Abstract
Background: Advances of allogeneic hematopoietic cell transplantation (allo-HCT) have brought long-term survival to the patients with hematologic malignancies. Chronic graft-versus-host disease (GVHD) is one of major problems for the long- term survivors after allo-HCT. Dysregulation of immune reconstitution has been reported to be involved in the pathogenesis of chronic GVHD. Differences of immune reconstitution between cord blood transplantation (CBT) and unrelated bone marrow transplantation (UBMT) remain unclear in long-term survivors. We investigated immune reconstitution in patients surviving for more than 2 years after CBT (n=21) or UBMT (n=20) without relapse of underlying disease.
Materials and Methods: Using flow cytometric analysis of peripheral blood, we investigated immune reconstitution of T cells, B cells, and NK cells between CBT and UBMT patients. We collected clinical data regarding allo-HCT and examined the relation of immune reconstitution to the development of chronic GVHD.
Results: Between CBT and UBMT patients, we found significant differences in absolute cell number of CD8+ as well as CD19+ cell and CD4/CD8 ratio even more than 2 years after allo-HCT. Among UBMT patients, absolute cell number of naive CD4+ cell was significantly lower in patients with chronic GVHD. In addition, we found significant differences in absolute cell number of CD19+ cell, especially naive B cell between patients with and without chronic GVHD in both CBT and UBMT patients.
Conclusion: These results suggest that differences of immune recovery between CBT and UBMT patients may exist even in patients surviving for more than 2 years and might be related to the development of chronic GVHD.
Key Words: Immune reconstitution, Cord blood transplantation, Unrelated bone marrow transplantation, Chronic GVHD
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) has improved the prognosis of the patients with hematologic malignancies. Advances of conditioning regimens and immunosuppressive therapy contribute to the improvement of the prognosis. In addition, the introduction of unrelated bone marrow transplantation (UBMT) and cord blood transplantation (CBT) increased the chance to receive allo-HCT. These progresses have led to the result that some patients survive for more than a decade. Concomitantly, there have increased various problems to disturb the quality of life of long-term survivors after allo-HCT1, 2.
Chronic graft-versus-host disease (GVHD) is one of major problems for long-term survivors after allo-HCT, leading to reduced patient-reported quality of life and non-relapse mortality3-5. Risk factors for chronic GVHD include prior acute GVHD, donor peripheral blood stem-cell grafts, HLA disparity, female donors for male recipients, and recipient age6. Concerning the graft of allo-HCT, the incidence and severity of chronic GVHD are recently reported to be lower in CBT than UBMT patients7.
Acute GVHD is thought to be mediated primarily by mature donor T cells in the allogeneic stem cell product. By contrast, chronic GVHD is now considered to be more complex immune reaction. Both donor-derived effector T and B cells contribute to the pathology of chronic GVHD8, 9. In addition, regulatory elements within T and B cell lineages play important roles in the development and maintenance of immune tolerance after allo-HCT10, 11. Several reports have shown that the differences in immune reconstitution exist between the patients who received cord blood and other hematopoietic stem cell sources. In CBT patients, delayed recovery of T cells has been reported, by contrast, B cell numbers were higher compared to the patients received HLA-matched sibling or unrelated peripheral blood stem cell transplantation12-14. However, the observation duration was up to 2 years post allo-HCT. The differences in immune reconstitution more than 2 years after allo-HCT between CBT and UBMT have not been elucidated.
In this study, we investigated the differences of immune reconstitution between CBT and UBMT patients, who survive for more than 2 years after allo-HCT without relapse of underlying disease, in relation to the development of chronic GVHD in our institute.
MATERIALS AND METHODS
Study design
To determine whether the differences in immune reconstitution would exist between CBT and UBMT patients in long-survivors, we selected patients who had attended to our outpatient clinic for more than 2 years after allo-HCT and showed no symptoms of infections and relapse of underlying disease. Twenty-one patients who had received CBT (CBT group) and 20 patients who had received HLA-matched UBMT (UBMT group) from January 2002 to January 2014 were enrolled in this study. We collected peripheral blood for flow cytometric analysis to investigate immune reconstitution and clinical symptoms regarding allo-HCT at the time after allo-HCT described in duration in Table 1 after informed consent was given. We examined whether the differences in the immune reconstitution between CBT and UBMT patients who survive for more than 2 years after allo-HCT and any relations to the development of cGVHD could exist. This study was approved by the ethical committee of our institute.
Table 1.
