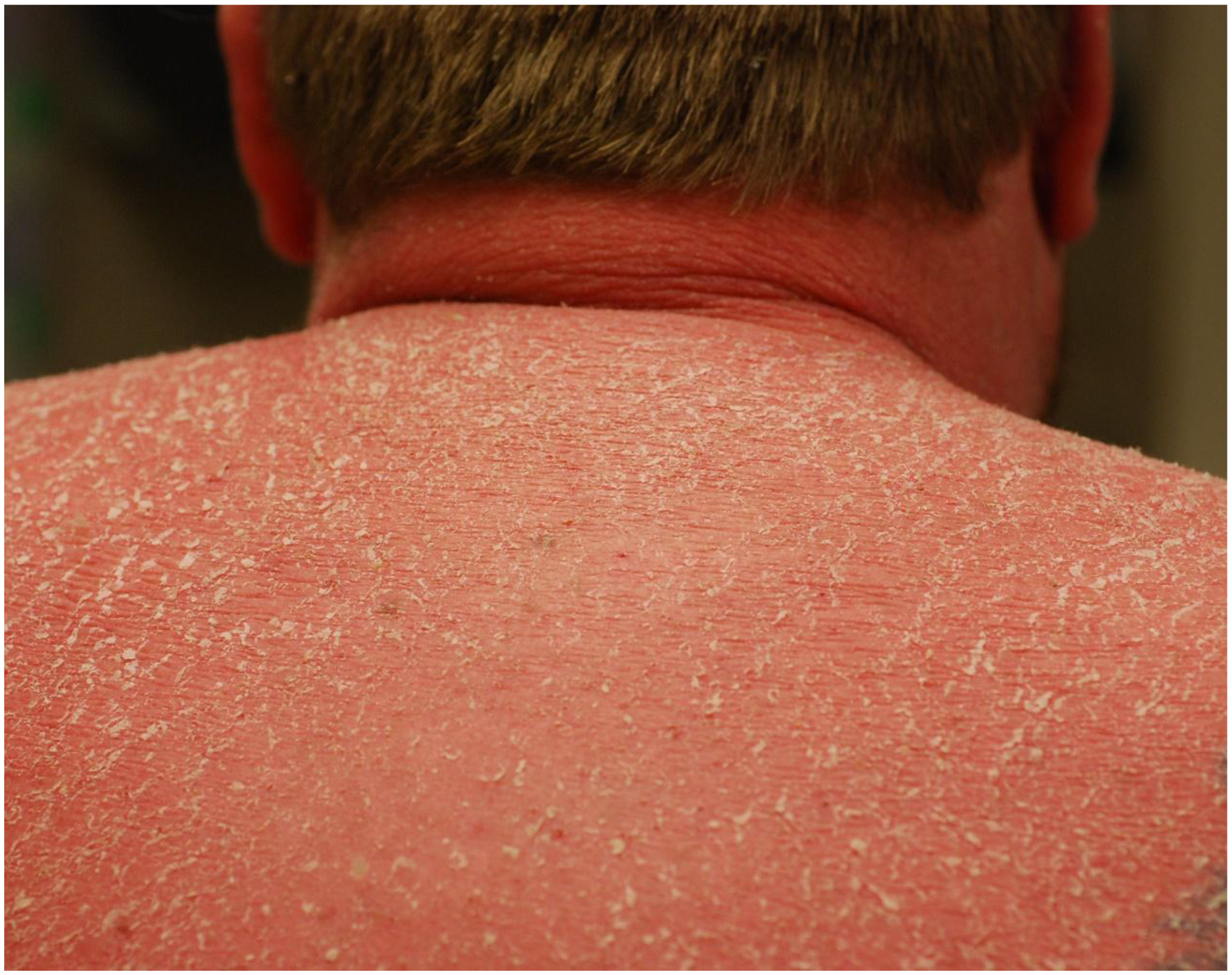
Erythrodermic psoriasis involves over 80–90% of the total body surface area. EP differs clinically in several ways from chronic plaque with the lesions lacking the sharp demarcation from uninvolved skin. The lesions frequently lack the thickness seen in chronic plaque psoriasis and the scale is different, being finer and “powdery”..
Although EP is considered to be a subtype of psoriasis, the pathogenic mechanisms involved are not identical to plaque psoriasis (summarized in Table 1). In 2005, it was demonstrated that serum immunoglobulin E level was significantly higher in EP patients compared to patients with plaque psoriasis, a finding typically associated with Th2 immune responses (11). Similarly, and consistent with increased Th2 responses playing a role in EP, several studies showed that the serum level of interleukin (IL)-13 and IL-4 were significantly higher in EP patients compared to patients with plaque psoriasis (12, 13). While the association with IL-13 is highly intriguing as mentioned above, one case report of a patient with history of AD treated with the anti-IL-4R antagonist dupilumab, which targets both IL-4 and IL-13, led to development of erythrodermic psoriasis (14), suggesting more of a regulatory role for IL-13 in the pathogenesis of erythrodermic psoriasis. Moreover, microarray analysis of biopsies of chronic plaque psoriasis and EP demonstrated that IL-17A was the major shared pathway for plaque psoriasis and EP (15), and consistent with this, Moy et al. observed via immunohistochemical staining that Th17 cell was the most predominant T cell subset in EP lesions (16). Consistently, published case series indicated that most EP patients achieved complete clearance with anti-IL-17A treatment (15), suggesting that IL-17A may be the dominant cytokine in EP development. Recent publications have reported the association of mutations of the CARD14 gene in a family with prominent presentation of EP (17), but this signaling pathway has been implicated in pustular forms of psoriasis (18), where IL-36 activation is prominent (19). Further in-depth mechanistic and functional studies are thus needed to better characterize the immunological network and crosstalk in the pathogenesis of EP.
Table 1.
Summary of the possible pathogenesis of erythrodermic psoriasis (EP)
| Study | Key Evidence | Implications |
|---|---|---|
| Th2 immune response | The serum immunoglobulin E level has been described to be higher in EP patients compared to plaque psoriasis patients (11). | EP may have a degree of Th2 skewing. |
| The serum level of IL-13 was significantly higher in EP patients compared to plaque psoriasis patients (12). | ||
| Levels of IL-4 and IL-10 in EP patients were higher compared to those of plaque psoriasis patients and healthy controls (12, 13). | ||
| Th1/Th2 ratio was dramatically lower in EP patients than that in plaque psoriasis patients (13). | ||
| Th17 signaling pathway | Microarray analysis of biopsies of chronic plaque psoriasis and EP demonstrated that IL-17A is a major shared pathway for plaque psoriasis and EP pathogenesis (15). |
Th17 signaling pathway can be therapeutically targeted in EP. |
| Th17 cells are the most predominant T cell subset in EP skin lesions by tissue immunofluorescence staining (16). | ||
| Predisposing genetic variants | The association of mutations of the CARD14 gene in a family with prominent presentation of EP was detected (17). | EP may have genetic background similar to pustular psoriasis. |
| Other factors | In erythroderma, circulating ICAM-1, VCAM-1, and E-selectin levels were significantly elevated (109). | Cell adhesion mechanisms may be part of EP pathogenesis. |
Abbreviations: EP: erythrodermic psoriasis; IL: interleukin; CARD14: caspase recruitment domain family member 14; ICAM-1: intercellular adhesion molecule 1; VCAM-1: vascular cell adhesion protein 1.
