Summary
Background
Hospitalized bronchiolitis imposes a significant burden among infants, particularly among Indigenous children. Traditional or known risk factors for severe disease are well described, but there are limited data on risks for prolonged hospitalization and persistent symptoms. Our aims were to determine factors (clinical and microbiological) associated with (i) prolonged length of stay (LOS); (ii) persistent respiratory symptoms at 3 weeks; (iii) bronchiectasis up to ∼24 months post‐hospitalisation; and (iv) risk of respiratory readmissions within 6 months.
Methods
Indigenous infants hospitalized with bronchiolitis were enrolled at Royal Darwin Hospital between 2008 and 2013. Standardized forms were used to record clinical data. A nasopharyngeal swab was collected at enrolment to identify respiratory viruses and bacteria.
Results
The median age of 232 infants was 5 months (interquartile range 3–9); 65% male. On multivariate regression, our 12 point severity score (including accessory muscle use) was the only factor associated with prolonged LOS but the effect was modest (+3.0 hr per point, 95%CI: 0.7, 5.1, P = 0.01). Presence of cough at 3 weeks increased the odds of bronchiectasis (OR 3.0, 95%CI: 1.1, 7.0, P = 0.03). Factors associated with respiratory readmissions were: previous respiratory hospitalization (OR 2.3, 95%CI: 1.0, 5.4, P = 0.05) and household smoke (OR 2.6, 95%CI: 1.0, 6.3, P = 0.04).
Conclusion
Increased severity score is associated with prolonged LOS in Indigenous children hospitalized with bronchiolitis. As persistent symptoms at 3 weeks post‐hospitalization are associated with future diagnosis of bronchiectasis, optimising clinical care beyond hospitalization is needed to improve long‐term respiratory outcomes for infants at risk of respiratory disease. Pediatr Pulmonol. 2016;51:613–623. © 2015 Wiley Periodicals, Inc.
Keywords: bronchiolitis, indigenous, risk factors, viruses, bacteria
Abbreviations
- HREC
human research ethics committee
- HRV
human rhinovirus
- IQR
interquartile range
- LOS
length of stay
- NPS
nasopharyngeal swab
- OR
odds ratio
- O2
oxygen
- RCT
randomised controlled trial
- RSV
respiratory syncytial virus
- SpO2
oxygen saturation
INTRODUCTION
Bronchiolitis is typically a self‐limiting illness, but causes considerable morbidity and remains a leading cause for hospitalization among infants worldwide.1 The cost of hospitalized bronchiolitis has risen substantially in the USA over the last decade (increase of 34%).2 There are many studies that have reported on the risk factors for severe disease and length of stay (LOS) in hospital, for example, prematurity and cardio‐respiratory disease.3 However, there is relatively little data on other factors (e.g. detection of bacteria with viruses) associated with LOS in hospital in an at‐risk population (e.g., Indigenous children who have more severe bronchiolitis)4 and future bronchiectasis.5
Factors associated with severe illness and/or prolonged LOS include clinical severity (assessed by scoring systems), viruses,1, 3, 6 and secondary bacterial infection.7 Respiratory syncytial virus (RSV) is implicated for 50–80% of cases and is associated with more severe disease in some studies8, 9, 10 but not in others.6 Whether single versus multiple viruses influence disease severity is also controversial.11, 12 Further, a number of non‐classical respiratory viruses (e.g. coronaviruses, bocaviruses) have been identified13 but the clinical relevance of these remains unclear, as these non‐classical viruses are commonly present in asymptomatic children.14 In a hospital‐based study, a virus (including non‐classical viruses) was detected (42%) in the nasopharynx of children who did not have any respiratory symptoms.14
Children with severe bronchiolitis requiring intensive care are likely to have a secondary bacterial infection.7 However, studies on bronchiolitis to date have not examined whether the presence of respiratory bacteria in the upper airways influences clinical outcomes in children hospitalized with bronchiolitis. Examination for bacteria is not routine in bronchiolitis management. However, it is plausible that secondary bacterial infection that occur post viral infections15 are more likely when the nasopharynx is colonized with bacteria, as found in some populations such as in Northern Territory Indigenous infants. In these children, early (at ∼2 weeks of age), common (up to 90% of infants) and dense acquisition of respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis) 16 have been documented. Thus, in study settings like ours, the presence of bacteria in the nasopharynx may be particularly relevant. Secondary bacterial infection may complicate bronchiolitis, and possibly contribute to poorer long‐term outcomes such as chronic wet cough17 and bronchiectasis.18 It has been shown in children aged ≤3 years hospitalized with first time wheezing, co‐detection of viruses (93%) and bacteria (60%) in nasopharyngeal aspirates resulted in prolonged LOS.19 Yet, it remains unknown whether co‐detection of bacteria with viruses in the nasopharynx at the point of bronchiolitis hospitalization contributes to LOS. This data is lacking and is especially important for populations at high risk of poorer outcomes (e.g. secondary bacterial pneumonia, chronic wet cough etc).4, 17 Further rationale for assessing viruses and bacteria in the infants involved in our studies was described in a previous paper.20
Beyond hospitalization, persistence of respiratory symptoms (i.e., post‐bronchiolitis syndrome) is increasingly appreciated.3, 21 Cohort studies report symptoms in up to 40% of infants, 14–25 days post‐hospitalization.3, 22 There are few data on what happens to these children in the medium term (4 weeks to 6 months). Determining the clinical relevance of symptoms beyond hospitalization is particularly important in populations, for example, Indigenous children who have a high risk of bronchiectasis.
In the absence of any data from Indigenous children, we combined data from three prospective studies that included 232 Indigenous infants hospitalized with a clinical diagnosis of bronchiolitis, to examine factors (clinical and microbiological) on admission that were associated with (i) prolonged LOS (Aim‐1); (ii) presence of persistent symptoms 3 weeks after hospital discharge (Aim‐2); (iii) whether presence of cough at 3 weeks was associated with bronchiectasis up to ∼24 months post‐hospitalisation (Aim‐3); and (iv) re‐hospitalization within 6 months for a respiratory illness (Aim‐4). We hypothesized that co‐detection of viruses and/or bacteria in the nasopharynx is associated with longer LOS, persistent respiratory symptoms and re‐hospitalization with a respiratory illness within 6 months of discharge.