Clinical data in the patients who had received cord blood transplantation (CBT) and unrelated bone marrow transplantation (UBMT)
| CBT |
|
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|
| Disease | Disease status | Age at allo-HCT | Gender | Source | CMV status | Conditioning | GVHD prophylaxis | Duration (years post allo-HCT) |
| AML | NC | 57 | F | CB | + | FLU+Mel+CA+TBI | CsA+sMTX | 2.8 |
| ATLL | CR1 | 57 | M | CB | + | FLU+Mel+TBI | CsA+sMTX | 3.4 |
| Ph+ALL | CR2 | 48 | M | CB | + | FLU+BU4+TBI | CsA+sMTX | 2.4 |
| DLBCL | CR2 | 51 | F | CB | + | FLU+Mel+TBI | CsA+sMTX | 2.4 |
| CMMoL | NC | 40 | M | CB | + | CY+TBI | CsA+sMTX | 3.9 |
| Ph+ALL | CR2 | 44 | M | CB | + | CY+TBI | CsA+sMTX | 6.1 |
| FL | NC | 56 | M | CB | + | FLU+Mel+TBI | CsA+sMTX | 7.9 |
| ALL | CR1 | 32 | F | CB | + | CY+TBI | CsA+sMTX | 9.5 |
| FL | CR3 | 51 | M | CB | + | FLU+Mel+TBI | CsA+sMTX | 5.5 |
| ALL | CR1 | 41 | M | CB | + | FLU+Mel+TBI | CsA+sMTX | 4.0 |
| AML | CR1 | 45 | M | CB | + | CY+AraC+TBI | CsA+sMTX | 7.3 |
| AML | CR2 | 45 | F | CB | + | FLU+BU+TBI | CsA+sMTX | 3.0 |
| Ph+ALL | CR1 | 39 | F | CB | - | CY+TBI | CsA+sMTX | 10.8 |
| AML | CR2 | 44 | F | CB | + | CY+TBI | CsA+sMTX | 7.1 |
| PTCL | NC | 33 | M | CB | + | CY+TBI | CsA+sMTX | 7.6 |
| AML | CR1 | 52 | F | CB | + | FLU+Mel+TBI | CsA+sMTX | 3.5 |
| AML | CR1 | 38 | F | CB | + | CY+TBI | CsA+sMTX | 2.1 |
| CML | CP1 | 24 | M | CB | + | CY+TBI | CsA+sMTX | 13.1 |
| ATLL | CR1 | 41 | F | CB | + | FLU+Mel+TBI | CsA+sMTX | 3.1 |
| MDS | RA | 62 | M | CB | + | FLU+CA+Mel+TBI | CsA+sMTX | 6.3 |
| Ph+ALL | CR1 | 63 | M | CB | + | FLU+Mel+TBI | CsA+sMTX | 6.2 |
| UBMT | ||||||||
| AML | CR1 | 25 | F | uBM | + | CY+TBI | CsA+sMTX | 8.8 |
| MDS | RAEB | 54 | M | uBM | + | CY+TBI | CsA+sMTX | 7.8 |
| AML | CR1 | 34 | M | uBM | + | CY+TBI | CsA+sMTX | 3.4 |
| ALCL | CR2 | 56 | M | uBM | + | FLU+Mel+TBI | CsA+sMTX | 4.3 |
| AML | CR2 | 48 | F | uBM | - | FLU+BU4+TBI | CsA+sMTX | 5.0 |
| MM | sCR | 39 | F | uBM | - | FLU+Mel+TBI | CsA+sMTX | 2.1 |
| DLBCL | CR2 | 44 | F | uBM | + | FLU+BU2 | CsA+sMTX | 6.8 |
| FL | CR2 | 46 | F | uBM | + | FLU+Mel | CsA+sMTX | 9.3 |
| MDS | RAEB2 | 26 | M | uBM | - | CY+TBI | CsA+sMTX | 10.3 |
| AML | CR1 | 26 | F | uBM | + | CY+TBI | CsA+sMTX | 13.6 |
| MDS | RAEB | 38 | M | uBM | + | CY+TBI | CsA+sMTX | 6.2 |
| AML | CR1 | 38 | F | uBM | + | CY+TBI | CsA+sMTX | 8.0 |
| PNH/AA | 39 | F | uBM | + | CY+FLU+ATG | CsA+sMTX | 3.8 | |
| AML | CR1 | 49 | M | uBM | + | CY+TBI | CsA+sMTX | 10.2 |
| MDS | RAEB2 | 29 | M | uBM | + | CY+TBI | CsA+sMTX | 8.0 |
| CML | BC | 35 | F | uBM | + | CY+TBI | CsA+sMTX | 11.7 |
| NHL | CR2 | 32 | M | uBM | + | CY+TBI | CsA+sMTX | 14.1 |
| CML | CR2 | 38 | M | uBM | + | CY+TBI | CsA+sMTX | 6.6 |
| AML | CR2 | 20 | F | uBM | + | FLU+BU2+TBI | Tac+sMTX | 2.2 |
| DLBCL | NC | 48 | F | uBM | + | FLU+BU2+TBI | CsA+sMTX | 2.2 |
Allo-HCT; allogeneic hematopoietic cell transplantation, CMV; cytomegalovirus, GVHD; graft-versus-host disease, AML; acute myelogenous leukemia, ATLL; adult T cell leukemia/lymphoma, Ph+ALL; Philadelphia chromosome positive acute lymphoblastic leukemia, DLBCL; diffuse large B cell lymphoma, CMMoL; chronic myelomomocytic leukemia, FL; follicular lymphoma, ALL; acute lymphoblastic leukemia, PTCL; peripheral T cell lymphoma, CML; chronic myelogenous leukemia, MDS; myelodysplastic syndrome, ALCL; anaplastic large cell lymphoma, MM; multiple myeloma, PNH/AA; paroxysmal nocturnal hematuria/aplastic anemia, NHL; non-Hodgkin lymphoma, NC; non-complete remission, CR1; first complete remission, CR2; second complete remission, CR3; third complete remission, RA; refractory anemia, RAEB; refractory anemia with excess blast, sCR; stringent complete remission, BC; blastic crisis, CB; cord blood, UBMT; unrelated bone marrow transplantation, FLU; fludarabine, Mel; melphalan, CA; cytosine-arabinocyde, TBI; total body irradiation, BU; buslfan, CY; cyclophosphamide, CsA; cyclosporine A, sMTX; short-term methotrexate.
