Abstract
Recent evidence has shown that exposure to airborne particulate matter (PM) is associated with cognitive delay, depression, anxiety, autism, and neurodegenerative diseases; however, the role of PM in the etiology of these outcomes is not well-understood. Therefore, there is a need for controlled animal studies to better elucidate the causes and mechanisms by which PM impacts these health outcomes. We assessed the effects of gestational and early life exposure to traffic-related PM on social- and anxiety-related behaviors, cognition, inflammatory markers, and neural integrity in male rat juveniles. Gestating and lactating rats were exposed to PM from a Boston (MA, USA) traffic tunnel for 5 hours/day, 5 days/week for 6 weeks (3 weeks gestation, 3 weeks lactation). The target exposure concentration for the fine fraction of nebulized PM, measured as PM2.5, was 200 μg/m3. To assess anxiety and cognitive function, F1 male juveniles underwent elevated platform, cricket predation, nest building, social behavior and marble burying tests at 32–60 days of age. Upon completion of behavioral testing, multiple cytokines and growth factors were measured in these animals and their brains were analyzed with diffusion tensor MRI to assess neural integrity. PM exposure had no effect on litter size or weight, or offspring growth; however, F1 litters developmentally exposed to PM exhibited significantly increased anxiety (p =0.04), decreased cognition reflected in poorer nest-organization (p =0.04), and decreased social play and allogrooming (p= 0.003). MRI analysis of ex vivo brains revealed decreased structural integrity of neural tissues in the anterior cingulate and hippocampus in F1 juveniles exposed to PM (p<0.01, p=0.03, respectively). F1 juvenile males exposed to PM also exhibited significantly decreased plasma levels of both IL-18 (p=0.03) and VEGF (p=0.04), and these changes were inversely correlated with anxiety-related behavior. Chronic exposure of rat dams and their offspring to traffic-related PM during gestation and lactation decreases social behavior, increases anxiety, impairs cognition, decreases levels of inflammatory and growth factors (which are correlated with behavioral changes), and disrupts neural integrity in the juvenile male offspring. Our findings add evidence that exposure to traffic-related air pollution during gestation and lactation is involved in the etiology of autism spectrum disorder (ASD) and other disorders including social and cognitive deficits and/or increased anxiety.
Keywords: particulate matter, behavior, inflammation, development, rodent
Introduction
The 2015 State of Global Air Report concluded that 92% of the world’s population lived in areas where particulate matter size ≤ 2.5 μm (PM2.5) concentrations exceeded the US EPA’s annual average guideline of 12 μg/m3. Substantial epidemiological and experimental evidence links PM2.5 with hypertension, atherosclerosis, myocardial infarction, ischemic stroke, and respiratory and neurological diseases (Araujo and Nel, 2009; Pun et al., 2017; Tonne et al., 2007; Wright and Ding, 2016). The Global Burden of Disease (GBD) project ranked exposure to PM2.5 as the fifth highest risk factor for death, causing 4.2 million deaths from heart disease, lung cancer, chronic lung disease, and respiratory infection (Cohen et al., 2017).
While air pollution is a complex mixture of gases and aerosolized PM, the most adverse effects on human health are linked with the particulate matter component (Anderson et al., 2012; Araujo and Nel, 2009). The primary mechanism by which PM is believed to damage the heart and lungs is through induction of both local and systemic oxidative stress and inflammation. Upon inhalation, PM causes direct free radical damage to cells, stimulating inflammatory cytokine release (MohanKumar et al., 2008). PM is also recognized by receptors on respiratory macrophages, causing a release of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) (Dai et al., 2016). Ultrafine PM (aerodynamic diameter < 100 nm ) are also known to cross the pulmonary endothelium into the bloodstream and distribute to other organs (Dai et al., 2016; MohanKumar et al., 2008), amplifying systemic superoxide radical production through secondary neutrophil activation, and disrupting endothelial cell integrity (Wang et al., 2012; Wright and Ding, 2016).
Concern for the impact of PM on neurological health has recently increased and evidence suggests a role of physical environmental factors in the pathogenesis of neurological disease, as the brain is particularly susceptible to oxidative stress and damage (Halliwell, 2006; Pun et al., 2017). In response to xenobiotics or inflammatory cytokines, the brain’s resident macrophage-type cells, microglia, produce reactive oxygen species and glutamate (Block and Calderon-Garciduenas, 2009; Wang et al., 2017). When activated, microglia produce more IL-6, TNF-α, and other inflammatory cytokines, leading to a cycle of neuroinflammation and oxidative damage, termed reactive microgliosis (Block and Calderon-Garciduenas, 2009; Wang et al., 2017).
Numerous studies indicate that this persistent microglial activation is toxic to CNS tissue, especially pre-myelinating oligodendrocytes (another type of glial cell), and can lead to white matter injury (Allen et al., 2017; Liu et al., 2013). In addition to this cytokine-mediated CNS damage, particulate matter itself can enter the brain and exert direct detrimental effects. Ultrafine PM in the bloodstream can cross the blood-brain barrier (BBB) as well as pass through the cribriform plate in the olfactory mucosa into the brain (Maher et al., 2016a; Oberdorster et al., 2004; Oberdorster et al., 2002). Once in the brain, PM can initiate reactive microgliosis and has been shown to cause decreased neuronal growth even at low concentrations, and neuronal death at higher concentrations (Allen et al., 2014; Allen et al., 2017; Gillespie et al., 2013; Maher et al., 2016a).
Accumulating evidence suggests that PM also impacts mental health through these neuroinflammatory and oxidative stress mechanisms. Compared with children and dogs living in clean air, those living in the highly polluted area of Mexico city exhibit breakdown of the BBB, systemic and brain inflammation, neural accumulation of combustion-related metals, attention and short-term memory deficits, as well as specific hallmarks of Alzheimer’s and Parkinson’s diseases (Calderon-Garciduenas et al., 2015; Calderón-Garcidueñas et al., 2016a; Calderón-Garcidueñas et al., 2016b; Maher et al., 2016b). In related mouse studies, exposure to PM2.5 for 9 months induced an Alzheimer’s disease-like inflammation profile, including increased beta-amyloid 1–40 protein levels, beta-site amyloid precursor protein cleaving enzyme, cyclooxygenase 1 and 2, and proinflammatory cytokines (Bhatt et al., 2015). Epidemiological studies have reported associations between air pollution and attention deficit/hyperactivity disorder in children (Newman et al., 2013; Saez et al., 2018) and anxiety and depression in adults even when correcting for socioeconomic status (Pun et al., 2017).
Accumulating evidence also suggests a role for PM in the pathogenesis of autism spectrum disorder (ASD). Two studies performed in California by Volk et. al found an association between autism and residential proximity to a freeway during the third trimester and also exposure to traffic-related air pollution during gestation (Volk et al., 2011; Volk et al., 2013). Another study in mice showed that prenatal and early-life exposure to diesel exhaust particles led to increased behaviors similar to those present in humans with ASD, including higher levels of motor activity and repetitive self-grooming (Thirtamara Rajamani et al., 2013).
In the current study, maternal rats and their offspring were exposed to PM derived from aged traffic-related air pollution (TRAP) collected from a highway tunnel in Boston, Massachusetts, while animals exposed to HEPA-filtered ambient laboratory air for 6 weeks during gestation (3 weeks) and lactation (3 weeks) served as controls. We hypothesized that developmental exposure to TRAP PM would increase anxiety, impair cognition, decrease social behavior, induce systemic increases in circulating markers of inflammation, and reduce neural integrity (assessed with diffusion tensor imaging, DTI) similar to previous ASD-related epidemiological observations and associated mouse studies. This current work enhances the rodent PM exposure literature through the use of a naturalistic home cage exposure protocol (without chronic restraint, repeated novel cage exposures, and/or instillation), which includes both gestation and lactation, novel behavioral assessments, inflammatory measures, and DTI MRI in an integrative outbred rat paradigm. Rat dams are excellent behavioral models for perinatal manipulations (Nephew and Murgatroyd, 2013) and rat brains are large enough for high resolution DTI MRI.
