Abstract
Integrins are the major family of cell adhesion receptors in humans and essential for a wide range of normal physiology, including formation and maintenance of tissue structure integrity, cell migration, proliferation and differentiation. Integrins also play a prominent role in tumor growth and metastasis. Translational research has tried to define the contribution of integrins to the phenotypic aggressiveness of melanoma because such knowledge is clinically useful. For example, differential expression of integrins in primary cutaneous melanoma can be used to distinguish indolent from aggressive, prometastatic melanoma. Recent studies have shown that gene expression–based testing of patient-derived melanoma tissue is feasible and molecular tests may fully replace interventional surgical methods such as sentinel lymph node biopsies in the future. Because of their central role in mediating invasion and metastasis, integrins are likely to be useful biomarkers. Integrins are also attractive candidate targets for interventional therapy. This article focuses on the role of integrins in melanoma and highlights recent advances in the field of translational research.
Introduction
Metastasis is the major cause of death in patients with melanoma. Tumor metastasis is a sequential, multistep process resulting in the spread of tumor cells from the site of origin. This involves a complex interplay of cellular events, including loss of cell adhesion at the primary site, transmigration through the extracellular matrix (ECM) into the bloodstream, extravasation at the metastatic site, seeding, and proliferation. Translational research on melanoma aims to better understand the molecular events that culminate to cause metastasis. Integrins have been of interest to the field of melanoma molecular biology for some time. Their key functions in melanoma pathogenesis include but are not limited to cell adhesion, intracellular signaling, ECM remodeling, and cell migration, proliferation, survival, and differentiation; these factors mediate metastasis. They are also key molecules in predictive and prognostic testing and therapeutic interventional studies. This article focuses on the role of integrins in melanomagenesis.
Structure of Integrins
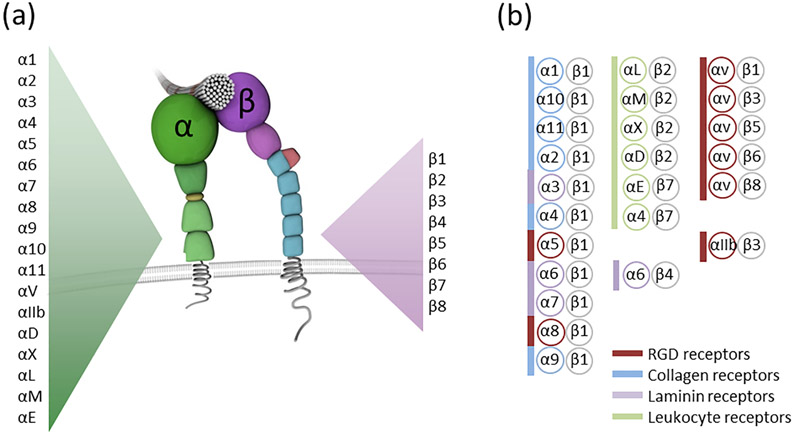
Integrins are heterodimeric proteins composed of noncovalently associated ɑ and β subunits. Different combinations of 18 ɑ and 8 β subunits make up the 24 integrin heterodimers encountered in mammals.1 The integrin structure is depicted as a molecule with a head and two tails.2 The head is the ligand-binding extracellular component made up of the ectodomains of the ɑ and β subunits; intracellular domains represent the legs anchoring to cytoskeletal proteins, with other domains traversing the transmembrane region in between (Fig. 1).3 When integrins bind extracellular ligands such as fibronectin their cytoplasmic domains recruit and locally enrich a myriad of cell surface receptors, scaffolding proteins and enzymes to form adhesion structures known as focal adhesions.4 Focal adhesions manipulate the extracellular space by transmitting cytoskeletal forces via integrins (inside-out signaling) while simultaneously translating external physicochemical cues into signaling that drives the transcription of secreted proteins (outside-in signaling; Fig. 2) such as those that inhibit tumor cytotoxic T cells.5,6 Integrins therefore function bidirectionally, meaning information can be transmitted from the outside environment to inside the cell and vice versa.7 This bidirectional signaling capability of integrins provides the cell with important information on its immediate extracellular environment and informs decisions on proliferation, apoptosis, or the remodeling of the ECM to facilitate metastasis.8,9
Figure 1.
Integrin Structure. (a) Integrins are transmembrane adhesion receptors consisting of an ɑ and β subunit. The mammalian genome encodes for 18 ɑ and eight β subunits. (b) ɑ and β subunits combine to form 24 distinct integrin heterodimers. These can be grouped based on ligand specificity and expression pattern. Some integrins such as the β2 and β7 integrins are only found on white blood cells (highlighted in green). Integrins which bind the tripeptide sequence Arg-Gly-Asp (RGD) which is encountered in extracellular matrix proteins like fibronectin and osteopontin as well as adhesion receptors such as cadherin 17 are highlighted in red. Collagen binding integrins are labeled in blue and laminin binding integrins such as the ɑ6β4 integrin are highlighted in purple.
Figure 2.
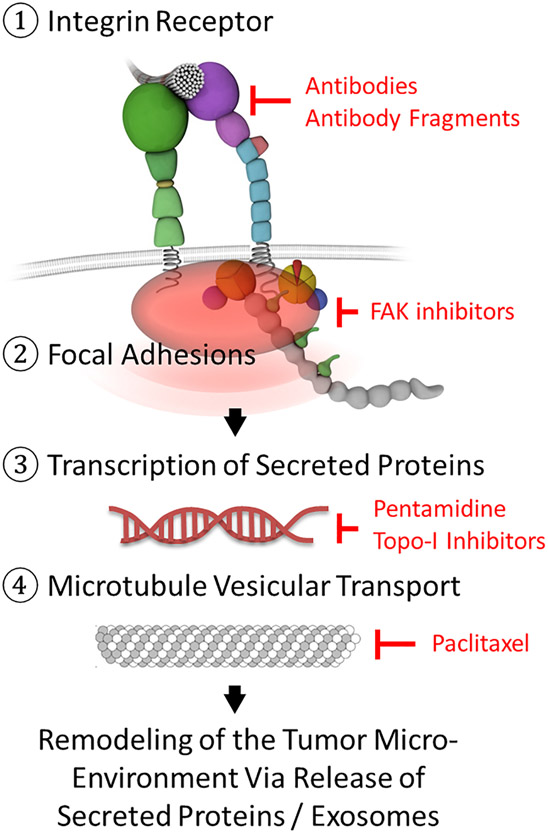
Targeting the integrin signaling and exocytosis pathway. Integrin function can be targeted at multiple levels by antibodies or small molecules (shown in red): at the level of the cell surface receptor (1), at the level of the adhesome, i.e. the multiprotein adhesion complex that links the integrin cytoplasmic tail to intracellular actin and contains many signaling proteins such as FAK (2), at the level of protein transcription (3) and at the level of microtubular vesicular transport (4). FAK, focal adhesion kinase; Topo-I, type I topoisomerase inhibitors.
