Key Points
New Gata3 reporter mice allow noninvasive monitoring of Th2 polarization.
Gata3-driven fluorescent marker expression highlights ILC2 progenitor cells.
Patterns of reporter expression disprove claims of monoallelic Gata3 expression.
Abstract
Accurately tuned expression levels of the transcription factor GATA-3 are crucial at several stages of T cell and innate lymphoid cell development and differentiation. Moreover, several lines of evidence suggest that Gata3 expression might provide a reliable molecular marker for the identification of elusive progenitor cell subsets at the earliest stages of T lineage commitment. To be able to faithfully monitor Gata3 expression noninvasively at the single-cell level, we have generated a novel strain of knock-in reporter mice, termed GATIR, by inserting an expression cassette encoding a bright fluorescent marker into the 3′-untranslated region of the endogenous Gata3 locus. Importantly, in contrast to three previously published strains of Gata3 reporter mice, GATIR mice preserve physiological Gata3 expression on the targeted allele. In this study, we show that GATIR mice faithfully reflect endogenous Gata3 expression without disturbing the development of GATA-3–dependent lymphoid cell populations. We further show that GATIR mice provide an ideal tool for noninvasive monitoring of Th2 polarization and straightforward identification of innate lymphoid cell 2 progenitor populations. Finally, as our reporter is non–gene-destructive, GATIR mice can be bred to homozygosity, not feasible with previously published strains of Gata3 reporter mice harboring disrupted alleles. The availability of hetero- and homozygous Gata3 reporter mice with an exceptionally bright fluorescent marker, allowed us to visualize allelic Gata3 expression in individual cells simply by flow cytometry. The unambiguous results obtained provide compelling evidence against previously postulated monoallelic Gata3 expression in early T lineage and hematopoietic stem cell subsets.
Introduction
The transcription factor GATA-3 is a key player in development and differentiation of several lymphocytic cell populations (1). Within the T cell lineage, Gata3 is expressed continuously from the earliest stages of thymopoiesis up to mature T cells in peripheral lymphoid tissues, playing critical roles at several stages of differentiation (2, 3). In mature CD4+ T cells, Gata3 expression is essential for the generation of Th2 cells (4–6). Gata3 is also required for the development and function of several innate lymphoid cell (ILC) subsets (7–9), including ILC2 progenitors (ILC2Ps), which express particularly high levels of Gata3 transcripts (10–13). Elevated levels of Gata3 expression have also been noted in a distinct subset of thymus-derived NK cells, termed thymic NK (tNK) cells (14, 15). Additionally, Gata3 transcripts are present in long-term hematopoietic stem cells (LT-HSCs) and at various levels in several downstream multipotent progenitor subsets. In contrast, cells committed to the erythroid, thrombocytic, myeloid, or B lymphocytic lineage lack Gata3 expression.
The function of GATA-3 is remarkably sensitive to gene dosage. Homozygous Gata3 inactivation in mice is invariably lethal around day 11–12 of embryogenesis. Knockout embryos exhibit severe defects in multiple organ systems, including adrenal glands, kidney, CNS, and fetal liver hematopoiesis (16, 17). Conditional inactivation of Gata3 in hematopoietic cells results in a complete block of T lymphopoiesis at the earliest intrathymic stage of development (18–20), complete ILC2P/ILC2 deficiency (10–12), and abrogation of tNK development (12, 14). However, even more modest experimental manipulations of Gata3 expression can have profound biological effects. For instance, mild overexpression of Gata3 in early T progenitors is either cytotoxic or redirects differentiation toward the mast cell lineage (21), and transgenic overexpression in thymocytes can promote oncogenesis (22). In contrast, reductions in Gata3 transcript levels impede early T lymphopoiesis (20, 23), and heterozygous loss of Gata3 elicits a marked reduction in the number of mature ILC2s (11). Moreover, haploinsufficiency of GATA3 in humans results in a severe autosomal-dominant disorder, referred to as hypoparathyroidism-deafness-renal dysplasia (24), with at least part of the pathological phenotype preserved in heterozygous Gata3 knockout mice (25). Tight control of physiologic Gata3 expression levels thus seems to be requisite for normal development and function of several cell lineages, including ILCs, tNK cells, and T cells in mice.
As a further complication to this theme, a tantalizing study recently reported allele-specific control of Gata3 expression at defined developmental stages during both early hematopoiesis as well as thymopoiesis (20). Using sophisticated techniques, such as single-cell transcript sequencing and single-cell in situ hybridization, the authors observed parent-of-origin–independent monoallelic Gata3 expression specifically in LT-HSCs and in the two developmentally earliest intrathymic T progenitor populations, termed early thymic progenitor (ETP) and double-negative (DN) stage 2 early (DN2E). Curiously, upon further differentiation, about half of these progenitors were found to switch from monoallelic to biallelic Gata3 expression. In a follow-up study, the same laboratories reported a provocative correlation between the allelic status of Gata3 expression (and thus GATA-3 protein abundance) and the V > (D)J rearrangement status of TCRβ alleles in developing thymocytes (26).
Unfortunately, the techniques used in the aforementioned studies are cell disruptive, precluding prospective purification of live cell populations with differential allelic Gata3 expression for further study. In this study, we describe the generation and analysis of a novel strain of knock-in Gata3 reporter mice (Gata3-IRESvYFP, termed GATIR), allowing noninvasive determination of the allelic Gata3 expression status in individual cells. We show that GATIR mice can be maintained as homozygous line with unperturbed endogenous Gata3 expression. We further show that GATIR mice provide a unique tool for noninvasive monitoring of specific cell subsets based on Gata3 transcript levels. Surprisingly, observed patterns of reporter expression in relevant cell populations from heterozygous and homozygous reporter mice were completely incompatible with monoallelic Gata3 expression, refuting key conclusions of two previous studies (20, 26).
Materials and Methods
Mice
GATIR mice were generated via classical gene targeting as described below. Genotyping of GATIR mice was performed by multiplex PCR as outlined in Supplemental Fig. 1. All data presented in this study were obtained with knock-in mice backcrossed for at least 20 generations onto C57BL/6. Wild-type littermates or C57BL/6 mice served as controls. All mice were bred and maintained in a specific pathogen–free facility of our animal research center (Tierforschungszentrum). All animal experiments were performed in accordance with legal guidelines and our Institutional Animal Care and Use Committee.
Generation of GATIR mice
The IRESvYFP reporter cassette (Fig. 1A) was inserted into the 3′-untranslated region of the endogenous Gata3 gene via classical gene targeting in embryonic stem (ES) cells. The final targeting vector (pGAT-CE), which was assembled using conventional recombinant DNA technology, consisted of the following individual sequence elements: 1) a short arm of homology spanning positions 9’860’360 to 9’858’337 of the Gata3 gene (5′-ACTTGGCTGTGTACATCTAG …. TGCTCCACATGCGTG AGGAG-3′); 2) an encephalomyocarditis virus–derived internal ribosomal entry site (IRES) followed by 3) a cDNA encoding Venus yellow fluorescent protein (vYFP), a very bright reporter optimized for fast and efficient maturation (27); 4) a loxP-flanked neomycin resistance gene for positive selection with G418; 5) a long arm of homology spanning positions 9’858’333 to 9’852’369 of the Gata3 gene (5′-CCAAGTGTGCGAAGAGTTCC …. CATGCCTCGTTGAATTGGGC-3′); 6) an HSV-derived thymidine kinase gene for negative selection with gancyclovir; and, finally, 7) the pBSK-derived vector. The complete nucleotide sequence of the final targeting construct (pGAT-CE) can be obtained by contacting the corresponding author upon request (see footnotes above). Gene targeting experiments were performed in E14.1 ES cells of 129/Ola origin using G418/gancyclovir double-selection as described previously (28). Six independent ES clones with correctly targeted Gata3 alleles were identified after screening a total of 400 doubly resistant colonies by PCR. ES clone 364 was used to generate corresponding knock-in mice following conventional methodology. In brief, targeted ES cells were injected into C57BL/6-derived blastocysts, giving rise to several chimeric males that were intercrossed with C57BL/6 females to generate offspring with agouti coat color and stable incorporation of the targeted allele in the germline. One male heterozygous for the Gata3-IRESvYFP-loxP-Neo-loxP knock-in allele (Fig. 1A) was subsequently intercrossed with a female of the ubiquitous deleter strain CMV-Cre (29) to excise the neomycin cassette with its strong enhancer/promoter elements that have been shown repeatedly to interfere in unpredictable ways with the physiological expression of targeted gene loci (30, 31). Knock-in mice carrying the GATIR allele but lacking the neo cassette were subsequently backcrossed onto C57BL/6, thereby removing the CMV-Cre transgene.
