Abstract
Angiotensin converting enzyme-2 (ACE2) receptors mediate the entry into the cell of three strains of coronavirus: SARS-CoV, NL63 and SARS-CoV-2. ACE2 receptors are ubiquitous and widely expressed in the heart, vessels, gut, lung (particularly in type 2 pneumocytes and macrophages), kidney, testis and brain. ACE2 is mostly bound to cell membranes and only scarcely present in the circulation in a soluble form. An important salutary function of membrane-bound and soluble ACE2 is the degradation of angiotensin II to angiotensin1-7. Consequently, ACE2 receptors limit several detrimental effects resulting from binding of angiotensin II to AT1 receptors, which include vasoconstriction, enhanced inflammation and thrombosis. The increased generation of angiotensin1-7 also triggers counter-regulatory protective effects through binding to G-protein coupled Mas receptors. Unfortunately, the entry of SARS-CoV2 into the cells through membrane fusion markedly down-regulates ACE2 receptors, with loss of the catalytic effect of these receptors at the external site of the membrane. Increased pulmonary inflammation and coagulation have been reported as unwanted effects of enhanced and unopposed angiotensin II effects via the ACE→Angiotensin II→AT1 receptor axis. Clinical reports of patients infected with SARS-CoV-2 show that several features associated with infection and severity of the disease (i.e., older age, hypertension, diabetes, cardiovascular disease) share a variable degree of ACE2 deficiency. We suggest that ACE2 down-regulation induced by viral invasion may be especially detrimental in people with baseline ACE2 deficiency associated with the above conditions. The additional ACE2 deficiency after viral invasion might amplify the dysregulation between the ‘adverse’ ACE→Angiotensin II→AT1 receptor axis and the ‘protective’ ACE2→Angiotensin1-7→Mas receptor axis. In the lungs, such dysregulation would favor the progression of inflammatory and thrombotic processes triggered by local angiotensin II hyperactivity unopposed by angiotensin1-7. In this setting, recombinant ACE2, angiotensin1-7 and angiotensin II type 1 receptor blockers could be promising therapeutic approaches in patients with SARS-CoV-2 infection.
Keywords: Coronavirus, SARS-CoV-2, ACE, ACE2, ACE-inhibitors, Angiotensin receptor blockers, Hypertension, Diabetes, Elderly, Heart failure
Abbreviations: ADAM17, disintegrin and metalloproteinase 17; ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; COVID-19, 2019 novel coronavirus disease; DABK, des-Arg9 bradykinin; IL, interleukin; NL63, human coronavirus NL63; RAAS, renin-angiotensin-aldosterone system; SARS, severe acute respiratory syndrome; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome novel coronavirus; TMPRSS2, transmembrane protease serine 2
1. Introduction
According to the Center for Systems Science and Engineering at The Johns Hopkins University, as of April 12, 2020, the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic caused 108,867 deaths worldwide, with a total of 1,777,666 infected people (https://coronavirus.jhu.edu/map.html). Just to make a comparison, the SARS-Cov virus in years 2002-2003 infested about 8,500 people in 27 countries and caused 866 deaths. [1]
The tremendous impact of SARS-Cov-2 infection and the paucity or lack of established therapeutic measures is generating basic and clinical studies to explore the mechanisms of viral entry into the human body and the subsequent pathophysiological and therapeutic implications.
The present review discusses the role of angiotensin converting enzyme 2 (ACE2) receptors which are not only the door through which the virus enters into cells, [2,3] but also the conductor of several pathophysiological reactions associated with the clinical features of the disease, with potential therapeutic implications.
2. Entry of SARS-CoV-2 into cells
The entry of SARS-CoV-2 into cells is mediated by the efficient binding of the spike (S) viral protein, a 1273 amino acid long protein which belongs to the viral envelope and protrudes outwards with a ‘corona’ like appearance, to the angiotensin converting enzyme 2 (ACE2) receptors. [2,3]
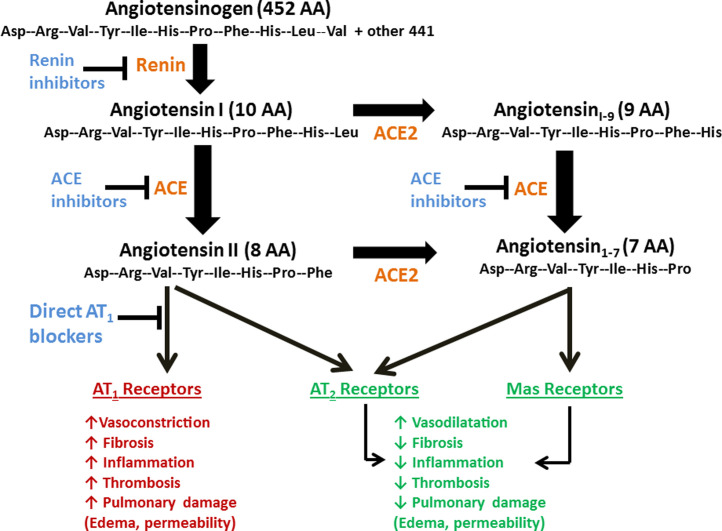
The ACE2 receptor, discovered by two independent groups in year 2000, [4,5] is a trans-membrane type I glycoprotein (mono-carboxypeptidase) composed by 805 amino acids which uses a single extracellular catalytic domain to remove one single amino acid from the octapeptide angiotensin II to generate angiotensin1-7. ACE2 receptor also converts angiotensin I into angiotensin1-9, which in turn is converted to angiotensin1-7 by ACE and neprilisin (Figure 1 ). The catalytic efficiency of ACE2 is 400 times higher on angiotensin II than on angiotensin I. [6] ACE2 shows a 40% structural identity to ACE, [4] although ACE-inhibitors do not block ACE2 because of the different conformational structure of the catalytic site. [4]
Figure 1.
