Cellular metal ion homeostasis is tightly regulated. When metal ion levels are imbalanced, or when one metal is at toxic levels, enzymes may bind to the wrong metal cofactor. Enzyme mismetallation can impair metabolism, lead to new and deleterious reactions, and cause cell death. Beginning with Bacillus subtilis strains genetically sensitized to metal intoxication through loss of efflux or by lowering intracellular iron, we identified mutations that suppress the deleterious effects of excess Mn(II) or Co(II). For both metals, mutations in mpfA, encoding a Mg(II) efflux pump, suppressed toxicity. These mutant strains have elevated intracellular Mg(II), suggesting that Mg(II)-dependent processes are very sensitive to disruption by transition metals.
KEYWORDS: magnesium efflux, cobalt intoxication, manganese intoxication, Bacillus subtilis, cobalt toxicity, manganese toxicity, metal homeostasis
ABSTRACT
Transition metals are essential for life but are toxic when in excess. Metal ion intoxication may result from the mismetallation of essential metal-dependent enzymes with a noncognate metal. To begin to identify enzymes and processes that are susceptible to mismetallation, we have selected for strains with increased resistance to Mn(II) and Co(II). In Bacillus subtilis, cells lacking the MntR metalloregulator are exquisitely sensitive to Mn(II) but can easily become resistant by acquiring mutations affecting the MntH Mn(II) importer. Using transposon mutagenesis, and starting with an mntR mntH strain, we recovered mariner insertions that inactivated the mpfA gene encoding a putative Mg(II) efflux system. Loss of MpfA leads to elevated intracellular Mg(II), increased sensitivity to high Mg(II), and reduced Mn(II) sensitivity. Consistently, we also recovered an insertion disrupting the mgtE riboswitch, which normally restricts expression of the major Mg(II) importer. These results suggest that Mn(II) intoxication results from disruption of a Mg(II)-dependent enzyme or process. Mutations that inactivate MpfA were also recovered in a selection for Co(II) resistance beginning with sensitized strains lacking the major Co(II) efflux pump, CzcD. Since both Mn(II) and Co(II) may mismetallate iron-dependent enzymes, we repeated the selections under conditions of iron depletion imposed by expression of the Listeria monocytogenes FrvA iron exporter. Under conditions of iron depletion, a wider variety of suppressor mutations were recovered, but they still point to a central role for Mg(II) in maintaining metal ion homeostasis.
IMPORTANCE Cellular metal ion homeostasis is tightly regulated. When metal ion levels are imbalanced, or when one metal is at toxic levels, enzymes may bind to the wrong metal cofactor. Enzyme mismetallation can impair metabolism, lead to new and deleterious reactions, and cause cell death. Beginning with Bacillus subtilis strains genetically sensitized to metal intoxication through loss of efflux or by lowering intracellular iron, we identified mutations that suppress the deleterious effects of excess Mn(II) or Co(II). For both metals, mutations in mpfA, encoding a Mg(II) efflux pump, suppressed toxicity. These mutant strains have elevated intracellular Mg(II), suggesting that Mg(II)-dependent processes are very sensitive to disruption by transition metals.
INTRODUCTION
Nearly 30% of all proteins require metals as cofactors that are necessary to support protein folding or to serve as electrophilic or redox active centers in catalysis. A variety of metal ion homeostatic processes enable cells to acquire essential metals, to export metals when in excess, and to store and properly distribute metals to nascent metalloproteins (1). The chemical properties of biologically relevant metal ions determine the affinity with which they bind ligands, and this can be succinctly represented in the Irving-Williams series (Mg = Ca < Mn < Fe < Co < Ni < Cu > Zn) (2). Ions such as Mg(II) and Ca(II) tend to be highly hydrated and mobile and are often at high concentrations in cells (3). Conversely, Zn(II) tends to bind to proteins with much greater affinity, often forming long-lasting complexes that may not dissociate on a biologically relevant timescale (4). The buffered concentration of labile metal ions, which are kinetically exchangeable and bioavailable, reflects these properties. The labile Mg(II) pool in bacteria is estimated to be close to 3 mM, Fe(II) and Mn(II) in the range of 10 μM, and Zn(II) in the subnanomolar range (5–7).
The cytosol can be modeled as a complex mixture of metal-binding sites, reflecting the many potential metal-binding ligands, with widely ranging affinities (8, 9). Nascent metalloproteins are thus confronted with a complex, mixed pool of metals and must acquire the appropriate cofactor to maintain their functionality. Mismetallation, in which a noncognate and often nonfunctional metal binds to a metal-binding site, can lead to enzyme inactivation and, potentially, cell death (1, 10, 11). Metal ion intoxication results when cells encounter an excess of a metal ion that overwhelms the metal ion homeostasis or defense mechanisms and interferes with cell physiology or even viability. Cells can be intoxicated by either essential nutrient metal ions [such as Zn(II), Mn(II), and Fe)] or by toxic metal ions [such as (Hg(II) and Cd(II)]. A primary defense mechanism against metal ion intoxication is the expression of efflux transporters to remove metal from the cytosol, although metal sequestration and storage proteins also play a protective role in many cases (1, 12).
Bacillus subtilis is a model system for the investigation of metal ion homeostasis mechanisms (1, 7, 13). We identified the key regulatory proteins that sense Zn(II), Mn(II), and Fe(II) levels and implement the appropriate changes in gene expression to maintain these essential ions at physiologically compatible concentrations (1). As a result, we revealed the processes by which cells adapt to both metal deficiency and excess. In the specific case of Zn(II) homeostasis, sufficiency is sensed by the Zur repressor (14–17), and excess is sensed by CzrA (13, 18). In response to excess Zn(II), CzrA dissociates from DNA, leading to expression of two parallel pathways for Zn(II) efflux, the CadA P-type ATPase and the CzcD cation diffusion facilitator. Using a forward genetics strategy, we then asked how cells can become resistant to Zn(II) intoxication, in both the presence and absence of the cognate efflux systems. In efflux-competent cells, Zn(II) intoxicates cells from the outside, resulting in poisoning of the electron transport chain (19), as also observed in mitochondria (20, 21). To identify cytosolic targets of Zn intoxication, we selected for suppressors of the high Zn(II) sensitivity of an efflux defective strain. In this case, we found that Zn(II) mismetallates a transcription factor, PerR, and that dysregulation of the PerR regulon leads to heme intoxication (19).
In this study, we focus on the transition metals manganese (Mn) and cobalt (Co) in an effort to define the targets of mismetallation during metal intoxication. Manganese is required as a cofactor for many enzymes that are involved in oxidative stress and DNA replication (22, 23). The Mn(II) biosensor, MntR, is the key regulator of manganese homeostasis in many bacteria (24–28). MntR in B. subtilis has dual roles: it functions as a repressor for two Mn(II) import systems (MntABCD and MntH) and as an activator for two Mn(II) export systems (MneP and MneS) (26). Mutants lacking MntR, or lacking the two efflux systems, are known to be highly sensitive to Mn(II) (25, 26). However, the basis of this toxicity is not yet clear. Mn(II) is relatively uncompetitive in protein binding compared to Fe(II) or Co(II) but much more competitive than Mg(II). In Bradyrhizobium japonicum, Mn(II) affects Mg(II)-dependent processes (29), most likely due to mismetallation interfering with Mg(II)-requiring enzymes or processes. Similarly, Mn(II) intoxication in the yeast Saccharomyces cerevisiae is reduced by Mg(II) supplementation (30).
Cobalt, another transition metal, is required as an integral part of cobalamin (vitamin B12) and as a cofactor for at least eight different types of non-corrin-dependent cobalt enzymes (31). Many bacteria require submicromolar cobalt for growth and encode specific uptake, efflux, and transcription regulatory systems to maintain cobalt homeostasis (32). In B. subtilis, no dedicated Co(II) importers have been documented. Two exporters generally serve to protect cells against intoxication by thiophilic metals. CadA, a CPx-type ATPase, is the major determinant for Cd(II) resistance and contributes to Zn(II) resistance but has a very limited effect on Co(II) resistance (13, 33). CzcD, a cation diffusion facilitator, confers resistance to Zn(II), Co(II), and Ni(II) (13, 34). Both exporters are under regulation of CzrA, a metal-sensing ArsR/SmtB family repressor (18). Consistent with the broad selectivity of its regulated transporters, CzrA responds in vivo to multiple metals, including Zn(II), Cd(II), Co(II), and Ni(II) (13, 33). However, at least in the case of Cd(II), this induction is an indirect effect of displacement of Zn(II) from intracellular, thiol-rich buffers (35).
Cobalt is toxic to cells at elevated levels. Cobalt toxicity may have several origins, including (i) production of reactive oxygen species, (ii) interference with sulfur assimilation, (iii) reduction of the free thiol pool, (iv) competition with iron during Fe-S cluster biogenesis, and (v) metalloporphyrin formation (36, 37). In Escherichia coli, iron-dependent processes are the most likely targets of Co(II) intoxication (38). These two metals possess very similar properties such as radii and oxidation states (2+ and 3+), which makes mismetallation of iron-containing proteins with cobalt possible. Furthermore, cobalt is more competitive than iron in protein binding, and the resulting cobalt complexes would be more stable than iron complexes. In fact, the ligand exchange rate of Co(III) is 8 orders of magnitude slower than that of Fe(III) [10−6 s−1 for Co(III) versus 102 s−1 for Fe(III)] (32), indicating that the misincorporation events will be long-lasting and likely beyond repair. Indeed, in cobalt-treated E. coli cells, many Fe-S enzymes, such as aconitase and ferrichrome reductase, are inactivated, resulting in drastic growth inhibition (39). In B. subtilis, however, the specific targets of cobalt intoxication are unknown.
