Abstract
abstract: Severe acute respiratory syndrome (SARS) is a newly found infectious disease that is caused by a novel human coronavirus, SARS coronavirus (SARS‐CoV). Because the mortality rate of SARS patients is very high, understanding the pathological mechanisms of SARS not only in vivo but in vitro is important for the prevention of SARS. Activation of signaling pathways caused by SARS‐CoV infection leads to the phosphorylation and activation of downstream molecules. Two conflicting cellular programs, apoptosis to eliminate virus‐infected cells and survival to delay apoptosis by producing antiviral cytokines, occur in SARS patients. Recent studies regarding SARS and SARS‐CoV have clarified that activation of mitogen‐activated protein kinases (MAPKs) plays important roles in upregulation of cytokine expression and apoptosis both in vitro and in vivo. Both Akt and p38 MAPK are keys for determination of cell survival or death in SARS‐CoV‐infected cells in vitro. Agents being developed to target these signaling cascades may be important for the design of anti‐SARS‐CoV drugs. This review highlights recent progress regarding SARS‐CoV biology, especially signal transduction in SARS‐CoV‐infected cells.
Keywords: SARS, apoptosis, MAPK
INTRODUCTION
Severe acute respiratory syndrome (SARS) is a newly found infectious disease that is caused by a novel coronavirus, SARS coronavirus (SARS‐CoV). 26 , 34 In late 2002, SARS spread from China to more than 30 countries, causing severe outbreaks of atypical pneumonia. Although the mechanism of SARS pathogenesis in vivo may involves both the effects of viral replication in the target cells and immune responses to viral antigens, there is a lack of molecular pathological data, including data regarding the signaling pathways of SARS‐CoV infection. As viral virulence and the mortality rate of SARS patients are very high, understanding the pathological mechanisms of SARS is important for the prevention of SARS.
Generally, both proapoptotic and prosurvival signaling pathways are activated during viral replication. Many pro‐ and antiapoptotic proteins are involved in these pathways in cells. Several reports indicated that activation of physiological intracellular signaling cascades caused by SARS‐CoV infection leads to the phosphorylation and activation of downstream molecules. For example, mitogen‐activated protein kinases (MAPKs) are well‐known signal transducers that respond to extracellular stimulation by cytokines, growth factors, viral infection, and stress, and in turn regulate cell differentiation, proliferation, survival, and apoptosis. 15 , 23 , 40 Recent studies regarding SARS and SARS‐CoV have clarified that activation of MAPKs plays important roles in upregulation of cytokine expression and apoptosis both in vitro and in vivo. This review highlights progress regarding SARS‐CoV biology, especially signal transduction in SARS‐CoV‐infected cells ( fig. 1).
Figure 1.
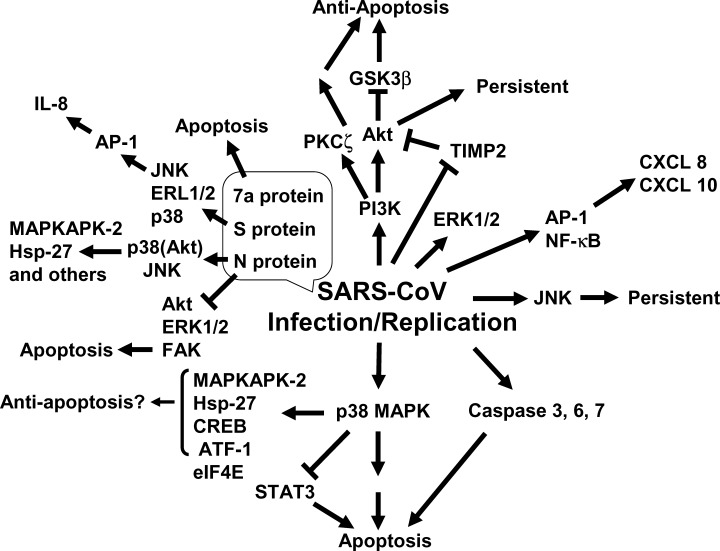
Schematic representation of signal transduction on SARS‐CoV infection.