Due to severity and high risk of comorbidities in EP, initial management should include a full medical assessment, including an evaluation for infection given the increased risk of bacteremia and sepsis (3); correction of body fluid, protein, and electrolyte abnormalities; and skin barrier restoration. Older conventional agents such as oral methotrexate, retinoids, and cyclosporine, are frequently partially or entirely ineffective in controlling EP (20), but may be used either in combination with biologic treatment or added on in recalcitrant cases. For example, in a case of EP induced by infliximab used to treat underlying plaque psoriasis, cyclosporine was effective in alleviating symptoms due to its specific inhibition of T cell functions (21). Topicals such as medium-to-high potency steroids, vitamin D analogs, and emollients may be helpful in combination with systemic therapy.
In recent years, a number of biologic treatments including anti-tumor necrosis factor (TNF) and anti-IL-17A agents have shown promising results for treating EP (22). Guidelines for EP released in 2010 by the National Psoriasis Foundation Medical Board suggested infliximab as first-line therapy, especially in life-threatening cases (2). Secukinumab, an anti-IL-17A agent, has been shown to be highly effective in EP and enhance life quality (23). However, major limitations remain, and there are few if any head-to-head studies or other prospective controlled trial data available to guide clinicians in managing EP, with the great majority of data and clinical evidence being based on small case series and individual case reports. Here, we review the latest data with regard to new targeted therapies of EP.
2. Efficacy of new targeted therapies
The immunopathogenesis of psoriasis is complex having strong genetic and environmental contribution with involvement of multiple different cytokines and inflammatory mediators and interplay between multiple different cell types including keratinocytes, T cells, and macrophages/dendritic cells (24). The critical inflammatory mediators are considered to be TNF and IL-23, which expands and maintains Th17 cells and promotes secretion of IL-17A. This in turns leads to a self-sustaining cycle of inflammation acting through multiple feed-forward mechanisms (25). Thus, TNF, IL-23, and IL-17A are considered to be key biologic targets for psoriasis treatments, and in recent years treatments targeting these cytokines have proven to be highly effective in the treatment of plaque psoriasis (26). Many of these treatments have also been shown to help control EP, but despite overall good short-term efficacy, treatment switches due to lack of efficacy or adverse events are frequently observed on a longer-term basis with only a third of the patients receiving the same drug after one year (27). Evidence for the use of biologic therapies for EP are summarized in Table 2.
Table 2.
Studies examining biologic monotherapy in erythrodermic psoriasis (EP) patients
| Biologic agent | Targets | Character istics | Types of study design | Total number of subjects | Efficacy (total responders/enr olled EP patients; observed earliest time) | Main adverse effects in EP patients in literatures |
|---|---|---|---|---|---|---|
| Anti-TNF agents | ||||||
| Etanercept | Anti-TNF | Recombin ant human fusion protein | Prospective clinical trial (33), prospective open-label uncontrolled trial (27), case reports (35, 36) | 21 | 17/21; within 12 weeks | Two with bacterial infection (27) |
| Infliximab | Anti-TNF | Mouse-human chimeric monoclona l antibody IgG1 | Prospective open-label, uncontrolled, trial (27), prospective post-marketing surveillance (40), case reports (39, 41, 42, 44–47) | 77 | 55/77; within 12 weeks | Seven with bacterial infection (27, 40); one with myocardial infarction; one with suicidal attempt; one with immunoallergic shock (27) |
| Adalimumab | Anti-TNF | Fully human, IgG1 | Prospective open-label, uncontrolled, trial (27), case report (51) | 8 | 4/8; within 12 weeks | One with nodal T-cell lymphoma (27) |
| Golimumab | Anti-TNF | Fully human, IgGlK | Case report (53) | 1 | 1/1; after the first administration | Not reported |
| Anti-IL-17 agents | ||||||
| Secukinumab | Anti-IL-17 A | Fully human, IgGlK | Multi-center, retrospective study (64), case series and reports (23, 60, 61) | 20 | 17/20; within 4 weeks | Not reported |
| Ixekizumab | Anti-IL-17 | Humanize | Open-label, | 18 | 16/18; within 4 | Four with |
| A | d, IgG4 | phase 3 study (67, 68), case series and reports (70–72)\ | weeks | injection-site discomfort (72); six with infections; two with allergic reactions/hyperse nsitivity; one with hepatic (68) | ||
| Brodalumab | Anti-IL-17R | Fully human, IgG2 | Open-label, multicenter, long-term phase III study (74) | 18 | 18/18; within 12 weeks | Six with nasopharyngitis; one with diarrhea; one with folliculitis; one with skin papilloma; one with dry skin; two with periarthritis; two with dental caries; two with tooth fracture; one with candidiasis (74) |
| Anti-IL-12/23 agents | ||||||
| Ustekinumab | Anti-p40 subunit of IL-23 and IL-12 | Fully human, IgG1K | Multicenter retrospective studies (27, 80), case series and reports (81–88) | 46 | 44/46; within 4 weeks | aOne with tuberculosis reactivation under treatment with ustekinumab and a low dose steroid (110); two with severe cutaneous bacterial infection (27) |
| Guselkumab | Anti-p19 subunit of IL-23 | Fully human, IgG1 | Multicenter, open-label study (89); case report (90) | 12 | 11/12; within 16 weeks | Five with infections and one with rib fracture (89) |
| Other agents | ||||||
| Apremilast | Inhibitor of PDE4 |
Small molecule inhibitor | Case reports (105–107) | 3 | 3/3; with in 20 days | One with upper respiratory infection (106); one with atrial fibrillation (107) |
| Alefacept (not currently in use) | Anti-LFA-3/CD2 interaction | Recombin ant human fusion protein | Case report (94) | 1 | 1/1; | Not reported |
| Efalizumab (not currently in use) | Anti-CD11a | Fully human, IgG1 | Multicenter, retrospective study (27), case reports (95) | 3 | 1/3; | One with erythroderma (96) |
| Panitumumab | Anti-EGFR | Fully human, IgG2 k | Case report (100) | 1 | 1/1; within approximately 10 days | Not reported |
Abbreviations: EP: erythrodermic psoriasis; TNF: tumor necrosis factor; IL: interleukin; IL-17R, IL-17 receptor; LFA: lymphocyte function-associated antigen; EGFR: epidermal growth factor receptor; PDE4: phosphodiesterase 4.
