Abstract
HAIR-AN—a syndrome of hyperandrogenism (HA), insulin resistance (IR) and acanthosis nigricans (AN)—is a specific subphenotype of polycystic ovary syndrome (PCOS), and it is seen in almost 5% of all women with hyperandrogenism. An adolescent girl aged 11 years old was referred with adrenarche, hyperandrogenism and obesity commencing at age 8. Clinical and biochemical investigations confirmed significant hyperandrogenism and insulin resistance, and a diagnosis of HAIR-AN syndrome was made after exclusion of other differential diagnoses. HAIR-AN syndrome is an important diagnosis for the adolescent gynaecologist to be aware of, and it requires a multidisciplinary approach, including endocrinology input, for optimal management. Weight loss, lifestyle modification and combined hormonal pill and metformin are considered first-line treatment.
Keywords: obstetrics and gynaecology, metabolic disorders
Background
HAIR-AN—a syndrome of hyperandrogenism (HA), insulin resistance (IR) and acanthosis nigricans (AN)—is a specific and rare subphenotype of the polycystic ovary syndrome (PCOS) characterised by the presence of severe insulin resistance.1 HAIR-AN syndrome can cause menstrual problems, hyperandrogenic symptoms and insulin resistance among adolescents. This syndrome is seen in almost 5% of women with hyperandrogenism. HAIR-AN can cause severe and potentially irreversible manifestations of obesity, acne, hirsutism and acanthosis nigricans in the affected adolescent around puberty, having a significant impact on the affected adolescent’s quality of life. This case describes an extreme phenotype of HAIR-AN syndrome and provides an overview of the clinical features, manifestations, diagnosis and management.
Case presentation
A girl patient aged 11 years 2 months was referred to the Queensland Paediatric and Adolescent Gynaecology Service with adrenarche, hyperandrogenism and obesity. History revealed adrenarche at age 8 years, with gradual onset of worsening hirsutism, acne and deepening of the voice over 2 years prior to referral. Menarche occurred at age 10 years and 11 months, with two light menses lasting 1 day at approximately a 28-day interval prior to presentation. The patient had a background of anxiety, mild attention-deficient hyperactivity disorder and learning problems, and chronic bronchitis. There was a family history of maternal gestational diabetes.
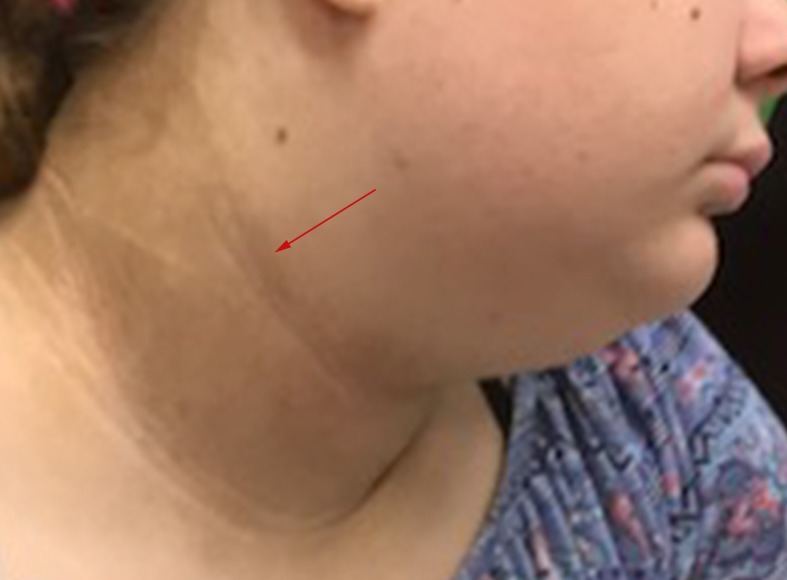
On examination, her findings were as follows: Blood pressure 138/87 (>99th centile), height 166 cm (>99th centile), weight 98.5 kg (>99th centile), body mass index (BMI) 35.3 kg/m2 (>99th centile) and waist circumference 108.5 cm (>99th centile). She had normal facial features, with severe acanthosis nigricans visible on her neck (figure 1), axillae and elbow creases and thick terminal hairs over her face, chest, abdomen, linea alba, lower back, legs, arms with a modified Ferriman–Gallwey score of 19. Acne was visible over her face, back, chest and inner thighs. Breast development to Tanner Stage 4 and Pubic Hair Stage 5 was noted. She had no clitoromegaly or virilisation of the external female genitalia. Her cardiorespiratory and abdominal examination was otherwise unremarkable.