p38 MAPK
Apoptosis, which is fundamentally different from necrosis, is an active and physiological type of cell death, which can be induced by viral infection, viral replication, and production of viral proteins. The monkey kidney cell line, Vero E6, is widely used in SARS‐CoV research due to its high sensitivity to infection with the virus. Several studies have shown that SARS‐CoV infection of Vero E6 cells induces apoptosis, detected by DNA fragmentation and caspase activation. 28 , 42 p38 MAPK is strongly activated by stress and inflammatory cytokines. Mouse hepatitis virus (MHV), a prototype coronavirus, was able to induce activation of p38 MAPK into virus‐infected cells. 1 p38 MAPK activation in CD14 monocytes was also observed in SARS patients. 24 Phosphorylation of p38 MAPK was significantly upregulated at 18 h postinfection (h.p.i.) in SARS‐CoV‐infected Vero E6 cells. 28 Cytopathic effects of SARS‐CoV‐infection were partially prevented by adding the p38 MAPK‐specific inhibitor, SB203580, to the cells. Although p38 MAPK can promote both cell death and survival, 10 the results of inhibitor studies indicated that the p38 MAPK signaling pathway may be involved mainly in cell death in SARS‐CoV‐infected Vero E6 cells. Several downstream targets of p38 MAPK were phosphorylated in virus‐infected cells, and SB203580 effectively inhibited phosphorylation of these proteins in SARS‐CoV‐infected cells. MAPK‐activated protein kinase (MAPKAPK)‐2, which is activated in response to stress and growth factors, 12 , 13 was phosphorylated in virus‐infected cells. Hsp‐27, which is a substrate of MAPKAPK‐2 and is known to show antiapoptotic activity by inhibiting apoptosome formation, 14 survival factors, 20 cAMP response element‐binding protein (CREB), and activation of transcription factor (ATF)‐1, was also phosphorylated in virus‐infected cells. Phosphorylation of Hsp‐27, CREB, and ATF‐1 may induce an antiapoptotic environment in SARS‐CoV‐infected cells. Nucleocapsid (N) protein was able to induce phosphorylation of Hsp‐27 and CREB in transfected cells as described below. 36 The translation initiation factor, eukaryotic initiation factor 4E (eIF4E), is known to enhance translation rates of cap‐containing mRNAs. 16 The phosphorylation of eIF4E was utilized to promote virus‐specific protein synthesis in the case of MHV. 1 Although the levels of phosphorylated eIF4E were increased by SARS‐CoV infection, the activated eIF4E was not advantageous for viral protein synthesis as demonstrated by the similar kinetics of viral protein accumulation in infected Vero E6 cells in the presence and absence of SB 203580. There may be other substrates of p38 MAPK that are inducible on apoptosis of Vero E6 cells caused by SARS‐CoV infection. As each downstream target molecule of p38 MAPK has a role in the induction of cell death or survival in response to SARS‐CoV infection, determination of the apoptosis‐inducible target molecules of p38 MAPK is important for the development of anti‐SARS‐CoV drugs.
ERK1/2
Extracellular signal‐regulated kinase (ERK) 1/2 was phosphorylated in SARS‐CoV‐infected Vero E6 cells, 27 whereas ERK1/2 was downregulated in N protein‐expressing COS‐1 cells as described below. 36
JNK
c‐Jun N‐terminal kinase (JNK) was phosphorylated in SARS‐CoV‐infected Vero E6 cells. 27 A recent study indicated that persistent infection was established after most of the SARS‐CoV‐infected Vero E6 cells had died by apoptosis. 30 SP600125, an inhibitor of JNK, and LY294002, an inhibitor of phosphatidylinositol 3‐kinase (PI3K), inhibited the establishment of persistence, whereas PD98059, an inhibitor of MEK1/2, and SB203580, an inhibitor of p38 MAPK, did not. Thus, two signaling pathways of JNK and PI3K are important for the establishment of persistence in Vero E6 cells.
