Abstract
Children with ADHD show developmentally abnormal levels of mirror overflow—unintentional movements occurring symmetrically opposite of intentional movements. Because mirror overflow correlates with ADHD behavioral symptoms, the study of disinhibition in motor control may shed light on physiologic mechanisms underlying impaired behavioral/cognitive control. This is a case-controlled study of EEG recording from 25 children with ADHD and 25 typically-developing (TD) controls performing unilateral sequential finger-tapping, with overflow movements measured using electronic goniometers. Consistent with previously published findings, children with ADHD showed increased mirror overflow as compared with TD peers. EEG findings revealed less lateralized alpha modulation (event-related desynchronization; ERD) and decreased magnitude of beta ERD in ADHD; both alpha and beta ERD reflect cortical activation. Moderation analysis revealed a significant association between beta ERD and overflow, independent of diagnosis; and an equivocal (p=0.08) effect of diagnosis on the relationship between alpha ERD and overflow, with a significant effect in children with ADHD but not TD children. These results suggest two mechanisms involved with mirror overflow: one reflected in beta ipsilateral to the intentional movement and relevant to both children with ADHD and controls, and the other seemingly more specific to ADHD (alpha, contralateral to movement).
Keywords: EEG, motor control, sensory-motor rhythm, moderation analysis
Graphical Abstract
Children with ADHD have unexpectedly large amounts of involuntary movement in one hand when they voluntarily move the other hand. During a finger-tapping task, children with ADHD showed atypical activation on EEG in two frequency bands, alpha and beta. Variability in beta activation was associated with involuntary movements in both controls and children with ADHD, whereas variability in alpha activity was relevant only in children with ADHD.
INTRODUCTION
Mirror overflow is a commonly observed developmental phenomenon, defined as the unintentional movement of a symmetrically homologous body part during intentional, unilateral movements (Denckla, 1985). Mirror overflow disappears by older adolescence and adulthood (Lazarus & Todor, 1987; Larson et al., 2007) in typically developing individuals. In children with attention-deficit/hyperactivity disorder (ADHD) however, overflow movements persist long after they would otherwise be expected to remit (Mostofsky et al., 2003b; Cole et al., 2008; MacNeil et al., 2011). Moreover, the magnitude of these motor atypicalities correlates with cognitive control tasks and measures of clinical severity (Mostofsky et al., 2003a), suggesting they have shared neurobiology with the other aspects of ADHD and therefore serve as a model system for the study of neurophysiological mechanisms contributing to ADHD.
Unilateral sequential finger tapping tasks have been well characterized as to their ability to elicit overflow movements in children with ADHD (Mostofsky et al., 2006; MacNeil et al., 2011). EEG, particularly in task-related contexts, has long been used to measure directly the activity of central motor regions. The mu rhythm, or sensory-motor rhythm, is comprised of alpha and beta components. Suppression of mu as a whole has been used to report on the level of activation of the motor system in a variety of contexts (Pfurtscheller, 1981; Pfurtscheller & Neuper, 1994; Neuper et al., 2006), but the two frequency components are dissociable in terms of generators (Salmelin et al., 1995) and in terms of functional implications.
Alpha-band activity is, within multiple brain regions and task conditions, often understood to be inhibitory and mechanistically related to the inhibition of task-irrelevant cortical areas (Jensen & Mazaheri, 2010). Suppressive modulation, or event-related desynchronization (ERD) of alpha, then, reflects cortical activation. In hemi-field sensory attentional contexts, cortical hemispheric lateralization of alpha ERD corresponds to lateralization of attentional deployment (Ikkai et al., 2014; Vollebregt et al., 2015; Blacker et al., 2016). Children with ADHD have shown an impaired ability to modulate alpha ERD asymmetrically, with corresponding deficits in lateralized attention (ter Huurne et al., 2013).
Within the context of motor function specifically, data show alpha oscillations originate around the central sulcus (Salmelin et al., 1995). Alpha ERD (motor cortical activation) during unilateral movement is bilateral but greater in the hemisphere that is contralateral to the movement (Jensen & Mazaheri, 2010).
Beta activity is pervasive throughout the motor system, and has been measured not only in cortex, but in brainstem, spinal cord and in muscle. Indeed, there is coherence between cortical beta and muscle beta (Keil et al., 2014). Such activity seems to be driven by cortical sources, as rTMS stimulation of M1 at beta frequencies results in time-locked activity measurable by EMG (Romei et al., 2016). Beta ERD in bilateral central motor regions during finger movement tasks is seen to increase in magnitude with age (Kurz et al., 2016), as is the related (but separate) phenomenon of post-movement beta rebound (Gaetz et al., 2010).
Our primary goal was to evaluate task-related modulation of inhibitory cerebral activity (alpha and beta oscillations) within the context of a unilateral sequential movement task which is known to manifest reduced inhibition of mirror overflow movements in children with ADHD. Given that unilateral movement in neurotypical individuals is accompanied by lateralized alpha ERD, maximal in the hemisphere contralateral to the movement, our primary prediction was that children with ADHD and mirror overflow would show decreased lateralization of alpha ERD driven by increased ERD in the hemisphere contralateral to the overflow movement (and ipsilateral to the intentional movement).
Beta ERD was examined in an exploratory fashion.