Materials and Methods
Animals
Sprague-Dawley rats in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee. Given the prevalence of ASD in males, male rat pups were used in this study. The rat dam/pup units were divided into the following two groups: (1) laboratory air (Control) and (2) airborne particulate matter (PM) exposure (Exposure). The study involved 12 dams (6 per group), which were mated with established breeders (Charles River), and 4 male and 4 female pups from each litter (weaned at day 21). All other pups were culled shortly after birth. All rats were kept on a 14:10 h light-dark cycle in virus-free sections of the animal facility at the Tufts Cummings School and were allowed ad libitum access to food and water. Behavioral testing was conducted between the ages of 32 and 40 days.
Traffic-Related Particulate Matter Exposure
Pregnant dams and their litters were housed in their standard home cages within larger exposure chambers during gestation and lactation to avoid novel environmental exposures and related disrupted stress induced disruption of maternal care. The exposure chambers were airtight, and equipped with HEPA filters at the distal end to prevent PM administered into the chamber from discharging out into the room/facility air (supplementary fig. 1).
Pollution exposure involved whole-body inhalant exposure to PM, which was collected with from a Boston highway tunnel exhaust plenum and has been used in a prior cardiovascular study (Carll et al., 2017), but not characterized in detail. Rats were exposed to re-aerosolized PM at a target concentration of 200 μg/m3 (measured as PM2.5) using a Heart nebulizer (Westmed, Tucson, AZ). Dams were exposed for 5 hours a day, 5 days a week for the duration of gestation (~22 days) and lactation (21 days) for a total of 29 days across 6 weeks (one missed day to accommodate for exposure chamber cleaning). The objective of this target concentration was to expose the rats to high, but epidemiologically observed levels from metropolitan areas throughout gestation and lactation (e.g. New Delhi and Beijing) (Lv et al., 2016; Sharma et al., 2018; Tiwari et al., 2013; Xie et al., 2015; Zhan et al., 2017). We considered that it would take time for the levels to reach 200 μg/m3 each day of exposure and that the exposures were not 24 hours/day, 7 days/week. Therefore, values normalized across the exposure period, similar to the calculation of daily averages in epidemiology studies and as presented in related animal studies (Fonken et al., 2011), would be substantially lower. Dams were kept with their pups throughout lactation.
Each day, 6 mg of PM that had been vortexed in 50ml of deionized water was added to deionized water within the nebulizer to reach a total volume of 240ml. PM concentration was monitored closely throughout the exposure period and adjusted as needed to stay within the 100 – 300 μg/m3 range; additional PM was added to the nebulizer to increase concentrations and deionized water was added to the nebulizer to lower concentrations. Control animals underwent the identical procedures with only nebulized deionized water. Temperature and humidity in both exposure and control chambers were assessed at 0, 0.5, 1, 2, 3, 4 and 5 hours during exposure sessions with a Thermopro TP55 digital hygrometer and thermometer. Humidity levels were controlled by pumping in room air that had first passed through two desiccators filled with Drierite desiccant. The desiccant was replenished whenever its color changed from blue to pink, indicating moisture saturation. PM2.5 concentrations in both exposure and control chambers were assessed by attaching polyethylene tubing to ports on the side of the chambers at 0, 0.5, 1, 2, 3, 4 and 5 hours after 1 minute of sampling using a TSI DRX 8532 aerosol monitor (Shoreview, USA). Preliminary testing indicated that PM levels were homogenous within the chambers 8–10 minutes after starting the nebulizers.
Body Weight and Milk Intake
The weights of the dams (12, 6 per treatment group) and all pups (4 males and 4 females per litter) were recorded on days 2, 9, and 16 of lactation. To quantify milk intake, the pups were removed from their mothers for one hour. Pup weights for each sex were measured at the time of removal, at time of pup return, and 2 hours after return, and the difference between the pup return and 2-hour after return weights was used as the value for milk intake.
Behavioral Testing
All F1 juvenile male (18 exposure and 18 control rats) offspring of F0 dams used for behavioral testing were between the ages of 32 and 40 days. Each rat was subjected to the following five behavioral tests in groups of 18 (9 exposure and 9 control) in a counterbalanced design. Behaviors were observed and scored by individuals blind to the exposure status.
In the cricket predation test (Kinsley et al., 2014; Pittet et al., 2017), the rat was removed from the home cage and placed in a clean breeding cage (16 × 20 × 8 in.) with bedding for 10 min to allow for locomotor acclimation to the novel environment. Two crickets were placed in the cage, and the behavior of the rat was video recorded for 15 minutes. The typical behavioral pattern is chasing, capture, and consumption of the cricket. Time to initiate chasing of the cricket as well as the durations and frequencies of pursuit of the cricket, consumption of the cricket, locomotor activity, and rearing were measured. Number of fecal boli produced during this test was also recorded as a measure of anxiety (Ferre et al., 1995; Hall, 1934; Ramos et al., 1997; Seibenhener and Wooten, 2015).
The marble burying test was performed to assess repetitive and perseverative behaviors (Murgatroyd et al., 2016b; Thomas et al., 2009). Male juveniles were placed in a clean cage for 15 minutes with 6 marbles evenly spaced on top of the bedding. The number of marbles at least 75% covered (only the top surface visible) was counted at the end of the 15-minute period.
An elevated platform test was performed to assess anxiety (Ennaceur, 2012), where the time required to climb down from an 8-inch high platform in a novel clean cage was recorded.
The nest building performance of the rats, as a measure of cognition (Deacon, 2012; Deacon, 2006; Lin et al., 2007; Yuan et al., 2018), over a 24-hour period in a clean cage with fresh nesting material was evaluated (score of 0–5) in the morning, with higher scores indicating more organized nests (0 = no nest, 5 = fully formed nest with walls and a roof) (Gaskill et al., 2013).
Social behavior at the cage level was initially assessed with 30-second scan sampling observations of all cages (6 control and 6 exposure cages with 3 males in each cage) every 2 minutes for a 60-minute undisturbed period. The number of cages with play behavior or allogrooming among any of the three rats in each cage at each two minute interval was recorded. This 60-minute period was video recorded and later scored for total frequency and durations of play behavior, allogrooming, locomotor activity, and self grooming at the cage level (Babb et al., 2014).
Cytokine Measurements
At the end of lactation (at 21 days of age), all 12 dams, one of the 4 male weanlings, and all 4 female weanlings from each litter were sacrificed and both blood and brain samples were taken. The remaining 3 juvenile males were weaned at this time. Upon completion of behavioral testing, these juvenile males were euthanized and both blood and brain samples were taken. Levels of IFN- γ, TIMP-1, ICAM-1, IL-18, IL-1a, VEGF, and AGP in the male juvenile plasma samples were quantified using single or multiplex (TIMP-1, ICAM, IL-18, IL-a, VEGF) Quantikine ELISA kits from R&D Systems (Minneapolis, MN, USA) following the manufacturer’s directions. All samples from both groups were analyzed together as single sets.