STAT3
In Vero E6 cells, signal transducer and activator of transcription (STAT) 3 is constitutively phosphorylated at tyrosine (Tyr)‐705 and is slightly phosphorylated at serine (Ser)‐727. 27 However, SARS‐CoV replication induced dephosphorylation of STAT3 at Tyr‐705. As STAT3 is a major transcription factor activated in response to cytokines, such as interleukin‐6 (IL‐6) and IL‐10, inhibition of STAT3 signaling by dominant negative and antisense oligonucleotides against STAT3 resulted in decreases in cell viability and apoptosis. 17 , 31 Thus, STAT3 is thought to act as an antiapoptotic transcription factor. Although inhibitors of MEK and JNK had no effect on the phosphorylation status of STAT3 in virus‐infected cells, two inhibitors of p38 MAPK (SB203580 and SB202190) partially inhibited dephosphorylation of STAT3 at Tyr‐705, suggesting that the p38 MAPK signaling pathway is upstream of Tyr‐705 dephosphorylation of STAT3 in Vero E6 cells. The level of STAT3 phosphorylation at Ser‐727 was increased in virus‐infected cells. Although the effect of Ser‐727 phosphorylation on the function of STAT3 remains unresolved, it was reported that phosphorylation of Ser‐727 of STAT3 negatively modulated its Tyr phosphorylation. 6 Interestingly, Tyr‐705 dephosphorylation and Ser‐727 phosphorylation showed almost the same timing in SARS‐CoV‐infected cells. STAT3 phosphorylated at Tyr‐705 was localized mainly in the nucleus in mock‐infected cells, whereas STAT3 disappeared from the nucleus in virus‐infected cells. As STAT3 acts as an activator of transcription in the nucleus, STAT3 may lack its transcriptional activity in SARS‐CoV‐infected Vero E6 cells, and thus result in a decrease in antiapoptotic activity in the cells.
As other STAT signal transduction pathways are involved in SARS‐CoV infection, there have been many reports that treatment with interferons (IFNs) can inhibit viral replication in vivo and in vitro. However, there have been no detailed studies of IFN signal transduction in SARS‐CoV‐infected cells. IFN‐alpha receptor recognizes STAT1 and STAT2 via Jak1 and Tyk2, whereas the IFN‐gamma receptor recognizes STAT1 via Jak1 and Jak2. Signal‐transducing adaptor molecule 1 (STAM1) was upregulated in SARS‐CoV‐infected Vero E6 cells. 25 As STAM1 is known to associate with Jak2 and 3 via the immunoreceptor Tyr‐based activation motif, it may play an important role in signal transduction of cytokine receptors by SARS‐CoV infection.
AKT
As mentioned above, the PI3K signaling pathway (including Akt) plays important roles in establishing persistent infection by SARS‐CoV. Akt is phosphorylated at both Ser‐473 and threonine (Thr)‐308 residues via a PI3K‐dependent mechanism on stimulation by growth factors, insulin, and hormones. 39 One of the most important functions of activated Akt in cells is the prevention of apoptosis. Akt has many downstream targets and it induces cell survival via phosphorylation of the forkhead transcription factor (FKHR) family, glycogen synthase kinase 3ß (GSK‐3ß), caspase‐9, and Bad. 2 , 8 , 9 In SARS‐CoV‐infected confluent Vero E6 cells, Ser‐473 of Akt was phosphorylated at 8 h.p.i. and maximal phosphorylation was observed at 18 h.p.i., whereas phosphorylation of Thr‐308 was not observed. As Akt is thought to show its full activity when phosphorylated at both Ser‐473 and Thr‐308, the total activity of Akt may be low in Vero E6 cells. Therefore, the phosphorylated Akt in SARS‐CoV‐infected cells cannot prevent apoptosis.
Interestingly, tissue inhibitor of metalloproteinase 2 (TIMP2), which activates Ras and leads to Ras/PI3K complex formation, was shown to be downregulated in virus‐infected Vero E6 cells at 12 h.p.i. by microarray analysis. 25 The acute Ser dephosphorylation of Akt at 24 h.p.i. after tentative phosphorylation in virus‐infected cells may be due to downregulation of TIMP2.
