Abstract
Peritoneal carcinomatosis (PC) is progression of the primary cancer to the peritoneum that is seen in only 1.2% of patients with lung cancer. It is associated with poor prognosis especially if present at the time of initial cancer diagnosis. The predisposing factors for peritoneal spread are not yet well understood. It has been suggested that the oncogene status of the tumour can influence the patterns of metastatic spread. There is not enough data about the role of c-ROS oncogene 1 (ROS1) mutation in the development of PC in non-small cell lung cancer. Here, we describe a case of a 56-year-old man who presented with new-onset ascites and was found to have PC. He was diagnosed with ROS1-rearranged lung adenocarcinoma. No obvious primary tumour was identified. Patient responded well to targeted therapy with crizotinib and remained 6 months free of disease progression.
Keywords: lung cancer (oncology), pathology, tyrosine kinase inhibitor
Background
Lung cancer is the leading cause of cancer-related death in adults.1 2 The most common sites of lung cancer metastasis include the pleura, lung parenchyma, bone, liver and brain.3 Peritoneal spread is rare in lung cancer with a reported incidence of 1.2% on clinical diagnosis.4 Peritoneal carcinomatosis (PC) usually develops late in the disease process and is rarely seen at the time of cancer diagnosis.5 6 There are limited data on the factors that predict peritoneal progression. It has been proposed that the oncogene profile of the tumour might predispose to different metastatic patterns.7 Several common mutations have been analysed including epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK) and Kirsten rat sarcoma (KRAS) viral oncogene, but no statistically significant association with PC was found.5 6 On review of the literature published in English, testing for c-ROS oncogene 1 (ROS1) was reported only in three patients with non-small cell lung cancer (NSCLC) with peritoneal spread and returned positive in one of them. Here, we described a case of a 56-year-old man who was diagnosed with PC and ROS1-rearranged NSCLC with no obvious primary tumour identified.
Case presentation
Patient is a 56-year-old man with a history of type I diabetes mellitus, alcohol abuse, never smoker, no intravenous drug use, who presented to the hospital with 1 month of fever up to 38.3°C and night sweats, shortness of breath, non-productive cough, nausea, diarrhoea, progressive abdominal pain and distention. His family history was unremarkable. Patient denied history of asbestos or other occupational exposure. He was initially evaluated as an outpatient, diagnosed with community-acquired pneumonia and treated with antibiotics with minimal improvement of symptoms. On presentation to the emergency room, patient was afebrile, normotensive, hypoxic (oxygen saturation of 93% on room air), with decreased bilateral breath sounds, abdominal distention and diffuse tenderness, with positive fluid wave, bilateral lower extremity oedema and no lymphadenopathy.
Investigations
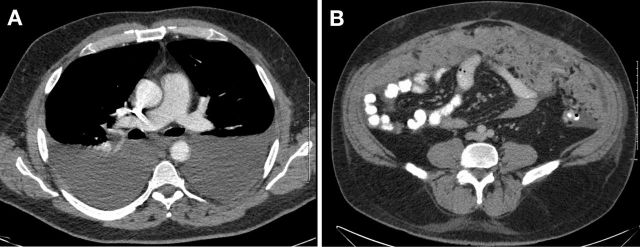
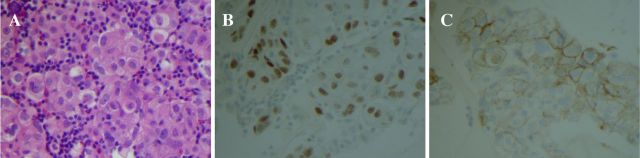
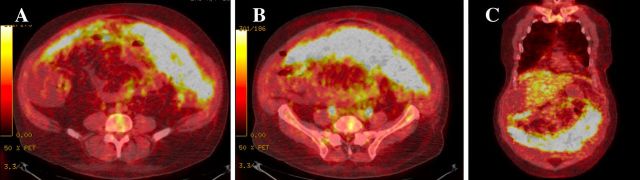
On initial presentation, patient’s lab work revealed leukocytosis, thrombocytosis, normal kidney and liver function tests. Paracentesis drained 3.5 L of greenish fluid. Peritoneal fluid analysis demonstrated 468 polymorphonuclear leukocytes/μl and serum-ascites albumin gradient of 0.8 g/dL. His hepatitis panel, HIV test as well as autoimmune workup came back unrevealing, and tuberculosis test was inconclusive. Chest X-ray revealed new left greater than right bilateral pleural effusions. Abdominal ultrasound demonstrated nodular cirrhotic liver with no vein thrombosis. Patient underwent thoracentesis and pleural fluid cell immunohistochemistry revealed carcinoma cells that were positive for cytokeratin 7 (CK7) and thyroid transcription factor-1 (TTF1) and negative for cytokeratin 20 (CK20) consistent with the diagnosis of pulmonary adenocarcinoma. CT angiography of the chest on admission and non-contrast CT chest after thoracentesis revealed