Patient characteristics
Patient characteristics concerning allo-HCT are listed in Table 1. Median age in CBT group (45 years old, range: 24-63) was significantly older than in UBMT group (38 years old, range: 20-56) (p<0.01). But there was no significant difference in median duration from allo-HCT to the time, when the investigation was performed, between CBT (5.5 years, range: 2.4-13.1) and UBMT group (7.3 years, range: 2.1-14.1). Most of underlying disease were hematologic malignancies, only one patient in UBMT group was non-malignant disease (paroxysmal nocturnal hematuria/ aplastic anemia).
All of CBT were performed using cord blood which matched a minimum of 4 of 6 HLA loci to the patient at antigen level for HLA-A, HLA-B, and HLA-DRB1. In UBMT group, all patients received bone marrow but not peripheral blood stem cell from HLA-matched unrelated donor.
In 9 patients in CBT group and 12 patients in UBMT group, cyclophosphamide (60mg/kg x2) and total body irradiation (TBI) (3Gy x4) had been performed as conditioning regimen (myeloablative conditioning; MAC) (Table 1). In 12 patients in CBT group and 8 patients in UBMT group, various reduced intensity conditioning (RIC) had been conducted (Table 1). For the prophylaxis of GVHD, calcineurine inhibitor plus short-term methotrexate had been introduced into all of patients.
Diagnosis of acute and chronic GVHD
We collected clinical data from medical charts and diagnosed acute and chronic GVHD according to the previously described criteria15.
Flow cytometry
Aliquots of whole peripheral blood were stained with various mAbs according to the manufacturer’s instructions. We had performed three-color flow cytometric analysis. mAbs used in this study were as follows: PC5-conjugated anti-CD4 mAb, PC5-conjugated anti-CD8 mAb, PE-conjugated anti-CD45RO mAb, FITC-conjugated anti-CD25 mAb, PE-conjugated anti-CD127 mAb, PC5-conjugated anti-CD19 mAb purchased from Beckman Coulter. FITC-conjugated anti-CD27 mAb and PE-conjugated anti-IgM mAb were purchased from Pharmingen.
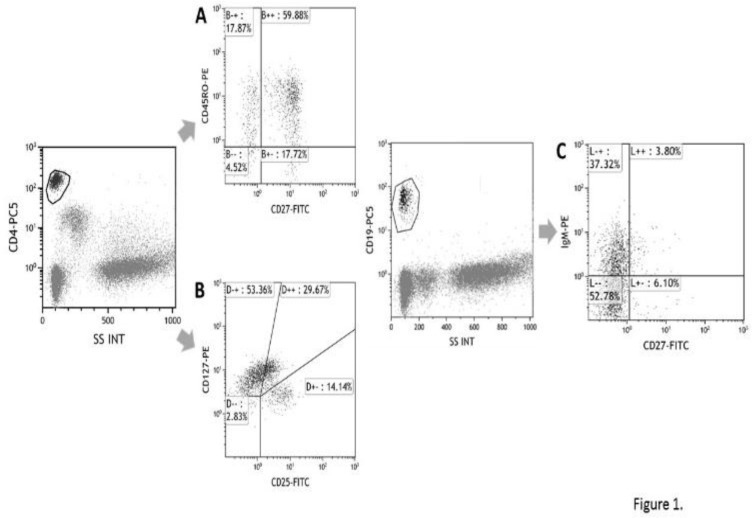
In CD4+ or CD8+ cells, CD27+CD45RO- cells have been designated as naive T cell (Figure 1A), and CD25+CD127-~+- cells have been designated as regulatory T cell16 (Figure 1B). In CD19+ cells, CD27- cells have been designated as naive B cell and CD27+ B cells as memory B cell (Figure 1C). CD3-CD56+ cells have been determined as NK cell.
Figure 1.
Representative data of flow cytometric analysis of the peripheral blood
After gating CD4+ cells, we investigated the expression of CD45RO and CD27 (A). Similar analysis had been done in CD8+ cells. In addition, CD25+CD127negatvie to weekly positive cells in CD4+ cells were detected as regulatory T cells (B). In CD19+ cells, we investigated the expression of CD27 and IgM (C).
Statistical analysis
Immunologic parameters, described in Flow cytometry, were compared between the patients who received CBT and UBMT. In some investigation, immunologic parameters of the patients without versus with chronic GVHD were compared. A comparison between the two groups was performed using Mann-Whitney U test.
