Abstract
Propofol is a clinically important intravenous anesthetic. We previously reported that it directly inhibited 5-lipoxygenase (5-LOX), a key enzyme for leukotriene biosynthesis. Because the hydroxyl group in propofol (propofol 1-hydroxyl) is critical for its anesthetic effect, we examined if its presence would be inevitable for 5-lipoxygenase recognition. Fropofol is developed by substituting the hydroxy group in propofol with fluorine. We found that propofol 1-hydroxyl was important for 5-lipoxygenase recognition, but it was not absolutely necessary. Azi-fropofol bound to 5-LOX at one of the two propofol binding sites of 5-LOX (pocket around Phe-187), suggesting that propofol 1-hydroxyl is important for 5-LOX inhibition at the other propofol binding site (pocket around Val-431). Interestingly, 5-hydroperoxyeicosatetraenoic acid (5-HpETE) production was significantly increased by stimulation with calcium ionophore A23187 in HEK293 cells expressing 5-LOX, suggesting that the fropofol binding site is important for the conversion from 5-HpETE to leukotriene A4. We also indicated that propofol 1-hydroxyl might have contributed to interaction with wider targets among our body.
Keywords: Propofol, Fropofol, Propofol 1-Hydroxyl, 5-lipoxygenase
Introduction
Propofol (2,6-diisopropylphenol) is the most widely used intravenous drug for general anesthesia and targets γ-aminobutyric acid type A (GABAA) receptor [1, 2]. It also possesses non-anesthetic targets [3, 4]. We previously reported that propofol directly inhibited 5-lipoxygenase (5-LOX), a critical enzyme for leukotriene production in neutrophils and macrophages. 5-LOX converts arachidonic acid (AA) to 5-hydroperoxyeicosatetraenoic acid (5-HpETE) by oxidization, and then 5-HpETE to leukotriene A4 (LTA4) by dehydration (Fig. 1A). Then, LTA4 is converted to LTB4 by leukotriene A4 hydrolase (LTA4H) and to LTC4 by leukotriene C4 synthase (LTC4S). LTC4 is then converted to LTD4 and LTE4 by peptidases [5]. 5-LOX consists of the C2-like domain (residue 1–112) and the catalytic domain (residue 126–673), the latter of which possesses the active site [6]. Our previous investigation demonstrated that propofol directly bound to 5-LOX at two different pockets near the active site [7].
Figure 1. The effect of propofol and fropofol on 5-lipoxygenase derivative production in HEK cells system.
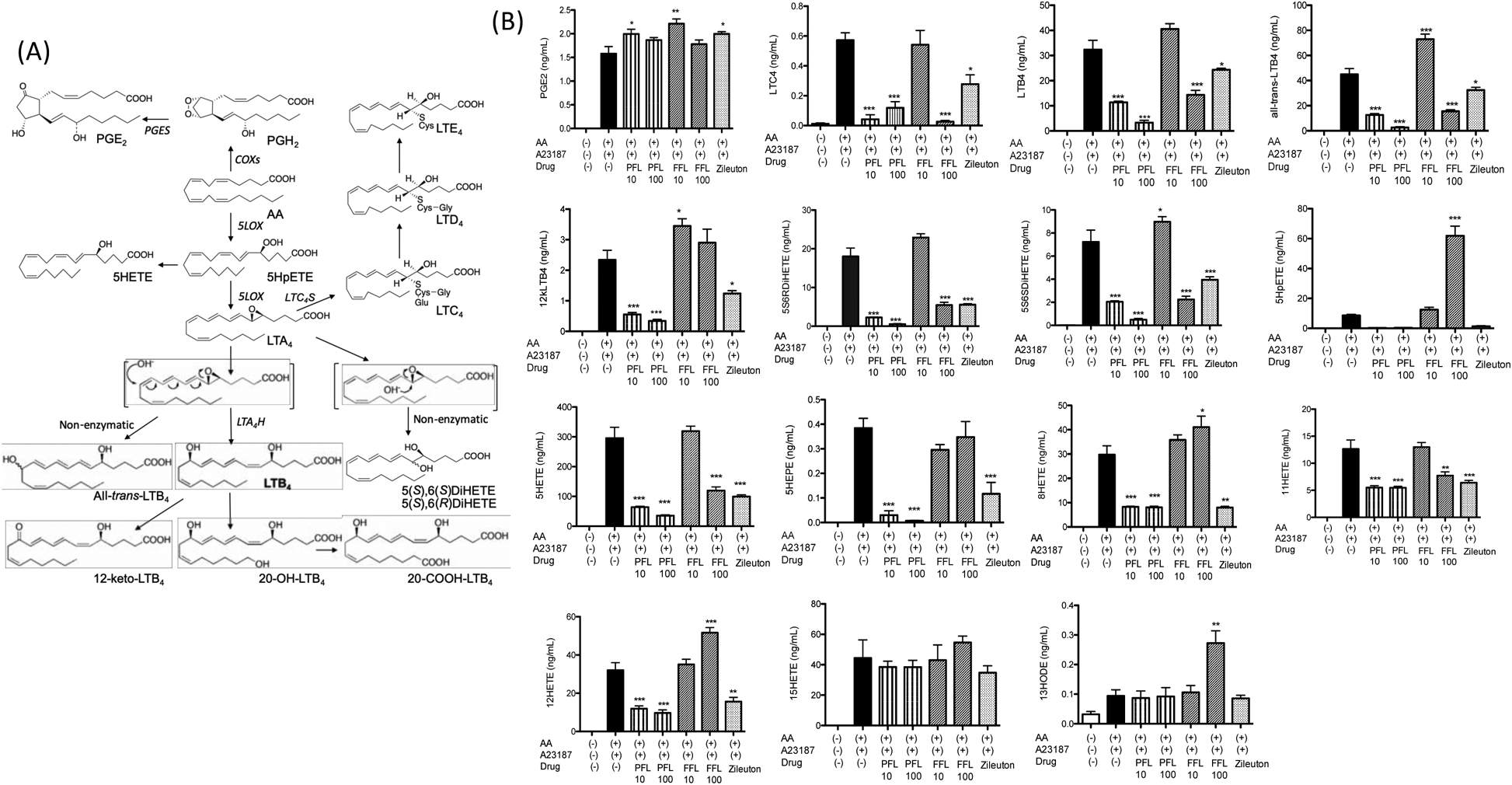
(A) Diagram of 5-lipoxygenase derivatives.