MATERIALS AND METHODS
Subjects
We analyzed data from 25 children, aged 8–12 years, with ADHD (18 male) and 25 age-matched typically-developing (TD) controls (19 male). All included subjects were recruited between January, 2015 and January, 2018 from local schools and community organizations (TD controls), and from several ADHD-focused clinics, from general pediatricians and from local schools (ADHD group). Written informed consent was obtained from the legal guardians of the 50 pediatric participants in this study, which was approved by the Johns Hopkins Medicine Institutional Review Board. Parent interviews were used for an initial screening, and the Conner’s Rating Scale-Revised (CPRS-R) (Conners et al., 1998) was administered to quantify clinical ADHD symptoms. Children were included in the ADHD group if they met criteria on the rating scale. Then the diagnosis of ADHD, as well as any comorbidities, was confirmed using a structured interview, the Diagnostic Interview for Children and Adolescents, Fourth Edition; (DICA-IV) (Reich et al., 1997) or Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS) (Kaufman, 2013), administered by a master’s-level psychologist, and by the clinical impression of a board-certified pediatric neurologist with extensive experience in ADHD research (SHM). Participants regularly taking stimulant medication had at least a 24-hour wash-out period prior to testing. Children were included in the typically developing (TD) control group only if they did not meet diagnostic criteria on either the CPRS-R or the DICA-IV. Additionally, children were excluded from both groups if they had histories of neurological illness or injury, seizures, intellectual disability or left-handedness/mixed dominance, as assessed by the Edinburgh Handedness Inventory (≤ 0.5) (Oldfield, 1971). All children were screened with the Wechsler Intelligence Scale for Children (WISC-IV (Wechsler, 2003) or WISC-V(Wechsler, 2014)). Children a full-scale IQ < 80 were excluded. Between-group comparisons in IQ were made using the General Ability Index (GAI), as it does not take into account processing speed, a known deficit in ADHD (Thaler et al., 2013).
Behavioral paradigm
Following a 1-second baseline period for each trial, subjects sequentially approximated their fingers (d2-d3-d4-d5) to their thumb with self-paced timing while visually fixating on a computer monitor. Joint displacements were measured using electronic goniometers (Biopac Systems Inc., Goleta, CA). Subjects performed five blocks of 20 trials lasting seven seconds each, with trials alternating between right- and left-hand finger tapping (RHFT, LFHT). Whether the first trial was LHFT or RHFT was randomized across participants.
Behavioral overflow measurement
Overflow was calculated in line with previous reports from our laboratory (MacNeil et al., 2011). Total overflow was calculated as the sum of angular displacement from the baseline resting hand position in the hand contralateral to voluntary tapping. Total overflow was averaged across RHFT and LHFT blocks separately. Because two different goniometers were used to measure left-hand movements over the course of the study, and because technical factors prevented precise between-device calibration, only right-handed overflow during LHFT was used for overflow correlations to EEG measures.
EEG collection
EEG was collected during finger tapping from 47 equidistant electrodes with full scalp coverage using Advanced Neuro Technologies (Netherlands) asa-lab system, at a 1024 Hz sampling rate and using an anti-aliasing filter (138 Hz cut-off). The electrode cap used active cable-shielding technology. Each channel was referenced to an average of all channels during recording. Electrode impedance was kept below 15 kΩ in all channels. Trials for each subject were excluded during a video analysis if children were observed not to be paying attention, moved out of compliance with visually displayed instructions, or did not complete at least 5 seconds of tapping within that trial. Trials were also excluded if the participant was clenching their hand(s) into fists, scratching, rubbing their face, or making any similar non-task-related movements during a period when they should have been tapping or resting. This criterion was to ensure two seconds of true rest before the trial and to avoid any movements during the tapping period to be mistaken for taps or overflow. TD controls had significantly more trials included in the analysis over the course of the task than children with ADHD (ADHD: 89±11; TD: 95±9; p=0.03).
EEG preprocessing
EEG data were preprocessed using asa-lab version 4 software. Data were high-pass filtered at 0.2 Hz and visually inspected for eye-blinks, horizontal eye movements, and muscle activity. These artifacts could all be identified visually based on well-defined morphology. A Principal Component Analysis (PCA)-based method of removing artifact components within asa-lab was used to remove artifact components that account for >90% of the variance of the artifact subspace. Not a single trial from any subject was removed in the artifact rejection step. To minimize effects of volume conduction, signals were then converted to current source density (CSD) estimates from CSD toolbox (Kayser & Tenke, 2006) in MATLAB (Mathworks, Natick, MA).
ERD analysis
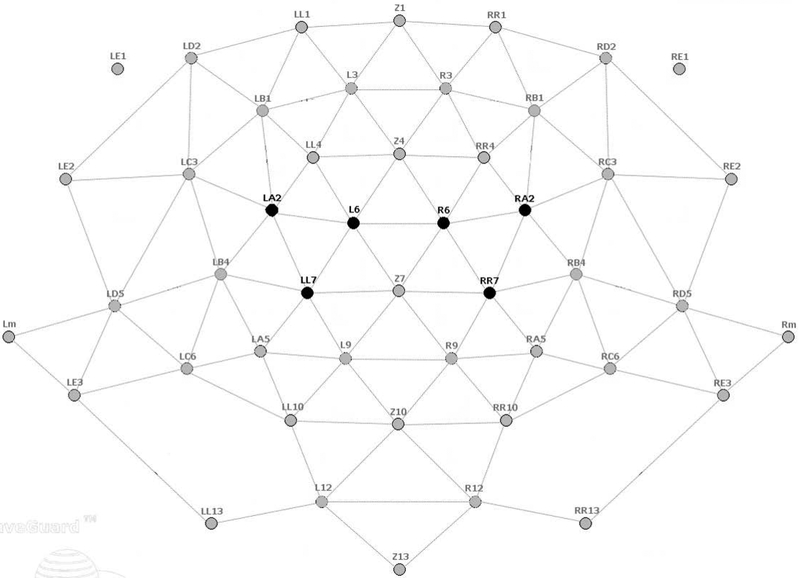
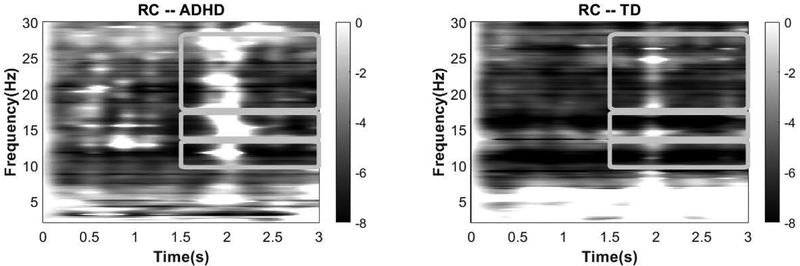
We selected regions of interest (ROI) targeting the left and right primary cortex based on experience with prior motor-control datasets (Pillai et al.; Ewen et al., 2014; Ewen et al., 2016a; Ewen et al., 2016b) (Fig. 1). For ease of language, we will refer to brain activity relative to the side of the intentional movement. Therefore, for LHFT, the right ROI will be referred to as contralateral, and the left ROI will be referred to as ipsilateral. For the RHFT condition, these labels will be opposite. The alpha frequency band was confirmed using spectrograms from both groups (Fig. 2). The beta band is generally defined from 13–30 Hz. ERD was seen in a low-beta band (14–17 Hz). A higher-frequency portion of this range (referred to hereafter simply as “beta”), beginning around 18 or 20 Hz, is typically associated with motor function (Wheaton et al., 2005; Cannon et al., 2014). The spectrogram confirmed ERD within this broad band (18–28 Hz) as well. Analysis parameters were selected before dependent variables were calculated.