Diffusion Tensor MRI
Ex vivo brain MRI analysis of 12 F1 male juveniles (6 from each group) was performed. Isolated brains were immersed in 4% paraformaldehyde and phosphate-buffered saline for 8 weeks until imaged. Fixed brains were positioned in a 50 mL polypropylene tube filled with 3M Fluorinert FC-770 (Parallax Technology, Waltham, MA, USA). Fluorinert has no hydrogen nuclei, is invisible to proton MRI, and commonly used to reduce signal susceptibility changes between the brain and its immediate environment in ex vivo imaging (Hikishima et al., 2015; Molet et al., 2016). Each brain was further secured in a folded length of MR-invisible mesh to prevent movement. All MR images were acquired on a recently upgraded 4.7T/40 cm horizontal MRI magnet (Oxford, UK) interfaced with a Biospec Bruker console (Bruker, Germany) and equipped with a 20G/cm magnetic field gradient. A custom-built rat 1H radiofrequency (RF) volume and head coil was used. For anatomical images, a T2-weighted high-resolution scan was collected using TurboRARE sequence with the following parameters: repetition time TR=4060 ms, echo time TE=36 ms, RARE factor=8, field of view FOV=32 mm x 32 mm, slice number=35; slice thickness=0.5 mm, 256 × 256 data matrix, number of averages=8. DTI data were acquired in 30 directions with an EPI sequence and B value of 650/0, TR=4000 ms, TE=43 ms, number of averages =26, 192 × 192 data matrix; other geometry parameters as with the T2 anatomical image. Images were converted to Analyze 7.5 format using ImageJ (Schneider et al., 2012) (QuickVol plugin (Schmidt et al., 2004) and re-oriented using a custom Matlab routine.
Statistics
All data from the two groups were compared using one (treatment, for behavioral data) and two-way (treatment + time, for exposure and environmental data) ANOVA’s and t-tests. Analyses of DTI data to generate axial and radial diffusivity and FA maps used established methods and were conducted at the Center for Comparative Neuroimaging (CCNI). A representative FA is presented in figure 6. DTI data were analyzed in native space using DTIstudio (Jiang et al., 2006) including eddy current correction, motion correction, and generation of tensor metrics including axial diffusivity, radial diffusivity, and fractional anisotropy (FA) maps. Probabilistic tractography was applied for the corpus callosum (thresholds of 0.2 FA to start and stop, 70° curvature). Regions of interest identification was guided by the Paxinos and Watson rat brain atlas to generate 3D renderings. The ROIs from each animal were then placed onto their respective axial and radial diffusivity and FA maps. Percentage changes or differences reported are relative to control or initial values.
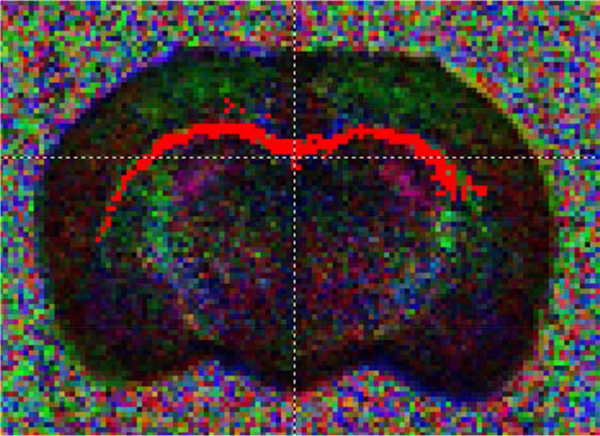
Figure 6.
Representative fractional anisotropy image of the corpus callosum.
Results
PM2.5 concentrations, humidity, and temperature
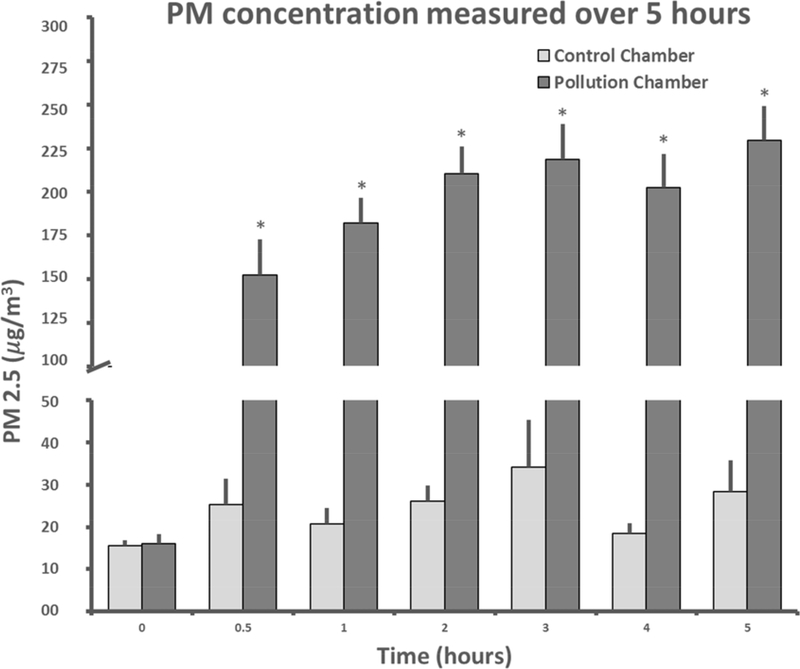
The concentration of PM2.5 was significantly greater in the exposure chamber than in the control chamber over the course of the experiment with significant effects of treatment (F1,405 = 473.4, p<0.01), time (F6,405 = 18.6, p<0.01) and an interaction between treatment and time (F6,405 = 387.2, p<0.01, fig. 1). PM2.5 reached a mean of 230 μg/m3 at hour five compared to 28 μg/m3 in the control chamber at the same time point (p < 0.001, a 721% increase). The mean concentration across the 7 timepoints over the exposure period was 173.0 ± 27.9 μg/m3 in the exposure chamber and 23.9 ± 2.4 μg/m3 in the control chamber (a 624% increase). When normalized across the total hours in the 6 weeks of the experiment, the PM2.5 levels were 25.8 μg/m3 in the exposure chamber and 3.6 μg/m3 in the control chamber (a 617% increase), not accounting for exposures outside the 5-hour experimental periods due to lack of data from these times. The relatively elevated concentrations in the control chamber during the experimental period were due to bedding-related PM (investigated and reported in a related study (Hudda et al., 2019)). A portion of the total PM in the exposure chamber was also presumably bedding-generated; we cannot quantify it but expect the magnitude of bedding-generated PM to be of the same order as that observed in control group.
Figure 1.
PM2.5 Concentrations (mean ± standard error for all 29 exposure sessions) at seven time points in the control and exposure chambers. The fluctuation in control PM is due to the aperiodic/random nature of PM generation from rat locomotor and burrowing activity. * indicates that the exposure concentration was statistically significantly greater than the control concentration.
