Abstract
Dementia has a wide range of reversible causes. Well known among these is depression, though other psychiatric disorders can also impair cognition and give the appearance of neurodegenerative disease. This phenomenon has been known historically as “pseudodementia.” Although this topic attracted significant interest in the 1980s and 1990s, research on the topic has waned. In this paper, we consider reasons for this decline, including objections to the term itself and controversy about its distinctness from organic dementia. We discuss limitations in the arguments put forward and existing research, which, crucially, does not support inevitable progression. We also discuss other neglected masquerades, such as of pseudodementia itself (“pseudo‐pseudodementia”) and depression (“pseudodepression”). Based on this reappraisal, we argue that these terms, while not replacing modern diagnostic criteria, remain relevant as they highlight unique groups of patients, potential misdiagnosis, and important, but neglected, areas of research.
Keywords: Alzheimer's disease, apathy, cognitive impairment, dementia, depression, pseudodementia, reversible dementia
1. INTRODUCTION
When evaluating a patient who has evidence of cognitive decline, a number of reversible causes need to be excluded before one can diagnose a neurodegenerative pathology. One well‐known cause that needs to be excluded is depression. Depression can have significant deleterious effects on cognition, especially in older people and if the depression is severe, to the point where it may be confused with Alzheimer's disease (AD) and other neurodegenerative pathologies. 1 , 2 Less commonly, other psychiatric conditions, such as mania, other psychoses, and conversion disorder, can also impair cognition and produce a similar clinical picture. 1 , 3 Historically, this general phenomenon has been known as “pseudodementia”—defined as a psychiatric condition masquerading as neurodegenerative disease but which is largely reversible when the psychiatric condition resolves or is successfully treated. 1 , 2 The term was attributed to Wernicke, 4 although this diagnostic possibility was known well before then. 5 Despite a flurry of academic activity on the topic in the 1980s and 1990s, interest has now largely subsided (see Figure 1). In this article, we reconsider the empirical evidence for the construct and reasons for this decline. We argue, in particular, that pseudodementia and other related terms, while not supplanting modern diagnostic criteria, serve an important need by identifying unique groups of patients with atypical presentations of their disorders and highlighting potential pitfalls in clinical decision‐making. We also argue that the terms reflect a number of important, but currently neglected, areas of research at the interface between psychiatric and neurodegenerative disease. As such, we call for renewed research on these topics.
FIGURE 1.
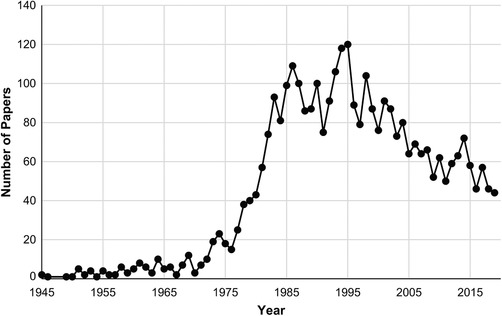
Number of published papers with “pseudodementia” as a keyword each year. Data from PubMed < https://www.ncbi.nlm.nih.gov/pubmed/?term = pseudodementia > (retrieved on 17 February 2020)
2. PSEUDODEMENTIA
2.1. Previous research
Much of the modern interest in pseudodementia can be traced to a 1961 paper by Kiloh, which has been extensively cited. Kiloh 1 presented a case series of 10 patients with an apparent organic dementia that was instead due a psychiatric disorder, including, in current terminology, depression, mania, psychosis, conversion disorder, and malingering. The article highlighted the importance of careful clinical assessment and the reversibility of the condition with appropriate treatment. This was particularly significant given that, at the time, dementia was largely considered to be progressive and irreversible. 6 Recognizing the disorder was thus critical to preventing psychiatric patients from being misdiagnosed as having dementia and being left untreated and/or chronically institutionalized. Importantly, Kiloh argued that the term pseudodementia did not replace existing medical and psychiatric criteria—the correct diagnosis for the patient remained the underlying psychiatric condition from which the patient suffered—but merely described situations in which the clinical picture was potentially misleading and suggested organic dementia. Since Kiloh's paper, many further case reports and group studies of pseudodementia have been published. 2 , 5 , 7
Patients with pseudodementia are consistently found in clinical populations. One population‐based study that recruited patients from primary care practices found depressive pseudodementia in 0.6% of people aged 65 years or older. 8 Higher rates may be found in patients who specifically present for assessment of cognitive decline: Depression alone accounts for between 0.9% and 4.5% of such patients depending on clinical setting. 9 Even higher rates may be found in younger patients who present with cognitive decline, with up to 13% of those younger than 65 years of age with cognitive impairment identified as having pseudodementia. 10 Given this prevalence and the condition's severe cognitive and functional impairments, pseudodementia would appear to pose a significant burden at a population level. Regardless of demographics or setting, the findings indicate that clinicians who see patients with cognitive decline are likely to encounter pseudodementia during their career.
