Campylobacter jejuni causes millions of human gastrointestinal infections annually, with poultry a major source of infection. Due to the emergence of multidrug resistance in C. jejuni, there is need to identify alternative ways to control this pathogen. Genes encoding the high-affinity phosphate transporter PstSCAB are highly expressed by C. jejuni in chickens and humans. In this study, we address the role of PstSCAB on chicken colonization and other C. jejuni phenotypes. PstSCAB is required for colonization in chicken, metabolism and survival under different stress responses, and during growth on lactate, a potential growth substrate in chickens. Our study highlights that PstSCAB may be an effective target to develop mechanisms for controlling bacterial burden in both chicken and human.
KEYWORDS: Campylobacter jejuni, host-pathogen interactions, phosphate metabolism
ABSTRACT
Campylobacter jejuni causes acute gastroenteritis worldwide and is transmitted primarily through poultry, in which it is often a commensal member of the intestinal microbiota. Previous transcriptome sequencing (RNA-Seq) experiment showed that transcripts from an operon encoding a high-affinity phosphate transporter (PstSCAB) of C. jejuni were among the most abundant when the bacterium was grown in chickens. Elevated levels of the pstSCAB mRNA were also identified in an RNA-Seq experiment from human infection studies. In this study, we explore the role of PstSCAB in the biology and colonization potential of C. jejuni. Our results demonstrate that cells lacking PstSCAB survive poorly in stationary phase, in nutrient-limiting media, and under osmotic conditions reflective of those in the chicken. Polyphosphate levels in the mutant cells were elevated at stationary phase, consistent with alterations in expression of polyphosphate metabolism genes. The mutant strain was highly attenuated for colonization of newly hatched chicks, with levels of bacteria at several orders of magnitude below wild-type levels. Mutant and wild type grew similarly in complex media, but the pstS::kan mutant exhibited a significant growth defect in minimal medium supplemented with l-lactate, postulated as a carbon source in vivo. Poor growth in lactate correlated with diminished expression of acetogenesis pathway genes previously demonstrated as important for colonizing chickens. The phosphate transport system is thus essential for diverse aspects of C. jejuni physiology and in vivo fitness and survival.
IMPORTANCE Campylobacter jejuni causes millions of human gastrointestinal infections annually, with poultry a major source of infection. Due to the emergence of multidrug resistance in C. jejuni, there is need to identify alternative ways to control this pathogen. Genes encoding the high-affinity phosphate transporter PstSCAB are highly expressed by C. jejuni in chickens and humans. In this study, we address the role of PstSCAB on chicken colonization and other C. jejuni phenotypes. PstSCAB is required for colonization in chicken, metabolism and survival under different stress responses, and during growth on lactate, a potential growth substrate in chickens. Our study highlights that PstSCAB may be an effective target to develop mechanisms for controlling bacterial burden in both chicken and human.
INTRODUCTION
Campylobacter jejuni is a microaerophilic, Gram-negative bacterium that causes acute gastroenteritis worldwide (1). Common campylobacteriosis symptoms are self-limiting diarrhea (which may be watery to bloody), with nausea, fever, and abdominal cramping in human. As C. jejuni is a common member of the chicken gastrointestinal microbiota, poultry meat is a major source of human campylobacteriosis, either through direct consumption of poultry or through cross-contamination of other food (2, 3). As consumption of poultry meat worldwide over the last 20 years has approximately doubled (4), the threat of campylobacteriosis remains high.
To explore mechanisms by which Campylobacter colonizes the chicken gastrointestinal tract, we used transcriptome sequencing (RNA-Seq) to determine the transcriptome of C. jejuni strain 81-176 harvested from ceca of seven-day-old chickens after experimental orogastric inoculation on day of hatch (5). Among the more highly abundant transcripts in this population were those encoding a predicted phosphate transporter (pstSCAB), suggesting that phosphate transport is important for the in vivo fitness of C. jejuni and perhaps that inorganic phosphate plays a key role for chicken colonization (5).
Phosphorus, or inorganic phosphate (Pi), is one of the most important macronutrients for microbial growth. It is required for biosynthesis of cellular components such as nucleic acid, phospholipids, and proteins, and is critical for maintaining a cellular energy source in ATP. It is also involved in regulating carbon and nitrogen metabolism (6). Uptake and storage of inorganic phosphate, a growth-limiting nutrient, is heavily regulated in bacteria. Generally, in Gram-negative bacteria, a two-component sensor kinase/response regulator system controls phosphate transport in response to levels of available inorganic phosphate. In C. jejuni, a two-component regulatory system, PhosSR, controls the high-affinity phosphate transporter operon (pstSCAB), as well as other genes, in phosphate-limiting conditions (7). PstSCAB comprises an ATP binding cassette (ABC) transporter system in the inner membrane of many Gram-negative bacteria, with PstS as the periplasmic phosphate binding protein, PstAC comprising transmembrane protein permeases, and PstB as an ATP binding and hydrolyzing subunit. PstS bound to Pi is stimulated to associate with the PstA/C transmembrane proteins. This association triggers ATP hydrolysis in the PstB dimer, which induces the conformational changes that facilitate release of Pi into the cytosol (8). In many bacteria, a PstSCAB-associated protein, PhoU, acts to reduce expression of the Pho regulon under conditions of phosphate sufficiency (6), but it was previously reported that C. jejuni does not harbor a PhoU homolog (7) and our more recent BLAST searches confirm that such a protein is not widely distributed within the genus (unpublished results).
Phosphate transporters contribute to virulence in other microbes, although the molecular mechanisms by which this occurs vary across species and are incompletely understood (9). For example, inoculation into chickens of an avian pathogenic Escherichia coli mutant strain lacking pst led to fewer extraintestinal lesions than did inoculation of wild type, and the mutant also showed higher sensitivity to rabbit serum (though not to chicken serum), polymyxin B, and acid shock (10). A pstS mutant of virulent enterohemorrhagic E. coli (EHEC) and enteropathogenic E. coli (EPEC) showed significant adherence and colonization defects in intestinal epithelial cells, as well as in animal models, resulting from altered expression of adhesin proteins (9, 11). Mycobacterium tuberculosis contains two different phosphate transporters which regulate replication in macrophages and provide protection from antimicrobial stress response (12, 13).
Given the high abundance of transporter mRNA during in vivo growth of C. jejuni, it seemed likely that the transporter is a determinant of colonization. We analyzed a mutant lacking pstSCAB for several growth phenotypes as well as chicken colonization. We demonstrate a clear colonization defect in mutants lacking the pstSCAB operon and we also uncover metabolic deficiencies in the mutant strain, particularly its inability to thrive on l-lactate at concentrations approximating those in the chicken cecum, which likely contributes to loss of fitness in vivo.
RESULTS
Mutagenesis of and characterization of the C. jejuni pstSCAB operon.
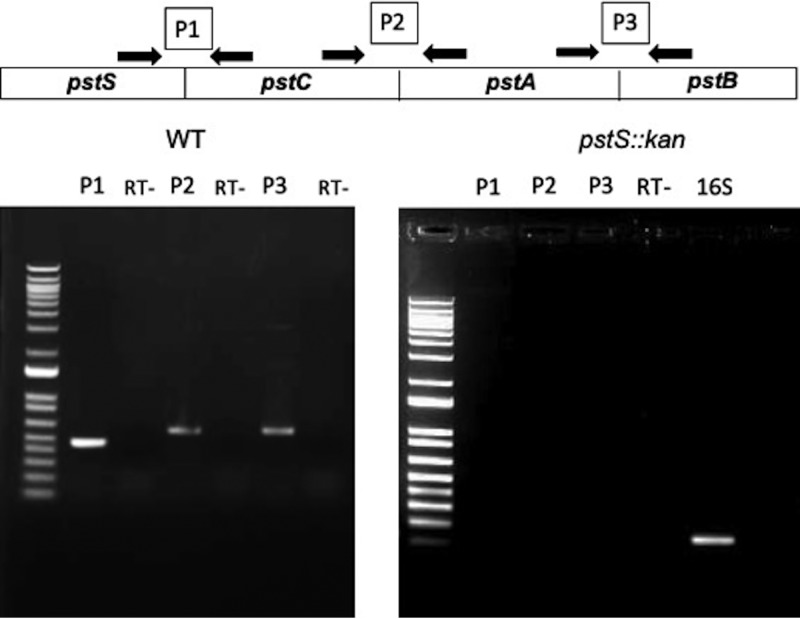
We constructed an insertion allele using a cassette encoding resistance to kanamycin inserted into the pstS gene of C. jejuni strain DRH212, a streptomycin-resistant derivative of the widely used strain 81-176 (see Fig. S1 in the supplemental material). The resulting kanamycin-resistant strain was used in real-time PCR (RT-PCR) experiments designed to analyze transcription of the pstSCAB locus. Consistent with the earlier RNA-Seq study (5), RT-PCR of the wild-type locus using primers specific for the junctions between open reading frames confirmed that the four genes are cotranscribed, a conclusion further supported by the observation that insertion in the pstS gene, the most upstream gene of the locus, resulted in loss of detectable downstream transcription (Fig. 1).