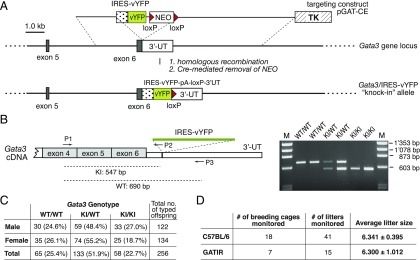
FIGURE 1.
Generation of Gata3 knock-in reporter (GATIR) mice that can be maintained as a homozygous line. (A) Targeting strategy. TK indicates the gene encoding herpes simplex thymidine kinase; NEO indicates the neomycin resistance-encoding gene. 3′-UT indicates the 3′-untranslated region of the Gata3 gene; IRES (stippled box) indicates the IRES; vYFP (green box) indicates the vYFP-encoding cDNA. (B) Multiplex RT-PCR reveals robust expression of the targeted allele and confirms insertion of the reporter cassette at the predicted site. Left panel, Exon assignment of the Gata3 cDNA template (partial), including the position of the three primers (P1, P2, and P3) used for multiplex RT-PCR analysis. P1 anneals in exon 4–encoded sequences, P2 anneals within a short artificial linker region at the 5′-end of the IRES cassette, and P3 anneals in the 3′-untranslated region downstream of the IRESvYFP insertion site. The dotted lines below indicate the predicted fragment size for transcripts from wild-type (WT) and knock-in (KI) alleles, respectively; right panel: result of a representative RT-PCR analysis with total RNA isolated from unfractionated thymocytes of two different WT (WT/WT), heterozygous (KI/WT), and homozygous (KI/KI) GATIR mice, respectively. Please note that primer combination P1/P3 does not give rise to an amplified PCR product with cDNA derived from the KI allele under our RT-PCR conditions, which were optimized for amplification products <1 kb. The theoretical P1/P3–primed amplification product from message of the targeted allele would be 2.24 kb. M indicates the DNA size marker. (C) Genotype distribution of KI and WT alleles among offspring of heterozygous GATIR breeders. Data were obtained with a total of 49 litters from 16 independent breeding cages over a period of 2 y (July 17, 2017–July 2, 2019). Genotyping was performed at the time of weaning (i.e., 4–5 wk after birth). (D) There was no difference in average litter size among offspring of homozygous GATIR versus WT C57BL/6 breeders. Breeding performance was monitored over a period of ∼15 mo (January 1, 2017–April 10, 2018) and determined at the time of weaning. Homozygous GATIR breeders were on 20th generation C57BL/6 backcross. Breeders of both genotypes (GATIR and WT C57BL/6) were housed in the same room of our animal facility.
Flow cytometry
Preparation of single-cell suspensions from relevant mouse organs (thymus, spleen, and bone marrow [BM]) and subsequent staining for cytofluorometric analysis were done as described previously (32). A complete list of all Abs used in the study is given in Supplemental Table I. Stained cells were acquired on a BD LSRFortessa and analyzed using BD FACSDiva software.
In vitro polarization studies
Naive CD4+ T cells were isolated from pooled splenocytes and lymph node cells of wild-type C57BL/6 and homozygous GATIR mice, respectively, using the Miltenyi Biotec Naive CD4+ T Cell Isolation Kit (catalog no. 130-104-453) and the autoMACS Pro Separator (Miltenyi Biotec) according to the provider’s instructions. Polarization experiments were performed in triplicate with 2.5 × 105 cells per well in 96-well plates precoated with 5 μg/μl anti-CD3 (catalog no. 100302; BioLegend) and 5 μg/μl anti-CD28 (catalog no. 102102; BioLegend), Abs in TexMACS Medium (catalog no. 130-097-196; Miltenyi Biotec) supplemented with 10% FBS and 0.01mM 2-ME (catalog no. 31350-010; Life Technologies), 1× penicillin–streptomycin (catalog no. 15140; Life Technologies), and various cytokines to induce polarization following the protocols of CytoBox Th1 mouse (catalog no. 130-107-761; Miltenyi Biotec), Th2 (catalog no. 130-107-760), and Th17 (catalog no. 130-107-758). For Th1 polarization, we used 10 ng/ml IL-2, 10 μg/ml anti–IL-4 and 10 ng/ml IL-12. For Th2 polarization, we used 10 ng/ml IL-2, 10 ng/ml IL-4, and 10 μg/ml anti–IFN-γ. For Th17 polarization, 20 ng/ml IL-6, 10 ng/ml IL-23, 10 ng/ml IL-1β, 2 ng/ml TGF-β1, 10 μg/ml anti–IL-2, 10 μg/ml anti–IL-4, and 10 μg/ml anti–IFN-γ were used. Cells were incubated at 37°C in 5% CO2, split on day 2 and 4 at a ratio of 1:3, and analyzed on day 4 and day 6 by flow cytometry. For intracellular quantification of cytokines, cells were restimulated with 25 ng/ml PMA (catalog no. P1585; Sigma-Aldrich) and 500 ng/ml ionomycin (catalog no. I0634; Sigma-Aldrich) in the presence of 10 μg/ml brefeldin A (catalog no. B7651; Sigma-Aldrich) for 5 h. After restimulation, cells were stained with Fixable Viability Dye (catalog no. 565388; BD Biosciences or catalog no. 65-0863-14; Invitrogen), fixed and permeabilized (catalog no. 554722; BD Biosciences or catalog no. 00-5523-00; Invitrogen), blocked with 2 μl of Normal Rat Serum (catalog no. 13552; STEMCELL Technologies), stained with anti-mouse GATA-3–eFluor 660 (catalog no. 50-9966-42, 1:20; eBioscience), anti–IFN-γ–BV421 (catalog no. 505830, 1:400; BioLegend), anti–IL-13–PE (catalog no. 12-7133-71, 1:20; eBioscience) or anti–IL-17A–PerCPCy5.5 (catalog no. 45-7177-80, 1:400; eBioscience) Abs, and analyzed on a BD LSRFortessa. Expression of the vYFP reporter was determined in unstained cells without prior ionomycin/PMA/brefeldin treatment and without fixation. On the day of analysis, Th0 cells were freshly isolated as controls from pooled splenocytes and lymph node cells of a C57BL/6 and a homozygous GATIR mouse, respectively, using again the Naive CD4+ T cell isolation Kit (catalog no. 130-104-453; Miltenyi Biotec) and the autoMACS Pro Separator (Miltenyi Biotec).
Quantification of Gata3 transcript levels by real-time quantitative PCR
RNA was extracted from cells using the ReliaPrep RNA Cell Miniprep Kit (catalog no. Z6011; Promega). The reverse transcription was performed using SuperScript III First-Strand Synthesis System (catalog no. 18080051; Invitrogen) or SuperScript VILO Master Mix (catalog no. 11756050; Invitrogen). Real-time quantitative PCR (RT-qPCR) was performed with TaqMan Gene Expression Master Mix (catalog no. 4369016; Applied Biosystems) and TaqMan assays for Gata3, Actin, and CyclophilinA (catalog no. Mm00484683_m1, catalog no. 4352663, and catalog no. Mm02342429_g1; Applied Biosystems) using the StepOnePlus RealTime PCR system.
Quantification of Gata3 protein levels by intracellular flow cytometry
Intracellular stainings were performed using the Foxp3 Transcription Factor Staining Buffer Set (catalog no. 00-5523; Thermo Fisher Scientific) and following the provided protocol. Briefly, after cell surface staining with anti-CD4 and anti-CD8 Abs, cells of interest (thymocytes, lymph node cells, and splenocytes) were permeabilized for 15 min in 100 μl of permeabilization buffer (catalog no. 00-5523; Thermo Fisher Scientific) containing 2 μl of Normal Rat Serum (catalog no. 13552; STEMCELL Technologies). After permeabilization, 5 μl of anti-mouse GATA-3–eFluor 660 Ab (catalog no. 50-9966-42; eBioscience) was added to each sample, followed by overnight incubation at 4°C. On the next day, cells were washed twice in permeabilization buffer, resuspended in PBS containing 5% FBS, and analyzed on a BD LSRFortessa.
Statistical analysis
Statistical analyses were performed using the unpaired Student t test (GraphPad Prism 8.2.1). Data are presented throughout as mean with error bars indicating SD.
Results
Generation of a novel, non–gene-destructive Gata3 reporter mouse
Using classical gene targeting in ES cells, we inserted an IRES–vYFP expression cassette into the 3′-untranslated region of the endogenous Gata3 gene locus (Fig. 1A), thus generating a novel strain of Gata3 reporter mice, termed GATIR. RT-PCR analysis of total RNA isolated from thymocytes of GATIR mice revealed robust expression of the targeted allele and the presence of chimeric transcripts of the predicted size, confirming correct insertion of the reporter cassette at the desired genomic site (Fig. 1B).