Counter-regulatory effects of angiotensin1-7 on angiotensin II.
ACE2 mediates the cell entry of three strains of coronavirus: SARS-CoV, NL63 and SARS-CoV-2. [7] Notably, SARS-CoV and Sars-CoV2 share a 76% identity in the amino acid sequence, [8] thereby explaining the propensity of these viruses for binding with ACE2. Some structural variations of human ACE2 have been identified that are characterized by a lower binding affinity with the spike viral protein, with potential protective implications. [9] The first step of viral entry process is the binding of the N-terminal portion of the viral protein unit S1 to a pocket of the ACE2 receptor. The second step, which is believed to be of utmost importance for viral entry, is the protein cleavage between the S1 and S2 units, which is operated by the receptor transmembrane protease serine 2 (TMPRSS2), a member of the Hepsin/TMPRSS subfamily. [10] TMPRSS2 is stechiometrically contiguous to ACE2 receptor. [10] The cleavage of the viral protein by TMPRSS2 is a crucial step because, after S1 detachment, the remaining viral S2unit undergoes a conformational rearrangement which drives and completes the fusion between the viral and cellular membrane, with subsequent entry of the virus into cell, release of its content, replication, and infection of other cells. The importance of TMPSRR2 is supported by the evidence that entry of SARS-CoV and SARS-CoV-2 into cells is partially blocked by camostat mesylate, an inhibitor of TMPSRR2. [2]
3. Site of ACE2 receptors
ACE2 genes map to the X chromosome, [11] its expression seems to be higher in Asian than in white and African-American people, [12] and receptors are ubiquitous. In particular, ACE2 receptors are expressed in the heart (endothelium of coronary arteries, myocites, fibroblasts, epicardial adipocites), vessels (vascular endothelial and smooth cells), gut (intestinal epithelial cells), lung (tracheal and bronchial epithelial cells, type 2 pneumocytes, macrophages), kidney (luminal surface of tubular epithelial cells), testis, brain. [13], [14], [15], [16] In the lung, the wide surface of alveolar epithelial cells might explain the vulnerability of this organ to the consequences of virus invasion. ACE2 is mostly bound to cell membranes and only scarcely present in the circulation in a soluble form.
The disintegrin and metalloproteinase 17 (ADAM17), upregulated by angiotensin II through its type 1 receptors (AT1 receptors), cleaves the membrane-anchored ACE2, thereby releasing a circulating active form of ACE2 with loss of the catalytically activity of the remaining part of the enzyme anchored to membrane. [17] Elevated circulating levels of soluble ACE2 are markers of different disease states characterized by increased activity of the renin-angiotensin system and associated with a worse prognosis. [18,19]