Here, we use a forward genetic selection to identify mutations in B. subtilis that can alleviate Mn(II) and Co(II) toxicity in efflux-defective strains. We repeatedly isolated suppressor mutations in yhdP, which encodes a homolog of the Staphylococcus aureus magnesium protection factor, MpfA (40). Our data revealed that YhdP (here renamed MpfA) is induced by, and confers resistance to, high Mg(II). Analysis of intracellular metal levels supports the assignment of MpfA as a Mg(II) exporter. Elevated intracellular Mg(II) levels in the mpfA null mutant strains suppressed Mn(II) and Co(II) toxicity, suggesting that Mg(II)-dependent processes are one target of intoxication when these ions are present in excess.
RESULTS
Isolation of Mn(II)- and Co(II)-resistant suppressors.
To identify potential targets of Mn(II) intoxication, we selected for mutants resistant to Mn(II) using sensitized strains. A B. subtilis mntR mutant is very sensitive to Mn(II) due to constitutive expression of Mn(II) import systems and reduced expression of Mn(II) exporters (26). This sensitivity is further enhanced in strains that additionally carry null mutations of the two MntR-activated efflux systems, MneP and MneS (26). Indeed, we found it very easy to select for Mn(II)-resistant mutants using these sensitized strains. Previous selections for Mn(II) resistance in an mntR null mutant yielded almost exclusively mutations in mntH (25), which encodes the major Mn(II) transporter under high Mn(II) conditions. We therefore sequenced the mntH locus in the isolated, spontaneous suppressors. In every case tested, there were mutations affecting mntH that led to substantial increases in Mn(II) resistance (see Table S1 and Fig. S1 in the supplemental material). This confirms that MntH is the major pathway of Mn(II) import in these strains, even under conditions of excess Mn(II), and the simplest way of avoiding Mn(II) intoxication is to reduce uptake. However, we did not recover strains retaining wild-type MntH that might instead allow identification of specific targets of Mn(II) intoxication.
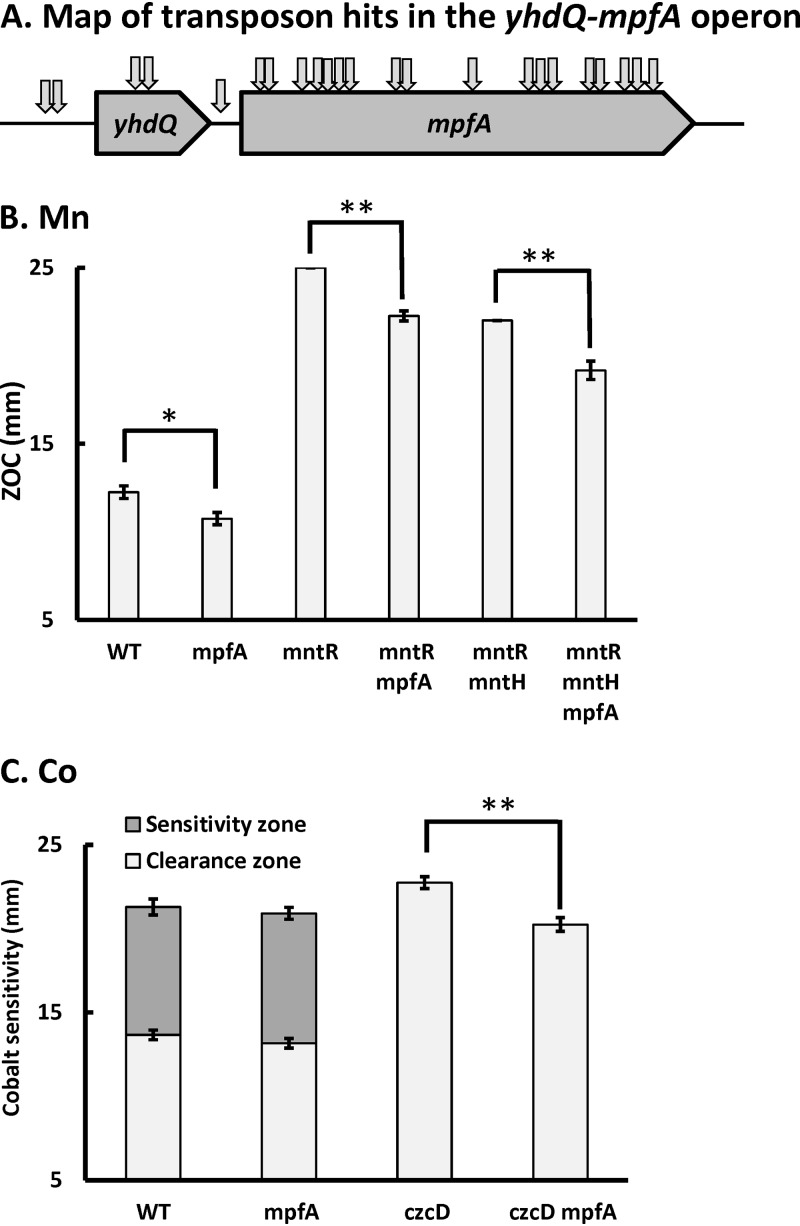
Next, we began with an mntR mntH double mutant, which is still highly sensitive to Mn(II) intoxication (26), and used mariner transposon (mTn) mutagenesis to select for increased Mn(II) resistance. Among nine Mn(II)-resistant suppressors, seven contained transposon insertions in the yhdQ-mpfA operon. The other insertions were in yeaD, which encodes an unknown protein, and in the 5′ region of mgtE, disrupting the Mg(II)-sensing riboswitch that serves to restrict mgtE transcription (Table 1 and Fig. 1A). MgtE is the major Mg(II) importer in B. subtilis (41). YhdQ is a MerR family transcriptional regulator of unknown function. YhdQ was erroneously implicated as a regulator of Cu(I) efflux and renamed CueR (42), but subsequent work clarified that the correct regulator is CsoR, and the gene designation has since reverted to yhdQ (43, 44). MpfA is a homolog of the putative Mg(II) efflux transporter CorC in E. coli and S. aureus MpfA, which confers high Mg(II) tolerance (40).
TABLE 1.
Isolated cobalt- and manganese-resistant suppressorsa
| Metal | Background | Selection conditions | No. of hits (no. of unique hits) | Target | Function |
|---|---|---|---|---|---|
| Manganese | mntR mntH | 200 μM MnCl2 | 5 (5) | mpfA | Putative magnesium exporter |
| Manganese | mntR mntH | 200 μM MnCl2 | 2 (2) | yhdQ | MerR family regulator |
| Manganese | mntR mntH | 200 μM MnCl2 | 1 | yeaD | Unknown |
| Manganese | mntR mntH | 200 μM MnCl2 | 1 | 5′ UTRb MgtE riboswitch | Regulation of magnesium uptake |
| Cobalt | czcD | 400 μM CoCl2 | 16 (12) | yhdQ-mpfA | YhdQ, MerR family regulator |
| MpfA, putative magnesium exporter |
15 μg ml−1 kanamycin was used to select the specific transposon insertion suppressors.
UTR, untranscribed region.
FIG 1.
Loss of MpfA alleviates Co(II) and Mn(II) intoxication in efflux-deficient mutants. (A) Map of suppressor mutants [Mn(II) and Co(II)] with transposon insertions in the yhdQ-mpfA operon. (B and C) The sensitivity of the wild type and its derived mutants to Mn (B) and Co (C) was monitored using a disk diffusion assay. The mutant strains tested in panel B were mpfA::mls, mntR::tet, mntR::tet mpfA::mls, mntR::tet ΔmntH, and mntR::tet ΔmntH mpfA::mls. The mutant strains tested in panel C were mpfA::mls, czcD::tet, and czcD::tet mpfA::mls. Ten microliters of 250 mM MnCl2 or 250 mM CoCl2 was applied to each disk. Data are expressed as the diameter of the zone of clearance (ZOC) (mean ± standard error [SE]; n = 3) and zone of sensitivity (mean ± SE; n = 3). * (P < 0.05) and ** (P < 0.01) indicate statistically significant differences between the indicated groups as judged using a paired Student t test.
To further explore the relevance of MpfA to Mn(II) resistance, we tested the effect of an mpfA::mls null mutation in wild-type, mntR, and mntR mntH strain backgrounds using a disk diffusion assay (Fig. 1B). Deletion of mpfA reduced Mn(II) sensitivity in all three strain backgrounds. Indeed, an mntR mpfA double mutant is similar in Mn(II) resistance to the mntR mntH double mutant, and the effects of the mntH and mpfA mutations are additive (Fig. 1B).
In parallel, we selected for mTn insertions that suppressed Co(II) sensitivity in the efflux-deficient czcD strain. Although not anticipated at the outset, there was considerable overlap between the suppressors recovered in the Mn(II) and Co(II) resistance selections; 12 unique mTn insertions mapped to the yhdQ-mpfA operon (Table 1 and Fig. 1A). The effect of an mpfA null mutation on reducing Co(II) sensitivity was also apparent in a disk diffusion assay when tested in a czcD background, but there was little effect in a wild-type strain (Fig. 1C).
Loss of MpfA alleviates Mn(II) and Co(II) intoxication in B. subtilis.
