Abstract
Background
Outbreaks of enterovirus D68 (EV‐D68) respiratory infections in children were reported globally in 2014. In Japan, there was an EV‐D68 outbreak in the autumn of 2015 (September–October). The aim of this study was to compare EV‐D68‐specific polymerase chain reaction (PCR)‐positive and EV‐D68‐specific PCR‐negative patients.
Methods
Pediatric patients admitted for any respiratory symptoms between September and October 2015 were enrolled. Nasopharyngeal swabs were tested for multiplex respiratory virus PCR and EV‐D68‐specific reverse transcription‐PCR. EV‐D68‐specific PCR‐positive and ‐negative patients were compared regarding demographic data and clinical information.
Results
A nasopharyngeal swab was obtained from 76 of 165 patients admitted with respiratory symptoms during the study period. EV‐D68 was detected in 40 samples (52.6%). Median age in the EV‐D68‐specific PCR‐positive and ‐negative groups was 3.0 years (IQR, 5.5 years) and 3.0 years (IQR, 4.0 years), respectively. The rates of coinfection in the two groups were 32.5% and 47.2%, respectively. There was no significant difference in the history of asthma or recurrent wheezing, length of hospitalization, or pediatric intensive care unit admission rate between the groups. The median days between symptom onset and admission was significantly lower for the EV‐D68‐positive group (3.0 days vs 5.0 days, P = 0.001). EV‐D68 was identified as clade B on phylogenetic analysis. No cases of acute flaccid myelitis were encountered.
Conclusions
More than half of the samples from the children admitted with respiratory symptoms were positive for EV‐D68‐specific PCR during the outbreak. Asthma history was not associated with the risk of developing severe respiratory infection.
Keywords: asthma, children, disease outbreak, enterovirus 68
Enterovirus D68 (EV‐D68) was first detected in 1962 in four American children with respiratory infections.1, 2 EV‐D68 is a type of non‐polio enterovirus sharing biological characteristics with both EV and rhinoviruses (RV). In fact, RV87 was reclassified as EV‐D68 in 2002.3 EV‐D68 was a rare serotype with only 26 cases reported between 1970 and 2005 under the voluntary surveillance system in the USA.3 After 2005, EV‐D68 infections began to be sporadically reported worldwide. In Japan, several cases detected in respiratory samples have been reported annually since 2005 except in 2010 and 2013, when the number of cases increased dramatically to 129 and 122, respectively.4 EV‐D68 drew global attention in 2014 after a huge outbreak of respiratory infections was reported in the USA: 1,152 cases were confirmed between August and December 2014.5 EV‐D68 infection mostly occurs in children presenting with cough and wheezing with or without a history of asthma or reactive airway disease.6 Of the 574 patients who were hospitalized in the USA, 59% required intensive care and 28% received mechanical ventilator support.6 EV‐D68 became a worldwide epidemic with a total of >2,000 cases reported in 20 countries in 2014, although only nine cases were reported in Japan in the same year.7 Concurrently, clusters of acute flaccid myelitis with or without evidence of EV‐D68 infection were also increasingly being reported.8, 9, 10, 11, 12 Due to the similarity of EV‐D68 to poliovirus and enterovirus A71, which are known to cause acute flaccid paralysis, EV‐D68 was strongly suspected of causing paralysis in some infected patients. This possibility remains under investigation.11, 13
In September 2015, the number of pediatric patients presenting cough and wheezing suddenly increased at Tokyo Metropolitan Children's Medical Center in Japan. The number of patients allocated under the Diagnosis Procedure Combination (DPC) codes for wheeze and asthma treatment doubled (Fig. 1). Although an EV‐D68 outbreak was not observed in 2014 in Japan, an infectious pathogen was suspected as the cause of the respiratory symptoms. Possible etiological agents included EV‐D68. Immediately, active surveillance was implemented and a prospective collection of respiratory samples was made in order to identify the pathogens in the hospitalized patients. Five samples obtained in the early stage of the investigation were sent for laboratory testing for several viruses including EV‐D68 to determine whether the further investigation and sampling from patients were warranted. In this sentinel testing, four out of five samples were positive on reverse transcription–polymerase chain reaction (RT‐PCR) for EV‐D68.14 The aim of this study was therefore to compare EV‐D68‐specific PCR‐positive and EV‐D68‐specific PCR‐negative patients regarding clinical manifestations during the 2015 surge in patients with respiratory symptoms.
Figure 1.
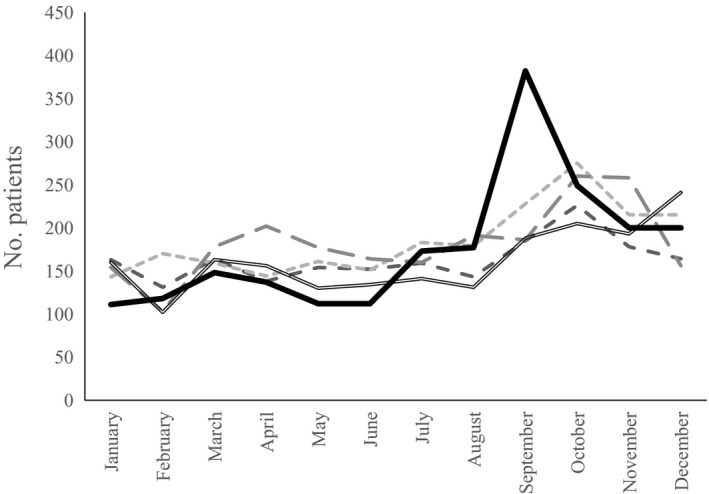
Annual distribution of inpatients and outpatients with asthma or reactive airway disease at Tokyo Metropolitan Children's Medical Center. ( ) 2011; (
) 2011; ( ) 2012; (
) 2012; ( ) 2013; (
) 2013; ( ) 2014; (
) 2014; ( ) 2015.