Three previously described strains of Gata3 reporter mice cannot be bred to homozygosity because of the loss of Gata3 expression from the targeted gene locus (18, 33) or formation of a hypomorphic allele (34). Our targeting strategy was designed to minimize potential effects of reporter insertion on endogenous Gata3 expression. Indeed, mating of heterozygous GATIR mice gave rise to homozygous offspring with Mendelian frequencies (Fig. 1C). Heterozygous and homozygous GATIR mice appeared healthy and fertile and were outwardly indistinguishable from wild-type littermates. Importantly, GATIR mice could be maintained as a homozygous line, with litter sizes not differing from those of wild-type C57BL/6 breeders (Fig. 1D). These findings demonstrate unbiased embryonic development and unimpaired fertility of heterozygous as well as homozygous GATIR mice.
Unperturbed T lymphopoiesis attests to physiologic Gata3 expression in GATIR reporter mice
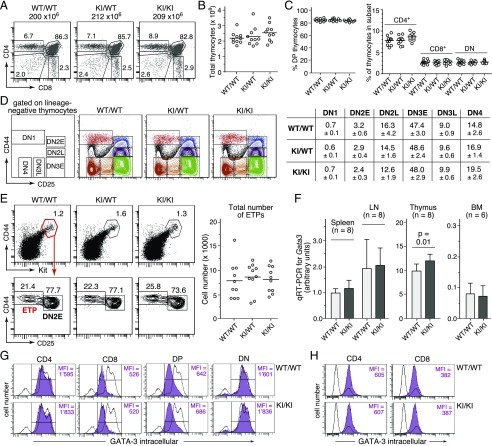
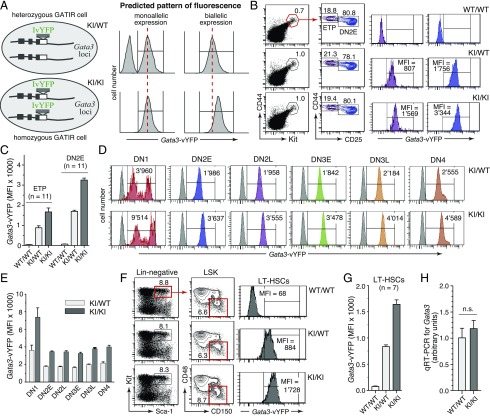
Most developmental stages in T lymphopoiesis are exquisitely dependent on physiological Gata3 expression, and experimental manipulation of Gata3 transcript levels can have profound effects on early thymopoiesis (reviewed in Ref. 3, 35). It thus seemed mandatory to comprehensively assess thymopoiesis in GATIR mice as a sensitive indicator for potential knock-in–associated detrimental effects. Importantly, thymopoiesis in neither heterozygous nor homozygous GATIR mice showed any significant deviation from wild-type controls. CD4/CD8 staining profiles (Fig. 2A), total number of thymocytes (Fig. 2B), and CD4/CD8 subset composition (Fig. 2C) were indistinguishable in all three genotypes. CD4/CD8 DN thymocytes can be further subdivided into several successive developmental stages (DN1–DN4) based on differential CD25 and CD44 expression (36). All of these developmentally early cell subsets were present in mice with targeted Gata3 alleles at frequencies comparable to those in wild-type mice (Fig. 2D). Also the total number of ETPs, which are currently considered the developmentally earliest intrathymic T cell progenitor population (37, 38), was unaffected in GATIR mice (Fig. 2E). In line with these findings, RT-qPCR analysis revealed highly similar Gata3 transcript levels in representative hematopoietic cell populations from homozygous GATIR and wild-type mice, with just a slight, statistically significant increase in GATIR thymocytes (Fig. 2F). Finally, equivalent GATA-3 protein levels in CD4/CD8 thymocyte (Fig. 2G) and splenic T cell subsets (Fig. 2H) from GATIR and wild-type mice indicated unimpaired translation of the chimeric, IRES–vYFP–encoding message. In conclusion, T lymphopoiesis proceeds normally in GATIR mice, consistent with unperturbed Gata3/GATA-3 expression.
FIGURE 2.
Unaltered thymopoiesis and intact Gata3 expression in GATIR reporter mice. (A) Representative CD4/CD8 profiles of total thymocytes from wild-type (WT) (WT/WT), heterozygous (knock-in [KI]/WT) and homozygous (KI/KI) GATIR mice. The total number of thymocytes is given on top of each panel. Numbers in dot plots indicate the percentage of cells in the respective gate. (B) No difference in total number of thymocytes between Gata3 genotypes (n = 10 mice of each genotype). Unless stated otherwise in this and all subsequent figures, each circle represents data from one individual mouse. (C) No difference in thymic CD4/CD8 subset composition between Gata3 genotypes; double-positive (DP) (i.e., CD4+CD8+) thymocytes; double-negative (DN) (i.e., CD4−CD8−) thymocytes. (D) Unaltered DN subset composition in heterozygous and homozygous GATIR mice. Leftmost panel, Definition of gates based on CD25 and CD44 surface expression; dot plots, representative CD25/CD44 staining profiles from mice of all three genotypes. Cells were pregated on Lin− (negative for CD4, CD8α, CD11b, CD11c, CD19, Gr1, NK1.1, TCRβ, TCRγδ, and TER119). Table to the right, Average percentage of cells in respective gate ± SD (n = 10 per genotype). (E) The total number of ETPs, defined as Lin−, CD25−CD44+Kithigh thymocytes is unaffected in heterozygous and homozygous GATIR mice. Left panels, Representative flow cytometric staining profiles and gating strategy. Depicted cell populations were pregated on Lin− thymocytes (negative for same marker combination as in (D). Right, Total number of ETPs in thymi from all three Gata3 genotypes (n = 10). (F) Comparison of Gata3 transcript levels in unfractionated splenocytes, lymph node (LN) cells, thymocytes, and total BM cells isolated from homozygous GATIR and WT control mice. Total RNA was isolated from indicated cell populations, transcribed in cDNA, and quantified for Gata3 transcript levels by RT-qPCR relative to actin expression. The average expression level in WT splenocytes was arbitrarily set as 1 (n = number of independent cell samples from different mice that were assessed by RT-qPCR in triplicate). No statistically significant differences were observed in samples from spleen, LN, and BM. (G and H) Highly similar intracellular GATA-3 protein levels in CD4/CD8 thymocyte (G) and splenic T cell (H) subsets from homozygous GATIR and WT control mice, as determined by intracellular flow cytometry. Respective cell populations were defined by electronic gating on CD4/CD8 thymic cell subsets (G) and CD3+CD4+ or CD3+CD8+ splenocytes (H). Purple histograms: GATA-3 protein levels in electronically gated cell subsets. Open histogram overlays in (G): GATA-3 protein level in total thymocytes from the same mice but depleted for CD4-, CD8-, and CD3-expressing cells to increase the relative abundance of GATA-3–negative thymocytes and thus better document the specificity of the staining for thymocytes. Open histogram overlays in (H): GATA-3 protein levels in total splenocytes.
Bright vYFP fluorescence faithfully reflects endogenous Gata3 expression in GATIR reporter mice
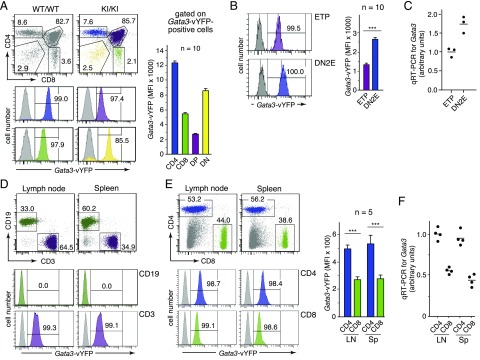
Several studies have provided significant information on highly dynamic Gata3 transcript levels in specific T lineage–committed cell subsets (reviewed in Ref. 1, 3, 35), allowing us to readily evaluate the reliability of GATIR mice for noninvasive monitoring of Gata3 expression. For instance, flow cytometric analysis of CD4/CD8 thymocyte subsets revealed strong marker expression in virtually all cells of CD4- and CD8-expressing subpopulations (Fig. 3A). Importantly, vYFP reporter expression is markedly upregulated from double-positive to single-positive thymocytes, with highest levels of fluorescence in CD4 single-positive cells, fully in line with published RT-qPCR data (39, 40). We also noted marked upregulation of vYFP fluorescence at the transition from the ETP to the DN2E stage of development (Fig. 3B), faithfully mimicking increased Gata3 transcript levels in wild-type mice (Fig. 3C), in full agreement with previous reports (23, 41, 42). In contrast, peripheral B cells in GATIR mice are completely devoid of vYFP expression (Fig. 3D), as expected from previous studies (23, 43–45). In contrast, peripheral T cell populations are fully labeled (Fig. 3D), consistent with GATA-3 being a critical T lineage determinant (reviewed in Ref. 1, 35). Again, markedly increased levels of vYFP fluorescence in electronically gated CD4+ versus CD8+ T cell subsets perfectly correlate with Gata3 transcript levels in wild-type mice (Fig. 3E, 3F).