Figure 1. The Duke equidistant electrode layout.
The darkened electrodes were used in analysis.
Figure 2. Task-related spectrograms measured in the right central (RC) regions during left-handed finger tapping.
Darker colors indicate greater magnitude of ERD. All bands were measured from 1.5 to 3 sec following tapping onset. The alpha band was measured in the pass-band of 10–13 Hz; low beta at 14–17 Hz (no group differences either in ipsilateral or contralateral ROIs); and beta in 18–28 Hz.
Data were down-sampled to 256 Hz. The EEG analysis of the “active period” was time-locked to the onset of tapping for each trial, as measured by initial goniometer deflection in the tapping hand. The baseline was defined as the 1s prior to the onset of the start-tapping cue. ERD was calculated for each channel as follows: at each time-frequency point during the task, a z-score was calculated relative to a distribution created from the baseline period. We limited our analysis to a 1.5-second window (1.5 – 3s compared with onset of tapping) in the middle of each tapping block, as visual inspection of participant videos in this and previous datasets showed that overflow movements are most likely to start at about 1.5 seconds. By ending the ERD analysis prior to movement offset, we avoided offset-related EEG effects. ERD-related z-scores for each channel were integrated over 384 time-samples (in 1.5 seconds) × 8 frequency bins per Hz. Alpha was calculated as 10–13 Hz (therefore 24 frequency bins); beta, as 18–28, therefore 80 bins. Alpha ERD units (α.u.) are therefore the sum of 9,216 z-scores, and beta units (β.u.) as 30,720 z-scores. To calculate the ERD value for each ROI, the ERD from each of the three channels was averaged across LHFT and RHFT in the respective contralateral and ipsilateral ROI.
To assess task-related alpha ERD laterality, we calculated an asymmetry index (AI) of alpha ERD as (contralateral-ipsilateral)/(contralateral+ipsilateral), and performed a two-sample Student’s t-test between groups as well as within each group (one sample, with respect to H0: AI=0). To assess beta ERD magnitude between groups, we compared the contralateral and ipsilateral between using a two-sample Student’s t-test across diagnoses groups.
A sample size estimation conducted prior to data collection was based on a simple between-group comparison of a single-ROI ERD measurement and assumed a 20% difference in ERD magnitude. Further, setting (1-β)=0.8 and α=0.05, the required sample size was 26 subjects per group.
Clinical correlations
In order to assess the relationship between alpha/beta ERD and mirror overflow, we computed Pearson’s r between the ERD measures and the goniometer measures of overflow in the right hand during LHFT independently in the two groups. To explore the relationship between ERD and broader symptoms of ADHD, we computed Pearson’s r between ERD and CPRS-R DSM ADHD inattention and CPRS-R DSM ADHD hyperactive-impulsive scores independently in each group.
Moderation analysis
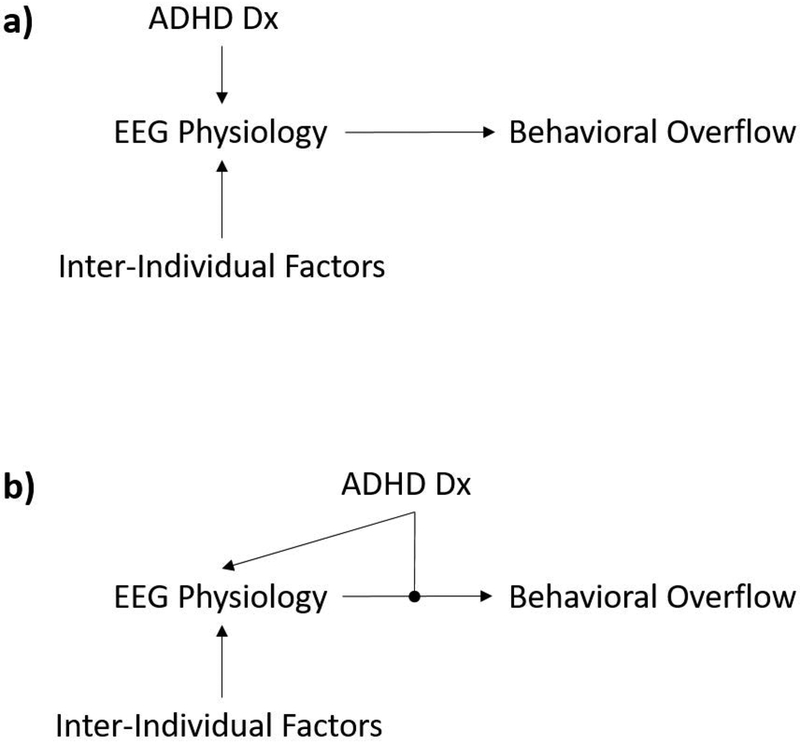
The primary strategy of this analysis was to determine whether children with ADHD have a relationship between physiology (alpha/beta ERD) and mirror overflow movements. A simple moderation analysis (Hayes & Rockwood, 2017) is a rigorous statistical method for determining whether diagnosis (the moderator) influences the relationship between physiology and behavior. Examining where the diagnosis exerts influence is fundamentally important in establishing the proper causal model linking the diagnosis and clinical phenomenology (Fig. 3).