This case is not included in the total patients in this Table, because it is not treated by a single agent.
2.1. Anti-TNF agents
For many years, psoriasis was considered to be a Th1-dominant disease in which IFN-γ and TNF were thought to be the predominant pathogenic cytokines (28, 29). TNF can activate several critical signaling pathways including nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase to regulate cellular differentiation, proliferation/apoptosis, and secretion, thus contributing to the development of psoriasis and a variety of other inflammatory conditions (24, 30). Therefore, TNF was the first cytokine to be targeted for the treatment of patients with moderate-to-severe psoriasis. Notably, anti-IFN-γ therapy did not work in psoriasis (31).
Etanercept is a soluble and recombinant human fusion protein that acts as a competitive inhibitor of endogenous TNF, thus inhibiting the inflammatory cascade of TNF. It was approved by the Food and Drug Administration (FDA) in 1999 for treating patients with psoriatic arthritis and moderate-to-severe plaque psoriasis (32). In 2006, it was demonstrated in a prospective clinical trial that etanercept could also be effectively used to treat EP (33). This trial, was a prospective 24-week open-label uncontrolled trial that enrolled 10 patients with EP, 6 of which achieved a PASI75 (greater than 75% decrease in disease activity) within 24 weeks, demonstrating that etanercept is an effective treatment option for EP (33). In another monotherapy study using etanercept, 4 out of 6 EP patients reached PASI75 within 12–14 weeks (27). In addition, several case reports have also demonstrated the efficacy of etanercept in EP (34–36). In one of these reports, a child with EP had a near complete response to etanercept together with methotrexate without any adverse events after treatment failure with cyclosporine and methotrexate (35).
Infliximab was approved by the FDA for treatment of psoriasis in 2006 (37), while the first report of its successful use in psoriasis dates back to 2000 (38). Infliximab is a mouse-human chimeric monoclonal antibody that can bind to both soluble and membrane-bound TNF with high affinity, thus interrupting the downstream inflammatory cascade (37). The earliest report of infliximab in EP was on a patient who had an excellent response despite having failed all previous conventional therapies over a 12-year period (39). An open-label, uncontrolled, multicenter clinical trial revealed that 48% of 24 EP patients achieved PASI75 score after 12–14 weeks of infliximab treatment (27). A larger prospective post-marketing surveillance assessing the efficacy of infliximab in 746 Japanese patients with different subtypes of psoriasis, including plaque psoriasis, psoriatic arthritis, pustular psoriasis, and EP, confirmed that infliximab is a highly effective and well tolerated option in treating refractory EP (40). A follow-up study confirmed that 3 of 5 EP patients reached the PASI75 or more (41). Other studies have reported rapid responses to infliximab in EP patients with complicated life-threatening disease (42–45). Erythrodermic flares caused by increased psoriasis activity after discontinuation of prior treatment (efalizumab) have also been shown to respond dramatically to infliximab therapy (46). In addition, infliximab can be used to gain rapid control of EP to help transition patients to other agents, such as was the case with a patient diagnosed with EP and ankylosing spondylitis who was treated with infliximab initially for 48 weeks and then switched over to a low-dose etanercept monotherapy for additional 34 months with excellent control (47). To data, infliximab is the most frequently reported biologic therapy for EP (table 2). Moreover, combination of infliximab with other conventional medications, including acitretin and methotrexate, have also been explored, with several studies showing excellent results and minimal adverse events (43, 48, 49).
Adalimumab is another high affinity and specific fully human monoclonal antibody against TNF that can block the interaction of TNF with its cell-surface receptors. Adalimumab is a well-established treatment of psoriatic arthritis (37) and moderate-to-severe plaque psoriasis (50). Due to its late arrival on the market, only the previously mentioned multicenter, retrospective study (27) and several case reports have focused on its efficacy in EP patients. One example is a report on a patient with EP presenting in the setting combination pegylated interferon alpha-2a ribavirin therapy for hepatitis-C responded who responded well to adalimumab (51).
Other anti-TNF agents such as golimumab, another fully human anti-TNF monoclonal antibody approved by the FDA in 2009 for treatment of rheumatoid arthritis, ulcerative colitis, psoriatic arthritis, and ankylosing spondylitis (52), have also been successfully used to treat EP in one case report (53).
Certolizumab Pegol (CZP) is a PEGylated anti-TNF biologic approved for the treatment of psoriatic arthritis and plaque psoriasis (54, 55). A phase 2/3, prospective, randomized study to assess the efficacy and safety of CZP in 127 Japanese participants with moderate to severe plaque psoriasis, generalized pustular psoriasis, or EP, recently completed (Clinicaltrials.gov, NCT03051217), and results for EP patients are awaited.
However, given the risk of bacteremia and sepsis in EP (20), it is important to note that anti-TNF agents can increased the risk of opportunistic infections and malignancy (56). In one EP patient, a lethal bacterial endocarditis was reported following infliximab treatment (56).
2.2. Anti-IL-17 agents
IL-17A, a pro-inflammatory cytokine secreted by Th17 cells and innate lymphoid cells (ILC) 3 (57), has been implicated as one of the central cytokines in the pathogenesis of psoriasis (58). To date, two anti-IL-17A agents, secukinumab and ixekizumab, as well as an IL-17R antagonist, brodalumab, have been approved for treatment of psoriasis. All three have been reported to be useful for treating EP.
Secukinumab is a fully human anti-IL-17A monoclonal antibody approved for the treatment of moderate-to-severe plaque psoriasis (59). Then secukinumab has been shown in several case reports to induce long-term remission in EP (23, 60–63). Moreover, a multi-center, retrospective study on the efficacy of secukinumab enrolled 13 EP patients, and showed that 10/13 patients responded to secukinumab with clearance in 4 weeks and were then followed up to 52 weeks, concluding that secukinumab is a safe and effective therapeutic option for EP. Notably, the patients that failed to respond were actively using alcohol, smoking, or cannabis (64). Other reports, including a case report, on EP arising after discontinuation of topical steroid showed successful treatment with secukinumab (65). Interestingly, a small case series of 10 EP patients, showed the suboptimal response to secukinumab compared to plaque psoriasis, but it was noted that the majority of the EP patients had history of prior failure to biologics (66).
