Abstract
Background
Korea is no longer safe from the risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL); the first reported case was a Korean woman in her 40s who had a 7-year-history of receiving an implant-based augmentation mammaplasty using a textured implant. We conducted this study to discuss the emerging crisis of stakeholders in implant-based augmentation mammaplasty and to propose a multi-disciplinary approach to early detection of its complications.
Methods
We analyzed medical examination data that was collected from patients who visited us between August 12 and September 27, 2019. We evaluated a total of 114 women (n = 114) in the current study. They were evaluated for whether they were in healthy condition. Moreover, their baseline characteristics were also examined; these included age, gender, height (cm), weight (kg), duration since surgery (years), possession of a breast implant card, the site of surgical incision, side of symptoms and reasons for outpatient visit. Furthermore, the patients were also evaluated for their subjective awareness of the manufacturer, surface and shape of the breast implant. Potential complications include malrotation, folding, seroma, capsule thickening, upside-down rotation, rupture, capsule mass and breast mass.
Results
A majority of the patients had a past history of receiving textured implants. The corresponding percentage was 78.95% (90/114) and 85.09% (97/114) based on their subjective awareness of a breast implant and sonographic findings, respectively. That is, it was slightly increased with the use of a breast ultrasound.
Conclusion
Here, we propose the following approaches. First, patient data should be prospectively collected. By tracking outcomes and complications of an implant-based augmentation mammaplasty, both high-quality care and patient safety can be ensured. Second, stakeholders in implant-based augmentation mammaplasty should collaborate with customers and regulatory authorities. Third, surgeons should consider applying imaging modalities for early detection of postoperative complications.
Keywords: Breast, Breast Implants, Breast Implantation, Mammaplasty, Safety
Graphical Abstract
INTRODUCTION
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is an extremely rare, non-Hodgkin's lymphoma whose characteristics include abnormal growth of T lymphocytes and over-expression of protein cytokine receptor CD30. It annually occurs at an estimated incidence of approximately 3/100 million women in the United States.1 Possible relationship between vulnerability to BIA-ALCL and placement of a textured breast implant was recently suggested. Cordeiro et al.2 prospectively enrolled a cohort of 3,546 women receiving 6,023 textured implants between 1993 and 2017, all of whom were surgically treated at a median age of 48 (range, 18–89) years old by the same single surgeon and then followed up during a median period of 7 years (range, 3 days to 24.7 years). According to these authors, a total of 8 women developed BIA-ALCL after receiving textured implants during a median period of 11.2 (range, 8.3–15.8) years. The reported incidence corresponds to 1/433 women. According to these authors, 96.7% of textured implants were the BioCell® (Allergan Inc., Irvine, CA, USA); it has been recalled in some countries for safety reasons.2
In March 2017, the US Food and Drug Administration (FDA) released a report of 359 cases and 9 deaths of BIA-ALCL. This was also highlighted by the New York Times article titled “Nine Deaths are Linked to Rare Cancer From Breast Implants.”3
According to the US FDA plan for review of the safety of breast implants dated March 25 and 26, 2019, BIA-ALCL and other systemic symptoms were discussed in relation to implant-based augmentation mammaplasty.4 This was preceded by a compulsory recall request from the Agence Nationale de Sécurité du Médicament, prohibiting the clinical use of the BioCell® (Allergan Inc.).5 Moreover, recall of textured implants in association with BIA-ALCL was also discussed; controversial opinions existed regarding a ban on textured implants.6,7,8,9,10,11,12
A series of patients diagnosed with BIA-ALCL were reported between 2011 and 2017, which influenced viewpoints of the US FDA on it. In 2008, de Jong et al.13 analyzed a population of 8 million people during a 17-year period from 1990 to 2006 and then reported that 389 of them were diagnosed with breast lymphoma, 11 (2.83%) and 5 (1.29%) had a histopathologically-proven diagnosis of ALCL and association with a breast implant, respectively. Then, a total of 173 histopathologically-proven cases of BIA-ALCL were seen in women with a past history of receiving a textured implant.14 Thus, Srinivasa et al.14 performed an analysis of the published incidences of BIA-ALCL and then identified 363 reported cases, including 258 entries in the US Manufacturer and User Facility Device Experience (MAUDE) database, only 130 of which were histopathologically-proven cases of BIA-ALCL. This was followed by published studies about 72 cases in Australia, 41 cases in the UK, 22 cases in Italy, 43 in the Netherlands, 19 in France, 7 cases in Germany and 149 cases in the US.15,16,17,18,19,20,21
Korea is no longer safe from a risk of BIA-ALCL. Recently, it also occurred in Korea; the first reported case was a Korean woman in her 40s who had a 7-year-history of receiving an implant-based augmentation mammaplasty using the BioCell® (Allergan Inc.).22 It is allegedly reported that 222,470 textured breast implants have been circulated in Korea between 2007 and 2018. Of these, 114,365 breast implants were the Allergan products and only 4,560 were manufactured from a Korean company.23
Given the above background, we conducted this study to discuss the emerging crisis of stakeholders in implant-based augmentation mammaplasty and to propose a multi-disciplinary approach to an early detection of its complications.