HIGHLIGHTS
Pseudodementia is a psychiatric condition masquerading as neurodegenerative disease
The term refers to a unique group of patients, but is not a formal diagnosis per se
Although historically of great interest, research has waned in recent years
Existing research shows that it poses a large burden and increases risk of dementia
Renewed research is needed to clarify nosology, mechanisms, and management
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the research literature on pseudodementia using online databases. They identified 18 longitudinal studies on clinical outcomes of pseudodementia. Details of the search are cited in the text.
Interpretation: Although pseudodementia presenting later in life (eg, after 73 years of age) is associated with an increased likelihood of subsequently developing organic dementia, pseudodementia presenting earlier in life does not show this effect. Many patients remained burdened by their condition independent of organic dementia, supporting the value and distinctiveness of the construct. Findings were limited by the poor quality of early studies and the lack of recent research.
Future directions: There is a clear need to re‐examine pseudodementia with modern investigative tools. Neuroimaging (eg, positron emission tomography with amyloid and tau ligands), biomarkers of neuroinflammation, and genetic sequencing could help clarify nosology and underlying mechanisms. Clinical trials could help determine effective treatments.
As Kiloh noted, distinguishing pseudodementia from other causes of cognitive decline is important clinically in order to provide appropriate management and to be able to offer accurate information about prognosis to patients and their families. Patients with depressive pseudodementia, in particular, have been reported to respond to a variety of interventions, including antidepressants and electroconvulsive therapy, 7 that are not efficacious in dementia. These studies have been limited by small sample sizes, the lack of a control group, and lack of blinding. 7 Nevertheless, the positive findings—in some cases with extensive follow‐up—and the possibility of reversing impairments with commonly available psychiatric interventions are promising. For patients with pseudodementia due to other psychiatric conditions, management strategies that are effective for the underlying conditions, rather than for dementia, may also be beneficial. 7
Given the differences in management, there has been significant interest in identifying clinical features that differentiate pseudodementia from dementia due to an organic pathology. An influential paper by Wells 11 proposed 22 criteria, suggesting, for example, that patients with pseudodementia were more likely to complain about their cognition, make less effort to complete tasks, and have a pattern of memory loss whereby both recent and remote events were affected equally compared to patients with AD. Other authors have suggested at least a further 20 features, including a past history of affective disturbance, a family history of psychiatric disorders, and response to psychiatric treatment. 3 , 12 The evidence for all these proposals, however, is mixed and limited by the relatively small sample sizes from which they were derived (Wells based his criteria on 10 patients). Nevertheless, one study verified the psychometric properties of 18 features from the literature in a separate sample that included 22 patients with the condition. 12 On this basis, these authors produced a scale capable of distinguishing depressive pseudodementia from dementia with high sensitivity and specificity on initial testing, 12 although further validation has not been completed in the subsequent 20 years.
Other assessments and investigations may be useful in identifying likely pseudodementia. Neuropsychological testing has found that patients with depressive pseudodementia show less effect of cognitive load or test delay on memory performance, 13 less severe short‐term visual memory impairment, 13 and greater proneness to false‐positive errors in memory tasks than patients with dementia. 14 Neuroimaging studies indicate more focal reduction in cerebral blood flow in depressive pseudodementia compared to the more diffuse reductions in blood flow in AD. 15 Other studies have reported differences in event‐related potentials to unexpected stimuli; 16 electroencephalography 17 and amount of rapid eye movement during sleep; 18 and plasma cortisol levels in response to the dexamethasone suppression test, 19 though the latter result was not replicated in another study. 20 Altogether, while certainly not conclusive, the findings suggest that a number of clinical features and investigations could be useful in identifying patients with a higher likelihood of having an underlying psychiatric disorder.
2.2. Reasons for decline in interest in pseudodementia
Despite these suggestive findings, interest and research in pseudodementia have waned in recent times and the term has fallen into disfavor (Figure 1). There appear to be at least three main reasons. The first stems from resistance to the term itself. Critics contend that it is potentially confusing and not helpful diagnostically because other existing labels, such as depression, suffice. 21 Others similarly suggest that the term is misleading because the condition itself constitutes a form of dementia, as there is no longer any requirement for dementia to be progressive or irreversible as it was in Kiloh's time. 22 Such objections, however, miss the point that the term was not intended to replace existing diagnoses. Instead, it was merely considered to be a useful descriptive shorthand for a group of patients who have the outward phenotypic features of an organic dementia but where the underlying diagnosis turns out to be psychiatric. 23 Kiloh 1 noted: “The name can of course have no place in any nosological system; it is purely descriptive and carries no diagnostic weight” (p. 336). The formal diagnosis remains the underlying psychiatric condition responsible for the dementia‐like symptoms. Objections to the term also overlook the non‐depressive causes of pseudodementia, 3 for which there is no obvious alternative descriptor, and the fact that the term, given its long history, is widely understood among clinicians. Regardless of the label affixed, the term refers to a unique group of patients with features and responses to treatment that are distinct from more common presentations of depression, dementia, and other psychiatric disorders.