Altered Integrin Expression in Melanoma
Multiple studies have shown altered expression of integrins in normal melanocytes compared with malignant melanocytes or benign nevi compared with malignant melanoma. In vivo and in vitro studies have noted a pathogenic role of this altered integrin expression (Table 1).10-27
Table 1.
Integrin expression in indolent versus aggressive melanocytes.
| Study | Integrin (Direction of Regulation in Aggressive Melanocytes) |
Material Studied |
Method | Findings |
|---|---|---|---|---|
| Danen et al10 | ɑ2β1 (↑) ɑ6β1 (↑) | Human cells | FACS, IHC | Expression of laminin receptor ɑ6β1 and laminin/collagen receptor ɑ2β1 was low on non-metastatic or poorly metastatic cell lines but strongly expressed on highly metastatic cell lines. |
| Etoh et al11 | ɑ2β1 (↑) | Human cells | FACS | Enhanced migration on laminin and type IV collagen of several human melanoma cell lines is largely mediated by ɑ2β1 integrin. |
| Yoshinaga et al12 | ɑ2β1 (↑) ɑ3β1 (↑) | Human cells | FACS | A metastatic melanoma (MM) cell line expressed markedly increased levels of the β1, ɑ2, and ɑ3 subunits, but not the ɑ6 subunit, compared with a primary melanoma (PM) cell line. MM and PM cell migration was significantly inhibited by function-blocking anti-β1 and anti-ɑ3 MAbs but not by the anti-ɑ6 MAb tested. In contrast, the anti-ɑ2 MAb significantly inhibited MM but not PM cell migration. |
| Natali et al13 | ɑ3β1 (↑) | Human cells and tissue | IHC | Increased ɑ3β1 expression correlates with the degree of dermal invasion in primary lesions and is detectable in 82% of metastatic foci but only weakly expressed in benign nevi. |
| Vizkeleti et al14 | ɑ3β1 (↑) ɑ4β1 (↑) ɑvβ8 (↑) | Human cells and tissue | qRT-PCR | Analysis of select integrins (ɑ2, ɑ3, ɑ4, ɑ9, β5, β8, ɑ6, β1, and β3) highlighted the possible importance of ɑ3β1, ɑ4β1 and ɑvβ8 in the metastatic process and in distinguishing regional and distant metastases. |
| Danen et al15 | ɑ5β1 (↑) ɑvβ3 (↑) ɑ6β4 (↓) | Human tissue | IHC | ɑ5β1 and ɑvβ3 integrin are exclusively expressed in melanoma but not in nevi; expression of ɑ6β4 integrin is decreased in melanoma. |
| Ziober et al16 | ɑ6β1 (↑) ɑ7β1 (↓) | Murine cells | FACS, Northern & Western Blots | The study showed that highly metastatic murine melanoma cells lose ɑ7β1 integrin expression and upregulate ɑ6β1 integrin. |
| Kramer et al17 | ɑ7β1 (↑) | Human and murine cells | Western Blots | Laminin binding of ɑ7β1 integrin was detected in melanoma cells but not in normal melanocytes. |
| Hieken et al18 | β1 (↑) | Human tissue | IHC | β1 integrin expression in primary cutaneous melanoma was associated with occult regional lymph node metastasis. |
| Hieken et al19 | β1 (↑) ɑvβ3 (↑) | Human tissue | IHC | Integrin β1 and ɑvβ3 expression in intermediate thickness primary cutaneous melanoma was associated with an increased likelihood of disease recurrence and decreased long term survival. |
| Nikkola et al20 | β1 (↑) | Human tissue | IHC | Elevated integrin β1 expression was associated with shorter DFS and increased anti-apoptotic protein Bcl-2 expression in metastatic melanoma patients. |
| Albelda et al21 | ɑvβ3 (↑) | Human tissue | IHC, Western Blot | ɑvβ3 expression exclusively restricted to invasive vertical growth phase melanoma cells and melanoma metastases. |
| Felding-Habermann22 | ɑvβ3 (↑) | Human cells | Western Blot | Lack of ɑvβ3 expression strongly inhibits tumorigenicity of human melanoma cells in mice. |
| Montgomery23 | ɑvβ3 (↑) | Human cells | Functional Assays | ɑvβ3 melanoma cells have a growth and survival advantage in collagen. |
| Hsu24 | ɑvβ3 (↑) | Human cells and tissue | FACS, IHC, Western Blot | ɑvβ3 associates with the progression from radial growth to vertical invasive growth in primary cutaneous melanoma. |
| Van Belle et al25 | ɑvβ3 (↑) | Human tissue | IHC | ɑvβ3 is mostly absent in nevi but expressed in nearly all melanoma metastases. ɑvβ3 associates with the progression from radial growth to vertical invasive growth in primary cutaneous melanoma. |
| Voura et al26 | ɑvβ3 (↑) | Human cells | Functional Assays | Interaction of ɑvβ3 on melanoma cells with the L1 Cell Adhesion Molecule on endothelial cells plays and important role in the transendothelial migration of melanoma cells. |
| Meves et al27 | ɑvβ3 (↑) | Human tissue | IHC, qRT-PCR, RNAseq | ɑvβ3 expression in thin and intermediate thickness primary cutaneous melanoma was associated with an increased likelihood of sentinel lymph node metastasis within 90 days of diagnosis. |
Abbreviations: FACS, Fluorescence-activated cell sorting; IHC, immunohistochemistry; MAb, monoclonal antibody; qRT-PCR, qualitative reverse transcriptase polymerase chain reaction; DFS, disease free survival; RNAseq, next-generation RNA sequencing.