Results
Reconstitution in T cell between CBT and UBMT group
We first examined the differences in the reconstitution of T cells between CBT and UBMT group. As shown in Figure 2A, there was no significant difference in absolute number of CD4+ T cell, but significant differences were observed in absolute number of CD8+ cell and CD4/CD8 ratio between the two groups. Absolute cell number of CD8+ cell was significantly higher in UBMT patients (Figure 2B). As a result, CD4/CD8 ration was significantly lower in UBMT patients (Figure 2C). We next examined the ratio and cell count of subpopulations in T cells. In CD4+ and CD8+ cells, no significant difference in ratio and absolute cell number of naive cell (CD27+CD45RO-) was observed between the two groups as well as memory cell (CD27-CD45RO+) (data not shown). Furthermore, we have observed no significant difference in absolute cell number of regulatory T cell, which has been reported to be involved in the pathogenesis of chronic GVHD (Figure 2D).
Figure 2.
Differences in T cell recovery between CBT and UBMT group Absolute count of CD4+ cell (A), CD8+ cell (B), CD4/CD8 ratio (C), and regulatory T cell (D) of peripheral blood were compared between CBT and UBMT group. * indicates differences that are statistically significant (p<0.05).
Reconstitution in B cell and NK cell
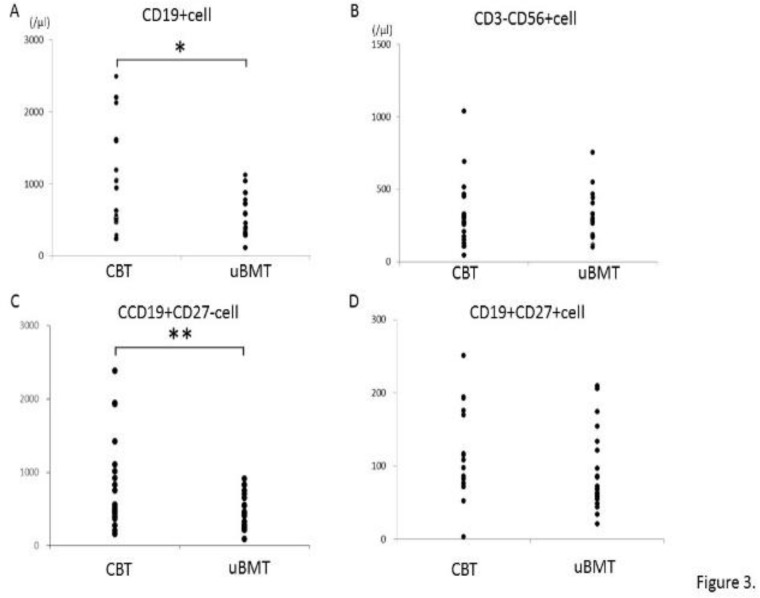
We next examined the recovery of B cells. As shown in Figure 3A, absolute cell number of CD19+ cell in UBMT group was significantly lower than in CBT group (p<0.05), although there was no significant difference in serum levels of immunoglobulins (IgG, IgA, and IgM) (data not shown). By contrast, no significant difference was observed in NK cell counts between the two groups (Figure 3B). Absolute cell number of naive B cell (CD19+CD27-) was also lower in UBMT group without statistical significance (P=0.05) (Figure 3C). In memory B cells (CD19+CD27+), absolute cell number tended to be lower in UBMT than CBT group without statistical significance (Figure 3D, p=0.10). These results suggest that the differences in the reconstitution of B cells between CBT and UBMT patients at a long-term period after allo-HCT could exist.
Figure 3.
Differences in B cell recovery between CBT and UBMT group
Complications after allo-HCT including chronic GVHD in CBT and UBMT group
Complications after allo-HCT have been summarized in Table 2. Acute GVHD (grade II-IV) was observed in 7 and 6 patients in CBT and UBMT groups, respectively. CMV antigenemia was more frequently detected in CBT group but cleared in all of cases within 6 months after allo-HCT. In addition, bacterial infections such as sepsis and febrile neutropenia were more frequently complicated in CBT group, as described previously17, 18. Chronic GVHD was observed in 7 of 21 patients in CBT group and 8 of 20 patients in UBMT group. Three of 7 patients in CBT group and 5 of 8 patients in UBMT group were diagnosed as extended type of chronic GVHD. Continuation of immnosuppressive therapy has been required in 2 and 3 patients in CBT and UBMT group, respectively. We have found no clear difference in the frequency and the severity of chronic GVHD between the two groups in our institute, although the incidence of chronic GVHD has been reported to be higher in UBMT than CBT patients7.
Table 2.