METHODS
Study Design
Data from three prospective studies were combined (Fig. 1) for the different aims; two studies were randomized controlled trials (RCT)5, 23 and one a cohort study.24 Here, we briefly describe these studies as the methods have been published.5, 23, 24 Both RCTs aimed to determine if different durations of azithromycin, compared to placebo, improved clinical outcomes for infants hospitalized with bronchiolitis (i.e., LOS, oxygen requirement and respiratory readmissions within 6 months of hospital discharge). The cohort study aimed to determine the validity and reliability of a severity scoring system among infants presenting to Royal Darwin Hospital (RDH) with bronchiolitis (Fig. 1).24 RDH is a 363 bed referral center, servicing ∼150,000 people with geographical coverage of ∼400,000 km.2 Studies were approved by the Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research (RCT‐1:HREC 07/60; RCT‐2 and cohort study: HREC‐2010‐1324). Written informed consent was obtained from the carer of each infant. Most children were involved in the two RCTs; Australian and New Zealand Clinical Trials Register: Clinical trials numbers: ACTRN12608000150347 (RCT‐1) and ACTRN12608000150347 (RCT‐2).
Figure 1.
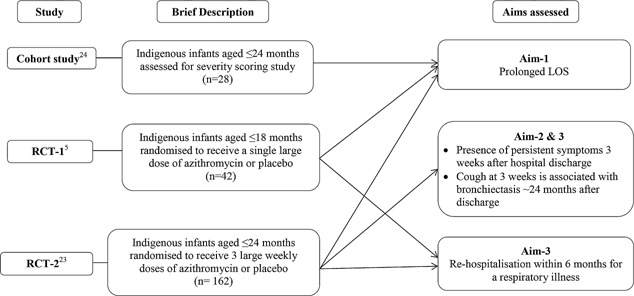
Studies where Indigenous infants were recruited at Royal Darwin Hospital.
Study Population
In this study, only Indigenous infants were included as our previous work showed being Indigenous as an independent risk factor for more severe bronchiolitis.5 Infants were eligible if they were from Darwin, ≤18 months (RCT‐1)5 or ≤24 months old (RCT‐2 and cohort study),23, 24 and hospitalized with bronchiolitis. Infants were excluded if they had very severe disease (admitted to intensive care), chronic lung disease, congenital heart disease, contraindications to macrolide use (RCT‐1 and RCT‐2), received macrolides (in last 7 days), diarrhoea, or clinical and radiological features consistent with a primary diagnosis of pneumonia (as diagnosed by the attending medical team). Infants were similar and contributed data only once for this study.
Clinical Assessment and Specimen Collection at Enrolment
Standardized data collection forms were used to record demographic, medical history and clinical data (Table 1). Other therapies and routine investigations were also documented. Infants who received additional trial medication (e.g., azithromycin) were treated the same for this analysis as those who received placebo, as no clinically significant differences in either RCT were observed.5, 23 Severity was assessed using a score we validated in our setting.24 The score comprised of four components (respiratory rate, accessory muscle use, degree of wheezing and SpO2). Each component scored between 0 and 3, providing a composite score between 0‐12. LOS was defined by the treating medical team as time from admission, to time for “ready for discharge” (Sp02 consistently >94% in air for >16 hr) and feeding adequately.
Table 1.
Demographic and Clinical Characteristics of (n = 232) Indigenous Infants
| Demographics | |
| Age (months) | 5 (3–9) |
| Boys | 151 (65%) |
| Gestational age (weeks) | 38 (36–39) |
| Birth weight (kg) | 3.0 (2.5–3.3) |
| Premature (≤37 weeks) | 59 (25%) |
| Remote | 193 (83%) |
| Currently breastfed | 195 (84%) |
| Previous respiratory hospitalisation | 51 (22%) |
| Mother smoked during pregnancy 1 | 130 (56%) |
| Exposed to household smoke 1 | 145 (63%) |
| Symptoms leading up to admission (parent reported) | |
| Days with respiratory symptoms 1 | 3 (2–4) |
| Nasal discharge | 196 (85%) |
| Cough | 228 (98%) |
| Breathing difficulties | 228 (98%) |
| Poor feeding | 95 (41%) |
| Lethargy | 136 (59%) |
| Severity score (composite score (0–12))23 | 5 (3–7) |
| Enrolment observations | |
| Number on oxygen | 144 (62%) |
| Level of supplemental O2 (L/min) | 1 (0.5, 2) |
| Heart rate (beats/min) | 143 (132–155) |
| Temperature (°C) | 37.0 (36.0–37.0) |
| Antibiotics prescribed prior to hospital 1 | 116 (57%) |
| Antibiotics prescribed during hospital | 201 (87%) |
| Supplemental IV fluid administered | 64 (28%) |
| Chest X‐ray taken | 217 (94%) |
| Any virus detected 1 | 175 (76%) |
| Any bacteria present 1 | 155 (67%) |
| Any co‐morbidity 1 , 2 | 131 (56%) |
| Hospitalisation | |
| Length of stay (hours) | 58 (43–85) |
| Supplemental O2 required | 144 (62%) |
| Time on supplemental O2 (hours) | 43 (23–74) |
| Post‐hospitalisation data | |
| Cough present at 3 weeks post‐hospitalisation 1 | 31/157 (20%) |
| Presence of any respiratory abnormality 3 weeks post‐hospitalisation 1 | 32/157 (20%) |
| Presence of otitis media 3 weeks post‐hospitalisation 1 | 21/143 (15%) |
| Re‐hospitalisation for any respiratory illness within 6 months | 48/204 (24%) |
| CT‐confirmed bronchiectasis | 30/157 (19%) |
Baseline demographics and clinical characteristics of indigenous infants hospitalized with bronchiolitis.
Data presented as median and IQR for continuous variables, and actual numbers for categorical variables and percentages.
Missing data–; Gest age, 16; Weight, 22; Mother smoke, 4; Household smoke, 2; Days with respiratory symptoms, 3; Poor feeding, 1; Lethargy, 3; Pulse, 2; Temperature, 5; Ox (L/min), 25; Nasopharyngeal swab, 3 refused/did not collect. Severity score, 42 infants (one study did not collect). Three‐week post hospital, 157 (only one study collected this data); n = 5 did not complete review.