Integrin Role in Primary Tumor Aggressiveness
In situ melanomas that exhibit the radial growth phase (RGP) have a better prognosis than melanomas that show the vertical growth phase (VGP).28 The depth of invasion by melanoma cells (Breslow depth) is a major determinant in patient prognosis, and the switch from RGP (indolent) to VGP (aggressive) is associated with poor prognosis. Multiple studies have shown that alteration in integrin expression is associated with this conversion. Herlyn et al24 showed conversion of RGP to VGP in melanoma cells by forced expression of integrin β3. Integrin β3 heterodimerizes with integrin ɑv (Fig 1), and increased expression of functional integrin ɑvβ3 in RGP cells was associated with invasive growth into the dermis, inhibition of apoptosis, and tumor growth. The role of integrin β3 in melanoma growth was also supported by another study by Herlyn et al25 in human melanoma biopsy tissue. In this study, expression was low to absent in melanomas in RGP, whereas it was high in melanomas with VGP and in metastasis.
In addition to their role in primary tumor growth, integrins are also important mediators of metastasis.29 They are involved in multiple steps that help in tumor spread: 1) degradation of the basement membrane barrier for tumor cells,30-36 2) angiogenesis for tumor survival at the primary site,37-40 3) as integral components of exosomes (small extracellular molecules detached from the primary tumor into the circulation),41-44 4) intravasation of tumor cells into the circulation,37 and 5) implantation at the metastatic niche.45,46
One study30 showed that integrins have an important role in overcoming the initial basement membrane barrier at the primary tumor site through matrix metalloproteinases (MMPs). The basement membrane and ECM are primarily composed of type IV collagen, type I collagen, and fibronectin.31 This barrier is degraded primarily by MMPs. It is not surprising that expression of MMP-1, MMP-2, and MMP-9 are increased in invasive melanoma phenotypes. The expression of these MMPs is regulated by integrins. Integrins directly bind to MMPs and stimulate their expression and function, which results in degradation of collagen and fibronectin. This degradation is critical for tumor cell invasion and progression. Multiple studies32-34 have shown the role of integrins, especially ɑ2β1, ɑ5β1, and ɑvβ3, in tumor invasion through their effects on MMPs. The degradation of collagen and fibronectin in the ECM exposes the Arg-Gly-Asp (RGD) tripeptide sequence of these proteins, which is a ligand for integrin, especially ɑvβ3. Binding of ɑvβ3 to the exposed RGD motif further stimulates MMP-2 expression, thereby perpetuating the cycle and promoting tumor invasion. Zeng et al35 noted that blocking integrins β1 and ɑvβ3 with antibodies resulted in decreased tumor cell adhesion and migration. MMP-9 has been shown to have a crucial role in melanoma metastasis. Sil et al36 noted in the highly metastatic murine B16F10 melanoma cell line that the integrin ɑ5β1-fibronectin interaction resulted in the expression of MMP-9. They also showed that blockage of ɑ5 integrin receptor reduces fibronectin stimulation of MMP-9 and its downstream effects. These studies reinforce again the crucial role of integrins in melanoma pathogenesis.
Integrins also are important for angiogenesis, providing both blood supply to the rapidly growing tumor cells and a pathway for hematogenous spread to distant organs.37 Melanoma tumor cells produce multiple growth factors, including fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), placental growth factor (PIGF), and transforming growth factors (TGF), among others. These growth factors stimulate angiogenesis to maintain blood supply to the rapidly dividing cells. Integrins mediate angiogenesis by interacting with the tumor-secreted growth factors, specifically by promoting endothelial cell migration and survival. Integrins may contribute to signal transduction that promotes angiogenesis. For example, integrin ɑvβ3 mediates angiogenesis induced by basic FGF (FGF-2) in vivo and integrin ɑvβ5 mediates angiogenesis induced by VEGF-A. Anti-ɑvβ3 antibodies block angiogenesis promoted by FGF, whereas anti-ɑvβ5 blocks VEGF-mediated angiogenesis.38 Given their major role in tumor angiogenesis, the ɑv integrins have been targeted in clinical trials of melanoma and other cancer types by antibodies and small molecules.39 In the future such therapies might work synergistically with immunotherapies to help recruit immune cells to metastatic tumors, including melanoma.40
Another interesting protein in the realm of ECM involved in melanoma pathogenesis is osteopontin (OPN). Osteopontin is a secreted glycophosphoprotein that has diverse roles in tumor metastasis, including cell adhesion, tumor cell proliferation, angiogenesis, and invasion.47 OPN contains the RGD motif, which is a major binding site for integrins. OPN binds to several integrins, including αvβ1, αvβ3, αvβ5, αvβ6, α4β1, α5β1, α8β1, and α9β1.48 One study49 has shown that experimental overexpression of OPN activated αvβ3 and αvβ5, and knockdown inactivated αvβ3 and αvβ5 in melanoma cell lines, thereby altering tumor aggressiveness. Given the difference in the expression of OPN in aggressive vs indolent melanoma, OPN has been considered as a biomarker in the gene expression–based risk stratification of melanoma.27,50
Although the biology and behavior of tumor cells at the primary site have been studied extensively, an interesting area of upcoming research focuses on the cellular events in the metastatic niche. As recently discussed by Huang and Rofstad45 it is now well known that the site of distant metastasis is not random. A complex interaction between the mediators released by the primary tumor and changes at the metastatic niche determine the site of metastasis. After implantation the trophic organ/site also exhibits changes that promote tumor cell survival and proliferation. Studies show that integrins are important for priming the premetastatic niche for metastasis. Kaplan et al46 studied the molecular changes involved at the trophic site in mouse models. They noted that injection of melanoma cells with high metastatic potential into mice resulted in a sequence of events that determined the metastatic site. The injection stimulated release of bone marrow–derived hematopoietic progenitor cells into the bloodstream. These cells were then mobilized to the premetastatic site and formed clusters there, where they prominently expressed VEGF receptor 1 and integrins ɑ4β7, ɑ4β1 (VLA-4), and ɑ6β4. Expression of ɑ4β1 integrin caused upregulation of fibronectin in the resident fibroblasts and induced MMP-promoted ECM degradation. These changes caused by integrins at the metastatic site create a favorable environment for the circulating tumor cells to implant and proliferate.