) 2015.
Methods
This was a single‐center outbreak investigation conducted between September 3 and October 5, 2015, at Tokyo Metropolitan Children's Medical Center (561 beds) in Japan. The study was a prospective design with pre‐defined inclusion and exclusion criteria with respect to study implementation, but patient characteristics and clinical information were collected retrospectively. All patients admitted to the division of general pediatrics are given a written document containing all the required elements of informed consent that asks guardians to allow the physician to collect and utilize anonymized clinical data in research in the future and gives guardians the opportunity and sufficient time to opt out of providing permission when they are admitted to the hospital. Consent forms for research enrollment were obtained from guardians. Inclusion criteria were all hospitalizations with any respiratory symptoms and informed consent from guardians for sample collection and molecular analysis. Demographic and clinical information including the length of hospitalization were collected from the electronic medical records. Respiratory symptoms were defined as cough, rhinorrhea, wheezing, and hypoxia without cardiac etiology. Axillary temperature >38°C (100.4°F) was defined as a fever. Nasopharyngeal swabs were tested on multiplex respiratory virus PCR and EV‐D68‐specific RT‐PCR. All samples obtained were routinely tested on PCR for viruses, but mycoplasma was tested only in selected cases. DNA and RNA were extracted from the nasopharyngeal swabs for processing using the QIAamp MinElute virus spin kit (Qiagen, Hilden, Germany). RT‐PCR was performed at Tokyo Metropolitan Children's Medical Center for amplification using a commercial multiplex virus PCR assay (ScyMed, Bunkyo, Tokyo, Japan) for the following viruses: respiratory syncytial viruses (RSV) A and B, influenza viruses A, B and A pdm2009, human metapneumovirus, human bocavirus, adenovirus, rhinovirus, cytomegalovirus, and coronavirus. Mycoplasma pneumonia was tested for using the loop‐mediated isothermal amplification method (Eiken Chemical, Tokyo, Japan)15 when physicians in charge considered it as a potential etiologic pathogen based on patient age and symptoms regardless of the patient's eligibility for this study. EV‐D68‐specific real‐time RT‐PCR was performed at the National Institute of Infectious Diseases in Tokyo.16 Sequence analysis of the partial viral structural protein (VP)1 region was performed for several EV‐D68‐specific real‐time RT‐PCR positive samples. The partial VP1 region of EV‐D68 was amplified from the clinical samples on semi‐nested RT‐PCR,17 and a phylogenetic tree was generated using the neighbor‐joining method based on the partial VP1 sequences (340 nucleotides in length) of four EV‐D68 strains in this study (accession numbers LC413952–LC 413955 in the DDBJ database) and the other 77 strains representing all clades and sub‐clades of EV‐D68 strains. Blood samples were collected when physicians thought the procedure appropriate regardless of whether patients were eligible for this study. Consolidation or ground‐glass opacity, hyperlucent lung such as air trapping on chest X‐ray were defined as abnormal, and all chest X‐rays were checked and interpreted by a pediatric radiologist.
Statistical analysis
Patients with positive EV‐D68 PCR were compared with a control group of patients who had a negative EV‐D68 PCR. Continuous variables are described as median (IQR) and were analyzed using Wilcoxon rank‐sum test. Nominal variables are described as n (%) and were analyzed using the Pearson chi‐squared test, or Fisher's exact test, to determine the significance between groups. In the EV‐D68 PCR‐positive patients, the association between explanatory variables and severity was analyzed using univariate logistic regression. Severity was defined as the presence of either intensive care unit (ICU) admission, the use of magnesium sulfate, non‐invasive positive pressure or mechanical ventilation.18, 19 Hospitalization duration was treated as a binary variable and classified according to the median on univariate analysis. Two‐sided P‐values were used for all analyses. All statistical analyses were performed using Stata Statistical Software Release 14. (StataCorp 2015; College Station, TX, USA). An institutional review board granted approval for this study (no. H27b‐149).
Results
During the study period, 165 hospitalized patients were eligible for enrollment, and a nasopharyngeal swab was obtained from 76 patients (46.1%; Fig. 2). One sample was taken from a tracheostomy tube. EV‐D68 was detected in 40 samples (52.6%). The number of positive samples peaked in mid‐September 2015 (Fig. 3). Three children with EV‐D68 required admission to the pediatric intensive care unit (PICU). No case of acute flaccid paralysis or mortality was encountered in the study cohort. On phylogenetic analysis of several EV‐D68 strains in the study cohort, clade B was identified, as in the strains mainly detected in other parts of Asia and the USA in 2014 (Fig. 4).20
Figure 2.
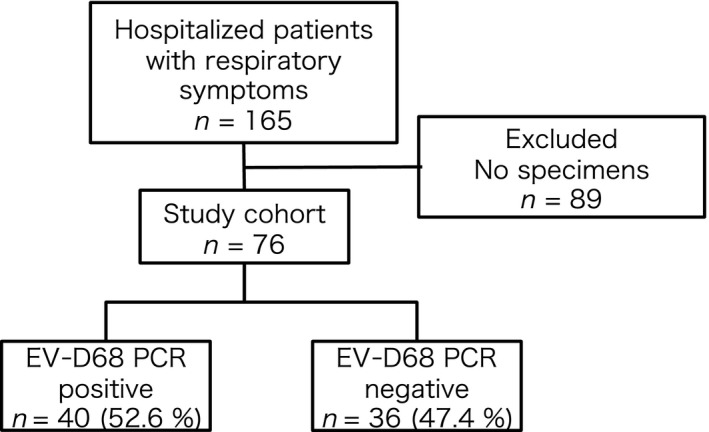
Subject selection. EV‐D68, enterovirus D68; PCR, polymerase chain reaction.