METHODS
Study patients and setting
The current single-center study analyzed medical examination data that was collected from patients who visited us for further evaluation and treatment between August 12 and September 27, 2019 since the report of the first Korean case of a woman diagnosed with BIA-ALCL.22
Inclusion criteria for the current study are as follows:
1) Women aged 18 years or older
2) Women with a past history of receiving an implant-based augmentation mammaplasty at other hospitals before visiting us.
Exclusion criteria for the current study are as follows:
1) Women lost to follow-up
2) Women who were deemed to be ineligible for the current analysis according to our judgment.
We therefore evaluated a total of 114 women (n = 114) in the current study; it was conducted in compliance with the relevant ethics guidelines; all procedures performed in it were in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Patient evaluation and criteria
On history taking and physical examination, the patients were evaluated for whether they were in healthy condition. Moreover, their baseline characteristics were also examined; these included age, gender, height (cm), weight (kg), duration since surgery (years), possession of a breast implant card, the site of surgical incision, side of symptoms and reasons for outpatient visit. Furthermore, the patients were also evaluated for their subjective awareness of the manufacturer, surface and shape of a breast implant.
The patients underwent breast ultrasound, as previously described.24 We used the Aplio i600 (Canon Medical System, Otawara, Tochigi, Japan) system with a 7–18-MHz linear transducer. Thus, they were further evaluated for the manufacturer, types and shape of the breast implant, any notable signs associated with an implant-based augmentation mammaplasty in the pocket or breast parenchyma and presence of nodules on the breast imaging-reporting and data system category. Potential complications include malrotation, folding, seroma, capsule thickening, upside-down rotation, rupture, capsule mass and breast mass (BM).
Analysis of the patient data
All data was expressed as mean ± standard deviation or the number of patients with percentage, where appropriate.
Ethics statement
The current study was approved by the Internal Institutional Review Board (IRB) of the Korea National Institute of Bioethics Policy (IRB No. 2017-0324-001). Informed consent was waived due to its retrospective nature.
RESULTS
Baseline characteristics of the patients
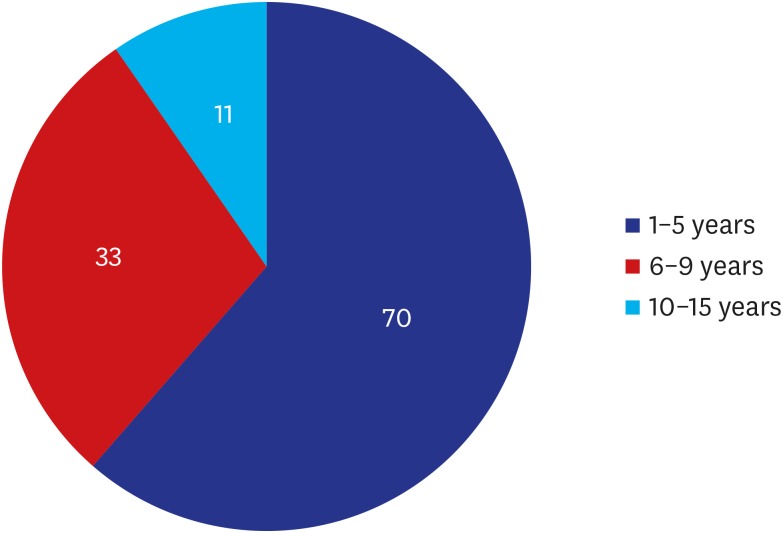
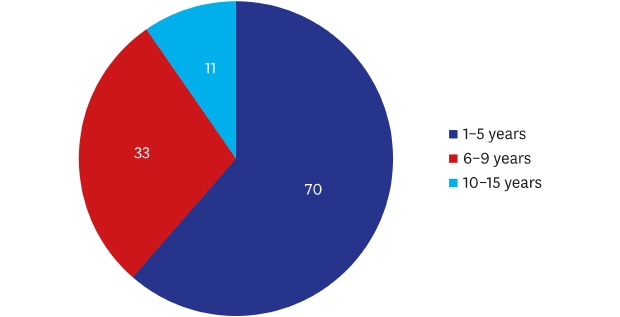
A total of 114 patients (n = 114) were evaluated, all of whom were women with a mean age of 36.60 ± 8.20 years old and had a mean duration of 4.93 ± 2.95 years since surgery. Their baseline characteristics are represented in Table 1. Moreover, distribution of years since surgery is shown in Fig. 1.