Integrins as Diagnostic Tools for Melanoma
Because of their altered expression in melanoma and their role in tumor behavior, integrins have become an important component in molecular-based studies for risk stratification and prognosis. Meves et al27,51 noted that addition of cell adhesion–linked gene expression variables to standard clinicopathologic variables increased the predictive ability to identify patients who present with sentinel lymph node (SLN) metastasis within 90 days of melanoma of their primary diagnosis. This was based on their extensive study of biopsy samples from patients with melanoma that showed differential expression of integrin β3 in addition to other ECM proteins in indolent versus aggressive melanoma. It is promising to note that these molecular-based tests help better risk-stratify patients, and they may obviate the need for SLN biopsy in the future as an invasive surgical staging procedure.27,50,52 It is not surprising that integrin molecules are included in some of these test kits given their significant role in melanoma aggressiveness. Although it is not the standard of care to include molecular testing in melanoma risk stratification, awareness of molecular-based testing among patients and clinicians is increasing.53 However, in addition to the added cost of these tests there continue to be questions about the clinical utility, especially if they are purely prognostic.54 There are no definite guidelines available to date to determine a treatment course on the basis of prognostic molecular testing and gene expression profiling is not recommended by the National Comprehensive Cancer Network outside of clinical research. Given the multiple caveats associated with testing,53,55 clinicians should educate themselves on the accuracy and validity of commercially available molecular tests before recommending them to patients.
Other research in molecular diagnostics focuses on metastatic risk and prognosis based on biomarkers such as circulating tumor cells or exosomes in the peripheral blood.41-44 Circulating tumor cells are cells that have detached from the primary tumor and have reached peripheral blood, and exosomes are extracellular vesicles formed from the primary tumor that contain proteins, growth factors, cytokines, and integrins, which are also seen in the circulation.41,42 Integrins are expressed in cytotoxic T lymphocytes and exosomes mediating cell adhesion at the metastatic site.43 Integrin expression in exosomes has been shown to influence the site of metastasis. One study44 showed a correlation of α6β4 and α6β1 upregulation in exosomes with lung metastasis and αvβ5 with liver metastasis. Exosomes also highly express CD44 and α6β4 integrin, which mediate adhesion at the metastatic site. There is hope for the availability of “liquid biopsy” in the future quantifying exosomes and cytotoxic T lymphocytes in the blood as well as other molecular markers. Their presence in the peripheral blood provides an easily accessible source for molecular studies. Although practical difficulties exist in successful isolation and detection of these molecules, their significance as biomarkers in melanoma metastasis is increasingly recognized, and thus, they may represent a valuable tool for risk prediction and prognosis in the future.
Innovative advances have also been made in the field of medical imaging. Notni et al56 developed pseudopeptides that could selectively target particular integrins and thus be used as radiotracers. These molecules were used in positron emission tomography (PET) to identify the presence of integrins in vivo. The possibility of in vivo integrin detection would allow for a better understanding of integrin expression in melanoma metastasis. They designed PET radiopharmaceuticals 68Ga-aquibeprin and 68Ga-avebetrin, which showed high selectivity for integrin ɑ5β1 and integrin ɑvβ3, respectively. Both 68Ga-aquibeprin and 68Ga-avebetrin were stable in mouse models, with no metabolites detectable in urine, bloodstream, liver, or kidney (centrifuged) 30 minutes after injection. Radiolabeled RGD peptides and other nanoparticles have also been designed to use as tracers for integrin αvβ3 in PET, with the hopes of having a better understanding of its role in metastasis and angiogenesis.57 These findings highlight the future potential for combined tissue and imaging biomarker studies in staging cancers.58
Integrin-Targeted Therapeutics
Researchers have found promising therapeutic targets in both integrins and molecules that interact with integrins. These include integrin blockers, integrin active site analogues, and disintegrins,59 which are naturally occurring peptides in viper snake venom that are known to inhibit integrin-dependent cell adhesion.
One experimental therapy is intetumumab (CNTO 95). Intetumumab is a monoclonal antibody against the integrin ɑv subunit which acts as a blocker. Although it was able to decrease angiogenesis and tumor growth in preclinical models of melanoma, metastatic melanoma was not susceptible to this therapy in clinical trials. This introduces the idea that integrin function needs to be targeted at more than just the receptor level as integrin monotherapy does not prolong survival in patients with advanced melanoma (Fig. 2).60
Integrins αIIbβ3, αvβ3, and α5β1 recognize the RGD motif, which is known for mediating cell-substratum and cell-cell adhesion. As previously mentioned, OPN and fibronectin are able to bind to integrins using this RGD sequence.61 Other integrins may recognize the Leu-Asp-Val (LDV) or Ile-Asp-Ser (IDS) motifs.62
Expression of the RGD motif in the adhesion molecule cadherin, another important molecule in cell adhesion, has been associated with aggressive disease during late stages of metastasis. Cadherin 17 (CDH17) and vascular endothelial cadherin reportedly have important roles in the development of metastasis.63,64 The RGD motif binds to integrins, which leads to changes in cell adhesion, invasion, and cell proliferation. Casal et al63 reported that monoclonal antibodies targeting the RGD motif of CDH17 can block integrin ɑ2β1 from being activated by CDH17. The study was performed using mouse models of lung metastasis and showed a decrease in metastatic colonization in the presence of the anti-CDH17 RGD monoclonal antibodies.
Furthermore, Karageorgis et al65 described a new and sophisticated method of inducing cellular apoptosis by using integrins. They synthesized a RGD peptide targeting integrins and reported its internalization and the subsequent release of a mitochondrial disruption peptide derived from the proapoptotic Bax protein. The goal was to take advantage of the overexpression of integrins in malignant cells and target them using this toxic peptide, resulting in mitochondrial membrane destabilization by creating pores and inducing apoptosis in cells.
Additional RGD-based drugs have been tested in preclinical studies. The venom of certain snake species contains disintegrin that contains the RGD motif. In nature, the purpose of this molecule is that when a snake bites its prey, disintegrin is released into the bloodstream of the victim and inhibits cell adhesion between platelets. Thus, the prey is unable to form blood clots, which results in continuous bleeding. Tzabcanin is a small 7.1 kDa disintegrin protein isolated from the Yucatan Rattlesnake (Crotalus simus tzabcan). Because of tzabcanin’s ability to bind integrin ɑvβ3 via the RGD motif, experiments were performed to study the effects of this protein on cancer cells.59 Results showed that this molecule inhibited cell-cell and cell-ECM interactions in A549 (lung epithelial carcinoma) and A375 (malignant melanoma) cell lines. This highlights the potential for tzabcanin to be used as a marker for tumors with high integrin ɑvβ3 expression, and for the development of antimetastatic drugs.