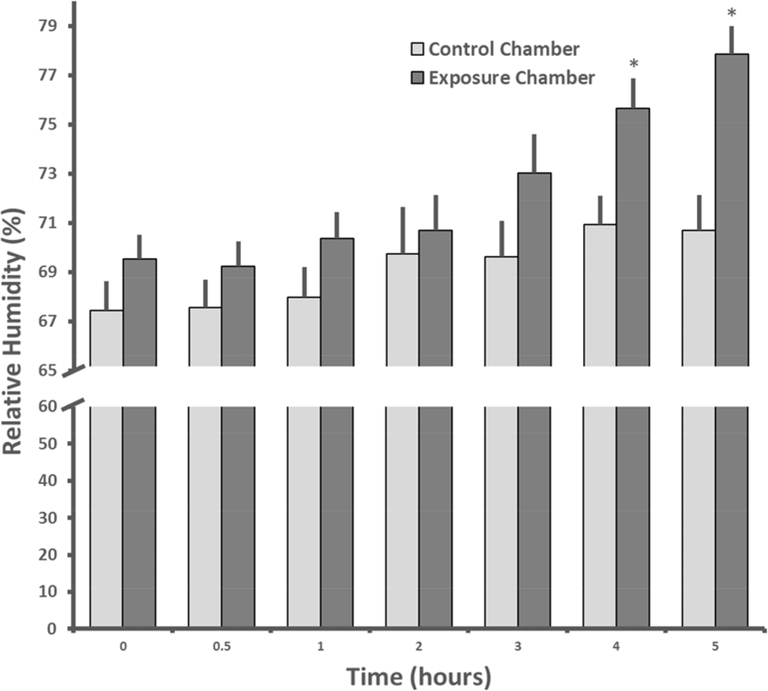
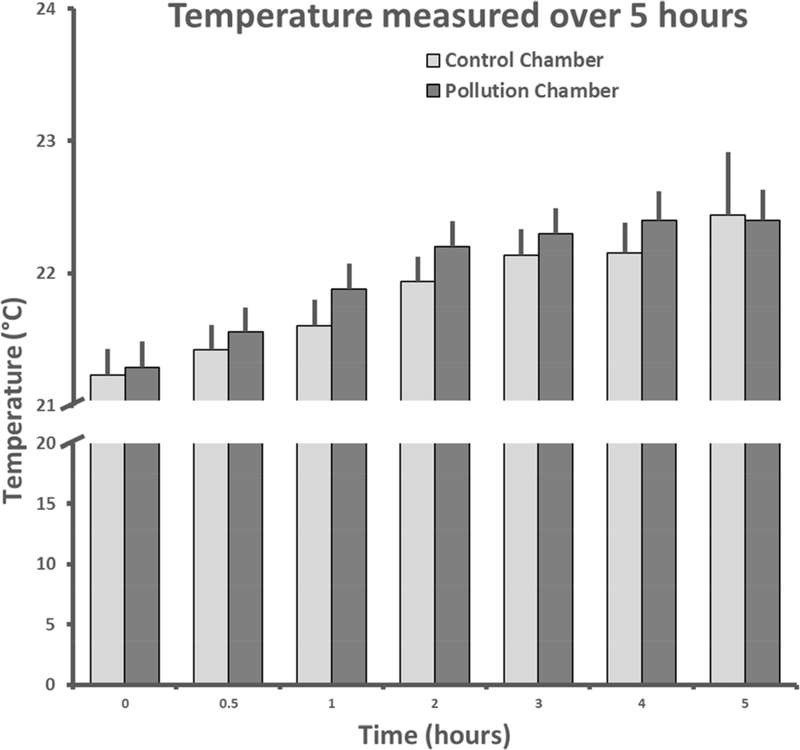
The relative humidity (RH) was marginally greater (4.6%) in the exposure chamber (mean = 72.3±1.2 %) than in the control chamber (mean = 69.1±0.6 %) over the course of the experiment with significant effects of treatment (F1,405 = 2.5, p<0.05) and time (F1,405 = 21.6, p<0.01, fig. 2). RH was not correlated with PM2.5 values over hours 2–5 in the exposure chamber, the period when RH increased in the exposure chamber (r2 = 0.2, p=0.6). There was no statistically significant group difference in temperature during the exposure period (p>0.5), but temperature did increase slightly across time (3%) from 21.3 to 22.4 °C (F6,405 = 22.9, p<0.01, fig. 3).
Figure 2.
Mean + SEM relative humidity levels (%) across the 29 days of exposure at seven time points in the control and exposure chambers. * indicates that the relative humidity in the exposure chamber was significantly higher than in the control chamber.
Figure 3.
Mean +SEM temperature across the 29 days of exposure at seven time points in the control and exposure chambers.
Growth and Milk Intake
Dam weight, pup weight, litter size, and milk intake did not statistically differ between exposure and control groups (all p-values >0.2, Table 2).
Table 2:
Mean dam weight, pup weight, and milk intake ± SEM over the course of lactation (n=6, all values in grams).
| Experiment Day | Dam Weight | Male Pup Weight | Female Pup Weight | Male Milk Intake | Female Milk Intake | |
|---|---|---|---|---|---|---|
| Exposure | 2 | 249.3 ± 7.6 | 8.1 ± 0.4 | 7.6 ± 0.4 | 2.8 ± 0.4 | 4.1 ± 0.63 |
| 9 | 278.8 ± 7.0 | 24.7 ± 0.7 | 24.0 ± 0.6 | 0.63 ± 0.51 | 0.92 ± 0.47 | |
| 16 | 291.2 ± 7.2 | 44.6 ± 1.1 | 43.2 ± 1.1 | 0.60 ± 0.13 | 0.75 ± 0.27 | |
| Control | 2 | 260.7 ± 4.1 | 8.0 ± 0.3 | 7.7 ± 0.3 | 2.6 ± 0.7 | 3.1 ± 0.74 |
| 9 | 290.7 ± 4.0 | 24.2 ± 0.9 | 23.5 ± 0.8 | 0.92 ± 0.46 | 0.70 ± 0.39 | |
| 16 | 282.0 ± 14.4 | 44.8 ± 1.5 | 41.6 ± 2.6 | 0.34 ± 0.43 | 0.43 ± 0.33 |
Behavior Data
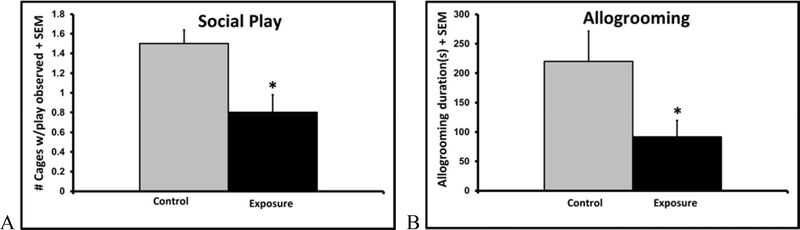
Rats in the exposure group displayed lower levels of whole cage social play (47% less, p=0.003) and allogrooming (13% less, p<0.05) among the three littermates, indicating deficits in social behavior compared to controls (fig. 4). There were no treatment differences in social play, self grooming, or locomotor activity (all p’s > 0.3).
Figure 4.
Mean ± SEM social play observations from scan sampling (A) and total allogrooming duration (B) during a one hour observation of juveniles in same treatment cages of three juvenile littermate males (n=6).
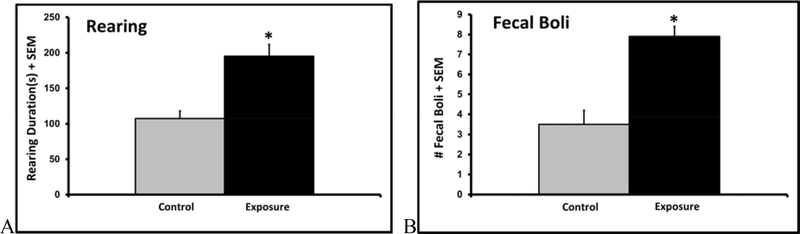
Latencies to climb down in the elevated platform test were 46% greater in the exposure group (141.5 13.6 vs. 97.2 15.1, p=0.04). While there were no statistically significant differences in latencies to attack and consume the crickets (p<0.3), the pollution-exposed males spent almost twice (82%) as much time rearing (p<0.01) and produced over 2 times (126%) as many fecal boli (p<0.01) and during the 15-minute predation test (fig. 5A+B). There was no difference in locomotor activity (p>0.2).
Figure 5.
Mean + SEM rearing duration (A) and number of fecal boli dropped (B) during the cricket predation test in control and TRAP-exposed rats (n=18).
Similarly, although there was no significant difference in number of marbles buried (p>0.1); rats in the exposure group also produced 34% more fecal boli (3.9 0.4 vs. 2.9±0.4, p<0.05) during this test of repetitive and perseverative behavior in a novel environment. As a functional and ecologically relevant measure of cognition, rats exposed to TRAP PM had 31% lower nest-building scores (2.2 0.3 vs. 3.2 0.4, p= 0.04, 1-tailed t-test).