PKC
Protein kinase C (PKC) is one of the major cellular mediators of biological functions. The PKC superfamily is classified as subsuperfamilies based on activation profiles: conventional PKC (cPKC α, βI, βII, γ novel PKC (nPKC δ, ε, η, θ), atypical PKC (aPKC ζ, ι/ ) PKCμ PKD and PKCν. PKCζ, which was discovered as a unique PKC isotype,
32
is thought to be one of the most important PKC, because PI3K‐dependent activation of PKCζ mediates B‐cell survival by nerve growth factor.
22
Akt has been reported to interact with PKCζ.
21
PKCζ was phosphorylated in SARS‐CoV‐infected Vero E6 cells,
27
suggesting that this molecule is activated as an antiapoptotic response to SARS‐CoV infection.
) PKCμ PKD and PKCν. PKCζ, which was discovered as a unique PKC isotype,
32
is thought to be one of the most important PKC, because PI3K‐dependent activation of PKCζ mediates B‐cell survival by nerve growth factor.
22
Akt has been reported to interact with PKCζ.
21
PKCζ was phosphorylated in SARS‐CoV‐infected Vero E6 cells,
27
suggesting that this molecule is activated as an antiapoptotic response to SARS‐CoV infection.
TRANSCRIPTION FACTORS
Human intestinal epithelial Caco‐2 cells, which are highly permissive to SARS‐CoV, have been used for analysis of cellular gene expression by microarray analysis. The proinflammatory chemokines, interleukin 8 (CXCL8), and interferon‐γ‐inducible protein 10 (CXCL10), were upregulated in SARS‐CoV‐infected Caco‐2 cells. 7 These two chemokines are regulated by AP‐1 and NF‐κB, which were also activated by viral infection. CXCL10 levels were significantly elevated in the blood of SARS patients 38 , 41 and in macrophages in vitro. 5 As described above and below, CREB was phosphorylated in virus‐infected Vero E6 cells and N protein‐expressing COS‐1 cells.
APOPTOSIS‐INDUCIBLE VIRAL GENES
The genome of SARS‐CoV is approximately 30 kb in length and contains 14 potential open‐reading frames (ORFs), including nine viral proteins with no homologues in other coronaviruses. The N protein, which is a 423 amino acid predicted phospho‐protein of 46 kDa with a short Ser‐rich stretch and a putative bipartite nuclear localization signal, is known to show little homology with molecules in other coronaviruses. The N protein has been shown to undergo self‐association through the C‐terminal 209 amino acid region 35 and was able to induce apoptosis of COS‐1 cells in the absence of growth factors. 36 Both JNK and p38 MAPK were upregulated in the transfected cells, whereas ERK and Akt were downregulated. Activated p38 MAPK by N protein induced actin reorganization into cells in the absence of growth factors. The downstream targets of p38 MAPK, MAPKAPK‐2, and Hsp‐27, were phosphorylated in the cells as well as in the virus‐infected Vero E6 cells. Downregulation of focal adhesion kinase (FAK) activity and fibronectin expression was observed due to N protein expression, supporting the hypothesis that apoptosis induced by N protein is caused by interference with the integrin signaling pathway, and that serum factors are able to inhibit apoptosis by maintaining the activity of FAK through alternative pathways. Caspase‐3 and ‐7 were activated only in the absence of growth factors in N‐expressing cells. However, SB203580, an inhibitor of p38 MAPK, failed to inhibit caspase‐3 activation. Thus, there are two independent functional properties of N protein, p38 MAPK‐dependent actin reorganization, and caspase activation. Another group showed that the levels of transcription factors, c‐Fos, ATF2, CREB, and FosB, are increased by expression of N in Vero and Huh‐7 cells. 18 Spike (S) protein of SARS‐CoV also induced activation of AP‐1 and IL‐8 promoter, possibly via activation of MAPKs, in A549 and HFL‐1 cells. 4 As high serum levels of IL‐8 were observed in patients in the acute stage of SARS, 19 activation of the IL‐8 promoter via AP‐1 induced by S protein may explain these clinical observations. The binding of S protein to a viral receptor, angiotensin‐converting enzyme (ACE)‐2, may be a trigger for activation of these MAPKs.