Figure 1.
Severe acanthosis nigricans on the neck of the affected adolescent.
Investigations
Investigations had found a normal full blood count (FBC), luteinising hormone (LH) 9.7 U/L, Follicular stimulating hormone (FSH) 4.6 U/L and oestradiol 140 pmol/L. Serum testosterone and free testosterone were found to be markedly elevated at 3.9 nmol/L (normal <2.5) and 122 pmol/L (range 10–45), respectively, androstenedione 10 nmol/L (range 0.8–6.1) and low sex hormone-binding globulin 13 nmol/L (range 40–90). Adrenal hormone investigations found normal cortisol, normal adrenocorticotropic hormone (ACTH), normal 17-hydroprogesterone 2.8 nmol/L (0.5–4.1) and mildly elevated dehydroepiandrosterone sulfate (DHEAS) 5.5 μmol/L (normal <4.0). Thyroid function was normal. A 24-hour urinary steroid profile showed high androgen levels and cortisone but not cortisol, consistent with PCOS but not congenital adrenal hyperplasia (CAH) or Cushing syndrome. Fasting insulin levels were grossly elevated at 266 mU/L (normal 2.0–23) with a Haemoglobin A1c (HbA1c) 6.1% (43.2 mmol/mol). SRY gene, fluorescence in situ hybridization (FISH) and karyotype were performed and consistent with 46 XX. Insulin-like Growth Hormone 1 (IGF1) was slightly elevated at 76 nmol/L (9.9–71) and growth hormone (GH) was low normal at 0.24 mcg/L (0.05–8.00) which along with a normal MRI brain with pituitary slices excluded gigantism and pituitary adenoma as a cause for early puberty (figure 2). Imaging with MRI and CT adrenals were performed to exclude adrenal pathology (figure 3) and both were unremarkable.
Figure 2.
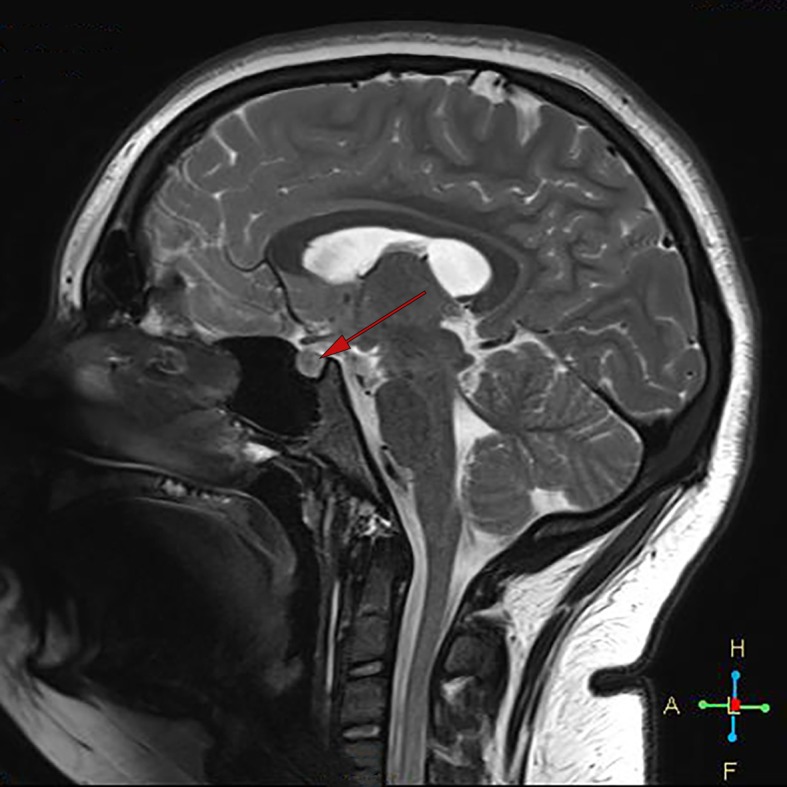
MRI brain sagittal view showing normal pituitary gland (see arrow).
Figure 3.