4. ACE2: angels or devils?
In the current SARS-CoV-2 pandemic, ACE2 receptors can be considered ‘devils’, being the ‘entry door’ for the virus. Evidence about this phenomenon is now strong and convincing. Hoffmann et al [2] and Walls et al [3] provided unequivocal evidence that SARS-CoV-2 gain access to cells through ACE2 receptors, as it happened with SARS-Cov. [7] Other evidences complete the picture. For example, in a mouse model of SARS-CoV infection, the virus entry is enhanced by overexpression of ACE2. [20] Anti-ACE2 antibodies, but not anti-ACE antibodies are able to block SARS-Cov viral invasion, [7] which is also blocked by N-(2-aminoethil)-1 aziridine-ethamine, a specific ACE-2 inhibitor. [21] The pulmonary lesions induced by experimental SARS-Cov infection are less aggressive in ACE2-knockout mice than in wild-type mice. [22]
In the same time, however, ACE2 receptors exert salutary biological functions that turn them as ‘angels’ under several aspects. A pivotal protective function of ACE2 is the degradation of angiotensin II to angiotensin1-7., although ACE2 is able to metabolize other biological peptides including (des-Arg9)-bradykinin. [15] The degradation of angiotensin II to angiotensin1-7 is blocked by selective ACE2 inhibitors like MLN-4760. [23]
To understand the relevance of angiotensin II degradation by ACE2 results, it is important to review the biological effects of angiotensin II. Angiotensin II serves not only as a potent vasoconstrictor and stimulant of aldosterone release. In different experimental and clinical models, angiotensin II triggered a variety of important adverse reactions which included myocardial hypertrophy and dysfunction, interstitial fibrosis, endothelial dysfunction, enhanced inflammation, obesity-associated hypertension, oxidative stress and increased coagulation. [13], [14], [15], [16] A detailed discussion of the mechanisms through which angiotensin II mediates the above reactions is out of the purposes of this review. In the current pandemic of SARS-CoV-2 infection with associated pulmonary inflammation and Acute Respiratory Distress Syndrome (ARDS), it is interesting to note that angiotensin II also interferes with adaptive immunity by activating machrophages [24] and other cells of the immune system, with consequent increased production of IL-6, [25] TNFα and other inflammatory citokynes. [26,27]
It is important to remark that the deleterious effects of angiotensin II summarized above almost entirely result from the stimulation of AT1 receptors. This chain of events can be defined as the ACE→Angiotensin II→AT1 receptor axis.
The ACE2 receptors reduce the adverse effects of angiotensin II not only by degrading angiotensin II, thereby eliminating or limiting its deleterious potential, but also by generating angiotensin1-7. Angiotensin1-7 exerts numerous salutary and opposite (‘counter-regulatory’) effects to those of angiotensin II through an efficient binding with the G protein-coupled receptor Mas and angiotensin II type 2 receptors (AT2 receptors). Therefore, the ACE2→Angiotensin1-7→Mas receptor axis is counter-regulatory to the ACE→Angiotensin II→AT1 receptor axis.
Santos et al provided an excellent review of the multiple effects of the ACE2→Angiotensin1-7→Mas receptor axis. [28]
4.1. ACE2→Angiotensin1-7→Mas receptor axis and the lung
Studies addressing the pulmonary effects of angiotensin1-7 appear particularly appealing. Mas receptors are expressed at the surface of bronchial smooth muscle cells and alveolar epithelium. [29,30] In experimental and clinical models of lung inflammation, angiotensin1-7 exerted anti-inflammatory effects with less infiltrates of lymphocytes and neutrophils, reduced perivascular and peri-bronchial inflammation, and prevention of subsequent fibrosis. [29,[31], [32], [33]
ACE2 is expressed on the luminal side of the bronchial ciliated epithelia, where it removes a single amino acid residue also from the polypeptide des-Arg [9] bradykinin (DABK), [6] thereby preventing the binding of DABK on the bradykinin receptor B1 receptor. [34] In the presence of reduced ACE2 function in the lung induced by endotoxins there is an increase of free DABK, which in turn activates B1 receptors with release of pro-inflammatory cytokines and intense lung inflammatory and injury. [34]
4.2. ACE2→Angiotensin1-7→Mas receptor axis and thrombosis
The ACE2→Angiotensin1-7→Mas receptor axis exerts anti-thrombotic effects [35], [36], [37], [38]. Mas receptors are expressed on platelets. [39] Stimulation of Mas receptors by angiotensin1-7 increases prostacyclin and NO release. [35,36] Animals knockout for Mas receptors have a shorter bleeding time and increased size of thrombi. [36] In these animals, administration of angiotensin1-7 induces a marked antithrombotic effect which is directly related to the plasma levels of angiotensin1-7 [39] and is inhibited by A-779, an antagonist of Mas receptors. [35] Thus, angiotensin1-7 plays an important role in opposing the pro-thrombotic and pro-inflammatory effects of angiotensin II. [40,41]
4.3. ACE2→Angiotensin1-7→Mas receptor axis and the endocrine system
The ACE2→Angiotensin1-7→Mas receptor axis is well expressed in the pancreas where it improves insulin secretion possibly by improving peri-insular blood flow and inhibiting fibrosis as a result of increased NO release. [28,42] ACE2 receptors are also expressed in the adipose tissue [43,44] and a reduction of ACE2 has been noted in the adipose tissue of obese animals [44] In animal experiments, diets rich of fats decreased ACE2 activity and angiotensin1-7, and increased angiotensin II and blood pressure levels in male, but not in female, animals and these reactions were inhibited by AT1 blockade with losartan. [45] After ovariectomy, female animals showed similar reactions as in males. [45] These data suggest that ACE2 deficiency may favor obesity-induced hypertension. [45] ACE2 is also expressed in the cardiac adipocytes. [46] Obese patients with heart failure have an increased amount of epicardial adipose tissue [46] and it has been suggested that ACE2 deficiency can induce heart failure with preserved ejection fraction in animals. [47] This phenomenon has been attributed to adipose tissue inflammation through local activation of macrophages, which possess AT1 receptors on their cellular membrane. [26]
5. What does it happen to ACE2 after SARS-Cov binding?
SARS-Cov and SARS-CoV2 bind to ACE2 receptors, with the subsequent membrane fusion and virus entry into the cell, leads to down-regulation of these receptors. [16,22,48] In other terms, the virus appears to entry into the cell along with the membrane receptor, which is functionally removed from the external site of the membrane.