Complications after allo-HCT in the patients who had received cord blood transplantation (CBT) and unrelated bone marrow transplantation (UBMT)
| CBT |
|
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|
| Disease | Disease status | Age at allo-HCT | Gender | aGVHD (grade) | infection | CMV antigenemia | cGVHD | Sites of cGVHD |
| AML | NC | 57 | F | I | - | + | L | skin |
| ATL | CR1 | 57 | M | II | sepsis | + | L | skin |
| Ph+ALL | CR2 | 48 | M | I | FN | + | L | mouth |
| DLBCL | CR2 | 51 | F | I | sepsis | + | E | lung |
| CMMoL | NC | 40 | M | II | Tbc | + | E | skin, liver |
| Ph+ALL | CR2 | 44 | M | 0 | FN | + | - | |
| FL | NC | 56 | M | 0 | FN | + | E | lung |
| ALL | CR1 | 32 | F | I | - | + | - | |
| FL | CR3 | 51 | M | 0 | FN | + | L | |
| ALL | CR1 | 41 | M | 0 | sepsis | + | - | |
| AML | CR1 | 45 | M | 0 | - | + | - | |
| AML | CR2 | 45 | F | III | sepsis | - | - | |
| Ph+ALL | CR1 | 39 | F | II | sepsis | + | - | |
| AML | CR2 | 44 | F | II | sepsis | + | - | |
| PTCL | NC | 33 | M | II | sepsis | + | - | |
| AML | CR1 | 52 | F | 0 | FN | + | - | |
| AML | CR1 | 38 | F | II | - | + | - | |
| CML | CP1 | 24 | M | 0 | FN | + | - | |
| ATL | CR1 | 41 | F | 0 | FN | + | - | |
| MDS | RA | 62 | M | I | hemorrhagic cystitis | + | - | |
| Ph+ALL | CR1 | 63 | M | I | - | + | - | |
| UBMT | ||||||||
| Disease | Disease status | Age at allo-HCT | Gender | aGVHD (grade) | infection | CMV antigenemia | cGVHD | Sites of cGVHD |
| AML | CR1 | 25 | F | 0 | - | + | - | |
| MDS | RAEB2 | 54 | M | I | - | + | L | mouth |
| AML | CR1 | 34 | M | II | FN | + | E | lung |
| ALCL | CR2 | 56 | M | II | sepsis | + | E | mouth, eye |
| AML | CR2 | 48 | F | 0 | sepsis | - | - | |
| MM | sCR | 39 | F | I | FN | - | - | |
| DLBCL | CR2 | 44 | F | 0 | - | - | - | |
| FL | CR2 | 46 | F | I | pneumonia | - | E | eye, lung |
| MDS | RAEB2 | 26 | M | 0 | FN | - | - | |
| AML | CR1 | 26 | F | I | - | + | - | |
| MDS | RAEB2 | 38 | M | 0 | FN | - | - | |
| AML | CR1 | 38 | F | I | - | + | - | |
| PNH/AA | 39 | F | 0 | pneumonia | + | - | ||
| AML | CR1 | 49 | M | I | - | + | - | |
| MDS | RAEB2 | 29 | M | III | hemorrhagic cystitis | + | L | skin |
| CML | BC | 35 | F | I | FN | + | E | eye, skin, lung |
| NHL | CR2 | 32 | M | I | FN | + | - | |
| CML | CR2 | 38 | M | III | - | + | E | skin, mouth, eye |
| AML | CR2 | 20 | F | I | - | - | L | skin, eye |
| DLBCL | NC | 48 | F | III | FN | + | - |
Allo-HCT; allogeneic hematopoietic cell transplantation, CMV; cytomegalovirus, GVHD; graft-versus-host disease, AML; acute myelogenous leukemia, ATLL; adult T cell leukemia/lymphoma, Ph+ALL; Philadelphia chromosome positive acute lymphoblastic leukemia, DLBCL; diffuse large B cell lymphoma, CMMoL; chronic myelomomocytic leukemia, FL; follicular lymphoma, ALL; acute lymphoblastic leukemia, PTCL; peripheral T cell lymphoma, CML; chronic myelogenous leukemia, MDS; myelodysplastic syndrome, ALCL; anaplastic large cell lymphoma, MM; multiple myeloma, PNH/AA; paroxysmal nocturnal hematuria/aplastic anemia, NHL; non-Hodgkin lymphoma, NC; non-complete remission, CR1; first complete remission, CR2; second complete remission, CR3; third complete remission, RA; refractory anemia, RAEB; refractory anemia with excess blast, sCR; stringent complete remission, BC; blastic crisis, CB; cord blood, UBMT; unrelated bone marrow transplantation, FN; febrile neutropenia, Tbc; tuberculosis, L; limited type, E; extensive type.
To investigate whether the differences of immune reconstitution would exist between CBT and UBMT group in relation to the development of chronic GVHD, we further divided the patients into 4 groups according to the presence or absence of chronic GVHD as follows: the patients with cGVHD in CBT group (n=7), without cGVHD in CBT group (n=14), without cGVHD in UBMT group (n=12), with cGVHD in UBMT group (n=8).