A second reason for disfavor has been the view that the term obscures the complex relationship between dementia and depression. 21 , 24 In addition to frequent overlap, 25 research has found that depression may be a risk factor for dementia 26 and a prodrome in the months or years prior to dementia's clinical presentation. 27 Other neuropsychiatric symptoms, such as apathy, may similarly presage the onset of dementia. 28 Furthermore, late‐onset depression is itself associated with neurobiological changes—specifically, white matter hyperintensities on magnetic resonance imaging (MRI), 29 so called vascular depression 30 —that are, in turn, also associated with cerebrovascular disease and dementia. 31 Other research has shown that patients with late‐life depression are more likely to have cerebrospinal fluid biomarkers of amyloid peptides resembling those of AD than patients without depression. 32 More recent research also suggests that the loss of biogenic amine nuclei in the early stages of neurodegenerative disease could contribute to depression. 33 Altogether, these findings suggest that depression and dementia are not always clearly distinct and many patients may have overlapping pathologies. In addition, the findings suggest possible biological underpinnings for late‐life depression. These issues, however, are not in themselves problematic for the construct. The term pseudodementia simply describes the clinical phenomenon where the outward appearance of dementia is mimicked by a psychiatric disorder—it does not commit to biological mechanisms, deny the possibility of comorbidity, or require that there be no other relationships between the two disorders. As already noted, the term also encompasses other psychiatric disorders beyond depression. 3
The final and perhaps most important reason for the decline in interest in pseudodementia has been longitudinal studies that have more directly questioned its validity as a clinical construct. A number of studies have shown that the neuropsychological deficits in depression persist, albeit to a lesser extent, even after the depression has remitted. 34 In particular, deficits in memory and executive function, which are present at first presentation, 35 have been found to persist in late‐life depression following treatment. 36 , 37 Although based on depression generally and involving cognitive deficits that are relatively modest compared to those in dementia, these studies raise questions about the supposed reversibility of pseudodementia. More critically, other studies have followed up patients with pseudodementia and examined their cognitive status several years later. Some of these have shown that a high proportion of patients initially diagnosed with pseudodementia subsequently develop frank dementia. 7 This finding seemingly challenged the defining features of pseudodementia as being reversible and distinct from dementia. Not all studies, however, are consistent with this pattern. Sachdev and colleagues followed up 19 patients originally identified by Kiloh with pseudodementia 12 years later and found that only one patient had developed dementia (as a result of Huntington disease, a hereditary condition that was not diagnosed at baseline and which was mistaken for schizophrenia; for a second patient, dementia could not be excluded). 38
2.3. Reconsidering the evidence
As these longitudinal studies have been arguably the main impetus for rejecting the construct, we examined this evidence in more detail. A systematic review we recently conducted 7 identified 15 separate studies that followed up patients with depression as a cause of pseudodementia (see Table 1). Across studies, whereas around 38% of patients developed irreversible dementia, 48% did not. So although pseudodementia may confer an increased risk of irreversible dementia, a significant proportion of patients improved, while many remain burdened with their psychiatric condition, independent of organic dementia. Large differences were noted, however, across studies. Six studies reported that 30% or more of their patients developed irreversible dementia at follow‐up, whereas eight studies reported that none of their patients with depressive pseudodementia developed frank dementia at follow‐up. These differences in outcome were largely independent of duration of follow‐up.
TABLE 1.