Cytokine and Growth Factor Levels
Cytokine levels were below the detection limits of the IFN- γ, and IL-1a ELISA assays, and above the detection maximum for TIMP 1. There were no statistically significant differences between the groups in levels ICAM-1, or AGP. Basal concentrations of both IL-18 (104.6±10.1 vs. 135.0±8.2, p=0.03) and VEGF ( 22.7±1.8 vs. 28.9±2.3, p=0.04) were lower (22.5% and 21.5%, respectively) in the rats developmentally exposed to pollution.
Behavior-cytokine correlations
Levels of IL-18 (r2=0.21, p=0.01) and VEGF (r2=0.18, p=0.03) were inversely correlated to rearing duration during the cricket predation test at the individual level. When levels of cytokines were averaged at the cage level, IL-18 was correlated with cage level observations of allogrooming (r2=0.25, p=0.05, 1-tailed t-test), and nest score was correlated with VEGF (r2=0.22, p=0.05, 1-tailed t-test).
Diffusion Tensor MRI
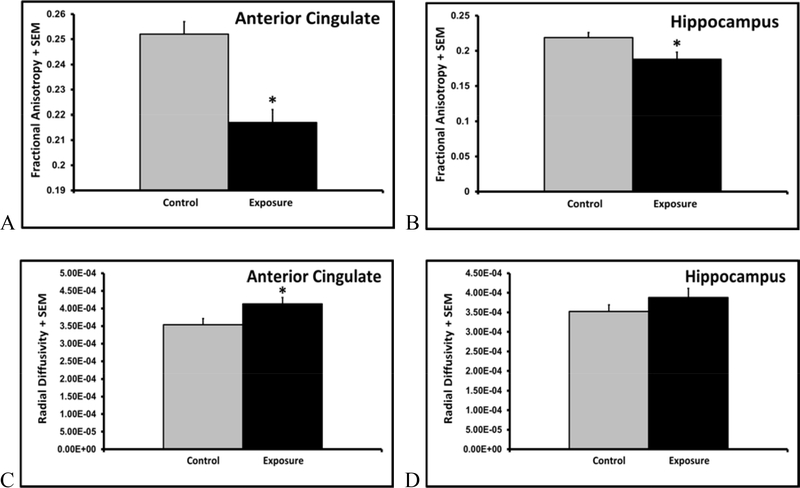
MRI analysis of ex vivo brains revealed 14% lower fractional anisotropy in both the anterior cingulate (p<0.01, fig. 7A) and hippocampus (p<0.05, fig. 7B) of PM exposed juveniles, but FA levels were similar in the corpus callosum (p=0.23). While axial diffusivity in the anterior cingulate (p=0.31), hippocampus (p=0.49), or corpus callosum (p=0.50) did not differ, radial diffusivity was 17% greater in the anterior cingulate of rats exposed to pollution (p<0.05, fig. 7C). There were no differences in radial diffusivity in the hippocampus (p=0.23, fig. 7D), or corpus callosum (p=0.45).
Figure7A-D.
Mean + SEM fractional anisotropy (A+B) and radial diffusivity (C+D) values in the anterior cingulate and hippocampus of control and TRAP exposed juvenile male rats (n=6).
Discussion
The present study describes a novel, effective, and ethologically- and environmentally-relevant rat model of the role of TRAP-related developmental PM exposure in ASD etiology. The findings also pertain to other PM associated disorders involving cognitive and social deficits and/or increased anxiety. This work supports and augments previous rodent work (Allen et al., 2017; Fonken et al., 2011) through the use of a novel home cage exposure paradigm which includes undisturbed gestation and lactation, novel behavioral assessments, cytokine levels, and DTI MRI in an integrative design. Developmental exposure to PM impaired both cognition and social behavior, increased anxiety, decreased levels of an inflammatory cytokine and endothelial growth factor, and disrupted neural integrity in juvenile male rats. PM exposure induced increases in anxiety, cognition, and social behavior that might be mediated by IL-18 and/or VEGF, which have previously been implicated in ASD etiology (Businaro et al., 2016; Emanuele et al., 2010).
Nebulization of Boston tunnel PM resulted in a consistent increase in the exposure chamber to concentrations that we were able to reliably maintain over the five hour treatment periods. Substantially elevated levels of PM air pollution have been reported for traffic tunnels in Boston (Herndon et al., 2005; Perkins et al., 2013), California (Geller et al., 2005), Pennsylvania (Gertler et al., 2002), Sweden (Gidhagen et al., 2003), and China (Zhou et al., 2014) as well as subway tunnels in Barcelona (Moreno et al., 2015). Similar methodology of collecting TRAP-enriched PM and re-aerosolizing it for exposures has been used in other rodent studies, but most have focused on respiratory or cardiovascular outcomes (Carll et al., 2017; Gerlofs-Nijland et al., 2005) while the brain and neurological effects remain understudied (Bos et al., 2012; Woodward et al., 2017a). Use of a traffic derived heterogeneous composition PM may have been key to the reported effects, as neonatal exposure to ultrafine elemental carbon particles does not affect cognition, anxiety, or neuroinflammation (Morris-Schaffer et al., 2019), contrasting with the related rodent literature in general. Concentrations of PM2.5 in the exposure chamber were kept at concentrations similar to the levels found in highly polluted cities in developing countries (Lv et al., 2016; Sharma et al., 2018; Tiwari et al., 2013; Xie et al., 2015; Zhan et al., 2017), while the concentrations in the control chamber were substantially lower. The normalized value of the exposure corresponds to levels experienced by greater than 90% of the population of China (Zhan et al., 2017) Nonetheless, the control chamber concentrations were elevated compared to the typical levels in the animal facility (5–10 μg/m3). This was due to the generation of bedding-related PM due to rat activity, which has been documented and investigated in a related study (Hudda et al., 2019).
The fact that there were no significant differences between the dam weight, pup weight, litter size, and milk intake between the exposure and control groups suggests that PM exposure in our experiment did not result in acute toxicity, which could have adversely affected overall development and the current outcomes of interest. Although relative humidity was slightly increased in the exposure chamber, likely due to variation in nebulizer production and/or efficacy of the desiccators, the difference was minimal (3%) and the humidity levels were only marginally above the recommended range of 40–70% for a few hours/week in total (Council, 2011). There is no evidence that such a marginal difference is associated with neurological and/or behavioral effects. However, this marginal difference in RH between the exposure versus control chamber could potentially have biased the measurements because the photometric instrument we used over-estimates mass concentration readings at higher humidity. A lack of correlation between PM2.5 and RH between hours 2–5, when the difference in RH appeared, indicates that RH did not significantly bias the exposure PM2.5 data
Behavioral results of this study support previous research looking at the association between ASD and/or impaired cognition and exposure to PM. The combined cognition and social deficits seen in the juvenile rats exposed to PM parallel symptom combinations commonly seen in children with ASD (Costa et al., 2017; Levy et al., 2009). Multiple epidemiological studies have identified a significant relationship between autism and exposure to traffic-related air pollution during gestation (Volk et al., 2011; Volk et al., 2013). Two additional studies reported that higher maternal exposure to PM2.5 during pregnancy was associated with greater odds of having a child diagnosed with autism (Becerra et al., 2013; Raz et al., 2015). In China, a study that assessed cognitive, motor, sensory, and psychomotor neurological functions identified a significant relationship between chronic low-level traffic-related air pollution exposure and poorer neurobehavioral function in exposed children (Wang et al., 2009). Children living in highly polluted areas of Mexico City with no known risk factors for cognitive disorders exhibited significant deficits in attention, short-term memory, and cognitive tasks compared to children living in clean air (Calderón-Garcidueñas et al., 2008).
