Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic has become a major challenge to public health in China and other countries, considering its pathogenicity across all age groups. Pregnancy is a unique physiological condition, and is characterized by altered immunity and elevated hormone levels to actively tolerate the semi-allogeneic fetus, which undergoes a sudden and substantial fluctuation during the immediate postpartum period. Changes in clinical features, laboratory characteristics, and imaging features of pregnant women during the pre-partum and post-partum periods require further elucidation. Here, we retrospectively analyzed the clinical features, laboratory characteristics, and imaging features of eight pregnant cases of SARS-CoV-2 infection during the pre-partum and post-partum periods. Our results showed that four of the eight pregnant women were asymptomatic before delivery but became symptomatic post-partum. Correspondingly, white blood cell (WBC) counts increased and lymphocyte (LYMPH) counts decreased. C-reactive protein (CRP) levels in the serum also increased to a higher level than those in general pregnancy. Therefore, it is imperative to closely monitor laboratory parameters including the WBC count, LYMPH count, and CRP, along with other imaging features in chest CT scans, to promptly prevent, diagnose, and treat a SARS-CoV-2 infection during pregnancy.
Electronic supplementary material
The online version of this article (10.1007/s12250-020-00227-0) contains supplementary material, which is available to authorized users.
Keywords: SARS-CoV-2, Pregnant women, Clinical manifestation, Laboratory characteristics, Immunity
Introduction
Since the first report of pneumonia associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, Hubei Province, China in December 2019, the SARS-CoV-2 epidemic has become a major public health challenge in China and other countries (Huang et al. 2020; Hui et al. 2020; Lu et al. 2020).
People of all ages are susceptible to SARS-CoV-2; however, current data have confirmed that adults, specifically older men with comorbidities, are more likely to be affected, suffering subsequently from severe pneumonia, pulmonary edema, acute respiratory distress syndrome, multiple organ failure, and death (Chen N et al. 2020; Huang et al. 2020; Wang et al. 2020). Pregnancy is a special physiological condition with unique characteristics, including altered immunity and hormone levels which is required to tolerate and support the development and survival of the placenta and fetus in the hostile maternal immune system environment (Robinson and Klein 2012). Compared with non-pregnant women, pregnant women are more severely affected by pathogenic infections, particularly those caused by respiratory pathogens, including the influenza and SARS viruses (Kourtis et al. 2014; Sappenfield et al. 2013; Wong et al. 2004). A study had previously reported SARS-CoV-2 infection in nine pregnant women (Chen H et al. 2020). However, the study primarily focused on the clinical characteristics and vertical transmission potential of SARS-CoV-2 infection in pregnant women. The changes in clinical features, laboratory characteristics, and imaging features of the pregnant women both before and after the delivery were not explored.
We retrospectively collected and analyzed detailed clinical data from eight pregnant patients, including six laboratory-confirmed SARS-CoV-2 infections and two highly suspected SARS-CoV-2 infection cases at the Maternal and Child Health Hospital of Hubei Province, Wuhan, China. The differences in clinical features, laboratory characteristics, and imaging features of pregnant women at pre-partum and post-partum were determined by examining the clinical features, laboratory characteristics, and imaging features of these patients.
Methods
Study Design and Patients
We conducted a retrospective review of medical records of eight pregnant patients, including six laboratory-confirmed SARS-CoV-2 infections and two highly suspected SARS-CoV-2 infection cases, and five pregnant cases excluding SARS-CoV-2 infection admitted to the Maternal and Child Health Hospital of Hubei Province, Wuhan, China, from January 23 to February 10, 2020. The diagnosis was based on the World Health Organization interim guidance, and the New Coronavirus Pneumonia Prevention and Control Program published by the National Health Commission of China (National Health Commission of the PRC 2020; WHO 2020).
Data Collection
The clinical records, laboratory findings, chest CT scans, and treatment and outcome data for all patients were reviewed and collected with data collection forms. Two study investigators independently reviewed the data collection forms to verify the data accuracy. The date of disease onset was defined as the day when the symptoms were identified.
Throat swab samples were collected and dispatched to the Hubei Provincial Center for Disease Control and Prevention for testing for SARS-CoV-2. A positive test result indicated a confirmed diagnosis of SARS-CoV-2 infection.