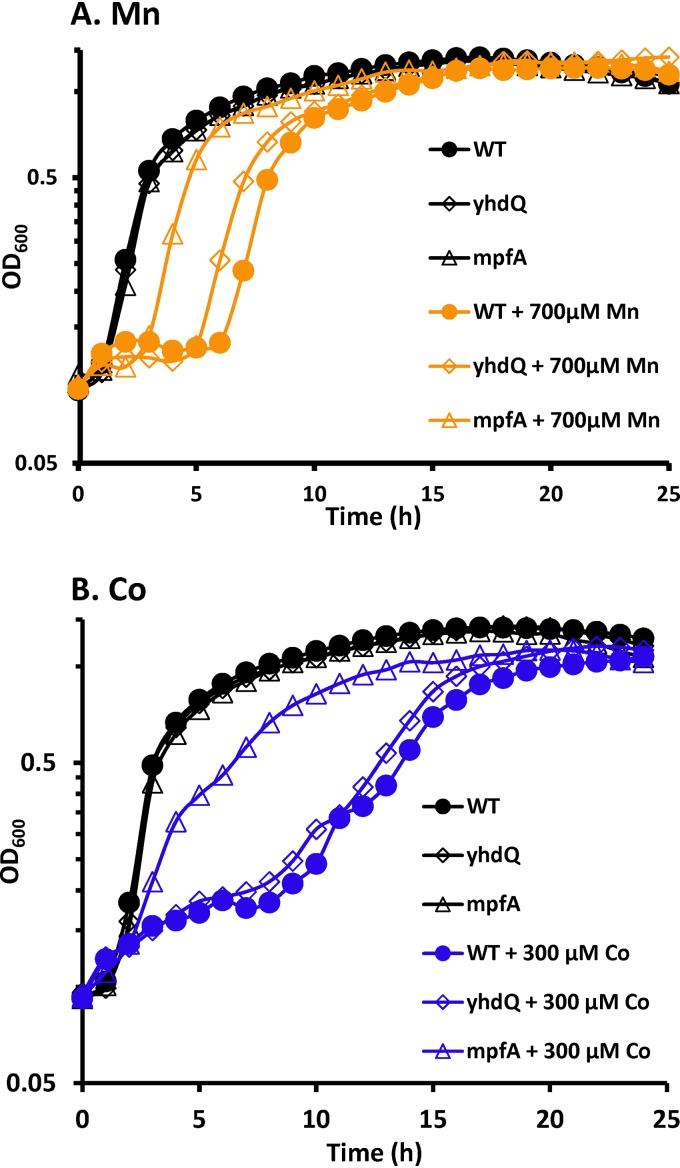
To better define the impact of mutations in the yhdQ-mpfA operon on metal ion sensitivity, we monitored growth in LB medium supplemented with intoxicating levels of metals (Fig. 2). Loss of MpfA significantly increased tolerance to Mn(II) and Co(II) (Fig. 2) but had little effect on tolerance to other metals tested (Fig. S2). In the case of Mn(II) intoxication, wild-type cells started to grow after a 6-h lag phase, while the mpfA null mutant resumed growth with only a 3-h lag phase (Fig. 2A). Under conditions of Co(II) intoxication, the mpfA null mutant grew without a significant lag phase, but at a reduced rate, while the wild-type strain became growth inhibited after one doubling and only resumed growth after a prolonged lag phase (Fig. 2B). This lag is likely due to acclimation to high Co(II) levels rather than to the emergence of suppressor mutations, since cells recovered from the end of the growth experiment still showed the same lag when tested again for Co(II) sensitivity. To reduce the impact of polar effects, we generated an unmarked, in-frame deletion mutation in yhdQ using the BKE collection of strains (45). The nonpolar yhdQ deletion mutation led to a much less pronounced increase in fitness with high Mn(II) compared to the mpfA mutation and did not confer a growth advantage under Co(II) stress conditions (Fig. 2). Mutation of yhdQ or mpfA did not result in large changes in fitness when cells were grown with inhibitory levels of other metals (Fe, Zn, Cu, and Ni), although small effects were sometimes noted (Fig. S2).
FIG 2.
Deletion of MpfA attenuates Co(II) and Mn(II) toxicity. A growth assay was carried out in LB medium to evaluate sensitivity of the wild type and ΔyhdQ and mpfA null mutants to Mn(II) (A) and Co(II) (B) intoxication. The concentrations of MnCl2 and CoCl2 used were 700 μM and 300 μM, respectively. Experiments were performed with three biological replicates. Representative growth curves are shown.
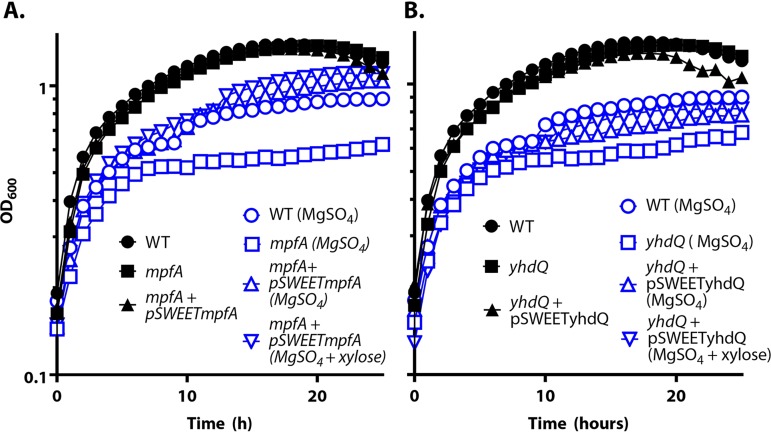
MpfA plays an important role in high Mg(II) tolerance.
Consistent with a role for MpfA in Mg(II) efflux, an mpfA null mutant is severely compromised in growth, and its growth yield is significantly lower (optical density at 600 nm [OD600], ∼0.6) than that of wild-type cells (OD600, ∼0.9) grown in the presence of 300 mM Mg(II) (Fig. 3A). This phenotype was complemented by introduction of an ectopic copy of the mpfA gene controlled by the Pxyl promoter. A yhdQ null mutant was also more sensitive to high Mg(II) than the wild-type cells (Fig. 3B), and this sensitivity could also be complemented by an ectopic copy of yhdQ. These results suggest that both YhdQ, presumably acting as a transcription factor, and MpfA can contribute to growth in the presence of excess Mg(II).
FIG 3.
MpfA confers high Mg(II) tolerance. WT, ΔmpfA, ΔydhQ, and complemented strains were grown in LB with or without the addition of 300 mM MgSO4; 0.1% xylose was amended to induce expression of MpfA and YhdQ. Experiments were performed at least three times with three biological replicates. Representative plots are shown.
Building on prior reports (40), we hypothesized that MpfA was functioning in Mg(II) efflux and that the mpfA null mutant might have elevated Mg(II) levels. We therefore used inductively coupled plasma mass spectrometry (ICP-MS) to monitor intracellular metal levels in cells grown in LB medium with or without supplementation with 10 mM Mg(II). The mpfA null mutant accumulated ∼50% higher levels of Mg(II) than the wild-type cells under both conditions (Table 2). The intracellular levels of Mn, Co, and Fe were also monitored, but no significant differences were observed between the mpfA null and wild-type cells (Table 2). These results are consistent with the idea that MpfA functions as a Mg(II) efflux transporter in B. subtilis.
TABLE 2.
Intracellular metal levels quantified by ICP-MSa
| Growth conditions | Strain description | Mg (mg g−1 protein) | Mn (μg g−1 protein) | Co (μg g−1 protein) | Fe (μg g−1 protein) |
|---|---|---|---|---|---|
| LB | WT | 2.0 ± 0.1 | 60 ± 1.8 | 4.2 ± 1.2 | 54 ± 3.6 |
| LB | mpfA | 3.2 ± 0.1 | 59 ± 3.6 | 4.5 ± 1.4 | 55 ± 4.7 |
| LB + 10 mM MgCl2 | WT | 3.1 ± 0.1 | 63 ± 3.0 | 4.3 ± 1.8 | 56 ± 5.3 |
| LB + 10 mM MgCl2 | mpfA | 4.6 ± 0.1 | 63 ± 3.1 | 4.9 ± 0.6 | 58 ± 4.8 |
All values are mean ± SD for biological replicates (n = 3).
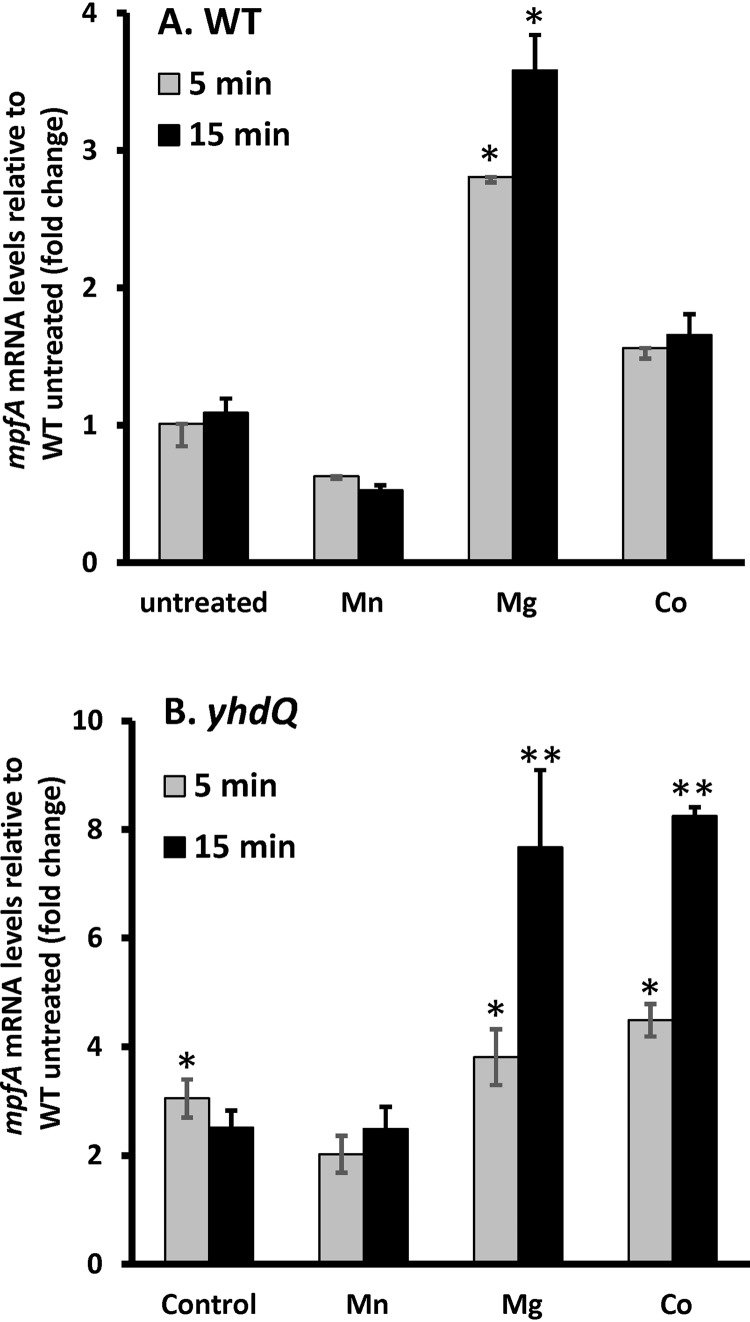
Expression of mpfA is induced by Mg(II), and induction is independent of YhdQ.