As a result, the ACE→Angiotensin II→Mas receptor axis is markedly attenuated, with amplification of the ACE→Angiotensin II→AT1 receptor axis.
5.1. Pulmonary implications of ACE2 down-regulation
Since the pulmonary inflammation and the resulting Acute Respiratory Distress Syndrome (ARDS) are potentially deadly complications of SARS-CoV and SARS-CoV-2, studies addressing the lung complications of ACE2 down-regulation are of outmost importance. Studies using different models of lung injury showed that the down-regulation of ACE2 receptors triggers important inflammatory lesions in the respiratory tree (alveolar wall thickening, edema, infiltrates of inflammatory cells, bleeding) which appear to be mediated by angiotensin II. [22,[48], [49], [50]
Tracheal instillation of cigarette smoke, [49] or particulate matter of aerodynamic diameter of less than 2,5 ɥm, [50] induces acute lung injury with release of inflammatory cytokines IL-6, TNF-α and TGF-β1 and increased expression of ACE, consistent with ACE→Angiotensin II→AT1 receptor axis over-activity. [50] These reactions are increased in ACE2 knockout mice. [50] In a model of acid aspiration, which induces acute lung injury, lung inflammatory lesions were more severe and lethal in ACE2 knock-out animals. [48] In these animals, injection of recombinant ACE2 as well as AT1 receptor blockers attenuate the degree of lung injury. [48] These findings strongly suggest that ACE2 protects from lung injury induced by acid aspiration. [48]
Notably, lung injury has been induced by the isolated spike viral protein of the SARS-Cov, the ligand for ACE2 binding, in the absence of other viral components. [22] This model has the merit to investigate the impact of ACE2 down-regulation in the absence of confounding effects of viral invasion and replication. The authors found that even the isolated spike viral protein induced down-regulation of ACE2 receptors with concomitant increase of angiotensin II in the lung tissue and precipitation of severe pulmonary inflammatory lesions. [22] Also in this model, AT1 receptor blockers attenuated the pulmonary lesions induced by the spike viral protein. [22]
A key point to remark is that ACE2 are mainly expressed in pneumocytes type II, small cylindrical cells which represent 5% of all pneumocytes. [51] Pneumocytes type 2 are responsible for the production of alveolar surfactant, and in the same time they function as ‘stem’ cells, progenitors of pneumocytes type I (95% of all pneumocytes) which are responsible of gas exchanges. [52] Therefore, the damage of pneumocytes type II due to the binding of coronavirus to ACE2 receptors is devastating for at least three reasons: 1) local unopposed ACE→Angiotensin II→AT1 receptor axis over-activity; 2) reduced production of alveolar surfactant by injured pneumocytes type II leading to reduced lung elasticity; 3) reduced repair of pneumocytes type I leading to impaired gas exchanges and fibrosis. [53]
6. Clinical characteristics of patients infected with SARS-CoV and SARS-CoV-2
Studies from China and Italy have shown that hypertension, diabetes and history of cardiovascular disease are the most frequent comorbidities in patients infected with SARS-CoV-2. [54], [55], [56] Older age and male sex are two additional factors associated with SARS-CoV-2 infection. [54], [55], [56] A similar picture emerged a few years ago with the SARS-CoV infection. [57,58]
In a study conducted in 201 patients infected with SARS-CoV-2, most patients were men (63.7% of patients), the mean age was 51 years and the most frequent comorbidities were hypertension (19.4%), diabetes (10.9%) and history of cardiovascular disease (4.0). [55] Notably, the patients who developed ARDS were older and had a higher prevalence of hypertension (27.4% Vs. 13.7%), diabetes (19.0% Vs. 5.1%) when compared with those who did not develop ARDS. [55] In a multivariate analysis, the factors associated with progression from ARDS syndrome to death included older age, neutrophilia and hyper-coagulation, mainly reflected by a higher D-dimer. [55] Abnormal coagulation parameters and enhanced thrombosis predict a poor prognosis in patients with SARS-CoV-2. [59] A meta-analysis of 8 studies conducted in China on a total of 46,248 patients infected with SARS-CoV2 confirmed that hypertension, diabetes and history of cardiovascular disease were the most frequent comorbidities in these patients. [56] Again, hypertension and history of cardiovascular disease were significantly more prevalent among the more severe patients. [56]
In a recent analysis of 1591 infected patients from Italy, the mean age of patients was 63 years, men were 82% and the prevalence of patients with hypertension, diabetes and previous cardiovascular disease was 49%, 17% and 21%, respectively. [54] Patients with hypertension were older that those without hypertension (66 vs 62 years, p=0.005). When comparing the patients who died in the Intensive Care Unit with those who survived, the former were older and had a higher prevalence of hypertension (63% Vs. 40%, p<0.001). [54]