Moreover, an open-label study in Japanese verified the efficacy and safety of ixekizumab, as demonstrated by all patients achieved PASI75 response by week 12 (67). Longitudinal studies of these patients revealed that most of the satisfactory clinical responses were maintained over 52 weeks (68, 69). A report of a Caucasian patient with EP demonstrated similar results with clearance noted after 6 weeks of ixekizumab, and maintenance of response through week 24 (70). Other studies assessing the long-term (>3 years) efficacy and safety of ixekizumab in EP have also reported a quick onset and sustained improvement (69, 71). Finally, a case series demonstrated rapid response to ixekizumab in patients with secukinumab-refractory EP (72).
Other IL-17 directed agents such as brodalumab have also been explored as a therapeutic option for EP. Brodalumab is a human anti-IL-17 receptor A (IL-17RA) monoclonal antibody that inhibits the biological activity of IL-17A, IL-17F, along with IL-17C and IL-17E (IL-25). It was approved for treatment of all subtypes of psoriasis in Japan (73). In an open-label, multi-center, long-term phase III study, subcutaneous brodalumab significantly improved the symptoms of 18 (100%) Japanese EP patients throughout the 52 weeks observation period (74).
2.3. Anti-IL-12/23 agents
IL-23 is considered to be a critical cytokine for the expansion and maintenance of Th17 cells, through binding to the IL-23R, activation of Janus kinase (JAK)2/Tyrosine kinase (TYK)2-Signal transducer and activator of transcription (STAT)3 and NF-κB pathways, and upregulation of RORγt transcripts and subsequent IL-17A production (75). IL-23 is a heterodimeric cytokine composed of the p19 and the p40 subunit (76), therefore it can be targeted through its unique p19 subunit, or through targeting of the p40 subunit, which it shares with IL-12. Drugs targeting IL-23 have shown enormous therapeutic potential in psoriasis (77).
Ustekinumab is a fully human monoclonal antibody that binds to the common p40 subunit shared between IL-12 and IL-23, thus is effective at neutralizing both IL-12 and IL-23. Ustekinumab is approved by FDA for the treatment of moderate-to-severe plaque psoriasis and psoriatic arthritis (78, 79). Several multicenter retrospective studies and case reports have validated the efficacy of ustekinumab as monotherapy in treatment a total of 46 EP patients, including recalcitrant cases, with rapid clearance and sustained remission (80–84). For instance, an Italian multicenter retrospective analysis of 22 EP patients revealed that ustekinumab provided a rapid clinically significant response in most patients after 4 weeks, which could be maintained for 60 weeks or more (80). Furthermore, EP patients that have failed anti-TNF agents have shown responses to ustekinumab (81–83, 85–88). Notably, in EP patients who carry CARD14 gene mutations and tend to be relatively treatment refractory have been shown to respond well to ustekinumab (17). Compared with the anti-TNF agents, ustekinumab is well tolerated in the treatment of EP, with a relatively low risk of infection.
Guselkumab, a p19-directed anti-IL-23-specific biologic approved for the treatment of psoriasis in 2017, demonstrated robust efficacy in 11 Japanese patients with EP during a 52-week, phase 3, multicenter, open-label study, in which 10 EP patients achieved clinically significant responses at week 16 and improved the quality of life through the observed 52 weeks, suggesting guselkumab may have a long term efficacy in the prevention of EP recurrence (89). Another report was on a Caucasian male with EP who was successfully treated with guselkumab, reaching PASI100 after 20 weeks of treatment and was still maintaining this response at week 48 (90).
Risankizumab, another humanized IgG monoclonal antibody that targets the p19 subunit of IL-23, has been approved for the treatment of moderate-to-severe plaque psoriasis (91). An ongoing phase 3 clinical trial in Japan aims to evaluate the efficacy and safety of risankizumab in Japanese patients with plaque psoriasis, generalized pustular psoriasis, or EP (Clinicaltrials.gov, NCT0322045).
Lastly, compared to other biologics, anti-IL-12/IL-23 inhibitors have been associated with a reduced risk of serious infections in biologically naïve patients with plaque psoriasis or psoriatic arthritis. Interestingly, in biologically experienced individuals no difference in infection risk was seen across different biologics (92). Whether this also extends to EP remains to be seen.
2.4. Other biologic treatments
Several targeted therapeutics have been reported to have efficacy in EP. For example, alefacept is a CD2-directed lymphocyte function-associated antigen 3 (LFA-3)/Fc fusion protein, which can block the LFA-3/CD2 interaction, thus interfering with lymphocyte activation. It was the first biologic agent approved by the FDA for moderate-to-severe chronic plaque psoriasis in 2003 (93), and has been successfully used in a patient with EP and palmoplantar psoriasis (94). This drug was discontinued in 2011.
Efalizumab is a humanized monoclonal IgG1 antibody against CD11a, one of the subunits of LFA-1. One case report explored the successful use of efalizumab in one EP patient (95), but the previously mentioned multicenter, retrospective study demonstrated that no clinical benefit was observed in the two treated EP patients (27). Notably, EP was reported to develop either during efalizumab therapy or with discontinuation (96). Moreover, efalizumab was associated with development of progressive multifocal leukoencephalopathy, a deadly complication of treatment (97, 98) and is currently not in clinical use after being withdrawn in 2009.
Previous reports have demonstrated that epidermal growth factor receptor (EGFR) ligands and vascular endothelial growth factor (VEGF) contributed to the development of psoriasis (99). Panitumumab, an anti-EGFR antibody, showed remarkable therapeutic effect on skin symptoms in one EP patient, who was receiving the drug for rectal cancer metastases (100). In addition, one case report has suggested the use of low-dose naltrexone (LDN) as an alternative treatment for treating EP (101).
2.5. Apremilast
Apremilast is a small molecule inhibitor of phosphodiesterase 4 (PDE4), which has broad anti-inflammatory effects including reducing expression of adhesion molecules, and down-regulating the expression of multiple pro-inflammatory cytokines in psoriasis, including TNF, IL-17, and IL-23 (102, 103). Apremilast was approved by FDA in 2014 for plaque psoriasis and psoriatic arthritis (104). Several case reports have shown that apremilast may be an effective option for EP patients with comparatively minor adverse events (105–107). For instance, one EP patient with high PASI score and several comorbidities was treated with cyclosporine, methotrexate, and adalimumab before, but responded to apremilast with total clearance of skin lesions after only 20 days (105). It has therefore been suggested by some studies that apremilast can be considered as a first-line option for the treatment of EP. However, one patient discontinued its use because of apremilast-induced atrial fibrillation after initial response (107).