Figure 3.
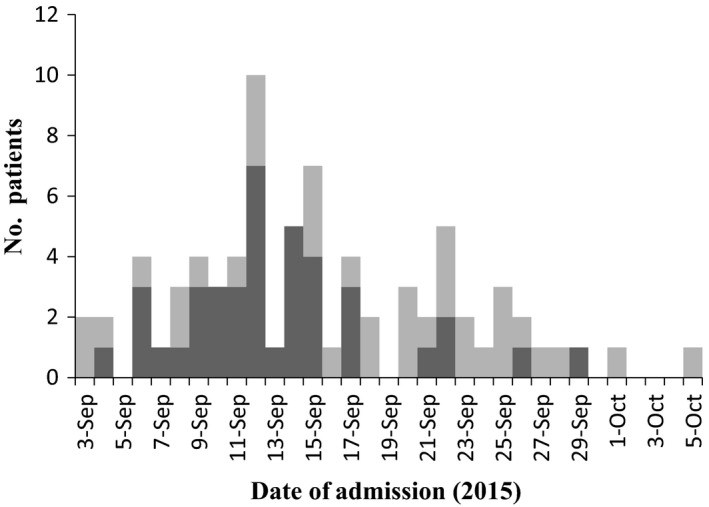
Distribution of enterovirus D68 (EV‐D68) polymerase chain reaction ( ) negative and (
) negative and ( ) positive patients at Tokyo Metropolitan Children's Medical Center, September and October 2015.
) positive patients at Tokyo Metropolitan Children's Medical Center, September and October 2015.
Figure 4.
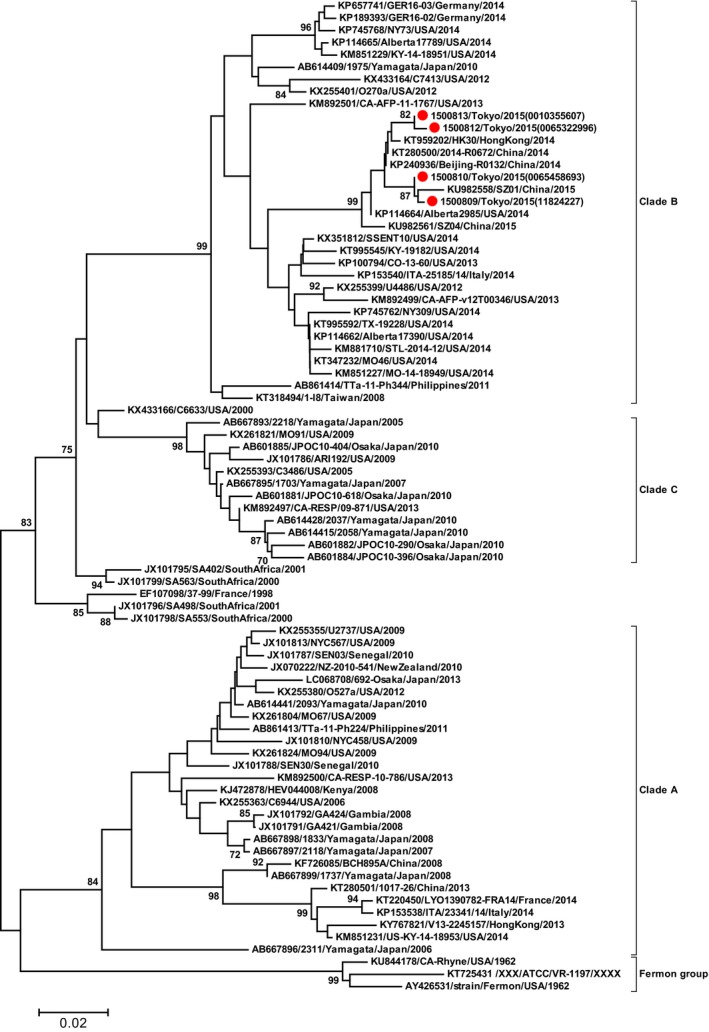
Phylogenetic tree based on partial viral structural protein (VP)1 sequences including the ( ) four Japanese enterovirus D68 (EV‐D68) strains in this study and another 77 representative EV‐D68 strains for each genetic clade (n=81 in total). The tree was constructed using the Neighbor joining and Tamura 3‐parameter included in MEGA639 with bootstrap values after 1,000 replicate trials. Genetic distance, 0.02. Percent bootstrap support, value at each node when ≥70%.
) four Japanese enterovirus D68 (EV‐D68) strains in this study and another 77 representative EV‐D68 strains for each genetic clade (n=81 in total). The tree was constructed using the Neighbor joining and Tamura 3‐parameter included in MEGA639 with bootstrap values after 1,000 replicate trials. Genetic distance, 0.02. Percent bootstrap support, value at each node when ≥70%.
Table 1 lists the participant characteristics and symptoms. The duration between the onset of symptoms and the timing of the sampling (P < 0.01) as well as that between the onset of symptoms and admission (P < 0.01) was significantly shorter in the EV‐D68‐specific PCR‐positive group. All blood cultures were negative for the tested patients (0/75).
Table 1.