Studies that followed‐up patients with depressive pseudodementia (organized by patient's age at baseline)
| Study | n | Mean age at baseline (yrs) a | Follow‐up (years) | Proportion with frank dementia at follow‐up |
|---|---|---|---|---|
| Tsiouris and Patti (1997) | 4 b | 44.0 (4.2) | 0.5‐3.0 | 0 (0%) |
| Sachdev et al. (1990) | 8 | 57.8 (6.1) | 7.9 | 0 (0%) c |
| Allen (1982) | 3 | 60.7 (4.0) | <1.0 | 0 (0%) |
| Stoudemire et al. (1995) | 8 | 67.0 (7.6) | 4.0 | 0 (0%) |
| Reynolds et al. (1987) | 8 | 71.8 (7.7) | 0.1 | 0 (0%) |
| Pearlson et al. (1989) | 15 | 71.9 (1.5) | 2.0 | 1 (7%) |
| Rapinesi et al. (2013) | 20 | 72.7 (5.3) | 0.2 | 0 (0%) |
| Alexopoulous et al. (1993) | 23 | 73.7 (6.8) | 2.7 | 10 (43%) |
| Bulbena and Berrios (1986) | 10 | 75.4 (7.9) | 1.3‐3.9 | 3 (30%) |
| McNeil (1999) | 13 | 76.2 (7.1) | 3.0 | 0 (0%) c |
| Kral and Emery (1989) | 44 | 76.5 (N/R) | 4.0‐18.0 | 39 (89%) |
| Sáez‐Fonseca et al. (2007) | 21 | 77.6 (N/R) | 1.0‐7.0 | 15 (71%) |
| Copeland et al. (1992) | 6 | N/R | 3.0 | 2 (33%) |
| Reding et al. (1985) | 31 | N/R | 2.5 | 16 (52%) |
| Rabins et al. (1984) | 18 | N/R | 2.0 | 2 (11%) |
| Wells (1979) | 6 | N/R | <1.0 | 0 (0%) |
Note. See Connors et al. 7 for more details and references. Only patients with depressive pseudodementia are included in this table.
N/R, not reported.
Standard deviation is shown in brackets.
These four patients also had Down syndrome.
Dementia could not be excluded in one patient at follow‐up.
Age of the sample appears to be a critical factor for these divergent findings (see Table 1). Whereas studies that reported patients with pseudodementia developing frank dementia had samples with a mean age over 73, those that did not tended to have samples with a mean age under 73. 7 This sharp divergence is somewhat surprising and may be due, at least in small part, to the higher rates of dementia with increasing age and in those with depression. A potentially more significant factor may be the imperfect screening of patients in many studies. Most studies reporting that a high proportion of their patients developed dementia did not describe how they excluded dementia at baseline and none required neuroimaging. 7 Given the difficulty in reliably distinguishing pseudodementia from comorbid dementia and depression on clinical grounds, it is possible that a proportion of patients in these studies could have had pre‐existing neurodegenerative disease at baseline. An additional possibility is that there are distinct forms of pseudodementia that are associated with different outcomes and which predominate in different age groups. Regardless of the reasons for the discrepancies, the existing evidence does not support the notions of an inevitable progression from pseudodementia to irreversible dementia or that the construct should be rejected. On the contrary, given the large burden of the condition in its own right and the potential for reversibility, the findings would seem to corroborate the utility of the construct and the need to distinguish it from organic dementia early in its course. 7
A further limitation of existing longitudinal research on pseudodementia is that the vast majority has focused on depression as a cause of pseudodementia and overlooked less common causes (eg, mania, psychosis, conversion disorder). Four of the five longitudinal studies that included patients with other such diagnoses (psychosis, bipolar, conversion disorder) showed a relatively low progression to a non‐reversible dementia (see Table 2). A fifth study found that 25% of non‐depressive pseudodementia—psychosis, bipolar, and personality dementia—developed frank dementia at follow‐up, though the average age of these patients was more than 70 and almost all patients were noted to have comorbid depression. While limited by the sample sizes, the low conversion rate overall seems to support the appropriateness of the initial designation “pseudodementia” for many cases and likewise does not support the view that the construct should be abandoned. In addition, the generally poor quality of existing research points to a clear need for further studies on the prognosis and treatment of psychiatric disorders when they manifest specifically in the form of cognitive impairment.
TABLE 2.
Studies that followed‐up patients with non‐depressive pseudodementia
| Study | n | Mean age at baseline (years) a | Follow‐up (years) | Proportion with frank dementia at follow‐up |
|---|---|---|---|---|
| Conversion disorder | ||||
| Hepple (2004) | 10 | 66.6 (N/R) | 13.4 b | 0 (0%) |
| Liberini et al. (1993) | 6 | 65.5 (4.6) | 2.0 | 1 (17%) |
| Wells (1979) | 2 | N/R | <1.0 | 0 (0%) |
| Psychosis | ||||
| Allen et al. (1982) | 2 | 43.5 (21.9) | <1.0 | 0 (0%) |
| Sachdev et al. (1990) | 6 | 52.3 (13.7) | 11.8 | 1 (17%) |
| Bulbena and Berrios (1986) | 5 c | 82.2 (7.4) | 1.3‐3.9 | 1 (20%) |
| Wells (1979) | 1 | N/R | <1.0 | 0 (0%) |
| Bipolar disorder | ||||
| Allen et al. (1982) | 1 | 34.0 | <1.0 | 0 (0%) |
| Sachdev et al. (1990) | 5 | 52.6 (7.0) | 11.8 | 0 (0%) |
| Bulbena and Berrios (1986) | 5 c | 63.0 (9.3) | 1.3‐3.9 | 2 (40%) |
| Post‐traumatic neurosis | ||||
| Wells (1979) | 1 | N/R | <1.0 | 0 (0%) |
Note. See Connors et al. 7 for more details and references.