Integrin inhibitors can also be non-proteinaceous small molecules. A 2015 article66 described an orally active integrin ɑvβ3 small molecule inhibitor called MK-0429, which showed effectiveness in preventing metastasis in melanoma. The study was performed in female B6D2F1 mice, which were injected with murine syngeneic B16F10 melanoma, known to cause lung metastases. MK-0429 in doses of 100 and 300 mg/kg decreased the quantity of tumor colonies by more than 50%, although the current chemotherapeutic agent cyclophosphamide decreased colonies by 99%. Still, the adverse effects of cyclophosphamide limit its clinical usefulness. In this preclinical experiment, MK-0429 had an excellent safety profile. Patients with hormone refractory prostate cancer and metastatic bone disease tolerated MK-0429 well at high doses. Clinical efficacy was however limited.67
Volociximab (M200),68 a monoclonal antibody that targets integrin ɑ5β1, is involved in blocking angiogenesis by inhibiting the proliferation of endothelial cells. In a phase II randomized controlled trial with 40 participants, the safety of M200 at 10 mg/kg every 2 weeks was tolerable, with 87% of patients having stable disease. Similar results were obtained for ovarian, primary peritoneal,69 and non-small-cell lung cancer.70 Clinical development of M200 was stopped due to lack of efficacy in phase II trials.39 Novel treatment strategies are needed to make anti-integrin ɑ5β1 therapy work, e.g. as part of combination therapies (Fig. 2) and in synergy with novel immunotherapy approaches.
MEDI-522 (Abegrin, etaracizumab, Vitaxin), another angiogenesis inhibitor, is a monoclonal antibody that targets integrin ɑvβ3. It was derived from the murine antibody LM609,71 humanized and subsequently affinity maturated.72 In a phase II clinical trial involving 112 participants with metastatic melanoma, MEDI-522 (8 mg/kg/wk) was shown to be well tolerated with or without dacarbazine (1,000 mg/m2 once every 3 weeks). Dacarbazine, FDA-approved since 1975, and its orally absorbed analog temozolomide are alkylating agents used in the treatment of metastatic melanoma.73,74 When MEDI-522 was combined with dacarbazine it was no more effective than dacarbazine alone. Neither tumor response rate nor progression free survival was improved by MEDI-522. A follow-up phase III trial was therefore considered unreasonable and clinical development was stopped pending new insights into the mechanism of action of MEDI-522.75
Despite recent progress in the area of immunotherapy, many patients with advanced melanoma still need additional effective treatment options. Drugs which target integrins showed promise in preclinical studies but proved not to be effective as monotherapy or in combination with standard chemotherapy.76,77 Just as the dual targeting of the BRAF pathway via BRAF/MEK combination therapy improves efficacy78 integrin signaling may need to be targeted at multiple levels to achieve meaningful effects (Fig. 2). Integrin-targeting antibodies may be combined with inhibitors of focal adhesion kinase (FAK), an important downstream effector of integrins. Moreover, stabilized interphase microtubules provide an exocytosis pathway for integrin-induced proteins and exosomes from the trans-Golgi network79. Microtubules may be targeted by paclitaxel (from the Pacific yew tree Taxus brevifolia), a FDA-approved antineoplastic agent with anti-melanoma activity.80,81 Integrin-induced transcription of secreted proteins may be suppressed by type I topoisomerase (topo-I) inhibitors or pentamidine. Interestingly, the naturally occurring topo-I inhibitor camptothecin and its clinically available synthetic derivative, topotecan hydrochloride (trade name Hycamtin) were recently identified as top hits in a screen for compounds that increase T-cell-mediated killing of melanoma cells.82 As monotherapy in melanoma however, topotecan is inactive at concentrations that induce significant myelosuppression.83 Pentamidine isethionate (trade name Pentam) is a synthetic amidine derivative that interacts with the minor groove of AT-rich DNA regions thereby interfering with DNA replication and function.84 Pentam is FDA-approved for the treatment of pneumonia due to Pneumocystis carinii and has been shown to possess anti-melanoma activity in preclinical models.85
Conclusion
The role of integrins in melanoma is an area of active ongoing research. Studies have found associations between integrin expression and the degree of dermal invasion in melanoma and risk of metastasis. Primary melanoma risk stratification is an area in which integrins have been found to be useful because the metastasis risk of melanoma has been tied to integrin expression. Integrin-targeted therapeutics are being developed to take advantage of these findings. Targeting strategies that combine integrin monotherapy via function blocking antibodies or small molecules and standard chemotherapy have failed in clinical trials. New and innovative treatment strategies are needed to make anti-integrin medications work for patients. Specifically, combination therapies are needed that target integrins not just at the receptor level but at multiple levels (Fig. 2). Moreover, integrin-directed therapies need to be optimized to work in concert with targeted BRAF/MEK inhibition and the highly successful PD-1 immune checkpoint inhibitors.
Educational Challenge
- The following is true about the structure and function of integrins:
- Integrins are homodimers involved in cell adhesion.
- Integrins are composed of one ɑ and one β subunit which mediate extracellular adhesion but are devoid of an intracellular signaling function.
- Integrins are heterodimers involved in the remodeling of extracellular matrix, apoptosis and proliferation.
- Integrins are heterodimeric proteins expressed in benign nevi but not melanoma.
C
- The following is true regarding the effects of integrins on tumor cells, except:
- Adhesion, intracellular signaling and extracellular matrix remodeling.
- Adhesion, proliferation, and apoptosis.
- Proliferation, apoptosis, and survival.
- Cell migration, cell adhesion, transmembrane transport.
D
- The following is true regarding the role of integrins, except:
- Integrins aid in the degradation of the basement membrane by regulating the expression of metalloproteinases which degrade collagen and fibronectin.
- Integrins aid in the detachment of primary tumor into circulation.
- Integrins are involved in the intravasation of tumor cells into circulation but play no role in their extravasation.
- Integrins prime the metastatic niche allowing for implantation of primary tumor.
C
- The following is true regarding integrin ɑvβ3, except:
- Increased ɑvβ3 expression is associated with progression from radial to vertical growth.
- Decreased ɑvβ3 expression is associated with increased tumorigenicity.
- Increased ɑvβ3 expression is associated with growth and survival of melanoma cells.
- Expression of ɑvβ3 may inhibit tumor cell apoptosis thereby promoting tumor growth.
B
- The following is true regarding integrins and angiogenesis, except:
- Integrin signaling leads to the activation and phosphorylation of vascular endothelial growth factor receptors.
- Integrins and transforming growth factor receptors crosstalk.
- Integrins bind some growth factors through their ectodomains.
- Binding to growth factors activates integrins (‘inside-out’ activation).