We hypothesized that if MpfA functions in Mg(II) efflux, its expression might be induced by high Mg(II). We therefore used quantitative reverse transcription-PCR (qRT-PCR) to monitor mpfA expression before and after treatment of cells with different metals. The mRNA level of mpfA was elevated ∼3-fold within 15 min of treatment of high Mg(II) but not by exposure to Mn(II) or Co(II) at levels known to induce expression of their cognate efflux systems (13, 26, 33) (Fig. 4). Since yhdQ encodes a MerR family regulator and deletion of yhdQ also affected resistance to Mg(II), we hypothesized that YhdQ may serve as a positive regulator for the yhdQ-mpfA operon. To test this idea, we quantified the mRNA levels of mpfA in the ΔyhdQ null mutant using qPCR. Expression of mpfA was upregulated ∼3-fold in both the wild-type and ΔyhdQ strains within 5 min of Mg(II) shock (Fig. 4). Moreover, mpfA expression was higher in the yhdQ null strain than in the wild type after 15 min of Mg(II) shock. Strikingly, in the yhdQ null strain, induction of the yhdQ-mpfA operon by Co(II) was enhanced relative to the wild-type strain (Fig. 4B). These results are not consistent with the hypothesis that YhdQ serves as an activator for the yhdQ-mpfA operon by high Mg(II). Instead, we suggest that induction of the yhdQ-mpfA operon by Mg(II) increases Mg(II) efflux, mediated by MpfA, and also elevates expression of one or more other unidentified efflux pumps activated by the YhdQ transcription factor.
FIG 4.
Transcription of mpfA is induced by high Mg(II). To understand the regulation of mpfA, quantitative PCR (qPCR) was carried out to evaluate mpfA mRNA expression levels in WT (A) and ΔyhdQ null mutant (B) strains in resonse to different metal treatments. The concentrations of metals used were 250 μM MnCl2, 200 mM MgCl2, and 250 μM CoCl2. The expression levels of mpfA in WT untreated cells are set as 1 in both panels. B. subtilis 23S rRNA was used as a housekeeping control gene. The data are expressed as the mean ± SE (n = 3). * (P < 0.05) and ** (P < 0.01) indicate statistically significant differences between the treated groups and the untreated WT as judged using a paired Student t test.
Supplemental Mg(II) restores resistance to Mn(II) and Co(II) toxicity.
Since an mpfA null mutation led to an increase in intracellular Mg(II) (Table 2) and also increases Mn(II) and Co(II) tolerance (Fig. 2), we hypothesized that Mg(II) supplementation might also suppress Mn(II) and Co(II) intoxication. Although only recently appreciated, LB and related peptide-based growth media may be growth limiting for Mg(II) even under conditions of carbon excess (46). Indeed, supplementation of LB with 25 mM Mg(II) greatly reduced sensitivity to Mn(II) intoxication in both wild-type and Mn(II) efflux-deficient (i.e., mneP mneS) strains (Fig. S3). Remarkably, supplemental Mg(II) also markedly reduced Co(II), Fe, and Zn(II) [but not Cd(II)] toxicity in both wild-type and efflux-deficient strains lacking the CadA and CzcD or the PfeT efflux system (13, 33, 47) (Fig. S3).
In addition to mutations inactivating MpfA, we also recovered a transposon insertion in the riboswitch of mgtE (Table 1), encoding the primary high-affinity Mg(II) importer in B. subtilis (41). We hypothesized that this insertion was likely leading to an upregulation of mgtE expression and therefore also increasing cytosolic Mg(II). To further explore the impact of MgtE on metal sensitivity, we constructed strains containing a second, inducible copy of mgtE or containing a CRISPR interference (CRISPRi) knockdown to reduce mgtE expression. Repression of mgtE expression led to a significant increase in sensitivity to both Mn(II) and Co(II), whereas induction of an ectopic copy of mgtE led to a small but significant increase in Mn(II) tolerance. However, induction of mgtE led to a significant increase in Co(II) sensitivity, consistent with prior suggestions that MgtE may also import Co(II) (Fig. S4) (48).
Effects of Fe(II) limitation on Mn(II) and Co(II) toxicity.
Prior work has suggested that enzymes that employ nonheme Fe(II) as a catalytic cofactor may be particularly susceptible to mismetallation, especially under conditions of oxidative stress (10). Moreover, inactivation of iron-sulfur-containing enzymes has been suggested to be one mechanism of Co(II) toxicity (36). We therefore hypothesized that reducing the levels of intracellular iron might reveal those Fe-containing enzymes or processes most sensitive to mismetallation.
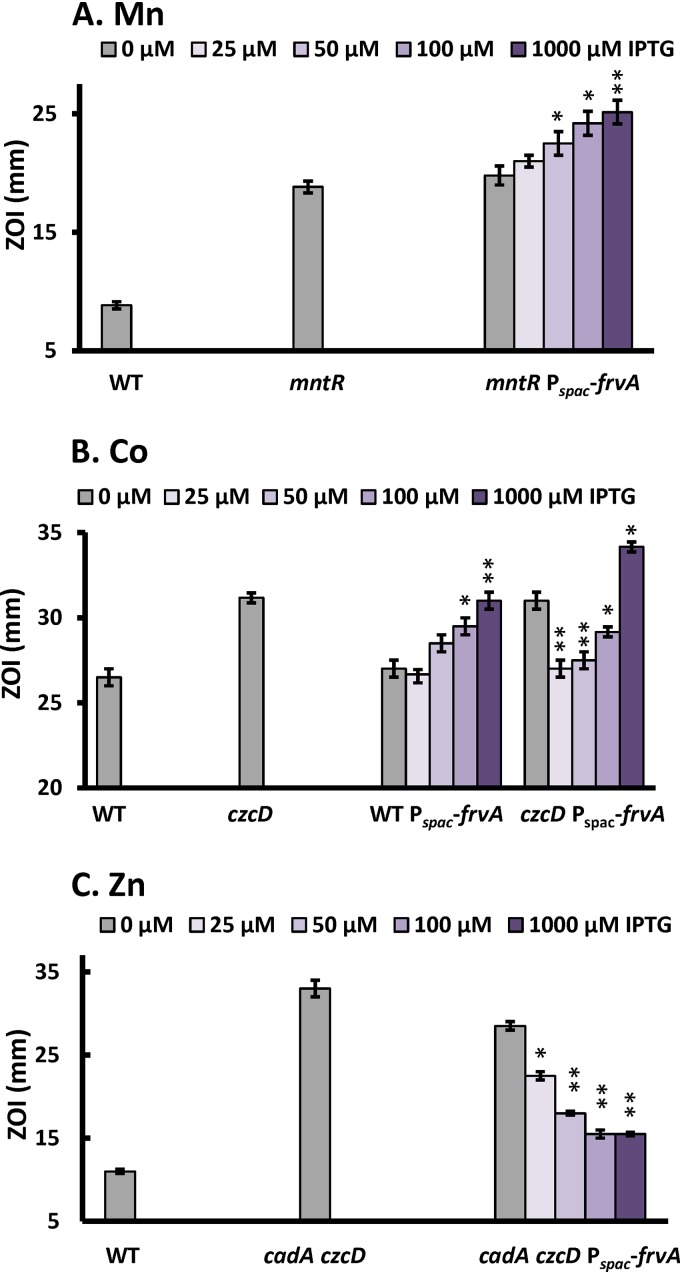
To explore the effect of iron limitation on metal intoxication, we took advantage of a recently developed system for the inducible depletion of cytosolic iron pools. Induction of the Listeria monocytogenes high-affinity Fe(II) efflux transporter FrvA leads to severe iron deprivation in B. subtilis (49, 50), which might thereby increase sensitivity to metal intoxication. Indeed, induction of frvA increased Mn(II) sensitivity in a concentration-dependent manner (Fig. 5A). A similar result was seen with Co(II) sensitivity, as monitored in wild-type cells (Fig. 5B). We conclude that depletion of cytosolic iron by FrvA can sensitize cells to the intoxicating effects of both Mn(II) and Co(II). Consistent with this hypothesis, supplementation with 100 μM iron largely rescued the Mn(II) and Co(II) sensitivity that results from FrvA-dependent iron depletion (Fig. S5). Conversely, induction of FrvA increased resistance to Zn(II) in a cadA czcD efflux-defective strain (Fig. 5C). This is consistent with the prior observation that the FrvA ATPase is weakly activated by Zn(II), and induction leads to a modest decrease in intracellular Zn(II) levels in wild-type cells (49).
FIG 5.
Effects of iron limitation mediated by FrvA on sensitivity to Co(II), Mn(II), and Zn(II). To test the effects of iron limitation on sensitivity to Mn (A), Co (B), and Zn (C), a disk diffusion assay was performed using the L. monocytogenes high-affinity Fe(II) efflux transporter FrvA for the inducible depletion of cytosolic iron pools. Mutant strains tested in panel A were mntR::tet and mntR::tet amyE::Pspac-frvA::cm, in panel B were czcD::tet and czcD::tet amyE::Pspac-frvA::cm, and in panel C were cadA::kan czcD::tet and cadA::kan czcD::tet amyE::Pspac-frvA::cm. Ten microliters of 100 mM MnCl2 (A), 750 mM CoCl2 (B), or 100 mM ZnCl2 (C) was applied to each disk. Varied concentrations of IPTG were amended to induce the expression of FrvA. The data are expressed as the diameter (mean ± SE; n = 3) of the clearance zone (ZOI). * (P < 0.05) and ** (P < 0.01) indicate statistically significant differences between the indicated treated and untreated groups as judged using a paired Student t test.