(B) HEK cells were transfected with 5-lipoxygenase wild-type plasmids. Cells were stimulated with A23187 in the presence of AA as described in the Method. Propofol (PFL) 10 μM and 100 μM, fropofol (FFL) 10 μM and 100 μM were tested. Data were shown as mean +/− S.D. of 4 replicates. Two independent experiments were performed. For statistical analysis, one-way ANOVA with Bonferroni post hoc analysis was used. *, ** and *** denote p< 0.05, p< 0.01 and p< 0.001, respectively.
The hydroxyl group in propofol (propofol 1-hydroxyl) plays an indispensable role in the recognition of GABAA receptor via forming a hydrogen bond. Fropofol (2-fluoro-1,3-diisopropylbenzene), a compound developed by substituting the hydroxyl group in propofol with fluorine, no longer possesses anesthetic activity [8]. To test if the propofol 1-hydoxyl plays a significant role in the recognition of non-anesthetic targets, we studied the effect of fropofol on 5-LOX function under the hypothesis that propofol 1-hydoxyl would be necessary for the recognition of 5-LOX. Although fropofol bound to one (Pocket 1; pocket near Phe-187) of the two propofol binding pockets (Pocket 1, Pocket 2-pocket near Val-431) on 5-LOX, it attenuated 5-LOX function significantly less compared with propofol, indicating that the propofol 1-hydroxyl is crucial to bind to pocket 2, but not indispensable to bind to pocket 1.
Material and Methods
Production of 5-LOX related AA derivatives by human embryonic kidney (HEK) cells transfected with 5-LOX
The pcDNA3.1_5-LOX wild-type (WT) plasmid was kindly provided by Dr. Dieter Steinhilber (University of Frankfurt, Frankfurt, Germany). HEK cells (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI1640/10% fetal bovine serum at 37°C in 5% CO2. The plasmid was transiently transfected into HEK cells using Lipofectamine 3000 (Thermo Fischer Scientific, Waltham, MA, USA) per the company’s protocol. Transfected HEK cells were suspended in PGC buffer (PBS, 0.1% glucose, 1 mM CaCl2) and stimulated with 2 μM of calcium ionophore A23187 (Sigma-Aldrich, St. Louis, MO, USA) and 3 μM AA in the presence or absence of propofol (10 μM, 100 μM) and fropofol (10 μM, 100 μM) at 37°C for 10 min. The reaction was stopped with equal volume of methanol, and samples were stored at −80°C until use. Eicosanoids were quantitated as below.
Measurement of eicosanoids
The reversed-phase mass spectrometry (MS)-based quantitation technique for eicosanoids was used as we previously described [7]. Briefly, the samples were diluted with 2 mL of methanol and 7 mL of water containing 0.1% formic acid, with a mixture of deuterium-labeled eicosanoids as an internal standard, and then loaded on an Oasis HLB cartridge (Waters, Milford, MA, USA). The column was washed with 1 mL of water, 1 mL of 15% methanol, and 1 mL of petroleum ether and then eluted with 0.2 mL of methanol containing 0.1% formic acid. Eicosanoids were quantitated by reverse-phase high performance liquid chromatography (HPLC)-electrospray ionization-tandem mass spectrometry (MS/MS).
Photolabeling of 5-LOX using azi-fropofol
Stable 5-LOX expression plasmid was kindly provided by Dr. Marcia Newcomer (Louisiana State University, Louisiana, LA, USA). The expression of stable 5-LOX was performed as previously described [9]. Photolabeling of stable 5-LOX protein using azi-fropofol was performed. Azi-fropofol is a fropofol photoaffinity probe that contains the photoactivatable group. Azi-fropofol (final concentration 25 μM) was equilibrated with stable 5-LOX protein (1 mg/mL) in a reaction volume of 300 μL for 10 min and then exposed to 350-nm light under a Rayonet RPR-3500 Lamp (Southern New England Ultraviolet Co., Branford, CT, USA). The protein was separated on an SDS-polyacrylamide gel and stained with Coomassie G-250. The protein gel band was excised for LC-MS/MS. After trypsin digestion, samples were injected into a nano-LC column with online electrospray into a LTQ linear ion trap (Thermo Fischer Scientific). Raw data were acquired with Xcalibur (Thermo Fischer Scientific), and Sequest (Scripps Research Institute, La Jolla, CA, USA) was used to search b and y ions against the sequence of 5-LOX for an azi-fropofol mass modification on any amino acid of every peptide. To confirm specificity of azi-fropofol binding, competition was done by photolabeling 5-LOX with azi-fropofol in the presence of 100 μM of fropofol.