7. ACE2 deficiency: a central role in SARS-CoV-2 infection?
It is interesting to note that several conditions associated with viral infection and severity of the disease share a variable degree of ACE2 deficiency. For example, ACE2 expression in the lungs markedly decreases with ageing, [60] to a greater extent in men than women. [60] Diabetes mellitus has been associated with reduced ACE2 expression, possibly as effect of glycosylation. [61], [62], [63] Several experimental and clinical studies indicate that ACE2 deficiency obtained through deletion or inhibition may be a causative factor for hypertension. [14,64] Treatment with soluble recombinant ACE2 reduces the blood pressure rise provoked by angiotensin II, increases angiotensin1-7 and reduces angiotensin II. [65] ACE2 deficiency has been associated with exacerbation of hypertension and cardiac hypertrophy induced by angiotensin II, [66] and maladaptive left ventricular remodeling after myocardial infarction. [67] Furthermore, deficiency of ACE2 enhances the susceptibility to heart failure. [14] A heterozygote loss of ACE2 is believed sufficient to increase the susceptibility to heart disease. [68]
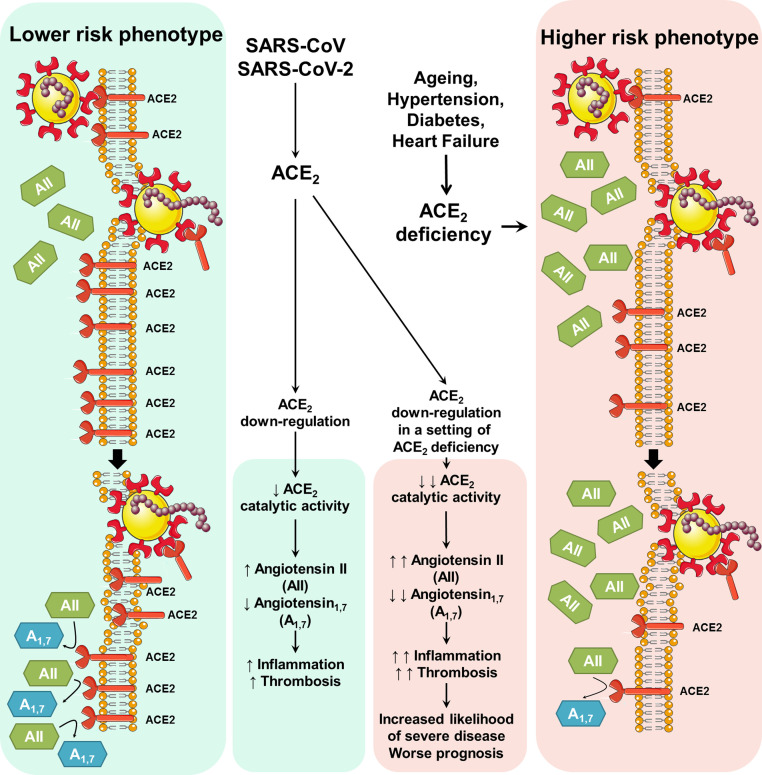
Given the above premises, it is tempting to speculate (Figure 2 ) that ACE2 deficiency may play a central role in the pathogenesis of SARS-CoV-2 infection. The down-regulation of ACE2 induced by viral invasion could be especially detrimental in individuals with baseline ACE2 deficiency due, for example, to older age, diabetes, hypertension and prior heart diseases including heart failure.
Figure 2.
Potential impact of ACE2 down-regulation induced by viral entry in a setting of pre-existing ACE2 deficiency.
The possibility that a mild or moderate ACE2 deficiency may protect from viral invasion seems unlikely because of the intrinsically high affinity of SARS-CoV-2 to ACE2 receptors. [2,3] By contrast, in a setting of ACE2 deficiency, the ACE2 down-regulation induced by virus could amplify the imbalance between the ACE→Angiotensin II→AT1 receptor axis (adverse) and the ACE2→Angiotensin1-7I→Mas receptor axis (protective). At lung level, such dysregulation would much facilitate the progression of inflammatory and hyper-coagulation processes which share dependency upon local angiotensin II hyper-activity insufficiently opposed by angiotensin1-7. This chain of events would not deny the concomitant role of other mechanisms including an impaired immune response to initial viral invasion, or a genetic susceptibility to hyper-inflammation and thrombosis. [69,70]
In this context, administration of soluble recombinant ACE2 [12] or angiotensin1-7 [71] could be promising therapeutic approaches, requiring urgent evaluation in clinical trials. Two trials of losartan as additional treatment for SARS-CoV-2 infection in hospitalized (NCT04312009) or not hospitalized (NCT04311177) patients have been announced, supported by the background of the huge adverse impact of the ACE→Angiotensin II→AT1 receptor axis over-activity in these patients.