Table 1. Baseline characteristics of the patients (n = 114).
| Variables | Values | |
|---|---|---|
| Age, yr | 36.60 ± 8.20 | |
| 20–29 | 25 (21.93) | |
| 30–39 | 48 (42.11) | |
| 40–49 | 31 (27.19) | |
| 50–59 | 9 (7.89) | |
| > 60 | 1 (0.88) | |
| Gender | ||
| Men | 0 (0.00) | |
| Women | 114 (100.00) | |
| Height, cm | 162.88 ± 4.25 | |
| Weight, kg | 52.59 ± 6.82 | |
| Duration since surgery, yr | 4.93 ± 2.95 | |
| 1–5 | 70 (61.40) | |
| 6–9 | 33 (28.95) | |
| 10–15 | 11 (9.65) | |
| Possession of a breast implant card | ||
| Yes | 52 (45.61) | |
| No | 61 (53.51) | |
| Unknown | 1 (0.88) | |
| Site of surgical incision | ||
| Axilla | 68 (59.65) | |
| Peri-areolar region | 10 (8.77) | |
| Inframammary fold | 36 (31.58) | |
| Presence of symptoms | 0 (0.00) | |
| Reasons for outpatient visita | ||
| Deformation or dissatisfaction with shape | 4 (3.51) | |
| Dissatisfaction with softness | 10 (8.77) | |
| Pain | 29 (25.44) | |
| Routine check-up | 94 (82.46) | |
Values are presented as mean ± standard deviation or the number (%).
aMultiple responses allowed.
Fig. 1. Years since surgery. Of the total patients, 38.60% (44/114) had a duration since surgery of 6-15 years during which breast implant-associated anaplastic large cell lymphoma may occur.
The patients' subjective awareness of the breast implant
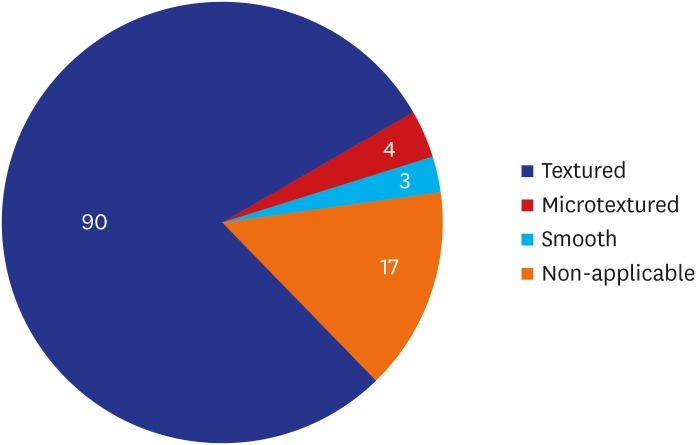
As shown in Table 2, the most prevalent manufacturer, surface and shape of a breast implant were ‘Allergan Inc. (53.50%, 61/114),’ ‘Textured (78.95%, 90/114)’ and ‘Anatomical (50.88%, 58/114),’ respectively. Moreover, distribution of the patients' subjective awareness of the surface of the breast implant is shown in Fig. 2.