Immune reconstitution in T and B cells in relation to chronic GVHD
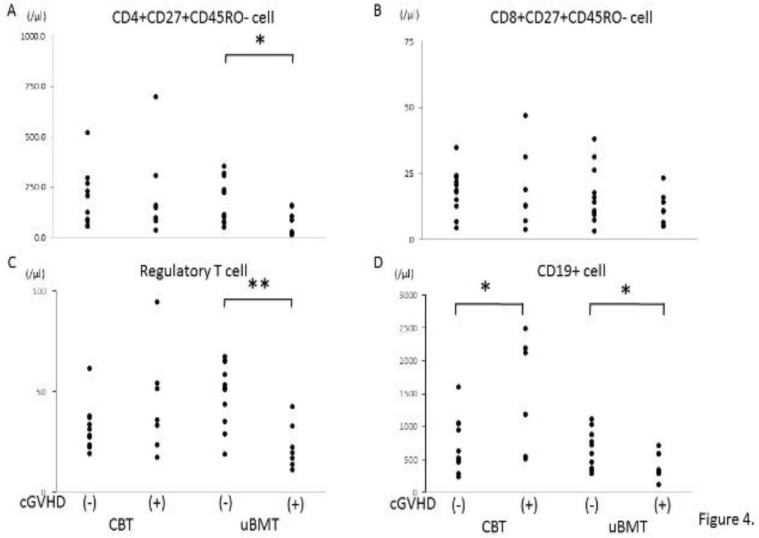
We examined T cell recovery between 4 groups described above. There was no significant difference in CD4+ and CD8+ cell number and CD4/CD8 ratio between 4 groups (data not shown). As shown in Figure 4A and B, absolute cell number in peripheral blood of naive CD4+ but not naive CD8+ T cells was significantly lower in the patients with cGVHD in UBMT group. Absolute cell number of regulatory T cell was lower in the patients with cGVHD in UBMT group without statistical significance (p=0.08, Figure 4C).
Figure 4.
Comparison of immune recovery in T and B cells between the patients with and without chronic GVHD
Absolute cell count of naïve CD4+ cell (A), naïve CD8+ cell (B), regulatory T cell (C), and CD19+ cell (D) of peripheral blood were compared between the patients with and without chronic GVHD in CBT and UBMT group. * indicates differences that are statistically significant (p<0.05). ** indicates p=0.08.
In B cells, absolute cell number of CD19+ cell in peripheral blood was higher in the patients with cGVHD in CBT group, but conversely lower in the patients with cGVHD in UBMT group, as shown in Figure 4D.
These results suggest that there exist some differences in immune reconstitution of both T and B cells in relation to the development of chronic GVHD between CBT and UBMT group
B cell subpopulations in relation to chronic GVHD among CBT and UBMT group
To more precisely investigate the differences in B cell reconstitution between the two groups in relation to chronic GVHD, we next examined the subpopulation of B cells.
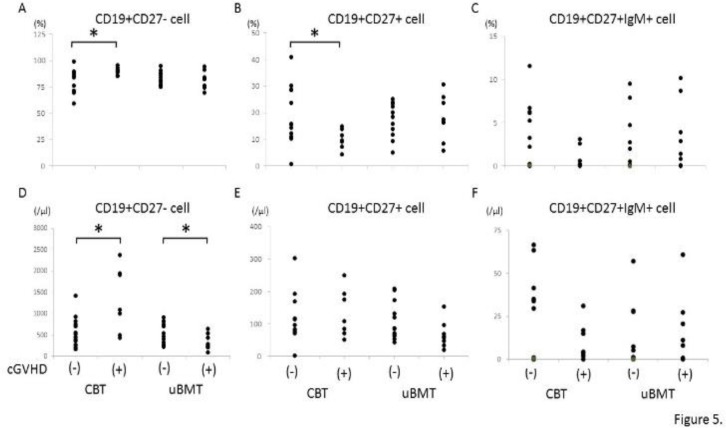
Both ratio and absolute cell number of naive B cell, which express CD19 but not CD27, were significantly higher in the patients with than without cGVHD in CBT group (Figure 5A and D). By contrast, absolute cell number but not ratio of naive B cell was significantly lower in the patients with than without cGVHD in UBMT group (Figure 5A and D). Concerning memory B cells, ratio but not absolute cell number of CD19+CD27+ cell in peripheral blood was significantly lower in the patients with than without cGVHD in CBT group, while there was no significant difference in memory B cells between the patients with and without chronic GVHD in UBMT group (Figure 5B and E).
Figure 5.
Comparison of immune recovery in B cell subpopulations between the patients with and without chronic GVHD
The ratio of naïve B cell (A), memory B cell (B), and IgM memory B cell (C) of peripheral blood were compared between the patients with and without chronic GVHD in CBT and UBMT group (upper column). Absolute cell number of naïve B cell (D), memory B cell (E), and IgM memory B cell (F) of peripheral blood were compared between the patients with and without chronic GVHD in CBT and UBMT group (lower column). * indicates differences that are statistically significant (p<0.05).
Regulatory B cells, which are also suggested to be involved in the pathogenesis of chronic GVHD, have been reported to be enriched in the IgM memory and transitional subsets11, 19. Therefore, we next examined the IgM memory B cells, designated as the cells which express CD19, CD27, and IgM on cell surface. As shown in Figure 5C and F, both ratio and absolute cell count were lower in the patients with cGVHD in CBT group, but the differences were not statistically significant. In UBMT group, we observed no significant difference in IgM memory B cells between the patients with and without chronic GVHD.
These results suggest that different immune reconstitution in T and B cells, which would be related to the development of chronic GVHD, could exist between CBT and UBMT patients.