Two unique proteins of SARS‐CoV, 3a (U274, X1) and 7a (U122, X4), have been studied. The 3a protein is located mainly in the Golgi apparatus and contains three transmembrane regions, 43 whereas 7a encodes a protein of 122 amino acids containing a probable cleaved signal sequence and a C‐terminal transmembrane helix. As the C‐terminal tail also contains a typical endoplasmic reticulum (ER) retrieval motif, 7a is localized to the perinuclear region in both SARS‐CoV‐infected and ‐transfected cells. 11 The overexpression of 7a, but not of 3a, induced apoptosis via a caspase‐dependent pathway. 37
CONCLUSIONS
In SARS patients, two conflicting cellular programs occur: “apoptosis” to eliminate virus‐infected cells and “survival” to delay apoptosis by producing antiviral cytokines. The control mechanisms balancing cell survival against cell death in vitro are important for understanding the pathology in vivo. As mentioned above, activation of p38 MAPK is involved in the regulation of IL‐8 in vivo. Both Akt and p38 MAPK are keys for determination of cell survival or death in SARS‐CoV‐infected cells in vitro. Activation of the p38 MAPK signaling pathway and dephosphorylation of STAT3 via p38 MAPK induced by SARS‐CoV infection have partially proapoptotic roles in Vero E6 cells. On the other hand, the levels of Akt, which inactivates proapoptotic pathways, are too low to block apoptosis signaling pathways. As Akt activation is necessary to establish persistently infected cells that escape from apoptotic cell death on viral infection, Akt plays an important role in delaying apoptosis in SARS‐CoV‐infected cells. Agents being developed to target p38 MAPK and its downstream molecules (for downregulation) and Akt (for upregulation) may be important for the design of anti‐SARS‐CoV drugs.
ADDENDUM
In recent papers, Surjit et al. reported that phosphorylated N protein shuttled from nucleus to cytoplasm by binding to 14‐3‐3 (J. Virol. 2005. 79: 11476–11486) and N protein blocked S phase progression (J. Biol. Chem. 2006. 281: 10669–10681). Yuan et al. reported that 7a protein blocked cell cycle progression at G0/G1 phase (Virology 2006. 346. 74–85). Inhibition of cell proliferation was caused by dephosphorylation of Akt (by us; FEMS Immunol. Med. Microbiol. 2006. 46: 236–243). Okabayashi et al. reported that production and response of IFN were not suppressed by SARS‐CoV‐infection (J. Med. Virol. 2006. 78: 417–424). We reported regulation 90 kDa ribosomal S6 kinase phosphorylation (FEBS Lett. 2006. 580: 1417–1424), importance of Bcl‐2 and Bcl‐xL for the establishment of persistent infection (http://Biochem.Biophys.Res.Commun. 2006. 347: 261–265), and enhancement of cytotoxity against Vero E6 cells persistently infected with SARS‐CoV by Mycoplasma fermentans (Arch. Virol. In press).
ACKNOWLEDGMENTS
I thank Drs. S. Fukushi, M. Saijo, M. Ogata, K. Sakai, I. Kurane, and S. Morikawa (National Institute of Infectious Diseases, Japan) for useful suggestions. We acknowledge funding from a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, a grant‐in‐aid from the Ministry of Health, Labor, and Welfare of Japan, and the Japan, Health Science Foundation, Tokyo, Japan.