bilateral pleural effusions, but no evidence of primary tumour (figure 1A). Contrast-enhanced CT abdomen and pelvis demonstrated extensive omental nodularity (figure 1B). Brain MRI was negative for metastasis. Peritoneal fluid analysis and core omental mass biopsy supported the initial diagnosis of pulmonary adenocarcinoma, immunohistochemistry was positive for CK7, TTF1, napsin A and negative for CK20, calretinin, hepar-1 stains (figure 2). Flow cytometry of pleural fluid revealed no immunophenotypic evidence of a lymphoproliferative disorder. The analysis of the driving mutations was negative for EGFR and ALK and positive for ROS1 rearrangement. Patient had high programmed death ligand 1 (PD-L1) expression of more than 50%. Positron emission tomography – computed tomography (PET/CT) scan showed extensive F-18-fluorodeoxyglucose (FDG)-avid omental carcinomatosis (standardized uptake value (SUV) measuring up to 7.2), 5 cm peritoneal left abdominal mass (SUV of 6.2), two left axillary/left chest wall small soft tissue nodules mildly FDG avid with SUV of 3.0 (figure 3).
Figure 1.
(A) CT angiogram at initial presentation: bilateral pleural effusions. (B) CT abdomen and pelvis with intravenous contrast at the time of diagnosis: peritoneal carcinomatosis.
Figure 2.
Pathology. Core omental mass biopsy. (A) Clusters of carcinoma cells with prominent nucleoli and cytoplasmic vacuolisation. H&E stain, ×400. (B) The tumour cells are immunoreactive for thyroid transcription factor immunostain, ×400. (C) Programmed death ligand 1 (PD-L1) immunostain positivity in tumour cells, ×400.
Figure 3.
Positron emission tomography – computed tomography (PET/CT) scan at the time of diagnosis demonstrating extensive F-18-fluorodeoxyglucose (FDG) -avid omental carcinomatosis (standardized uptake value (SUV) measuring up to 7.2). (A, B) Axial view. (C) Coronal view.
Differential diagnosis
On initial evaluation, patient was suspected to have alcoholic cirrhosis with portal hypertension and ascites complicated by spontaneous bacterial peritonitis. Nevertheless, his serum-ascites albumin gradient returned less than 1.1 g/dL, pointing away from the diagnosis of cirrhosis-related ascites and portal hypertension. Patient was evaluated for non-portal hypertensive causes of ascites including tuberculosis, autoimmune serositis, nephrotic syndrome, pancreatitis and PC. Extensive omental nodularity seen on abdominal CT with contrast supported the diagnosis of PC. The most common malignancies associated with PC in men include tumours of gastrointestinal and urinary origin as well as peritoneal mesothelioma.8 Lung cancer was initially low on our differential especially in light of no primary tumour apparent on the CT angiography or CT chest. The diagnosis of lung adenocarcinoma was confirmed by omental biopsy and analysis of pleural and peritoneal fluid. The second pathology review at National Cancer Institute-designated Cancer Center confirmed diagnosis of metastatic lung adenocarcinoma.
Treatment
Patient completed antibiotic course for spontaneous bacterial peritonitis and was discharged on secondary prophylaxis with ciprofloxacin as well as furosemide and spironolactone for treatment of his ascites and pulmonary effusions. As patient’s driver mutation analysis returned positive for ROS1 rearrangement, he was started on a tyrosine kinase receptor inhibitor crizotinib 250 mg two times per day.
Outcome and follow-up
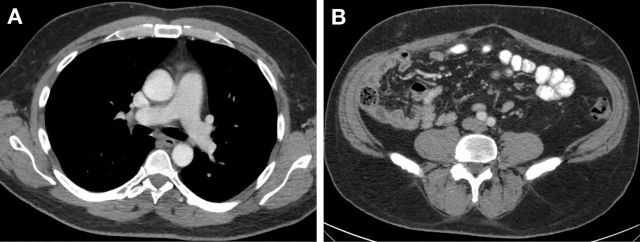
Patient had good clinical response to crizotinib treatment with resolution of cough, shortness of breath, abdominal pain and distention and decrease in lower extremity oedema. He continued to have mild nausea and diarrhoea. Contrast-enhanced CT chest, abdomen and pelvis done 2 months into crizotinib therapy revealed markedly decreased pleural effusions, resolution of ascites and peritoneal thickening, a fine nodularity throughout the greater omentum, greatly improved from the extensive omental involvement at the time of diagnosis. Follow-up imaging at 6 months showed no evidence of residual disease and patient remains on crizotinib therapy (figure 4A, B).