Results
The eight pregnant women were in their third trimester, six underwent cesarean section, and two underwent eutocia (Table 1). Their ages ranged from 26 to 35 years, and the range of gestational weeks at admission was from 33 weeks (+ 6 days) to 40 weeks (+ 4 days).
Table 1.
Baseline characteristics of 8 pregnant women infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 28 | 31 | 30 | 30 | 30 | 26 | 29 | 35 |
| Gestational age on admission | 39 weeks | 38 weeks, 1 day | 39 weeks, 1 day | 36 weeks, 4 days | 37 weeks, 6 days | 40 weeks, 3 days | 40 weeks, 4 days | 33 weeks, 6 days |
| Onset to delivery (days) | Post-partum 1 | Post-partum 1 | No | No | No | No | Post-partum 2 | Pre-partum 1 |
| Fever on admission | No | No | No | No | No | No | No | Yes |
| Post-partum fever | Yes | Yes | No | No | No | No | Yes | No |
| Cough | No | No | No | No | No | No | No | No |
| Other symptoms | No | No | No | No | No | No | No | No |
| CT evidence of pneumonia | ||||||||
| Pre-partum typical signs of viral infection | No | No | No | Yes | Yes | Yes | No | Yes |
| Post-partum typical signs of viral infection | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| SARS-CoV-2 RNA | Yes | Yes | Yes | Yes | Yes | Yes | NA | NA |
| Delivery | ||||||||
| Method of Delivery | C-section | C-section | C-section | C-section | Vaginal delivery | C-section | Vaginal delivery | C-section |
| Indication for C-section | History of C-section | PROM | Pre-eclampsia | PROM | Fetal distress | History of C-section | ||
| Treatment | ||||||||
| Intensive unit care | No | No | No | No | No | No | No | No |
| Mechanical ventilation | No | No | No | No | No | No | No | No |
NA, not available; C-section; cesarean section; PROM, premature rupture of membrane.
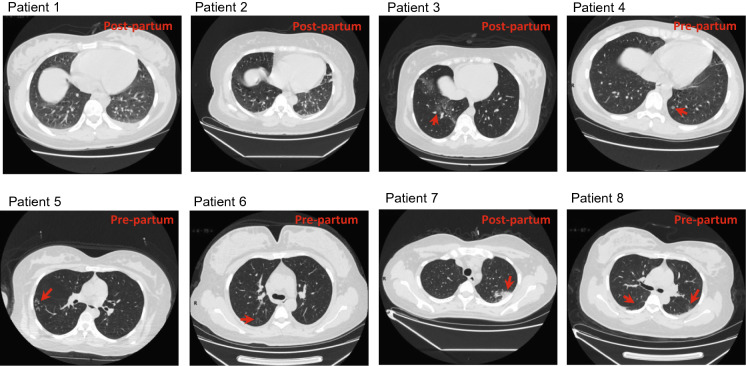
Seven of the eight patients had no fever, cough, or other symptoms before delivery. Three of the seven patients developed fever at the 1 or 2 days postpartum, and one demonstrated the typical ground-glass opacities in the lungs on chest CT scans as described previously (Huang et al. 2020) (Table 1; Fig. 1). The remaining four patients did not present with symptoms after delivery. Among which, three had typical ground-glass opacities in the lungs in chest CT scans before delivery and one developed ground-glass opacities in chest CT scans after delivery (Table 1; Fig. 1). One of the eight patients had fever and typical ground-glass opacities on admission at 1-day pre-partum. None of the patients had a high fever (body temperature > 39 °C) or developed a severe pneumonia, requiring intensive unit care or mechanical ventilation (Table 1).
Fig. 1.
Chest CT scans (transverse plane) of eight pregnant women with confirmed SARS-CoV-2 infection. Patients 1 and 2: no visible ground-glass opacities or small plaque; Patient 3–8: ground-glass opacities or/and small plaque are indicated by red arrow.