N/R, not reported.
Standard deviation is shown in brackets.
This case series reported the duration of time since the onset of patients’ symptoms; the actually clinical follow‐up was unclear from the description.
The majority of these patients were reported to have comorbid depression.
3. PSEUDO‐PSEUDODEMENTIA
A limitation of much existing research on pseudodementia is the cursory screening for organic dementia and hence the difficulty in excluding those with pre‐existing neurodegenerative disease. This highlights another potential scenario whereby neurodegenerative disease is misdiagnosed as a psychiatric disorder, a possibility also noted by Kiloh (1961). This can perhaps be considered as the reverse of pseudodementia—it involves organic dementia masquerading as a purely psychiatric condition. As patients may appear to have pseudodementia, the condition has sometimes been referred to with the term “pseudo‐pseudodementia”. 24 , 39 While again not intended to replace existing diagnoses, the term serves to highlight a more general clinical situation whereby psychiatric symptoms that are typical of a primary psychiatric disorder can be misleading and mask the actual underlying diagnosis of neurodegenerative disease. Irrespective of the label, it appears to be a relatively common occurrence. One study found that around 28% of patients with a neurodegenerative disease had received a primary psychiatric diagnosis between the onset of their symptoms and their final diagnosis. 40 Patients with behavioral variant frontotemporal dementia (bvFTD) seem to be at particular risk, with 51% receiving a prior psychiatric diagnosis compared to 23% with AD. 40 For both groups, the most common psychiatric diagnosis given was depression, followed, in the case of bvFTD, by bipolar disorder and schizophrenia. 40 The frequency of misdiagnosis, even in AD, highlights the diagnostic challenges involved and raises questions about whether participants with the condition were inadvertently included in the longitudinal studies of pseudodementia.
Neurobiological underpinnings for the psychiatric symptoms in these patients have been described. Depression in later life, for example, has been associated with cerebrovascular damage, visible as white matter hyperintensities on MRI. 29 This has led some to propose a causal relationship, 30 whereby damage to white matter and microvasculature in subcortical regions disrupts neural connectivity and produces clinical symptoms. 41 Other explanations, such as a common underlying mechanism that contributes to both depression and cerebrovascular changes, are also possible. Other behavioral changes, such as disinhibition and apathy, have been associated with deterioration of a number of specific brain regions, such as prefrontal and temporal cortices, suggesting a role for these regions. 42 Altogether, rather than undermining the construct of pseudodementia, these findings and the existence of pseudo‐pseudodementia indicate greater complexity than the simple dichotomy of dementia and pseudodementia. It also points to the challenges in distinguishing pseudodementia from pseudo‐pseudodementia and determining whether psychiatric symptoms are a mimic or consequence of neurodegeneration. With the increasing recognition that other neurological disorders, such as epilepsy and autoimmune encephalitis, can present with stereotypical psychiatric symptoms, further research into underlying mechanisms and the role of discriminatory investigations is clearly needed.
4. PSEUDODEPRESSION
The binary opposition between depression and neurodegenerative disease is further complicated by the fact that other conditions can mimic both disorders. Chief among these conditions is apathy—defined primarily as a loss or absence of motivation in combination with associated behavioral and affective changes. 43 , 44 It can be distinguished from depression by the fact that depression predominantly affects mood, whereas apathy predominantly affects volition. In practice, though, the distinction in not always easy to make. The two syndromes often co‐occur and can share overlapping features, including flattened mood, anhedonia, and loss of interest in activities. Both are also among the most common neuropsychiatric symptoms in dementia 25 and after stroke. 45 As a result, it may mimic or co‐exist with both depression and neurodegenerative disease. Nevertheless, a large body of evidence points to the separateness of the symptoms in terms of clinical manifestations, neurobiology, time course, and treatment response. 46 , 47 Possible clinical features that may distinguish apathy and depression are listed in Table 3.
TABLE 3.