Subject characteristics and symptoms†
| Characteristic |
EV‐D68 PCR positive n = 40 n (%) or ‡median (IQR) |
EV‐D68 PCR negative n = 36 n (%) or ‡median (IQR) |
P‐value | OR (95%CI) |
|---|---|---|---|---|
| Male | 25 (62.5) | 23 (63.9) | 1.00§ | 0.94 (0.33–2.65) |
| Median age, years (range)‡ | 3.0 (5.5) | 3.0 (4.0) | 0.36¶ | NA |
| Viral coinfection | 13 (32.5) | 17 (47.2) | 0.24§ | 0.54 (0.19–1.51) |
| Bacterial and other coinfection†† | 0 (0.0) | 3 (8.3) | 0.10§ | 0 (0–1.11) |
| Symptoms at presentation | ||||
| Cough | 40 (100) | 35 (97.2) | NA | NA |
| Missing | 0 (0.0) | 1 (2.8) | ||
| Rhinorrhea | 11 (27.5) | 19 (52.8) | 0.19§ | 0.36 (0.075–1.66) |
| Missing | 21 (52.5) | 12 (33.3) | ||
| Wheeze | 34 (85.0) | 29 (80.6) | 0.34§ | 2.05 (0.46–10.45) |
| Missing | 2 (5.0) | 0 (0.0) | ||
| Fever | 26 (65.0) | 28 (77.8) | 0.31§ | 0.53 (0.17–1.64) |
| Days between onset of symptoms and timing of sample‡ | 3.0 (5.0) | 5.0 (4.5) | 0.0097 ¶ | NA |
| Days between onset of symptoms and admission‡ | 1.0 (2.0) | 4.0 (3.0) | 0.0001 ¶ | NA |
| Underlying disease | ||||
| Asthma/repeated wheezing | 14 (35.0) | 14 (40.0) | 0.81§ | 0.81 (0.29–2.29) |
| Missing | 0 (0.0) | 1 (2.8) | ||
| NMD | 3 (7.5) | 3 (8.3) | 1.00§ | 0.89 (0.11–7.14) |
| Premature birth | 4 (10.3) | 3 (8.8) | 1.00§ | 1.18 (0.18–8.68) |
| Missing | 1 (2.5) | 2 (5.6) | ||
| CVD | 2 (5.0) | 3 (8.3) | 0.66§ | 0.57 (0.046–5.41) |
| Chromosome abnormality | 2 (5.0) | 1 (2.7) | 1.00§ | 1.84 (0.091–111.72) |
| LOH (days)‡ | 5.0 (4.0) | 5.5 (2.5) | 0.75¶ | NA |
| PICU admission | 3 (7.5) | 3 (8.3) | 1.00§ | 0.89 (0.11–7.14) |
| NIPPV | 1 (2.5) | 1 (2.8) | 1.00§ | 0.90 (0.011–72.43) |
| Saturation < 93% on room air | 35 (87.5) | 28 (77.8) | 0.36§ | 2 (0.51–8.61) |
| Supplemental oxygen | 39 (97.5) | 33 (91.7) | 0.34§ | 3.55 (0.27–190.78) |
| I.v. steroid | 31 (77.5) | 21 (58.3) | 0.088§ | 2.46 (0.82–7.59) |
| I.v. magnesium sulfate | 6 (15.0) | 2 (5.6) | 0.27§ | 3 (0.48–32.0) |
| WBC (/μL)‡ | 11 980.0 (4,0.0) | 12 585.0 (7,670.0) | 1.00¶ | NA |
| CRP (mg/L)‡ | 0.70 (1.57) | 1.88 (3.38) | 0.035 ¶ | NA |
| Eosinophilia > 500/μL at presentation | 5 (13.2) | 1 (2.9) | 0.20§ | 5.15 (0.52–250.64) |
| Missing | 2 (5.0) | 1 (2.8) | ||
| Abnormal chest X‐ray‡‡ | 13 (33.3) | 16 (45.7) | 0.34§ | 0.59 (0.21–1.68) |
| Missing | 1 (2.5) | 1 (2.8) | ||
Bold, P< 0.05. †Percentages were calculated based on total numbers excluding missing values for each characteristic. §Fisher's exact test, ¶Wilcoxon rank‐sum test. ††Positive for Mycoplasma pneumonia on loop‐mediated isothermal amplification or culture‐confirmed invasive bacterial infection (n = 3). ‡‡Consolidation or ground glass opacity, hyperlucent lung due to air trapping. CRP, C‐reactive protein; CVD, cardiovascular disease; EV‐D68, enterovirus D68; LOH, length of hospitalization; NMD, neuromuscular disease; NIPPV, non‐invasive positive pressure ventilation; PCR, polymerase chain reaction; PICU, pediatric intensive care unit; WBC, white blood cells.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Viral coinfection was detected for RSV types A and B, human metapneumovirus, human rhinovirus, adenovirus, cytomegalovirus, and human coronavirus (Table 2). Mycoplasma pneumoniae was tested in five patients when it was considered to be a plausible etiologic agent depending on patient age and clinical manifestation, and detected in three patients who were negative for EV‐D68 PCR. Only two sputum culture samples obtained from an intubated patient and a tracheostomy patient grew methicillin‐resistant Staphylococcus aureus and Pseudomonas aeruginosa, which were not considered to be pathogens but colonization. Table 3 lists potential univariate predictors of EV‐D68 positivity. Of the children hospitalized for EV‐D68, 14 (35.0%) had a previous history of asthma. On univariate analysis, however, the history of asthma, hospitalization in ICU or prolonged hospitalization as a determinant of severity were not statistically significant. Other previously suggested predictors including age, sex, and prematurity18 were also not predictive of severe infection, which was defined as the presence of either ICU admission, use of magnesium sulfate, non‐invasive positive pressure or mechanical ventilation in this study.
Table 2.