Table 2. Distribution of the patients' subjective awareness of a breast implant.
| Variables | Values | |
|---|---|---|
| Manufacturer | ||
| Groupe Sebbin SAS (Boissy-l'Aillerie, France) | 5 (4.39) | |
| HansBiomed Co. Ltd. (Seoul, Korea) | 4 (3.51) | |
| Establishment Labs Holdings Inc. (Alajuela, Costa Rica) | 3 (2.63) | |
| GC Aesthetics PLC (Apt Cedex, France) | 0 (0.00) | |
| Allergan Inc. (Irvine, CA, USA) | 61 (53.50) | |
| Mentor Worldwide LLC (Santa Barbara, CA, USA) | 10 (8.77) | |
| Polytech Health & Aesthetics (Dieburg, Germany) | 10 (8.77) | |
| Sientra, Inc. (Santa Barbara, CA, USA) | 2 (1.75) | |
| Unknown | 19 (10.67) | |
| Surface | ||
| Textured | 90 (78.95) | |
| Microtextured | 4 (3.51) | |
| Smooth | 3 (2.63) | |
| Non-applicable (saline breast implant) | 17 (14.91) | |
| Shape | ||
| Anatomical | 58 (50.88) | |
| Round | 46 (40.35) | |
| Unknown | 10 (8.77) | |
Values are presented as number (%).
Fig. 2. Distribution of the patients' subjective awareness of the surface of a breast implant.
Sonographic findings of a breast implant
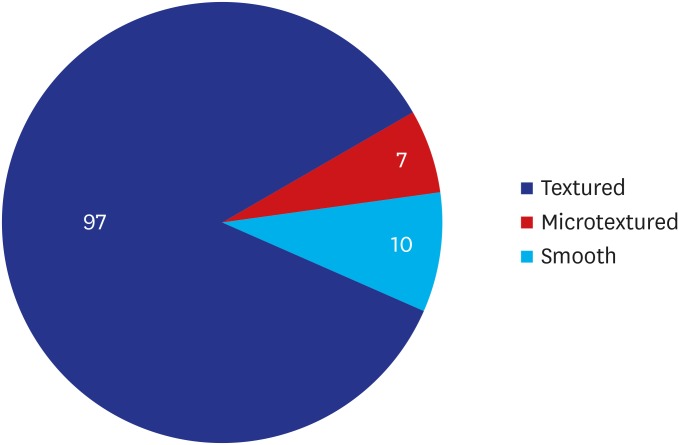
As shown in Table 3, the most prevalent manufacturer, surface and shape of a breast implant were ‘Allergan Inc. (30.70%, 35/114),’ ‘Textured (85.09%, 97/114)’ and ‘Round (51.75%, 59/114),’ respectively. Moreover, distribution of sonographic findings of the surface of a breast implant is shown in Fig. 3. Furthermore, there was an increase in the proportion of the patients receiving textured implants on sonographic findings as compared with their subjective awareness of it (78.95% vs. 85.09%). Sonographic findings of a textured implant are illustrated in Fig. 4.
Table 3. Distribution of sonographic findings of a breast implant.
| Variables | Values | |
|---|---|---|
| Manufacturer | ||
| Groupe Sebbin SAS (Boissy-l'Aillerie, France) | 4 (3.51) | |
| HansBiomed Co. Ltd. (Seoul, Korea) | 3 (2.63) | |
| Establishment Labs Holdings Inc. (Alajuela, Costa Rica) | 2 (1.75) | |
| GC Aesthetics PLC (Apt Cedex, France) | 0 (0.00) | |
| Allergan Inc. (Irvine, CA, USA) | 35 (30.70) | |
| Mentor Worldwide LLC (Santa Barbara, CA, USA) | 4 (3.51) | |
| Polytech Health & Aesthetics (Dieburg, Germany) | 15 (13.16) | |
| Sientra, Inc. (Santa Barbara, CA, USA) | 1 (0.88) | |
| Unknown | 50 (43.86) | |
| Surface | ||
| Textured | 97 (85.09) | |
| Microtextured | 7 (6.14) | |
| Smooth | 10 (8.77) | |
| Non-applicable (saline breast implant) | 0 (0.00) | |
| Shape | ||
| Anatomical | 55 (48.25) | |
| Round | 59 (51.75) | |
| Unknown | 0 (0.00) | |
| Location of pocket | ||
| Subpectoral pocket | 108 (94.74) | |
| Subglandular pocket | 6 (5.26) | |
Values are presented as number (%).
Fig. 3. Distribution of sonographic findings of the surface of a breast implant.