REFERENCES
- 1. Banerjee, S. , Narayanan K., Mizutani T. & Makino S.. 2002. Murine coronavirus replication‐induced p38 mitogen‐activated protein kinase activation promotes interleukin‐6 production and virus replication in cultured cells. J. Virol. 76: 5937–5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cardone, M.H. , Roy N., Stennicke H.R., et al 1998. Regulation of cell death protease caspase‐9 by phosphorylation. Science 282: 1318–1321. [DOI] [PubMed] [Google Scholar]
- 3. Chang, L. & Karin M.. 2001. Mammalian MAP kinase signalling cascades. Nature 410: 37–40. [DOI] [PubMed] [Google Scholar]
- 4. Chang, Y.J. , Liu C.Y., Chiang B.L., et al 2004. Induction of IL‐8 release in lung cells via activator protein‐1 by recombinant baculovirus displaying severe acute respiratory syndrome‐coronavirus spike proteins: identification of two functional regions. J. Immunol. 173: 7602–7614. [DOI] [PubMed] [Google Scholar]
- 5. Cheung, C.Y. , Poon L.L., Ng L.H., et al 2005. Cytokine responses in severe acute respiratory syndrome coronavirus‐infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 79: 7819–7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung, J. , Uchida E., Grammer T.C. & Blenis J.. 1997. STAT3 serine phosphorylation by ERK‐dependent and ‐independent pathways negatively modulates its tyrosine phosphorylation. Mol. Cell. Biol. 17: 6508–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cinatl, J. Jr ., Michaelis M., Morgenstern B. & Doerr H.W.. 2005. High‐dose hydrocortisone reduces expression of the pro‐inflammatory chemokines CXCL8 and CXCL10 in SARS coronavirus‐infected intestinal cells. Int. J. Mol. Med. 15: 323–327. [PubMed] [Google Scholar]
- 8. Cross, D.A. , Alessi D.R., Cohen P., et al 1995. Inhibition of glycogen synthase kinase‐3 by insulin mediated by protein kinase B. Nature 378: 785–789. [DOI] [PubMed] [Google Scholar]
- 9. Datta, S.R. , Dudek H., Tao X., et al 1997. Akt phosphorylation of BAD couples survival signals to the cell‐intrinsic death machinery. Cell 91: 231–241. [DOI] [PubMed] [Google Scholar]
- 10. Dent, P. , Yacoub A., Fisher P.B., et al 2002. MAPK pathways in radiation responses. Oncogene 22: 5885–5896. [DOI] [PubMed] [Google Scholar]
- 11. Fielding, B.C. , Tan Y.J., Shuo S., et al 2004. Characterization of a unique group‐specific protein (U122) of the severe acute respiratory syndrome coronavirus. J. Virol. 78: 7311–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foltz, I.N. , Lee J.C., Young P.R. & Schrader J.W.. 1997. Hemopoietic growth factors with the exception of interleukin‐4 activate the p38 mitogen‐activated protein kinase pathway. J. Biol. Chem. 272: 3296–3301. [DOI] [PubMed] [Google Scholar]
- 13. Freshney, N.W. , Rawlinson L., Guesdon F., et al 1994. Interleukin‐1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell 78: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 14. Garrido, C. , Schmitt E., Cande C., et al 2003. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle 2: 579–584. [PubMed] [Google Scholar]
- 15. Garrington, T.P. & Johnson G.L.. 1999. Organization and regulation of mitogen‐activated protein kinase signaling pathways. Curr. Opin. Cell. Biol. 11: 211–218. [DOI] [PubMed] [Google Scholar]
- 16. Gingras, A.C. , Raught B. & Sonenberg N.. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68: 913–963. [DOI] [PubMed] [Google Scholar]
- 17. Grandis, J.R. , Drenning S.D., Zeng Q., et al 2000. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo . Proc. Natl. Acad. Sci. USA 97: 4227–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He, R. , Leeson A., Andonov A., et al 2003. Activation of AP‐1 signal transduction pathway by SARS coronavirus nucleocapsid protein. Biochem. Biophys. Res. Commun. 311: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang, K.J. , Su I.J., Theron M., et al 2005. An interferon‐gamma‐related cytokine storm in SARS patients. J. Med. Virol. 75: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jean, D. , Harbison M., McConkey D.J., et al 1998. CREB and its associated proteins act as survival factors for human melanoma cells. J. Biol. Chem. 273: 24884–24890. [DOI] [PubMed] [Google Scholar]
- 21. Konishi, H. , Shinomura T., Kuroda S., et al 1994. Molecular cloning of rat RAC protein kinase alpha and beta and their association with protein kinase C zeta. Biochem. Biophys. Res. Commun. 205: 817–825. [DOI] [PubMed] [Google Scholar]