D
- The following is true regarding osteopontin (OPN), except:
- OPN is an extracellular molecular that binds to integrins.
- OPN overexpression is associated with tumor cell transformation.
- OPN binds to integrins via the Leu-Asp-Val (LDV) motif.
- OPN facilitates cell-matrix interactions and promotes tumor progression.
C
- Which time interval between melanoma wide local excision and SLN biopsy in node-positive patients has been shown to be safe:
- Maximum of 30 days.
- Maximum of 45 days.
- Maximum of 60 days.
- It is safe and informative to perform a SLN biopsy >9 weeks after diagnosis.
D
- Trials have been conducted using integrins as biomarkers. The following statements are true, except:
- RGD radiotracers are being developed for melanomas that bind the integrin ɑ6β4 laminin receptor.
- Exosomes are used as melanoma biomarkers in the circulation.
- CD44 and ɑ6β4 are expressed on exosomes that help tumor cells establish a metastatic niche.
- RGD radiotracers are well suited to image tumors via positron emission tomography.
A
- The following statements on integrin therapeutics are true, except:
- A number of drugs targeting integrins have reached the clinical market, however, none for the treatment of cancer.
- Animal models and especially systemic knock-out mouse models have been highly predictive of outcomes of first in-human clinical trials in integrin-directed drug therapy.
- Six anti-integrin drugs on the market in 2016 generated revenues of some US$3.5 billion.
- Volociximab which targets integrin ɑ5β1 blocks angiogenesis in preclinical animal models.
B
- Of the following statements, which is true regarding gene expression profiling in melanoma?
- Gene expression profiling in melanoma is routinely recommended by the National Comprehensive Cancer Network outside of clinical studies.
- Integrins have been tried as biomarkers for primary cutaneous melanoma risk stratification but have not been found to be of value.
- Gene expression profiling of primary melanoma biopsies requires fresh frozen material and cannot be performed on paraffin embedded tissue.
- Gene expression profiling of primary melanoma biopsy tissue may be useful for identifying patients who can safely forgo SLN biopsy.
D
Acknowledgments
This work was funded by the National Cancer Institute (grant CA215105), the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (grant UL1TR000135), the Mayo Clinic Center for Individualized Medicine, the Mayo Clinic Cancer Center and the 5th District Eagles Cancer Telethon. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnotes
Conflicts of Interest: There are no conflicts of interest to report.
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. [DOI] [PubMed] [Google Scholar]
- 2.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wegener KL, Partridge AW, Han J, et al. Structural basis of integrin activation by talin. Cell. 2007;128(1):171–182. [DOI] [PubMed] [Google Scholar]
- 4.Winograd-Katz SE, Fässler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nature Reviews Molecular Cell Biology. 2014;15(4):273–288. [DOI] [PubMed] [Google Scholar]
- 5.Gu Y, Liu Y, Fu L, et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat Med. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Serrels A, Lund T, Serrels B, et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015;163(1):160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moser M, Legate KR, Zent R, Fassler R. The tail of integrins, talin, and kindlins. Science. 2009;324(5929):895–899. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. [DOI] [PubMed] [Google Scholar]
- 9.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danen EH, van Muijen GN, van de Wiel-van Kemenade E, Jansen KF, Ruiter DJ, Figdor CG. Regulation of integrin-mediated adhesion to laminin and collagen in human melanocytes and in non-metastatic and highly metastatic human melanoma cells. Int J Cancer. 1993;54(2):315–321. [DOI] [PubMed] [Google Scholar]
- 11.Etoh T, Thomas L, Pastel-Levy C, Colvin RB, Mihm MC Jr., Byers HR. Role of integrin alpha 2 beta 1 (VLA-2) in the migration of human melanoma cells on laminin and type IV collagen. J Invest Dermatol. 1993;100(5):640–647. [DOI] [PubMed] [Google Scholar]
- 12.Yoshinaga IG, Vink J, Dekker SK, Mihm MC Jr., Byers HR. Role of alpha 3 beta 1 and alpha 2 beta 1 integrins in melanoma cell migration. Melanoma Res. 1993;3(6):435–441. [DOI] [PubMed] [Google Scholar]
- 13.Natali PG, Nicotra MR, Bartolazzi A, Cavaliere R, Bigotti A. Integrin expression in cutaneous malignant melanoma: association of the alpha 3/beta 1 heterodimer with tumor progression. Int J Cancer. 1993;54(1):68–72. [DOI] [PubMed] [Google Scholar]
- 14.Vizkeleti L, Kiss T, Koroknai V, et al. Altered integrin expression patterns shown by microarray in human cutaneous melanoma. Melanoma Res. 2017;27(3):180–188. [DOI] [PubMed] [Google Scholar]
- 15.Danen EH, Ten Berge PJ, Van Muijen GN, Van ‘t Hof-Grootenboer AE, Brocker EB, Ruiter DJ. Emergence of alpha 5 beta 1 fibronectin- and alpha v beta 3 vitronectin-receptor expression in melanocytic tumour progression. Histopathology. 1994;24(3):249–256. [DOI] [PubMed] [Google Scholar]
- 16.Ziober BL, Chen YQ, Ramos DM, Waleh N, Kramer RH. Expression of the alpha7beta1 laminin receptor suppresses melanoma growth and metastatic potential. Cell Growth Differ. 1999;10(7):479–490. [PubMed] [Google Scholar]
- 17.Kramer RH, Vu MP, Cheng YF, Ramos DM, Timpl R, Waleh N. Laminin-binding integrin alpha 7 beta 1: functional characterization and expression in normal and malignant melanocytes. Cell Regul. 1991;2(10):805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hieken TJ, Ronan SG, Farolan M, Shilkaitis AL, Das Gupta TK. Beta 1 integrin expression: a marker of lymphatic metastases in cutaneous malignant melanoma. Anticancer Res. 1996;16(4b):2321–2324. [PubMed] [Google Scholar]
- 19.Hieken TJ, Ronan SG, Farolan M, Shilkaitis AL, Das Gupta TK. Molecular prognostic markers in intermediate-thickness cutaneous malignant melanoma. Cancer. 1999;85(2):375–382. [DOI] [PubMed] [Google Scholar]