FIG 1.
Characterization of pstSCAB operon. RT-PCR was performed to determine whether the four genes (pstS, pstC, pstA and pstB) are cotranscribed. Three intergenic primer sets (P1, P2, and P3) were designed to amplify transcripts crossing the gene boundaries. The product sizes in base pairs were correct as predicted in all cases. RT-PCR with total RNAs were extracted from both the WT DRH212 (left) and pstS::kan mutant (right) using the designed three primer sets. RT-, negative control; 16S, positive control.
The earlier RNA-Seq study demonstrated that pstS mRNA is at significantly greater abundance than mRNA from the distal genes in the operon (5). Differential stability within a polycistronic mRNA can be controlled through a conserved internal RNA hairpin that protects the transcript from RNA decay (14). The protective RNA structure is often present in the open reading frame of the downstream gene to protect the essential upstream gene from decay (14). Consistent with such a mechanism acting on the pstSCAB operon, we identified a thermodynamically stable hairpin loop structure (ΔG = −27.70) within the protein-coding region of the pstC gene (Fig. S2A and B). We hypothesize that this RNA structure in pstC protects pstS mRNA from decay, leading to its elevated abundance relative to the other genes in the operon. Because of polarity from the pstS::kan insertion, for further experiments we used a complementing plasmid carrying the entire pstSCAB operon.
Wild-type survival in stationary phase and under osmotic stress requires pstSCAB.
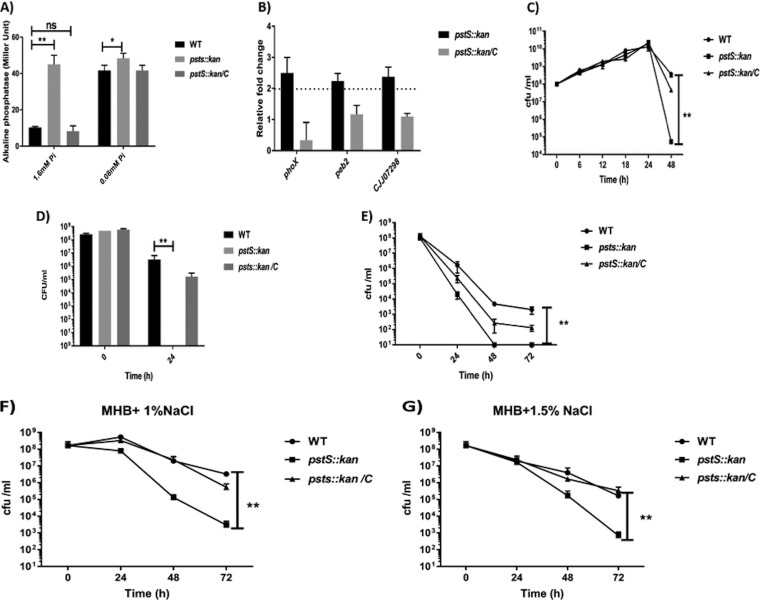
Expression of pstS is elevated approximately 400-fold in 0.08 mM inorganic phosphate (Pi) compared with 1.6 mM Pi, through activation of the PhoRS system at the more limiting phosphate concentration (7). The PhoRS-regulated alkaline phosphatase gene of C. jejuni (phoX) is also induced at the lower phosphate concentration, providing Pi for transport into the cell by the PstSCAB system (15). We hypothesized that the pstS::kan mutant strain would signal phosphate limitation to the cell, even at a concentration (1.6 mM) that does not typically result in phoX expression. We measured alkaline phosphatase activity in wild type and pstS::kan mutant cells grown in 0.08 mM and 1.6 mM phosphate. Supporting our hypothesis, the mutant expressed significantly elevated alkaline phosphatase activity at both the lower (0.08 mM) and higher (1.6 mM) concentrations, whereas wild type (and the complemented mutant) expressed high levels of alkaline phosphatase activity only at the lower phosphate concentration (Fig. 2A). General dysregulation of the phosphate regulon in the pstS::kan mutant was confirmed by analyzing RNA levels of PhoSR-regulated genes phoX, peb2a, and CJJ07298 (encoding an ATP-dependent DNA helicase) (7) from cells grown in 1.6 mM phosphate. Expression of each was elevated in the pstS::kan mutant two to three times over that of wild type (Fig. 2B). We conclude that, even at elevated phosphate concentrations, the pstS::kan mutant strain behaves as though it is phosphate starved.
FIG 2.
Characterization of C. jejuni pstS::kan strain in different in vitro conditions. (A) Alkaline phosphatase activity was determined in pyruvate-minimal media with 1.6 mM or 0.08 mM Pi (n = 3). Statistical analysis was done using two way ANOVA with Sidak’s multiple-comparison test: *, P < 0.05; **, P < 0.005; ns, not significant. (B) Transcription analysis of PhoRS-regulated genes phoX, peb2, and CJJ07298 at 3 h after growth of WT DRH212 and pstS::kan in pyruvate-minimal media with 1.6 mM Pi by quantitative RT-PCR. Changes in gene expression in the pstS::kan mutant and complemented strain compared with wild-type DRH212 were determined by the 2−ΔΔCT method. Data are represented as the mean value from three independent experiments ± standard deviation (SD). (C) C. jejuni strains were grown in Mueller-Hinton (MH) broth in microaerobic conditions for 48 h and CFU/ml were determined after specified time intervals. (D) C. jejuni DRH212 was grown in MH broth for 48 h. After 48 h, sterile spent media were collected by filter sterilization. Mid-log-phase cultures of DRH212, pstS::kan, pstS::kan/C strains were inoculated (108 CFU/ml) in spent media and survival was measured at different time intervals by CFU count. (E) In a nutrient downshift assay, DRH212, pstS::kan, pstS::kan/C strains grown to mid-log phase were collected and used to inoculate in minimal medium lacking carbon and phosphate sources. The survival assay was measured by CFU count at specified time intervals. (F and G) Osmotic stress tolerance was assessed by inoculating strains into MH broth containing 1% NaCl (F) or 1.5% NaCl (G) and determining growth by CFU counts. Results represent the mean value from three independent experiments. Statistical significance was assessed by Student's t test: **, P < 0.005.
To assess the effect of loss of pstSCAB on growth of C. jejuni, we cultured wild type, mutant, and complemented mutant strains in Mueller-Hinton broth (MHB), a complex medium of protein hydrolysate and infusion. All grew to similar levels for 24 h, peaking at 1010 CFU/ml (Fig. 2C). By 48 h, the wild type and complemented mutant strains had dropped about 2 orders of magnitude in plating efficiency, while the mutant strain had dropped by over 5 orders of magnitude in plating efficiency, suggesting a severe defect in survival in stationary phase. Assessing survival in nutrient-limited medium would provide a more direct examination of the challenge posed by loss of pstSCAB. To do this, we performed two experiments. In one, the wild-type strain was grown for 48 h in MHB to reach stationary phase, after which the growth medium was collected by filter sterilization. We inoculated mid-log-phase wild-type, pstS::kan, and complemented mutant strains in the sterilized depleted medium with initial inoculum (5 × 108 CFU/ml) and assessed viable cells at 24 h. In contrast to wild type and the complemented mutant, which were still recoverable at high numbers in depleted medium, the mutant was completely unrecoverable (Fig. 2D), confirming that loss of the phosphate uptake system causes a significant defect in growth physiology. The second experiment involved more direct nutrient depletion, growing cells to mid-logarithmic phase in rich media, collecting them by centrifugation, and resuspending in minimal media lacking carbon and phosphate sources. Plating efficiency of the pstS::kan mutant was more rapidly lost, and to a significantly greater degree, than that observed for the wild type and complemented mutant strains (Fig. 2E).