FIGURE 3.
Robust vYFP reporter-derived fluorescence levels faithfully reflect endogenous Gata3 expression. (A) Analysis of CD4/CD8 thymocyte subsets for vYFP reporter expression. Left panels, Representative profiles of reporter expression in thymic CD4/CD8 subpopulations. Dot plots on top show the gating strategy. Gray histograms correspond to respective thymocyte subsets from the wild-type control mouse. Bar graph: average mean fluorescent intensity (MFI) of vYFP reporter expression in respective thymocyte subsets from 10 homozygous GATIR mice. (B) Gata3-vYFP reporter expression in ETP and DN2E thymocyte subsets. Left panels, Representative histograms are shown. Electronic gates for sorting of ETP and DN2E cells were as shown in Fig. 2E. Gray histograms correspond to respective cell populations from a wild-type control mouse. Bar graph: Average MFI of vYFP reporter expression in ETP and DN2E thymocyte subsets from 10 homozygous GATIR mice. (C) Quantification of Gata3 transcript levels in wild-type ETP and DN2E cells by RT-qPCR relative to cyclophilin A expression. ETP and DN2E cells were obtained from pooled thymocytes of two wild-type mice in each of three separate FACS sorts. Electronic gates for sorting of ETP and DN2E cells were as shown in Fig. 2E. All cDNAs obtained from individual FACS sorts were then analyzed in triplicate in the same RT-qPCR amplification experiment. Hence, each circle corresponds to the average signal in the respective cell population (ETP or DN2E) from an individual FACS sort. The average signal intensity for ETPs was arbitrarily set as 1. (D) Representative histograms of vYFP reporter expression in gated B (CD19+; green) and T lymphocytes (CD3+; purple) from lymph node (LN) and spleen (Sp). Dot plots on top show gating strategy. Gray histograms correspond to the respective cell population from a wild-type mouse. (E) Gata3-vYFP reporter expression in CD4+ and CD8+ T cell populations from lymph node and spleen. Left panels, Representative profiles of vYFP reporter expression in indicated T cell subsets. Dot plots on top show gating strategy. All cells shown are pregated on CD3-positive cells. Gray histograms correspond to the respective cell population from a wild-type mouse. Bar graph: average MFI of vYFP reporter expression in respective T cell subsets from five homozygous GATIR mice. (F) Quantification of Gata3 transcript levels in CD4+ and CD8+ T cell subsets FACS-sorted from LN and Sp of wild-type mice. Corresponding cell subsets from two mice were pooled in each of four independent FACS sorts. All cDNAs obtained from individual FACS sorts were then analyzed in triplicate in the same RT-qPCR amplification experiment relative to actin expression. Hence, each circle corresponds to the average signal in the respective cell population from an individual FACS sort. The average signal intensity for splenic CD4+ T cells was arbitrarily set as 1. ***p < 0.001.
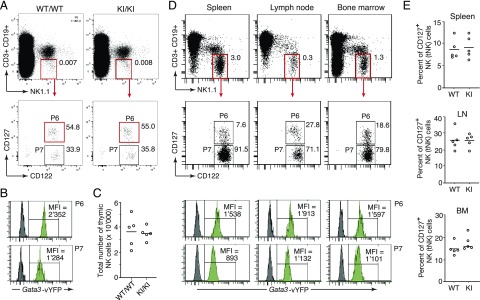
Finally, we assessed the fidelity of reporter expression by focusing on classical NK (cNK) and tNK cells, which are developmentally different subpopulations with distinctive levels of Gata3 expression (46, 47). Following the gating scheme published in the original description of tNK cells (14) (Fig. 4A), we find significantly elevated reporter expression in tNK versus cNK cells (Fig. 4B), faithfully reflecting the reported differences in Gata3 expression. Importantly, the total number of tNK cells was unaltered in thymi from homozygous GATIR mice compared with wild-type controls (Fig. 4C), demonstrating again the developmental neutrality of Gata3 knock-in alleles, this time for the tNK lineage, which is strictly dependent on Gata3 expression (12, 14). Also tNK cells present in spleen, lymph nodes, and BM of GATIR mice exhibited elevated levels of vYFP-derived fluorescence compared with cNK cells from the same tissue (Fig. 4D). Relative frequencies of tNK cells were highly similar in GATIR and wild-type mice (Fig. 4E) and comparable to those reported previously for the respective tissue (14). Of note, although β-galactosidase activity in heterozygous lacZ/Gata3-reporter mice (18) used in previous studies was too low to be detectable in most cNK cells (14), GATIR mice allowed visualization of Gata3 expression in virtually all cNK cells, in line with an important role of GATA-3 in cNK cell migration and function (48).
FIGURE 4.
Monitoring Gata3 expression in thymic versus conventional NK cell subsets. (A) Gating strategy for flow cytometric discrimination of tNK (gate P6) and cNK cells (gate P7) based on differential IL-7R (CD127) expression. Dot plots show total thymocytes from C57BL/6 wild-type (WT) (WT/WT) and homozygous GATIR knock-in (knock-in [KI]/KI) mice. (B) Representative histograms showing significantly increased Gata3-vYFP reporter expression in tNK (gate P6) versus cNK (gate P7) cells from the thymus of a homozygous GATIR mouse (green histograms). Dark gray histograms represent corresponding cell populations from a WT control mouse. The histograms are derived from the same cells shown in the gating scheme above. (C) Thymi from homozygous GATIR mice (KI/KI) have similar tNK cell numbers as WT (WT/WT) C57BL/6 control mice. Each circle represents data from one individual mouse. (D) Top panels show gating scheme to discriminate tNK (gate P6) and cNK (gate P7) cell subsets in spleen, lymph node (LN), and BM from a representative homozygous GATIR mouse. The green histograms below show Gata3-vYFP reporter expression in the respective tNK or cNK cell subset. Dark gray histograms represent corresponding cell populations from a WT control mouse. (E) Homozygous GATIR (KI) and C57BL/6 WT control mice (WT) have similar frequencies of tNK cells (CD127+ NK cells) in spleen, LN, and BM. Each circle represents data from one individual mouse (in total, five mice of each genotype were analyzed). Gating for CD127+ NK cells (tNK cells) as shown in (D).
GATIR mice as a novel tool for noninvasive monitoring of Th2 differentiation
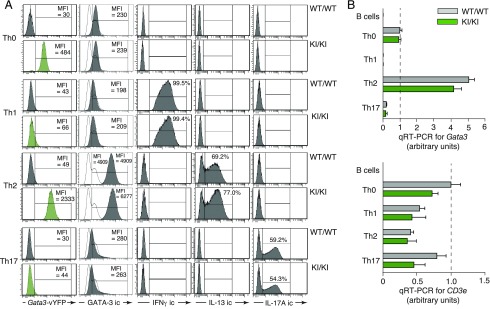
Elevated Gata3 expression is a lineage determinant for Th2-type helper cells (4–6). We therefore tested the usefulness of our fluorescent reporter for monitoring Th polarization. To this end, we cultured naive splenic T cells from homozygous GATIR mice and wild-type controls in vitro under conditions known to promote differentiation into Th1, Th2, or Th17 cells. After 6 d of culture, the differentiation status of in vitro–primed Th cells was confirmed by intracellular staining for the subtype-specific cytokines IFN-γ (Th1), IL-13 (Th2), and IL-17A (Th17). As documented in Fig. 5A, differentiation into Th2 cells resulted in strong upregulation of reporter-mediated fluorescence, in line with published RT-qPCR results (49) and the important role of Gata3 expression in Th2-type cells (reviewed in Ref. 3, 50, 51). In contrast, reporter expression decreased to near-background levels in Th1 and Th17 cells, indicating downregulation of Gata3 expression. Importantly, neither kinetics nor the pattern of cytokine expression differed between cells from homozygous GATIR and wild-type control mice, demonstrating that reporter insertion into the endogenous Gata3 locus did not influence Th polarization (Fig. 5A). Interestingly, although GATA-3 protein levels increased drastically in Th2-type cells, there was no appreciable decline in Th1 and Th17 cells despite strong reduction of vYFP expression (Fig. 5A, second column), highlighting the fact that our reporter monitors transcript but not protein levels. Importantly, RT-qPCR assays with total RNA isolated from in vitro–polarized cells of GATIR and wild-type origin confirmed strict correlation of reporter expression with Gata3 transcript levels (Fig. 5B). GATIR mice thus provide a powerful tool for noninvasive monitoring of Th polarization at the single-cell level.