Results with Co(II) were more complex when assayed in a strain lacking the CzcD cation diffusion facilitator. As reported previously, low-level induction of FrvA (with 25 μM IPTG [isopropyl-β-d-thiogalactopyranoside]) confers resistance to Co(II) in a czcD mutant (49). This suggests that FrvA can function in Co(II) efflux, consistent with the ability of Co(II) to activate FrvA ATPase activity (49). Indeed, Co(II) is roughly 10 times as potent an activator of the FrvA ATPase as Fe(II) on a concentration basis (attractant concentration necessary to trigger the half maximal response [K1/2] of 12 versus 116 μM), although the maximal velocity is greatest with Fe(II) (49). As a result, low-level induction of FrvA complements the efflux mutant strain and restores Co(II) resistance to the level seen in wild-type cells (Fig. 5B). However, with higher levels of IPTG, intracellular Fe(II) levels are depleted (49), and this leads to Co(II) sensitivity, as seen also in wild-type cells (Fig. 5B).
Together, these data suggest that excess Co(II) or Mn(II) may result in mismetallation of iron-requiring enzymes, particularly under iron-limited conditions. We then asked whether the sensitivity to Co(II) or Mn(II) triggered by iron limitation is additive. The three following pairs of strains were tested by monitoring their growth in medium amended with toxic levels of either one or both metals: (i) wild type (WT) versus Pspac-frvA, (ii) mntR versus mntR Pspac-frvA, and (iii) czcD versus czcD Pspac-frvA (Fig. S6). All of these strains showed an increased sensitivity when both metals were present compared to either one alone, particularly when FrvA was induced to deplete intracellular iron pools (Fig. S6). These results suggest that the specific intracellular targets of Co(II) and Mn(II) intoxication are likely to be distinct.
Altered Mg(II) homeostasis also suppresses Mn(II) and Co(II) toxicity under Fe(II) limitation.
To understand how iron limitation increases sensitivity to Mn(II) and Co(II), we repeated our selection for suppressor mutations in strains expressing the FrvA iron efflux pump. With an mntR strain harboring an ectopic copy of frvA with moderate induction (100 μM IPTG), all 16 resistant suppressors had mTn insertions within mntH (Table 3), consistent with previous screens performed under iron sufficiency (25). Selection for Mn(II) resistance mutants (using 200 μM MnCl2) in an mntR mntH mutant with induction of FrvA led primarily to insertions that inactivated Pspac-frvA. However, we also recovered a transposon insertion in the mgtE riboswitch (at a different site from the one mentioned above) and one in yhdQ (Table 3 and Fig. 1A). Thus, even with iron depletion, Mn(II) appears to intoxicate cells by disruption of a Mg(II)-dependent enzyme or process.
TABLE 3.
Isolated cobalt- and manganese-resistant suppressors under iron-limited conditions
| Metal | Background | Selection conditions | No. of hits (no. of unique hits) | Target | Function |
|---|---|---|---|---|---|
| Manganese | mntR Pspac-frvA | 75 μM MnCl2, 100 μM IPTG | 16 (11) | mntH | Manganese importer |
| Manganese | mntR mntH Pspac-frvA | 200 μM MnCl2, 100 μM IPTG | 1 | 5′ UTRa MgtE riboswitch | Regulation of magnesium uptake |
| Manganese | mntR mntH Pspac-frvA | 200 μM MnCl2, 100 μM IPTG | 1 | yhdQ | MerR family regulator |
| Manganese | mntR mntH Pspac-frvA | 200 μM MnCl2, 100 μM IPTG | 18 | Pspac-frvA | Ferrous iron efflux transporter |
| Cobalt | czcD Pspac-frvA | 400 μM CoCl2, 100 μM IPTG | 1 | putP | High-affinity proline permease |
| Cobalt | czcD Pspac-frvA | 400 μM CoCl2, 100 μM IPTG | 1 | ymfF | Putative multidrug-efflux transporter |
| Cobalt | czcD Pspac-frvA | 400 μM CoCl2, 100 μM IPTG | 1 | queF | Queosine biosynthesis |
| Cobalt | czcD Pspac-frvA | 100 μM CoCl2, 100 μM IPTG | 1 | mpfA | Putative magnesium exporter |
| Cobalt | czcD Pspac-frvA | 100 μM CoCl2, 100 μM IPTG | 1 | ybaF | Putative cobalt ABC transporter permease |
| Cobalt | czcD Pspac-frvA | 400 μM CoCl2, 1 mM IPTG | 12 | Pspac-frvA | Ferrous iron efflux transporter |
| Cobalt | czcD Pspac-frvA | 400 μM CoCl2, 1 mM IPTG | 1 | mpfA | Putative magnesium exporter |
| Cobalt | czcD Pspac-frvA | 400 μM CoCl2, 1 mM IPTG | 1 | yvrB | Putative vitamin B12 permease |
| Cobalt | czcD Pspac-frvA | 400 μM CoCl2, 1 mM IPTG | 1 | fur | Ferric uptake regulator |
| Cobalt | czcD Pspac-frvA | 400 μM CoCl2, 1 mM IPTG | 1 | ybcF | Putative carbonic anhydrase |
UTR, untranscribed region.
With the Co(II)-efflux-deficient mutant, czcD, and a moderate level of FrvA induction (100 μM IPTG), five suppressors were isolated with mTn insertions in putP, ymfF, queF, mpfA, and ybaF (Table 3). The gene putP encodes a high-affinity proline permease, ymfF encodes a putative multidrug-efflux transporter, queF encodes a queuosine biosynthesis enzyme, and ybaF encodes a putative cobalt ABC transporter permease. In all cases, these mTn insertions were linked to the observed Co(II) resistance phenotype. The mpfA::mTn insertion likely leads to elevated cytosolic Mg(II), as noted above. The mechanisms underlying the other suppressor mutations await further investigation, although we suggest that the ybaF::mTn insertion may simply decrease Co(II) import.
We reasoned that induction of FrvA with 1 mM IPTG might be required to more severely deplete intracellular iron pools and thereby enhance Co(II) sensitivity (Fig. 5). We performed the selection again using 1 mM IPTG to induce FrvA. Most of the mTn insertions (12 out of 16) were in the Pspac-frvA locus, consistent with the notion that preventing iron depletion helps prevent Co(II) intoxication. Other mTn insertions were identified in mpfA, yvrB, fur, and ybcF (Table 3). The gene yvrB encodes a putative vitamin B12 permease [which might thereby contribute to Co(II) influx], and ybcF encodes a putative carbonic anhydrase. Fur is the master regulator of iron homeostasis in B. subtilis, and inactivation of fur may counteract the severe iron deprivation imposed by overexpression of the ferrous iron exporter FrvA.
DISCUSSION
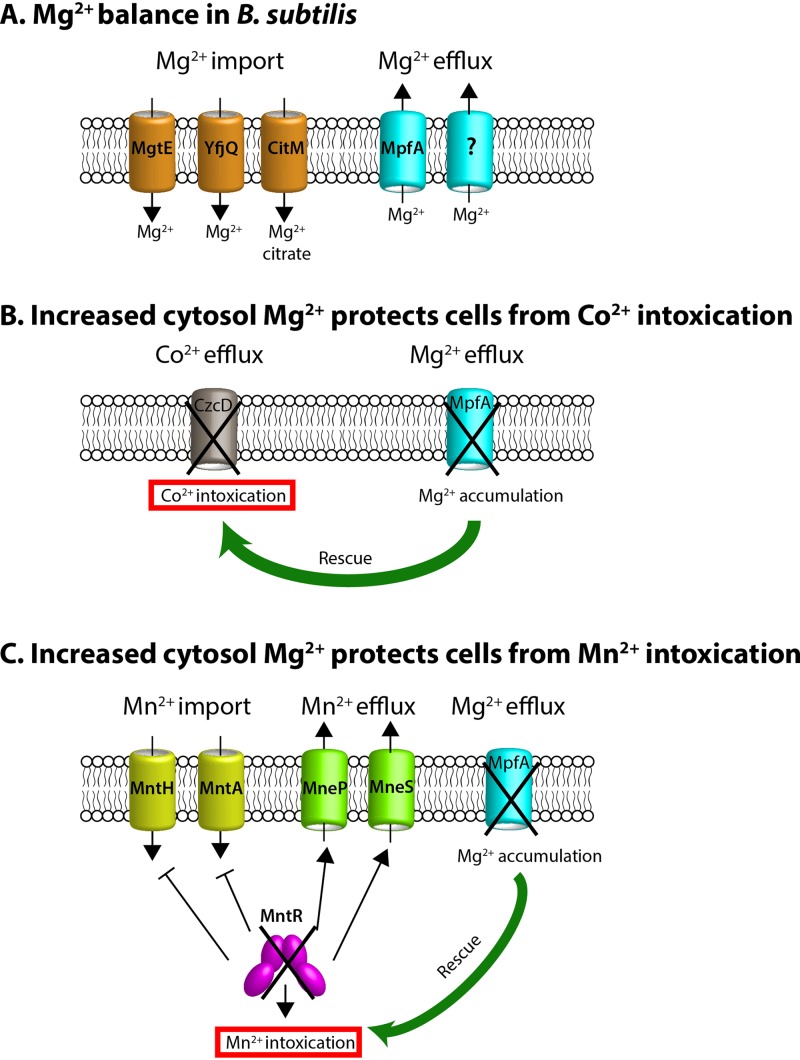
The essential transition metals Mn(II) and Co(II) are toxic for cells when in excess, yet the specific mechanisms that lead to intoxication are poorly understood. Prior results suggest that Mn(II) intoxication may interfere with Mg(II)-dependent process in both Bradyrhizobium japonicum (29) and the yeast Saccharomyces cerevisiae (30). Co(II) is a more thiophilic metal and is thought to more specifically interfere with iron-dependent processes. Here, using a forward genetics approach, we demonstrate that Mg(II)-dependent processes are susceptible to disruption by both Mn(II) and Co(II), and intoxication can be suppressed by either loss of MpfA or increased expression of MgtE, both of which elevate cytosolic Mg(II) (Fig. 6).