Discussion
Recent advances of allo-HCT improve the prognosis of the patients with hematopoietic malignancies and result in the long-term survivors, while there have appeared several problems such as chronic GVHD in long-term survivors. Development of chronic GVHD results in the decrease of quality of life and increase non-relapse mortality in long-term survivors3-5. Recently, the pathogenesis of chronic GVHD has been elucidated8, 9. In the development of chronic GVHD, effector cells as well as regulatory cells have been reported to be involved10, 11, 19. Several reports have shown the differences in immune recovery between the patients transplanted with cord blood and peripheral blood stem cells from HLA-matched sibling or unrelated donor12-14. However, observation period for immune recovery was up to 2 years in these reports. It has been elucidated whether the differences of immune reconstitution between CBT and UBMT patients surviving more than 2 years would exist and relate to the development of chronic GVHD, although the incidence and the severity of chronic GVHD have been reported to be lower in CBT than UBMT patients among Japanese patients7.
In this study, we investigated the immunologic reconstitution by flow cytometric analysis in the patients who received CBT or UBMT, although the duration from allo-HCT to investigating time was varied in each patient. We observed several differences in both T- and B-cell lineages between the patients who received CBT and UBMT. Absolute cell number of CD8+ T cell was significantly higher in UBMT group, while no difference was observed in CD4+ T cell. As a result, the ratio of CD4/CD8 was significantly lower in UBMT group (Figure 2). In respect to B cells, absolute cell number of B cell was significantly lower in UBMT group (Figure 3A). In addition, absolute cell number of naive B cell was lower in UBMT group, but not significantly (Figure 3C, p=0.05). These results suggest that different immune reconstitution between CBT and UBMT patients could exist even more than 2 years after allo-HCT.
Nine of 21 patients in CBT group and 12 of 20 patients in UBMT group had received MAC as conditioning regimen. The difference of conditioning regimen (MAC versus RIC) would possibly affect the immune reconstitution in the patients surviving more than 2 years after allo-HCT. However, Wike et al. investigated the effect of conditioning regimen on marrow damage and hematopoietic recovery in adult leukemia patients. They reported that MAC regimen including TBI leads to prolonged reduction in marrow cellularity, but does not show additional histological long-term injury and differences in immunologic recovery20. In addition, Geyer et al. also showed that immune cell subset recovery, immunoglobulin reconstitution, and the incidence of opportunistic infections did not differ significantly between MAC and RIC regimen in pediatric CBT cohort21. These results can suggest that differences of conditioning regimen would not affect immune reconstitution in our investigation.
Regarding the relation to the occurrence of chronic GVHD, several differences have found between the patients with and without chronic GVHD in both CBT and UBMT group.
In respect to T cells, absolute cell number of naive CD4+ T cell was significantly lower in the patients with chronic GVHD in UBMT group (Figure 4A), while no significant difference was observed in naive CD8+ T cells and regulatory T cells (Figure 4B and C). Although regulatory T cells have been reported to be involved in the pathogenesis in chronic GVHD10, we have no significant difference between the patients with and without chronic GVHD in both groups. Although it has been observed that absolute cell number of regulatory T cell was lower in the patients with chronic GVHD in UBMT group, the difference was not statistically significant (p=0.08) (Figure 4C). Immune reconstitution of T cells may be different between CBT and UBMT patients even in long-term survivors and might be related to the pathogenesis of chronic GVHD at least in UBMT patients.
Concerning B cells, significant differences of absolute cell number of CD19+ B cell between the patients with and without chronic GVHD were observed in both CBT and UBMT group (Figure 4D). In addition, absolute cell number of naive B cell significantly differed between the patients with and without chronic GVHD in both groups. Absolute cell number of naive B cell was significantly higher in the patients with chronic GVHD in CBT group, while significantly lower in UBMT group (Figure 5D). As we could not examine regulatory B cells in this study, we compared IgM memory B cells, which have been reported to contain regulatory B cells11, between CBT and UBMT patients in relation to the development of chronic GVHD. There was no significant difference between the patients with and without chronic GVHD in both groups, although IgM memory B cells were lower in the patients with chronic GVHD in CBT group, but not statistically significant. However, our results suggest that immune reconstitutions of B cells play important roles in the pathogenesis of chronic GVHD in both CBT and UBMT patients,being consistent to previous reports22, 23.
Taken together, our results suggest that the differences in immune reconstitution of T and B cells may exist between CBT and UBMT patients even in long-term survivors and might be related to the differences in the development of chronic GVHD between CBT and UBMT patients.
Treatment for chronic GVHD has been still difficult and not satisfactory, but new strategies for the treatment of chronic GVHD have been recently evolved24-26. Low-dose infusion of interleukin-2 to stimulate regulatory T cells has been reported to improve symptoms of chronic GVHD27. Infusion of rituximab targeting for B cells has also been reported to be effective to chronic GVHD26, 28. Investigating the immunological differences in the development of chronic GVHD between CBT and UBMT would provide the understanding of the pathogenesis of chronic GVHD and the clue to introduce more effective treatment modalities for chronic GVHD.