In addition to phoX of C. jejuni contributing to survival during osmotic stress response, the phoX mutant showed less survival in osmotic stress response (15). To assess whether phosphate transport per se contributes to osmotic survival, we analyzed wild type and pstS::kan mutant growth and survival at 1% and 1.5% NaCl, which approximate concentrations within the chicken gastrointestinal tract (16). The pstS::kan mutant strain demonstrated lower plating efficiency than wild type on 1% and 1.5% NaCl (Fig. 2F and G). The mutant strain was restored to wild-type levels of plating by complementing with pstSCAB in trans, confirming that loss of viability at elevated osmolarity is due to loss of phosphate transport. Thus, similar to mutant strains lacking ppx1 and ppx2 (exopolyphosphatase genes) (17), which are unable to degrade polyphosphate to produce Pi, the phosphate transporter mutant survives poorly under osmotic stress conditions that are similar to those found in the chicken gastrointestinal tract.
Expression of phosphate storage and phosphate metabolism genes is altered in the pstS::kan mutant.
Survival of C. jejuni in stationary phase and nutrient limiting condition is dependent on polyphosphate (polyP) metabolism (17). C. jejuni ppk1 and ppk2 encode polyP kinases, with the former mediating polyP synthesis using ATP generated from inorganic phosphate (18) and the latter generating GTP from polyP (19). In some microbes, Ppk2 can produce polyP from GTP (19), but this activity has not been reported in C. jejuni. C. jejuni also encodes two exopolyphosphatases, Ppx1 and Ppx2, which degrade polyP into inorganic phosphate and have guanosine pentaphosphate hydrolase activity to generate guanosine tetraphosphate (ppGpp) (20). With the regulatory gene product SpoT, ppGpp regulates the stringent response of C. jejuni, contributing to survival in stationary phase (21). Another key gene of phosphate metabolism is ppa, which encodes an inorganic pyrophosphatase that hydrolyzes pyrophosphate (PPi) to inorganic phosphate (PPi→Pi) (21).
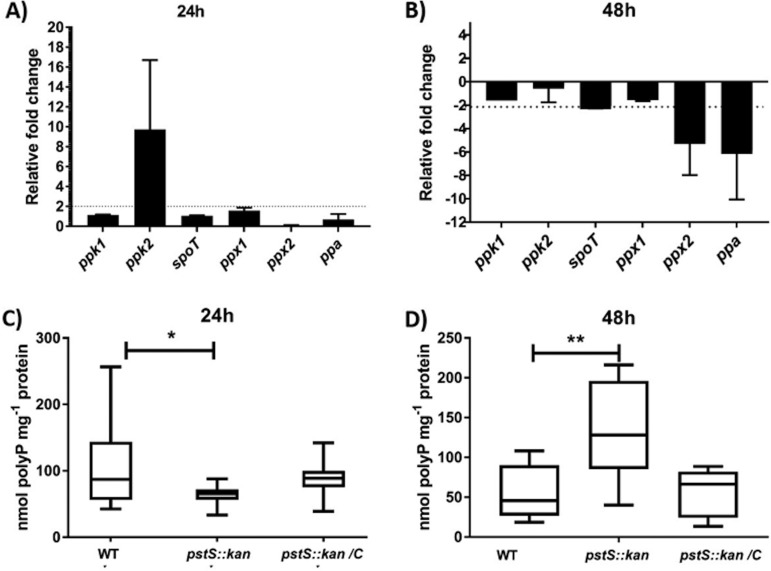
We investigated the effect of the pstS::kan mutation on these phosphate storage and stress response mechanisms, examining expression in wild type, mutant, and complemented mutant strains after 24 h and 48 h of growth in MHB. The gene largely responsible for polyP synthesis, ppk1, was unchanged in its level of expression in the mutant versus the wild type at either time point. Expression of ppk2, which converts GDP to GTP using polyP as a substrate—and is reported in some microbes (although not in C. jejuni) to produce polyP from GTP—was elevated up to 10-fold in the mutant strain at 24 h, returning to wild type expression levels by 48 h (Fig. 3A). At 48 h, expression levels of an exopolyphosphatase gene (ppx2), the inorganic pyrophosphatase gene ppa, and the stringent regulatory gene spoT were significantly reduced in the mutant compared to wild type (Fig. 3B). As ppx2 and spoT of C. jejuni regulate synthesis of ppGpp, important for survival in stationary phase, reduced expression of these two genes would likely result in the decreased levels of ppGpp observed in the pstS::kan mutant strain and could also account for the stationary-phase survival defects (Fig. 2).
FIG 3.
Comparative analysis of polyP metabolism regulatory genes and polyP level between WT DRH212 and the pstS::kan mutant. (A and B) Transcription analysis by quantitative RT-PCR of stress regulatory genes ppk1, ppk2, spoT, and ppA at 24 h (A) and 48 h (B) after growth of DRH212 and the pstS::kan mutant. Changes in gene expression in the pstS::kan mutant compared with wild type DRH212 were determined by the 2−ΔΔCT method. Data are represented as the mean value from three independent experiments ± standard deviation. (C and D) Levels of intracellular polyphosphate (polyP) of three strains (DRH212, pstS::kan, and pstS::kan/C strains) were measured at 24 h (CD) and 48 h (D). Statistical analysis used an unpaired t test and one-way ANOVA with Holm-Sidak’s multiple test (n = 8, ±SD): *, P < 0.05; **, P < 0.005.
At 24 h, polyP levels in the pstS::kan mutant were roughly half of those observed in the wild type. At 48 h, by which time the mutant had diminished in viability by several orders of magnitude (Fig. 2), polyP was elevated to about three times the levels observed in wild type (Fig. 3C and D). With elevated ppk2 expression at 24 h in the pstS::kan mutant, decreased intracellular polyP might be expected, as the Ppk2 enzyme consumes polyP to convert GDP to GTP. The higher levels of polyP observed in the pstS::kan mutant compared to wild type at 48 h is readily explained by decreased expression of the exopolyphosphatase gene ppx2 in the mutant background and is consistent with a similar report of a pstS::kan mutant of Pseudomonas aeruginosa (22).
Loss of PstSCAB compromises C. jejuni chicken colonization and fitness.
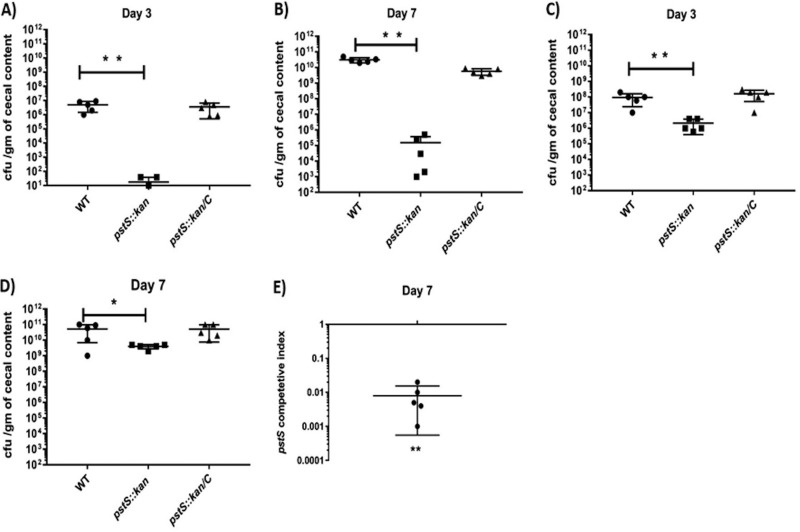
We investigated whether elevated abundance of pstS mRNA in C. jejuni harvested from chick ceca, compared to C. jejuni grown in vitro (5), correlates to actual requirement for pstSCAB during growth in the chicken. We orally inoculated day-of-hatch chickens with two different doses (103 or 106 CFU) of wild type C. jejuni 81-176, the pstS::kan mutant derivative, and the complemented mutant and assessed C. jejuni loads in cecal contents on days 3 and 7 after inoculation. Irrespective of the inoculum dose, the mutant strain exhibited a large colonization defect at both days 3 and 7 compared with wild type (Fig. 4A to D), with recovery at approximately 5 orders of magnitude less than wild type when introduced at the lower inoculum and at roughly 2 orders of magnitude when introduced at the higher inoculum. In all cases, the complemented strain colonized at levels similar to wild type.