Figure 3. Alternative causal models for the effect of diagnosis on the relationship between physiology (as indexed by ERD) and behavior (mirror overflow movements).
In panel (a), the effect of ADHD diagnosis occurs solely upstream of the physiology indexed by ERD. In panel (b), the effect of ADHD diagnosis occurs upstream of the physiology (as evidenced by group differences in distribution of ERD values), but also has a moderating effect on the link between physiology and behavior.
RESULTS
Demographics
Table 1 shows sex, IQ, and ADHD clinical severity scores. There were no statistical between-group differences in age or sex proportion. Because the groups differed statistically on GAI, the statistical analyses that follow were, post hoc, adjusted for GAI, and the results of these post hoc analyses were presented if controlling for GAI changed the interpretation (statistical significance or direction of results). One subject had an Edinburgh score < 0.5 but was included because his PANESS testing revealed a right-hand preference.
Table 1.
Participant Demographics
| TD (mean ± SD) | ADHD (mean ± SD) | p | |
|---|---|---|---|
| Age (years) | 10.7 ± 1.3 | 10.3 ± 1.3 | 0.36 |
| GAI | 113± 12 | 103±14 | 0.008 |
| Conners ADHD Inattention | 4±4 | 20± 6 | <0.0001 |
| Conners ADHD Hyperactivity/Impulsiveness | 3.4±3 | 18±8 | <0.0001 |
| Total n (males) | 25 (19 M) | 25 (18 M) |
Behavioral overflow measures
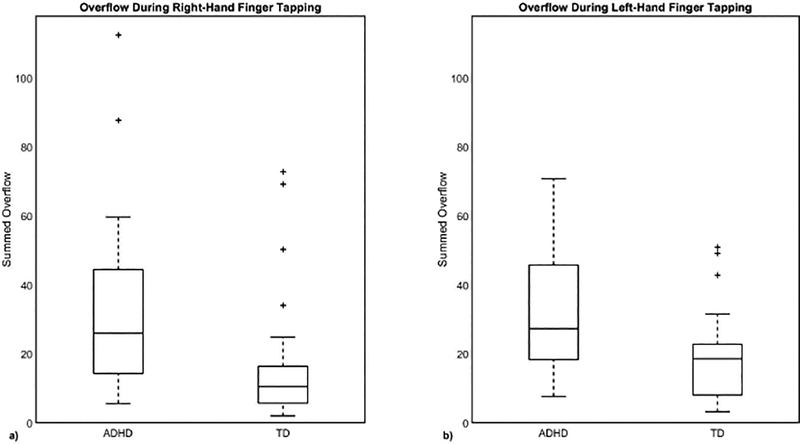
Consistent with previously published findings (Mostofsky et al., 2003b; Cole et al., 2008; MacNeil et al., 2011), children with ADHD showed significantly more mirror overflow movements than did TD children, with effect size greater for LHFT (95% confidence interval of the difference of means, TD minus ADHD: −23.7° to −5.4°, Cohen’s d=0.90, p=0.003) than RHFT (95% confidence interval: −29.9° to −3.9°, Cohen’s d=0.74, p=0.012) (Fig. 4).
Figure 4. Children with ADHD showed more overflow on average than controls.
This was true in both (a) right (p = 0.02) and (b) left-handed finger tapping (p = 0.006).
Alpha ERD asymmetry
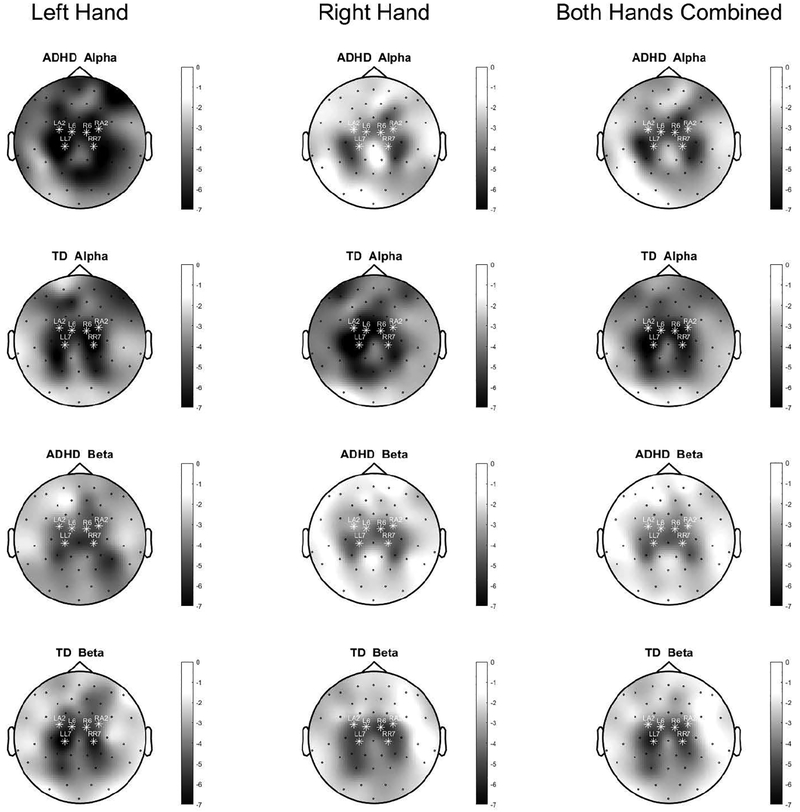
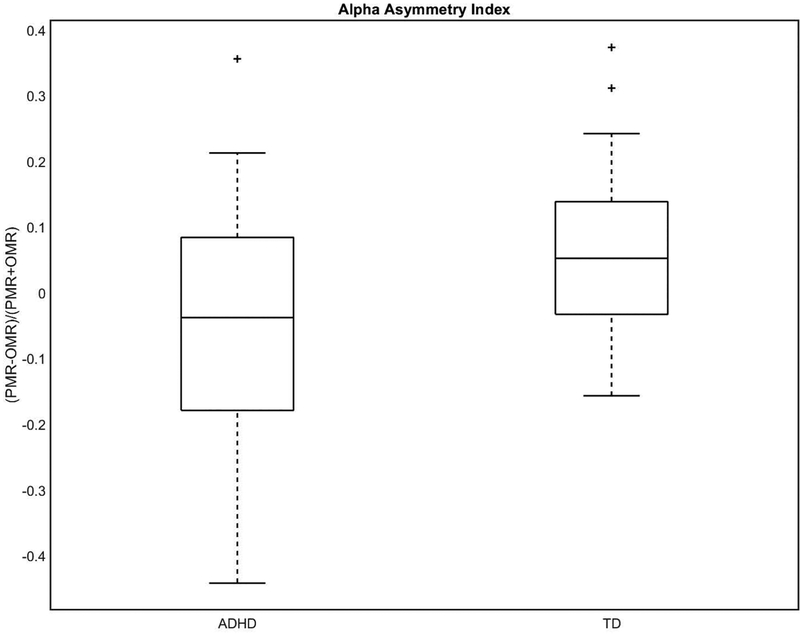