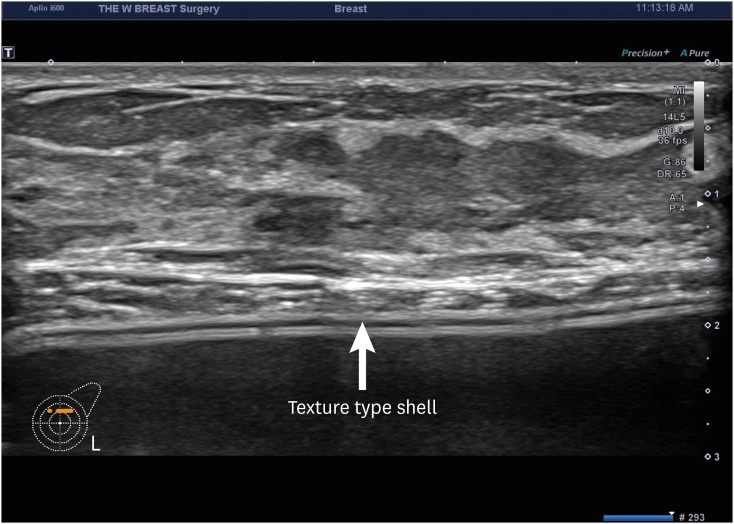
Fig. 4. Sonographic findings of a textured implant. Textured implants are visualized as hypoechoic images on breast ultrasound.
Sonographic findings of complications of an implant-based augmentation mammaplasty
In our series, there were six cases of malrotation, 27 cases of folding, eight cases of seroma, 15 cases of capsule thickening, three cases of upside-down rotation, seven cases of rupture, one case of capsule mass and 76 cases of BM (Table 4).
Table 4. Postoperative complications on breast ultrasound.
| Variables | Values | |
|---|---|---|
| Malrotation | 6 (5.26) | |
| Folding | ||
| Right side | 7 (6.14) | |
| Left side | 8 (7.02) | |
| Both sides | 12 (10.53) | |
| Seroma | ||
| Right side | 1 (0.88) | |
| Left side | 5 (4.39) | |
| Both sides | 2 (1.75) | |
| Capsule thickening | ||
| Right side | 7 (6.14) | |
| Left side | 5 (4.39) | |
| Both sides | 3 (2.63) | |
| Upside-down rotation | ||
| Right side | 1 (0.88) | |
| Left side | 1 (0.88) | |
| Both sides | 1 (0.88) | |
| Rupture | ||
| Right side | 5 (4.39) | |
| Left side | 2 (1.75) | |
| Both sides | 0 (0.00) | |
| Capsule mass | ||
| Right side | 1 (0.88) | |
| Left side | 0 (0.00) | |
| Both sides | 0 (0.00) | |
| Breast mass | ||
| Right side | 16 (14.04) | |
| Left side | 14 (12.28) | |
| Both sides | 46 (40.35) | |
Values are presented as number (%).
DISCUSSION
The recent Allergan breast implant scandal reflects a chronic problem that may inevitably occur as a result of the interaction between the stakeholders in implant-based augmentation mammaplasty. In June of 2016, the Korean Ministry of Food and Drug Safety (KMFDS) approved the clinical use of the Motiva Ergonomix™ (Establishment Labs Holdings Inc., Alajuela, Costa Rica) whose safety has not been yet confirmed in Korean women. This was considered serious because medical devices would not be used for humans unless their safety is confirmed by the FDA in the US. The FDA approval process is well known for its rigorousness. The KMFDS routinely checks if a medical device is approved for its safety by the US FDA during the regulatory approval process. It is also no doubt that the FDA approval is not mandatory for the KMFDS one. But a sufficient level of safety should be ensured for the KMFDS approval of clinical use of a medical device in Korea.25 Lack of safety studies also remains a serious problem in the US. As of March, 2019, the US FDA warned two manufacturers of a silicone gel-filled breast implant, the Mentor Worldwide LLC (Irvine, CA, USA) and the Sientra (Santa Barbara, CA, USA), of not conducting long-term safety studies although they were requirements for the regulatory approval by the US FDA.26 This indicates that there would be a long way for both plastic surgeons and manufactures of breast implants to go if its safety is ensured for patients. On the other hand, the only Korean manufacturer of a silicone gel-filled breast implant, HansBiomed Co. Ltd. (Seoul, Korea), deserves special attention in that they dedicate themselves to assessing the safety of their products, the BellaGel®, in Korean women.27,28
So-called “something-for-something relationship” between plastic surgeons and manufacturers of a breast implant could be found in the US. Shaped, textured breast implants belong to one of the best examples in this case.9 Disadvantages of shaped breast implants include a high rate of malrotation (42%), palpable margins, the possibility of causing double capsule and seroma and high cost as compared with their smooth round counterparts.29,30 Moreover, a high degree of vulnerability to BIA-ALCL and a lack of aesthetic advantage have also been reported to be problems due to the use of textured breast implants.31,32,33,34 Over decades, however, textured, shaped implants have been reported to be better as compared with their lower-cost counterparts. This is in agreement with a previous published study showing that some plastic surgeons form a favorable relationship with manufacturers of a textured, shaped breast implant and are misled by them.35
According to Swanson and Brown, the relationship with manufacturers of a breast implant could also be found in an industry-sponsored peer-reviewed article.12 The authors of that article reported that complications (only one case of hematoma but no cases of malposition, pain, rippling, rupture, erythema and capsular contracture) occurred at an overall incidence of 0.3% and a reoperation rate of < 1% following the use of nano-textured, micro-textured implant in 4,103 cases of augmentation mammaplasty.36 Although the authors of that article declared no conflict of interest relationship with the manufacturer, the corresponding author was designated as a medical advisor immediately after submission to a journal.12