3. Conclusion
EP is a rare and severe clinical form of psoriasis. While the pathogenesis of EP is incompletely understood, it shares TNF and IL-17A inflammatory pathways with plaque psoriasis. Due to its rarity and lack of well-designed clinical studies, EP treatment guidelines have not been updated in recent years (2). The numerous EP case reports that have been published need to be interpreted with caution given publication bias towards positive results, which is a problem for rare diseases that can spontaneously fluctuate in activity. However, it appears that a preponderance of the evidence supports the use of various biologic and systemic therapies as first line treatments for EP (108). Selection of which agent to use should be based on the patient comorbidities, clinical scenario, and disease severity. Physicians may need to balance their selection based on the need to obtain a rapid clinical response, versus avoiding more immunosuppressive agents given the high frequency of bacteremia and sepsis in EP. The limited studies and case reports available indicate that options to treat this rare subtype of EP are rapidly expanding, but given the lack of larger studies and head-to-head trials, there is incomplete evidence available to recommend one therapy over the other. Therefore, there is an urgent need for a better understanding of EP pathophysiology and high-quality clinical studies with extended follow-ups periods that can provide greater insights into this form of psoriasis and determine the optimal treatment approach.
Key Points.
-
1.
Erythrodermic psoriasis is a rare and severe form of psoriasis of unknown etiology, associated with various comorbidities.
-
2.
Most biological drugs that are approved for moderate to severe plaque psoriasis have also been tried in EP patients and show some advantages, but data from randomized clinical trials are non-existent.
-
3.
More research on pathogenesis of EP and its therapeutic management is needed for determination of optimal treatment of these patients.
Acknowledgments
No external support was used for the writing of this manuscript. JEG received research grants from AbbVie, AnaptysBio, Pfizer, Novartis, Celgene, and Eli Lilly, and serves as advisory board in Novartis, AbbVie, Eli Lilly, MiRagen, and Almirall.
Footnotes
Conflict of Interest
JG has received research support from AnaptysBio, AbbVie, Novartis, and Almirall. He has served on the consultant/advisory board of AbbVie, Novartis, Arthem Therapeutics. Other authors have no declared conflicts.
References
- 1.Yamada M, Kubo H, Kobayashi S, Ishizawa K, He M, Suzuki T, et al. The increase in surface CXCR4 expression on lung extravascular neutrophils and its effects on neutrophils during endotoxin-induced lung injury. Cellular & molecular immunology. 2011;8(4):305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbach M, Hsu S, Korman NJ, Lebwohl MG, Young M, Bebo BF Jr., et al. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62(4):655–62. [DOI] [PubMed] [Google Scholar]
- 3.Green MS, Prystowsky JH, Cohen SR, Cohen JI, Lebwohl MG. Infectious complications of erythrodermic psoriasis. J Am Acad Dermatol. 1996;34(5 Pt 2):911–4. [DOI] [PubMed] [Google Scholar]
- 4.Cox NH, Gordon PM, Dodd H. Generalized pustular and erythrodermic psoriasis associated with bupropion treatment. Br J Dermatol. 2002;146(6):1061–3. [DOI] [PubMed] [Google Scholar]
- 5.Oda T, Sawada Y, Yamaguchi T, Ohmori S, Omoto D, Haruyama S, et al. Psoriatic Erythroderma Caused by Terbinafine: A Possible Pathogenetic Role for IL-23. J Investig Allergol Clin Immunol. 2017;27(1):63–4. [DOI] [PubMed] [Google Scholar]
- 6.Evans AV, Parker JC, Russell-Jones R. Erythrodermic psoriasis precipitated by radiologic contrast media. J Am Acad Dermatol. 2002;46(6):960–1. [DOI] [PubMed] [Google Scholar]
- 7.Kamaria M, Liao W, Koo JY. How Long Does the Benefit of Biologics Last? An Update on Time To Relapse and Potential for Rebound of Biologic Agents for Psoriasis. Psoriasis forum. 2010;16(2):36–42. [PMC free article] [PubMed] [Google Scholar]
- 8.Potter KA, Motaparthi K, Schoch JJ. Erythrodermic psoriasis after discontinuation of ixekizumab. JAAD Case Rep. 2018;4(1):22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdallah MA, Abdel-Hamid MF, Kotb AM, Mabrouk EA. Serum interferon-gamma is a psoriasis severity and prognostic marker. Cutis. 2009;84(3):163–8. [PubMed] [Google Scholar]
- 10.Bressan AL, Picciani BLS, Azulay-Abulafia L, Fausto-Silva AK, Almeida PN, Cunha KSG, et al. Evaluation of ICAM-1 expression and vascular changes in the skin of patients with plaque, pustular, and erythrodermic psoriasis. International journal of dermatology. 2018;57(2):209–16. [DOI] [PubMed] [Google Scholar]
- 11.Li LF, Sujan SA, Yang H, Wang WH. Serum immunoglobulins in psoriatic erythroderma. Clin Exp Dermatol. 2005;30(2):125–7. [DOI] [PubMed] [Google Scholar]
- 12.Deeva I, Mariani S, De Luca C, Pacifico V, Leoni L, Raskovic D, et al. Wide-spectrum profile of inflammatory mediators in the plasma and scales of patients with psoriatic disease. Cytokine. 2010;49(2):163–70. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P, Chen HX, Duan YQ, Wang WZ, Zhang TZ, Li JW, et al. Analysis of Th1/Th2 response pattern for erythrodermic psoriasis. J Huazhong Univ Sci Technolog Med Sci. 2014;34(4):596–601. [DOI] [PubMed] [Google Scholar]
- 14.Tracey EH, Elston C, Feasel P, Piliang M, Michael M, Vij A. Erythrodermic presentation of psoriasis in a patient treated with dupilumab. JAAD Case Rep. 2018;4(7):708–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing X, Liang Y, Sarkar MK, Wolterink L, Swindell WR, Voorhees JJ, et al. IL-17 Responses Are the Dominant Inflammatory Signal Linking Inverse, Erythrodermic, and Chronic Plaque Psoriasis. J Invest Dermatol. 2016;136(12):2498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moy AP, Murali M, Kroshinsky D, Duncan LM, Nazarian RM. Immunologic Overlap of Helper T-Cell Subtypes 17 and 22 in Erythrodermic Psoriasis and Atopic Dermatitis. JAMA Dermatol. 2015;151(7):753–60. [DOI] [PubMed] [Google Scholar]