Co‐morbidity, any otitis media, skin infection, or lobar pneumonia/collapse.
Infants were clinically reviewed by research nurses (urban‐based infants) or at their local health clinic (remote‐based infants) 3 weeks after hospital discharge, to determine presence of persistent respiratory symptoms and signs. Remoteness was described as more than 100 km from a tertiary hospital. Any respiratory readmission and investigations for bronchiectasis were monitored by a hospital based study (HREC 07/63). As RDH is the only hospital our target population accesses, all hospitalizations were accurately captured.
A nasopharyngeal swab (NPS) at enrolment was tested for common respiratory bacterial pathogens (Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and Staphylococcus aureus) were identified on culture,25 and C. pneumoniae and M. pneumoniae on PCR. A broad panel of viruses; RSV (A and B), adenovirus, parainfluenza (1, 2, 3), influenzavirus (A and B), human rhinovirus (HRV) and enterovirus, coronaviruses, bocavirus, human metapneumovirus (hMPV), KI (KIPyV) and WU (WUPyV) polyomaviruses were identified by PCR.5
Statistical Analysis
Data were entered on an Access database and analyzed using Stata version 13 (StataCorp College Station, TX). As data were skewed, we log‐transformed our main outcome measure LOS. Exponentiating linear regression coefficients of log LOS gave us the multiplicative effect of a risk factor on geometric mean LOS, which we re‐expressed (by assuming the median LOS for the reference group was 58 hr) as the approximate increase in median LOS in hours. Logistic regression was used to examine the association of independent variables on presence of cough at 3 weeks, and respiratory readmissions within 6 months. All factors with univariate P < 0.2 were included in multivariable regression models (except for viruses—RSV was entered and other variables relating to viruses were not). A two‐tailed P ≤ 0.05 was considered significant. As this was a secondary analysis of available data, sample size calculation was not undertaken.
RESULTS
Demographic data are summarized in Table 1 for the 232 Indigenous infants included in this study (70% of the 232 were from RCT‐1,23 RCT‐218%5 and Cohort‐12%,24 Fig. 1). Most children were aged ≤12 months (90%), not born premature (75% born ≥37 weeks) and required O2 supplementation (62%). No child required intensive care transfer.
From the NPS of 229 Indigenous infants (one refused, two did not provide consent for NPS), 175 (76%) had one or more viruses identified. RSV was the most common followed by HRV, WUPyV and adenovirus (Table 2). At least one type of respiratory bacteria was detected in 155 (67%) infants. The most common bacteria cultured was Moraxella catarrhalis, followed by Haemophilus influenzae, and Streptococcus pneumoniae. The NPS from 24 (10%) infants was negative for both bacteria and virus.
Table 2.
Distributionb of Viruses, Atypical Bacteria and Bacteria Detected at Enrolment With NPS
| Virus and atypical bacteria | Number of children with micro‐organism detected (% of N = 229) * |
|---|---|
| No virus detected | 54 (24%) |
| 1 Virus detected | 123 (54%) |
| 2 Or more viruses detected | 52 (23%) |
| RSV | 98 (43%) |
| HRV | 61 (27%) |
| Adenovirus | 15 (7%) |
| WUPyV | 15 (7%) |
| Influenza_AB | 10 (4%) |
| hMPV | 7 (3%) |
| Bocavirus | 5 (2%) |
| Coronavirus | 3 (1%) |
| Parainfluenza_123 | 4 (2%) |
| Enterovirus | 4 (2%) |
| KIPyV | 4 (2%) |
| C. pneumoniae | 4 (2%) |
| M. pneumoniae | 0 (0%) |
| RSV and HRV | 144 (63%) |
| Bacteria | |
| No bacteria detected | 76 (33%) |
| 1 Type of bacteria detected | 90 (39%) |
| 2 Or more bacteria detected | 65 (28%) |
| Moraxella catarrhalis | 89 (39%) |
| Haemophilus influenzae | 88 (34%) |
| Streptococcus pneumoniae | 50 (22%) |
| Staphylococcus aureus | 24 (10%) |
Detection of viruses, atypical bacteria and bacteria detected at enrolment in NPS.
Many children had more than one organism detected.
Factors on Admission Associated With Prolonged LOS (Aim‐1)
On univariate analysis, factors on admission significantly associated with prolonged LOS were age and severity score (Table 3). Notably, antibiotics prior to hospitalization, any virus and co‐detection of virus/bacteria were not associated with longer LOS. On multivariate regression, the only factor that remained significant was severity score. Detection of RSV increased LOS by 10 hr (P = 0.06), but this did not reach statistical significance. We then examined which component of the severity score contributed the most to LOS (Table 4). In the univariate analysis, (considering each of the four components as single factors) all components except wheeze were significantly associated with prolonged LOS. On multivariate regression, only the “accessory muscle use” component remained significant.
Table 3.