FIG 6.
Effects of reduced Mg(II) efflux on resistance to Co(II) or Mn(II). (A) Magnesium homeostasis is achieved by both Mg(II) import and export systems. (B) The cobalt efflux-defective mutant (czcD null) is very sensitive to Co(II) intoxication. This sensitive phenotype is substantially rescued by increased cytosol Mg(II) resulting from mpfA null mutation. (C) The mntR null mutant is very sensitive to Mn(II) intoxication due to the constitutive expression of Mn(II) import and inactivation of Mn(II) efflux systems. This sensitive phenotype is significantly rescued by increased cytosol Mg(II) resulting from mpfA deletion.
Mg(II) is the most abundant divalent cation in the cell and is vitally important for a variety of cellular processes, including stabilization of ribosomes and membranes and neutralization of the charge of nucleic acids, and as a cofactor for diverse enzymes (51, 52). The relatively low affinity of Mg(II) for metalloenzymes correlates with its high abundance. Mg(II) binding to enzymes is rapid and reversible, which makes mismetallation by tighter-binding Co(II) or Mn(II) a potentially deleterious event. If enzymatic function is compromised by association with the wrong metal cofactor and the enzyme is involved in a crucial cellular process, mismetallation can be lethal.
Here, we have shown that Mg(II)-dependent processes are targets of toxicity by elevated levels of Co(II) and Mn(II). In several different selection conditions (Tables 1 and 3), we recovered insertions in mpfA (formerly yhdP), a putative Mg(II) efflux pump gene. An mpfA insertion was also recovered in a selection for Mn(II) resistance in an mneP mneS Mn(II) efflux-defective strain (53). Cells lacking MpfA have a 50% increase in intracellular Mg(II) (Table 2) and an increased sensitivity to Mg(II) (Fig. 3). Mutations affecting MgtE, the primary high-affinity transporter responsible for most Mg(II) import (41), were also recovered in two different selections for Mn(II) resistance (Tables 1 and 3). Furthermore, cells grown in medium containing supplemental Mg(II) exhibit an increased resistance to metal ion intoxication (see Fig. S2 in the supplemental material).
We also recovered insertions in yhdQ, encoding a MerR class transcription factor. The original recovery of these mTn insertions may have resulted, in part, from polarity on the downstream mpfA gene. However, analysis of an in-frame yhdQ deletion mutation reveals that YhdQ also has a modest effect on Mn(II) [but not Co(II)] sensitivity (Fig. 2), affects Mg(II) sensitivity (Fig. 3), and impacts the regulatory response of the yhdQ-mpfA operon to metals (Fig. 4). Since MerR homologs often function as transcription activators, we initially hypothesized that YhdQ might autoregulate its own operon. However, YhdQ is not required for Mg(II)-dependent induction of the yhdQ-mpfA operon (Fig. 4), and we suggest that it may activate expression of another system that plays a role in Mg(II) tolerance and thereby affects Mn(II) sensitivity. Possible candidates for such a function include the four paralogs of MpfA encoded in B. subtilis. Alternatively, YhdQ may regulate other genes that directly or indirectly impact Mg(II) tolerance. The regulatory mechanism that enhances transcription of the yhdQ-mpfA operon in response to elevated Mg(II) is not yet understood. However, the observation that this operon can also be induced by Co(II) in a yhdQ null strain (Fig. 4) suggests that perhaps YhdQ is an activator for a transporter that can export Co(II), and in the absence of this function, Co(II) can accumulate to levels that activate the yhdQ-mpfA operon.
The nature of the Mg(II)-dependent enzyme or process that is inhibited by excess Mn(II) is not known and will likely vary depending on the metabolic state of the cell. One candidate is ribosome assembly and function. Ribosomes are highly abundant in rapidly growing cells and are associated with large numbers of Mg(II) ions which likely play roles at all stages of assembly and function (52). It has been estimated that bacterial cells contain ∼100 mM total Mg(II), of which ∼12 mM may be ribosome-associated. The labile Mg(II) content is estimated to be in the range of 3 mM (52). Thus, ribosome assembly and function are Mg(II)-intensive processes. Indeed, cells grown under Mg(II) restriction may adapt, in part, by reducing their synthesis of ribosomes (54).
In B. subtilis, previous genetic screens have highlighted the importance of Mg(II) homeostasis for ribosome assembly and function. For example, cells lacking L34 are defective in 70S ribosome assembly, and this defect can be suppressed by either loss of MpfA or overexpression of MgtE (52, 55). Moreover, the total cytosolic Mg(II) content was found to correlate with the number of ribosomes. In follow-up work, it was shown that elevated Mg(II) also suppressed growth defects and/or ribosome assembly defects associated with loss of other ribosomal proteins, including L1, L23, L36, and S6 (56). Ribosome disassembly can be induced by high hydrostatic pressure, which thereby leads to transient growth arrest (57). By monitoring recovery from high hydrostatic pressure, it is possible to follow the synchronized reassociation of ribosome subunits to regenerate the 70S complex. This process requires Mg(II), as expected, and both Mn(II) and Zn(II) impeded recovery. These findings suggest that elevated levels of Mn(II) and other metals can impede the normally Mg(II)-dependent process of ribosome assembly (57). Further work will be required to ascertain whether the intoxication by high Mn(II) and Co(II) in our strains is due to defects in ribosome assembly or function or to interference with other Mg(II)-dependent processes.
Iron-dependent processes are also potential candidates for mismetallation during intoxication by Mn(II) or Co(II). Iron is essential for growth and viability in most bacteria yet poses distinct challenges owing to its capacity to readily participate in Fenton chemistry. This makes Fe(II)-dependent enzymes vulnerable to oxidative stress. Replacement of the iron with other transition metals can occur in response to oxidative damage, and this can block or alter the activity of the proteins (10). Here, we imposed iron-restriction by induction of the potent Fe(II) efflux pump, FrvA, as described previously (49, 58). Depletion of intracellular iron levels indeed increases the sensitivity of cells to elevated Co(II) or Mn(II), particularly in strains deficient in exporting either metal. This would suggest that iron-dependent processes are also potential targets of Co(II) and Mn(II) intoxication under our growth conditions. Furthermore, Co(II) and Mn(II) appear to target different iron-dependent enzymes since their sensitivities are additive. Mn(II) has been known to replace Fe(II) as a cofactor when the cell is under oxidative stress, due to its lower susceptibility to Fenton chemistry (59). This does not always result in a functional enzyme, and mismetallation of Fe(II) enzymes is one likely cause of Mn(II) intoxication.
In our mTn suppressor screen, only one metal-resistant isolate contained a transposon in a gene involved in iron homeostasis, the global regulator Fur (58, 60–62). This Co(II)-resistant mutant should leave Fe(II) import constitutively on, thereby increasing the intracellular iron pool and possibly contributing to the observed resistance. Alternatively, disruption of fur will also lead to derepression of the FsrA-mediated iron-sparing response which downregulates expression of a large number of Fe-containing enzymes (63, 64). Thus, another possibility is that downregulation of an Fe-dependent enzyme has removed a target of Co(II) mismetallation that negatively affects cell fitness. The mechanism of suppression due to the fur mutation, and also for several of the other mutations identified in these selections, will be subject to further studies.
In general, it is widely appreciated that metal intoxication can result from the mismetallation of critical enzymes (9, 10, 12). However, identification of the targets of mismetallation is challenging and likely varies depending on the specific growth conditions. In E. coli, under oxidative stress conditions, zinc-dependent mismetallation of iron-dependent biosynthetic enzymes can lead to amino acid auxotrophy (65). In B. subtilis, mismetallation of PerR by excess Zn(II) can lead, indirectly, to heme intoxication (19). Mismetallation of Fur by Mn(II) can impose iron starvation in perR mutant strains with elevated Fur protein levels (66, 67). Under the specific growth conditions tested here (rich medium), our genetic results indicate that Mg(II)-dependent processes are one primary target of Mn(II) and Co(II) intoxication.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains used in the study are derivatives of B. subtilis strain CU1065 (WT) and are listed in Table S2 in the supplemental material. Cells were grown in medium with vigorous shaking or on solid LB agar plates with appropriate antibiotic selection at 37°C. The concentrations of antibiotics used are ampicillin (amp), 100 μg ml−1; spectinomycin (spec), 100 μg ml−1; tetracycline (tet), 5 μg ml−1; chloramphenicol (cam), 10 μg ml−1; kanamycin (kan), 15 μg ml−1; neomycin (neo), 8 μg ml−1; and macrolide lincosoamide-streptogramin B (MLS), 1 μg ml−1 erythromycin and 25 μg ml−1 lincomycin.
Growth curves.
Cells were grown overnight in LB medium, subcultured at a 1:100 ratio into fresh LB medium, and grown to the early logarithmic phase (OD600, ∼0.2 to 0.3). Cell growth (OD600) was monitored every 15 min for 25 h using a Bioscreen growth analyzer (Growth Curves USA, Piscataway, NJ) at 37°C with continuous shaking. The data shown are representative growth curves and experiments that were conducted at least three times with three biological replicates each time.
Strain construction.
All strains used in this study were verified with PCR using the primers listed in Table S3. Complementation strains of mpfA and mgtE were constructed using Gibson assembly (68). Briefly, mpfA and mgtE were amplified using primers 8976/8977 and 8978/8979, respectively, and purified using an Omega Bio-Tek PCR cleanup kit. The vector template pSWEET was amplified using primers 8966/8967 and purified (69). The insert was cloned into the vector using Gibson assembly. The product was transformed in E. coli DH5α, purified, and transformed into B. subtilis for integration into amyE under selection. The MpfA overexpression construct was integrated at the amyE locus of an mpfA mutant, and the MgtE overexpression construct was integrated into a wild-type strain. The complementation strain of yhdQ was constructed by traditional cloning. yhdQ was amplified using primers 1190/1191 and digested along with the pSWEET vector using PacI and BamHI. The fragments were joined using T4 DNA ligase, and the ligation was transformed in DH5α, purified, and transformed into B. subtilis for integration into amyE under selection.