In our series, a majority of the patients had a past history of receiving textured implants. The corresponding percentage was 78.95% (90/114) and 85.09% (97/114) based on their subjective awareness of a breast implant and sonographic findings. That is, it was slightly increased with the use of a breast ultrasound. This is of significance in that 38.60% (44/114) of the patients had a duration since surgery of 6–15 years during which BIA-ALCL may occur. Therefore, such patients should be further evaluated for a risk of BIA-ALCL through our multi-disciplinary algorithm-based approach to an early detection of complications of an implant-based augmentation mammaplasty.
Currently, manufacturers of a breast implant face a crisis from the emergence of BIA-ALCL; they have been subject to myriads of external forces. To overcome this crisis, possible impacts of BIA-ALCL should be rigorously analyzed and appropriate measures should be taken as promptly as possible. We therefore propose the following approaches:
1) Patient data should be prospectively collected. By tracking outcomes and complications of an implant-based augmentation mammaplasty, both high-quality care and patient safety can be ensured.
2) Stakeholders in implant-based augmentation mammaplasty should collaborate with customers and regulatory authorities.
3) Surgeons should consider applying imaging modalities for early detection of complications of an implant-based mammaplasty. From this context, our multi-disciplinary, algorithm-based approach deserves special attention. In compliance with the National Comprehensive Cancer Network (NCCN) guidelines, we recommend that patients with an enlarged breast undergo ultrasound at an initial work-up. Thus, we evaluate them for whether they have fluid collection, a BM or enlarged regional lymph nodes (axillary, supraclavicular and internal mammary). Moreover, we consider the possibility of BIA-ALCL in patients with seroma of non-infectious or non-traumatic origin with a duration of > 1 year after an implant-based augmentation mammaplasty. In such patients, an ultrasound-guided fine-needle aspiration biopsy of seroma or surgical biopsy of capsule and seroma should be performed and then evaluated using immunohistochemistry for the purposes of establishing a diagnosis of BIA-ALCL. This should be followed by a positron emission tomography-computed tomography for the prevention of regional or systemic spread. According to the NCCN, both capsulectomy and implant removal should be considered as first line of treatment for the appropriate management of the affected capsule and a resectable mass. Moreover, chemotherapy should also be performed for patients with an advanced stage of BIA-ALCL involving local lymph or organ metastasis.
ACKNOWLEDGMENTS
This study was supported by the Korean Society of Breast Implant Research.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Data curation: Kim JH.
- Formal analysis: Kim JH.
- Investigation: Kim JH, Cho Y.
- Methodology: Kim JH, Cho Y.
- Project administration: Paik NS.
- Resources: Paik NS.
- Supervision: Park HK.
- Validation: Kim JH.
- Writing - original draft: Kim JH, Paik NS.
- Writing - review & editing: Nam SY.
References
- 1.Ezekwudo DE, Ifabiyi T, Gbadamosi B, Haberichter K, Yu Z, Amin M, et al. Breast implant-associated anaplastic large cell lymphoma: a case report and review of the literature. Case Rep Oncol Med. 2017;2017:6478467. doi: 10.1155/2017/6478467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cordeiro PG, Ghione P, Ni A, Qunying H, Ganesan N, Galasso N, et al. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstr Aesthet Surg. 2020 doi: 10.1016/j.bjps.2019.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook JA, Sasor SE, Tholpady SS, Chu MW, Momeni A. Complexity of health news reporting on breast implant-associated anaplastic large cell lymphoma. Breast J. 2019;25(1):163–165. doi: 10.1111/tbj.13189. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. General and plastic surgery devices panel of the medical devices advisory committee meeting announcement. [Updated 2019]. [Accessed October 5, 2019]. https://www.fda.gov/advisory-committees/advisory-committee-calendar/may-30-31-2019-general-and-plastic-surgery-devices-panel-medical-devices-advisory-committee-meeting.