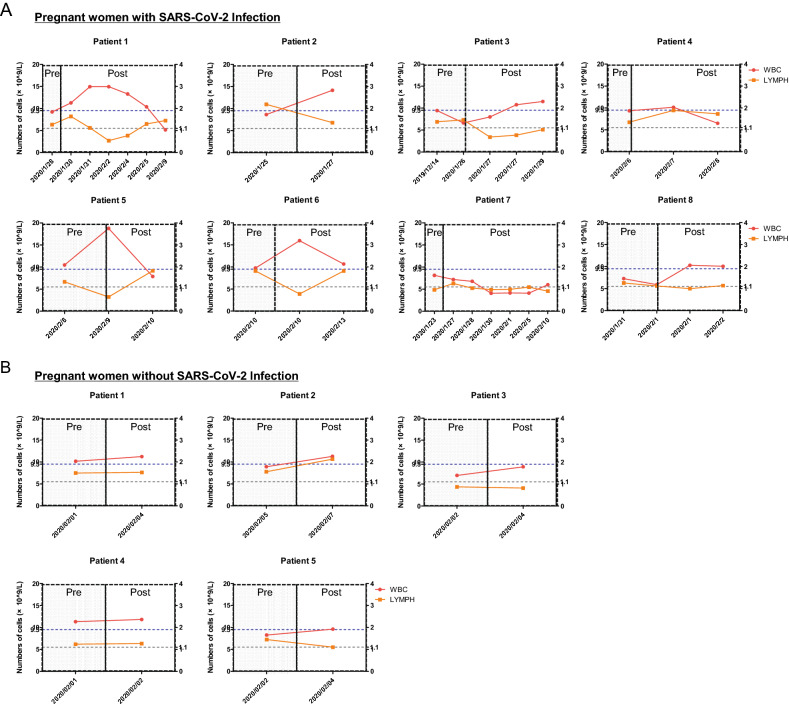
Laboratory test outcomes suggested that the white blood cell (WBC) counts of all eight patients were normal (patients 1–4 and 6–8) or slightly higher than the upper normal limit (patient 5) before delivery (Fig. 2A). However, the WBC counts of six patients drastically increased (patients 1–3, 5–6, and 8) after delivery, specifically for patients 1–2 and 5–6 (Fig. 2A). In contrast, a slight increase in WBC counts was observed in pregnant patients, excluding those with SARS-CoV-2 infection after delivery (Fig. 2B). The lymphocyte (LYMPH) counts were normal (patients 1–6 and 8) or slightly lower than the normal lower limit (patient 7) before delivery (Fig. 2A). However, the LYMPH counts of five patients drastically decreased (patients 1–3, 5–6) lower than the normal lower limit after delivery (Fig. 2A). In contrast, there were no obvious changes in the LYMPH counts of the pregnant patients without the SARS-CoV-2 infection between pre-partum and post-partum (Fig. 2B).
Fig. 2.
Dynamic profile of white blood cells and lymphocyte count in pregnant women with or without severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Considering pregnant women with SARS-CoV-2 infection, patients 1–6 are pregnant patients with laboratory-confirmed SARS-CoV-2 infection, and patients 7–8 are pregnant patients with highly suspected SARS-CoV-2 infection. The left y-axis shows the number of white blood cells (WBC). The right y-axis shows the number of lymphocytes (LYMPH). The dotted lines in blue show the upper normal limit of the WBC count, and the dotted line in grey shows the lower normal limit of LYMPH count. Pre, pre-partum; Post, post-partum.