Topographical plots of ERD are shown in Fig. 5. TD controls showed a significant asymmetry (95% confidence interval of mean AI, 0.0028 to 0.12 α.u., Cohen’s d=0.43, p=0.04), with contralateral > ipsilateral (i.e., greater activation in the hemisphere contralateral to intentional movement). In contrast, children with ADHD showed no significant alpha activation laterality (p=0.2). There was a significant between-group AI difference (95% confidence interval of the difference in means, TD minus ADHD 0.013 α.u. to 0.20 α.u., Cohen’s d=0.64, p=0.02) (Fig. 6). On post hoc analysis, it was group differences in contralateral rather than, as predicted, differences in ipsilateral which drove the group differences in AI (contralateral alpha ERD TD>ADHD; p=0.02). As a consequence, contralateral alpha ERD was used for subsequent correlations. Controlling for GAI did not change the interpretation regarding between-group AI.
Figure 5. Topographical plot of ERD associated with left-handed finger tapping.
The greatest degree of ERD is in bilateral motor regions. White stars indicate the channels which were used for statistical analyses. The first column is restricted to left-handed finger tapping; the second, to right-handed finger tapping; and the third averages the two task conditions.
Figure 6. TD controls showed greater alpha asymmetry (distance from zero) than children with ADHD (p=0.02).
Further, the TD group showed an alpha asymmetry that was statistically different from zero (p=0.04), whereas the ADHD group did not (p=0.2).
Beta ERD
Low-beta was not statistically different between groups in either ipsilateral or contralateral ROIs and was not analyzed further. Children with ADHD showed significantly less ipsilateral beta ERD compared with TD controls (95% confidence interval of the difference, TD minus ADHD −3630 to −182.9 β.u., p=0.031). There was no between-group difference in contralateral beta ERD (p=0.208). Within the ADHD group, ipsilateral beta ERD correlated weakly (not statistically significantly) with contralateral alpha ERD (r=0.36; p=0.081).
ERD-overflow correlations
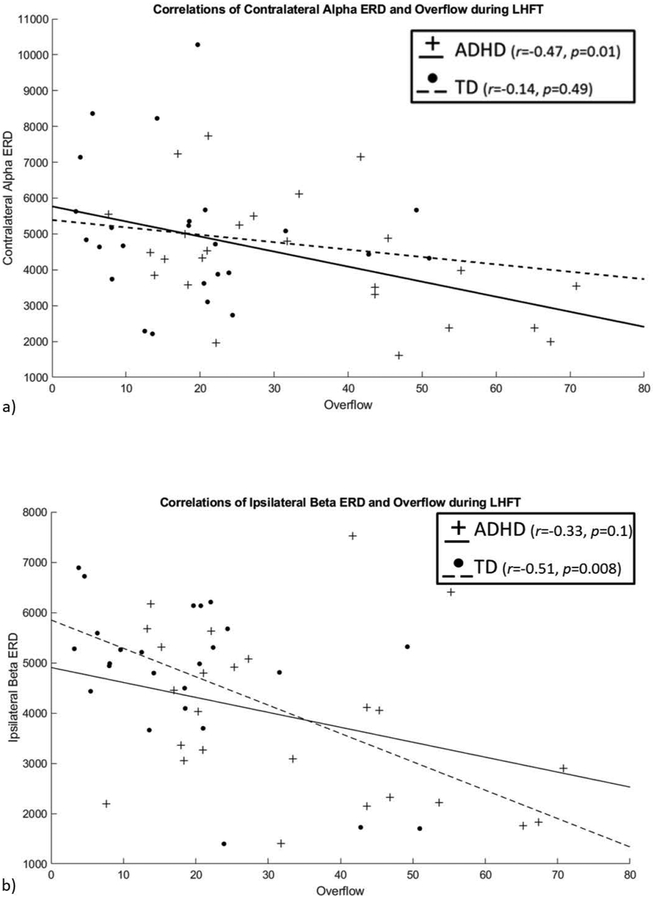
For contralateral alpha ERD, there was a significant correlation with overflow in children with ADHD (r=−0.47, p=0.01); this relationship was not present in the TD group (r=−0.14, p=0.49) (Fig. 7(a)). There was no relationship between alpha AI and overflow in either group (ADHD: r=0.11, p=0.5; TD: r=−0.16, p=0.4). For ipsilateral beta ERD, there was a significant correlation with overflow (r=−0.51, p=0.008) in TD children; a similar relationship was present, but not significant in the ADHD group (r=−0.33, p=0.1) (Fig. 7(b)). Controlling for GAI did not affect the interpretation of any of these correlations.
Figure 7. Scatterplot of (a) alpha and (b) beta ERD by overflow, by group.