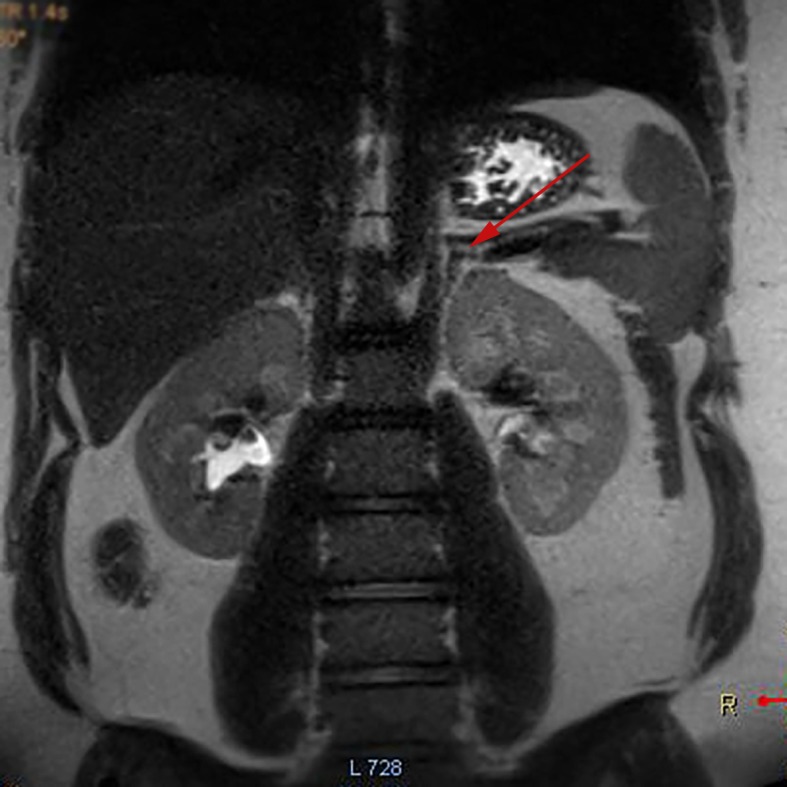
MRI adrenals coronal view showing normal adrenal glands (see arrow).
Pelvic ultrasound showed a normal uterus and bilaterally enlarged ovaries, and MRI pelvis confirmed significantly enlarged ovaries for her age measuring 51×25×36 mm on the left and 23×35×33 mm right ovary with no obvious features suspicious for tumours (figure 4). Ovarian tumour markers were negative.
Figure 4.
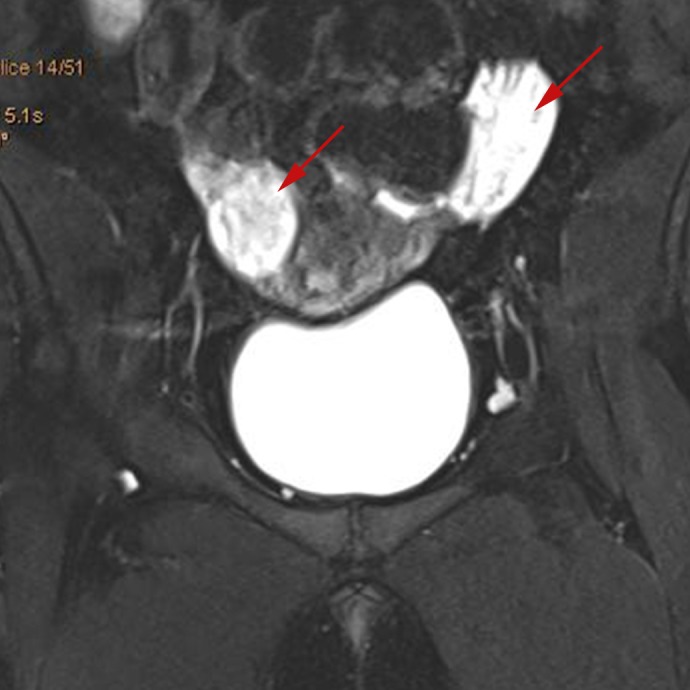
MRI pelvis coronal view showing normal uterus with enlarged ovaries (see arrows).