Past animal research into PM exposure and cognition has shown similar results to human studies. Prenatal and early-life exposure of mice to diesel exhaust particles leads to increased behaviors similar to those present in humans with ASD, including increased motor activity and repetitive self-grooming (Thirtamara Rajamani et al., 2013). Ultrafine TRAP exposure from gestation to adulthood results in decreased neurogenesis in hippocampus, microbleeds in the blood brain barrier, memory deficits, and increased depressive behavior (Woodward et al., 2018). Similarly, gestational and neonatal exposure to high levels of diesel exhaust decreases social interaction, alters social communication, and increases repetitive behavior (Chang et al., 2018) and similar results were reported from exposure to concentrated PM2.5(Church et al., 2018). UFP exposure of mice only during the prenatal period impairs neuronal differentiation and increases depressive behavior (Davis et al., 2013). Increased repetitive and impulsive behaviors were documented following neonatal exposure to UFP, and these changes were linked to increased measures of neuroinflammation (Allen et al., 2017). Neonatal exposure to PM also impairs short term memory (Allen et al., 2014), and effects on learning and memory and cognitive flexibility (Morris-Schaffer et al., 2018) are more likely to be observed in males (Cory-Slechta et al., 2018). Administration of UFP to juvenile mice increases levels of TNF and induced neurite atrophy in the hippocampus (Woodward et al., 2017b), and mice exposed to airborne PM2.5 for 10 months exhibit impairments in spatial learning and memory (Fonken et al., 2011). The current study adds to this literature with the use of an ecological measure of cognition and an early sign of dysfunction, nesting behavior (Deacon, 2012; Jirkof, 2014; Lin et al., 2007). The decreases in the social behavior of the current PM exposed group may have implications for later life social interactions critical to survival, such as mating, aggression, and the adult behavioral response to social stress.
Interestingly, autistic patients show various signs that fit with the air pollution-generated neuroinflammation and oxidative stress mechanisms proposed to underlie neurodegeneration. Markers of inflammation are increased in amniotic fluid and throughout life in children with ASD (Abdallah et al., 2013; Allen et al., 2017; Gesundheit et al., 2013). Increased microglia activation in multiple brain regions has been frequently observed in individuals with ASD (Allen et al., 2017), and levels of asthma are 35% more common in those with ASD after controlling for confounding factors, consistent with common inflammatory mechanisms in the lung and brain (Kotey et al., 2014).
Basal plasma levels of IL-18 were significantly decreased in F1 juvenile male rats within the exposure group when compared to control F1 juveniles. These findings are consistent with those from a previous study examining IL-18 levels in both autistic patients compared to healthy controls and Reeler mice, which serve as an experimental murine model of autism, compared to wild-type mice (Businaro et al., 2016). IL-18 was decreased in the sera of autistic patients and Reeler mice when compared to healthy subjects or wild-type mice, respectively. In fact, the greatest decrease in IL-18 was present in patients with the most severe autism (Businaro et al., 2016). Expanding on these results, this previous study also quantified brain levels of IL-18, finding significantly increased amounts of IL-18 in the brains of both the autistic patients and the Reeler mice, evidence of an inverse relationship between peripheral and central IL-18 (Businaro et al., 2016). There was an inverse correlation between IL-18 levels and duration of rearing during the cricket predation test in the present study. While brain levels of IL-18 were not quantified in the present study, future PM experiments should obtain both peripheral and central IL-18 levels in order to determine whether they mirror the inverse relationship seen in autistic patients and Reeler mice. Taken together with epidemiological associations between TRAP and ASD (Volk et al., 2011; Volk et al., 2013), these findings support further investigation of IL-18 in the potential role of PM exposure in ASD etiology.
Basal concentrations of VEGF were significantly lower in rats developmentally exposed to pollution compared to control rats. These results support the findings of a previous study in which circulating levels of VEGF and other pro-angiogenic growth factors were significantly decreased in healthy young adults exposed to periods of elevated PM2.5 levels (Pope et al., 2016). In studies of autistic patients, decreases in VEGF have been reported, and overall VEGF dysfunction has been implicated in ASD etiology (Emanuele et al., 2010). Furthermore, this growth factor may mediate neurodevelopmental cognitive deficits associated with preeclampsia (Lara et al., 2018). Sex differences have also been reported for VEGF, with lower levels in males (Murgatroyd et al., 2016a), and further study of the role of sex differences in the inflammatory and behavioral responses to PM is warranted.
MRI analysis of ex vivo brains revealed structural integrity deficits in the anterior cingulate and hippocampus in F1 litters exposed to PM. The decreases in fractional anisotropy could be due to decreased axonal fiber density, axonal diameter, and/or altered myelination (decreased or damaged) which has been reported in previous developmental PM studies of neuroinflammation and myelination (Klocke et al., 2018). An increase in radial diffusivity specifically in the anterior cingulate suggests that the decreased FA in this region is due to myelin damage (Winklewski et al., 2018), or given the developmental paradigm, deficient developmental myelination (Gao et al., 2009; Hammelrath et al., 2016). In general, FA is increasing and radial diffusivity is decreasing in rats during the developmental window targeted in the present study (Bockhorst et al., 2008). PM2.5 induces learning and memory deficits associated with morphological abnormalities in the hippocampus (Li et al., 2018). The present results parallel findings in a previous study in which 10 months of exposure to fine particulate matter (94 ug/m3 for 6h/day, 5 days/week) altered the morphological characteristics of hippocampal neurons and impaired the cognitive abilities of mice (Fonken et al., 2011). Allen et al. found that postnatal exposures of mice to ultrafine particles produced a pattern of developmental and biochemical changes and delays similar to hypothesized mechanistic underpinnings of ASD, including inflammation, microglial activation, reductions in the size of the corpus callosum, aberrant white matter development, and elevated glutamate (Allen et al., 2014; Allen et al., 2017). These disruptions in neural connectivity and morphology support the hypothesis that PM exposure is toxic to the developing and adult brain. It is not yet clear whether the mechanism is direct damage from particles entering CNS tissue or initiation of reactive microgliosis from inflammatory signaling molecules that enter the brain from systemic circulation.
One limitation of the present study is the increased humidity in the exposure chamber. While this absolute difference of 3% in relative humidity was statistically significant and could represent a stress related confound, it was small, typical of variations within animal facilities, and unlikely to be biologically significant with regards to the reported behavioral and physiological differences between the groups. However, this marginal difference in RH between the exposure versus control chamber could potentially have biased the measurements because the photometric instrument we used over-estimates mass concentration readings at higher humidity. A lack of correlation between PM2.5 and RH at hours 2–5, when the difference in RH appeared, indicates that RH did not significantly bias the exposure PM2.5 data. The size distribution and chemistry of the PM exposure is unknown, and the material used was aged rather than freshly generated. Since only PM2.5 was monitored, it is likely we underestimated total PM exposure. In addition, a portion of the total PM in the exposure chamber was also presumably bedding-generated; we cannot quantify it but expect the magnitude of bedding-generated PM to be of the same order as that observed in control group. The present findings are most relevant to regions with high levels of PM. Plasma samples were only taken at the end of lactation and at the end of juvenile behavioral testing, meaning that any cytokine differences in early gestation or lactation were missed. The correlation analyses of the seven significant behavioral differences and two cytokines were not corrected for multiple comparisons and brains were not sampled, preventing detection of any neuroinflammation that may have been present. Another limitation of this study was that behavioral testing was only performed on male rats due to ASD being more common in males. Results of this experiment, however, suggest a role for PM in the etiology of other neurologic disorders without a male sex bias, therefore, future studies should examine effects on both males and females.