Figure 4.
(A) CT chest with intravenous contrast at 6 months after crizotinib: resolution of pleural effusions. (B) CT abdomen and pelvis with intravenous and oral contrast 6 months after crizotinib.
Discussion
PC is rarely encountered at the time of initial lung cancer diagnosis. In the study by Satoh et al, only one out of 1041 patients with lung cancer was found to have PC at the time of initial lung cancer diagnosis.4 It is unclear what factors predispose to the peritoneal spread of lung cancer. The most common histological type associated with the peritoneal metastasis is adenocarcinoma.5 Many patients with PC have malignant pleural effusions suggesting that peritoneal spread may be related to serosal communication with metastatic seeding, however, cases of isolated PC without pleural or other site metastasis have been reported.6 9 It has been suggested that oncogene status of the tumour can influence the patterns of metastatic spread.7 There are several case reports documenting association between EGFR-mutation and PC in the setting of primary lung cancer.10 11 In the study by Hsu and colleagues, all three identified patients with PC had EGFR-mutation including one patient with PC at the time of initial cancer diagnosis.11 It could be that presence of driver mutation can be related to faster disease progression and development of PC. Nevertheless, larger studies did not identify statistically significant association between development of PC and presence of EGFR, KRAS and ALK mutations.5 6 There is very limited data on impact of the rare mutations including ROS1, MET and BRAF on development of PC in lung cancer patients. Among the reviewed literature in English, only three patients with PC and NSCLC were tested for ROS1 and only one tested positive. Similarly, only four patients were evaluated for MET expression and two of them were positive.5 BRAF mutation was described only in one case report in coexistence with oncogenic KRAS G12A mutations in a patient with lung squamous cell carcinoma and PC.12 Regarding the immunotherapy markers, Nassereddine et al demonstrated high PD-L1 expression in 37.5% of patients with lung adenocarcinoma and PC identified over 10-year period.13
The survival of patients with peritoneal spread has historically been poor with median survival of only 2 months after PC diagnosis.3 6 However, most of the studies were done before the introduction of targeted therapy and immunotherapy. Abbate et al demonstrated better overall survival in PC patients with EGFR mutation treated with EGFR tyrosine kinase inhibitor compared with EGFR wild-type/unknown population (8 months since PC diagnosis vs 3.5 months, respectively).5 To the best of our knowledge, there are no reports available on treatment of patients with PC with targeted therapy for ROS1 rearrangement or with immunotherapy in the setting of high PD-L1 expression. ROS1-rearrangement is seen in only 1% of lung adenocarcinoma. ROS1-rearranged NSCLC has high response rate (up to 80%) to targeted therapy with crizotinib, nevertheless, the development of resistance and tumour progression is extremely common over time.14 15 Alternative targeted therapy for ROS rearranged NSCLC treatment consists of entrectinib and ceritinib.15 High expression of PD-L1 is generally associated with a good response to immune checkpoint inhibitor and may be indication for monotherapy with pembrolizumab.15 However, it has been demonstrated that immunotherapy has weak activity in NSCLC with a driver mutation. Immune checkpoint inhibitors are currently not recommended as a single agent in patients with ROS1, EGFR, ALK or RET rearrangements.16 Our case demonstrates an interesting presentation of ROS1-rearranged lung adenocarcinoma with high expression of PD-L1. Our patient was diagnosed with PC on initial presentation while primary lung tumour has not been identified. He had an excellent response to crizotinib monotherapy with resolution of initial tumour burden and no evidence of disease progression and with no major side effects at 6 month follow-up.
Learning points.
This is a rare case of c-ROS oncogene 1 (ROS1) rearranged lung adenocarcinoma presenting as peritoneal carcinomatosis (PC) with no obvious primary tumour identified.
Based on the literature review, no statistically significant association between development of PC and presence of epidermal growth factor receptor, Kirsten rat sarcoma and anaplastic lymphoma kinase mutations has been established. The data about the role of ROS1 mutation in peritoneal spread are very limited due to rareness of this mutation and still require further investigation.