Analysis of Risk Factors for LOS (nmax = 232)
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Risk factors present on admission | Difference in median LOS (hours) * | 95%CI | P‐value | Difference in median LOS (hours) * | 95%CI | P‐value | |
| Continuous | Spearman correlation | ||||||
| Age (months) | −0.2 | −1.2 | (−2.1, −0.2) | 0.02 | −0.6 | (−1.6, 0.5) | 0.3 |
| Gestational age (weeks) | 0.09 | 0.7 | (−0.7, 2.0) | 0.3 | |||
| Birth weight (kg) | 0.03 | −0.0 | (−5.7, 6.2) | 0.9 | |||
| Severity score on admission (points) | 0.2 | 3.1 | (1.1, 5.1) | 0.002 | 3.0 | (0.7, 5.1) | 0.01 |
| Categorical | Geometric mean LOS (hours) | ||||||
| Gender | |||||||
| Female | 61 | −1.4 | (−9.4, 8.0) | 0.8 | |||
| Male | 62 | (reference) | |||||
| Currently breastfed | |||||||
| Yes | 62 | 1.9 | (−8.9, 15.1) | 0.7 | |||
| No | 60 | (reference) | |||||
| Previous respiratory hospitalisation | |||||||
| Yes | 60 | −2.0 | (−11.1, 9.0) | 0.7 | |||
| No | 62 | (reference) | |||||
| Mother smoked during pregnancy | |||||||
| Yes | 62 | 1.7 | (−6.6, 11.4) | 0.7 | |||
| No | 60 | (reference) | |||||
| Exposed to household smoke | |||||||
| Yes | 62 | 0.2 | (−8.0, 9.8) | 0.9 | |||
| No | 61 | (reference) | |||||
| Antibiotics before hospitalisation | |||||||
| Yes | 61 | −7.1 | (−14.4, 1.4) | 0.09 | −2.4 | (−11, 7.6) | 0.6 |
| No | 69 | (reference) | |||||
| Any virus detected | |||||||
| Yes | 64 | 8.7 | (−1.9, 21.3) | 0.11 | |||
| No | 55 | (reference) | |||||
| RSV | |||||||
| Yes | 66 | 7.0 | (−2.2, 17.2) | 0.14 | 10.0 | (−0.6, 22.3) | 0.06 |
| No | 58 | (reference) | |||||
| HRV | |||||||
| Yes | 64 | 3.4 | (−6.1, 14.6) | 0.5 | |||
| No | 61 | (reference) | |||||
| RSV and HRV | |||||||
| Yes | 64 | 5.0 | (−3.8, 15.1) | 0.3 | |||
| No | 59 | (reference) | |||||
| Adenovirus | |||||||
| Yes | 57 | −4.4 | (−18.3, 14.3) | 0.6 | |||
| No | 62 | (reference) | |||||
| Any bacteria detected | |||||||
| Yes | 63 | 3.1 | (−5.7, 13.4) | 0.5 | |||
| No | 59 | (reference) | |||||
| Virus/bacteria interaction | 0.3 | ||||||
| Virus negative/Bacteria negative | 58 | (reference) | |||||
| Virus negative/Bacteria positive | 53 | −5.0 | (−19.1, 14.2) | 0.6 | |||
| Virus positive/Bacteria negative | 60 | 1.5 | (−13.2, 21.0) | 0.9 | |||
| Virus positive/Bacteria positive | 65 | 7.0 | (−7.9, 26.0) | 0.4 | |||
On univariate analysis, factors at enrolment associated with prolonged LOS were age and severity score. On multivariate analysis, only severity score remained significant.
Arising as a re‐expression of the multiplicative effect on the geometric mean from a linear regression on log (LOS).
Table 4.
Analysis of the Different Components of the Severity Score Contributing to LOS (n = 190)
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Points | Component | n | Geometric mean LOS | Difference in median LOS (hours)* | 95%CI | P‐value | Difference in median LOS (hours)* | 95%CI | P‐value |
| Respiratory rate | |||||||||
| 0 | <30 | 3 | 58 | (reference) | |||||
| 1 | 30–45 | 73 | 54 | −4 | (−29.1, 42.0) | 0.8 | |||
| 2 | 45–60 | 70 | 60 | 2 | (−26.0, 53.2) | 0.9 | |||
| 3 | >60 | 44 | 73 | 16 | (−18.8, 80.0) | 0.5 | |||
| Wheeze | |||||||||
| 0 | None | 85 | 63 | (reference) | |||||
| 1 | Expiration only | 37 | 56 | −6 | (−15.7, 6.4) | 0.3 | |||
| 2 | Entire expiration and inspiration with stethoscope | 30 | 52 | −10 | (−20.0, 1.9) | 0.09 | |||
| 3 | Entire expiration and inspiration without stethoscope | 38 | 66 | 3 | (−8.4, 17.2) | 0.6 | |||
| SpO2 | |||||||||
| 0 | >95 | 148 | 58 | (reference) | |||||
| 1 | 94–95 | 14 | 59 | 2 | (−13.8, 22.2) | 0.9 | |||
| 2 | 90–93 | 24 | 69 | 11 | (−3.1, 29.7) | 0.1 | |||
| 3 | <89 | 4 | 97 | 40 | (−1.1, 109.3) | 0.06 | |||
| Accessory muscle use | |||||||||
| 0 | None | 15 | 50 | (reference) | |||||
| 1 | + | 64 | 53 | 4 | (−12.0, 26.0) | 0.6 | |||
| 2 | ++ | 66 | 60 | 12 | (−5.9, 36.5) | 0.2 | |||
| 3 | +++ | 45 | 76 | 31 | (7.6, 63.9) | 0.007 | |||
| Per one point increase per component | |||||||||
| Respiratory rate | 9.0 | (2.7, 15.4) | 0.004 | 4.7 | (−1.9, 12.0) | 0.2 | |||
| Wheeze | −0.2 | (−3.9, 3.7) | 0.9 | −1.8 | (−5.3, 2.0) | 0.4 | |||
| SpO2 | 7.0 | (0.5, 13.2) | 0.03 | 4.5 | (−1.4, 11.0) | 0.14 | |||
| Accessory muscle use | 10.0 | (4.4, 15.7) | 0.0001 | 7.0 | (0.7, 13.8) | 0.03 | |||
In the univariate analysis, all four components except wheeze contributed to prolonged LOS. On multivariate regression, only accessory muscle use remained significantly associated with prolonged LOS.
Arising as a re‐expression of the multiplicative effect on the geometric mean from a linear regression on log (LOS).
We repeated the main analyses, restricting the cohort to the n = 181 infants who had no previous respiratory hospitalizations. On multivariate analysis, the only factor which significantly prolonged LOS was the severity score (P = 0.02).
Presence of Cough 3 Weeks After Discharge (Aim‐2)
One hundred and fifty seven Indigenous infants who had a clinical review at 3 weeks contributed to this analysis (Fig. 1). Persistent respiratory symptoms were frequent beyond hospitalization (Table 1). On univariate analysis, factors significantly associated with presence of cough 3 weeks after discharge was age and presence of “any bacteria” cultured on NPS. No factors remained significant on multivariate regression.
Presence of Cough 3 Weeks After Discharge and the Relationship to Bronchiectasis (Aim‐3)
As of 7th May 2015, 30/157 (19%) infants had a chest CT scan requested by their treating pediatrician for clinical reasons when seen 7 months (interquartile range (IQR) 2–13) post‐hospitalization for bronchiolitis. All 30 (100%) children had bronchiectasis documented in the CT scan, performed at median 13 months (IQR 7–18) post the index bronchiolitis hospitalization. Infants with persistent cough at 3 weeks after hospitalization were significantly more likely to have bronchiectasis compared to those without a cough (OR 3.0, 95%CI: 1.1–7.0, P = 0.03).