Rigid docking of propofol and fropofol onto 5-LOX
Using the reported structure of stable 5-LOX protein (Protein Data Bank ID number: 3O8Y), we performed a rigid docking simulation of propofol and fropofol on 5-LOX in the vicinity of the adducted residues in the photolabeling experiments. Adducted residues with azi-propofol (azi-Pm) were previously reported [7]. The docking program Glide (Schrodinger, Cambridge, MA, USA) was used, and the calculations were done on an SBGrid workstation. Docking grid (12 × 12 × 12 cubic angstroms) was centered to the adducted residues, and the XP docking protocol with default settings was applied without any additional positional constraints. Docked configurations with the highest affinity based on the Glide score was chosen.
Mutagenesis
Alanine scanning mutagenesis was performed using QuikChange mutagenesis kit (Agilent Technologies; Santa Clara, CA, USA) as we previously described [7] and sequencing was confirmed.
Prediction of 5-LOX mutant structure
The structure of 5-LOX single amino acid mutants was predicted using Iterative threading assembly refinement (I-TASSER) [10].
Production of 5-LOX related AA derivatives in human whole blood
Peripheral blood was obtained from healthy donors in a tube containing heparin. Heparinized blood was preincubated with lipopolysaccharide (LPS; final concentration 1 μg/mL) for 30 min at 37°C and then stimulated with N-formylmethionine-leucyl-phenylalanine (fMLP) (final concentration 0.1 mM) for 37°C as we previously reported [11]. Some samples were exposed to propofol or fropofol. The reaction was immediately stopped on ice. Samples were immediately centrifuged at 200 × g for 5 min. Serum was collected for eicosanoid analysis.
Statistical analysis
Data were statistically analyzed as indicated in the corresponding figure legends. Statistical analyses were performed with PRISM 5 software (GraphPad Software, La Jolla, CA, USA). Statistical significance was defined as P < 0.05.
Results
Propofol attenuated the production of both 5-HpETE and LTB4, while fropofol increased 5-HpETE.
We previously showed that propofol blocked 5-LOX (IC50 of 1.37 μM) in HEK cells transfected with 5-LOX [7]. Here we tested the effect of propofol and fropofol on the production of 5-LOX derivatives at two different doses (10 μM and 100 μM). Both concentrations would be high enough for propofol to block the production of 5-LOX derivatives. 5-LOX derivatives are illustrated in Fig. 1A. As predicted, propofol significantly attenuated the production of LTB4, all-trans-LTB4, LTC4, 5-hydroxyeicosatetraenoid acid (5-HETE), 5(S),6(R)-dihydroxy-eicosatetraenoic acid (DiHETE) and 5(S),6(S)-DiHETE at both concentrations (Fig. 1B). Zileuton (N-(1-benzo-[b]-thien-2-ylethyl)-N-hydroxyurea) (10 μM) was included as an example of 5-LOX inhibitor.
Fropofol at 10 μM did not affect the production of 5-LOX derivatives at all, which was in line with our hypothesis. However, at 100 μM, fropofol attenuated the production of LTB4, all-trans-LTB4, LTC4, 5-HETE, 5(S),6(R)-DiHETE and 5(S),6(S)-DiHETE, suggesting that fropofol could affect 5-LOX function. Unexpectedly, 5-HpETE level was significantly increased in the presence of 100 μM fropofol. These results might indicate that fropofol impaired the 5-LOX function to convert 5-HpETE to LTA4, but not the one to convert AA to 5-HpETE. It is unclear why the level of 5-HpETE significantly increased, but 5-HETE level was less in samples treated with fropofol. Because peroxidase reduces 5-HpETE to 5-HETE [12], it is possible that fropofol might have also attenuated peroxidase activity. In fact, the reduction of peroxidase activity by propofol has been reported [13].
Zileuton is not a highly specific 5-LOX inhibitor [14], as it attenuated the production of 8-HETE and 12-HETE, mediated by 8-lipoxynase and 12-lipoxygenase, respectively, and 11-HETE mediated by cyclooxygenases. Propofol also inhibited the production of 8-HETE, 11-HETE and 12-HETE. Fropofol blocked 11-HETE production, but this did not block 8-HETE or 12-HETE.