There was a significant correlation in the ADHD group but not the TD group between alpha ERD and mirror overflow. There was a significant correlation within the TD group but not the ADHD group between beta ERD and mirror overflow.
ERD-symptom correlations
Table 2 shows correlations between ERD and CPRS-R DSM ADHD scales. For ipsilateral beta ERD, the TD group showed correlations with the CPRS-R DSM ADHD Hyperactivity/Impulsive scale (r=−0.42, p=0.03). We also note statistical uncertainty in trend-level correlations with the Inattentive scale (r=−0.35, p=0.09). For contralateral alpha ERD, there was statistical uncertainty in trend-level evidence for correlation within the ADHD group with CPRS-R DSM ADHD scales (Hyperactive/Impulsive: r=−0.38, p=0.06; Inattentive: r= −0.34; p=0.095).
Table 2. Correlations between ERD and CPRS-R scores.
H/I = Hyperactive; Inatt = Inattentive
| TD | ADHD | |
|---|---|---|
| CPRS-R ADHD H/I and Contralateral Alpha ERD | r = −0.152, p = 0.468 | r = −0.38, p = 0.06 |
| CPRS-R ADHD Inatt and Contralateral Alpha ERD | r = −0.059, p = 0.778 | r = −0.35, p = 0.095 |
| CPRS-R ADHD H/I and Ipsilateral Beta ERD | r = −0.42, p = 0.03* | r = 0.287, p = 0.165 |
| CPRS-R ADHD Inatt and Ipsilateral Beta ERD | r = −0.34, p = 0.09 | r = 0.077, p =0.720 |
Moderation analysis
To examine the moderating effect of diagnosis on the relationship between contralateral alpha ERD and overflow, we performed a regression analysis including an interaction between contralateral alpha ERD and diagnosis. For the ADHD group, the association between contralateral alpha ERD and overflow was significant, while for the TD group (see Fig. 7(a)), the association was insignificant. The ERD × diagnosis interaction term (which reports on the moderating role of diagnosis) had a p-value of 0.08. For ipsilateral beta ERD, both groups showed a significant association between ERD and overflow within this model (Fig. 7(b); note that while the Pearson’s correlation within the ADHD group was not significant, the association between ERD and overflow in the ADHD group in this model was significant, likely because the model reported in this section takes into account the variance from participants from both groups, and power is effectively increased). The interaction term however was not significant (p=0.73), suggesting that the regression line was statistically indistinguishable for both groups, and there was no moderating effect of diagnosis. Controlling for GAI did not markedly affect the interpretation in either alpha or beta, though it did increase the p-value of the interaction term in the alpha ERD model to 0.12.
DISCUSSION
Consistent with our prediction, we found that alpha ERD during sequential finger tapping was less lateralized in the ADHD group as compared with TD children. This reduced asymmetry, however, was driven by decreased alpha ERD contralateral to the intentional movement. We further found that children with ADHD showed reduced magnitude of beta ERD, specifically in the hemisphere ipsilateral to the intentional movements.
Additionally, we found that these ADHD-associated differences in contralateral alpha ERD and ipsilateral beta ERD were both associated with greater magnitudes of mirror overflow movements. Specifically, among children with ADHD (but not TD children), reduced contralateral alpha ERD was associated with a greater magnitude of mirror overflow movements.
We also note that ipsilateral beta ERD differed between groups and correlated with overflow magnitude in the TD group, without a group moderation effect on the ipsilateral beta ERD-overflow relationship, suggesting that with an increased sample size, we might see associations with overflow movements in both groups.
Moderation analyses (Pearl, 2009), in the context of the current experimental design, allow us to refine the causal models that relate physiology and behavior in ADHD (Morton, 2005). Validating causal models is central to the mechanistic understanding that is fundamental to the scientific enterprise but is also practically important in predicting how putative treatments are likely to be effective or ineffective. Causal understanding is also critical for designing biomarkers that validly report on what they are understood to reflect (Ewen et al., 2019).
In the current dataset, we may consider whether the effect of the ADHD diagnosis is upstream of the physiology that is measured by EEG (Fig. 3a), or whether the effect of diagnosis also moderates the relationship between EEG and behavior (Fig. 3b). The moderation analyses applied to the current data revealed that, for both children with ADHD and TD children, the statistical association between beta ERD and mirror overflow was indistinguishable between groups. Phrased another way, a child with ADHD and a certain magnitude of beta ERD would be expected to have the same amount of overflow as a typically developing peer with equivalent beta ERD. None of these results suggests a difference-of-kind relationship between behavior (overflow) and physiology in ADHD vs. controls.
By contrast, there was trend-level, statistically uncertain evidence that the relationship between alpha ERD and overflow is different in ADHD group than in the TD group (moderation analysis interaction term: p=0.08). This could be due to lack of statistical power or lack of a true effect. There is still uncertainty about which causal model is more valid for alpha ERD. Based on the differential results of the moderation effect and the poor within-group correlation (r=0.36), ERD in the two frequency bands seems to index two different processes.
How might we understand the mechanisms indexed by alpha and beta ERD? Any discussion is speculative, given relatively little knowledge regarding the cortical generators of involuntary movements. One framework that has the potential to encompass our results is that of Hoy and colleagues (Hoy et al., 2004), who explicate two competing hypotheses regarding the generation of mirror overflow movements: the “bilateral cortical activation hypothesis” and the “ipsilateral corticospinal tract (CST) hypothesis.” The first hypothesis proposes that mirror overflow is due to the alteration of the transcallosal influence of the contralateral (to intentional movement) cortical motor regions upon the ipsilateral motor regions; the ipsilateral motor regions then generate the mirror overflow movement through decussating CST. The second hypothesis proposes that mirror overflow movements are generated through overactivity of CST that run without decussation from motor cortex contralateral to the intentional movement to the hand that is also contralateral to the intentional movement. (The CST that run without decussation from primary motor cortex to muscles on the same side of the body are typically referred to as ipsilateral CST, and we try not to confuse the reader with the use of the term “ipsilateral” in a sense that is different from how it is used elsewhere in this manuscript.) The evidence from Hoy et al. indeed suggests that the first hypothesis is true in some disorders and the second in others.