FIG 4.
Colonization ability of the pstS::kan mutant in chickens. (A to D) Day-of-hatch white Leghorn chicks were infected with either WT DRH212, pstS::kan, or pstS::kan/C C. jejuni (at two different doses of 103 and 106 CFU/ml) and cecal loads were measured at day 3 and day 7. Shown is the comparative colonization ability between DRH212 and pstS::kan strains at days 3 and 7 after oral inoculation with 103 CFU/ml (A and B) and 106 CFU/ml (C and D). Statistical analysis used one-way ANOVA with Tukey’s multiple-comparison test (n = 5, ±SD). (E) In competition analysis between DRH212 and the pstS::kan mutant, day-of-hatch chicks were infected with equal ratios of DRH212 and pstS::kan mutant strains. At day 7, the ratio of pstS::kan mutant to DRH212 was determined and is presented as a competitive index. Statistical analysis was performed using a one-simple t test against a hypothetical value of 1 (n = 5 for each group). *, P < 0.05; **, P < 0.005.
In addition to individual colonization assays with wild-type and pstS::kan strains, we carried out a competition assay between the wild-type strain 81-176 and the pstSCAB derivative, mixing the strains at 1:1 concentration in the inoculum and introducing the combination at 106 CFU/ml by oral gavage to day-of-hatch chickens. We harvested cecal contents on day 7 postinfection and determined the ratios of the mutant and wild-type strains by measuring relative numbers of kanamycin resistant (pstS::kan mutant) and sensitive (wild type) C. jejuni in the output (Fig. 4E). The wild-type strain exhibited greater fitness in this experiment than the mutant by 100-fold. The wild-type strain also outcompeted the mutant when grown together in nutrient-rich media or in minimal media supplemented with 80 μM phosphate (Fig. S4A and B). These findings indicate the important requirement of pstSCAB for chick colonization and extend the earlier observation of high-level in vivo expression of this operon in the transcriptome study (5).
The phosphate transporter is critical for lactate-dependent growth.
Lactate is present in both d and l isomers at various concentrations in the chicken gastrointestinal tract, generally decreasing from the upper to the lower intestine and ceca (23). Although at high concentrations lactate inhibits growth of C. jejuni (24) and reduces expression of some colonization traits (23), many strains, including 81-176, have pathways for uptake of and growth in lactate, including a transporter for l-lactate encoded by lctP (CJJ81176_0113) and two NAD-independent membrane-associated l-lactate dehydrogenase complexes (CJJ81176_0112-0110) and (CJJ81176_1182), which convert l-lactate to pyruvate (25). Mechanisms for uptake and assimilation of d-lactate are less understood (25).
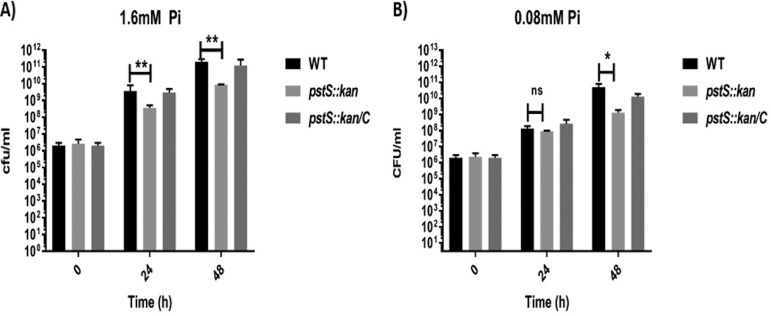
Given the reduced fitness of the pstS::kan mutant in the cecum, we investigated the growth of the mutant on this potentially relevant carbon source in that environment. We cultured wild type, mutant, and complemented mutant strains on minimal medium containing a mixture of d- and l-lactate (10 mM total lactate) in both low (80 μM) and high (1.6 mM) phosphate concentrations. In both phosphate concentrations, the mutant grew to levels 100 to 1,000 times lower than wild type at 24 h and 48 h of growth (Fig. 5). This contrasts to cells cultured in medium with pyruvate as the carbon source, where mutant and wild type grew to similar levels in high phosphate media (1.6 mM) and differed by only an order of magnitude at the lower phosphate concentration (80 μM) (Fig. S5A and B). When cultured on l-lactate alone, the pstS::kan mutant strain was significantly limited compared to wild type, regardless of phosphate concentration, while on d-lactate alone, the mutant was diminished for growth during initial exponential growth phase (regardless of phosphate levels) but ultimately attained the same level of growth as the wild type and the complemented mutant (Fig. 6). Collectively these data suggest that the phosphate transport mutant is challenged for growth on l-lactate in particular, for even when both d- and l-lactate are available in vitro, the mutant is at a significant disadvantage compared to wild type (Fig. 5). Given that d- and l-lactate may serve as growth substrates for C. jejuni growth in the chick cecum (25), these data also contribute to explaining the fitness disadvantage of the mutant in vivo.
FIG 5.
PstSCAB transporter of C. jejuni is critical for growth in lactate. C. jejuni strains were grown in minimal medium containing both d- and l-lactate (each 5 mM) with two concentrations of inorganic phosphate. Growth of WT DRH212 and pstS::kan mutant strain were measured by CFU count in minimal medium (d- and l-lactate) with 1.6 mM Pi (A) and 0.08 mM Pi (B) at 24 h and 48 h. n = 3; error bars indicate SD. Statistical analysis was done by two-way analysis of variation (ANOVA) with Sidak’s multiple comparision test: *, P < 0.05; **, P < 0.005; ns, not significant.
FIG 6.
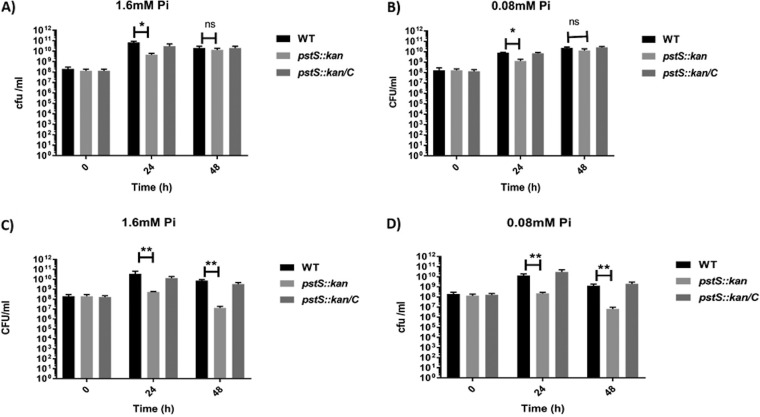
Comparative growth analysis of C. jejuni strains in minimal medium with d- or l-lactate. C. jejuni strains were grown in minimal medium with d-lactate or l-lactate as a carbon source and a high or low concentration of inorganic phosphate. (A and B) Growth of C. jejuni strains was measured in d-lactate minimal medium with 1.6 mM (A) and 0.08 mM (B) phosphate by CFU counts at 24 h and 48 h. (C and D) Growth of these three strains was also determined in l-lactate minimal medium with 1.6 mM (C) and 0.08 mM (D) phosphate. n = 3; error bars indicate SD. Statistical analysis was done by two-way analysis of variation (ANOVA) with Sidak’s multiple-comparison test: *, P < 0.05; **, P < 0.005; ns, not significant.