FIGURE 5.
Noninvasive monitoring of Th2 cell polarization. (A) Flow cytometric analysis of Gata3-vYFP reporter expression (first column) and of intracellular GATA-3 protein levels (second column) in splenic CD4+ T cell populations from homozygous GATIR (KI/KI) and C57BL/6 control (wild-type [WT]/WT) mice before (Th0) and 6 d after in vitro polarization into Th1, Th2, and TH17 cells, respectively. Intracellular (ic) stainings for IFN-γ, IL-13, and IL-17A were used to confirm efficient polarization. Data are representative of three independent experiments. (B) Quantification of Gata3 transcript levels in Th cell populations before (Th0) and 6 d after in vitro polarization into Th1, Th2, or Th17 cells, respectively (top panel). Concomitant analysis of the same RNA samples for CD3e expression served as quality control for the presence of template RNA (lower panel). Data were normalized for β-actin expression, and values obtained with RNA from wild-type Th0 cells were arbitrarily set as 1 (dashed lines). RNA from FACS-sorted B cells served as control for primer specificity in both RT-qPCR analyses. RNA samples were analyzed in triplicate. Data are derived from a single polarization experiment distinct from the one shown in (A).
BM analysis highlights GATIR mice as a convenient source for straightforward identification of ILC2Ps
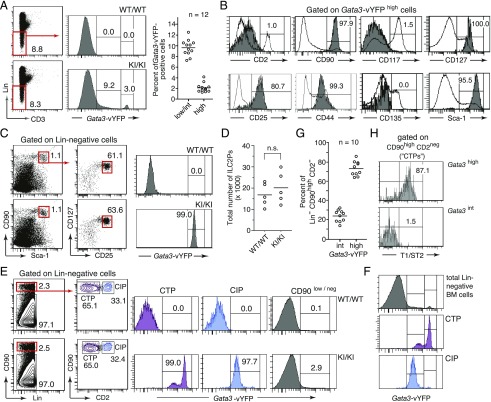
Gata3 transcripts have been detected at highly various levels in several hematopoietic progenitor cell subsets within the lineage marker–negative (Lin−) BM fraction [see the Immunological Genome Project at http://www.immgen.org (52)]. Flow cytometric analysis of Lin− BM cells from GATIR mice revealed a concordant pattern of vYFP-derived fluorescence. Although the majority of cells (>85%) were clearly Gata3-reporter–negative, ∼10% exhibited a continuum of fluorescence intensities reflecting very low-to-intermediate levels of reporter expression (Fig. 6A). More striking, a small group of cells (typically 1–4%) stood out as a discrete subset with extraordinarily bright fluorescence (Fig. 6A). Comprehensive surface immunophenotyping identified these cells as committed ILC2Ps, which have been described previously as discrete Lin− CD90high Sca-1high CD127high CD25+ population with high Gata3 expression (10). Indeed, when using this five-parameter scheme for Gata3-independent identification of ILC2Ps, all cells gated accordingly from GATIR mice uniformly exhibited the highest levels of Gata3 reporter expression (Fig. 6C). Importantly, although ILC2P development is exquisitely GATA-3–dependent (10–12), total numbers of ILC2Ps were identical in GATIR and wild-type mice (Fig. 6D), again in line with unimpaired Gata3 expression in reporter mice.
FIGURE 6.
Monitoring Gata3 expression in immature BM cell subsets. (A) Flow cytometric analysis of Gata3-vYFP reporter expression in Lin− BM cells. Left panels, Gating strategy and representative histograms of vYFP reporter expression. Scatter plot: percentage of Lin− BM cells with low to intermediate (low/int) and high reporter expression in homozygous GATIR mice. Classification in low/int and high cells is based on the gating shown in the histogram to the left. (B) Immunophenotypic characterization of vYFPhigh cells from homozygous GATIR mice for eight informative cell surface markers (dark histograms). As internal reference for staining intensities, open histogram overlays represent the corresponding staining pattern on Lin− cells (CD25, CD90, CD117, CD135, and Sca-1 panels), on all live cells (CD127 panel), on cells with low-to-intermediate reporter expression (CD2 panel), and on BM Lin− (CD11b−, CD11c−, CD19−, NK1.1−, GR1−, and TER119−) CD3+ T cells (CD44 panel), analyzed in the same sample. (C) All ILC2Ps identified as Lin−, CD90high, Sca-1high, CD127+, and CD25+ cells following a published gating scheme (10) are homogenously Gata3-vYFPhigh cells. (D) No difference in total number of ILC2Ps in BM of wild-type (WT) (WT/WT) and homozygous GATIR (knock-in [KI]/KI) mice. ILC2Ps were identified based on the gating scheme shown in (C) (i.e., independent of reporter expression). (E) GATIR mice allow identification of ILC2Ps within a previously defined CTP population based on high vYFP reporter expression. Dot plots show the gating scheme that has been described for the identification of CTPs and CIPs as discrete developmental stages of extrathymic T lymphopoiesis (53). Histograms show Gata3-vYFP reporter expression in gated cell subsets. (F) Comparison of Gata3-vYFP reporter expression intensities in CIP, CTP, and total Lin− BM cells. (G) Percentage of Gata3-vYFPint and Gata3-vYFPhigh (ILC2P) cells within the population gated as CTP. Data are from 10 homozygous GATIR mice. (H) Selective expression of the ILC2 marker T1/ST2 (IL-33Rα) on Gata3-vYFPhigh CTPs.
Long before the identification of ILC2Ps (10), Lin− CD90high CD2− cells were shown to contain a T lineage–committed precursor population, termed committed T cell progenitor (CTP) that would give rise in vitro and in vivo to Lin− CD90high CD2+ committed intermediate progenitors (CIPs), with both CTPs and CIPs belonging to an extrathymic pathway of T lymphopoiesis (53, 54). The fact that ILC2Ps are also Lin− CD90high CD2− cells (10) (see also Fig. 6B) suggested that the previously described CTP population might be heterogeneous, comprising not just T-committed CTPs, but also ILC2Ps. This suspicion was readily confirmed in GATIR mice. Following the published staining procedure for CTPs/CIPs (32, 53), we observed in the gated CTP subset of GATIR mice two discrete subpopulations of intermediate and high Gata3 expression, respectively (Fig. 6E), with fluorescence intensities corresponding to those in Gata3int and Gata3high subsets among Lin− cells (Fig. 6F). In contrast, CIPs exhibited a single peak of Gata3 reporter expression at the intermediate level, consistent with a developmentally more homogenous, T lineage-restricted population. Notably, as GATIR mice unveil, CTPs contain about four times as many ILC2Ps as genuine T lineage–committed precursors (Fig. 6G). That Gata3high but not Gata3int CTPs are authentic ILC2Ps is supported by the fact that only the former express T1/ST2 (Fig. 5H), a well-established marker for ILC2 lineage cells (55).
GATIR mice refute the concept of monoallelic Gata3 expression
Monoallelic Gata3 expression has been reported in hematopoietic stem cells and specific T cell progenitor subsets, including the ETP and DN2E stages (20, 26). The availability of heterozygous as well as homozygous Gata3 reporter mice provide the unique opportunity to easily distinguish monoallelic and biallelic patterns of Gata3 expression in live cell populations simply by flow cytometry. As outlined in Fig. 7A (top panels), in heterozygous GATIR mice, monoallelic Gata3 expression would be expected to generate a biphasic pattern of reporter expression in the cell population of interest, as only a fraction (typically 50%) of cells would express the labeled or unlabeled allele, respectively. In contrast, biallelic Gata3 expression would give rise to a single peak of fluorescence. When homozygous GATIR mice are included in the analysis (Fig. 7A, lower panels), the allelic pattern of Gata3 expression would be revealed additionally by the intensity of reporter-derived fluorescence. Surprisingly, unequivocal data obtained with GATIR mice were completely incompatible with monoallelic Gata3 expression in any of the analyzed cell subsets. Neither ETP or DN2E cells (Fig. 7B, 7C) nor any other DN cell subset defined by differential CD25 and CD44 expression (Fig. 7D, 7E) exhibited a pattern of reporter expression indicative of monoallelic Gata3 expression. Instead, the complete lack of unlabeled cells in heterozygous cell populations and the virtually exact doubling of fluorescent intensities in cells from homozygous reporter mice provided conclusive evidence for uniform biallelic Gata3 expression at all stages of thymocyte development. Similarly, LT-HSCs, also claimed to exhibit monoallelic Gata3 expression (20), were observed to express Gata3 from both reporter alleles (Fig. 7F, 7G). To explore the remote possibility that reporter insertion into the Gata3 locus might have destroyed the molecular machinery safeguarding monoallelic expression, we compared Gata3 transcript levels in combined ETP plus DN2E cell subsets from homozygous GATIR and wild-type mice by RT-qPCR. Experimentally provoked loss of monoallelic expression in GATIR mice would be expected to increase Gata3 transcript levels exactly 2-fold, which was clearly not the case (Fig. 7H).