- 17.Signa S, Campione E, Rusmini M, Chiesa S, Grossi A, Omenetti A, et al. Whole exome sequencing approach to childhood onset familial erythrodermic psoriasis unravels a novel mutation of CARD14 requiring unusual high doses of ustekinumab. Pediatr Rheumatol Online J. 2019;17(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berki DM, Liu L, Choon SE, David Burden A, Griffiths CEM, Navarini AA, et al. Activating CARD14 Mutations Are Associated with Generalized Pustular Psoriasis but Rarely Account for Familial Recurrence in Psoriasis Vulgaris. J Invest Dermatol. 2015;135(12):2964–70. [DOI] [PubMed] [Google Scholar]
- 19.Johnston A, Xing X, Wolterink L, Barnes DH, Yin Z, Reingold L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140(1):109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh RK, Lee KM, Ucmak D, Brodsky M, Atanelov Z, Farahnik B, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruzzese V, Pepe J. Efficacy of cyclosporine in the treatment of a case of infliximab-induced erythrodermic psoriasis. Int J Immunopathol Pharmacol. 2009;22(1):235–8. [DOI] [PubMed] [Google Scholar]
- 22.Stinco G, Errichetti E. Erythrodermic psoriasis: current and future role of biologicals. BioDrugs. 2015;29(2):91–101. [DOI] [PubMed] [Google Scholar]
- 23.Galluzzo M, D’Adamio S, Campione E, Mazzilli S, Bianchi L, Talamonti M. A clinical case of severe disease burden: an erythrodermic psoriatic patient treated with secukinumab. J Dermatolog Treat. 2018:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J Immunol. 2018;201(6):1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong J, Goldenberg G. New biologics in psoriasis: an update on IL-23 and IL-17 inhibitors. Cutis. 2017;99(2):123–7. [PubMed] [Google Scholar]
- 27.Viguier M, Pages C, Aubin F, Delaporte E, Descamps V, Lok C, et al. Efficacy and safety of biologics in erythrodermic psoriasis: a multicentre, retrospective study. Br J Dermatol. 2012;167(2):417–23. [DOI] [PubMed] [Google Scholar]
- 28.Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181(7):4733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lew W, Bowcock AM, Krueger JG. Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and “Type 1” inflammatory gene expression. Trends Immunol. 2004;25(6):295–305. [DOI] [PubMed] [Google Scholar]
- 30.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harden JL, Johnson-Huang LM, Chamian MF, Lee E, Pearce T, Leonardi CL, et al. Humanized anti-IFN-gamma (HuZAF) in the treatment of psoriasis. J Allergy Clin Immunol. 2015;135(2):553–6. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez Carazo JL, Mahiques Santos L, Oliver Martinez V. Safety of etanercept in psoriasis: a critical review. Drug Saf. 2006;29(8):675–85. [DOI] [PubMed] [Google Scholar]
- 33.Esposito M, Mazzotta A, de Felice C, Papoutsaki M, Chimenti S. Treatment of erythrodermic psoriasis with etanercept. Br J Dermatol. 2006;155(1):156–9. [DOI] [PubMed] [Google Scholar]
- 34.O’Quinn RP, Miller JL. The effectiveness of tumor necrosis factor alpha antibody (infliximab) in treating recalcitrant psoriasis: a report of 2 cases. Arch Dermatol. 2002;138(5):644–8. [DOI] [PubMed] [Google Scholar]
- 35.Fraga NA, Paim Mde F, Follador I, Ramos AN, Rego VR. Refractory erythrodermic psoriasis in a child with an excellent outcome by using etanercept. An Bras Dermatol. 2011;86(4 Suppl 1):S144–7. [DOI] [PubMed] [Google Scholar]
- 36.Pique-Duran E, Perez-Cejudo JA. [Psoriatic erythroderma treated with etanercept]. Actas Dermosifiliogr. 2007;98(7):508–10. [PubMed] [Google Scholar]
- 37.Cordoro KM, Feldman SR. TNF-alpha inhibitors in dermatology. Skin Therapy Lett. 2007;12(7):4–6. [PubMed] [Google Scholar]
- 38.Oh CJ, Das KM, Gottlieb AB. Treatment with anti-tumor necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J Am Acad Dermatol. 2000;42(5 Pt 1):829–30. [DOI] [PubMed] [Google Scholar]
- 39.Rongioletti F, Borenstein M, Kirsner R, Kerdel F. Erythrodermic, recalcitrant psoriasis: clinical resolution with infliximab. J Dermatolog Treat. 2003;14(4):222–5. [DOI] [PubMed] [Google Scholar]
- 40.Torii H, Terui T, Matsukawa M, Takesaki K, Ohtsuki M, Nakagawa H, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: Results from the prospective post-marketing surveillance. J Dermatol. 2016;43(7):767–78. [DOI] [PubMed] [Google Scholar]
- 41.Poulalhon N, Begon E, Lebbe C, Liote F, Lahfa M, Bengoufa D, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156(2):329–36. [DOI] [PubMed] [Google Scholar]
- 42.Lewis TG, Tuchinda C, Lim HW, Wong HK. Life-threatening pustular and erythrodermic psoriasis responding to infliximab. J Drugs Dermatol. 2006;5(6):546–8. [PubMed] [Google Scholar]
- 43.Takahashi MD, Castro LG, Romiti R. Infliximab, as sole or combined therapy, induces rapid clearing of erythrodermic psoriasis. Br J Dermatol. 2007;157(4):828–31. [DOI] [PubMed] [Google Scholar]
- 44.Fiehn C, Andrassy K. Case number 29: hitting three with one strike: rapid improvement of psoriatic arthritis, psoriatic erythroderma, and secondary renal amyloidosis by treatment with infliximab (Remicade). Ann Rheum Dis. 2004;63(3):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tridico LA, Antonio JR, Mathias CE, Pozetti EMO. Effectiveness and safety of infliximab for 11 years in a patient with erythrodermic psoriasis and psoriatic arthritis. An Bras Dermatol. 2017;92(5):743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yip L, Harrison S, Foley P. From biologic to biologic to biologic: lessons to learn for erythrodermic and recalcitrant chronic plaque psoriasis. Australas J Dermatol. 2008;49(3):152–5. [DOI] [PubMed] [Google Scholar]
- 47.Romero-Mate A, Garcia-Donoso C, Martinez-Moran C, Hernandez-Nunez A, Borbujo J. Long-term management of erythrodermic psoriasis with anti-TNF agents. Dermatol Online J. 2010;16(6):15. [PubMed] [Google Scholar]
- 48.Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84(3):247–8. [PubMed] [Google Scholar]