Lactate-derived pyruvate is converted to acetate in C. jejuni via phosphotransacetylase (Pta) and acetate kinase (AckA); this acetogenesis pathway is critical for establishing chicken colonization (23). Genes controlled by this acetogenesis pathway may contribute to fitness in the chick ceca, including those encoding γ-glutamyl transferase (Ggt), a component of an amino acid transporter complex (Peb1c), and a putative di/tri-peptide transporter (Cjj81176_0683) (23). The higher l-lactate levels in the upper intestine, compared to those of the lower intestine and ceca, lead to reduced expression of these genes through an undefined regulatory mechanism that correlates to relatively lower levels of C. jejuni in the upper intestine (23). We hypothesized that the poor growth on lactate of the pstS::kan mutant might also correlate with diminished expression of acetogenesis-regulated colonization traits. To test this, we carried out quantitative RT-PCR to measure transcript levels of ggt, peb1c, and Cjj81176_0683. Expression of each was reduced by 2- to 4-fold in the mutant compared to wild type (Fig. 7). This reduction in expression is similar in magnitude to the reduced expression of these genes in the upper intestine where C. jejuni colonizes poorly (23). Thus, we conclude that, in addition to poor growth on l-lactate by the mutant lacking PstSCAB, the strain also expresses potentially key colonization determinants at levels apparently insufficient for cecal colonization.
FIG 7.
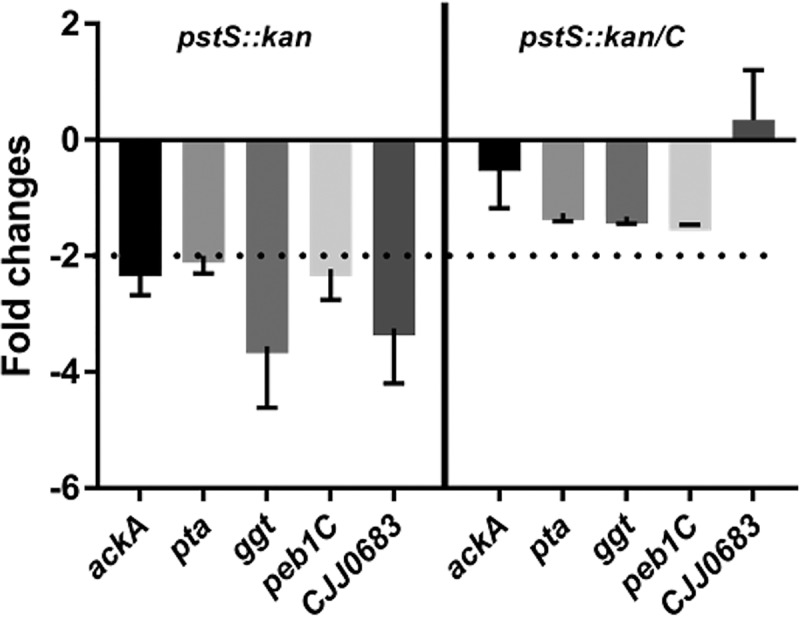
Transcript analysis of acetogenesis regulatory and acetogenesis-dependent gene expression in wild type, pstS::kan, and complemented strains. Bacterial strains were grown in minimal medium containing l-lactate with 1.6 mM Pi up to 18 h, after which cells were harvested for RNA isolation. Acetogenesis regulatory genes (ackA and pta) and acetogenesis-dependent genes (ggt, peb1C, and CJJ0683) were measured in the pstS::kan mutant and pstS::kan/C strains relative to WT DRH212 by real-time PCR. n = 3; error bars indicate SD.
Metabolomic analysis of wild-type and pstS::kan mutant C. jejuni.
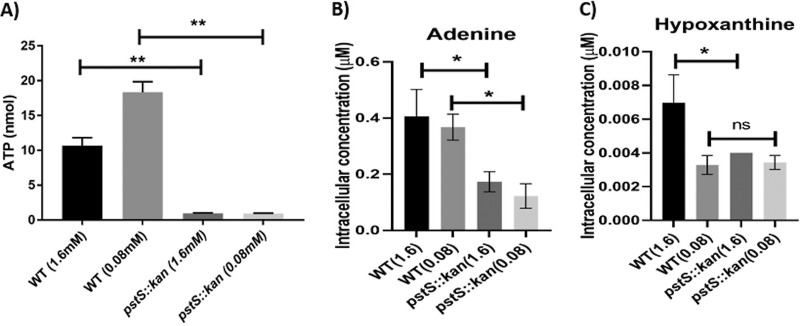
To further explore the growth physiology of wild type versus the pstS::kan mutant on lactate, we measured intracellular metabolites using mass spectrometry and ATP concentrations using a commercially available assay. We observed no significant differences in intracellular lactate or pyruvate between wild type and the pstS::kan mutant strain (Fig. S6A), indicating that the phosphate transport system has no effect on lactate uptake or its conversion to pyruvate. ATP levels in the mutant were significantly below those of wild type (Fig. 8A). Additionally, adenine and its deamination product hypoxanthine were significantly lower in the mutant compared with the wild type (Fig. 8B and C), which likely accounts for the lowered ATP levels. C. jejuni does not encode a purine/pyrimidine or nucleotide/nucleoside transporter (26), and, indeed, adenine supplementation (1 μM or 10 μM) of l-lactate medium did not restore growth or ATP levels in the mutant (Fig. S7).
FIG 8.
Intracellular ATP and metabolites in WT DRH212 and pstS::kan mutant grown in l-lactate minimal medium. DRH212 and pstS::kan strains were grown in l-lactate minimal medium with 1.6 mM and 0.08 mM Pi at 37°C. (A) After 18h, ATP levels in DRH212, pstS::kan were detetermined. n = 3; error bars indicate SD. (B and C) The intracellular concentrations of adenine (B) and hypoxanthine (C) were measured by liquid chromatography mass spectroscopy analysis. Data represent the mean value from two independent experiments. The statistical analysis was done by one-way ANOVA: *, P < 0.05; **, P < 0.005.
DISCUSSION
The pstSCAB phosphate transporter locus of C. jejuni is among the more significantly expressed transcripts identified by RNA-Seq from microbes harvested from the chick cecum (5) and during infection of humans (27). A transcriptomic study of C. jejuni colonizing chicks using microarray analyses (28) did not report high level expression of pstSCAB, although there are significant differences in the way the RNA-Seq and microarray studies were carried out with regard to chicken inoculation and colonization. The RNA-Seq study (5) used an inoculum of 103 CFU of C. jejuni, and harvested cecal contents for RNA preparation after 7 days of colonization. In contrast, the microarray study used a much higher inoculum of 109 CFU and harvested cecal contents after only 12 h of colonization (28). It is conceivable that these differences in inocula and time of colonization would result in different physiological outcomes in the C. jejuni recovered from the cecal contents.
Generally, elevated levels of expression of the high-affinity phosphate transporter in bacteria are observed in low-phosphate environments. For example, transcription of pstS in C. jejuni is significantly increased at 0.08 mM concentration compared to 1.6 mM (7). We determined phosphate concentrations within the chicken cecum as relatively high, at 0.5 to 0.8 mM/100 mg of cecal content (see Fig. S3 in the supplemental material). Further, previous metabolite studies estimated that the inorganic phosphate (Pi) concentrations in the intestinal lumen of healthy adults varied between 15 and 30 mM on a Western diet (29). At these in vivo levels of inorganic phosphate, we would not expect elevated expression of pstSCAB during colonization in chicks and humans, so it remains unclear what drives this outcome during host colonization. We hypothesize that, although present at high detectable levels, phosphate is nevertheless unavailable to C. jejuni, perhaps due to competition from other microbes. We previously demonstrated in vivo competition for zinc, resulting in poor colonization of a C. jejuni mutant lacking the znuA high-affinity zinc transporter. In that case, the colonization defect of the znuA mutant was mitigated in chicks reared in germfree conditions harboring a limited microbiota (30).
The pstS::kan mutant of C. jejuni showed a significant colonization defect in chickens at both inocula we tested (103 and 106 CFU), with the most significant defect at day 3 after infection. From these data, we hypothesize that pstSCAB enables C. jejuni to survive initial conditions after reaching the chicken gut. We also observed a dose-dependent colonization defect of the mutant at day 7. A previous study reported similar colonization levels between wild type C. jejuni and a mutant lacking phoRS, which encodes a regulator of pstSCAB expression (6). That study used a different strain of C. jejuni and the animal data were not shown, so we do not know which infection dose was used or what time points were assessed postinoculation. Another study demonstrated that mutants lacking the alkaline phosphatase PhoX have diminished capacity for colonizing chickens compared to wild type (15) and that this is a dose-dependent outcome with a somewhat greater colonization defect of the mutant observed after a low dose (103) inoculum compared to after a higher dose inoculum (105). In other bacteria, phosphate transporter mutations (such as the pstS::kan allele) cause constitutive expression of genes controlled by the phosphate two-component system, which is PhoSR in C. jejuni (6), and we observed similar dysregulation of PhoSR-regulated genes in the pstS::kan mutant (Fig. 2). Thus, we must consider the possibility that the colonization defect of the mutant strain and other phenotypes we report are due to disrupted regulation of the PhoSR regulon. The molecular basis for how mutation in pstSCAB might alter PhoSR function in C. jejuni is less clear and requires further investigation given that PhoU, proposed in other microbes to be the regulatory link between the transporter and the Pho two-component system, is not broadly identified in BLAST searches of C. jejuni (7, our results).