The possibility that ACE inhibitors and angiotensin II receptor blockers (ARBs) may be discontinued even temporarily, because these drugs appear to increase the expression of ACE2 receptors, the site of viral entry into the human organism, [72], [73], [74], [75] is actively debated. [76], [77], [78], [79], [80], [81], [82], [83], [84] Several Scientific Societies and various experts in this area expressed the position that discontinuation of these drugs is not justified by evidence and could be dangerous. [76,77,81,82] On the other hand, several experimental data discussed above suggest the potential utility of ARBs, particularly to limit lung inflammation during viral invasion. [80,83] It is hoped that the results of the above mentioned NCT04312009 and NCT04311177 trials will answer this question.
8. Conclusions
We suggest that ACE2 down-regulation induced by the cell entry of SARS-CoV, NL63 and SARS-CoV-2 may be particularly detrimental in subjects with pre-existing ACE2 deficiency. Some degree of ACE2 deficiency has been associated with a variety of conditions including older age, hypertension, diabetes and cardiovascular disease, which also characterize people more likely to be infected and to present more severe complications. In a setting of enhanced ACE2 deficiency produced by the viral invasion, the marked dysregulation between the ‘adverse’ ACE→Angiotensin II→AT1 axis and the ‘protective’ ACE2→Angiotensin1-7→Mas axis would contribute to enhance the progression of inflammatory and thrombotic processes. These considerations provide a rationale for investigating the role of therapeutic approaches conceptually linked to ACE2 receptor activity. These include the use of soluble recombinant ACE2, angiotensin1-7, and angiotensin II type 1 receptor blockers, which are currently being evaluated.
SOURCES OF FUNDING
Study supported in part by the no-profit Fondazione Umbra Cuore e Ipertensione-ONLUS, Perugia, Italy
DISCLOSURES
None
Declaration of competing interest
The authors declare they have no conflict of interest.
References
- 1.Chan-Yeung M, Xu RH. SARS: epidemiology. Respirology. 2003;8(Suppl:S9-14) doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 5.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 6.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 7.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain M, Jabeen N, Raza F, Shabbir S, Baig AA, Amanullah A, Aziz B. Structural Variations in Human ACE2 may Influence its Binding with SARS-CoV-2 Spike Protein. J Med Virol. 2020 doi: 10.1002/jmv.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glowacka I, Bertram S, Muller MA, Allen P, Soilleux E, Pfefferle S, Steffen I, Tsegaye TS, He Y, Gnirss K, Niemeyer D, Schneider H, Drosten C, Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 12.Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 13.Kuba K, Imai Y, Penninger JM. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013;77:301–308. doi: 10.1253/circj.cj-12-1544. [DOI] [PubMed] [Google Scholar]
- 14.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M, Lazartigues E. Clinical Relevance and Role of Neuronal AT1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ Res. 2017;121:43–55. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bitker L, Burrell LM. Classic and Nonclassic Renin-Angiotensin Systems in the Critically Ill. Crit Care Clin. 2019;35:213–227. doi: 10.1016/j.ccc.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wysocki J, Goodling A, Burgaya M, Whitlock K, Ruzinski J, Batlle D, Afkarian M. Urine RAS components in mice and people with type 1 diabetes and chronic kidney disease. Am J Physiol Renal Physiol. 2017;313:F487–F494. doi: 10.1152/ajprenal.00074.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XH, Deng W, Tong Z, Liu YX, Zhang LF, Zhu H, Gao H, Huang L, Liu YL, Ma CM, Xu YF, Ding MX, Deng HK, Qin C. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med. 2007;57:450–459. [PubMed] [Google Scholar]
- 21.Huentelman MJ, Zubcevic J, Hernandez Prada JA, Xiao X, Dimitrov DS, Raizada MK, Ostrov DA. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44:903–906. doi: 10.1161/01.HYP.0000146120.29648.36. [DOI] [PubMed] [Google Scholar]
- 22.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trask AJ, Averill DB, Ganten D, Chappell MC, Ferrario CM. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1-7) in transgenic Ren-2 hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H3019–H3024. doi: 10.1152/ajpheart.01198.2006. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein KE, Khan Z, Giani JF, Cao DY, Bernstein EA, Shen XZ. Angiotensin-converting enzyme in innate and adaptive immunity. Nat Rev Nephrol. 2018;14:325–336. doi: 10.1038/nrneph.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Recinos A, 3rd, LeJeune WS, Sun H, Lee CY, Tieu BC, Lu M, Hou T, Boldogh I, Tilton RG, Brasier AR. Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis. 2007;194:125–133. doi: 10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto S, Yancey PG, Zuo Y, Ma LJ, Kaseda R, Fogo AB, Ichikawa I, Linton MF, Fazio S, Kon V. Macrophage polarization by angiotensin II-type 1 receptor aggravates renal injury-acceleration of atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2856–2864. doi: 10.1161/ATVBAHA.111.237198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YB, Nagai A, Kim SU. Cytokines, chemokines, and cytokine receptors in human microglia. J Neurosci Res. 2002;69:94–103. doi: 10.1002/jnr.10253. [DOI] [PubMed] [Google Scholar]