In this study, there is no significant difference in the observation period from allo-HCT between two groups, although age at allo-HCT was older in CBT group. Moreover, in all of patients, GVHD prophylaxis was calcineurine inhibitor and short-term methotrexate.
CONCLUSION
Our results showed that the differences of immune reconstitution may exist between CBT and UBMT patients even in long- term survivors and might be correlated to the development of chronic GVHD, regardless of the small number of patients examined and the fact that longitudinal analysis was not used in our study. Studies in larger scale and more precise examinations of T and B cells would more clearly reveal the differences in the immune reconstitution and the roles of the pathogenesis of chronic GVHD in CBT and UBMT patients.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kurosawa S, Oshima K, Yamaguchi T, et al. Quality of Life after Allogeneic Hematopoietic Cell Transplantation According to Affected Organ and Severity of Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2017;23(10):1749–1758. doi: 10.1016/j.bbmt.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Majhail NS. Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2017;10(4):220–227. doi: 10.1016/j.hemonc.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117(17):4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman JM, Majhail NS, Klein JP, et al. Relapse and Late Mortality in 5-Year Survivors of Myeloablative Allogeneic Hematopoietic Cell Transplantation for Chronic Myeloid Leukemia in First Chronic Phase. J Clin Oncol. 2010;28(11):1888–1895. doi: 10.1200/JCO.2009.26.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin PJ, Counts Jr GW, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28(6):1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flowers MED, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda J, Nakasone H, Atsuta Y, et al. Risk factors and organ involvement of chronic GVHD in Japan. Bone Marrow Transplant. 2014;49(2):228–35. doi: 10.1038/bmt.2013.151. [DOI] [PubMed] [Google Scholar]
- 8.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–384. doi: 10.1182/blood-2014-01-514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeiser R, Sarantopoulos S, Blazar BR. B-cell targeting in chronic graft-versus-host disease. Blood. 2018;131(13):1399–1405. doi: 10.1182/blood-2017-11-784017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127(5):646–657. doi: 10.1182/blood-2015-10-672345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoder A, Sarvaria A, Alsuliman A, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124(13):2034–2045. doi: 10.1182/blood-2014-04-571125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson CA, Turki AT, McDonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(4):565–74. doi: 10.1016/j.bbmt.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda J, Chiou LW, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(11):1664–1676. doi: 10.1016/j.bbmt.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bejanyan N, Brunstein CG, Cao Q, et al. Delayed immune reconstitution after allogeneic transplantation increases the risks of mortality and chronic GVHD. Blood Adv. 2018;2(8):909–922. doi: 10.1182/bloodadvances.2017014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21(3):389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JA, Stevenson K, Kim HT, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood. 2010;115(20):4111–9. doi: 10.1182/blood-2009-09-244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballen K, Woo Ahn K, Chen M, et al. Infection Rates among Acute Leukemia Patients Receiving Alternative Donor Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(9):1636–1645. doi: 10.1016/j.bbmt.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Masson A, Bouaziz JD, Le Buanec H, et al. CD24 (hi) CD27⁺ and plasmablast-like regulatory B cells in human chronic graft-versus-host disease. Blood. 2015;125(11):1830–9. doi: 10.1182/blood-2014-09-599159. [DOI] [PubMed] [Google Scholar]
- 20.Wilke C, Holtan SG, Sharkey L, et al. Marrow damage and hematopoietic recovery following allogeneic bone marrow transplantation for acute leukemias: Effect of radiation dose and conditioning regimen. Radiother Oncol. 2016;118(1):65–71. doi: 10.1016/j.radonc.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geyer MB, Jacobson JS, Freedman J, et al. A comparison of immune reconstitution and graft-versus-host disease following myeloablative conditioning versus reduced toxicity conditioning and umbilical cord blood transplantation in paediatric recipients. Br J Haematol. 2011;155(2):218–34. doi: 10.1111/j.1365-2141.2011.08822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Gao Q, Feng Y, et al. Developing role of B cells in the pathogenesis and treatment of chronic GVHD. Br J Haematol. 2019;184(3):323–336. doi: 10.1111/bjh.15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarantopoulos S, Blazar BR, Cutler C, et al. B cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(1):16–23. doi: 10.1016/j.bbmt.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald KP, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129(1):13–21. doi: 10.1182/blood-2016-06-686618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler CS, Koreth J, Ritz J. Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood. 2017;129(1):22–29. doi: 10.1182/blood-2016-08-686659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakasone H, Sahaf B, Miklos DB. Therapeutic benefits targeting B-cells in chronic graft-versus-host disease. Int J Hematol. 2015;101(5):438–51. doi: 10.1007/s12185-015-1782-4. [DOI] [PubMed] [Google Scholar]
- 27.Koreth J, Kim HT, Jones KT, et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128(1):130–7. doi: 10.1182/blood-2016-02-702852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharfan-Dabaja , Cutler CS. Rituximab for prevention and treatment of graft-versus-host disease. Int J Hematol. 2011;93(5):578–585. doi: 10.1007/s12185-011-0855-2. [DOI] [PubMed] [Google Scholar]