To better understand how the PstSCAB system contributes to growth in the chicken, we explored growth on lactate, which may serve as a growth substrate in chickens (24) but also has deleterious growth effects at higher concentrations. While the wild-type strain grows to high levels in 10 mM lactate, the pstS::kan mutant failed to thrive and even lost viability at this concentration. The mutant strain also exhibits reduced expression of the pta-ackA genes in the acetogenesis pathway when grown on lactate, which could be partly responsible for the decreased ATP levels in the mutant compared to the wild type (23). Membrane damage to C. jejuni, caused by high levels of lactate, is the basis for suggesting that Lactobacillus strains producing high levels of lactic acid might be useful as probiotics to reduce C. jejuni loads in poultry (25). Concentrations required for killing C. jejuni are much higher than 10 mM, but in the context of loss of pstSCAB, perhaps the cell is sensitized to lactate toxicity. A mutant of avian pathogenic E. coli lacking pst was demonstrated to be more sensitive to membrane-targeting polymyxin B, but we did not observe this with the C. jejuni pstS::kan mutant.
As a proxy for analyzing fitness of wild type (WT) and pstS::kan mutant in humans, we measured growth of WT and the pstS::kan mutant strain in vitro in minimal medium with 10% human fecal extract, in which the phosphate concentration was approximately 500 μM (Fig. S8A). The mutant strain grew in this medium after 24 h but nevertheless grew to levels 10× lower than wild type and the complemented mutant (Fig. S8B). Taking these results together with previous human transcriptomic data demonstrating elevated levels of pst messages during human infection (27), we conclude that the phosphate transporter of C. jejuni contributes to fitness in both chickens and humans.
The pstSCAB operon is transcribed as a full-length polycistronic mRNA, demonstrated both in the earlier RNA-Seq study and in our qRT-PCR presented in this work. Transcripts encoded by the first gene in this operon, pstS, are present at much higher levels than those of the three downstream genes (5). We postulate that a potential stem-loop structure encoded just downstream of the pstS gene serves to stabilize pstS mRNA from degradation, resulting in higher steady-state levels compared to the other genes of the operon. A similar mechanism is proposed for the pstS2 operon of Vibrio cholerae (31).
In complex media, PstSCAB is dispensable until late in growth, after which it is required for cells to remain viable in the nutrient-depleted environment (Fig. 2). As transcription of the locus is elevated during mid-logarithmic growth (compared with stationary-phase growth) (5), we conclude that assembly of the system and Pi uptake occur early during growth and enable survival later when the locus is far less transcriptionally active. Accumulation of polyP in the pstS::kan mutant late in growth may be the result of decreased expression of the exopolyphosphatase genes ppx1 and ppx2, which encode enzymes that metabolize polyP. Consistent with a need to balance polyP production and metabolism for wild-type fitness in C. jejuni, others have demonstrated that phoX and ppk1/ppk2 mutants also have survival defects in nutrient-limiting conditions (18). Increased polyP levels were also observed in phosphate transport mutants of M. tuberculosis (32).
Our results demonstrate that the PstSCAB transporter plays a pivotal role in the physiology of C. jejuni. Its effect extends beyond phosphate storage and metabolism to growth on lactate, which may be key to thriving in the chicken gastrointestinal tract. How phosphate uptake and metabolism contribute to lactate-dependent growth will be the subject of future research.
MATERIALS AND METHODS
Bacterial strains and media.
All strains and plasmids used in the study are listed in Table 1. The wild-type strain used in all experiments is DRH212, a streptomycin-resistant derivative of C. jejuni 81-176 (33). All C. jejuni strains were routinely grown under microaerobic conditions (85% N2,10% CO2, 5% O2) on Mueller-Hinton (MH) agar or in broth under necessary antibiotics at the following concentrations: chloramphenicol, 15 μg ml−1; kanamycin, 50 μg ml−1; trimethoprim (TMP), 10 μg ml−1; streptomycin, 2 mg ml−1. C. jejuni strains were stored in MH broth with 20% glycerol at −80°C.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| C. jejuni DRH212 | Streptomycin-resistant wild-type 81-176 (Smr) | 34 |
| DRH212 pstS::kan | pstS insertion mutant of DRH212 (Knr Smr) | This study |
| DRH212 pstS::kan/C | pstS insertion mutant of DRH212 content pstSCAB cloned pECO212 (Knr Smr Cmr) | This study |
| E. coli DH5α | Strain used for cloning | |
| E. coli DH5α/pRK212.1 | Strain with conjugative plasmid for the transfer of plasmid DNA into Campylobacter | 36 |
| Plasmids | ||
| pGEMT | Subcloning vector (Apr) | |
| pECO102 | pRY112 derivative with cat promoter in XhoI-BamHI | 36 |
| pILL600 | Contains the Campylobacter kanamycin cassette | 36 |
Construction of a pstS::kan insertion mutant and complementation.
The C. jejuni pstS::kan mutant was constructed by insertion of a kanamycin resistance cassette into the gene as previously described (34). Briefly, the 500 bp of both upstream and downstream regions of the pstS locus was amplified by PCR with primers containing SmaI restriction sites. The amplified upstream and downstream regions were joined by PCR. The 1,000-bp amplified PCR product was cloned into the plasmid pGEM-T Easy. The Campylobacter kanamycin resistance cassette was excised from PILL600 using SmaI and ligated between the upstream and downstream cloned regions of the pGEM-T construct. The plasmid was electroporated into the DRH212 strain and grown on MH agar overnight at 37°C under microaerobic conditions. Cells were then plated and grown on MH agar containing kanamycin for 2 to 3 days, and successful integration of pstS::kan into the chromosome was confirmed by PCR. As we demonstrate in Results, this insertion is polar on the pst genes downstream of pstS. Therefore, for complementation, the coding sequences of the whole pstSCAB operon—from the second codon of pstS to the stop codon of pstB—were amplified by PCR and cloned into the BamHI site of the plasmid pECO102 (35), which was introduced into the E. coli DH5α/pRK212.1 strain (35). Conjugation to C. jejuni was performed as previously described by Guerry et al. (36).
Growth analysis in MH broth and minimal media.
WT, pstS::kan, and complemented (pstS::kan/C) C. jejuni strains were grown from freezer stocks on MH agar containing the desired antibiotics for 24 h under microaerobic condition. The strains were restreaked and grown for 16 h. The bacteria were suspended in MH broth and inoculated in 25 ml of MH broth containing the desired antibiotics. The initial optical density at 600 nm (OD600) was approximately 0.05 and bacterial numbers were measured at different times up to 48 h. To determine the growth of C. jejuni strains in different concentrations of inorganic phosphate, strains were grown in MCLMAN minimal medium containing different carbon sources, including sodium l-pyruvate, d-lactate, and l-lactate (37), as noted. Strains were washed twice with minimal medium and inoculated with 25 ml of minimal medium containing different phosphate concentrations. Growth was measured via absorbance at 600 nm (OD600) up to 48 h after inoculating at an initial OD600 of 0.05.
ATP measurements.
ATP levels were determined by BacTitre-Glo assay kit (Promega). Briefly, strains were grown for 24 h in minimal media (l-lactate) with higher (1.6 mM) and lower (0.08 mM) concentrations of Pi and normalized to an OD600 of 0.3. Then ATP levels were measured according to the manufacturer’s protocol and ATP concentrations were measured by standard curve (26).
Measurement of intracellular metabolites.