- 28.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7) Physiol Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magalhaes GS, Rodrigues-Machado MG, Motta-Santos D, Silva AR, Caliari MV, Prata LO, Abreu SC, Rocco PR, Barcelos LS, Santos RA, Campagnole-Santos MJ. Angiotensin-(1-7) attenuates airway remodelling and hyperresponsiveness in a model of chronic allergic lung inflammation. Br J Pharmacol. 2015;172:2330–2342. doi: 10.1111/bph.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Hashim AZ, Renno WM, Raghupathy R, Abduo HT, Akhtar S, Benter IF. Angiotensin-(1-7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-kappaB-dependent pathways. Br J Pharmacol. 2012;166:1964–1976. doi: 10.1111/j.1476-5381.2012.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Yang Y, Huang Y, Pan C, Liu L, Qiu H. Angiotensin-(1-7) attenuates lung fibrosis by way of Mas receptor in acute lung injury. J Surg Res. 2013;185:740–747. doi: 10.1016/j.jss.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Cao Y, Zeng Z, Liang M, Xue Y, Xi C, Zhou M, Jiang W. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis prevents lipopolysaccharide-induced apoptosis of pulmonary microvascular endothelial cells by inhibiting JNK/NF-kappaB pathways. Sci Rep. 2015;5:8209. doi: 10.1038/srep08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng Y, Yu CH, Li W, Li T, Luo W, Huang S, Wu PS, Cai SX, Li X. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-kappaB pathway. Am J Respir Cell Mol Biol. 2014;50:723–736. doi: 10.1165/rcmb.2012-0451OC. [DOI] [PubMed] [Google Scholar]
- 34.Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, McCray PB, Jr., Chappell M, Hackam DJ, Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. 2018;314:L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang C, Stavrou E, Schmaier AA, Grobe N, Morris M, Chen A, Nieman MT, Adams GN, LaRusch G, Zhou Y, Bilodeau ML, Mahdi F, Warnock M, Schmaier AH. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121:3023–3032. doi: 10.1182/blood-2012-09-459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraga-Silva RA, Pinheiro SV, Goncalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med. 2008;14:28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucharewicz I, Pawlak R, Matys T, Pawlak D, Buczko W. Antithrombotic effect of captopril and losartan is mediated by angiotensin-(1-7) Hypertension. 2002;40:774–779. doi: 10.1161/01.hyp.0000035396.27909.40. [DOI] [PubMed] [Google Scholar]
- 38.Pai WY, Lo WY, Hsu T, Peng CT, Wang HJ. Angiotensin-(1-7) Inhibits Thrombin-Induced Endothelial Phenotypic Changes and Reactive Oxygen Species Production via NADPH Oxidase 5 Downregulation. Front Physiol. 2017;8:994. doi: 10.3389/fphys.2017.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, Alenina N, Bader M, Sinisterra RD, Santos RA. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect. Clinics (Sao Paulo) 2011;66:837–841. doi: 10.1590/S1807-59322011000500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang B, Wang X, Zhang N, Yang H, Bai R, Liu M, Bian Y, Xiao C, Yang Z. Angiotensin-(1-7) Attenuates Angiotensin II-Induced ICAM-1, VCAM-1, and MCP-1 Expression via the MAS Receptor Through Suppression of P38 and NF-kappaB Pathways in HUVECs. Cell Physiol Biochem. 2015;35:2472–2482. doi: 10.1159/000374047. [DOI] [PubMed] [Google Scholar]
- 41.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 42.Yuan L, Li Y, Li G, Song Y, Gong X. Ang(1-7) treatment attenuates beta-cell dysfunction by improving pancreatic microcirculation in a rat model of Type 2 diabetes. J Endocrinol Invest. 2013;36:931–937. doi: 10.3275/8951. [DOI] [PubMed] [Google Scholar]
- 43.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss HP, Siems WE, Walther T. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26:1270–1277. doi: 10.1016/j.peptides.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, Zhang X, Karounos M, Cassis LA. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol. 2012;32:1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel VB, Basu R, Oudit GY. ACE2/Ang 1-7 axis: A critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte. 2016;5:306–311. doi: 10.1080/21623945.2015.1131881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, Parajuli N, Penninger JM, Grant MB, Lopaschuk GD, Oudit GY. ACE2 Deficiency Worsens Epicardial Adipose Tissue Inflammation and Cardiac Dysfunction in Response to Diet-Induced Obesity. Diabetes. 