Multiplex PCR results
| Pathogen |
EV‐D68 positive n = 40 n (%)† |
EV‐D68 negative n = 36 n (%)† |
P‐value‡ | OR (95%CI) |
|---|---|---|---|---|
| RSVa | 2 (5.0) | 5 (13.9) | 0.25 | 0.33 (0.030‐2.19) |
| RSVb | 1 (2.5) | 2 (5.6) | 0.60 | 0.43 (0.0072–8.81) |
| Influenza virus A | 0 (0) | 0 (0) | NA | NA |
| Influenza virus B | 0 (0) | 0 (0) | NA | NA |
| Influenza virus A pdm2009 | 0 (0) | 0 (0) | NA | NA |
| hMPV | 0 (0) | 1 (2.8) | 0.47 | NA |
| Human bocavirus | 0 (0) | 0 (0) | NA | NA |
| HRV | 8 (20.0) | 8 (22.2) | 1.00 | 0.88 (0.25–3.07) |
| Adenovirus | 0 (0) | 1 (2.8) | 0.47 | NA |
| CMV | 1 (2.5) | 1 (2.8) | 1.00 | 0.90 (0.011–72.43) |
| HCoV | 1 (2.5) | 0 (0) | 1.00 | NA |
†Percentages were calculated based on total numbers excluding missing values for each pathogen. ‡Fisher's exact test. CMV, cytomegalovirus; EV‐D68, enterovirus D68; HCoV, human coronavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; PCR, polymerase chain reaction; RSV, respiratory syncytial virus.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 3.
Univariate indicators of severe disease in pediatric EV‐D68 infection, TMCMC, September–October 2015 (n = 40)
| Severity† | ICU admission | Hospitalization duration (days)‡ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | OR (95%CI) | P‐value | No | Yes | OR (95%CI) | P‐value | ≤5 | >5 | OR (95%CI) | P‐value | |
| Sex | ||||||||||||
| Male | 20 | 5 | 1.0 (−0.16 to 7.61) | 0.65 | 23 | 2 | 1.22 (0.058–76.88) | 1.00 | 15 | 10 | 0.44 (0.098–1.96) | 0.18 |
| Female | 12 | 3 | 14 | 1 | 6 | 9 | ||||||
| Prematurity | ||||||||||||
| Yes | 4 | 0 | NA | 0.44 | 4 | 0 | NA | 0.80 | 1 | 3 | 4.0 (0.28–220.25) | 0.32 |
| No | 28 | 7 | 33 | 2 | 20 | 15 | ||||||
| History of asthma | ||||||||||||
| Yes | 11 | 3 | 0.59 (0.15–7.22) | 1.00 | 13 | 1 | 0.92 (0.015–19.39) | 1.00 | 9 | 5 | 0.48 (0.098–2.17) | 0.33 |
| No | 21 | 5 | 24 | 2 | 12 | 14 | ||||||
| History of CVD | ||||||||||||
| Yes | 1 | 1 | 4.43 (0.05–358.49) | 0.36 | 1 | 1 | 18.0 (0.15–1,448.79) | 0.15 | 0 | 2 | NA | 0.22 |
| No | 31 | 7 | 36 | 2 | 21 | 17 | ||||||
| History of NMD | ||||||||||||
| Yes | 2 | 1 | 2.14 (0.032–45.87) | 0.50 | 3 | 0 | NA | 1.00 | 1 | 2 | 2.35 (0.11–145.62) | 0.60 |
| No | 30 | 7 | 34 | 3 | 20 | 17 | ||||||
| Age (years) | ||||||||||||
| <2 | 9 | 1 | ref | ref | 10 | 0 | NA | ref | 4 | 6 | ref | ref |
| 2–5 | 12 | 4 | 3.0 (0.28–31.63) | 0.36 | 14 | 2 | 1.00 | 8 | 8 | 0.67 (0.13–3.30) | 0.62 | |
| >5 | 11 | 3 | 2.45 (0.21–27.84) | 0.47 | 13 | 1 | 1.00 | 9 | 5 | 0.37 (0.070–1.97) | 0.24 | |
†ICU admission, use of magnesium sulfate, non‐invasive positive pressure or mechanical ventilation. ‡Dichotomized according to the median. CVD, cardiovascular disease; EV‐D68, enterovirus D68; ICU, intensive care unit; NA, not applicable because of the small number of reports; NMD, neuromuscular disease; TMCMC, Tokyo Metropolitan Children's Medical Center.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Discussion
We encountered an outbreak of EV‐D68 in the autumn of 2015 (September–October), in which the number of patients with respiratory symptoms who required treatment for wheezing increased dramatically compared with previous years. Similar increases were reported in several locations throughout Japan during the same period following our first report,14 and a nationwide EV‐D68 outbreak was subsequently confirmed.21, 22, 23
Enterovirus are generally endemic to the temperate zone, occur most frequently during the summer, and cause hand‐foot‐mouth disease, herpangina, and aseptic meningitis.3 Interestingly, EV‐D68 shows a distinct seasonal preference judging by the increase in reports during late summer and autumn.24, 25 During the global outbreak in 2014, North America and Europe reported cases from August to December. In Japan as well, a major outbreak of EV‐D68 peaked in the autumn of 2015.4 The reason for this seasonal circulation of EV‐D68, which often occurs later than typical EV outbreaks, remains unknown.5 In an exceptional report from Australia in 2010 and 2013, EV‐D68 was detected during the winter–spring months of July–October.26 Although one article suggested an endemic circulation of EV‐D68 in Taiwan,27 whether EV‐D68 is endemic in Japan or globally remains to be clarified.7
In the present study, the duration from the onset of symptoms to admission and sampling was shorter for the EV‐D68 PCR‐positive group than for the ‐negative group. There are two possible explanations. First, the EV‐D68 PCR‐positive group may have experienced rapid progression of symptoms requiring earlier hospitalization than the EV‐D68 PCR‐negative group. Second, although some patients in the PCR‐negative group may have been infected with EV‐D68, patients with slow progression of symptoms could have been sampled later in the clinical course, resulting in a viral load undetectable on PCR. In the acute flaccid myelitis patients, the EV‐D68 PCR detection rate was higher when sampling was performed ≤7 days from onset (47%) compared with the overall rate (20%).12, 28
The percentage of patients who required more intensive treatment such as PICU admission, supplemental oxygen, or i.v. magnesium sulfate did not significantly differ between the EV‐D68‐positive and ‐negative groups. EV generally cause severe illnesses in young infants such as viral sepsis, aseptic meningitis, encephalitis, or myocarditis.3 Unlike other EV, EV‐D68 typically affects older children with asthma‐like respiratory diseases. The present findings were also in line with a previous report from the USA on the outbreak of 2014 in which the median age was 5 years6 for the EV‐D68‐positive group, a relatively higher age distribution than seen in other common infectious agents such as RSV.
