The coronavirus disease COVID19 is a global health pandemic emergency. The disease, named coronavirus disease 2019 (COVID-19) by the WHO, presents symptoms that range from mild to severe and fatal respiratory conditions. As the epidemy emerged and spread rapidly worldwide, more than 400,000 confirmed cases of COVID-19 and around 20,000 related deaths have been reported globally [1]. Clinical presentation might range from mild to severe lower respiratory tract symptoms, resulting in life-threatening complication such as acute respiratory distress syndrome (ARDS). Up to now, two serious coronavirus-related outbreaks occurred in the past two decades: severe acute respiratory syndrome (SARS) in 2003 and Middle East respiratory syndrome (MERS) in 2012 [2]. Since SARS-CoV-2 has shown analogous mechanisms to SARS, existing antiviral drugs used in the past epidemies - such as ribavirin, interferon, lopinavir-ritonavir - were primarily proposed. Drugs repositioning lists several benefits: the drugs are already available on the market and therefore promptly accessible and their safety profile and dosage are already known. Based on promising preliminary data against a wide range of viruses, chloroquine sulfate (CQ) – a drug used for a long time for the prophylaxis of malaria - was recommended at the dosage of 500 mg BID for the treatment of SARS-CoV-2. The other choice was hydroxychloroquine (HCQ) sulfate. First synthesized in 1946 as the hydroxyl derivative of CQ, HCQ has shown a less toxic profile than CQ in animal models and, more crucially, it is available worldwide since it has been used to treat autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus [3], [4], [5]. Since the two molecules share similar structures and immunomodulating functions, HCQ was also recommended and included in the therapeutic armamentarium to treat the infection of SARS-CoV-2, at the dosage of 200 mg BID [6]. Thus, the protease inhibitor combination lopinavir/ritonavir – already approved for the treatment of the human immunodeficiency virus (HIV) type 1 – in association with either CQ or HCQ represents the standard of care for COVID-19 symptomatic patients so far [6,7].
Caution in prescribing CQ/HCQ treatment in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency is generally observed due to the pro-hemolytic effect of these molecules [3], [4], [5]. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common worldwide distributed hereditary red cells enzymatic defect, with a prevalence of 400 million affected subjects [8,9]. Although G6PD deficiency is a benign haematologic disorder, acute haemolytic crisis triggered by exposition to oxidative agents such as fava beans, drugs or infections might be its most common life-threatening clinical presentation.
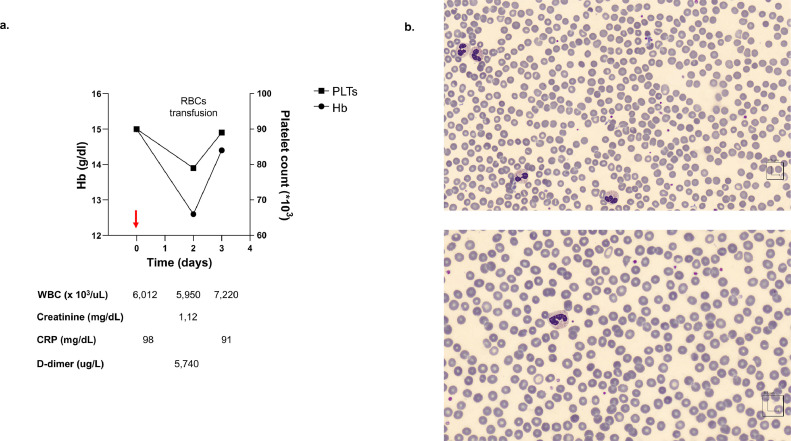
A 72-year-old caucasian man was admitted to ED for fatigue, dyspnea, dizziness and fever. No significant elements were present in patient's history, in particular no neurologic disorders, haemolytic anemia nor recent introduction of new drugs. He was suffering of ischemic cardiomyopathy with recent stenting of the interventricular coronary artery. Physical examination revealed significant reduction of peripheral saturation in ambient air (87%), and the chest X-Ray documented the presence of bilateral interstitial pneumonia. Laboratory tests showed normal hemoglobin (Hb: 15 g/dL) and mild reduction of platelet count as observed in COVD19 affected patients [10]. The presence of COVID19 infection was documented by RT-PCR analysis of the nose-throat swab. The patient was hospitalized in the COVID19 Unit and treatment with lopinavir plus HCQ and oxygen support was stared. At 48 hours after hospitalization, we observed an acute drop in Hb (Figure 1 a) associated with the appearance of hemoglobinuria. No change in leukocyte count was observed, while reactive protein C was increased. Renal tests were normal. LDH levels were undetermined due to the intravascular haemolysis (Fig. 1A). Both direct and indirect antiglobulin tests were negative, excluding an immune-mediated acute haemolysis. The peripheral blood smears showed (i) anisopoikilocytosis; reticulocytes as large and round-shaped cells; (ii) some “hemi-ghost” cells characterized by packed hemoglobin from one side and large vacuum cytoplasm; (iii) microspherocytes; (iv) schistocytes (2-3%), suggesting a blistering process (Figure 1b). HCQ was withdrawn and the patient was transfused. The trigger of acute haemolytic crisis was HCQ in a patient with suspected G6PD deficiency affected by COVID19 lung disease.