- 20.Nikkola J, Vihinen P, Vlaykova T, Hahka-Kemppinen M, Heino J, Pyrhonen S. Integrin chains beta1 and alphav as prognostic factors in human metastatic melanoma. Melanoma Res. 2004;14(1):29–37. [DOI] [PubMed] [Google Scholar]
- 21.Albelda SM, Mette SA, Elder DE, et al. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990;50(20):6757–6764. [PubMed] [Google Scholar]
- 22.Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest. 1992;89(6):2018–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery AM, Reisfeld RA, Cheresh DA. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci U S A. 1994;91(19):8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu MY, Shih DT, Meier FE, et al. Adenoviral gene transfer of beta3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma. Am J Pathol. 1998;153(5):1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Belle PA, Elenitsas R, Satyamoorthy K, et al. Progression-related expression of beta3 integrin in melanomas and nevi. Hum Pathol. 1999;30(5):562–567. [DOI] [PubMed] [Google Scholar]
- 26.Voura EB, Ramjeesingh RA, Montgomery AM, Siu CH. Involvement of integrin alpha(v)beta(3) and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol Biol Cell. 2001;12(9):2699–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meves A, Nikolova E, Heim JB, et al. Tumor Cell Adhesion As a Risk Factor for Sentinel Lymph Node Metastasis in Primary Cutaneous Melanoma. J Clin Oncol. 2015;33(23):2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elder D Tumor progression, early diagnosis and prognosis of melanoma. Acta Oncol. 1999;38(5):535–547. [DOI] [PubMed] [Google Scholar]
- 29.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000;115(3):337–344. [DOI] [PubMed] [Google Scholar]
- 31.Walker C, Mojares E, Del Rio Hernandez A. Role of Extracellular Matrix in Development and Cancer Progression. Int J Mol Sci. 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitra A, Chakrabarti J, Chatterjee A. Binding of alpha5 monoclonal antibody to cell surface alpha5beta1 integrin modulates MMP-2 and MMP-7 activity in B16F10 melanoma cells. J Environ Pathol Toxicol Oncol. 2003;22(3):167–178. [DOI] [PubMed] [Google Scholar]
- 33.Vuoriluoto K, Hognas G, Meller P, Lehti K, Ivaska J. Syndecan-1 and −4 differentially regulate oncogenic K-ras dependent cell invasion into collagen through alpha2beta1 integrin and MT1-MMP. Matrix Biol. 2011;30(3):207–217. [DOI] [PubMed] [Google Scholar]
- 34.Jiao Y, Feng X, Zhan Y, et al. Matrix metalloproteinase-2 promotes alphavbeta3 integrin-mediated adhesion and migration of human melanoma cells by cleaving fibronectin. PLoS One. 2012;7(7):e41591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng DF, Chen F, Wang S, et al. Autoantibody against integrin alphav beta3 contributes to thrombocytopenia by blocking the migration and adhesion of megakaryocytes. J Thromb Haemost. 2018;16(9):1843–1856. [DOI] [PubMed] [Google Scholar]
- 36.Sil H, Sen T, Chatterjee A. Fibronectin-integrin (alpha5beta1) modulates migration and invasion of murine melanoma cell line B16F10 by involving MMP-9. Oncol Res. 2011;19(7):335–348. [DOI] [PubMed] [Google Scholar]
- 37.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8(8):604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct αv integrins. Science. 1995;270(5241):1500–1502. [DOI] [PubMed] [Google Scholar]
- 39.Raab-Westphal S, Marshall JF, Goodman SL. Integrins as therapeutic targets: successes and cancers. Cancers. 2017;9(9):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwan BH, Zhu EF, Tzeng A, et al. Integrin-targeted cancer immunotherapy elicits protective adaptive immune responses. J Exp Med. 2017;214(6):1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tucci M, Mannavola F, Passarelli A, Stucci LS, Cives M, Silvestris F. Exosomes in melanoma: a role in tumor progression, metastasis and impaired immune system activity. Oncotarget. 2018;9(29):20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alix-Panabieres C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. 2012;63:199–215. [DOI] [PubMed] [Google Scholar]
- 43.Paolillo M, Schinelli S. Integrins and Exosomes, a Dangerous Liaison in Cancer Progression. Cancers (Basel). 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang R, Rofstad EK. Integrins as therapeutic targets in the organ-specific metastasis of human malignant melanoma. J Exp Clin Cancer Res. 2018;37(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao C, Cui Y, Owen S, Li W, Cheng S, Jiang WG. Human osteopontin: Potential clinical applications in cancer (Review). Int J Mol Med. 2017;39(6):1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K, Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol. 2005;24(6):418–427. [DOI] [PubMed] [Google Scholar]
- 49.Yin M, Soikkeli J, Jahkola T, Virolainen S, Saksela O, Holtta E. Osteopontin promotes the invasive growth of melanoma cells by activating integrin alphavbeta3 and down-regulating tetraspanin CD9. Am J Pathol. 2014;184(3):842–858. [DOI] [PubMed] [Google Scholar]
- 50.Gerami P, Cook RW, Wilkinson J, et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin Cancer Res. 2015;21(1):175–183. [DOI] [PubMed] [Google Scholar]
- 51.Bellomo D, Arias-Mejias SM, Ramana C, et al. A model combining tumor molecular and clinicopathologic risk factors predicts sentinel lymph node metastasis in primary cutaneous melanoma JCO Precis Oncol. 2020:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brunner G, Reitz M, Heinecke A, et al. A nine-gene signature predicting clinical outcome in cutaneous melanoma. J Cancer Res Clin Oncol. 2013;139(2):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grossman D, Kim CC, Hartman RI, et al. Prognostic gene expression profiling in melanoma: necessary steps to incorporate into clinical practice. Melanoma Management. 2019;6(4):MMT32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchetti MA, Bartlett EK, Dusza SW, Bichakjian CK. Use of a prognostic gene expression profile test for T1 cutaneous melanoma: Will it help or harm patients? J Am Acad Dermatol. 2019;80(6):e161–e162. [DOI] [PubMed] [Google Scholar]