Regarding host factors, a history of asthma has been reported as a risk factor for severe EV‐D68 respiratory disease.19, 29, 30 In the present study, EV‐D68 infection with a history of asthma was not associated with either severity (defined as ICU admission, magnesium sulfate use, or ventilation support) nor prolonged hospitalization. While none of the three patients who were EV‐D68 PCR positive and admitted to the ICU had a history of asthma, one patient had a history of milk allergy and chromosome abnormality. In contrast, one of three patients who were EV‐D68 PCR negative and admitted to the ICU had a history of asthma and preterm delivery. Although these results are not in line with some previous reports and the present retrospective design might have meant that thorough history taking was not possible, it should be noted that the reliability and validity of asthma diagnosis are likely to differ across different studies. One study from the Netherlands compared three methods, namely, self‐reported asthma, ICD‐10 diagnosis from a hospitalization registry, and data on anti‐asthmatic medication use from a prescription registry to explore the prevalence of asthma and examine the agreement between different methods, and found a substantial non‐overlap between the methods regarding asthma prevalence.31 Another article reported that dependence on parental report might result in underestimation of the prevalence of serious asthma, especially among poor children in the USA.32 This suggests that different methods produce different prevalences of asthma even in the same cohort of children, and therefore it is plausible that this would happen across different cohorts. Moreover, a diagnosis of asthma in early childhood may not be accurate due to the frequency of viral infections resembling asthmatic symptoms.33, 34 In short, whether or not the presence of asthma influences the severity of EV‐D68 respiratory infection requires further research.
On phylogenetic analysis of EV‐D68 in the present study and in other reports from Japan, the EV‐D68 strains in 2015 were identified as genetic clade B of EV‐D68.21, 22, 35, 36 In the USA, EV‐D68 respiratory infections were suspected in connection with 120 cases of pediatric acute flaccid paralysis/myelitis.12 Although we encountered no cases of acute flaccid paralysis/myelitis in the present study, several cases of acute flaccid myelitis following the confirmation of EV‐D68 infection were reported in Japan in 2015.28 A national, active, symptom‐based surveillance for acute flaccid myelitis conducted between August and December 2015 in Japan identified 59 cases.28 Although EV‐D68 was detected in the cerebrospinal fluid specimen in only one adult patient, a significant temporal correlation between the acute flaccid myelitis epidemic curve and the number of EV‐D68 detections was noted on national pathogen surveillance.28
The present study has a number of limitations. First, although we collected information from all patients with defined respiratory symptoms who were admitted during the study period, we were able to recruit and obtain samples for EV‐D68 and multiplex PCR in only 46% of the hospitalized patients because patients with short‐term admission were discharged before providing informed consent and samples. This low recruitment rate could have biased the results for missing mild cases. This potential selection bias, however, is less likely to have distorted the result because no one, including physicians, investigators, or patients, knew the EV‐D68 case status at the time of recruitment. Second, several respiratory pathogens such as parainfluenza virus or Bordetella pertussis were not tested for in the study and might have been missed. Third, mild cases of EV‐D68 infection might have been overlooked because one of the inclusion criteria was hospitalization. The restriction of recruitment to patients who were admitted precluded us from obtaining samples from patients who visited the outpatient ward. As a result, we could not infer to what degree EV‐D68 was associated with symptoms in patients who had wheezing but in whom the symptoms were not severe enough to require hospitalization during this period, although we observed a surge in patients with wheezing, which was unprecedented (Fig. 1) and the same phenomenon was observed nationally.23 Fourth, in the EV‐D68 PCR‐negative group, some patients infected with EV‐D68 might have been missed on PCR due to the long interval between sampling and symptom onset. Centers for Disease Control and Prevention recommend collecting samples 0–7 days after symptom onset for real‐time PCR to detect non‐polio Enterovirus.37 In addition, according to nationwide surveillance in the USA, respiratory specimens collected ≤7 days after respiratory illness/fever onset compared with any time had a higher detection rate of EV‐D68 (47% vs 20%).12 Thus, for the additional analyses, we chose 7 days as the cut‐off point from the onset of any symptoms. We found a statistically significant difference in EV‐D68 positivity between patients whose sample were collected ≤7 days after onset and ≥8 days (P = 0.044, Fisher's exact test). Nonetheless, we did not find a significant difference in baseline and clinical characteristics and concluded that the misclassification had not substantially biased the result (Tables 1, 2). Finally, there might remain a potential misclassification, stemming from the fact that the EV‐D68‐negative group could contain both patients with viral infection and those with asthma attack due to non‐infectious causes. Thus, we conducted an additional analysis by dividing the EV‐D68‐negative group into two groups: EV‐D68 negative but positive on multiplex RT‐PCR, and negative for both EV‐D68 and multiplex RT‐PCR. Then, we compared baseline and clinical characteristics between the three groups, namely, EV‐D68 PCR positive group, EV‐D68 PCR negative but positive on multiplex RT‐PCR, and negative for both EV‐D68 and multiplex RT‐PCR, and confirmed that there was no significant difference between the results in Table 1 and that of the additional analysis. Furthermore, it may be reasonable that the potential misclassification regarding the disease status among EV‐D68‐negative group was likely to be non‐differential, and multiplex PCR is faster and more sensitive for viral detection than traditional viral culture methods.38
In conclusion, more than half of the samples from the children admitted with respiratory symptoms were positive for EV‐D68‐specific PCR during the surge in the number of patients with respiratory symptoms. In contrast to previous research, asthma history was not associated with the risk of developing severe respiratory infections in the present study.