Diagnosis of peritoneal spread on initial presentation is associated with poor prognosis. Identification of target mutation can lead to dramatic improvement in patient’s condition and markedly prolong survival. Our case demonstrates a patient treated with ROS1 targeted therapy with crizotinib who experienced symptomatic improvement and regression of PC. He remains free of disease progression for 6 months. This case highlights the importance of somatic mutation sequencing even if there is no primary lung tumour identified.
Footnotes
Contributors: VK collected history and data, wrote the manuscript. SVAV and CSL participated in correcting the manuscript and writing discussion section. AP prepared the pathology slides, established the diagnosis and prepared images. All authors reviewed and approved the final version of the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Howlader N, Noone A, Krapcho M, et al. . SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD [Internet]. Available: https://seer.cancer.gov/csr/1975_2016/index.html [Accessed 23 Sep 2019].
- 2.Wao H, Mhaskar R, Kumar A, et al. . Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev 2013;2:10 10.1186/2046-4053-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.H-T S, Tsai C-M, Perng R-P. Peritoneal carcinomatosis in lung cancer. Respirol Carlton Vic 2008;13:465–7. [DOI] [PubMed] [Google Scholar]
- 4.Satoh H, Ishikawa H, Yamashita YT, et al. . Peritoneal carcinomatosis in lung cancer patients. Oncol Rep 2001;8:1305–7. 10.3892/or.8.6.1305 [DOI] [PubMed] [Google Scholar]
- 5.Abbate MI, Cortinovis DL, Tiseo M, et al. . Peritoneal carcinomatosis in non-small-cell lung cancer: retrospective multicentric analysis and literature review. Future Oncology 2019;15:989–94. 10.2217/fon-2018-0469 [DOI] [PubMed] [Google Scholar]
- 6.Patil T, Aisner DL, Noonan SA, et al. . Malignant pleural disease is highly associated with subsequent peritoneal metastasis in patients with stage IV non-small cell lung cancer independent of oncogene status. Lung Cancer 2016;96:27–32. 10.1016/j.lungcan.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 7.Doebele RC, Lu X, Sumey C, et al. . Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012;118:4502–11. 10.1002/cncr.27409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai JP, Moustarah F. Cancer, Peritoneal Metastasis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2019. Available: http://www.ncbi.nlm.nih.gov/books/NBK541114/ [Accessed 29 Oct 2019].
- 9.Tanriverdi O, Barutca S, Meydan N. Relapse with isolated peritoneal metastasis in lung adenocarcinoma: and review of the literature. Współczesna Onkologia 2012;6:586–9. 10.5114/wo.2012.32495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sereno M, RODRÍGUEZ-ESTEBAN I, GÓMEZ-RAPOSO C, et al. . Lung cancer and peritoneal carcinomatosis. Oncol Lett 2013;6:705–8. 10.3892/ol.2013.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu J-F, Lee Y-L, Chang H-L, et al. . Clinical efficacy of concurrent bevacizumab for malignant ascites in nonsquamous cell carcinoma of the lung. Asia Pac J Clin Oncol 2019;15:e126–31. 10.1111/ajco.13131 [DOI] [PubMed] [Google Scholar]
- 12.Li B, Lu JC, He D, et al. . Rapid onset lung squamous cell carcinoma with prominent peritoneal carcinomatosis and an eosinophilic leukemoid reaction, with coexistence of the BRAF V600E and oncogenic KRAS G12A mutations: a case report. Oncol Lett 2014;8:589–93. 10.3892/ol.2014.2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassereddine H, Sannier A, Brosseau S, et al. . Clinicopathological and molecular study of peritoneal carcinomatosis associated with non-small cell lung carcinoma. Pathol. Oncol. Res. 2019;136 10.1007/s12253-019-00713-1 [DOI] [PubMed] [Google Scholar]
- 14.Mazières J, Zalcman G, Crinò L, et al. . Crizotinib Therapy for Advanced Lung Adenocarcinoma and a ROS1 Rearrangement: Results From the EUROS1 Cohort. J Clin Oncol Off J Am Soc Clin Oncol 2015;33:992–9. 10.1200/JCO.2014.58.3302 [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network Non-Small Cell Lung Cancer (Version 7.2019) [Internet], 2019. Available: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf [Accessed 26 Sep 2019].
- 16.Mazières J, Drilon A, Lusque A, et al. . Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol Off J Eur Soc Med Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]