FIGURE 7.
Direct visualization of the allelic Gata3 expression status in select cell populations. (A) Scheme showing the predicted pattern of Gata3 reporter expression in cells from heterozygous (top panels) or homozygous (lower panels) GATIR mice in case of monoallelic (left histograms) or biallelic (right histograms) Gata3 expression. For instance, complete absence of a reporter-negative cell subset in heterozygous cells and a 2-fold increase in fluorescence intensity in homozygous cells would reveal perfect biallelic expression. (B) Representative pattern of Gata3 reporter expression in ETP (purple histograms) and DN2E (blue histograms) cells, attesting perfect biallelic expression of Gata3. Thymocytes were pregated for Lin− cells. (C) Quantification of reporter expression in ETPs and DN2E cells from 11 mice of each genotype (four separate experiments). (D) Representative pattern of Gata3 reporter expression in DN thymocyte subsets defined by differential CD25 and CD44 expression. Respective DN cell subsets are defined as shown in Fig. 2D. Gray histograms correspond to respective cell subsets from a wild-type mouse lacking reporter expression. (E) Quantification of reporter expression in indicated DN subsets, revealing perfect biallelic expression of Gata3 in all subsets analyzed (n = 11 for each genotype) in four separate experiments. (F) Representative pattern of Gata3 reporter expression in LT-HSCs, demonstrating perfect biallelic Gata3 expression. Notably, the gating scheme used to identify LT-HSCs is as described in the original publication purporting monoallelic Gata3 expression in LT-HSCs (20). (G) Quantification of reporter expression in LT-HSCs from seven mice of each genotype (three independent experiments). (H) Similar Gata3 transcript levels in FACS-sorted Lin− CD44high Kithigh thymocytes (combined ETP plus DN2E subsets) from wild-type and homozygous GATIR mice exclude the possibility of experimentally induced loss of monoallelic Gata3 expression specifically in GATIR mice. Sorting gates for Lin− CD44high Kithigh thymocytes were as shown in (B). Transcript levels were determined by RT-qPCR relative to actin expression with separate RNA samples from five mice of each genotype (three independent experiments).
Discussion
The intriguing pattern of Gata3 expression has long been recognized as a potentially valuable marker for the identification of specific lymphocytic cell subsets at critical stages of development and differentiation. However, expression of a transcription factor cannot be quantified directly in living cells. That is a problem that has prompted the development of knock-in reporter mice. The first such animals described carried a lacZ expression cassette within the endogenous Gata3 locus (18). Although these mice provided a useful tool to visualize Gata3 expression, particularly in tissue sections, they were not suitable for the isolation of living cells, as detection of β-galactosidase activity required prior cell permeabilization. Moreover, signal intensities were often quite low, leaving significant fractions of Gata3-positive cells unlabeled, including many thymocytes (18) or cNK cells (14). A much-improved reporter strain, carrying a GFP cassette within the endogenous Gata3 locus, allowed for the first-time, to our knowledge, monitoring of Gata3 expression in living cells (33). GFP/Gata3 reporter mice have helped to address a number of important questions (10, 56); however, they share with lacZ mice the disadvantage of disrupted Gata3 expression on the targeted allele. Consequently, these mice cannot be made homozygous, impeding reliable detection of Gata3low cells. Moreover, as GATA-3 function is highly dosage sensitive, loss of one functional Gata3 allele may result in problematic phenotypic abnormalities. The marked reduction of ILC2P/ILC2 cells in tissues of lacZ/Gata3 reporter mice provides a striking example (11). Finally, a third line of reporter mice, engineered to express an EGFP–GATA-3 fusion protein, turned out to be hypomorphic, exhibiting homozygous lethality and severely impaired T lymphopoiesis, even in heterozygous mice (34).
As shown in this study, GATIR mice are completely devoid of such problems, as endogenous Gata3 expression remains intact. Consequently, GATIR mice can be maintained as homozygous line affording twice the intensity of reporter expression. Importantly, neither T lymphopoiesis, nor development of tNK or ILC2 cell populations, known to be highly sensitive to GATA-3 dosage, are noticeably affected in homozygous GATIR mice, highlighting the developmental neutrality of our genetic modification. Several advantageous features of our fluorescent reporter vYFP, such as optimal excitation/emission spectra for flow cytometry, extrafast maturation kinetics, insensitivity to intracellular pH, and its exceptional brightness (27) further contribute to the usefulness of GATIR mice.
Although our comprehensive flow cytometric analysis of reporter expression in representative hematopoietic cell populations from GATIR mice documents faithful recapitulation of endogenous Gata3 expression, a prerequisite for a reliable reporter mouse, our data clearly go beyond mere confirmation of previously published expression data. Notably, GATIR mice provide information on Gata3 expression at the single-cell level, whereas methods used in most previous studies (RT-PCR, microarray technology, and heterozygous reporter mice with suboptimal marker expression) lacked this ultimate degree of resolution, revealing just average expression values for a particular population. For instance, whereas Gata3 expression has been validly detected in the ETP subset previously (57, 58), the exact fraction of Gata3-positive ETPs has remained obscure. GATIR mice now reveal that at least 99% of ETPs are uniformly Gata3 expressing (Fig. 3B), a remarkable observation, as Gata3 transcription is thought to be induced just at or shortly before the ETP stage, following Notch signaling (57, 59, 60). Furthermore, single-cell resolution of Gata3 expression in GATIR mice revealed a previously unappreciated developmental heterogeneity of BM CTPs (Fig. 6E–H) and disclosed for the first time, to our knowledge, uniform Gata3 expression in virtually all LT-HSCs (Fig. 7F), a finding of some emphasis, as a role for GATA-3 in LT-HSC maintenance is still under dispute (61, 62).
We also show that GATIR mice provide a perfect tool for noninvasive monitoring of Th2 differentiation in single cells (Fig. 5) and the identification of interesting cell subsets based on distinctive levels of Gata3 expression. BM ILC2Ps provide an eminent example (Fig. 6A, 6C). Whereas traditional methods of cytometric identification require at least five distinct parameters (Lin−, CD90high, Sca-1high, CD25+, and CD127+) (10), in GATIR mice, two parameters suffice (Lin−, vYFP/Gata3high). In a recent study, comprehensive characterization of BM-resident ILC progenitors using polychrome knock-in reporter mice and single-cell transcriptome analysis neatly confirms high Gata3 expression as a key marker for ILC2Ps (13). The usefulness of GATIR mice for the identification and quantification of Th2 and ILC2 cell populations has also been highlighted in a recent collaborative study investigating the heterogeneity of ILC2 subsets in mouse models of allergic airway inflammation (63).
A high-impact publication has reported monoallelic Gata3 expression in LT-HSCs and thymic ETP/DN2E cell subsets, purportedly followed by a “switch to bi-allelic Gata3 transcription abruptly at midthymopoiesis” (20). These weighty conclusions were based on technically demanding single-cell assays and a hypomorphic Gata3 reporter mouse. The availability of heterozygous as well as homozygous GATIR mice has allowed us to faithfully monitor the allelic Gata3 expression status in aforementioned cell populations in a noninvasive manner (Fig. 7). Notably, we did not find any evidence for monoallelic Gata3 expression. In contrast, patterns of reporter expression were fully congruent with biallelic expression in all cells analyzed. Our results thus also dispute the contention “that developing T cells in which allelic exclusion is maintained at the Tcrb locus bear one repressed and one active Gata3 allele” (26). Of note, in contrast to the complex techniques required in previous studies, our data are based on simple flow cytometry. The potential caveat of compromised allelic regulation in GATIR mice was ruled out experimentally, as Gata3 transcript levels were not markedly elevated in relevant cell populations of GATIR versus wild-type mice (Fig. 7H). Our data thus completely negate the concept of monoallelic Gata3 expression evoked in previous publications (20, 26).