Our findings add evidence that exposure to traffic-related particulate air pollution during gestation and lactation is involved in the etiology of ASD and other disorders which include social and cognitive deficits and/or increased anxiety. F1 juvenile males developmentally exposed to high concentrations of PM2.5 of traffic origin exhibited higher anxiety, impaired cognition, and decreased social behavior when compared to juveniles in the control group. The PM-induced decrease in VEGF suggests that air pollution impairs angiogenesis, highlighting a possible common mechanism for damage to both the central nervous and cardiovascular systems. Decreased plasma levels of IL-18 in exposed juveniles mirror those previously observed in autistic patients and animal models of ASD, suggesting that IL-18 dysregulation may constitute part of an immunological mechanism underlying the association between ASD and pollution. Brain MRI analysis of the juveniles in the exposure group revealed disrupted neural integrity within the hippocampus and anterior cingulate, consistent with the findings in previous studies that air pollution is both directly neurotoxic and able to initiate a secondary cascade of neurotoxic events within the brain. This work supports and augments previous work through the use of a novel ethologically- and environmentally-relevant exposure protocol that includes gestation and lactation, novel behavioral assessments, cytokine levels, and DTI MRI in an integrative outbred rat paradigm. The continued use of environmentally relevant models of PM exposure will generate valuable insight into the adverse immune and neurobehavioral impacts of PM in ASD and related neurodevelopmental disease etiology.
Supplementary Material
Supplementary Figure 1
Exposure apparatus.
Highlights.
Rats were developmentally exposed to Boston area traffic related particulate matter (PM).
PM exposure increased anxiety and impaired cognition and social behavior.
IL-18 and VEGF levels were inversely correlated with anxiety.
PM exposure decreased neural integrity assessed with diffusion tensor imaging.
Traffic related PM is involved in the etiology of anxiety, cognitive, and social disorders.
Acknowledgements
The PM exposure chambers were custom designed and built by Mina Akdogan, Baris Erdemli, Freddy Davaris, Georgios Pesmazoglou, and Faris Shamsi. The Cummings School of Veterinary Medicine Laboratory Animal Medicine Service provided exceptional logistical support and consultation throughout the project.
Funding Sources
We would like to thank Tufts University for an Inflammation-Based Seed Grant, which initiated and supported this work. DB, JD, and NH were supported by NIEHS R01 ES026980. GP and JK were supported by NIH S10 OD018132-01.
Footnotes
The authors have no conflicts of interest.
Animal Research Approval
Sprague-Dawley rats in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah MW, et al. , 2013. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J Biol Psychiatry. 14, 528–38. [DOI] [PubMed] [Google Scholar]
- Allen JL, et al. , 2014. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci. 140, 160–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, et al. , 2017. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 59, 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JO, et al. , 2012. Clearing the air: a review of the effects of particulate matter air pollution on human health. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 8, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo JA, Nel AE, 2009. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Particle and Fibre Toxicology. 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb JA, et al. , 2014. Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm Behav. 65, 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra TA, et al. , 2013. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect. 121, 380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DP, et al. , 2015. A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS One. 10, e0127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderon-Garciduenas L, 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32, 506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhorst KH, et al. , 2008. Early postnatal development of rat brain: In vivo diffusion tensor imaging. Journal of Neuroscience Research. 86, 1520–1528. [DOI] [PubMed] [Google Scholar]
- Bos I, et al. , 2012. Changed gene expression in brains of mice exposed to traffic in a highway tunnel. Inhal Toxicol. 24, 676–86. [DOI] [PubMed] [Google Scholar]
- Businaro R, et al. , 2016. Interleukin-18 modulation in autism spectrum disorders. J Neuroinflammation. 13, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, et al. , 2015. Air pollution and your brain: what do you need to know right now. Prim Health Care Res Dev. 16, 329–45. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, et al. , 2016a. Air pollution, a rising environmental risk factor for cognition, neuroinflammation and neurodegeneration: The clinical impact on children and beyond. Revue Neurologique. 172, 69–80. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, et al. , 2008. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain and Cognition. 68, 117–127. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, et al. , 2016b. Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environmental Research. 146, 404–417. [DOI] [PubMed] [Google Scholar]
- Carll AP, et al. , 2017. Inhaled ambient-level traffic-derived particulates decrease cardiac vagal influence and baroreflexes and increase arrhythmia in a rat model of metabolic syndrome. Particle and Fibre Toxicology. 14, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, et al. , 2018. Prenatal and early-life diesel exhaust exposure causes autism-like behavioral changes in mice. Part Fibre Toxicol. 15, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JS, et al. , 2018. Perinatal exposure to concentrated ambient particulates results in autism-like behavioral deficits in adult mice. NeuroToxicology. 65, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, et al. , 2017. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet. 389, 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, et al. , 2018. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology. 69, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, et al. , 2017. Developmental Neurotoxicity of Traffic-Related Air Pollution: Focus on Autism. Curr Environ Health Rep. 4, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR, 2011. Guide for the Care and Use of Laboratory Animals. National Academies Press, Washington DC. [PubMed] [Google Scholar]
- Dai J, et al. , 2016. Exposure to concentrated ambient fine particulate matter disrupts vascular endothelial cell barrier function via the IL-6/HIF-1α signaling pathway. FEBS open bio. 6, 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DA, et al. , 2013. Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depression-like responses. PloS one. 8, e64128–e64128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon R, 2012. Assessing burrowing, nest construction, and hoarding in mice. Journal of visualized experiments : JoVE. e2607–e2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, 2006. Assessing nest building in mice. Nat Protoc. 1, 1117–9. [DOI] [PubMed] [Google Scholar]
- Emanuele E, et al. , 2010. Serum levels of vascular endothelial growth factor and its receptors in patients with severe autism. Clin Biochem. 43, 317–9. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, 2012. Open space anxiety test in rodents: the elevated platform with steep slopes. Methods Mol Biol. 829, 177–91. [DOI] [PubMed] [Google Scholar]
- Ferre P, et al. , 1995. Behavior of the Roman/Verh high- and low-avoidance rat lines in anxiety tests: relationship with defecation and self-grooming. Physiol Behav. 58, 1209–13. [DOI] [PubMed] [Google Scholar]
- Fonken LK, et al. , 2011. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry. 16, 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, et al. , 2009. Temporal and Spatial Development of Axonal Maturation and Myelination of White Matter in the Developing Brain. American Journal of Neuroradiology. 30, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill BN, et al. , 2013. Nest building as an indicator of health and welfare in laboratory mice. Journal of visualized experiments : JoVE. 51012–51012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller MD, et al. , 2005. Measurements of Particle Number and Mass Concentrations and Size Distributions in a Tunnel Environment. Environmental Science & Technology. 39, 8653–8663. [DOI] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, et al. , 2005. Effects of particulate matter on the pulmonary and vascular system: time course in spontaneously hypertensive rats. Particle and Fibre Toxicology. 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler AW, et al. , 2002. Real-world particulate matter and gaseous emissions from motor vehicles in a highway tunnel. Res Rep Health Eff Inst. 5–56; discussion 79–92. [PubMed] [Google Scholar]
- Gesundheit B, et al. , 2013. Immunological and autoimmune considerations of Autism Spectrum Disorders. J Autoimmun. 44, 1–7. [DOI] [PubMed] [Google Scholar]
- Gidhagen L, et al. , 2003. Model simulation of ultrafine particles inside a road tunnel. Atmospheric Environment. 37, 2023–2036. [Google Scholar]
- Gillespie P, et al. , 2013. Particulate matter neurotoxicity in culture is size-dependent. Neurotoxicology. 36, 112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CS, 1934. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. Journal of Comparative Psychology. 18, 385–403. [Google Scholar]
- Halliwell B, 2006. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 97, 1634–58. [DOI] [PubMed] [Google Scholar]
- Hammelrath L, et al. , 2016. Morphological maturation of the mouse brain: An in vivo MRI and histology investigation. NeuroImage. 125, 144–152. [DOI] [PubMed] [Google Scholar]