- 5.CISION PR Newswire. Allergan suspends sales and withdraws supply of textured breast implants in European markets. [Updated 2018]. [Accessed October 5, 2019]. https://www.prnewswire.com/news-releases/allergan-suspends-sales-and-withdraws-supply-of-textured-breast-implants-in-european-markets-300768847.html.
- 6.Rohrich RJ. A review of breast-implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2019;143(3 Suppl):1S–2S. doi: 10.1097/PRS.0000000000005561. [DOI] [PubMed] [Google Scholar]
- 7.Clemens MW. Current controversies in breast implant–associated anaplastic large cell lymphoma. Aesthet Surg J. 2019;39(Suppl 1):S1–S2. doi: 10.1093/asj/sjy308. [DOI] [PubMed] [Google Scholar]
- 8.Deva AK. A perspective on the never-ending cycle of breast implant crises. Aesthet Surg J. 2019;39(4):NP85–NP86. doi: 10.1093/asj/sjz001. [DOI] [PubMed] [Google Scholar]
- 9.Swanson E. Textured breast implants, anaplastic large-cell lymphoma, and conflict of interest. Plast Reconstr Surg. 2017;139(2):558e–559e. doi: 10.1097/PRS.0000000000002966. [DOI] [PubMed] [Google Scholar]
- 10.Swanson E. Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL): why the search for an infectious etiology may be irrelevant. Aesthet Surg J. 2017;37(9):NP118–21. doi: 10.1093/asj/sjx108. [DOI] [PubMed] [Google Scholar]
- 11.Swanson E, Mackay DR. Why the micromort concept falls short in breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) risk analysis. Aesthet Surg J. 2018;38(3):NP68–NP70. doi: 10.1093/asj/sjx237. [DOI] [PubMed] [Google Scholar]
- 12.Swanson E, Brown T. A discussion of conflicts of interest in plastic surgery and possible remedies. Plast Reconstr Surg Glob Open. 2018;6(12):e2043. doi: 10.1097/GOX.0000000000002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jong D, Vasmel WL, de Boer JP, Verhave G, Barbé E, Casparie MK, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300(17):2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasa DR, Miranda RN, Kaura A, Francis AM, Campanale A, Boldrini R, et al. Global adverse event reports of breast implant-associated ALCL: an international review of 40 government authority databases. Plast Reconstr Surg. 2017;139(5):1029–1039. doi: 10.1097/PRS.0000000000003233. [DOI] [PubMed] [Google Scholar]
- 15.ElHawary H, Ghazawi N, Efanov J, Izadpanah A. Abstract 31: a systematic review of breast-implant associated anaplastic large cell lymphoma (BIA-ALCL): past and current knowledge. Plast Reconstr Surg Glob Open. 2019;7(4) Suppl:22–23. [Google Scholar]
- 16.Johnson L, O'Donoghue JM, McLean N, Turton P, Khan AA, Turner SD, et al. Breast implant associated anaplastic large cell lymphoma: The UK experience. Recommendations on its management and implications for informed consent. Eur J Surg Oncol. 2017;43(8):1393–1401. doi: 10.1016/j.ejso.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Campanale A, Boldrini R. BIA-ALCL incidence: the variable to be included in the denominator. Plast Reconstr Surg. 2018;141(5):779e. doi: 10.1097/PRS.0000000000004278. [DOI] [PubMed] [Google Scholar]
- 18.de Boer M, van Leeuwen FE, Hauptmann M, Overbeek LI, de Boer JP, Hijmering NJ, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. 2018;4(3):335–341. doi: 10.1001/jamaoncol.2017.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzal F, Turner SD, Kenner L. Is breast implant-associated anaplastic large cell lymphoma a hazard of breast implant surgery? Open Biol. 2019;9(4):190006. doi: 10.1098/rsob.190006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kricheldorff J, Fallenberg EM, Solbach C, Gerber-Schäfer C, Rancsó C, Fritschen UV. Breast implant-associated lymphoma. Dtsch Arztebl Int. 2018;115(38):628–635. doi: 10.3238/arztebl.2018.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doren EL, Miranda RN, Selber JC, Garvey PB, Liu J, Medeiros LJ, et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017;139(5):1042–1050. doi: 10.1097/PRS.0000000000003282. [DOI] [PubMed] [Google Scholar]
- 22.Lim J. Are ‘water drop’ breast implants a ticking time bomb in the chest? [Updated 2019]. [Accessed September 10, 2019]. http://www.koreaherald.com/view.php?ud=20190820000734.