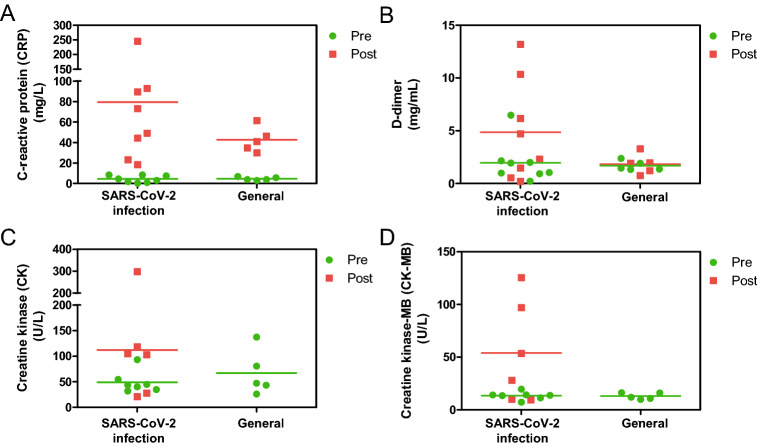
Furthermore, we closely monitored the C-reactive protein (CRP) levels, since it is an important marker and gauge of inflammation in the body (Wu et al. 2015). Its levels were normal and comparable between pregnant women with SARS-CoV-2 infection and pregnant women without the SARS-CoV-2 infection before delivery (Fig. 3A). Patient CRP levels drastically increased postpartum. However, the mean serum CRP level was higher in patients with SARS-CoV-2 infection than in those without (Fig. 3A and Supplementary Table S1). D-dimer is one of the most important procoagulant markers, which may be elevated during viral infections (Subramaniam and Scharrer 2018). The plasma D-dimer levels during the prepartum period were normal and comparable between pregnant women with SARS-CoV-2 infection and those without (Fig. 3B). However, D-dimer levels of four patients with SARS-CoV-2 infection increased significantly postpartum, but did not change in the five patients without SARS-CoV-2 infection (Fig. 3B and Supplementary Table S1). Both creatine kinase (CK) and creatine kinase-MB (CK-MB) levels increased in four pregnant women with SARS-CoV-2 infection after delivery (Fig. 3C, 3D and Supplementary Table S1). In terms of other laboratory tests, all eight patients with confirmed or highly suspected SARS-CoV-2 infection had normal levels of liver enzymes.
Fig. 3.
The levels of inflammatory factors and kinase in pregnant women at pre-partum and post-partum. The pregnant women with SARS-CoV-2 infection included six pregnant patients with laboratory-confirmed SARS-CoV-2 infection and two pregnant patients with highly suspected SARS-CoV-2 infection. The participants without the SARS-CoV-2 infection. The levels of C-reactive protein (CRP), D-dimer, creatine kinase (CK), and creatine kinase-MB (CK-MB) in the serum or plasma were detected.
All patients received empirical antibiotic treatment and supportive care. None of the eight patients required intensive care or mechanical ventilation.
Discussion
This study reported new SARS-CoV-2 infected pregnant patients and revealed distant clinical presentations during the pre- and post-partum periods. Comprehensive data from clinical manifestations, laboratory examinations, and chest imaging were subsequently analyzed.
During pregnancy, several mechanical and pathophysiological adaptive changes occur in the respiratory system, including a decrease in respiratory volumes, increased oxygen consumption, and edema of the respiratory tract mucosa, which can render them intolerant to hypoxia. Moreover, immune adaptations are required to accommodate the fetus (Kourtis et al. 2014). Therefore, pregnant women are particularly susceptible to respiratory pathogens and severe pneumonia. Previous studies have reported relatively more severe cases with higher mortality rates among pregnant women upon contracting respiratory viruses such as influenza virus and SARS virus (Gottfredsson 2008; Jamieson et al. 2009; Wong et al. 2004). Pregnant SARS-CoV-2 patients reportedly presented with a similar pattern of clinical characteristics to that of non-pregnant adult patients (Chen H et al. 2020; Huang et al. 2020). An investigation in 138 hospitalized patients with COVID showed that 98.6% patients had clinical manifestations of fever, 69.6% patients had fatigue, and 59.4% patients had dry cough. In addition, 70.3% patients had lymphopenia and 39.9% had elevated lactate dehydrogenase. Chest computed tomographic (CT) scans showed that all patients presented bilateral patchy shadows or ground glass opacity in the lungs of all patients (Wang et al. 2020). Another descriptive study in 99 cases with COVID reported that 83% patients had fever, 82% patients had cough, and 31% patients had shortness of breath. Other clinical manifestations include muscle ache, confusion, headache, sore throat and so on. The imaging examination showed that 75% patients presented bilateral pneumonia, 14% patients showed multiple mottling and ground-glass opacity (Chen N et al. 2020). Here, four patients had no symptoms during the observation period; additionally, the only symptom observed in the remaining patients was fever. The liver enzyme levels in all eight patients were also normal. As far as the imaging features were concerned, the typical signs of viral infection-ground-glass opacity in the lungs were also mild. Therefore, according to our study, SARS-CoV-2 infected pregnant patients appeared to present with relatively milder clinical symptoms than non-pregnant adult patients.