Respiratory Readmissions 6 Months After Discharge (Aim‐4)
On univariate analysis, factors that significantly increased the odds of respiratory readmission 6‐months after discharge were previous respiratory hospitalization and exposure to household smoke. Factors that significantly reduced the odds of readmission were detection of “any virus,” “any bacteria,” RSV and “RSV and HRV” on NPS (Table 5). On multivariate regression, previous respiratory hospitalization and exposure to household smoke were factors that significantly increased the odds of readmission but the presence of “any bacteria” and RSV reduced the odds of readmission (Table 5). The presence of any respiratory abnormality (i.e., presence of cough, wheeze, or crackles) at 3 weeks was not associated with the odds of respiratory readmissions (OR 1.2, 95%CI: 0.6–3.0, P = 0.6).
Table 5.
Analysis of Risk Factors for Respiratory Readmissions Within 6 Months (nmax = 204)
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Factors present on admission | OR | 95%CI | P‐value | OR | 95%CI | P‐value | |
| Continuous | Median (respiratory readmit vs. first hospitalisation) | ||||||
| Age (months) | 5.0 vs. 5.5 | 1.0 | (0.9, 1.1) | 0.9 | |||
| Birth weight (kg) | 3.0 vs. 3.0 | 0.8 | (0.5, 1.3) | 0.4 | |||
| Gestational age (weeks) | 38 vs. 38 | 0.9 | (0.8, 1.0) | 0.11 | 1.0 | (0.9, 1.1) | 0.5 |
| Severity score on admission (points) | 5 vs. 5 | 1.0 | (0.9, 1.2) | 0.6 | |||
| Categorical | n/N (%) | ||||||
| Gender | |||||||
| Female | 15/72 (21%) | 0.8 | (0.4, 1.6) | 0.5 | |||
| Male | 33/132 (25%) | (reference) | |||||
| Currently breastfed | |||||||
| Yes | 42/173 (24%) | 1.3 | (0.5, 3.5) | 0.6 | |||
| No | 6/31 (19%) | (reference) | |||||
| Previous respiratory hospitalisation | |||||||
| Yes | 16/41 (39%) | 2.6 | (1.3, 5.5) | 0.01 | 2.3 | (1.0, 5.4) | 0.05 |
| No | 32/163 (20%) | (reference) | |||||
| Mother smoked during pregnancy | |||||||
| Yes | 25/114 (22%) | 0.8 | (0.4, 1.5) | 0.5 | |||
| No | 23/87 (26%) | (reference) | |||||
| Exposed to household smoke | |||||||
| Yes | 37/127 (29%) | 2.4 | (1.1, 5.1) | 0.02 | 2.6 | (1.0, 6.3) | 0.04 |
| No | 11/76 (14%) | (reference) | |||||
| Antibiotics before hospitalisation | |||||||
| Yes | 26/116 (22%) | 0.9 | (0.5, 1.7) | 0.7 | |||
| No | 22/88 (25%) | (reference) | |||||
| Any virus detected | |||||||
| Yes | 31/154 (20%) | 0.5 | (0.2, 1.0) | 0.05 | |||
| No | 16/47 (34%) | (reference) | |||||
| RSV | |||||||
| Yes | 9/89 (10%) | 0.2 | (0.1, 0.5) | 0.0001 | 0.3 | (0.1, 0.6) | 0.001 |
| No | 38/112 (34%) | (reference) | |||||
| HRV | |||||||
| Yes | 14/54 (26%) | 1.2 | (0.6, 2.5) | 0.6 | |||
| No | 33/147 (22%) | (reference) | |||||
| RSV and HRV | |||||||
| Yes | 23/128 (18%) | 0.4 | (0.2, 0.9) | 0.02 | |||
| No | 25/76 (33%) | (reference) | |||||
| Adenovirus | |||||||
| Yes | 3/12 (25%) | 1.1 | (0.3, 4.2) | 0.9 | |||
| No | 44/189 (23%) | (reference) | |||||
| Any bacteria detected | |||||||
| Yes | 25/138 (18%) | 0.4 | (0.2, 0.8) | 0.008 | 0.4 | (0.2, 0.9) | 0.02 |
| No | 23/65 (35%) | (reference) | |||||
| Virus/Bacteria interaction | 0.5 | ||||||
| Virus negative/Bacteria negative | 8/20 (40%) | (reference) | |||||
| Virus negative/Bacteria positive | 8/27 (30%) | 0.6 | (0.2, 2.1) | 0.5 | |||
| Virus positive/Bacteria negative | 14/44 (32%) | 0.7 | (0.2, 2.1) | 0.5 | |||
| Virus positive/Bacteria positive | 17/110 (15%) | 0.3 | (0.1, 0.8) | 0.01 | |||
On multivariate regression, previous respiratory hospitalisation and exposure to household smoke significantly increasing odds of readmission but the presence of any bacteria and RSV reduced the odds of readmission.
DISCUSSION
In our study involving 232 Indigenous infants hospitalized with bronchiolitis, we found that a higher severity score on admission, particularly use of accessory muscles, was associated with prolonged hospital stay. The presence of a virus or viruses detected on hospitalization was not associated with poorer outcomes. Beyond hospitalization, factors associated with presence of cough at 3 weeks were previous respiratory hospitalization and presence of bacteria. By 6 months, infants previously hospitalized with a respiratory illness and exposed to household smoke had an increased odds of re‐hospitalization for a respiratory illness. In contrast however, infants with presence of RSV and culturable bacteria detected in NPS had decreased odds of re‐hospitalization at 6 months. Further, infants who were coughing at 3 weeks post‐hospitalization were significantly more likely to be diagnosed with bronchiectasis a median of 13 months later.