- Herndon SC, et al. , 2005. Characterization of urban pollutant emission fluxes and ambient concentration distributions using a mobile laboratory with rapid response instrumentation. Faraday Discuss. 130, 327–39; discussion 363–86, 519–24. [DOI] [PubMed] [Google Scholar]
- Hikishima K, et al. , 2015. Parkinson Disease: Diffusion MR Imaging to Detect Nigrostriatal Pathway Loss in a Marmoset Model Treated with 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Radiology. 275, 430–7. [DOI] [PubMed] [Google Scholar]
- Hudda N, et al. , 2019. Bedding-generated particulate matter: implications for rodent studies. Inhalation Toxicology. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, et al. , 2006. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 81, 106–16. [DOI] [PubMed] [Google Scholar]
- Jirkof P, 2014. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 234, 139–46. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, et al. , 2014. The mother as hunter: significant reduction in foraging costs through enhancements of predation in maternal rats. Horm Behav. 66, 649–54. [DOI] [PubMed] [Google Scholar]
- Klocke C, et al. , 2018. Enhanced cerebellar myelination with concomitant iron elevation and ultrastructural irregularities following prenatal exposure to ambient particulate matter in the mouse. Inhal Toxicol. 30, 381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotey S, et al. , 2014. Co-occurrence of autism and asthma in a nationally-representative sample of children in the United States. J Autism Dev Disord. 44, 3083–8. [DOI] [PubMed] [Google Scholar]
- Lara E, et al. , 2018. Are the Cognitive Alterations Present in Children Born From Preeclamptic Pregnancies the Result of Impaired Angiogenesis? Focus on the Potential Role of the VEGF Family. Frontiers in Physiology. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SE, et al. , 2009. Autism. Lancet. 374, 1627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. , 2018. The neurotoxicity induced by PM(2.5) might be strongly related to changes of the hippocampal tissue structure and neurotransmitter levels. Toxicology research. 7, 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, et al. , 2007. Neural encoding of the concept of nest in the mouse brain. Proceedings of the National Academy of Sciences. 104, 6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, et al. , 2013. Vulnerability of premyelinating oligodendrocytes to white-matter damage in neonatal brain injury. Neurosci Bull. 29, 229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv B, et al. , 2016. A systematic analysis of PM2.5 in Beijing and its sources from 2000 to 2012. Atmospheric Environment. 124, 98–108. [Google Scholar]
- Maher BA, et al. , 2016a. Magnetite pollution nanoparticles in the human brain. Proceedings of the National Academy of Sciences. 113, 10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BA, et al. , 2016b. Magnetite pollution nanoparticles in the human brain. Proceedings of the National Academy of Sciences. 113, 10797–10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MohanKumar SM, et al. , 2008. Particulate matter, oxidative stress and neurotoxicity. Neurotoxicology. 29, 479–88. [DOI] [PubMed] [Google Scholar]
- Molet J, et al. , 2016. MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus. 26, 1618–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno T, et al. , 2015. Urban air quality comparison for bus, tram, subway and pedestrian commutes in Barcelona. Environmental Research. 142, 495–510. [DOI] [PubMed] [Google Scholar]
- Morris-Schaffer K, et al. , 2019. Effects of neonatal inhalation exposure to ultrafine carbon particles on pathology and behavioral outcomes in C57BL/6J mice. Part Fibre Toxicol. 16, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Schaffer K, et al. , 2018. Effect of neonatal hyperoxia followed by concentrated ambient ultrafine particle exposure on cumulative learning in C57Bl/6J mice. NeuroToxicology. 67, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, et al. , 2016a. Transgenerational Social Stress, Immune Factors, Hormones, and Social Behavior. Frontiers in Ecology and Evolution. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd CA, et al. , 2016b. Effects of Chronic Social Stress and Maternal Intranasal Oxytocin and Vasopressin on Offspring Interferon-γ and Behavior. Frontiers in Endocrinology. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew B, Murgatroyd C, 2013. The role of maternal care in shaping CNS function. Neuropeptides. 47, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman NC, et al. , 2013. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ Health Perspect. 121, 731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorster G, et al. , 2004. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 16, 437–45. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, et al. , 2002. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. Journal of Toxicology and Environmental Health-Part A. 65. [DOI] [PubMed] [Google Scholar]
- Perkins JL, et al. , 2013. Particle number emission factors for an urban highway tunnel. Atmospheric Environment. 74, 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet F, et al. , 2017. Chronic social instability in adult female rats alters social behavior, maternal aggression and offspring development. Developmental Psychobiology. 59, 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA 3rd, et al. , 2016. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ Res. 119, 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun VC, et al. , 2017. Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. Environmental Health Perspectives. 125, 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, et al. , 1997. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res. 85, 57–69. [DOI] [PubMed] [Google Scholar]
- Raz R, et al. , 2015. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect. 123, 264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez M, et al. , 2018. The association between exposure to environmental factors and the occurrence of attention-deficit/hyperactivity disorder (ADHD). A population-based retrospective cohort study. Environ Res. 166, 205–214. [DOI] [PubMed] [Google Scholar]
- Schmidt KF, et al. , 2004. Volume reconstruction techniques improve the correlation between histological and in vivo tumor volume measurements in mouse models of human gliomas. J Neurooncol. 68, 207–15. [DOI] [PubMed] [Google Scholar]
- Schneider CA, et al. , 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 9, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener ML, Wooten MC, 2015. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. Journal of visualized experiments : JoVE. e 52434–e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, et al. , 2018. Forecasting air pollution load in Delhi using data analysis tools. Procedia Computer Science. 132, 1077–1085. [Google Scholar]
- Thirtamara Rajamani K, et al. , 2013. Prenatal and early-life exposure to high-level diesel exhaust particles leads to increased locomotor activity and repetitive behaviors in mice. Autism Res. 6, 248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, et al. , 2009. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 204, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, et al. , 2013. Diurnal and seasonal variations of black carbon and PM2.5 over New Delhi, India: Influence of meteorology. Atmospheric Research. 125–126, 50–62. [Google Scholar]
- Tonne C, et al. , 2007. A case-control analysis of exposure to traffic and acute myocardial infarction. Environ Health Perspect. 115, 53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, et al. , 2011. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 119, 873–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, et al. , 2013. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 70, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, et al. , 2009. Association of traffic-related air pollution with children’s neurobehavioral functions in Quanzhou, China. Environ Health Perspect. 117, 1612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, et al. , 2012. Particulate matter air pollution disrupts endothelial cell barrier via calpain-mediated tight junction protein degradation. Part Fibre Toxicol. 9, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. , 2017. Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease. J Appl Toxicol. 37, 644–667. [DOI] [PubMed] [Google Scholar]
- Winklewski PJ, et al. , 2018. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Frontiers in neurology. 9, 92–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward NC, et al. , 2018. Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Translational psychiatry. 8, 261–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward NC, et al. , 2017a. Traffic-related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiology of Aging. 53, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward NC, et al. , 2017b. Traffic-related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiol Aging. 53, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J, Ding Y, 2016. Pathophysiological effects of particulate matter air pollution on the central nervous system. Environmental Disease. 1, 85–89. [Google Scholar]
- Xie Y, et al. , 2015. Daily Estimation of Ground-Level PM2.5 Concentrations over Beijing Using 3 km Resolution MODIS AOD. Environmental Science & Technology. 49, 12280–12288. [DOI] [PubMed] [Google Scholar]
- Yuan D, et al. , 2018. Nest-building activity as a reproducible and long-term stroke deficit test in a mouse model of stroke. Brain and behavior. 8, e00993–e00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, et al. , 2017. Spatiotemporal prediction of continuous daily PM2.5 concentrations across China using a spatially explicit machine learning algorithm. Atmospheric Environment. 155, 129–139. [Google Scholar]
- Zhou R, et al. , 2014. Study on the traffic air pollution inside and outside a road tunnel in Shanghai, China. PloS one. 9, e112195–e112195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Exposure apparatus.