Fropofol bound to 5-LOX at one of the propofol binding pockets
To test the hypothesis that fropofol would directly bind to 5-LOX, we performed photolabeling experiments using photoactivatable fropofol (azi-fropofol) (Fig. 2A). Because fropofol did not affect 5-LOX function at 10 μM, we decided to use 25 μM of azi-fropofol. Competition assay was done using 100 μM of fropofol to determine the specificity of photoactivatable fropofol binding to 5-LOX. In our previous propofol photolabeling experiment, we found that Phe-187 and Valine-431 adducted with photoactivatable propofol azi-Pm [7]. Photoactivatable fropofol adducted to Phe-187. No adduction was seen in the competition assay, indicating that the adducted residue was specific to fropofol. These results suggested the overlap of propofol and fropofol binding site (Fig. 2B). Placing the adducted residue in a docking center, we performed rigid docking stimulation of propofol and fropofol on 5-LOX (Fig. 2C). Glide score is the scoring system that predicts the affinity between a ligand and a receptor, and a docked model with the most negative value is considered as the model with the highest affinity between the ligand and the receptor. Here, we called binding pockets near Phe-187 and Valine-433 as pocket 1 and pocket 2, respectively. The binding of propofol and fropofol to the pocket 1 was shown in Fig. 2D. We defined amino acid residues within 4 angstroms from the docked propofol and fropofol as “nearby residues”. In pocket 1, nearby residues from propofol were Asn-185, Leu-186, Phe-187, Lys-211, Ile-212, Ser-213, Asn-214 and Trp-603 (Fig. 2C). Propofol 1-hydroxyl formed a hydrogen bond with Trp-603. Nearby residues from fropofol in pocket 1 were Asn-185, Leu-186, Phe-187, Lys-211, Ile-212, Ser-213, Asn-214, Tyr-556, and Trp-603. Hydrophobic interactions were responsible for its binding to 5-LOX. Based on Glide score, the affinity of fropofol to pocket 1 (score: −2.042) was weaker than that of propofol (score: −2.845) (Fig. 2D). The hydrogen bond between propofol and 5-LOX at the pocket 1 likely contributed to a stronger affinity between propofol and 5-LOX. Likewise, propofol 1-hydroxyl formed a hydrogen bond with Glu-415 in pocket 2. Propofol was predicted to bind to 5-LOX at both pockets with the similar affinity (pocket 1-score: −2.845, pocket 2-score: −2.899).
Figure 2. Photolabeling experiments of 5-lipoxygenase using azi-fropofol.
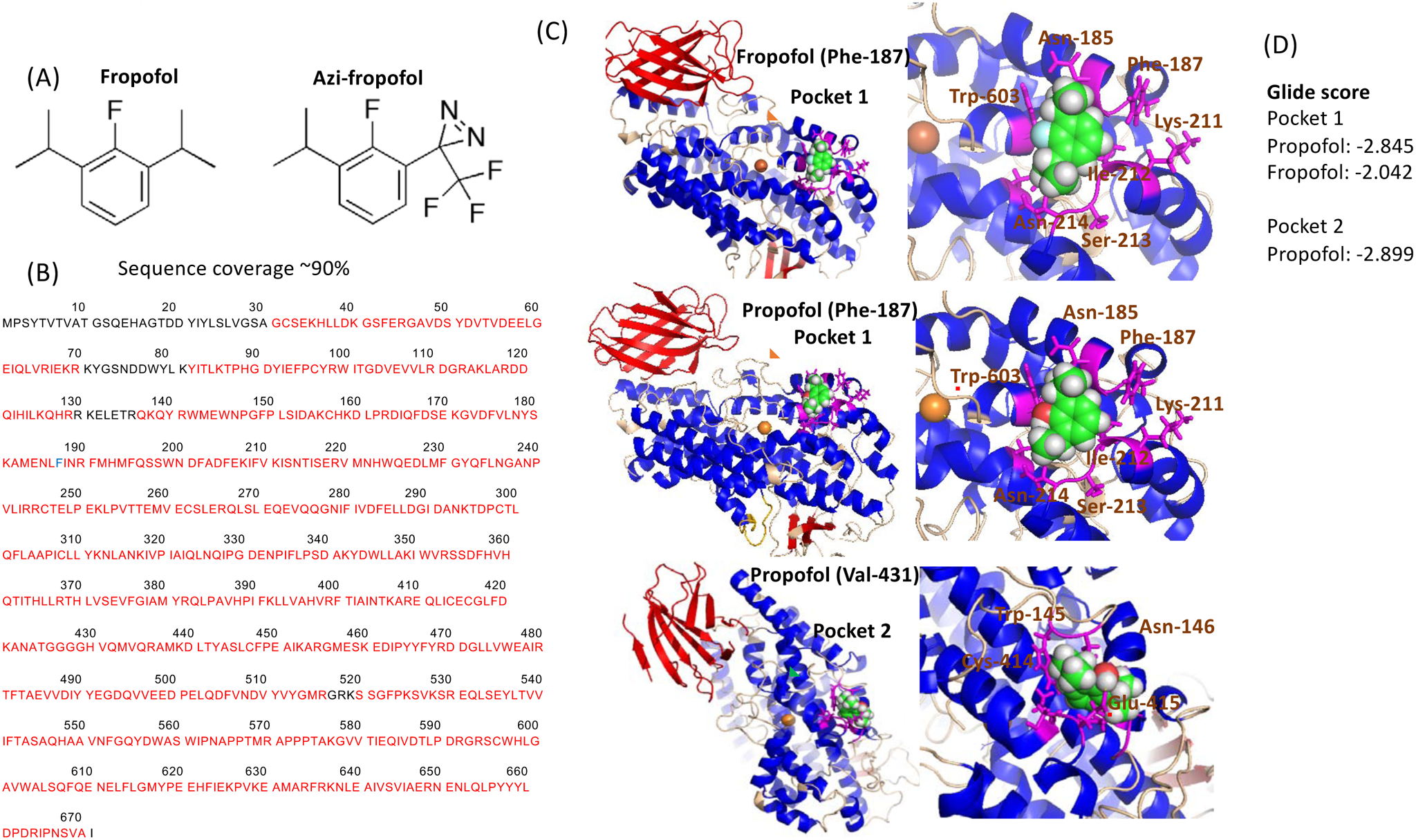
(A) Structure of fropofol and azi-fropofol.