Transposon (mTn) mutagenesis.
Mariner transposon (mTn) mutagenesis was performed under the conditions stated in Tables 1 and 3 using the protocol described previously (70). Briefly, cells were transformed with the pMarA plasmid, plated on LB plus 1 μg/ml erythromycin, and incubated at 30°C for 48 h (71). The resulting transformants were stored at 80°C (host for transposition). Cells containing the pMarA plasmid were then grown overnight at 30°C in LB supplemented with kanamycin and erythromycin. A new culture (1:40) was started in LB plus kanamycin (to select for the transposition events) and incubated for 4 h at 30°C; the cells were then transferred at 37°C, incubated for 3 additional hours, and finally plated on LB amended with 15 μg/ml kanamycin and the indicated concentrations of IPTG and CoCl2 or MnCl2. Plates were incubated at 42°C for loss of the plasmid, and then candidate mutant colonies resistant to cobalt or manganese were saved for further studies. To determine the site of the mTn insertion, chromosomal DNA was isolated using the DNeasy kit (Qiagen) and digested with the TaqI restriction enzyme, and the products were ligated using the T4 ligase. The resulting DNA was used as the template for an inverse PCR. The PCR products were in-column cleaned and analyzed by sequencing. The sequencing information was then used to map the transposon insertion site.
RNA extraction and qPCR.
Cells were grown at 37°C in LB medium overnight and subcultured at a 1:100 ratio in fresh LB medium. After the OD600 reached ∼0.3 to 0.4, aliquots of 2 ml of cells were harvested at different time points as indicated. Total RNA was extracted using an RNeasy minikit following the manufacturer’s instructions (Qiagen Sciences, Germantown, MD), treated with Turbo-DNA Free DNase (Ambion), and precipitated with 2 to 3 volumes of ethanol and 0.1 volume of 3M sodium acetate at –80°C overnight. RNA samples were washed with 70% ethanol and dissolved in nuclease-free water and then quantified using a NanoDrop spectrophotometer. Next, 200 ng of total RNA from each sample was subjected to cDNA synthesis using high-capacity cDNA reverse transcription kits (Applied Biosystems, Foster City, CA). Quantitative PCR (qPCR) was then conducted using iQ SYBR green Supermix in an Applied Biosystems 7300 real-time PCR system. The housekeeping gene 23S rRNA was used as an internal control. Statistical analysis was carried out with the paired Student t test using at least three independent replicates.
Quantification of intracellular metal content using ICP-MS.
Cells were grown in LB medium overnight and subcultured at a 1:100 ratio in fresh LB medium and grown to an OD600 of ∼0.4. Aliquots of 4 ml of cell culture were harvested, and levels of intracellular metals (Mg, Fe, Mn, and Co) were monitored before and after addition of 10 mM MgCl2 using inductively coupled plasma mass spectrometry (ICP-MS). All samples were washed once with buffer 1 (1× PBS buffer, 0.1 M EDTA) and then twice with buffer 2 (1× Chelex-treated PBS buffer). Cell pellets were resuspended in 400 μl of buffer 3 (1× Chelex-treated PBS buffer, 75 mM NaN3, 1% Triton X-100) and incubated at 37°C for 90 min to lyse the cells. Lysed samples were spun down by centrifugation, and the total protein content was quantified using a Bradford assay. Then, samples were mixed with 600 μl buffer 4 (5% HNO3, 0.1% [vol/vol] Triton X-100) and heated in a 95°C sand bath for 30 min. The debris was removed by centrifugation, and the total metal ions in the diluted samples were analyzed with a Perkin-Elmer ELAN DRC II ICP-MS instrument. Gallium was used as an internal standard. The total intracellular ion levels are expressed as μg ion per gram of protein content (mean ± standard deviation [SD]; n = 3). Fe, Mn, and Co levels were not significantly changed over the course of the experiment.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by a grant from the National Institutes of Health (R35GM122461) to J.D.H. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Chandrangsu P, Rensing C, Helmann JD. 2017. Metal homeostasis and resistance in bacteria. Nat Rev Microbiol 15:338–350. doi: 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irving H, Williams RJP. 1948. Order of stability of metal complexes. Nature 162:746–747. doi: 10.1038/162746a0. [DOI] [Google Scholar]
- 3.Bowman JC, Lenz TK, Hud NV, Williams LD. 2012. Cations in charge: magnesium ions in RNA folding and catalysis. Curr Opin Struct Biol 22:262–272. doi: 10.1016/j.sbi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Krężel A, Maret W. 2016. The biological inorganic chemistry of zinc ions. Arch Biochem Biophys 611:3–19. doi: 10.1016/j.abb.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alatossava T, Jutte H, Kuhn A, Kellenberger E. 1985. Manipulation of intracellular magnesium content in polymyxin B nonapeptide-sensitized Escherichia coli by ionophore A23187. J Bacteriol 162:413–419. doi: 10.1128/JB.162.1.413-419.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finney LA, O’Halloran TV. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931–936. doi: 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- 7.Helmann JD. 2014. Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. J Biol Chem 289:28112–28120. doi: 10.1074/jbc.R114.587071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osman D, Martini MA, Foster AW, Chen J, Scott AJP, Morton RJ, Steed JW, Lurie-Luke E, Huggins TG, Lawrence AD, Deery E, Warren MJ, Chivers PT, Robinson NJ. 2019. Bacterial sensors define intracellular free energies for correct enzyme metalation. Nat Chem Biol 15:241–249. doi: 10.1038/s41589-018-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster AW, Osman D, Robinson NJ. 2014. Metal preferences and metallation. J Biol Chem 289:28095–28103. doi: 10.1074/jbc.R114.588145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imlay JA. 2014. The mismetallation of enzymes during oxidative stress. J Biol Chem 289:28121–28128. doi: 10.1074/jbc.R114.588814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotruvo JA Jr, Stubbe J. 2012. Metallation and mismetallation of iron and manganese proteins in vitro and in vivo: the class I ribonucleotide reductases as a case study. Metallomics 4:1020–1036. doi: 10.1039/c2mt20142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldron KJ, Robinson NJ. 2009. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol 7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 13.Moore CM, Gaballa A, Hui M, Ye RW, Helmann JD. 2005. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol Microbiol 57:27–40. doi: 10.1111/j.1365-2958.2005.04642.x. [DOI] [PubMed] [Google Scholar]
- 14.Gaballa A, Helmann JD. 1998. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J Bacteriol 180:5815–5821. doi: 10.1128/JB.180.22.5815-5821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Z, Gabriel SE, Helmann JD. 2011. Sequential binding and sensing of Zn(II) by Bacillus subtilis Zur. Nucleic Acids Res 39:9130–9138. doi: 10.1093/nar/gkr625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prestel E, Noirot P, Auger S. 2015. Genome-wide identification of Bacillus subtilis Zur-binding sites associated with a Zur box expands its known regulatory network. BMC Microbiol 15:13. doi: 10.1186/s12866-015-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin JH, Helmann JD. 2016. Molecular logic of the Zur-regulated zinc deprivation response in Bacillus subtilis. Nat Commun 7:12612. doi: 10.1038/ncomms12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvie DR, Andreini C, Cavallaro G, Meng W, Connolly BA, Yoshida K, Fujita Y, Harwood CR, Radford DS, Tottey S, Cavet JS, Robinson NJ. 2006. Predicting metals sensed by ArsR-SmtB repressors: allosteric interference by a non-effector metal. Mol Microbiol 59:1341–1356. doi: 10.1111/j.1365-2958.2006.05029.x. [DOI] [PubMed] [Google Scholar]
- 19.Chandrangsu P, Helmann JD. 2016. Intracellular Zn(II) intoxication leads to dysregulation of the PerR regulon resulting in heme toxicity in Bacillus subtilis. PLoS Genet 12:e1006515. doi: 10.1371/journal.pgen.1006515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skulachev VP, Chistyakov VV, Jasaitis AA, Smirnova EG. 1967. Inhibition of the respiratory chain by zinc ions. Biochem Biophys Res Commun 26:1–6. doi: 10.1016/0006-291x(67)90242-2. [DOI] [PubMed] [Google Scholar]
- 21.Lorusso M, Cocco T, Sardanelli AM, Minuto M, Bonomi F, Papa S. 1991. Interaction of Zn2+ with the bovine-heart mitochondrial bc1 complex. Eur J Biochem 197:555–561. doi: 10.1111/j.1432-1033.1991.tb15944.x. [DOI] [PubMed] [Google Scholar]
- 22.Aguirre JD, Culotta VC. 2012. Battles with iron: manganese in oxidative stress protection. J Biol Chem 287:13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torrents E. 2014. Ribonucleotide reductases: essential enzymes for bacterial life. Front Cell Infect Microbiol 4:52. doi: 10.3389/fcimb.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant AT, Spatafora GA. 2014. A role for the DtxR family of metalloregulators in Gram-positive pathogenesis. Mol Oral Microbiol 29:1–10. doi: 10.1111/omi.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Que Q, Helmann JD. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol 35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Shin JH, Pinochet-Barros A, Su TT, Helmann JD. 2017. Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Mol Microbiol 103:253–268. doi: 10.1111/mmi.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey R, Russo R, Ghanny S, Huang X, Helmann J, Rodriguez GM. 2015. MntR(Rv2788): a transcriptional regulator that controls manganese homeostasis in Mycobacterium tuberculosis. Mol Microbiol 98:1168–1183. doi: 10.1111/mmi.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters LS, Sandoval M, Storz G. 2011. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol 193:5887–5897. doi: 10.1128/JB.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohle TH, O’Brian MR. 2014. Magnesium-dependent processes are targets of bacterial manganese toxicity. Mol Microbiol 93:736–747. doi: 10.1111/mmi.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwell KJ, Tobin JM, Avery SV. 1998. Manganese toxicity towards Saccharomyces cerevisiae: dependence on intracellular and extracellular magnesium concentrations. Appl Microbiol Biotechnol 49:751–757. doi: 10.1007/s002530051242. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi M, Shimizu S. 1999. Cobalt proteins. Eur J Biochem 261:1–9. doi: 10.1046/j.1432-1327.1999.00186.x. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto S, Eltis LD. 2011. The biological occurrence and trafficking of cobalt. Metallomics 3:963–970. doi: 10.1039/c1mt00056j. [DOI] [PubMed] [Google Scholar]