Disclosure
Y.H. reports grants from Asahi Kasei Pharm, Janssen Pharm, Fuji Film Pharm, BD, MSD Pharm, Abbvie GK, Astellas Pharm, Taisho Toyama Pharm, JCR Pharm, Maruho, Japan Vaccine, Sumitomo Dainippon Pharm, Pfizer Japan, Meiji Seika Pharm, outside the submitted work. Y.M. reports grants from Japan Foundation For Pediatric Research, grants from Tokyo Metropolitan Government, grants from Japan Kawasaki Disease Research Center, grants from Health Labour Sciences Research Grant, grants from Japan Agency for Medical Research and Development, grants from Center for Clinical Trials, Japan Medical Association, outside the submitted work. Dr H.S. reports grants from Japan Agency for Medical Research and Development (AMED), during the conduct of the study; and from The Research Foundation for Microbial Diseases of Osaka University (BIKEN), Japanese Society for Neuroinfectious Diseases, Japan Society for the Promotion of Science, Ministry of Health, Labour and Welfare of Japan, outside the submitted work. The other authors declare no conflict of interest.
Author contributions
Y.F. contributed to the conception and design of this study; Y.F. performed the statistical analysis and drafted the manuscript; A.S., T.C., T.A., Y.H.D., H.S., M.K., N.H., T.F. performed laboratory analysis and created the phylogenetic tree; K.I., S.M., K.K., Y.M., T.K., Y.T., K.W., N.S. gave technical support and conceptual advice, Y.H. critically reviewed the manuscript and supervised the whole study process. All authors read and approved the final manuscript.
Acknowledgments
We thank Mr James R Valera for assistance with editing, and the physicians at the Division of General Pediatrics, Tokyo Metropolitan Children’s Medical Center, for sample collection. This study was supported by Tokyo project research grant (H27‐28) and AMED Grant Number 18fk0108004j0003 and 17fk0108204j0702 to T.F., And by Tokyo project research grant (H27‐30) to Y.H., K.I. and MOH Science Research grant to Y.H.
References
- 1. Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am. J. Epidemiol. 1967; 85: 297–310. [DOI] [PubMed] [Google Scholar]
- 2. Bragstad K, Jakobsen K, Rojahn AE et al High frequency of enterovirus D68 in children hospitalised with respiratory illness in Norway, autumn 2014. Influenza Other Respir. Viruses 2015; 9: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khetsuriani N, Lamonte‐Fowlkes A, Oberst S, Pallansch MA. Enterovirus surveillance–United States, 1970‐2005. MMWR Surveill. Summ. 2006; 55 (8): 1–20. [PubMed] [Google Scholar]
- 4. National Institute of Infectious Diseases . Epidemiology of enterovirus D68 in Japan, 2005‐2015 (as of 20 January 2016). IASR; 2016; 33–5. Shinjuku, Japan [Google Scholar]
- 5. Oermann CM, Schuster JE, Conners GP, Newland JG, Selvarangan R, Jackson MA. Enterovirus d68A. Focused review and clinical highlights from the 2014 U.S. Outbreak. Ann. Am. Thorac. Soc. 2015;12:775–81. [DOI] [PubMed] [Google Scholar]
- 6. Midgley CM, Watson JT, Nix WA et al Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): A descriptive epidemiological investigation. Lancet. Respir. Med. 2015; 3: 879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holm‐Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: A systematic review. Lancet. Infect. Dis. 2016; 16: e64–75. [DOI] [PubMed] [Google Scholar]
- 8. Pfeiffer HC, Bragstad K, Skram MK et al Two cases of acute severe flaccid myelitis associated with enterovirus D68 infection in children, Norway, autumn 2014. Euro. Surveill. 2015; 20: 21062. [DOI] [PubMed] [Google Scholar]
- 9. Greninger AL, Naccache SN, Messacar K et al A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012‐14): A retrospective cohort study. Lancet. Infect. Dis. 2015; 15: 671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lang M, Mirand A, Savy N et al Acute flaccid paralysis following enterovirus D68 associated pneumonia, France, 2014. Euro. Surveill.. 2014;19 pii=20952. [DOI] [PubMed] [Google Scholar]
- 11. Messacar K, Schreiner TL, Maloney JA et al A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet 2015; 385 (9978): 1662–71. [DOI] [PubMed] [Google Scholar]
- 12. Sejvar JJ, Lopez AS, Cortese MM et al Acute flaccid myelitis in the United States, August–December 2014: Results of Nationwide Surveillance. Clin. Infect. Dis. 2016; 63: 737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N. Engl. J. Med. 1999; 341: 936–42. [DOI] [PubMed] [Google Scholar]
- 14. Ito K, Horikoshi Y, Funakoshi Y et al National Institute of Infectious Diseases Detection of enterovirus D68 from 4 pediatric cases, September 2015‐Tokyo. IASR; 2015; 36: 193–5. Shinjuku, Japan (In Japanese). [Google Scholar]
- 15. Yoshino M, Annaka T, Kojima T, Ikedo M. Sensitive and rapid detection of Mycoplasma pneumoniae by loop‐mediated isothermal amplification. Kansenshogaku Zasshi 2008; 82 (3): 168–76. [DOI] [PubMed] [Google Scholar]