Possible features that could distinguish apathy and depression
| Apathy | Depression | |
|---|---|---|
| Emotion | Lack of emotion | Dysphoric, sad, tearful |
| Cognitions and beliefs |
Generalized lack of caring Indifference |
Hopeless, helpless, and worthless Considers there to be no point to life |
| Behaviora | Passive, compliant | May avoid socialization or treatment |
| Vegetative symptoms | Usually absent except loss of interest in food and sex | Often present including changes in sleep, appetite, weight, libido |
| Suicidality | Not suicidal | May be suicidal or express that they would “rather be dead” |
| Rumination | Usually absent | May be present |
| Anxiety | Not usually anxious | May be anxious |
| Counter‐transference | No sadness or despair transmits to clinician | Clinician feels sadness and despair |
| Response to treatment | Poor response to antidepressants | May respond to antidepressants |
| Time courseb | Tends to increase over time in dementia due to the deterioration of brain regions responsible for motivation |
May remain stable, fluctuate, or resolve. In dementia, it may peak in middle stages and decrease in later stages as cognition declines |
Despite these findings, previous studies of pseudodementia have largely neglected this distinction and apathy has not been considered, as far as we are aware, as a cause of a pseudodementia presentation. It is unclear whether, given the similarity between depression and apathy, it could have confounded some previous outcome studies if apathy of dementia was misdiagnosed as depression. These issues point to the existence of a further diagnostic possibility, whereby patients with apparent depression turn out to instead have another condition, namely apathy. Adopting a similar nomenclature as that used to name “pseudodementia,” this alternative category has been referred to as “pseudodepression” 48 —disorders, particularly organic dementia but also other diseases manifesting in apathy, that outwardly masquerade as depression. As for analogous terms, there appears to be a clear need for further research into the underlying cognitive and neurobiological processes and ways of differentiating the disorders despite outwardly similar presentations.
5. CONCLUSION
Depression, apathy, and dementia are common disorders in older people and frequently overlap. Each of these disorders can be mistaken for the other two or confused with other psychiatric disorders altogether. In this regard, we suggest that pseudodementia, pseudo‐pseudodementia, and pseudodepression are useful constructs. Instead of replacing any existing diagnostic categories, the terms simply draw attention to and describe more general clinical situations where the apparent signs and symptoms of a patient are due to a less obvious cause. The terms refer to unique groups of patients whose underlying disorder and actual diagnosis take on a less typical and less recognizable form. Importantly, the terms also highlight potential errors of pattern recognition in clinicians’ decision‐making and serve as a useful reminder of what could be missed. We suggest that these terms have heuristic and clinical implications, especially for treatment and prognostication. In addition, we suggest that the terms point to challenges in applying current diagnostic criteria that rely heavily on patients’ symptoms. These issues may be of particular relevance to recent trends to focus on mechanisms in mental disorders (eg, the National Institute of Mental Health's Research Domain Criteria project, which seeks to organize research into mental disorders along general dimensions of neurobiology and behavior, such as positive and negative valence systems, cognitive systems, and systems for social processes), rather than relying on diagnostic criteria alone.
Given the limitations in existing research and the frequency with which these conditions present, future studies could better characterize their clinical and demographic correlates and longitudinal outcomes. Future research could also incorporate technological advances to investigate factors implicated in neurodegenerative diseases within these different groups of patients. This could include positron emission tomography with amyloid and tau ligands; cerebrospinal fluid biomarkers (eg, amyloid; tau; neurofilament light chain); genetic sequencing (eg, apoE and C9orf72); and other investigations used to assess markers of neuroinflammation. In late‐life depression without pseudodementia, such investigations have revealed associations with some biomarkers, 32 as well as subgroups at greater risk of developing dementia. 49 , 50 Patients with pseudo‐pseudodementia may well demonstrate stronger evidence of neuropathology—such as the presence of amyloid and tau, being APOE ε4 positive, and having high level of interleukin 6 and other inflammatory markers—while patients with true pseudodementia would not. Such research could help clarify issues of diagnosis and improve understanding of the mechanisms that contribute to the unusual presentations of pseudodementia, pseudo‐pseudodementia, and pseudodepression. This, in turn, may also inform management and help to ensure the accurate and timely identification of underlying disorders in the clinic.
ACKNOWLEDGMENTS
The Dementia Centre for Research Collaboration is funded by the National Health and Medical Research Council (NHMRC), Australia.
DECLARATION OF INTERESTS
In the last 3 years, Henry Brodaty has been a consultant and/or advisory board member for Nutricia. Michael Connors has no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Both authors were involved in conceptualizing, writing, and revising this paper for critically meaningful content. Both authors approved the final version.