(B) Photolabeling experiment was done as described in the Method. The adducted residue was shown in blue. Residues sequence-covered by mass spectrometry were shown in red.
(C) Rigid docking of propofol and fropofol. Blow-out images of docked sites were shown on the right. Red, blue and wheat colors showed beta-sheet, helix and loop, respectively. Gold sphere showed iron. Residues within 4 angstroms from the docked propofol or fropofol were shown in magenta. Red underlines indicate residues involved in hydrogen bond with propofol.
(D) Glide scores were shown.
Mutagenesis of residues at pocket 1 affected the conversion of 5-HpETE to LTB4
To understand the role of pocket 1 in 5-HpETE and LTB4 production, we examined the impact of 5-LOX mutants of residues at pocket 1 on the production of 5-LOX derivatives. The mutants did not affect 5-HpETE production, but LTB4 production was significantly diminished or enhanced in some of the mutants (Fig. 3A), indicating the possibility that pocket 1 would be more important for the conversion of 5-HpETE to LTA4 (i.e. LTB4). Next, we examined the predicted structure of our 5-LOX single amino acid mutants. Although the role of the α2 helix of 5-LOX (residue 175–191) in oxidization and dehydration has been not studied, it is known to be the critical area for 5-LOX function [15]. A part of residues consisting of the α2 helix was changed into a loop with the mutation of Lys-211 to alanine (Fig. 3B). Similarly, the mutation of Ser-214 to alanine also affected the structure of the α2 helix (Fig. 3B). Asn-185 and Leu-186 were located at the α2 helix (Fig. 3B). In summary, N185A, L186A, K211A, and N214A mutants affected the structure of the α2 helix, and they primarily affected LTB4 level, but not 5-HpETE level. This may indicate that the α2 helix plays a significant role in the conversion of 5-HpETE to LTA4. Further study is needed to validate this statement.
Figure 3. 5-lipoxygenase activity of mutants at pocket 1.
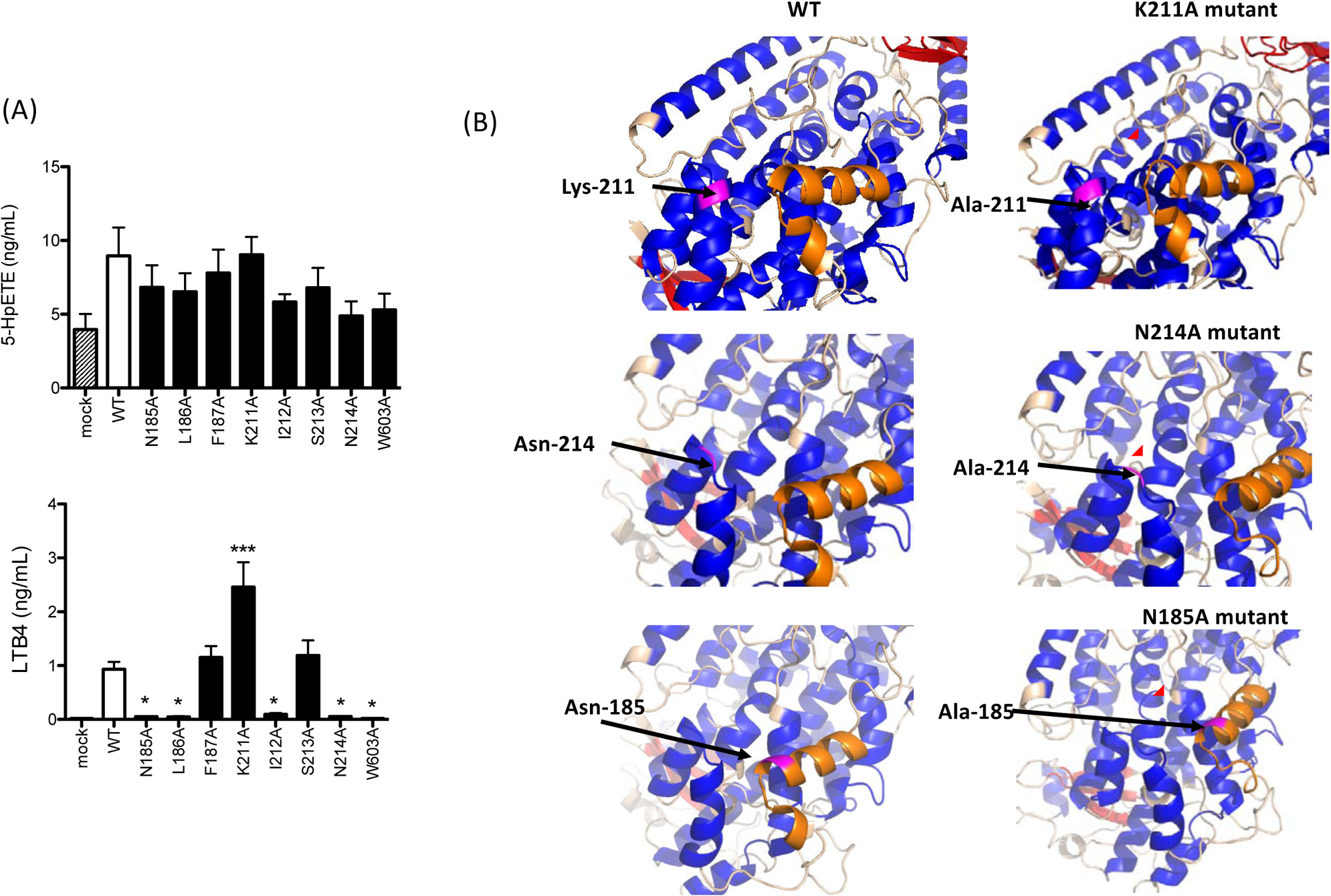
(A) Alanine mutants were transiently transfected into HEK cells as described in the Method. 5-lipoxygenase activity of alanine mutants was measured. Data were shown as mean +/− S.D. of 4 replicates. Two independent experiments were performed. Statistical analysis was performed using one-way ANOVA with Bonferroni post hoc analysis. * and *** denote p< 0.05 and p< 0.001, respectively.