Our a priori hypothesis regarding alpha ERD was in line with the “bilateral cortical activation” account. While we did find decreased alpha ERD asymmetry, these results were driven by decreased contralateral alpha ERD rather than increased ipsilateral alpha ERD; changes in ipsilateral alpha ERD would have been necessary to substantiate the “bilateral cortical activation” account. Similarly, decreased ipsilateral beta ERD in ADHD is not fully consistent with the “bilateral cortical activation” account, as one would have expected increased ipsilateral beta, if the “bilateral cortical activation” hypothesis were true.
On the other hand, although we cannot directly measure the activity of the ipsilateral CST pathway using scalp EEG, it seems more plausible that the scalp correlate of the “ipsilateral CST” hypothesis would have been increased contralateral alpha ERD. While our results do not seem to clearly inform the discussion regarding competing hypotheses around mirror overflow, we can point out that the results are at least convergent with results from a different imaging modality. The alpha results converge with fMRI studies of children with ADHD during a similar finger tapping task, where children with ADHD show a decrease in contralateral activation compared with TDs, and a correlation existed within the ADHD group and not the TD group between contralateral primary motor cortex activation and overflow movement (Gaddis et al., 2015).
Of further interest would be differences in the expression and possibly biology of ADHD in boys vs.girls, given that ADHD mechanisms are thought to differ substantially by sex (Mahone & Wodka, 2008). Our sample size is unfortunately too small to consider the potential role of sex.
In summary, children with ADHD showed increased mirror overflow movements and decreased alpha ERD asymmetry in the context of a unilateral finger tapping task. Alpha ERD differed between groups in the hemisphere contralateral to the intentional movement and correlated with overflow in the ADHD group only. Consistent with prior fMRI findings, these results are consistent with the notion that children with ADHD have a disorder-specific mechanism implicating reduced recruitment of inhibitory neural mechanisms in the contralateral hemisphere in the production of overflow movements. Moreover, children from both groups showed a similar relationship between ipsilateral beta ERD and overflow, consistent with the proposition that the ipsilateral hemisphere has a group-nonspecific mechanism relating to mirror overflow that is indexed by beta ERD.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Mental Health at the National Institutes of health (R01 MH078160-08S1 and R01 MH085328) to SHM.
ABBREVIATIONS
- ADHD
Attention-Deficit/Hyperactivity Disorder
- AI
Asymmetry Index
- α.u.
(investigator-defined) Alpha Units
- β.u.
(investigator-defined) Beta Units
- CPRS
Connors’ Parent Rating Scale
- CSD
Current Source Density
- CST
Corticospinal tract
- d2-d3-d4-d5
Second, third, fourth and fifth digits (i.e., index finger, middle finger, ring finger and pinky, respectively)
- DICA
Diagnostic Interview for Children and Adolescents
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EEG
Electroencephalogram
- EMG
Electromyogram
- ERD
Event-Related Desynchronization
- fMRI
functional Magnetic Resonance Spectroscopy
- GAI
General Ability Index
- Hz
Hertz
- IQ
Intelligence Quotient
- kΩ
Kilo-ohm
- KSADS
Kiddie Schedule for Affective Disorders and Schizophrenia
- LHFT
Left-hand finger tapping
- M1
Primary motor cortex
- PCA
Principal Components Analysis
- PANESS
Physical and Neurological Examination for Soft Signs
- RHFT
Right-hand finger tapping
- ROI
Region of Interest
- rTMS
repetitive Transcranial Magnetic Stimulation
- TD
Typically Developing
Footnotes
COMPETING INTERESTS
None of the authors repors real or apparent conflicts of interest.
DATA ACCESSIBILITY
We intend to make de-identified EEG and behavioral data available on the Open Science Framework, pending approval from NIH.
REFERENCES
- Blacker KJ, Ikkai A, Lakshmanan BM, Ewen JB & Courtney SM (2016) The Role of Alpha Oscillations in Deriving and Maintaining Spatial Relations in Working Memory. Cognitive, Affective, and Behavioral Neuroscience, 16, 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J, McCarthy MM, Lee S, Lee J, Borgers C, Whittington MA & Kopell N (2014) Neurosystems: brain rhythms and cognitive processing. Eur J Neurosci, 39, 705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole WR, Mostofsky SH, Larson JCG, Denckla MB & Mahone EM (2008) Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology, 71, 1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD & Epstein JN (1998) The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol, 26, 257–268. [DOI] [PubMed] [Google Scholar]
- Denckla MB (1985) Revised Neurological Examination for Subtle Signs (1985). Psychopharmacol Bull, 21, 773–800. [PubMed] [Google Scholar]
- Ewen JB, Lakshmanan BM, Hallett M, Mostofsky SH, Crone NE & Korzeniewska A (2014) Dynamics of functional and effective connectivity within human cortical motor control networks. Clin Neurophysiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen JB, Lakshmanan BM, Pillai AS, McAuliffe D, Nettles C, Hallett M, Crone NE & Mostofsky SH (2016a) Decreased Modulation of EEG Oscillations in High-Functioning Autism During a Motor Control Task Frontiers in Human Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen JB, Pillai AS, McAuliffe D, Lakshmanan BM, Ament K, Hallett M, Crone NE & Mostofsky SH (2016b) Practicing Novel, Praxis-Like Movements: Physiological Effects of Repetition. Front Hum Neurosci, 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen JB, Sweeney JA & Potter WZ (2019) Conceptual, Regulatory and Strategic Imperatives in the Early Days of EEG-Based Biomarker Validation for Neurodevelopmental Disabilities. Front. Integr. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddis A, Rosch KS, Dirlikov B, Crocetti D, MacNeil L, Barber AD, Muschelli J, Caffo B, Pekar JJ & Mostofsky SH (2015) Motor overflow in children with attention-deficit/hyperactivity disorder is associated with decreased extent of neural activation in the motor cortex. Psychiatry research, 233, 488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, MacDonald M, Cheyne D & Snead OC (2010) Neuromagnetic imaging of movement-related cortical oscillations in children and adults: Age predicts post-movement beta rebound. NeuroImage, 51, 792–807. [DOI] [PubMed] [Google Scholar]