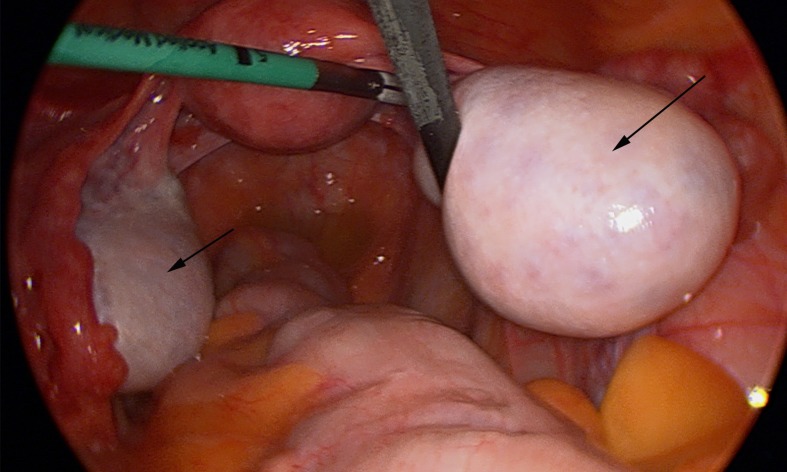
In view of the markedly elevated androgen levels and enlarged ovaries, an urgent laparoscopic wedge biopsy of the ovaries (figure 5) was performed to exclude an androgen-secreting tumour such as an androblastoma, the prevalence of which is 0.2% in hyperandrogenism resulting in virilisation.2 3 Androblastomas of the ovary may present as tumours of sertoli cells and/or Leydig cells (most often in combination), and comprise less than 0.5% of all ovarian tumours. The histopathology was consistent with polycystic ovaries.
Figure 5.
Laparoscopic view of pelvis showing bilateral enlarged ovaries (see arrows), prior to wedge biopsy.
Differential diagnosis
The differential diagnosis of hyperandrogenism in adolescents presents a wide spectrum of disease (box 1), including disorders of steroidogenesis such as classic and non-classic congenital adrenal hyperplasia, gonadal dysgenesis, Cushing syndrome, tumours and glucocorticoid resistance, and these need to be excluded before a diagnosis of PCOS or HAIR-AN can be made. Investigation of clinically significant hyperandrogenism should be individualised based on patient presentation and symptomatology, with investigations listed in box 2 to be considered. In our patient, after the exclusion of other pathology, a diagnosis of PCOS with HAIR-AN phenotype was made.
Box 1. Differential diagnosis of hyperandrogenism in adolescents.
Physiological adolescent anovulation.
Polycystic ovarian syndrome.
Thyroid dysfunction.
-
Disorders of steroidogenesis.
Including CAH, non-classical CAH (NCAH).
Androgen-secreting tumour.
Cushing syndrome.
Gonadal dysgenesis.
Hyperprolactinemia.
Exogenous androgen administration.
Insulin receptor mutation.
Box 2. Investigation of hyperandrogenism in adolescents.
Initial investigations
Full blood count (FBC), Electrolytes and Liver function Tests (ELFTs).
Luteinising hormone (LH), follicular stimulating hormone (FSH), oestradiol, progesterone.
Prolactin.
Thyroid stimulating hormone (TSH) and T4.
Serum 17-hydroxyprogesterone (17-OHP) - follicular phase, morning.
Serum total and free testosterone.
Sex hormone-binding globulin.
Dehydroepiandrosterone sulfate (DHEAs).
Serum cortisol (morning).
24-hour urinary steroid profile.
Fasting insulin levels, Haemoglobin 1Ac (Hb1Ac).
Targeted investigations
Karyotype.
Adrenocorticotropic hormone (ACTH) stimulation test to exclude non-classical congenital adrenal hyperplasia (CAH).
Dexamethasone suppression test to exclude Cushing syndrome
MRI ovaries and adrenals to detect mass or tumour.
MRI pituitary to detect mass or tumour.
Ovarian biopsy.
Treatment
A multidisciplinary approach to the management of this patient was taken involving a paediatric endocrinologist, paediatric and adolescent gynaecologist, dietician and psychologist with ongoing regular review. Strong encouragement to achieve weight loss was supported with ongoing dietician support and an exercise plan for weight reduction. Counselling regarding longer-term health consequences was given, including obesity, cardiovascular disease and type 2 diabetes mellitus. The patient was commenced on metformin-extended release which was titrated up to 2000 mg daily, a combined contraceptive pill (COCP) containing 35 μg ethinylestradiol (EE) and cyproterone 2 mg, and spironolactone 50 mg twice daily with multidisciplinary team follow-up.
