Kawasaki disease (KD) is a vasculitic illness of childhood associated with the development of coronary artery aneurysms. Since its recognition as a distinct syndrome in the late 1960s, incidence has risen and it is now the commonest cause of acquired cardiac disease amongst children in the developed world. 1 , 2 The cause of KD is unknown, but epidemiological characteristics suggest it is caused by one or more as‐yet‐undefined infectious agents. Attempts to identify the pathogen(s) responsible have been unsuccessful, but in small series the syndrome has been associated with infection by several different agents, raising the possibility that it represents a common aberrant immunological response to multiple pathogens. 3 Influenza A H1N1/09 is a novel strain that emerged in early 2009 and caused a global pandemic. Although highly infectious, its virulence was low compared to that of previous pandemic strains. 4 The majority of children who contracted it had a mild self‐limiting viral illness, but a few developed severe disease, and the high prevalence of shock amongst those requiring intensive care support appeared to be a novel feature. 5 We describe a case of KD coincident with influenza A H1N1/09 infection, indicating that KD is a potential phenotype of influenza infection.
Case Report
In November 2009, mid‐way through the “second wave” of influenza A H1N1/09 cases in London, a previously well five‐year‐old boy, born in the UK to Egyptian parents, presented with a febrile illness. Nine days previously he had developed a cough, sore throat and coryzal symptoms with fever to 39°C, and 48 h into the illness he developed a widespread urticarial rash. He was initially managed at home but after 3 days without improvement was prescribed oseltamivir by a general practitioner (without virological studies, in line with current policy), however this was discontinued by his mother after three doses due to the onset of vomiting. On day 5 of the illness he developed conjunctivitis and mucositis, and began complaining of pain in his hips. He was persistently pyrexial up to 40.5°C, had decreased appetite, a limp, and was progressively irritable and distressed at home.
On examination he was alert and upset. He was febrile to 39.6°C, had a pulse rate of 122/min, respiratory rate of 24/min, and oxygen saturation of 99% in room air. Prominent pharyngitis, a red tongue, and red, dry and swollen lips were noted, along with bilateral non‐exudative conjunctivitis and cervical lymphadenopathy. There was a confluent macular–papular rash over both hands and feet up to his elbows/knees with prominent excoriation. Hands and feet were tender and edematous, and he was unable to bend his fingers, toes or knees due to pain. No Bacillus Calmette–Guérin (BCG) scar was evident. The abdomen was soft with a tender liver edge 2 cm below the right costal margin and a palpable spleen tip. His chest was clear, heart sounds normal, and there was no evidence of neurological involvement. Urinalysis showed ketonuria (3+) with proteinuria (1+) but no cells, and a chest X‐ray showed minor peribronchial wall thickening bilaterally but no focal collapse or consolidation. Hip ultrasound showed bilateral small joint effusions.
The white cell count was 16.1 × 109/L with neutrophils 13.2 × 109/L and lymphocytes 2.0 × 109/L, and blood film showed left‐shift with toxic degranulation. His hemoglobin was 10.1 g/dl, platelets 191 × 109/L, and clotting studies were normal. His C‐reactive protein was 148 mg/L, erythrocyte sedimentation rate 134 mm/h, and lactate dehydrogenase 829 u/L. Sodium was 132 mEq/L, alanine transaminase 67 u/L, alkaline phosphatase 313 u/L, and albumin 24 g/L. Blood and urine cultures were negative, as was a throat swab. Polymerase chain reaction on his nasopharyngeal aspirate (NPA) from the time of admission was positive for influenza A H1N1/09. A presumptive diagnosis of Kawasaki disease was made, and he received i.v. immunoglobulin at 2 g/kg, and was started on aspirin at 7.5 mg/kg q.d.s. He was also started on ceftriaxone 80 mg/kg once daily and restarted on oseltamivir 45 mg twice daily once his NPA result was returned.
In the 48 h following i.v. immunoglobulin administration his fever, conjunctivitis, mucositis, and rash all settled. His white cell count fell to 8.3 × 109/L (5.3 × 109/L neutrophils, 2.2 × 109/L lymphocytes), C‐reactive protein to 116 mg/L, and alanine transaminase normalized. He remained in hospital for five days during which he developed thrombocytosis with a platelet count of 478 × 109/L (Fig. 1). His NPA remained positive for H1N1/09 when repeated after 48 h, but was negative after a 5‐day course of oseltamivir. An extended polymerase chain reaction panel to detect other respiratory viruses, including adenovirus, bocavirus and coronavirus (OC43 and 229E strains), was negative. Echocardiogram was normal on day 20 of the illness, but he remained on aspirin (at 5 mg/kg OD) until a second echo was performed 2 months later. This was also normal, along with a full set of blood results, and he was discharged from active follow up.
Figure 1.