Our study involved only Indigenous children as recurrent hospitalizations for respiratory infections and chronic suppurative lung disease is prevalent in our setting.18 Indigenous children living in USA, Canada, Australia and New Zealand share many similarities with respect to the burden of respiratory illness.26 Thus, we were interested in finding factors associated with poorer clinical outcomes and possible future intervention points. Studies on hospitalized bronchiolitis in Canadian children found ethnicity, apnoea or respiratory arrest prior to hospitalization or pulmonary consolidation to have more complicated hospitalization.27 Further, previous RSV bronchiolitis hospitalization was associated with chronic cough and recurrent respiratory infections in early childhood.17
Well known or “traditional risk factors” associated with severe bronchiolitis in infants include prematurity, cardio‐respiratory disease, and being Indigenous.3, 28 In contrast, there are a few prospective studies which examined other factors that prolonged LOS. In these studies, factors significantly associated with prolonged LOS were young age, increased work of breathing, heart rate, respiratory rate, dehydration, hypoxia on admission, SpO2 <94%, respiratory distress assessment instrument (RDAI) score >11, apnoea, weight, RSV, ethnicity, and winter season.27, 29, 30, 31 Retrospective studies have described that RSV, ethnicity, congenital syndromes, neuromuscular disorders and existing chronic respiratory diseases were associated with prolonged LOS.32 In our study, severity score on admission, particularly use of accessory muscles, was the only factor to prolong LOS, once other factors (n=21) were accounted for in the multivariate regression. None of the previous studies however had dissected which component of the scoring system contributed the most to LOS. Further, despite the many studies undertaken for bronchiolitis, most scoring systems used were not validated.6, 29, 32 Indeed the most commonly used system, the RDAI score, has limited validity when systematically examined.33 Within our study, we had validated the scoring system used and showed good inter‐ and intra‐rater reliability.24
The type and number of viruses (particularly RSV and HRV) did not significantly influence LOS in our study. Our results are similar to some studies,9, 11 but dissimilar to others where RSV and/or HRV influenced disease severity.8, 34, 35 Reasons for these variations are likely multi‐factorial including sample size, different methodologies, sampling frame, age, number and types of viruses investigated.11, 12 We also found that although respiratory bacteria (H. influenzae, S. pneumoniae, and M. catarrhalis) and/or viruses were common (88%), their presence did not prolong LOS. While data relating to LOS and type of virus detection has been previously examined, none of these studies have examined the influence of the presence of bacteria in the upper airways. We found that the presence of bacteria detected in the nasopharynx with or without concurrent viruses did not influence LOS. Our finding of high prevalence of concurrent bacteria with viruses (i.e., 88%) in the children's nasopharynx is similar to that reported by Bisgaard and colleagues among young children of asthmatic mothers with acute wheeze in a non‐hospitalised cohort.36
Beyond the hospital phase, our study reported several novel outcomes. At 3 weeks post‐hospitalisation, the prevalence of respiratory symptoms in our study (20%) was similar to other studies (18–25%).21, 22, 37 Few studies however have prospectively recorded respiratory symptoms post‐hospitalization, and no studies have examined for predictors of persistent respiratory symptoms otherwise known as “post‐bronchiolitis syndrome.” While presence of bacteria in the NPS on admission was not significantly associated with persistence of cough at 3 weeks,36 chronic cough is the most common symptom of an underlying lung disease and causes poorer quality of life.38 In this study, persistent cough was however a significant risk factor (OR 3.0, 95%CI 1.0–7.0, P = 0.03) for CT‐confirmed bronchiectasis 13 months later. Bronchiectasis is particularly common in Indigenous infants in the USA, Australia and New Zealand and hospitalization for respiratory disease is an independent risk factor.18
In the context that early treatment of bacterial infections in the lower airways prevents long‐term lung damage,39, 40 our post‐hospitalization data is both novel and important in high‐risk population groups (e.g., Indigenous children).18 Post‐bronchiolitis syndrome is likely highly important especially in high‐risk populations. Our data raises an important public health issue with respect to follow‐up of children with bronchiolitis, to optimise clinical care beyond the immediate hospitalization phase to improve long‐term respiratory outcomes for high‐risk populations. We suggest that children should be clinically reviewed between weeks 3–4 after hospital discharge to assess for persistent symptoms and signs and managed accordingly. There is currently no high quality evidence on the most appropriate intervention in any groups of children with post‐bronchiolitis syndrome.41 However, until further evidence is available, we suggest the use of antibiotics when the cough is wet and not improving in a group at high risk of chronic suppurative lung disease, in line with the treatment of protracted bacterial bronchitis.42
Our post‐hospitalization data also showed that children exposed to environmental tobacco smoke and those previously hospitalized with a respiratory illness were twice as likely to be readmitted with a respiratory illness within 6 months of hospital discharge. Surprisingly, we found that presence of RSV on admission and “any bacteria” significantly reduced the odds of re‐hospitalization by 6 months post index hospitalization. Whether respiratory readmissions in this population were related to the initial bronchiolitis episode, a new infection or other causes can only be postulated. One possible reason for this could be that RSV detection is a marker (as opposed to a cause) for future asthma and as the majority of paediatric asthma is mild, having asthma is less likely to lead to re‐hospitalisation compared to a respiratory infection in our setting.
Our study has several limitations. Firstly, our sampling frame is restricted to Indigenous children without the well‐known, traditional risk factors (e.g. chronic lung disease, cardiac disease) and who did not require intensive care. Reasons for our sampling frame were discussed above but it also means our data cannot be extrapolated to the general population or to non‐hospitalized infants. Secondly, we did not exclude infants that had been hospitalized previously; however, exclusion of these infants did not alter our main results. Thirdly, 87% of children received antibiotics during hospitalization. This may have impacted on nasopharyngeal bacterial carriage, however removing these children did not alter our main results. Fourthly, the children were systematically reviewed only at one time point, that is, at 3 weeks post‐hospitalization. The decision by the treating paediatricians who reviewed these infants at a median of 7 months post‐hospitalization to subject the child to a chest CT scan was based on clinical assessments, not systemized and thus subject to external biases. Thus, we could not systematically analyze the factors on admission that are associated with future development of bronchiectasis. Also, while long‐term, prospective, follow‐up studies in high‐risk populations are required, we cannot unethically subject all children to a chest CT (given the known risk of radiation in young children).