- Hayes AF & Rockwood NJ (2017) Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav Res Ther, 98, 39–57. [DOI] [PubMed] [Google Scholar]
- Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA & Georgiou-Karistianis N (2004) Investigating the cortical origins of motor overflow. Brain Res Brain Res Rev, 46, 315–327. [DOI] [PubMed] [Google Scholar]
- Ikkai A, Blacker KJ, Lakshmanan BM, Ewen JB & Courtney SM (2014) Maintenance of Relational Information in Working Memory Leads to Suppression of the Sensory Cortex. J Neurophysiol, 112, 1903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O & Mazaheri A (2010) Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Frontiers in Human Neuroscience, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, & Ryan N (2013) Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children – Lifetime Version (Kiddie-SADS-PL 2013 Working Draft), Pittsburgh Pennsylvania: Western Psychiatric Institute and Clinic and Yale University. [Google Scholar]
- Kayser J & Tenke CE (2006) Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin Neurophysiol, 117, 369–380. [DOI] [PubMed] [Google Scholar]
- Keil J, Timm J, Sanmiguel I, Schulz H, Obleser J & Schonwiesner M (2014) Cortical brain states and corticospinal synchronization influence TMS-evoked motor potentials. J Neurophysiol, 111, 513–519. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Proskovec AL, Gehringer JE, Becker KM, Arpin DJ, Heinrichs-Graham E & Wilson TW (2016) Developmental Trajectory of Beta Cortical Oscillatory Activity During a Knee Motor Task. Brain Topogr, 29, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson JC, Mostofsky SH, Goldberg MC, Cutting LE, Denckla MB & Mahone EM (2007) Effects of gender and age on motor exam in typically developing children. Dev Neuropsychol, 32, 543–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JA & Todor JI (1987) Age differences in the magnitude of associated movement. Dev Med Child Neurol, 29, 726–733. [DOI] [PubMed] [Google Scholar]
- MacNeil LK, Xavier P, Garvey MA, Gilbert DL, Ranta ME, Denckla MB & Mostofsky SH (2011) Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology, 76, 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM & Wodka EL (2008) The neurobiological profile of girls with ADHD. Dev Disabil Res Rev, 14, 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J (2005) Understanding Developmental Disabilities: A Causal Model Approach. Wiley-Blackwell, Singapore. [Google Scholar]
- Mostofsky SH, Newschaffer CJ & Denckla MB (2003a) Overflow movements predict impaired response inhibition in children with ADHD. Perceptual & Motor Skills, 97, 1315–1331. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ & Denckla MB (2003b) Overflow Movements Predict Impaired Response Inhibition in Children with ADHD. Perceptual and Motor Skills, 97, 1315–1331. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ & Denckla MB (2006) Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biological Psychiatry, 59, 48–56. [DOI] [PubMed] [Google Scholar]
- Neuper C, Wörtz M & Pfurtscheller G (2006) ERD/ERS patterns reflecting sensorimotor activation and deactivation In Klimesch C.N.a.W. (ed) Progress in Brain Research. Elsevier, pp. 211–222. [DOI] [PubMed] [Google Scholar]
- Oldfield R (1971) The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Pearl J (2009) Causality. Cambridge University Press, Cambridge. [Google Scholar]
- Pfurtscheller G (1981) Central beta rhythm during sensorimotor activities in man. Electroencephalography and Clinical Neurophysiology, 51, 253–264. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G & Neuper C (1994) Event-related synchronization of mu rhythm in the EEG over the cortical hand area in man. Neurosci Lett, 174, 93–96. [DOI] [PubMed] [Google Scholar]
- Pillai AS, McAuliffe D, Lakshmanan BM, Mostofsky SH, Crone NE & Ewen JB Altered task-related modulation of long-range connectivity in children with autism. Autism Research, n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z & Herjanic B (1997) The Diagnostic Interview for Children and Adolescents—IV. Multi-Health Systems, North Tonawanda. [Google Scholar]
- Romei V, Bauer M, Brooks JL, Economides M, Penny W, Thut G, Driver J & Bestmann S (2016) Causal evidence that intrinsic beta-frequency is relevant for enhanced signal propagation in the motor system as shown through rhythmic TMS. NeuroImage, 126, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R, Hamalainen M, Kajola M & Hari R (1995) Functional segregation of movement-related rhythmic activity in the human brain. Neuroimage, 2, 237–243. [DOI] [PubMed] [Google Scholar]
- ter Huurne N, Onnink M, Kan C, Franke B, Buitelaar J & Jensen O (2013) Behavioral consequences of aberrant alpha lateralization in attention-deficit/hyperactivity disorder. Biol Psychiatry, 74, 227–233. [DOI] [PubMed] [Google Scholar]
- Thaler NS, Bello DT & Etcoff LM (2013) WISC-IV profiles are associated with differences in symptomatology and outcome in children with ADHD. J Atten Disord, 17, 291–301. [DOI] [PubMed] [Google Scholar]
- Vollebregt MA, Zumer JM, ter Huurne N, Castricum J, Buitelaar JK & Jensen O (2015) Lateralized modulation of posterior alpha oscillations in children. NeuroImage, 123, 245–252. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003) Wechsler intelligence scale for children, fourth edition. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wechsler D (2014) Wechsler intelligence scale for children-fifth edition. PsychCorp, Bloomington, MN. [Google Scholar]
- Wheaton LA, Shibasaki H & Hallett M (2005) Temporal activation pattern of parietal and premotor areas related to praxis movements. Clin Neurophysiol, 116, 1201–1212. [DOI] [PubMed] [Google Scholar]