Bacteria were grown in the presence of l-lactate with higher and lower concentrations of phosphate for 18 h. Cells were collected by centrifugation and washed twice with normal saline. Then all samples were normalized approximately to an OD600 of 1.0 and were extracted for determination of metabolites. Samples on dry ice were combined with 800 μl of ice-cold acetonitrile-methanol-acetone (8:1:1, vol/vol/vol) and suitable stable isotope-labeled or synthetic internal standards, including D4-glycochenodeoxycholic acid and myristoyl phosphatidylcholine for estimation of metabolite recovery and relative quantitation across experimental groups. Samples were homogenized with zirconium oxide beads for 2 min in a Bullet Blender (Next Advance) at 4°C, incubated for 30 min at 4°C, then centrifuged for 10 min at 10,000 × g at 4°C. Supernatants were filtered through 0.2 μm syringe filters (Fisher Scientific) and evaporated to dryness in a SpeedVac. Samples were resuspended in 95% acetonitrile for use in liquid chromatography-mass spectrometry (LC-MS) analysis.
Liquid chromatography-mass spectrometry.
Untargeted metabolite identification used a Thermo model LTQ-Orbitrap Velos mass spectrometer operating in full scan negative ionization MS mode at 60,000 resolution with data-dependent high-energy collisional dissociation (HCD)-MS/MS over a mass range of 50 to 1,000 m/z. The mass spectrometer was coupled to a Shimadzu Prominence high-performance liquid chromatography (HPLC) system through an electrospray ionization source. Ten microliters of each metabolite extract were injected and separated on a Phenomenex HILIC LC column, 2.0 mm by 100 mm, 3.0-μm particle size, 100-Å pore size, using a gradient of water containing 50 mM ammonium formate and acetonitrile from 95% to 50% over 14 min as previously described. (38). A guard cartridge of matching chemistry was fitted to the analytical column.
Data analysis.
Compound identifications, isotope correction, chromatographic peak alignment, and peak area quantification were performed with MAVEN software (39). Due to the need to simultaneously analyze numerous disparate classes of metabolites in untargeted experiments, only relative quantitation of analytes against a selected internal standard were performed for comparison of values across experimental treatment groups. Multivariate statistical analysis was performed using MetaboAnalyst version 4.0 software (https://www.metaboanalyst.ca/).
Nutrient downshift survival.
C. jejuni were grown overnight at 37°C in MH broth under microaerobic condition. Bacteria were collected by centrifugation at 6,000 rpm for 10 min and washed twice with MCLMAN minimum medium with no carbon and no phosphate added. Strains were diluted to an OD600 of 0.05. After different time intervals, CFU were measured by plating on MH agar plates.
Osmotic stress survival.
C. jejuni strains were grown overnight at 37°C in MH broth under microaerobic condition. Bacteria were centrifuged and suspended in MH media containing 1% and 1.5% NaCl. The initial OD600 of of the culture was 0.05 and survival was measured by CFU count at different time intervals (16).
Real-time PCR.
Wild type strain DRH212, pstS::kan strains were grown in different condition in vitro and total RNA was isolated by the TRIzol method and RNeasy minikit (Qiagen). After digestion with TURBO Dnase treatment, cDNA was made by SuperScript III first-strand synthesis supermix (Invitrogen). Then real-time PCR was performed by the Sybr green method. Expression of different targeted genes was measured by the 2−ΔΔCT method. Primer sequences or primers themselves for all genes examined in this study are available by request.
Measurement of in vivo polyP levels.
Intracellular polyP levels were measured as described (40). Briefly, strains were grown in MH broth for 24 h and 48 h. Strains were collected at each time point by centrifugation and dissolved in 250 μl of GITC lysis buffer (4 M guanidine isothiocyanate, 50 mM Tris-HCl, pH 7) and lysed by incubating at 95°C for 10 min. Lysates were stored at –80°C. The protein concentration was determined by Bradford assay (Bio-Rad) of a 5-μl aliquot. polyP was isolated using EconoSpin silica spin columns (Epoch Life Science) and digested with 1 μg of PPX1 exopolyphosphatase from Saccharomyces cerevisiae. The resulting free phosphate was measured using a malachite green colorimetric assay and normalized to the total protein (40).
Chicken colonization assay and in vivo phosphate determination.
Day-of-hatch white Leghorn chickens were inoculated orally with two doses (approximately 1 × 103 and 1 × 106 CFU/ml) of indicated C. jejuni strains (wild-type strain 81-176, pstS::kan, and pstS::kan/C) (8). Birds were euthanized at days 3 and day 7 after infection, and cecal contents were collected as described (41). Cecal contents were homogenized in sterile phosphate-buffered saline (PBS) and serial dilutions were plated on Mueller-Hinton (MH) agar containing 10% sheep’s blood, cefoperazone (40 μg/ml), cycloheximide (100 μg/ml), trimethoprim (10 μg/ml), and vancomycin (100 μg/ml), selective for Campylobacter. Plates were grown for 48 h under microaerobic conditions and colonies were counted. For the competition experiment, the wild-type and pstS::kan strains were mixed equally (approximately 1 × 106 CFU/ml of each strain) and inoculated into day-of-hatch chicks by oral gavage. At day 7, cecal contents were collected and, after serial dilution, plated on Campylobacter-selective media with and without kanamycin (50 μg/ml). Competitive index values were calculated as previously described (42).
Inorganic free phosphate was measured in chicken cecal content (100 mg) at day 3 and day 7 by the inorganic free phosphate measurement ELISA kit (Bioassay) as per the manufacturer’s protocol. All chicken experiments and protocols were approved by the Institutional Animal Care and Use Committee in the Animal Care Program at Michigan State University.
Supplementary Material
ACKNOWLEDGMENTS
We thank Shannon Manning, Department of Microbiology and Molecular Genetics, Michigan State University, for providing human stool samples used in this study.
This work was supported in part by NIH award AI 111192 (to V.J.D.) and by the Rudolph Hugh Endowment of Michigan State University.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Burnham PM, Hendrixson DR. 2018. Campylobacter jejuni: collective components promoting a successful enteric lifestyle. Nat Rev Microbiol 16:551–565. doi: 10.1038/s41579-018-0037-9. [DOI] [PubMed] [Google Scholar]
- 2.Skarp CPA, Hänninen M-L, Rautelin H. 2016. Campylobacteriosis: the role of poultry meat. Clin Microbiol Infect 22:103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Marotta F, Garofolo G, Di Donato G, Aprea G, Platone I, Cianciavicchia S, Alessiani A, Di Giannatale E. 2015. Population diversity of Campylobacter jejuni in poultry and its dynamic of contamination in chicken meat. Biomed Res Int 2015:859845. doi: 10.1155/2015/859845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng L, Ruan M, Liu J, Wilde P, Naumova EN, Mozaffarian D, Zhang FF. 2019. Trends in processed meat, unprocessed red meat, poultry, and fish consumption in the United States, 1999–2016. J Acad Nutr Diet 119:1085–1098. doi: 10.1016/j.jand.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taveirne ME, Theriot CM, Livny J, DiRita VJ. 2013. The complete Campylobacter jejuni transcriptome during colonization of a natural host determined by RNAseq. PLoS One 8:e73586. doi: 10.1371/journal.pone.0073586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamarche MG, Wanner BL, Crépin S, Harel J. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev 32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- 7.Wösten M, Parker CT, van Mourik A, Guilhabert MR, van Dijk L, van Putten J. 2006. The Campylobacter jejuni PhosS/PhosR operon represents a non-classical phosphate-sensitive two-component system. Mol Microbiol 62:278–291. doi: 10.1111/j.1365-2958.2006.05372.x. [DOI] [PubMed] [Google Scholar]