2016;65:85–95. doi: 10.2337/db15-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung YH, Hsieh WY, Hsieh JS, Liu FC, Tsai CH, Lu LC, Huang CY, Wu CL, Lin CS. Alternative Roles of STAT3 and MAPK Signaling Pathways in the MMPs Activation and Progression of Lung Injury Induced by Cigarette Smoke Exposure in ACE2 Knockout Mice. Int J Biol Sci. 2016;12:454–465. doi: 10.7150/ijbs.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin CI, Tsai CH, Sun YL, Hsieh WY, Lin YC, Chen CY, Lin CS. Instillation of particulate matter 2.5 induced acute lung injury and attenuated the injury recovery in ACE2 knockout mice. Int J Biol Sci. 2018;14:253–265. doi: 10.7150/ijbs.23489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivellese F, Prediletto E. ACE2 at the centre of COVID-19 from paucisymptomatic infections to severe pneumonia. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102536:102536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, Network C-LI. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 58.Chan JW, Ng CK, Chan YH, Mok TY, Lee S, Chu SY, Law WL, Lee MP, Li PC. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS) Thorax. 2003;58:686–689. doi: 10.1136/thorax.58.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie X, Chen J, Wang X, Zhang F, Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pal R, Bhansali A. COVID-19, Diabetes Mellitus and ACE2: The conundrum. Diabetes Res Clin Pract. 2020 doi: 10.1016/j.diabres.2020.108132:108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tikellis C, Thomas MC. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. Int J Pept. 2012;2012 doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamagata R, Nemoto W, Nakagawasai O, Takahashi K, Tan-No K. Downregulation of spinal angiotensin converting enzyme 2 is involved in neuropathic pain associated with type 2 diabetes mellitus in mice. Biochem Pharmacol. 2020;174 doi: 10.1016/j.bcp.2020.113825. [DOI] [PubMed] [Google Scholar]
- 64.Patel SK, Velkoska E, Freeman M, Wai B, Lancefield TF, Burrell LM. From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol. 2014;5:227. doi: 10.3389/fphys.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wysocki J, Ye M, Rodriguez E, Gonzalez-Pacheco FR, Barrios C, Evora K, Schuster M, Loibner H, Brosnihan KB, Ferrario CM, Penninger JM, Batlle D. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55:90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong J, Basu R, Guo D, Chow FL, Byrns S, Schuster M, Loibner H, Wang XH, Penninger JM, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. 718 p following 728. [DOI] [PubMed] [Google Scholar]
- 67.Kassiri Z, Zhong J, Guo D, Basu R, Wang X, Liu PP, Scholey JW, Penninger JM, Oudit GY. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2:446–455. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Patel VB, Parajuli N, Fan D, Basu R, Wang Z, Ramprasath T, Kassiri Z, Penninger JM, Oudit GY. Heterozygote loss of ACE2 is sufficient to increase the susceptibility to heart disease. J Mol Med (Berl) 2014;92:847–858. doi: 10.1007/s00109-014-1149-y. [DOI] [PubMed] [Google Scholar]
- 69.Mehta P, MaAuley DF, Brown M, Sanchez E, Tattersall RS, Manson J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395:1–2. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akhmerov A, Marban E. COVID-19 and the Heart. Circ Res. 2020 doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peiro C, Moncada S. Substituting Angiotensin-(1-7) to Prevent Lung Damage in SARSCoV2 Infection? Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 73.Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol. 2008;295:C1169–C1174. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 75.Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 76.Bavishi C, Maddox TM, Messerli FH. Coronavirus Disease 2019 (COVID-19) Infection and Renin Angiotensin System Blockers. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1282. [DOI] [PubMed] [Google Scholar]
- 77.Danser AHJ, Epstein M, Batlle D. Renin-Angiotensin System Blockers and the COVID-19 Pandemic: At Present There Is No Evidence to Abandon Renin-Angiotensin System Blockers. Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15082. doi:10.1161/HYPERTENSIONAHA.120.15082:HYPERTENSIONAHA12015082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J. Hypertens. 2020;38:1–2. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 79.Fang L, Karakiulakis G, TRoth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30116-8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verdecchia P, Angeli F, Reboldi G. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and coronavirus. J Hypertens. 2020;38 doi: 10.1097/HJH.0000000000002469. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verdecchia P, Reboldi G, Cavallini C, Mazzotta G, Angeli F. ACE-inibitori, sartani e sindrome respiratoria acuta da coronavirus 2. G Ital Cardiol (Rome) 2020;21:1–7. doi: 10.1714/3343.33127. [DOI] [PubMed] [Google Scholar]