Outcome and follow-up
The adolescent continued to have regular follow-up with gynaecology, endocrinology, dietician and psychology teams. There was initially issues with medication compliance, which needed to be addressed and reinforced over several consultations. For 6 months after the COCP was commenced, the adolescent remained amenorrhoeic; however once compliance with the COCP was established, the adolescent began to have regular monthly menses. Repeat blood investigations showed a significant drop in testosterone levels from 3.9 to 2.3 nmol/L (normal <2.5), which correlated clinically to a noticeable reduction in hyperandrogenism; however the adolescent remains to have visible and distressing features.
Despite compliance with medication, regular dietician input, adhering to a healthy diet and 30 min of exercise daily, she was unable to achieve weight loss. On review at age 12 years 10 months, approximately 2 years after referral, her weight had increased to 113 kg (99.9th centile;+3.19 SD), height 168.1 cm (96th centile) and BMI 40.3 kg/m2 (99.6th centile;+2.65 SD). She was encouraged to maintain lifestyle changes, to consider a very low-calorie diet (VLCD) in consultation with a dietician and to increase her exercise to 30–60 min daily. The adolescent wanted the opinion of a bariatric surgeon, regarding surgical options for weight reduction, and after counselling she proceeded to a laparoscopic sleeve gastrectomy at age 12 years and 11 months which was uncomplicated. At her last review, aged 13 years and 1 month, with VLCD preoperatively, bariatric surgery and regular exercise she had lost over 20 kg, and her weight had reduced to 92.7 kg (99.5th centile, BMI 32.5 kg/m2 (98.7th centile)). Blood tests revealed normalisation of insulin levels to 20 mU/L, HbA1c 5.1% (32.2 mmol/mol) and ongoing elevation in testosterone 2.8 nmol/L. She was recommended to cease metformin and to continue with vitamin and mineral supplementation, the COCP and spironolactone, with next review planned for 3 months.
Discussion
HAIR-AN syndrome represents a rare subphenotype of PCOS characterised by severe insulin resistance and hyperandrogenism. Clinical manifestations of the HAIR-AN phenotype include obesity, moderate/severe acne, hirsutism, irregular menses and acanthosis nigricans. HAIR-AN syndrome should not be confused with ‘classic’ PCOS which is a common heterogeneous endocrine disorder that affects about 1 in 15 women worldwide.4 There remains considerable debate in the literature regarding the definitions for PCOS, the classification of phenotypes and diagnosis in adolescents.5–7 The HAIR-AN syndrome has been described as a variant of another syndrome, which is characterised by the presence of Seborrhoea, Acne, Hirsutism and Alopecia in women, otherwise known as SAHA syndrome. It can also be associated with PCOS, cystic mastitis, obesity and infertility.8
Global literature reports cases of adolescents with HAIR-AN syndrome.9–11 A cohort study of an outpatient adolescent clinic at a tertiary hospital showed that of the 1002 young women who attended the clinic over a 2-year period, 50 adolescents (5% of total and 36.8% of those with irregular menses) were diagnosed with HAIR-AN syndrome. The mean age of diagnosis was 15.5 years (range 10–19), initial mean weight at diagnosis was 94.5 kg and the mean BMI was 33.1 kg/m2.1
Patients with HAIR-AN syndrome have markedly elevated levels of insulin, testosterone and androstenedione. The primary abnormality in patients is thought to be insulin resistance with elevated insulin levels which is associated with mutations in the insulin receptor gene.12 13 High insulin levels directly stimulate steroidogenesis in the ovaries and androgen production, and insulin also amplifies the effect of LH on granulosa cell steroidogenesis.14 High insulin levels may cross-react with receptors for insulin-like growth factors leading to increased ovarian androgen production.15
Primary objectives of management of HAIR-AN are weight loss and lifestyle modification in overweight adolescents with HAIR-AN syndrome. This reduces the clinical manifestations of HAIR-AN syndrome by reducing peripheral oestrogen production, reducing insulin resistance and ovarian androgen production, consequently lowering the risk of diabetes and cardiovascular disease.16 Patients should be encouraged to engage in regular exercise and reduce caloric intake with the goal of normoglycaemia. To achieve this requires the involvement of a parent or carer, exercise physiologist and dietician. It is important to ensure that the adolescent with chronic illness understands her condition and is able to be compliant with recommended care. In cases of refractory obesity with metabolic complications, a referral to a bariatric surgeon may be considered, with surgical options including a laparoscopy sleeve gastrectomy and Roux-en-Y gastric bypass.