CONCLUSION
In the first prospective study of Indigenous infants hospitalized with bronchiolitis with post‐hospitalization data at 3 weeks and 6 months, we found that the severity score on admission, particularly accessory muscle use, was the sole factor associated with prolonged LOS once other factors (clinical and microbiological) were accounted for. Persistent respiratory symptoms at 3 weeks increased the risk of detecting bronchiectasis and factors associated with persistent symptoms were prior hospitalisation for a respiratory illness and presence of bacteria on the NPS on admission. Thus, clinical review, with early treatment when necessary, should be undertaken in populations at high risk for chronic respiratory disease. Follow‐up studies beyond 6 months are necessary to consolidate our findings and determine intervention points that can reduce the high burden of respiratory diseases in at‐risk populations.
AUTHOR'S CONTRIBUTION
Anne Chang conceptualized the study with modifications by Peter Morris and Gabrielle McCallum. Gabrielle McCallum designed the data collection instruments, collected data, coordinated the study, and carried out initial data analysis and manuscript. Mark Chatfield provided statistical support, carried out further data analysis, and edited the manuscript. All authors assisted in data interpretation, contributed to the manuscript and approved the manuscript as submitted.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supporting information Figure S1: Studies where Indigenous infants were recruited at Royal Darwin Hospital. Infants from the three studies conducted at Royal Darwin Hospital during 2008–2013 used to answer different aims. “n” refers to the number of infants included in this paper, rather than the actual number of infants in the original studies.
ACKNOWLEDGMENTS
We are grateful for all infants and families who participated in this study. We thank the remote health clinics for their support and attending the clinical review. We thank Lesley Versteegh and Clare Mckay for enrolling participants and collecting the clinical data. We thank Vanya Hampton, Donna Woltring, Jemima Beissbarth, Jane Gaydon and Rebecca Rockett for processing the viral and bacterial samples.
Conflict of interest: The authors declare that they have no conflicts of interest.
REFERENCES
- 1. Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, Johnson DW, Light MJ, Maraqa NF, Mendonca EA, Phelan KJ, Zorc JJ, Stanko‐Lopp D, Brown MA, Nathanson I, Rosenblum E, Sayles S, III. , Hernandez‐Cancio S. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502. [DOI] [PubMed] [Google Scholar]
- 2. Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA, Jr. Temporal trends in emergency department visits for bronchiolitis in the United States, 2006 to 2010. Pediatr Infect Dis J 2014; 33:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baumer JH. SIGN guideline on bronchiolitis in infants. Arch Dis Child Educ Pract Ed 2007; 92:ep49–e151. [DOI] [PubMed] [Google Scholar]
- 4. Bailey EJ, Maclennan C, Morris PS, Kruske SG, Brown N, Chang AB. Risks of severity and readmission of Indigenous and non‐Indigenous children hospitalised for bronchiolitis. J Paediatr Child Health 2009; 45:593–597. [DOI] [PubMed] [Google Scholar]
- 5. McCallum GB, Morris PS, Chatfield MD, Maclennan C, White AV, Sloots TP, Mackay IM, Chang AB. A single dose of azithromycin does not improve clinical outcomes of children hospitalised with bronchiolitis: a randomised, placebo‐controlled trial. PLoS ONE 2013; 8:e74316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ricart S, Marcos MA, Sarda M, Anton A, Munoz‐Almagro C, Pumarola T, Pons M, Garcia‐Garcia JJ. Clinical risk factors are more relevant than respiratory viruses in predicting bronchiolitis severity. Pediatr Pulmonol 2013; 48:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorburn K, Harigopal S, Reddy V, Taylor N, van Saene HK. High incidence of pulmonary bacterial co‐infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax 2006; 61:611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, Espinola JA, Camargo CA, Jr. Investigators M‐. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med 2012; 166:700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marguet C, Lubrano M, Gueudin M, Le Roux P, Deschildre A, Forget C, Couderc L, Siret D, Donnou MD, Bubenheim M, Vabret A, Freymuth F. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS ONE 2009; 4:e4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calvo C, Pozo F, Garcia‐Garcia ML, Sanchez M, Lopez‐Valero M, Perez‐Brena P, Casas I. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three‐year prospective study. Acta Paediatr 2010; 99:883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brand HK, de Groot R, Galama JM, Brouwer ML, Teuwen K, Hermans PW, Melchers WJ, Warris A. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol 2012; 47:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Single and multiple respiratory virus infections and severity of respiratory disease: a systematic review. Paediatr Respir Rev 2014; 15:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jartti T, Jartti L, Ruuskanen O, Soderlund‐Venermo M. New respiratory viral infections. Curr Opin Pulm Med 2012; 18:271–278. [DOI] [PubMed] [Google Scholar]
- 14. Advani S, Sengupta A, Forman M, Valsamakis A, Milstone AM. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J 2012; 31:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T. Sustained desensitization to bacterial Toll‐like receptor ligands after resolution of respiratory influenza infection. J Exp Med 2008; 205:323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith‐Vaughan H, Byun R, Nadkarni M, Jacques NA, Hunter N, Halpin S, Morris PS, Leach AJ. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord 2006; 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singleton RJ, Redding GJ, Lewis TC, Martinez P, Bulkow L, Morray B, Peters H, Gove J, Jones C, Stamey D, Talkington DF, DeMain J, Bernert JT, Butler JC. Sequelae of severe respiratory syncytial virus infection in infancy and early childhood among Alaska Native children. Pediatrics 2003; 112:285–290. [DOI] [PubMed] [Google Scholar]