- 49.Heikkila H, Ranki A, Cajanus S, Karvonen SL. Infliximab combined with methotrexate as long-term treatment for erythrodermic psoriasis. Arch Dermatol. 2005;141(12):1607–10. [DOI] [PubMed] [Google Scholar]
- 50.Wu JJ, Valdecantos WC. Adalimumab in Chronic Plaque Psoriasis: A Clinical Guide. J Drugs Dermatol. 2017;16(8):779–90. [PubMed] [Google Scholar]
- 51.Richetta AG, Maiani E, Carlomagno V, Carboni V, Mattozzi C, Giancristoforo S, et al. Treatment of erythrodermic psoriasis in HCV+ patient with adalimumab. Dermatol Ther. 2009;22 Suppl 1:S16–8. [DOI] [PubMed] [Google Scholar]
- 52.Boyce EG, Halilovic J, Stan-Ugbene O. Golimumab: Review of the efficacy and tolerability of a recently approved tumor necrosis factor-alpha inhibitor. Clin Ther. 2010;32(10):1681–703. [DOI] [PubMed] [Google Scholar]
- 53.Lee WK, Kim GW, Cho HH, Kim WJ, Mun JH, Song M, et al. Erythrodermic Psoriasis Treated with Golimumab: A Case Report. Ann Dermatol. 2015;27(4):446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campanati A, Benfaremo D, Luchetti MM, Ganzetti G, Gabrielli A, Offidani A. Certolizumab pegol for the treatment of psoriasis. Expert opinion on biological therapy. 2017;17(3):387–94. [DOI] [PubMed] [Google Scholar]
- 55.Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamata M, Tada Y. Safety of biologics in psoriasis. J Dermatol. 2018;45(3):279–86. [DOI] [PubMed] [Google Scholar]
- 57.Brembilla NC, Senra L, Boehncke WH. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front Immunol. 2018;9:1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, Cameron GS, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130(1):145–54 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38. [DOI] [PubMed] [Google Scholar]
- 60.Dogra S, Bishnoi A, Narang T, Handa S. Long-term remission induced by secukinumab in a 13-year-old boy having recalcitrant chronic erythrodermic psoriasis. Dermatol Ther. 2018;31(4):e12611. [DOI] [PubMed] [Google Scholar]
- 61.Mugheddu C, Atzori L, Lappi A, Pau M, Murgia S, Rongioletti F. Successful Secukinumab treatment of generalized pustular psoriasis and erythrodermic psoriasis. J Eur Acad Dermatol Venereol. 2017;31(9):e420–e1. [DOI] [PubMed] [Google Scholar]
- 62.Tichy M Arthropathic psoriasis complicated by a paradoxical reaction in the form of erythrodermic psoriasis following adalimumab and by an allergic reaction following infliximab which was successfully managed with secukinumab. Postepy Dermatol Alergol. 2019;36(4):495–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mateu-Puchades A, Santos-Alarcon S, Martorell-Calatayud A, Pujol-Marco C, Sanchez-Carazo JL. Erythrodermic psoriasis and secukinumab: Our clinical experience. Dermatol Ther. 2018;31(4):e12607. [DOI] [PubMed] [Google Scholar]
- 64.Damiani G, Pacifico A, Russo F, Pigatto PDM, Bragazzi NL, Bonifati C, et al. Use of Secukinumab in a Cohort of Erythrodermic Psoriatic Patients: A Pilot Study. J Clin Med. 2019;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shibata T, Muto J, Takama H, Yanagishita T, Ito T, Watanabe D. Case of psoriatic erythroderma induced by the discontinuation of the chronic use of topical steroid after dialysis initiation and successfully treated with secukinumab. J Dermatol. 2019;46(4):e119–e20. [DOI] [PubMed] [Google Scholar]
- 66.Weng HJ, Wang TS, Tsai TF. Clinical experience of secukinumab in the treatment of erythrodermic psoriasis: a case series. Br J Dermatol. 2018;178(6):1439–40. [DOI] [PubMed] [Google Scholar]
- 67.Saeki H, Nakagawa H, Ishii T, Morisaki Y, Aoki T, Berclaz PY, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29(6):1148–55. [DOI] [PubMed] [Google Scholar]
- 68.Saeki H, Nakagawa H, Nakajo K, Ishii T, Morisaki Y, Aoki T, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: Results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44(4):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okubo Y, Mabuchi T, Iwatsuki K, Elmaraghy H, Torisu-Itakura H, Morisaki Y, et al. Long-term efficacy and safety of ixekizumab in Japanese patients with erythrodermic or generalized pustular psoriasis: subgroup analyses of an open-label, phase 3 study (UNCOVER-J). J Eur Acad Dermatol Venereol. 2019;33(2):325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Megna M, Gallo L, Balato N, Balato A. A case of erythrodermic psoriasis successfully treated with ixekizumab. Dermatol Ther. 2019;32(2):e12825. [DOI] [PubMed] [Google Scholar]
- 71.Egawa G, Honda T, Kabashima K. Long-term efficacy of ixekizumab in erythrodermic and generalized pustular psoriasis patients. J Eur Acad Dermatol Venereol. 2019;33(2):259. [DOI] [PubMed] [Google Scholar]
- 72.Lo Y, Tsai TF. Clinical experience of ixekizumab in the treatment of patients with history of chronic erythrodermic psoriasis who failed secukinumab: a case series. Br J Dermatol. 2019;181(5):1106–7. [DOI] [PubMed] [Google Scholar]
- 73.Greig SL. Brodalumab: First Global Approval. Drugs. 2016;76(14):1403–12. [DOI] [PubMed] [Google Scholar]
- 74.Yamasaki K, Nakagawa H, Kubo Y, Ootaki K, Japanese Brodalumab Study G. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176(3):741–51. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki E, Mellins ED, Gershwin ME, Nestle FO, Adamopoulos IE. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13(4–5):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Girolomoni G, Strohal R, Puig L, Bachelez H, Barker J, Boehncke WH, et al. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(10):1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–28. [DOI] [PubMed] [Google Scholar]
- 79.McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780–9. [DOI] [PubMed] [Google Scholar]
- 80.Pescitelli L, Dini V, Gisondi P, Loconsole F, Piaserico S, Piccirillo A, et al. Erythrodermic psoriasis treated with ustekinumab: an Italian multicenter retrospective analysis. J Dermatol Sci. 2015;78(2):149–51. [DOI] [PubMed] [Google Scholar]