Combined hormonal contraceptives that contain both an oestrogen (EE) and a progestin or antiandrogen such as cytoproterone, and metformin, an insulin sensitising agent, are considered first-line therapy to reduce insulin resistance and regulate the menstrual cycle. COCPs decrease LH levels and decrease androgen synthesis while also increasing sex hormone-binding globulin levels and testosterone binding, thereby decreasing free testosterone.17 Metformin may reduce BMI and is an effective adjuvant to lifestyle modification and weight reduction.18 Evidence suggests that the combination of COCPs, metformin and spironolactone significantly reduces clinical hypandrogenism.19 Spironolactone is an aldosterone receptor antagonist that is similar in structure to progestins and it competitively blocks androgen receptors. In addition, spironolactone weakly inhibits enzymes involved in androgen production.20 Spironolactone is clinically indicated for patients with moderate or severe hirsutism (baseline Ferriman–Gallwey score higher than 15).21 22 Use of cosmetic methods such as laser hair removal can also be used to reduce the severity of existing hirsutism. Patients with acne may benefit from COCPs, and a daily skin cleansing regimen should be recommended. Topical antibiotics, retinoic acid, systemic antibiotics and isotretinoin may be required for cases of severe acne, in consultation with a dermatologist. Importantly, the psychological sequelae including depression, self-esteem issues and quality of life in affected adolescents should be considered and addressed, as studies have found a 24% prevalence of depression in adolescents with HAIR-AN syndrome.23
Patient’s perspective.
The adolescent’s mother has kindly contributed in her review and contribution to this article. She expressed that the ‘social, emotional, physical and mental effects that this condition has on a young girl is exhausting, making them feel socially awkward and isolated as well as gender confusion due to the excessive male characteristics are debilitating’.
Learning points.
Hyperandrogenism, insulin resistance and acanthosis nigricans (HAIR-AN) syndrome represents a rare subphenotype of polycystic ovary syndrome (PCOS) characterised by severe insulin resistance and hyperandrogenism which may present in the adolescent age group.
HAIR-AN syndrome can be associated with significant morbidity, adverse cosmetic and psychosocial effects for the adolescent.
Treatment of HAIR-AN syndrome involves lifestyle changes to promote weight reduction along with COCP, antiandrogen agents and insulin-sensitising agents. Bariatric surgery may be considered in treatment-resistant cases.
Increased awareness of this condition is necessary to facilitate early detection, diagnosis and treatment, reduce symptoms and morbidity and improve self-esteem to have a positive impact on the quality of life of affected young women.
Footnotes
Contributors: BO: patient care, first author, collection data and writing clinical case, literature review, drafting and revising clinical work, revision manuscript. RD: patient care, contribution and revision of manuscript. RK: patient care, critical review of paper, final approval for paper to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Omar HA, Logsdon S, Richards J. Clinical profiles, occurrence, and management of adolescent patients with HAIR-AN syndrome. ScientificWorldJournal 2004;4:507–11. 10.1100/tsw.2004.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera-Arkoncel MLC, Pacquing-Songco D, Lantion-Ang FL. Virilising ovarian tumour in a woman with an adrenal nodule. Case Rep Child Meml Hosp Chic 2010;2010:bcr0720103139 10.1136/bcr.07.2010.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veilleux-Lemieux M, DiVasta AD. Polycystic ovary syndrome mimicking an androgen-secreting tumor. J Pediatr Adolesc Gynecol 2012;25:e54–5. 10.1016/j.jpag.2011.12.016 [DOI] [Google Scholar]
- 4.Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. Lancet 2007;370:685–97. 10.1016/S0140-6736(07)61345-2 [DOI] [PubMed] [Google Scholar]