- 18. Singleton RJ, Valery PC, Morris P, Byrnes CA, Grimwood K, Redding G, Torzillo PJ, McCallum G, Chikoyak L, Mobberly C, Holman RC, Chang AB. Indigenous children from three countries with non‐cystic fibrosis chronic suppurative lung disease/bronchiectasis. Pediatr Pulmonol 2014; 49:189–200. [DOI] [PubMed] [Google Scholar]
- 19. Jartti T, Kuneinen S, Lehtinen P, Peltola V, Vuorinen T, Leinonen M, Ruuskanen O. Nasopharyngeal bacterial colonization during the first wheezing episode is associated with longer duration of hospitalization and higher risk of relapse in young children. Eur J Clin Microbiol Infect Dis J 2011; 30:233–241. [DOI] [PubMed] [Google Scholar]
- 20. Chang AB, Grimwood K, White AV, Maclennan C, Sloots TP, Sive A, McCallum GB, Mackay IM, Morris PS. Randomized placebo‐controlled trial on azithromycin to reduce the morbidity of bronchiolitis in Indigenous Australian infants: rationale and protocol. Trials 2011; 12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swingler GH, Hussey GD, Zwarenstein M. Duration of illness in ambulatory children diagnosed with bronchiolitis. Arch Pediatr Adolesc Med 2000; 154:997–1000. [DOI] [PubMed] [Google Scholar]
- 22. Petruzella FD, Gorelick MH. Duration of illness in infants with bronchiolitis evaluated in the emergency department. Pediatrics 2010; 126:285–290. [DOI] [PubMed] [Google Scholar]
- 23. McCallum GB, Morris PS, Grimwood K, Maclennan C, White AV, Chatfield MD, Sloots TP, Mackay IM, Smith‐Vaughan H, Mckay CC, Versteegh LA, Jacobsen N, Mobberley C, Byrnes CA, Chang AB. Three‐weekly doses of azithromycin for indigenous infants hospitalised with bronchiolitis: a multicentre, randomised, placebo‐controlled trial. Front Pediatr 2015; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCallum GB, Morris PS, Wilson CC, Versteegh LA, Ward LM, Chatfield MD, Chang AB. Severity scoring systems: are they internally valid, reliable and predictive of oxygen use in children with acute bronchiolitis? Pediatr Pulmonol 2013; 48:797–803. [DOI] [PubMed] [Google Scholar]
- 25. Stubbs E, Hare K, Wilson C, Morris P, Leach AJ. Streptococcus pneumoniae and noncapsular haemophilus influenzae nasal carriage and hand contamination in children—a comparison of two populations at risk of otitis media. Pediatr Infect Dis J 2005; 24:423–428. [DOI] [PubMed] [Google Scholar]
- 26. Chang AB, Brown N, Toombs M, Marsh RL, Redding GJ. Lung disease in indigenous children. Paediatr Respir Rev 2014; 15:325–332. [DOI] [PubMed] [Google Scholar]
- 27. Wang EE, Law BJ, Stephens D. Pediatric investigators collaborative network on infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr 1995; 126:212–219. [DOI] [PubMed] [Google Scholar]
- 28. Koehoorn M, Karr CJ, Demers PA, Lencar C, Tamburic L, Brauer M. Descriptive epidemiological features of bronchiolitis in a population‐based cohort. Pediatrics 2008; 122:1196–1203. [DOI] [PubMed] [Google Scholar]
- 29. Corneli HM, Zorc JJ, Holubkov R, Bregstein JS, Brown KM, Mahajan P, Kuppermann N. Bronchiolitis Study Group for the Pediatric Emergency Care Applied Research N. Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care 2012; 28:99–103. [DOI] [PubMed] [Google Scholar]
- 30. Walsh P, Rothenberg SJ, O'Doherty S, Hoey H, Healy R. A validated clinical model to predict the need for admission and length of stay in children with acute bronchiolitis. Eur J Emerg Med 2004; 11:265–272. [DOI] [PubMed] [Google Scholar]
- 31. Watt R, Jackson G, Turner S. G162(P) What predicts duration of hospital stay for bronchiolitis? Arch Dis Child 2013; 98:A75. [Google Scholar]
- 32. Weisgerber MC, Lye PS, Li SH, Bakalarski D, Gedeit R, Simpson P, Gorelick MH. Factors predicting prolonged hospital stay for infants with bronchiolitis. J Hosp Med 2011; 6:264–270. [DOI] [PubMed] [Google Scholar]
- 33. Lowell DI, Lister G, Von Koss H, McCarthy P. Wheezing in infants: the response to epinephrine. Pediatrics 1987; 79:939–945. [PubMed] [Google Scholar]
- 34. Bamberger E, Srugo I, Abu Raya B, Segal E, Chaim B, Kassis I, Kugelman A, Miron D. What is the clinical relevance of respiratory syncytial virus bronchiolitis?: findings from a multi‐center, prospective study. Eur J Clin Microbiol Infect Dis J 2012; 31:3323–3330. [DOI] [PubMed] [Google Scholar]
- 35. Garcia CG, Bhore R, Soriano‐Fallas A, Trost M, Chason R, Ramilo O, Mejias A. Risk factors in children hospitalized with RSV bronchiolitis versus non‐RSV bronchiolitis. Pediatrics 2010; 126:e1453–e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bisgaard H, Hermansen MN, Bonnelykke K, Stokholm J, Baty F, Skytt NL, Aniscenko J, Kebadze T, Johnston SL. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ 2010; 341:c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson M, Vodicka TA, Blair PS, Buckley DI, Heneghan C, Hay AD, Team TP. Duration of symptoms of respiratory tract infections in children: systematic review. BMJ 2013; 347:f7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang AB, Robertson CF, Van Asperen PP, Glasgow NJ, Mellis CM, Masters IB, Teoh L, Tjhung I, Morris PS, Petsky HL, Willis C, Landau LI. A multicenter study on chronic cough in children: burden and etiologies based on a standardized management pathway. Chest 2012; 142:943–950. [DOI] [PubMed] [Google Scholar]
- 39. Chang AB, Redding GJ, Everard ML. State of the Art—Chronic wet cough: protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatric Pulmonol 2008; 43:519–531. [DOI] [PubMed] [Google Scholar]
- 40. Finke W. Prospects for prevention of chronic bronchitis and bronchiectasis; rational management of bronchopulmonary infections by penicillin aerosol therapy. J Pediatr 1948; 33:29–42. [DOI] [PubMed] [Google Scholar]
- 41. McCallum GB, Morris PS, Chang AB. Antibiotics for persistent cough or wheeze following acute bronchiolitis in children. Cochrane Database Syst Rev 2012; 12:CD009834. [DOI] [PubMed] [Google Scholar]
- 42. Chang AB, Redding GJ, Everard ML. Chronic wet cough: protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr Pulmonol 2008; 43:519–531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supporting information Figure S1: Studies where Indigenous infants were recruited at Royal Darwin Hospital. Infants from the three studies conducted at Royal Darwin Hospital during 2008–2013 used to answer different aims. “n” refers to the number of infants included in this paper, rather than the actual number of infants in the original studies.