- 8.Vuppada RK, Hansen CR, Strickland KAP, Kelly KM, McCleary WR. 2018. Phosphate signaling through alternate conformations of the PstSCAB phosphate transporter. BMC Microbiol 18:8. doi: 10.1186/s12866-017-1126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chekabab SM, Harel J, Dozois CM. 2014. Interplay between genetic regulation of phosphate homeostasis and bacterial virulence. Virulence 5:786–793. doi: 10.4161/viru.29307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamarche MG, Dozois CM, Daigle F, Caza M, Curtiss R, Dubreuil JD, Harel J. 2005. Inactivation of the pst system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun 73:4138–4145. doi: 10.1128/IAI.73.7.4138-4145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chekabab SM, Jubelin G, Dozois CM, Harel J. 2014. PhoB activates Escherichia coli O157:H7 virulence factors in response to inorganic phosphate limitation. PLoS One 9:e94285. doi: 10.1371/journal.pone.0094285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tischler AD, Leistikow RL, Kirksey MA, Voskuil MI, McKinney JD. 2013. Mycobacterium tuberculosis requires phosphate-responsive gene regulation to resist host immunity. Infect Immun 81:317–328. doi: 10.1128/IAI.01136-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rengarajan J, Bloom BR, Rubin EJ. 2005. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci U S A 102:8327–8332. doi: 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dar D, Sorek R. 2018. Extensive reshaping of bacterial operons by programmed mRNA decay. PLoS Genet 14:e1007354. doi: 10.1371/journal.pgen.1007354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drozd M, Gangaiah D, Liu Z, Rajashekara G. 2011. Contribution of TAT system translocated PhoX to Campylobacter jejuni phosphate metabolism and resilience to environmental stresses. PLoS One 6:e26336. doi: 10.1371/journal.pone.0026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron A, Frirdich E, Huynh S, Parker CT, Gaynor EC. 2012. Hyperosmotic stress response of Campylobacter jejuni. J Bacteriol 194:6116–6130. doi: 10.1128/JB.01409-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Gangaiah D, Torrelles JB, Rajashekara G. 2016. Polyphosphate and associated enzymes as global regulators of stress response and virulence in Campylobacter jejuni. World J Gastroenterol 22:7402–7414. doi: 10.3748/wjg.v22.i33.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candon HL, Allan BJ, Fraley CD, Gaynor EC. 2007. Polyphosphate kinase 1 is a pathogenesis determinant in Campylobacter jejuni. J Bacteriol 189:8099–8108. doi: 10.1128/JB.01037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gangaiah D, Liu Z, Arcos J, Kassem II, Sanad Y, Torrelles JB, Rajashekara G. 2010. Polyphosphate kinase 2: a novel determinant of stress responses and pathogenesis in Campylobacter jejuni. PLoS One 5:e12142. doi: 10.1371/journal.pone.0012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malde A, Gangaiah D, Chandrashekhar K, Pina-Mimbela R, Torrelles JB, Rajashekara G. 2014. Functional characterization of exopolyphosphatase/guanosine pentaphosphate phosphohydrolase (PPX/GPPA) of Campylobacter jejuni. Virulence 5:521–533. doi: 10.4161/viru.28311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaynor EC, Wells DH, MacKichan JK, Falkow S. 2005. The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes. Mol Microbiol 56:8–27. doi: 10.1111/j.1365-2958.2005.04525.x. [DOI] [PubMed] [Google Scholar]
- 22.Peng Y-C, Lu C, Li G, Eichenbaum Z, Lu C-D. 2017. Induction of the pho regulon and polyphosphate synthesis against spermine stress in Pseudomonas aeruginosa. Mol Microbiol 104:1037–1051. doi: 10.1111/mmi.13678. [DOI] [PubMed] [Google Scholar]
- 23.Luethy PM, Huynh S, Ribardo DA, Winter SE, Parker CT, Hendrixson DR. 2017. Microbiota-derived short-chain fatty acids modulate expression of Campylobacter jejuni determinants required for commensalism and virulence. mBio 8:e00407-17. doi: 10.1128/mBio.00407-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neal-McKinney JM, Lu X, Duong T, Larson CL, Call DR, Shah DH, Konkel ME. 2012. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS One 7:e43928. doi: 10.1371/journal.pone.0043928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas MT, Shepherd M, Poole RK, van Vliet AHM, Kelly DJ, Pearson BM. 2011. Two respiratory enzyme systems in Campylobacter jejuni NCTC 11168 contribute to growth on L-lactate. Environ Microbiol 13:48–61. doi: 10.1111/j.1462-2920.2010.02307.x. [DOI] [PubMed] [Google Scholar]
- 26.Cameron A, Huynh S, Scott NE, Frirdich E, Apel D, Foster LJ, Parker CT, Gaynor EC. 2015. High-frequency variation of purine biosynthesis genes is a mechanism of success in Campylobacter jejuni. mBio 6:e00612-15. doi: 10.1128/mBio.00612-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crofts AA, Poly FM, Ewing CP, Kuroiwa JM, Rimmer JE, Harro C, Sack D, Talaat KR, Porter CK, Gutierrez RL, DeNearing B, Brubaker J, Laird RM, Maue AC, Jaep K, Alcala A, Tribble DR, Riddle MS, Ramakrishnan A, McCoy AJ, Davies BW, Guerry P, Trent MS. 2018. Campylobacter jejuni transcriptional and genetic adaptation during human infection. Nat Microbiol 3:494–502. doi: 10.1038/s41564-018-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodall CA, Jones MA, Barrow PA, Hinds J, Marsden GL, Kelly DJ, Dorrell N, Wren BW, Maskell DJ. 2005. Campylobacter jejuni gene expression in the chick cecum: evidence for adaptation to a low-oxygen environment. Infect Immun 73:5278–5285. doi: 10.1128/IAI.73.8.5278-5285.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton J, Gray TK. 1979. Absorption of inorganic phosphate in the human small intestine. Clin Sci (Lond) 56:407–412. doi: 10.1042/cs0560407. [DOI] [PubMed] [Google Scholar]
- 30.Gielda LM, DiRita VJ. 2012. Zinc competition among the intestinal microbiota. mBio 3:e00171-12. doi: 10.1128/mBio.00171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da C Leite DM, Barbosa LC, Mantuano N, Goulart CL, Veríssimo da Costa GC, Bisch PM, von Krüger W. 2017. Transcriptional and post-transcriptional regulation of pst2 operon expression in Vibrio cholerae O1. Infect Genet Evol 51:10–16. doi: 10.1016/j.meegid.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Namugenyi SB, Aagesen AM, Elliott SR, Tischler AD. 2017. Mycobacterium tuberculosis PhoY proteins promote persister formation by mediating Pst/SenX3-RegX3 phosphate sensing. mBio 8:e00494-17. doi: 10.1128/mBio.00494-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendrixson DR, Akerley BJ, DiRita VJ. 2001. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol Microbiol 40:214–224. doi: 10.1046/j.1365-2958.2001.02376.x. [DOI] [PubMed] [Google Scholar]
- 34.Johnson JG, Livny J, Dirita VJ. 2014. High-throughput sequencing of Campylobacter jejuni insertion mutant libraries reveals mapA as a fitness factor for chicken colonization. J Bacteriol 196:1958–1967. doi: 10.1128/JB.01395-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiesner RS, Hendrixson DR, DiRita VJ. 2003. Natural transformation of Campylobacter jejuni requires components of a type II secretion system. J Bacteriol 185:5408–5418. doi: 10.1128/jb.185.18.5408-5418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerry P, Yao R, Alm RA, Burr DH, Trust TJ. 1994. Systems of experimental genetics for Campylobacter species. Methods Enzymol 235:474–481. doi: 10.1016/0076-6879(94)35163-5. [DOI] [PubMed] [Google Scholar]
- 37.Alazzam B, Bonnassie-Rouxin S, Dufour V, Ermel G. 2011. MCLMAN, a new minimal medium for Campylobacter jejuni NCTC 11168. Res Microbiol 162:173–179. doi: 10.1016/j.resmic.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 38.Cai X, Li R. 2016. Concurrent profiling of polar metabolites and lipids in human plasma using HILIC-FTMS. Sci Rep 6:36490. doi: 10.1038/srep36490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clasquin MF, Melamud E, Rabinowitz JD. 2012. LC-MS data processing with MAVEN: a metabolomic analysis and visualization engine In Current protocols in bioinformatics. John Wiley & Sons, Inc., Hoboken, NJ, USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pokhrel A, Lingo JC, Wolschendorf F, Gray MJ. 2019. Assaying for inorganic polyphosphate in bacteria. J Vis Exp 21. doi: 10.3791/58818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendrixson DR, DiRita VJ. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol Microbiol 52:471–484. doi: 10.1111/j.1365-2958.2004.03988.x. [DOI] [PubMed] [Google Scholar]
- 42.Johnson JG, Gaddy JA, DiRita VJ. 2016. The PAS domain-containing protein HeuR regulates heme uptake in Campylobacter jejuni. mBio 7:e01691-16. doi: 10.1128/mBio.01691-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.