- 5.Rosenfield RL. The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics 2015;136:1154–65. 10.1542/peds.2015-1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witchel SF, Roumimper H, Oberfield S. Polycystic ovary syndrome in adolescents. Endocrinol Metab Clin North Am 2016;45:329–44. 10.1016/j.ecl.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 7.Rothenberg S, Beverley R, Barnard E, et al. Polycystic ovary syndrome in adolescents. Best Pract Res Clin Obstet Gynaecol 2017:1–12. [DOI] [PubMed] [Google Scholar]
- 8.Orfanos CE, Adler YD, Zouboulis CC. The SAHA syndrome. Horm Res 2000;54:251–8. 10.1159/000053267 [DOI] [PubMed] [Google Scholar]
- 9.Zemtsov A, Wilson L. Successful treatment of hirsutism in HAIR-AN syndrome using flutamide, spironolactone, and birth control therapy. Arch Dermatol 1997;133:431–3. 10.1001/archderm.1997.03890400023003 [DOI] [PubMed] [Google Scholar]
- 10.Esperanza LE, Fenske NA. Hyperandrogenism, insulin resistance, and acanthosis nigricans (HAIR-AN) syndrome: spontaneous remission in a 15-year-old girl. J Am Acad Dermatol 1996;34:892–7. 10.1016/S0190-9622(96)90074-2 [DOI] [PubMed] [Google Scholar]
- 11.Amesse LS, Ding X, Pfaff-Amesse T, et al. From HAIR-AN to eternity. J Pediatr Adolesc Gynecol 2002;15:235–40. 10.1016/S1083-3188(02)00162-6 [DOI] [PubMed] [Google Scholar]
- 12.Barbieri RL, Ryan KJ. Hyperandrogenism, insulin resistance, and acanthosis nigricans syndrome: a common endocrinopathy with distinct pathophysiologic features. Am J Obstet Gynecol 1983;147:90–101. 10.1016/0002-9378(83)90091-1 [DOI] [PubMed] [Google Scholar]
- 13.Musso C, Cochran E, Moran SA, et al. Clinical course of genetic diseases of the insulin receptor (type A and Rabson–Mendenhall syndromes): a 30-year prospective. Medicine 2004;83:209–22. 10.1097/01.md.0000133625.73570.54 [DOI] [PubMed] [Google Scholar]
- 14.Rojas J, Chávez M, Olivar L, et al. Polycystic ovary syndrome, insulin resistance, and obesity: Navigating the pathophysiologic labyrinth. Int J Reprod Med 2014;2014:719050:1–17. 10.1155/2014/719050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nestler JE, Strauss JF. Insulin as an effector of human ovarian and adrenal steroid metabolism. Endocrinol Metab Clin North Am 1991;20:807–23. 10.1016/S0889-8529(18)30245-7 [DOI] [PubMed] [Google Scholar]
- 16.Tang T, Glanville J, Hayden CJ, et al. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod 2006;21:80–9. 10.1093/humrep/dei311 [DOI] [PubMed] [Google Scholar]
- 17.Mendoza N, Simoncini T, Genazzani AD. Hormonal contraceptive choice for women with PCOS: a systematic review of randomized trials and observational studies. Gynecol Endocrinol 2014;30:850–60. 10.3109/09513590.2014.943725 [DOI] [PubMed] [Google Scholar]
- 18.Haas DA, Carr BR, Attia GR. Effects of metformin on body mass index, menstrual cyclicity, and ovulation induction in women with polycystic ovary syndrome. Fertil Steril 2003;79:469–81. 10.1016/S0015-0282(02)04800-8 [DOI] [PubMed] [Google Scholar]
- 19.Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovasc Dis 2014;24:132–9. 10.1016/j.numecd.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 20.Lobo RA, Shoupe D, Serafini P, et al. The effects of two doses of spironolactone on serum androgens and anagen hair in hirsute women. Fertil Steril 1985;43:200–5. 10.1016/s0015-0282(16)48373-1 [DOI] [PubMed] [Google Scholar]
- 21.Martin KA, Anderson RR, Chang RJ, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2018;103:1233–57. 10.1210/jc.2018-00241 [DOI] [PubMed] [Google Scholar]
- 22.Lanzo E, Monge M, Trent M. Diagnosis and management of polycystic ovary syndrome in adolescent girls. Pediatr Ann 2015;44:e223–30. 10.3928/00904481-20150910-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClanahan KK, Omar HA. Navigating adolescence with a chronic health condition: a perspective on the psychological effects of HAIR-AN syndrome on adolescent girls. ScientificWorldJournal 2006;6:1350–8. 10.1100/tsw.2006.242 [DOI] [PMC free article] [PubMed] [Google Scholar]