This is not the first time that the analysis of knock-in reporter mice contests reports of monoallelic gene expression based on single-cell assays. Prominent examples include the Il2 (64, 65) and Pax5 (66, 67) genes. In the latter case, the same research group critically revised initial findings in a later study with their own knock-in reporter mice, commenting as follows: “These discrepancies question whether negative results obtained by FISH and single-cell RT-PCR analyses always reflect the failure of an allele to be expressed or rather result from the limitation of these methods in reliably detecting the expression of both alleles, as discussed by Rhoades et al. (68)” (67). We fully subscribe to this view.
Supplementary Material
Acknowledgments
We thank Ramona Syhachak and the Tierforschungszentrum Ulm for expert care of our mouse facility and Alpaslan Tasdogan for manifold support.
This work was funded by a grant from the Deutsche Forschungsgemeinschaft (FE 578/3-2) and institutional resources.
The online version of this article contains supplemental material.
- BM
- bone marrow
- CIP
- committed intermediate progenitor
- cNK
- classical NK
- CTP
- committed T cell progenitor
- DN
- double-negative
- DN2E
- DN stage 2 early
- ES
- embryonic stem
- ETP
- early thymic progenitor
- GATIR
- Gata3-IRESvYFP
- ILC
- innate lymphoid cell
- ILC2P
- ILC2 progenitor
- IRES
- internal ribosomal entry site
- Lin−
- lineage marker–negative
- LT-HSC
- long-term hematopoietic stem cell
- RT-qPCR
- real-time quantitative PCR
- tNK
- thymic NK
- vYFP
- Venus yellow fluorescent protein.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Tindemans I., Serafini N., Di Santo J. P., Hendriks R. W. 2014. GATA-3 function in innate and adaptive immunity. Immunity 41: 191–206. [DOI] [PubMed] [Google Scholar]
- 2.Hosoya T., Maillard I., Engel J. D. 2010. From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation. Immunol. Rev. 238: 110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho I. C., Tai T. S., Pai S. Y. 2009. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 9: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng W., Flavell R. A. 1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89: 587–596. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J., Min B., Hu-Li J., Watson C. J., Grinberg A., Wang Q., Killeen N., Urban J. F., Jr., Guo L., Paul W. E. 2004. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 5: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 6.Pai S. Y., Truitt M. L., Ho I. C. 2004. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc. Natl. Acad. Sci. USA 101: 1993–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J. 2017. GATA3 regulates the development and functions of innate lymphoid cell subsets at multiple stages. Front. Immunol. 8: 1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Obaldia M. E., Bhandoola A. 2015. Transcriptional regulation of innate and adaptive lymphocyte lineages. Annu. Rev. Immunol. 33: 607–642. [DOI] [PubMed] [Google Scholar]
- 9.Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J. P., Eberl G., Koyasu S., Locksley R. M., McKenzie A. N. J., Mebius R. E., et al. 2018. Innate lymphoid cells: 10 years on. Cell 174: 1054–1066. [DOI] [PubMed] [Google Scholar]
- 10.Hoyler T., Klose C. S., Souabni A., Turqueti-Neves A., Pfeifer D., Rawlins E. L., Voehringer D., Busslinger M., Diefenbach A. 2012. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37: 634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein Wolterink R. G., Serafini N., van Nimwegen M., Vosshenrich C. A., de Bruijn M. J., Fonseca Pereira D., Veiga Fernandes H., Hendriks R. W., Di Santo J. P. 2013. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 110: 10240–10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagi R., Zhong C., Northrup D. L., Yu F., Bouladoux N., Spencer S., Hu G., Barron L., Sharma S., Nakayama T., et al. 2014. The transcription factor GATA3 is critical for the development of all IL-7Rα-expressing innate lymphoid cells. Immunity 40: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker J. A., Clark P. A., Crisp A., Barlow J. L., Szeto A., Ferreira A. C. F., Rana B. M. J., Jolin H. E., Rodriguez-Rodriguez N., Sivasubramaniam M., et al. 2019. Polychromic reporter mice reveal unappreciated innate lymphoid cell progenitor heterogeneity and elusive ILC3 progenitors in bone marrow. Immunity 51: 104–118.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vosshenrich C. A., García-Ojeda M. E., Samson-Villéger S. I., Pasqualetto V., Enault L., Richard-Le Goff O., Corcuff E., Guy-Grand D., Rocha B., Cumano A., et al. 2006. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. [Published erratum appears in 2006 Nat. Immunol. 7: 1343.] Nat. Immunol. 7: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro V. S., Hasan M., Wilson A., Boucontet L., Pereira P., Lesjean-Pottier S., Satoh-Takayama N., Di Santo J. P., Vosshenrich C. A. 2010. Cutting edge: thymic NK cells develop independently from T cell precursors. J. Immunol. 185: 4993–4997. [DOI] [PubMed] [Google Scholar]
- 16.Lim K. C., Lakshmanan G., Crawford S. E., Gu Y., Grosveld F., Engel J. D. 2000. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat. Genet. 25: 209–212. [DOI] [PubMed] [Google Scholar]
- 17.Pandolfi P. P., Roth M. E., Karis A., Leonard M. W., Dzierzak E., Grosveld F. G., Engel J. D., Lindenbaum M. H. 1995. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat. Genet. 11: 40–44. [DOI] [PubMed] [Google Scholar]
- 18.Hendriks R. W., Nawijn M. C., Engel J. D., van Doorninck H., Grosveld F., Karis A. 1999. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. Eur. J. Immunol. 29: 1912–1918. [DOI] [PubMed] [Google Scholar]
- 19.Ting C. N., Olson M. C., Barton K. P., Leiden J. M. 1996. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature 384: 474–478. [DOI] [PubMed] [Google Scholar]
- 20.Ku C. J., Lim K. C., Kalantry S., Maillard I., Engel J. D., Hosoya T. 2015. A monoallelic-to-biallelic T-cell transcriptional switch regulates GATA3 abundance. Genes Dev. 29: 1930–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taghon T., Yui M. A., Rothenberg E. V. 2007. Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nat. Immunol. 8: 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nawijn M. C., Ferreira R., Dingjan G. M., Kahre O., Drabek D., Karis A., Grosveld F., Hendriks R. W. 2001. Enforced expression of GATA-3 during T cell development inhibits maturation of CD8 single-positive cells and induces thymic lymphoma in transgenic mice. J. Immunol. 167: 715–723. [DOI] [PubMed] [Google Scholar]
- 23.Scripture-Adams D. D., Damle S. S., Li L., Elihu K. J., Qin S., Arias A. M., Butler R. R., III, Champhekar A., Zhang J. A., Rothenberg E. V. 2014. GATA-3 dose-dependent checkpoints in early T cell commitment. J. Immunol. 193: 3470–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Esch H., Groenen P., Nesbit M. A., Schuffenhauer S., Lichtner P., Vanderlinden G., Harding B., Beetz R., Bilous R. W., Holdaway I., et al. 2000. GATA3 haplo-insufficiency causes human HDR syndrome. Nature 406: 419–422. [DOI] [PubMed] [Google Scholar]
- 25.Bardhan T., Jeng J. Y., Waldmann M., Ceriani F., Johnson S. L., Olt J., Rüttiger L., Marcotti W., Holley M. C. 2019. Gata3 is required for the functional maturation of inner hair cells and their innervation in the mouse cochlea. J. Physiol. 597: 3389–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ku C. J., Sekiguchi J. M., Panwar B., Guan Y., Takahashi S., Yoh K., Maillard I., Hosoya T., Engel J. D. 2017. GATA3 abundance is a critical determinant of T cell receptor β allelic exclusion. Mol. Cell. Biol. 37: e00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai T., Ibata K., Park E. S., Kubota M., Mikoshiba K., Miyawaki A. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20: 87–90. [DOI] [PubMed] [Google Scholar]
- 28.Madan V., Madan B., Brykczynska U., Zilbermann F., Hogeveen K., Döhner K., Döhner H., Weber O., Blum C., Rodewald H. R., et al. 2009. Impaired function of primitive hematopoietic cells in mice lacking the mixed-lineage-leukemia homolog MLL5. Blood 113: 1444–1454. [DOI] [PubMed] [Google Scholar]