Brodaty H, Connors MH. Pseudodementia, pseudo‐pseudodementia, and pseudodepression. Alzheimer's Dement. 2020;12:e12027 10.1002/dad2.12027
REFERENCES
- 1. Kiloh LG. Pseudo‐dementia. Acta Psychiatr Scand. 1961;37:336‐351. [DOI] [PubMed] [Google Scholar]
- 2. Burns A, Jolley D. Pseudodementia: history, mystery and positivity In: Bhugra D, Malhi GS, eds. Troublesome Disguises: Managing Challenging Disorders in Psychiatry. 2nd ed Oxford, UK: John Wiley & Sons; 2015:218‐230. [Google Scholar]
- 3. Sachdev PS, Reutens S. The nondepressive pseudodementias In: Oxman TE, Emery VOB, eds. Dementia: Presentations, Differential Diagnosis and the Nosology. 2nd ed Baltimore, USA: The Johns Hopkins University Press; 2003:417‐443. [Google Scholar]
- 4. Bleuler E. Texbook of Psychiatry. New York: MacMillan; 1934. [Google Scholar]
- 5. Bulbena A, Berrios GE. Pseudodementia: facts and figures. Br J Psychiatry. 1986;148:87. [DOI] [PubMed] [Google Scholar]
- 6. Snowdon J. Pseudodementia, a term for its time: the impact of Leslie Kiloh's 1961 paper. Australas Psychiatry. 2011;19:391‐397. [DOI] [PubMed] [Google Scholar]
- 7. Connors MH, Quinto L, Brodaty H. Longitudinal outcomes of patients with pseudodementia: a systematic review. Psychological Medicine. 2019;59:727‐737. [DOI] [PubMed] [Google Scholar]
- 8. Copeland JR, Davidson IA, Dewey ME, et al. Alzheimer's disease, other dementias, depression and pseudodementia: prevalence, incidence and three‐year outcome in Liverpool. Br J Psychiatry. 1992;161:230‐239. [DOI] [PubMed] [Google Scholar]
- 9. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta‐analysis. Arch Intern Med. 2003;163:2219‐2229. [DOI] [PubMed] [Google Scholar]
- 10. Smith JS, Kiloh LG. The investigation of dementia: results in 200 consecutive admissions. The Lancet. 1981;317:824‐827. [DOI] [PubMed] [Google Scholar]
- 11. Wells CE. Pseudodementia. Am J Psychiatry. 1979;136:895‐900. [DOI] [PubMed] [Google Scholar]
- 12. Yousef G, Ryan WJ, Lambert T, Pitt B, Kellett J. A preliminary report: a new scale to identify the pseudodementia syndrome. Int J Geriatr Psychiatry. 1998;13:389‐399. [DOI] [PubMed] [Google Scholar]
- 13. Abas MA, Sahakian BJ, Levy R. Neuropsychological deficits and CT scan changes in elderly depressives. Psychol Med. 2009;20:507‐520. [DOI] [PubMed] [Google Scholar]
- 14. Gainotti G, Marra C. Some aspects of memory disorders clearly distinguish dementia of the Alzheimer's type from depressive pseudo‐dementia. J Clin Exp Neuropsychol. 1994;16:65‐78. [DOI] [PubMed] [Google Scholar]
- 15. Cho MJ, Lyoo IK, Lee DW, et al. Brain single photon emission computed tomography findings in depressive pseudodementia patients. J Affect Disord. 2002;69:159‐166. [DOI] [PubMed] [Google Scholar]
- 16. Gottlieb D, Wertman E, Bentin S. Passive listening and task related P300 measurement for the evaluation of dementia and pseudodementia. Clin Electroencephalogr. 1991;22:102‐107. [DOI] [PubMed] [Google Scholar]
- 17. Reynolds CF, III , Kupfer DJ, Houck PR, et al. Reliable discrimination of elderly depressed and demented patients by electroencephalographic sleep data. Arch Gen Psychiatry. 1988;45:258‐264. [DOI] [PubMed] [Google Scholar]
- 18. Buysse DJ, Reynolds CF, III, Kupfer DJ, et al. Electroencephalographic sleep in depressive pseudodementia. Arch Gen Psychiatry. 1988;45:568‐575. [DOI] [PubMed] [Google Scholar]
- 19. Grunhaus L, Dilsaver S, Greden JF, Carroll BJ. Depressive pseudodementia: a suggested diagnostic profile. Biol Psychiatry. 1983;18:215‐225. [PubMed] [Google Scholar]
- 20. Alexopoulos GS, Young RC, Haycox JA, Shamoian CA, Blass JP. Dexamethasone suppression test in depression with reversible dementia. Psychiatry Res. 1985;16:277‐285. [DOI] [PubMed] [Google Scholar]
- 21. Reifler BV. Arguments for abandoning the term pseudodementia. J Am Geriatr Soc. 1982;30:665‐668. [DOI] [PubMed] [Google Scholar]
- 22. Mahendra B. ‘Pseudodementia’ a misleading and illogical concept. Br J Psychiatry. 1983;143:202. [DOI] [PubMed] [Google Scholar]
- 23. Arie T. Pseudodementia. BMJ. 1983;286:1301‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shraberg D. The myth of pseudodementia: depression and the aging brain. Am J Psychiatry. 1978;135:601‐603. [DOI] [PubMed] [Google Scholar]