- 23.“In Korea between 2007 and 2018, women received a total of 222,470 textured breast implants with a risk of breast implant-associated analplastic large cell lymphoma”. [Updated 2019]. [Accessed October 5, 2019]. http://medigatenews.com/news/2630587104.
- 24.Park AY, Seo BK, Cho KR, Woo OH. The utility of MicroPure™ ultrasound technique in assessing grouped microcalcifications without a mass on mammography. J Breast Cancer. 2016;19(1):83–86. doi: 10.4048/jbc.2016.19.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sisa Journal. ‘Exceptional’ approval of a silicone gel-filled breast implant by the Korean Ministry of Food and Drug Safety. [Updated 2017]. [Accessed October 6, 2019]. https://www.sisajournal.com/news/articleView.html?idxno=170545.
- 26.U.S. Food and Drug Administration. FDA issues warning letters to two breast implant manufacturers for failure to comply with post-approval study requirements. [Updated 2019]. [Accessed September 25, 2019]. https://www.fda.gov/news-events/press-announcements/fda-issues-warning-letters-two-breast-implant-manufacturers-failure-comply-post-approval-study.
- 27.Han J, Jeong JH, Bang SI, Heo CY. BellaGel breast implant: 4-year results of a prospective cohort study. J Plast Surg Hand Surg. 2019;53(4):232–239. doi: 10.1080/2000656X.2019.1583572. [DOI] [PubMed] [Google Scholar]
- 28.Sung JY, Jeong JP, Moon DS, Kim MS, Kim HC, Choi WS, et al. Short-term safety of augmentation mammaplasty using the BellaGel implants in Korean women. Plast Reconstr Surg Glob Open. 2019;7(12):e2566. doi: 10.1097/GOX.0000000000002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieber DA, Stark RY, Chase S, Schafer M, Adams WP., Jr Clinical evaluation of shaped gel breast implant rotation using high-resolution ultrasound. Aesthet Surg J. 2017;37(3):290–296. doi: 10.1093/asj/sjw179. [DOI] [PubMed] [Google Scholar]
- 30.Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011;127(1):56–66. doi: 10.1097/PRS.0b013e3181fad34d. [DOI] [PubMed] [Google Scholar]
- 31.Brody GS, Deapen D, Taylor CR, Pinter-Brown L, House-Lightner SR, Andersen JS, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;135(3):695–705. doi: 10.1097/PRS.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 32.Hidalgo DA, Weinstein AL. Intraoperative comparison of anatomical versus round implants in breast augmentation: a randomized controlled trial. Plast Reconstr Surg. 2017;139(3):587–596. doi: 10.1097/PRS.0000000000003114. [DOI] [PubMed] [Google Scholar]
- 33.Friedman T, Davidovitch N, Scheflan M. Comparative double blind clinical study on round versus shaped cohesive gel implants. Aesthet Surg J. 2006;26(5):530–536. doi: 10.1016/j.asj.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Gahm J, Edsander-Nord A, Jurell G, Wickman M. No differences in aesthetic outcome or patient satisfaction between anatomically shaped and round expandable implants in bilateral breast reconstructions: a randomized study. Plast Reconstr Surg. 2010;126(5):1419–1427. doi: 10.1097/PRS.0b013e3181ef8b01. [DOI] [PubMed] [Google Scholar]
- 35.Hall-Findlay EJ. Discussion: late seromas and breast implants: theory and practice. Plast Reconstr Surg. 2012;130(2):436–438. doi: 10.1097/PRS.0b013e31825910cb. [DOI] [PubMed] [Google Scholar]
- 36.Sforza M, Zaccheddu R, Alleruzzo A, Seno A, Mileto D, Paganelli A, et al. Preliminary 3-year evaluation of experience with SilkSurface and VelvetSurface motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J. 2018;38(suppl_2):S62–S73. doi: 10.1093/asj/sjx150. [DOI] [PMC free article] [PubMed] [Google Scholar]