- 16. Wylie TN, Wylie KM, Buller RS, Cannella M, Storch GA. Development and evaluation of an Enterovirus D68 Real‐time reverse transcriptase PCR assay. J. Clin. Microbiol. 2015; 53: 2641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 2006; 44: 2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuffenecker I, Mirand A, Josset L et al Epidemiological and clinical characteristics of patients infected with enterovirus D68, France, July to December 2014. Euro. Surveill. 2016;21(19). https://doi.org.10.2807/1560-7917.ES.2016.21.19.30226. [DOI] [PubMed] [Google Scholar]
- 19. Schuster JE, Miller JO, Selvarangan R et al Severe enterovirus 68 respiratory illness in children requiring intensive care management. J. Clin. Virol. 2015; 70: 77–82. [DOI] [PubMed] [Google Scholar]
- 20. Tan Y, Hassan F, Schuster JE et al Molecular evolution and intraclade recombination of enterovirus D68 during the 2014 outbreak in the United States. J. Virol. 2015; 90: 1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ikuse I, Maruyama K, Fuse R et al Increase in asthma attacks and detection of EV‐D68, September 2015‐Tsuruoka City, Yamagata Prefecture. IASR; 2015; 36: 248–9. Shinjuku, Japan (In Japanese). [Google Scholar]
- 22. Ito T, Nakamura H, Azuma R et al Association between increased inpatient admission of children with asthma attacks and EV‐D68 activity in autumn 2015‐Tsu City, Mie Prefecture. IASR; 2015; 36: 250–2. [Google Scholar]
- 23. Korematsu S, Nagashima K, Sato Y et al “Spike” in acute asthma exacerbations during enterovirus D68 epidemic in Japan: A nation‐wide survey. Allergol. Int. 2018; 67: 55–60. [DOI] [PubMed] [Google Scholar]
- 24. Tokarz R, Firth C, Madhi SA et al Worldwide emergence of multiple clades of enterovirus 68. J. Gen. Virol. 2012; 93 (Pt 9): 1952–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Todd AK, Hall RJ, Wang J et al Detection and whole genome sequence analysis of an enterovirus 68 cluster. Virol. J. 2013; 10: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levy A, Roberts J, Lang J et al Enterovirus D68 disease and molecular epidemiology in Australia. J. Clin. Virol. 2015; 69: 117–21. [DOI] [PubMed] [Google Scholar]
- 27. Huang YP, Lin TL, Lin TH, Wu HS. Molecular and epidemiological study of enterovirus D68 in Taiwan. J. Microbiol. Immunol. Infect. 2017; 50: 411–7. [DOI] [PubMed] [Google Scholar]
- 28. Chong PF, Kira R, Mori H et al Clinical features of acute flaccid myelitis temporally associated with an enterovirus D68 outbreak: Results of a Nationwide Survey of Acute Flaccid Paralysis in Japan, August‐December 2015. Clin. Infect. Dis. 2018; 66: 653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drews SJ, Simmonds K, Usman HR et al Characterization of enterovirus activity, including that of enterovirus D68, in pediatric patients in Alberta, Canada, in 2014. J. Clin. Microbiol. 2015; 53 (3): 1042–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mertz D, Alawfi A, Pernica JM, Rutherford C, Luinstra K, Smieja M. Clinical severity of pediatric respiratory illness with enterovirus D68 compared with rhinovirus or other enterovirus genotypes. CMAJ 2015; 187 (17): 1279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hansen S, Strøm M, Maslova E, Mortensen EL, Granström C, Olsen SF. A comparison of three methods to measure asthma in epidemiologic studies: Results from the Danish National Birth Cohort. PLoS ONE 2012;7:e36328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roberts EM. Does your child have asthma? Parent reports and medication use for pediatric asthma. Arch. Pediatr. Adolesc. Med. 2003; 157: 449–55. [DOI] [PubMed] [Google Scholar]
- 33. Bush A, Fleming L. Is asthma overdiagnosed? Arch. Dis. Child. 2016; 101: 688–9. [DOI] [PubMed] [Google Scholar]
- 34. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N. Engl. J. Med. 1995; 332: 133–8. [DOI] [PubMed] [Google Scholar]
- 35. Kaida A, Iritani N, Yamamoto SP et al Distinct genetic clades of enterovirus D68 detected in 2010, 2013, and 2015 in Osaka City, Japan. PLoS ONE. 2017;12:e0184335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Itagaki T, Aoki Y, Matoba Y et al Clinical characteristics of children infected with enterovirus D68 in an outpatient clinic and the association with bronchial asthma. Infect. Dis. (Lond). 2018; 50: 303–12. [DOI] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention . Non‐Polio Enterovirus: Specimen Collection, Storage and Shipment. [updated 2018‐06‐26T02:23:01Z]. Available from: https://www.cdc.gov/non-polio-enterovirus/lab-testing/specimen-collection.html.
- 38. Mahony JB, Petrich A, Smieja M. Molecular diagnosis of respiratory virus infections. Crit. Rev. Clin. Lab. Sci. 2011; 48: 217–49. [DOI] [PubMed] [Google Scholar]
- 39. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013; 30: 2725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