(B) Predicted structures of alanine mutants were shown. Magenta showed residues to be mutated or mutated. Orange showed the α2 helix. Red circle highlighted the structural change of the α2 helix.
Fropofol attenuated the production of 5-LOX derivatives to a lesser extent than propofol in human whole blood
So far we have studied the effect of fropofol using 5-LOX overexpression system in HEK cells in the presence of excess arachidonic acids. HEK cells have limited expression of LTA4 hydroxylase. To test the effect of fropofol in a more physiological condition, we examined the production of 5-LOX derivatives in human whole blood. Fropofol at 100 μM attenuated LTB4, 20-COOH-LTB4, 20-OH-LTB4, and 5-HETE production to the same extent with propofol 10 μM (Fig. 4), which was consistent with our findings in our HEK assay system. Interestingly, in this whole blood assay, 5-HpETE production was not detected in any of the conditions. 11-HETE and 12-HETE production was not affected in whole blood by both propofol and fropofol, which was different from HEK assay system.
Figure 4. 5-lipoxygenase activity of whole blood under propofol and fropofol.
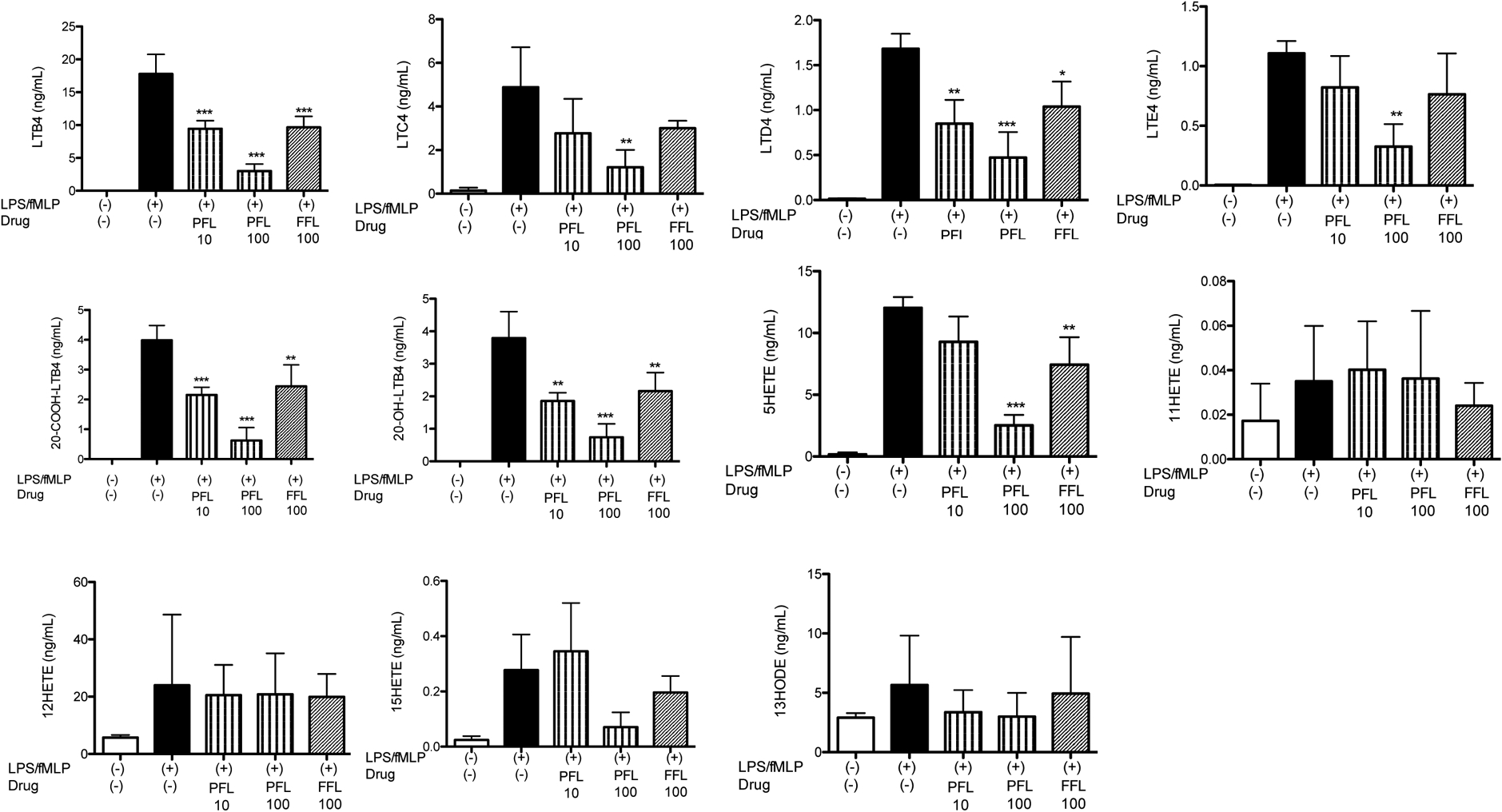
Whole blood was stimulated with LPS and fMLP in the presence or absence of propofol (PFL; 10, 100 μM) or fropofol (FFL; 100 μM). Data were shown as mean +/− S.D. of 4 replicates. Two independent studies were performed. Statistical analysis was performed using one-way ANOVA with Bonferroni post hoc analysis. *, **, and *** denote p< 0.05, p< 0.01, and p< 0.001, respectively.
Discussion
We have demonstrated that propofol 1-hydroxyl played a significant role to recognize and inhibit 5-LOX using fropopfol, a propofol derivative devoid of its hydroxyl group. Different from its role on GABAA receptor recognition, the presence of propofol 1-hydroxyl was not indispensable in 5-LOX recognition because fropofol also interacted with 5-LOX. The interaction between propofol and 5-LOX involved a hydrogen bond, which strengthened the effect on 5-LOX, indicating the importance of propofol 1-hydroxyl for protein recognition. The weaker effect of fropofol on 5-LOX function over propofol was demonstrated in both HEK cell system and human whole blood.
This study illustrated the importance of propofol 1-hydroxyl in 5-LOX recognition by propofol. The similarity between propofol structure and vitamin E has been described [16]. In line, vitamin E metabolites, long-chain ω-carboxylates also containing hydroxy groups, also inhibited 5-LOX activity [17]. Our study also suggested that the presence of propofol 1-hydroxyl might be responsible for many interactions between propofol and non-anesthetic related targets. Propofol attenuated the production of 8-HETE, 11-HETE, and 12-HETE. The