- 25. Brodaty H, Connors MH, Xu J, Woodward M, Ames D. The course of neuropsychiatric symptoms in dementia: a 3‐year longitudinal study. J Am Med Dir Assoc. 2015;16:380‐387. [DOI] [PubMed] [Google Scholar]
- 26. Cherbuin N, Kim S, Anstey KJ. Dementia risk estimates associated with measures of depression: a systematic review and meta‐analysis. BMJ Open. 2015;5:e008853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer's disease: a natural history study. J Am Geriatr Soc. 1996;44:1078‐1081. [DOI] [PubMed] [Google Scholar]
- 28. van Dalen J, van Wanrooij LL, Moll van Charante EP, Brayne C, van Gool WA, Richard E. Association of apathy with risk of incident dementia: a systematic review and meta‐analysis. JAMA Psychiatry. 2018;75:1012‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619‐624. [DOI] [PubMed] [Google Scholar]
- 30. Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915‐922. [DOI] [PubMed] [Google Scholar]
- 31. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nascimento KK, Silva KP, Malloy‐Diniz LF, Butters MA, Diniz BS. Plasma and cerebrospinal fluid amyloid‐β levels in late‐life depression: a systematic review and meta‐analysis. J Psychiatr Res. 2015;69:35‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Šimić G, Babić Leko M, Wray S, et al. Monoaminergic neuropathology in Alzheimer's disease. Prog Neurobiol. 2017;151:101‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bora E, Harrison BJ, Yücel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta‐analysis. Psychol Med. 2012;43:2017‐2026. [DOI] [PubMed] [Google Scholar]
- 35. Lee RSC, Hermens DF, Porter MA, Redoblado‐Hodge MA. A meta‐analysis of cognitive deficits in first‐episode major depressive disorder. J Affect Disord. 2012;140:113‐124. [DOI] [PubMed] [Google Scholar]
- 36. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late‐life depression. Am J Psychiatry. 2000;157:1949‐1954. [DOI] [PubMed] [Google Scholar]
- 37. Riddle M, Potter GG, McQuoid DR, Steffens DC, Beyer JL, Taylor WD. Longitudinal cognitive outcomes of clinical phenotypes of late‐life depression. Am J Geriatr Psychiatry. 2017;25:1123‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sachdev PS, Smith JS, Angus‐Lepan H, Rodriguez P. Pseudodementia twelve years on. J Neurol Neurosurg Psychiatry. 1990;53:254‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lishman WA. Organic Psychiatry: The Psychological Consequences of Cerebral Disorder. Oxford, UK: Blackwell Scientific; 1978. [Google Scholar]
- 40. Woolley JD, Khan BK, Murthy NK, Miller BL, Rankin KP. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease; rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72:126‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia. Neurology. 2008;71:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147:22‐30. [DOI] [PubMed] [Google Scholar]
- 44. Starkstein SE, Leentjens AFG. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry. 2008;79:1088‐1092. [DOI] [PubMed] [Google Scholar]
- 45. Hackett ML, Köhler S, O'Brien JT, Mead GE. Neuropsychiatric outcomes of stroke. Lancet Neurol. 2014;13:525‐534. [DOI] [PubMed] [Google Scholar]
- 46. Tagariello P, Girardi P, Amore M. Depression and apathy in dementia: same syndrome or different constructs? A critical review. Arch Gerontol Geriatr. 2009;49:246‐249. [DOI] [PubMed] [Google Scholar]
- 47. Mortby ME, Maercker A, Forstmeier S. Apathy: a separate syndrome from depression in dementia? A critical review. Aging Clin Exp Res. 2012;24:305‐316. [DOI] [PubMed] [Google Scholar]
- 48. Feinberg T, Goodman B. Affective illness, dementia, and pseudodementia. J Clin Psychiatry. 1984;45:99‐102. [PubMed] [Google Scholar]
- 49. Qiu WQ, Zhu H, Dean M, et al. Amyloid‐associated depression and ApoE4 allele: longitudinal follow‐up for the development of Alzheimer's disease. Int J Geriatr Psychiatry. 2016;31:316‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Butters MA, Klunk WE, Mathis CA, et al. Imaging Alzheimer pathology in late‐life depression with PET and Pittsburgh Compound‐B. Alzheimer Dis Assoc Disord. 2008;22:261‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]