- 55.Sominidi-Damodaran S, Pittelkow MR, Meves A. Gene Expression Profiling in Cutaneous Melanoma: Caveats for Clinicians. Paper presented at: Mayo Clin Proc 2016. [DOI] [PubMed] [Google Scholar]
- 56.Notni J, Steiger K, Hoffmann F, et al. Complementary, Selective PET Imaging of Integrin Subtypes alpha5beta1 and alphavbeta3 Using 68Ga-Aquibeprin and 68Ga-Avebetrin. J Nucl Med. 2016;57(3):460–466. [DOI] [PubMed] [Google Scholar]
- 57.Chen H, Niu G, Wu H, Chen X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin alphavbeta3. Theranostics. 2016;6(1):78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weissleder R Molecular imaging in cancer. Science. 2006;312(5777):1168–1171. [DOI] [PubMed] [Google Scholar]
- 59.Saviola AJ, Burns PD, Mukherjee AK, Mackessy SP. The disintegrin tzabcanin inhibits adhesion and migration in melanoma and lung cancer cells. Int J Biol Macromol. 2016;88:457–464. [DOI] [PubMed] [Google Scholar]
- 60.Alday P, Stupp, Rüegg. Are Integrins Still Practicable Targets for Anti-Cancer Therapy? Cancers. 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nieberler M, Reuning U, Reichart F, et al. Exploring the Role of RGD-Recognizing Integrins in Cancer. Cancers (Basel). 2017;9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dattoli SD, De Marco R, Baiula M, et al. Synthesis and assay of retro-alpha4beta1 integrin-targeting motifs. Eur J Med Chem. 2014;73:225–232. [DOI] [PubMed] [Google Scholar]
- 63.Casal JI, Bartolome RA. RGD cadherins and alpha2beta1 integrin in cancer metastasis: A dangerous liaison. Biochim Biophys Acta Rev Cancer. 2018;1869(2):321–332. [DOI] [PubMed] [Google Scholar]
- 64.Liao F, Li Y, O’Connor W, et al. Monoclonal antibody to vascular endothelial-cadherin is a potent inhibitor of angiogenesis, tumor growth, and metastasis. Cancer Res. 2000;60(24):6805–6810. [PubMed] [Google Scholar]
- 65.Karageorgis A, Claron M, Juge R, et al. Systemic Delivery of Tumor-Targeted Bax-Derived Membrane-Active Peptides for the Treatment of Melanoma Tumors in a Humanized SCID Mouse Model. Mol Ther. 2017;25(2):534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickarski M, Gleason A, Bednar B, Duong LT. Orally active alphavbeta3 integrin inhibitor MK-0429 reduces melanoma metastasis. Oncol Rep. 2015;33(6):2737–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenthal MA, Davidson P, Rolland F, et al. Evaluation of the safety, pharmacokinetics and treatment effects of an ανβ3 integrin inhibitor on bone turnover and disease activity in men with hormone-refractory prostate cancer and bone metastases. Asia Pac J Clin Oncol. 2010;6(1):42–48. [DOI] [PubMed] [Google Scholar]
- 68.Figlin RA, Kondagunta GV, Yazji S, Motzer RJ, Bukowski RM. Phase II study of volociximab (M200), an α5β1 anti-integrin antibody in refractory metastatic clear cell renal cell cancer (RCC). Journal of Clinical Oncology. 2006;24(18_suppl):4535–4535. [Google Scholar]
- 69.Bell-McGuinn KM, Matthews CM, Ho SN, et al. A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol Oncol. 2011;121(2):273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Besse B, Tsao L, Chao D, et al. Phase Ib safety and pharmacokinetic study of volociximab, an anti-α5β1 integrin antibody, in combination with carboplatin and paclitaxel in advanced non-small-cell lung cancer. Ann Oncol. 2012;24(1):90–96. [DOI] [PubMed] [Google Scholar]
- 71.Borst AJ, James ZM, Zagotta WN, et al. The Therapeutic Antibody LM609 Selectively Inhibits Ligand Binding to Human alphaVbeta3 Integrin via Steric Hindrance. Structure. 2017;25(11):1732–1739 e1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu H, Beuerlein G, Nie Y, et al. Stepwise in vitro affinity maturation of Vitaxin, an αvβ3-specific humanized mAb. Proceedings of the National Academy of Sciences. 1998;95(11):6037–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Velho TR. Metastatic melanoma - a review of current and future drugs. Drugs Context. 2012;2012:212242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serrone L, Zeuli M, Sega F, Cognetti F. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Cancer Res. 2000;19(1):21–34. [PubMed] [Google Scholar]
- 75.Hersey P, Sosman J, O’Day S, et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alpha(v)beta(3), + or − dacarbazine in patients with stage IV metastatic melanoma. Cancer. 2010;116(6):1526–1534. [DOI] [PubMed] [Google Scholar]
- 76.Stupp R, Hegi ME, Gorlia T, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated< i> MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 77.Hussain M, Le Moulec S, Gimmi C, Bruns R, Straub J, Miller K. Differential Effect on Bone Lesions of Targeting Integrins: Randomized Phase II Trial of Abituzumab in Patients with Metastatic Castration-Resistant Prostate Cancer. Clinical Cancer Research. 2016;22(13):3192–3200. [DOI] [PubMed] [Google Scholar]
- 78.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. [DOI] [PubMed] [Google Scholar]
- 79.Clague MJ, Urbe S. Multivesicular bodies. Curr Biol. 2008;18(10):R402–R404. [DOI] [PubMed] [Google Scholar]
- 80.Legha SS, Ring S, Papadopoulos N, Raber M, Benjamin RS. A phase II trial of taxol in metastatic melanoma. Cancer. 1990;65(11):2478–2481. [DOI] [PubMed] [Google Scholar]
- 81.Sonee M, Barrón E, Yarber FA, Hamm-Alvarez SF. Taxol inhibits endosomal-lysosomal membrane trafficking at two distinct steps in CV-1 cells. Am J Physiol. 1998;275(6):C1630–C1639. [DOI] [PubMed] [Google Scholar]
- 82.McKenzie JA, Mbofung RM, Malu S, et al. The Effect of Topoisomerase I Inhibitors on the Efficacy of T-Cell-Based Cancer Immunotherapy. J Natl Cancer Inst. 2018;110(7):777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kraut EH, Walker MJ, Staubus A, Gochnour D, Balcerzak SP. Phase II trial of topotecan in malignant melanoma. Cancer Invest. 1997;15(4):318–320. [DOI] [PubMed] [Google Scholar]
- 84.Reddy BP, Sondhi SM, Lown JW. Synthetic DNA minor groove-binding drugs. Pharmacol Ther. 1999;84(1):1–111. [DOI] [PubMed] [Google Scholar]
- 85.Pathak MK, Dhawan D, Lindner DJ, Borden EC, Farver C, Yi T. Pentamidine is an inhibitor of PRL phosphatases with anticancer activity. Mol Cancer Ther. 2002;1(14):1255–1264. [PubMed] [Google Scholar]