absence of propofol 1-hydroxyl obliterated the effect on 8-HETE and 12-HETE production. We should also note that zileuton attenuated the production of 8-HETE, 11-HETE and 12-HETE. Zileuton is widely used as a 5-LOX inhibitor, but it is not purely specific to 5-LOX [14], which was also supported by our findings here. Zileuton possesses a hydroxyl group and it is interesting to study in the future if the hydroxyl group is responsible for the effects of zileuton on additional targets. This is an important investigation to develop a 5-LOX specific inhibitor, particularly because 5-LOX is involved in a number of diseases. From anesthetic standpoint, the presence of propofol 1-hydroxyl was necessary for propofol to have an anesthetic effect [8]. However, our study suggested the possibility that its presence also enhanced its chance to interact with various non-anesthesia related proteins. Propofol has been used in a number of clinical settings including induction and maintenance of anesthesia as well as sedation in intensive care units. However, propofol also has side-effects. For example, propofol infusion syndrome is a condition caused by propofol infusion, leading into cardiac failure, rhabdomyolysis, metabolic acidosis. This can be fatal. The interaction of propofol with mitochondria is postulated [18, 19], and it is certainly possible that propofol interacts with a subset of proteins in the mitochondria. Given the importance of propofol 1-hydroxyl in protein recognition, it is critical to determine if the hydroxyl group is involved in this adverse reaction.
In our study using HEK assay system, we observed a significant increase in 5-HpETE levels when fropofol 100 μM was used. 5-LOX is responsible for two step reactions consisting of the conversion from AA to 5-HpETE and then from 5-HpETE to LTA4 (Fig. 1A). Our study indicated that the fropofol binding site on 5-LOX might be more important in the latter conversion, but further study is needed.
In conclusion, we demonstrated that propofol 1-hydroxyl was important for 5-LOX recognition, although the absence still allowed the compound to weakly interact with 5-LOX. We also demonstrated that the presence of propofol 1-hydroxyl might allow propofol to interact with other molecules, lessening its specificity.
Highlights.
Propofol 1-hydroxyl is not essential, but important for 5-lipoxygenase recognition
Propofol 1-hydroxyl helps propofol to recognize many proteins
The fropofol binding site could be important for 5-HpETE to LTA4 conversion
Financial Support:
This work was in part supported by CHMC Anesthesia Foundation (K.Y.), National Institute of Health R01GM118277 (K.Y.), P01GM55896 (R.G.E.) and MEXT/JSPS KAKENHI Grant Numbers, 16K08596, 19K07357 (T.O.), 15H05904, 15H04708, 18H02627 (T.Y.).