- 22. Kronfeld, I. , Kazimirsky G., Gelfand E.W. & Brodie C.. 2002. NGF rescues human B lymphocytes from anti‐IgM induced apoptosis by activation of PKC. Eur. J. Immunol. 32: 136–143. [DOI] [PubMed] [Google Scholar]
- 23. Kyriakis, J.M. & Avruch J.. 2001. Mammalian mitogen‐activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81: 807–869. [DOI] [PubMed] [Google Scholar]
- 24. Lee, C.H. , Chen R.F., Liu J.W., et al 2004. Altered p38 mitogen‐activated protein kinase expression in different leukocytes with increment of immunosuppressive mediators in patients with severe acute respiratory syndrome. J. Immunol. 172: 7841–7847. [DOI] [PubMed] [Google Scholar]
- 25. Leong, W.F. , Tan H.C., Ooi E.E., et al 2005. Microarray and real‐time RT‐PCR analyses of differential human gene expression patterns induced by severe acute respiratory syndrome (SARS) coronavirus infection of Vero cells. Microbes Infect. 7: 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marra, M.A. , Jones S.J., Astell C.R., et al 2003. The genome sequence of the SARS‐associated coronavirus. Science 300: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 27. Mizutani, T. , Fukushi S., Murakami M., et al 2004. Tyrosine dephosphorylation of STAT3 in SARS coronavirus‐infected Vero E6 cells. FEBS Lett. 577: 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mizutani, T. , Fukushi S., Saijo M., et al 2004. Phosphorylation of p38 MAPK and its downstream targets in SARS coronavirus‐infected cells. Biochem. Biophys. Res. Commun. 319: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mizutani, T. , Fukushi S., Saijo M., et al 2004. Importance of Akt signaling pathway for apoptosis in SARS‐CoV‐infected Vero E6 cells. Virology 327: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizutani, T. , Fukushi S., Saijo M., et al 2005. JNK and PI3K/Akt signaling pathways are required for establishing persistent SARS‐CoV‐infection in Vero E6 cells. Biochim. Biophys. Acta 1741: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mora, L.B. , Buettner R., Seigne J., et al 2002. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 62: 6659–6666. [PubMed] [Google Scholar]
- 32. Ono, Y. , Fujii T., Ogita K., et al 1989. Protein kinase C‐ subspecies from brain: its structure, expression and properties. Proc. Natl. Acad. Sci. USA 86: 3099–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajan, P. & McKay R.D.. 1998. Multiple routes to astrocytic differentiation in the CNS. J. Neurosci. 18: 3620–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rota, P.A. , Oberste M.S., Monroe S.S., et al 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300: 1394–1399. [DOI] [PubMed] [Google Scholar]
- 35. Surjit, M. , Liu B., Kumar P., et al 2004. The nucleocapsid protein of the SARS coronavirus is capable of self‐association through a C‐terminal 209 amino acid interaction domain. Biochem. Biophys. Res. Commun. 317: 1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Surjit, M. , Liu B., Jameel S., et al 2004. The SARS coronavirus nucleocapsid (N) protein induces actin reorganization and apoptosis in COS‐1 cells. Biochem. J. 383: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan, Y.J. , Fielding B.C., Goh P.Y., et al 2004. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase‐dependent pathway. J. Virol. 78: 14043–14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ward, S.E. , Loutfy M.R., Blatt L.M., et al 2005. Dynamic changes in clinical features and cytokine/chemokine responses in SARS patients treated with interferon alfacon‐1 plus corticosteroids. Antivir. Ther. 10: 263–275. [PubMed] [Google Scholar]
- 39. Welch, H. , Eguinoa A., Stephens L.R. & Hawkins P.T.. 1998. Protein kinase B and RAC are activated in parallel within a phosphatidylinositide 3OH‐kinase‐controlled signaling pathway. J. Biol. Chem. 273: 11248–11256. [DOI] [PubMed] [Google Scholar]
- 40. Whitmarsh, A.J. & Davis R.J.. 2000. A central control for cell growth. Nature 403: 255–256. [DOI] [PubMed] [Google Scholar]
- 41. Wong, C.K. , Lam C.W., Wu A.K., et al 2004. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yan, H. , Xiao G., Zhang J., et al 2004. SARS coronavirus induces apoptosis in Vero E6 cells. J. Med. Virol. 73: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yuan, X. , Li J., Shan Y., et al 2005. Subcellular localization and membrane association of SARS‐CoV 3a protein. Virus Res. 109: 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]