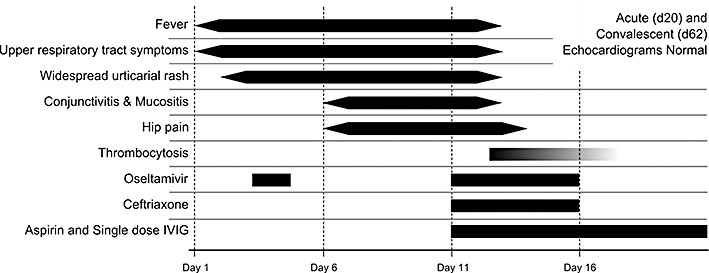
Timeline showing development of pertinent features and treatment strategy. IVIG, intravenous immunoglobulin.
Discussion
A wide variety of respiratory viruses have been isolated from the nasopharynx of children with KD, but careful case–control analyses have excluded all known candidates. 3 To our knowledge this is the first description of classical Kawasaki disease occurring coincident with influenza A infection. Jackson and Burry described a case of influenza B that met the diagnostic criteria for KD, however there was no involvement of the extremities, a relatively short time course, and no leukocytosis or raised inflammatory markers, which are characteristic. 6 The discovery of plasma cells in the bronchial epithelium of KD patients producing oligoclonal immunoglobulin A directed against distinct cytoplasmic inclusion bodies in ciliated epithelial cells suggests that KD is caused by an as‐yet undefined virus. 7 The novelty of the influenza A H1N1/09 strain effectively rules it out as a candidate for the causative agent in KD. However influenza infection is potently immunomodulatory at the bronchial epithelial surface, leading to sustained desensitization to subsequent pathogen challenge and is associated with changes in bacterial colonization patterns. 8 , 9 , 10 We believe the coincident development of KD with influenza infection supports the hypothesis that KD is caused by an endemic pathogen that usually causes a mild self‐limiting illness indistinguishable from other viral illnesses of childhood: development of the KD syndrome is dependent on a permissive genetic and environmental host phenotype that may be facilitated by previous or concurrent infection.
A single report has described the development of Reye's syndrome after KD in a case treated with aspirin, and the evidence base for routine high‐dose aspirin therapy in KD is inconclusive. 11 , 12 The importance of performing high‐quality randomized controlled trials will be heightened if association between influenza infection and KD is recognized in more children – an outcome for which clinicians should be vigilant.
Acknowledgments
KDJJ and BK are grateful for funding via the UK National Institute for Health Research's Comprehensive Biomedical Research Centre funding scheme.
References
- 1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 1967; 16: 178–222 (in Japanese). [PubMed] [Google Scholar]
- 2. Taubert KA, Rowley AH, Shulman ST. Seven‐year national survey of Kawasaki Disease and acute rheumatic fever. Pediatr. Infect. Dis. J. 1994; 13: 704–8. [DOI] [PubMed] [Google Scholar]
- 3. Burgner D, Harnden A. Kawasaki Disease: What is the epidemiology telling us about the etiology? Int. J. Infect. Dis. 2005; 9: 185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: A cross‐sectional serological study. Lancet 2010; 375: 1100–8. [DOI] [PubMed] [Google Scholar]
- 5. Lister P, Reynolds F, Parslow R et al Swine‐origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet 2009; 374: 605–7. [DOI] [PubMed] [Google Scholar]
- 6. Jackson MA, Burry VF. Influenza mimicking Kawasaki disease. Pediatr. Infect. Dis. J. 1993; 12: 787–8. [DOI] [PubMed] [Google Scholar]
- 7. Rowley AH, Baker SC, Shulman ST et al RNA‐containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PLoS One 2008; 3: e1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wadowsky RM, Mietzner SM, Skoner DP, Doyle WJ, Fireman P. Effect of experimental influenza A virus infection on isolation of Streptococcus pneumoniae and other aerobic bacteria from the oropharynges of allergic and nonallergic adult subjects. Infect. Immun. 1995; 63: 1153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walzl G, Tafuro S, Moss P, Openshaw PJ, Hussell T. Influenza virus lung infection protects from respiratory syncytial virus‐induced immunopathology. J. Exp. Med. 2000; 192: 1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Didierlaurent A, Goulding J, Patel S et al Sustained desensitization to bacterial Toll‐like receptor ligands after resolution of respiratory influenza infection. J. Exp. Med. 2008; 205: 323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei CM, Chen HL, Lee PI, Chen CM, Ma CY, Hwu WL. Reye's syndrome developing in an infant on treatment of Kawasaki syndrome. J. Paediatr. Child Health 2005; 41(5–6): 303–4. [DOI] [PubMed] [Google Scholar]
- 12. Baumer JH, Love SJ, Gupta A, Haines LC, Maconochie I, Dua JS. Salicylate for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 2006; 4: CD004175. [DOI] [PMC free article] [PubMed] [Google Scholar]