- 81.Kim YS, Kim HJ, Lee S, Park YL. Erythrodermic Psoriasis Improved by Ustekinumab: A Report of Two Cases. Ann Dermatol. 2016;28(1):121–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koutsoukou XA, Papadavid E, Theodoropoulos K, Rigopoulos D. Ustekinumab in severe complicated erythrodermic psoriasis: rapid clearing, safety, and sustained remission. Dermatol Ther. 2014;27(5):257–9. [DOI] [PubMed] [Google Scholar]
- 83.Wang TS, Tsai TF. Clinical experience of ustekinumab in the treatment of erythrodermic psoriasis: a case series. J Dermatol. 2011;38(11):1096–9. [DOI] [PubMed] [Google Scholar]
- 84.Stinco G, Piccirillo A, Errichetti E, Bergamo S, Patrone P. Treatment of recalcitrant erythrodermic psoriasis with ustekinumab. Eur J Dermatol. 2014;24(3):387–90. [DOI] [PubMed] [Google Scholar]
- 85.Saraceno R, Talamonti M, Galluzzo M, Chiricozzi A, Costanzo A, Chimenti S. Ustekinumab treatment of erythrodermic psoriasis occurring after physical stress: a report of two cases. Case Rep Dermatol. 2013;5(3):254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Santos-Juanes J, Coto-Segura P, Mas-Vidal A, Galache Osuna C. Ustekinumab induces rapid clearing of erythrodermic psoriasis after failure of antitumour necrosis factor therapies. Br J Dermatol. 2010;162(5):1144–6. [DOI] [PubMed] [Google Scholar]
- 87.Castineiras I, Fernandez-Diaz L, Juarez Y, Lueiro M. Sustained efficacy of ustekinumab in refractory erythrodermic psoriasis after failure of antitumor necrosis factor therapies. J Dermatol. 2012;39(8):730–1. [DOI] [PubMed] [Google Scholar]
- 88.Buggiani G, D’Erme AM, Krysenka A, Pescitelli L, Lotti T, Prignano F. Efficacy of ustekinumab in sub-erythrodermic psoriasis: when TNF-blockers fail. Dermatol Ther. 2012;25(3):283–5. [DOI] [PubMed] [Google Scholar]
- 89.Sano S, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: Efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45(5):529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Megna M, Ruggiero A, Camela E, Fabbrocini G, Marasca C. A case of erythrodermic psoriasis successfully treated with guselkumab. Dermatol Ther. 2020:e13238. [DOI] [PubMed] [Google Scholar]
- 91.McKeage K, Duggan S. Risankizumab: First Global Approval. Drugs. 2019;79(8):893–900. [DOI] [PubMed] [Google Scholar]
- 92.Li X, Andersen KM, Chang HY, Curtis JR, Alexander GC. Comparative risk of serious infections among real-world users of biologics for psoriasis or psoriatic arthritis. Ann Rheum Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wong VK, Lebwohl M. The use of alefacept in the treatment of psoriasis. Skin Therapy Lett. 2003;8(6):1–2, 7. [PubMed] [Google Scholar]
- 94.Prossick TA, Belsito DV. Alefacept in the treatment of recalcitrant palmoplantar and erythrodermic psoriasis. Cutis. 2006;78(3):178–80. [PubMed] [Google Scholar]
- 95.Piccirillo F, Stinco G, Patrone P. Erythrodermic psoriasis successfully treated with efalizumab. Eur J Dermatol. 2008;18(3):357–8. [DOI] [PubMed] [Google Scholar]
- 96.Hernandez Garcia I Severe erythroderma in a patient under intermittent therapy with efalizumab. J Drugs Dermatol. 2008;7(10):987–9. [PubMed] [Google Scholar]
- 97.Schwab N, Ulzheimer JC, Fox RJ, Schneider-Hohendorf T, Kieseier BC, Monoranu CM, et al. Fatal PML associated with efalizumab therapy: insights into integrin alphaLbeta2 in JC virus control. Neurology. 2012;78(7):458–67; discussion 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kothary N, Diak IL, Brinker A, Bezabeh S, Avigan M, Dal Pan G. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65(3):546–51. [DOI] [PubMed] [Google Scholar]
- 99.Anderson KS, Petersson S, Wong J, Shubbar E, Lokko NN, Carlstrom M, et al. Elevation of serum epidermal growth factor and interleukin 1 receptor antagonist in active psoriasis vulgaris. Br J Dermatol. 2010;163(5):1085–9. [DOI] [PubMed] [Google Scholar]
- 100.Nishizawa A, Satoh T, Yokozeki H. Erythrodermic psoriasis improved by panitumumab, but not bevacizumab. Acta Derm Venereol. 2012;92(4):360–1. [DOI] [PubMed] [Google Scholar]
- 101.Beltran Monasterio EP. Low-dose Naltrexone: An Alternative Treatment for Erythrodermic Psoriasis. Cureus. 2019;11(1):e3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficac y of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380(9843):738–46. [DOI] [PubMed] [Google Scholar]
- 103.Ratner M First PDE4 inhibitor for psoriasis hits the market but impact is uncertain. Nat Biotechnol. 2014;32(6):505–7. [DOI] [PubMed] [Google Scholar]
- 104.Otezla Fala L. (Apremilast), an Oral PDE-4 Inhibitor, Receives FDA Approval for the Treatment of Patients with Active Psoriatic Arthritis and Plaque Psoriasis. Am Health Drug Benefits. 2015;8(Spec Feature):105–10. [PMC free article] [PubMed] [Google Scholar]
- 105.Papadavid E, Kokkalis G, Polyderas G, Theodoropoulos K, Rigopoulos D. Rapid clearance of erythrodermic psoriasis with apremilast. J Dermatol Case Rep. 2017;11(2):29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krishnamoorthy G, Kotecha A, Pimentel J. Complete resolution of erythrodermic psoriasis with first-line apremilast monotherapy. BMJ Case Rep. 2019;12 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arcilla J, Joe D, Kim J, Kim Y, Truong VN, Jaipaul N. Erythrodermic Psoriasis Treated with Apremilast. Dermatol Reports. 2016;8(1):6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reynolds KA, Pithadia DJ, Lee EB, Liao W, Wu JJ. A systematic review of treatment strategies for erythrodermic psoriasis. J Dermatolog Treat. 2019:1–7. [DOI] [PubMed] [Google Scholar]
- 109.Groves RW, Kapahi P, Barker JN, Haskard DO, MacDonald DM. Detection of circulating adhesion molecules in erythrodermic skin disease. J Am Acad Dermatol. 1995;32(1):32–6. [DOI] [PubMed] [Google Scholar]
- 110.Errichetti E, Piccirillo A. Latent tuberculosis reactivation in a patient with erythrodermic psoriasis under treatment with ustekinumab and a low dose steroid, despite isoniazid chemoprophylaxis. Eur J Dermatol. 2014;24(4):508–9. [DOI] [PubMed] [Google Scholar]