- 29.Schwenk F., Baron U., Rajewsky K. 1995. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23: 5080–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kissel H., Timokhina I., Hardy M. P., Rothschild G., Tajima Y., Soares V., Angeles M., Whitlow S. R., Manova K., Besmer P. 2000. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 19: 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlot T., Alt F. W., Bassing C. H., Suh H., Pinaud E. 2005. Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc. Natl. Acad. Sci. USA 102: 14362–14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luche H., Nageswara Rao T., Kumar S., Tasdogan A., Beckel F., Blum C., Martins V. C., Rodewald H. R., Fehling H. J. 2013. In vivo fate mapping identifies pre-TCRα expression as an intra- and extrathymic, but not prethymic, marker of T lymphopoiesis. J. Exp. Med. 210: 699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grote D., Souabni A., Busslinger M., Bouchard M. 2006. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development 133: 53–61. [DOI] [PubMed] [Google Scholar]
- 34.Hosoya T., Kuroha T., Moriguchi T., Cummings D., Maillard I., Lim K. C., Engel J. D. 2009. GATA-3 is required for early T lineage progenitor development. J. Exp. Med. 206: 2987–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yui M. A., Rothenberg E. V. 2014. Developmental gene networks: a triathlon on the course to T cell identity. Nat. Rev. Immunol. 14: 529–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceredig R., Rolink T. 2002. A positive look at double-negative thymocytes. Nat. Rev. Immunol. 2: 888–897. [DOI] [PubMed] [Google Scholar]
- 37.Rothenberg E. V. 2019. Programming for T-lymphocyte fates: modularity and mechanisms. Genes Dev. 33: 1117–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S. L., Bhandoola A. 2014. Trafficking to the thymus. Curr. Top. Microbiol. Immunol. 373: 87–111. [DOI] [PubMed] [Google Scholar]
- 39.Hernández-Hoyos G., Anderson M. K., Wang C., Rothenberg E. V., Alberola-Ila J. 2003. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity 19: 83–94. [DOI] [PubMed] [Google Scholar]
- 40.Gimferrer I., Hu T., Simmons A., Wang C., Souabni A., Busslinger M., Bender T. P., Hernandez-Hoyos G., Alberola-Ila J. 2011. Regulation of GATA-3 expression during CD4 lineage differentiation. J. Immunol. 186: 3892–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yui M. A., Feng N., Rothenberg E. V. 2010. Fine-scale staging of T cell lineage commitment in adult mouse thymus. J. Immunol. 185: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taghon T. N., David E. S., Zúñiga-Pflücker J. C., Rothenberg E. V. 2005. Delayed, asynchronous, and reversible T-lineage specification induced by Notch/Delta signaling. Genes Dev. 19: 965–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothenberg E. V. 2013. GATA-3 locks the door to the B-cell option. Blood 121: 1673–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García-Ojeda M. E., Klein Wolterink R. G., Lemaître F., Richard-Le Goff O., Hasan M., Hendriks R. W., Cumano A., Di Santo J. P. 2013. GATA-3 promotes T-cell specification by repressing B-cell potential in pro-T cells in mice. Blood 121: 1749–1759. [DOI] [PubMed] [Google Scholar]
- 45.Banerjee A., Northrup D., Boukarabila H., Jacobsen S. E., Allman D. 2013. Transcriptional repression of Gata3 is essential for early B cell commitment. Immunity 38: 930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Male V., Brady H. J. M. 2017. Murine thymic NK cells: a case of identity. Eur. J. Immunol. 47: 797–799. [DOI] [PubMed] [Google Scholar]
- 47.Smyth M. J., Nutt S. L. 2006. IL-7 and the thymus dictate the NK cell ‘labor market’. Nat. Immunol. 7: 1134–1136. [DOI] [PubMed] [Google Scholar]
- 48.Samson S. I., Richard O., Tavian M., Ranson T., Vosshenrich C. A., Colucci F., Buer J., Grosveld F., Godin I., Di Santo J. P. 2003. GATA-3 promotes maturation, IFN-gamma production, and liver-specific homing of NK cells. Immunity 19: 701–711. [DOI] [PubMed] [Google Scholar]
- 49.Wei G., Abraham B. J., Yagi R., Jothi R., Cui K., Sharma S., Narlikar L., Northrup D. L., Tang Q., Paul W. E., et al. 2011. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 35: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yagi R., Zhu J., Paul W. E. 2011. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 23: 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker J. A., McKenzie A. N. J. 2018. TH2 cell development and function. Nat. Rev. Immunol. 18: 121–133. [DOI] [PubMed] [Google Scholar]
- 52.Immunological Genome Project Consortium 2013. Transcriptome analysis identifies regulators of hematopoietic stem and progenitor cells. Stem Cell Rep. 1: 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Ojeda M. E., Dejbakhsh-Jones S., Chatterjea-Matthes D., Mukhopadhyay A., BitMansour A., Weissman I. L., Brown J. M., Strober S. 2005. Stepwise development of committed progenitors in the bone marrow that generate functional T cells in the absence of the thymus. J. Immunol. 175: 4363–4373. [DOI] [PubMed] [Google Scholar]
- 54.Dejbakhsh-Jones S., Garcia-Ojeda M. E., Chatterjea-Matthes D., Zeng D., Strober S. 2001. Clonable progenitors committed to the T lymphocyte lineage in the mouse bone marrow; use of an extrathymic pathway. Proc. Natl. Acad. Sci. USA 98: 7455–7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neill D. R., Wong S. H., Bellosi A., Flynn R. J., Daly M., Langford T. K., Bucks C., Kane C. M., Fallon P. G., Pannell R., et al. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464: 1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frelin C., Herrington R., Janmohamed S., Barbara M., Tran G., Paige C. J., Benveniste P., Zuñiga-Pflücker J. C., Souabni A., Busslinger M., Iscove N. N. 2013. GATA-3 regulates the self-renewal of long-term hematopoietic stem cells. Nat. Immunol. 14: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambandam A., Maillard I., Zediak V. P., Xu L., Gerstein R. M., Aster J. C., Pear W. S., Bhandoola A. 2005. Notch signaling controls the generation and differentiation of early T lineage progenitors. [Published erratum appears in 2005 Nat. Immunol. 6: 852.] Nat. Immunol. 6: 663–670. [DOI] [PubMed] [Google Scholar]
- 58.Lai A. Y., Kondo M. 2007. Identification of a bone marrow precursor of the earliest thymocytes in adult mouse. Proc. Natl. Acad. Sci. USA 104: 6311–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heinzel K., Benz C., Martins V. C., Haidl I. D., Bleul C. C. 2007. Bone marrow-derived hemopoietic precursors commit to the T cell lineage only after arrival in the thymic microenvironment. J. Immunol. 178: 858–868. [DOI] [PubMed] [Google Scholar]
- 60.Del Real M. M., Rothenberg E. V. 2013. Architecture of a lymphomyeloid developmental switch controlled by PU.1, Notch and Gata3. Development 140: 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ku C. J., Hosoya T., Maillard I., Engel J. D. 2012. GATA-3 regulates hematopoietic stem cell maintenance and cell-cycle entry. Blood 119: 2242–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buza-Vidas N., Duarte S., Luc S., Bouriez-Jones T., Woll P. S., Jacobsen S. E. 2011. GATA3 is redundant for maintenance and self-renewal of hematopoietic stem cells. Blood 118: 1291–1293. [DOI] [PubMed] [Google Scholar]
- 63.Li B. W. S., Stadhouders R., de Bruijn M. J. W., Lukkes M., Beerens D. M. J. M., Brem M. D., KleinJan A., Bergen I., Vroman H., Kool M., et al. 2017. Group 2 innate lymphoid cells exhibit a dynamic phenotype in allergic airway inflammation. Front. Immunol. 8: 1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holländer G. A., Zuklys S., Morel C., Mizoguchi E., Mobisson K., Simpson S., Terhorst C., Wishart W., Golan D. E., Bhan A. K., Burakoff S. J. 1998. Monoallelic expression of the interleukin-2 locus. Science 279: 2118–2121. [DOI] [PubMed] [Google Scholar]
- 65.Naramura M., Hu R. J., Gu H. 1998. Mice with a fluorescent marker for interleukin 2 gene activation. Immunity 9: 209–216. [DOI] [PubMed] [Google Scholar]
- 66.Nutt S. L., Vambrie S., Steinlein P., Kozmik Z., Rolink A., Weith A., Busslinger M. 1999. Independent regulation of the two Pax5 alleles during B-cell development. Nat. Genet. 21: 390–395. [DOI] [PubMed] [Google Scholar]
- 67.Fuxa M., Busslinger M. 2007. Reporter gene insertions reveal a strictly B lymphoid-specific expression pattern of Pax5 in support of its B cell identity function. J. Immunol. 178: 8222–8228. [DOI] [PubMed] [Google Scholar]
- 68.Rhoades K. L., Singh N., Simon I., Glidden B., Cedar H., Chess A. 2000. Allele-specific expression patterns of interleukin-2 and Pax-5 revealed by a sensitive single-cell RT-PCR analysis. Curr. Biol. 10: 789–792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.