Our study revealed that four of the eight pregnant women were asymptomatic before delivery but became symptomatic after delivery. Correspondingly, WBC counts increased while LYMPH counts decreased in most pregnant patients with SARS-CoV-2 infection. Furthermore, CRP levels increased considerably higher than those observed during general pregnancy. During pregnancy, the concentrations of steroid hormones such as estrogens and progesterone progressively increase over the course of pregnancy (Vojtek et al. 2018). In addition, to ensure that the mother’s body can actively tolerate the semi-allogeneic fetus, the immune system is modulated (Vojtek et al. 2018). However, there is a sudden and substantial decrease in steroid hormone concentrations during the immediate postpartum period (Dennis et al. 2008). In comparison to the third trimester of pregnancy, there is a significant change in immune cells including the blood phagocytes, pDCs, NK cells, and T cells during the post-partum period (Kraus et al. 2012). It is possible that these alterations in both the hormones and immune system trigger the onset of SARS-CoV-2 infection following partum. Further investigations are needed to elucidate the immunological differences between pre- and post-partum in the context of combating a SARS-CoV-2 infection.
Summarily, our results suggest that compared to non-pregnant adult patients, pneumonia and other symptoms were mild in pregnant patients with SARS-CoV-2 infection. A number of pregnant women presented with asymptomatic SARS-CoV-2 infection pre-partum, but the disease onset may occur post-partum. Close monitoring of laboratory parameters such as WBC count, LYMPH count, and CRP as well as the imaging features in chest CT scans, may be helpful for the early prevention, diagnosis, and treatment of SARS-CoV-2 infection during pregnancy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Bo Nie, Zuliang Shi and Dan Lu in Department of Laboratory Medicine, Maternal and Child Health Hospital of Hubei Province for kindly helping us to collect all the data. We thank Dr. Quan Gan in Department of Intensive Care Unit, Maternal and Child Health Hospital of Hubei Province for kindly helping us to discuss clinical data.
Author Contributions
CW, WR and JX conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. WY, XW, TZ and YZ collected data and carried out the initial analyses.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This study was approved by the Medical Ethical Committee of the Maternal and Child Health Hospital of Hubei Province. Written informed consent was obtained from the spouses of each enrolled patient.
Contributor Information
Wei Ren, Email: ren33107@163.com.
Jianbo Xia, Email: xjb915@126.com.
References
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL, Ross LE, Herxheimer A. Oestrogens and progestins for preventing and treating postpartum depression. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD001690.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfredsson M. The Spanish flu in Iceland 1918. Lessons in medicine and history. Laeknabladid. 2008;94:737–745. [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ, Novel Influenza A (H1N1) Pregnancy Working Group H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus TA, Engel SM, Sperling RS, Kellerman L, Lo Y, Wallenstein S, Escribese MM, Garrido JL, Singh T, Loubeau M, Moran TM. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol. 2012;32:300–311. doi: 10.1007/s10875-011-9627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commission of the PRC (2020) New coronavirus pneumonia prevention and control program (trial version 7). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed 4 Mar 2020
- Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol. 2013;2013:752852. doi: 10.1155/2013/752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Scharrer I. Procoagulant activity during viral infections. Front Biosci (Landmark Ed) 2018;23:1060–1081. doi: 10.2741/4633. [DOI] [PubMed] [Google Scholar]
- Vojtek I, Dieussaert I, Doherty TM, Franck V, Hanssens L, Miller J, Bekkat-Berkani R, Kandeil W, Prado-Cohrs D, Vyse A. Maternal immunization: where are we now and how to move forward? Ann Med. 2018;50:193–208. doi: 10.1080/07853890.2017.1421320. [DOI] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, Ng PC, Lam PW, Ho LC, To WW, Lai ST, Yan WW, Tan PY. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Healthy Organization (WHO) (2020) Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 13 Mar 2020
- Wu Y, Potempa LA, El Kebir D, Filep JG. C-reactive protein and inflammation: conformational changes affect function. Biol Chem. 2015;396:1181–1197. doi: 10.1515/hsz-2015-0149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.