- 33.Gaballa A, Helmann JD. 2003. Bacillus subtilis CPx-type ATPases: characterization of Cd, Zn, Co and Cu efflux systems. Biometals 16:497–505. doi: 10.1023/a:1023425321617. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Guffanti AA, Wei Y, Ito M, Krulwich TA. 2000. Two types of Bacillus subtilis tetA(L) deletion strains reveal the physiological importance of TetA(L) in K(+) acquisition as well as in Na(+), alkali, and tetracycline resistance. J Bacteriol 182:2088–2095. doi: 10.1128/jb.182.8.2088-2095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Z, Chandrangsu P, Helmann TC, Romsang A, Gaballa A, Helmann JD. 2014. Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis. Mol Microbiol 94:756–770. doi: 10.1111/mmi.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorgersen MP, Downs DM. 2007. Cobalt targets multiple metabolic processes in Salmonella enterica. J Bacteriol 189:7774–7781. doi: 10.1128/JB.00962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranquet C, Ollagnier-de-Choudens S, Loiseau L, Barras F, Fontecave M. 2007. Cobalt stress in Escherichia coli. The effect on the iron-sulfur proteins. J Biol Chem 282:30442–30451. doi: 10.1074/jbc.M702519200. [DOI] [PubMed] [Google Scholar]
- 38.Barras F, Fontecave M. 2011. Cobalt stress in Escherichia coli and Salmonella enterica: molecular bases for toxicity and resistance. Metallomics 3:1130–1134. doi: 10.1039/c1mt00099c. [DOI] [PubMed] [Google Scholar]
- 39.Majtan T, Frerman FE, Kraus JP. 2011. Effect of cobalt on Escherichia coli metabolism and metalloporphyrin formation. Biometals 24:335–347. doi: 10.1007/s10534-010-9400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armitano J, Redder P, Guimaraes VA, Linder P. 2016. An essential factor for high Mg(2+) tolerance of Staphylococcus aureus. Front Microbiol 7:1888. doi: 10.3389/fmicb.2016.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakeman CA, Goodson JR, Zacharia VM, Winkler WC. 2014. Assessment of the requirements for magnesium transporters in Bacillus subtilis. J Bacteriol 196:1206–1214. doi: 10.1128/JB.01238-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaballa A, Cao M, Helmann JD. 2003. Two MerR homologues that affect copper induction of the Bacillus subtilis copZA operon. Microbiology 149:3413–3421. doi: 10.1099/mic.0.26225-0. [DOI] [PubMed] [Google Scholar]
- 43.Smaldone GT, Helmann JD. 2007. CsoR regulates the copper efflux operon copZA in Bacillus subtilis. Microbiology 153:4123–4128. doi: 10.1099/mic.0.2007/011742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma Z, Cowart DM, Scott RA, Giedroc DP. 2009. Molecular insights into the metal selectivity of the copper(I)-sensing repressor CsoR from Bacillus subtilis. Biochemistry 48:3325–3334. doi: 10.1021/bi900115w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koo BM, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, Wapinski I, Galardini M, Cabal A, Peters JM, Hachmann AB, Rudner DZ, Allen KN, Typas A, Gross CA. 2017. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst 4:291–305e7. doi: 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christensen DG, Orr JS, Rao CV, Wolfe AJ. 2017. Increasing growth yield and decreasing acetylation in Escherichia coli by optimizing the carbon-to-magnesium ratio in peptide-based media. Appl Environ Microbiol 83:e03034-16. doi: 10.1128/AEM.03034-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan G, Pinochet-Barros A, Gaballa A, Patel SJ, Argüello JM, Helmann JD. 2015. PfeT, a P1B4-type ATPase, effluxes ferrous iron and protects Bacillus subtilis against iron intoxication. Mol Microbiol 98:787–803. doi: 10.1111/mmi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattori M, Iwase N, Furuya N, Tanaka Y, Tsukazaki T, Ishitani R, Maguire ME, Ito K, Maturana A, Nureki O. 2009. Mg(2+)-dependent gating of bacterial MgtE channel underlies Mg(2+) homeostasis. EMBO J 28:3602–3612. doi: 10.1038/emboj.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pi H, Patel SJ, Arguello JM, Helmann JD. 2016. The Listeria monocytogenes Fur-regulated virulence protein FrvA is an Fe(II) efflux P1B4-type ATPase. Mol Microbiol 100:1066–1079. doi: 10.1111/mmi.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pi H, Helmann JD. 2017. Ferrous iron efflux systems in bacteria. Metallomics 9:840–851. doi: 10.1039/c7mt00112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romani AM, Scarpa A. 2000. Regulation of cellular magnesium. Front Biosci 5:D720–734. doi: 10.2741/Romani. [DOI] [PubMed] [Google Scholar]
- 52.Nierhaus KH. 2014. Mg2+, K+, and the ribosome. J Bacteriol 196:3817–3819. doi: 10.1128/JB.02297-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paruthiyil S, Pinochet-Barros A, Huang X, Helmann JD. 2019. Bacillus subtilis TerC family proteins help prevent manganese intoxication. J Bacteriol 202:e00624-19. doi: 10.1128/JB.00624-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pontes MH, Yeom J, Groisman EA. 2016. Reducing ribosome biosynthesis promotes translation during low Mg(2+) stress. Mol Cell 64:480–492. doi: 10.1016/j.molcel.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akanuma G, Kobayashi A, Suzuki S, Kawamura F, Shiwa Y, Watanabe S, Yoshikawa H, Hanai R, Ishizuka M. 2014. Defect in the formation of 70S ribosomes caused by lack of ribosomal protein L34 can be suppressed by magnesium. J Bacteriol 196:3820–3830. doi: 10.1128/JB.01896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akanuma G, Yamazaki K, Yagishi Y, Iizuka Y, Ishizuka M, Kawamura F, Kato-Yamada Y. 2018. Magnesium suppresses defects in the formation of 70S ribosomes as well as in sporulation caused by lack of several individual ribosomal proteins. J Bacteriol 200:e00212-18. doi: 10.1128/JB.00212-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen HTM, Akanuma G, Tu HTM, Nakai Y, Kimura K, Yamamoto K, Inaoka T. 2019. Ribosome reconstruction during recovery from high hydrostatic pressure-induced injury in Bacillus subtilis. Appl Environ Microbiol 86:e01640-19. doi: 10.1128/AEM.01640-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pi H, Helmann JD. 2017. Sequential induction of Fur-regulated genes in response to iron limitation in Bacillus subtilis. Proc Natl Acad Sci U S A 114:12785–12790. doi: 10.1073/pnas.1713008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Privalle CT, Fridovich I. 1992. Transcriptional and maturational effects of manganese and iron on the biosynthesis of manganese-superoxide dismutase in Escherichia coli. J Biol Chem 267:9140–9145. [PubMed] [Google Scholar]
- 60.Baichoo N, Wang T, Ye R, Helmann JD. 2002. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol Microbiol 45:1613–1629. doi: 10.1046/j.1365-2958.2002.03113.x. [DOI] [PubMed] [Google Scholar]
- 61.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann JD. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol 29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 62.Ollinger J, Song KB, Antelmann H, Hecker M, Helmann JD. 2006. Role of the Fur regulon in iron transport in Bacillus subtilis. J Bacteriol 188:3664–3673. doi: 10.1128/JB.188.10.3664-3673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaballa A, Antelmann H, Aguilar C, Khakh SK, Song KB, Smaldone GT, Helmann JD. 2008. The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proc Natl Acad Sci U S A 105:11927–11932. doi: 10.1073/pnas.0711752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smaldone GT, Revelles O, Gaballa A, Sauer U, Antelmann H, Helmann JD. 2012. A global investigation of the Bacillus subtilis iron-sparing response identifies major changes in metabolism. J Bacteriol 194:2594–2605. doi: 10.1128/JB.05990-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gu M, Imlay JA. 2013. Superoxide poisons mononuclear iron enzymes by causing mismetallation. Mol Microbiol 89:123–134. doi: 10.1111/mmi.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faulkner MJ, Ma Z, Fuangthong M, Helmann JD. 2012. Derepression of the Bacillus subtilis PerR peroxide stress response leads to iron deficiency. J Bacteriol 194:1226–1235. doi: 10.1128/JB.06566-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Z, Faulkner MJ, Helmann JD. 2012. Origins of specificity and cross-talk in metal ion sensing by Bacillus subtilis Fur. Mol Microbiol 86:1144–1155. doi: 10.1111/mmi.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 69.Bhavsar AP, Zhao X, Brown ED. 2001. Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant. Appl Environ Microbiol 67:403–410. doi: 10.1128/AEM.67.1.403-410.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rojas-Tapias DF, Helmann JD. 2019. Identification of novel Spx regulatory pathways in Bacillus subtilis uncovers a close relationship between the CtsR and Spx regulons. J Bacteriol 201:e00151-19. doi: 10.1128/JB.00151-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Breton Y, Mohapatra NP, Haldenwang WG. 2006. In vivo random mutagenesis of Bacillus subtilis by use of TnYLB-1, a mariner-based transposon. Appl Environ Microbiol 72:327–333. doi: 10.1128/AEM.72.1.327-333.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.