Figure 1.
(a) The graph shows lab exams. RBCs: red blood cells. (b) Upper and lower panels. Peripheral blood smears. Acute haemolysis induced by hydroxychloroquine is characterized by marked anisopoikilocytosis with erythrocytes of different shapes and sizes, hemi-ghost blister cells and microspherocytes.
Our patient might be affected by Mediterannean variant of G6PD deficiency, which is more sensitive to pro-oxidant drugs compared to African G6PD A variant. No conclusive data are available on the possible pro-hemolytic impact of CQ/HCQ on patient with G6PD deficiency [3], [4], [5]. In COVID-19 emergency we believe it is important to warning the possible hemolytic effects of CQ/HCQ in patients with G6PD deficiency. Thus, the acute drop in Hb levels in the early days of CQ/HCQ treatment of COVID19 symptomatic patient should be considered suspicious for possible G6PD deficiency. CQ/HCQ should be discontinued and the hemolysis is generally self-limiting when the CQ/HCQ is withdrawn.
1. Disclosures
The authors have nothing to disclose
Declaration of Compeying Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
No funding for this study
Acknowledgments
We would like to thank prof MD Cappellini and prof L Luzzatto for the fruitful discussion.
References
- 1.Wu P., Hao X., Lau E.H.Y., Wong J.Y., Leung K.S.M., Wu J.T., Cowling B.J., Leung G.M. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cauchemez S., Fraser C., Van Kerkhove M.D., Donnelly C.A., Riley S., Rambaut A., Enouf V., van der Werf S., Ferguson N.M. Middle East respiratory syndrome coronavirus: quantification of the extent of the epidemic, surveillance biases, and transmissibility. Lancet Infect Dis. 2014;14::50–56. doi: 10.1016/S1473-3099(13)70304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammad S., Clowse M.E.B., Eudy A.M., Criscione-Schreiber L.G. Examination of Hydroxychloroquine Use and Hemolytic Anemia in G6PDH-Deficient Patients. Arthritis Care Res (Hoboken) 2018;70::481–485. doi: 10.1002/acr.23296. [DOI] [PubMed] [Google Scholar]
- 4.Youngster I., Arcavi L., Schechmaster R., Akayzen Y., Popliski H., Shimonov J., Beig S., Berkovitch M. Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review. Drug Saf. 2010;33::713–726. doi: 10.2165/11536520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Ashley E.A., Recht J., White N.J. Primaquine: the risks and the benefits. Malar J. 2014;13::418. doi: 10.1186/1475-2875-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6::16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappellini M.D., Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371::64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 9.Luzzatto L., Arese P. Favism and Glucose-6-Phosphate Dehydrogenase Deficiency. N Engl J Med. 2018;378::60–71. doi: 10.1056/nejmra1708111. [DOI] [PubMed] [Google Scholar]
- 10.Qu R., Ling Y., Zhang Y.H., Wei L.Y., Chen X., Li X., Liu X.Y., Liu H.M., Guo Z., Ren H., Wang Q. Platelet-to-lymphocyte ratio is associated with prognosis in patients with Corona Virus Disease-19. J Med Virol. 2020 doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]