Abbreviation:
- GABAA
γ-aminobutyric acid type A
- 5-LOX
5-lipoxygenase
- AA
Arachidonic acid
- 5-HpETE
5-hydroperoxyeicosatetraenoic acid
- LTA4H
Leukotriene A4 hydrolase
- LTC4S
Leukotriene C4 synthase
- WT
Wild-type
- LPS
Lipopolysaccharide
- fMLP
N-formylmethionine-leucyl-phenylalanine
- HPLC
High performance liquid chromatography
- MS
Mass spectrometry
- azi-Pm
Azi-propofol
- I-TASSER
Iterative threading assembly refinement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Orser BA, Wang LY, Pennefather PS, MacDonald JF. Propofol modulates activation and desensitization of GABAA receptors in cultured murine hippocampal neurons. J Neurosci. 1994;14(12):7747–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeller A, Arras M, Lazaris A, Jurd R, Rudolph U. Distinct molecular targets for the central respiratory and cardiac actions of the general anesthetics etomidate and propofol. FASEB J. 2005;19(12):1677–9. doi: 10.1096/fj.04-3443fje. [DOI] [PubMed] [Google Scholar]
- 3.Weiser BP, Eckenhoff RG. Propofol inhibits SIRT2 deacetylase through a conformation-specific, allosteric site. J Biol Chem. 2015;290(13):8559–68. doi: 10.1074/jbc.M114.620732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng T, Bu W, Ren X, Chen X, Yu J, Eckenhoff RG, et al. Molecular mechanism of anesthetic-induced depression of myocardial contraction. FASEB J. 2016;30(8):2915–25. doi: 10.1096/fj.201600290RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jo-Watanabe A, Okuno T, Yokomizo T. The Role of Leukotrienes as Potential Therapeutic Targets in Allergic Disorders. Int J Mol Sci. 2019;20(14). doi: 10.3390/ijms20143580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim Biophys Acta. 2015;1851(4):331–9. doi: 10.1016/j.bbalip.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Okuno T, Koutsogiannaki S, Ohba M, Chamberlain M, Bu W, Lin FY, et al. Intravenous anesthetic propofol binds to 5-lipoxygenase and attenuates leukotriene B4 production. FASEB J. 2017;31(4):1584–94. doi: 10.1096/fj.201601095R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woll KA, Weiser BP, Liang Q, Meng T, McKinstry-Wu A, Pinch B, et al. Role for the propofol hydroxyl in anesthetic protein target molecular recognition. ACS Chem Neurosci. 2015;6(6):927–35. doi: 10.1021/acschemneuro.5b00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert NC, Bartlett SG, Waight MT, Neau DB, Boeglin WE, Brash AR, et al. The structure of human 5-lipoxygenase. Science. 2011;331(6014):217–9. doi: 10.1126/science.1197203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okuno T, Koutsogiannaki S, Hou L, Bu W, Ohto U, Eckenhoff RG, et al. Volatile anesthetics isoflurane and sevoflurane directly target and attenuate Toll-like receptor 4 system. FASEB J. 2019;33(12):14528–41. doi: 10.1096/fj.201901570R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skoog MT, Nichols JS, Harrison BL, Wiseman JS. Glutathione peroxidase is neither required nor kinetically competent for conversion of 5-HPETE to 5-HETE in rat PMN lysates. Prostaglandins. 1986;31(3):577–93. [DOI] [PubMed] [Google Scholar]
- 13.De La Cruz JP, Zanca A, Carmona JA, de la Cuesta FS. The effect of propofol on oxidative stress in platelets from surgical patients. Anesth Analg. 1999;89(4):1050–5. [DOI] [PubMed] [Google Scholar]
- 14.Rossi A, Pergola C, Koeberle A, Hoffmann M, Dehm F, Bramanti P, et al. The 5-lipoxygenase inhibitor, zileuton, suppresses prostaglandin biosynthesis by inhibition of arachidonic acid release in macrophages. Br J Pharmacol. 2010;161(3):555–70. doi: 10.1111/j.1476-5381.2010.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra S, Bartlett SG, Newcomer ME. Identification of the Substrate Access Portal of 5- Lipoxygenase. Biochemistry 2015;54(41):6333–42. doi: 10.1021/acs.biochem.5b00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008;14(2):95–106. doi: 10.1111/j.1527-3458.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pein H, Ville A, Pace S, Temml V, Garscha U, Raasch M, et al. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nat Commun. 2018;9(1):3834. doi: 10.1038/s41467-018-06158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finsterer J, Frank M. Propofol Is Mitochondrion-Toxic and May Unmask a Mitochondrial Disorder. J Child Neurol. 2016;31(13):1489–94. doi: 10.1177/0883073816661458. [DOI] [PubMed] [Google Scholar]
- 19.Vollmer JP, Haen S, Wolburg H, Lehmann R, Steiner J, Reddersen S, et al. Propofol Related Infusion Syndrome: Ultrastructural Evidence for a Mitochondrial Disorder. Crit Care Med. 2018;46(1):e91–e4. doi: 10.1097/CCM.0000000000002802. [DOI] [PubMed] [Google Scholar]


