Summary
Background
The diagnostic yield of bronchoalveolar lavage (BAL) in the Immunocompromised pediatric population has ranged from 28% to 68%. We hypothesized that the diagnostic yield of BALs would be higher in more recent years due to new diagnostic assays.
Methods
A retrospective case series was performed among immunocompromised children ≤18 years old who underwent BALs from 2001 to 2012, to assess the yield of microbiologic diagnostic studies and to determine the impact of BAL findings on antimicrobial management.
Results
In all, 123 subjects underwent 174 BALs (mean age 9.9 years). Underlying diagnoses included both malignant (n = 79) and non‐malignant (n = 44) disorders, and 75 (61.0%) subjects were hematopoietic stem cell transplant (HSCT) recipients. Fifty‐four (31.0%) of 174 BAL were positive for ≥1 potential pathogen (n = 58 microorganisms). The diagnostic yield of BALs performed from 2001 to 2006 versus2007–2012 was similar (40.5% vs. 26.6%, respectively, P = 0.07). Most subjects (86.2%) were on ≥1 antimicrobial at the time of BAL. Most (65.8%) negative BALs were associated with narrowing antimicrobial therapy, while most (74.1%) positive BALs were associated with continuing or changing to targeted antimicrobial therapy.
Conclusions
In this study population, the diagnostic yield of BAL was similar to that previously described and unchanged in more recent years. Both negative and positive BALs were associated with changes in antimicrobial management.
Summary
A 10‐year retrospective review of bronchoalveolar lavage in 123 immunocompromised children determined that the rate of isolation of potential pathogens was 31% in this population. The majority of BAL was associated with a change in antimicrobial therapy. Pediatr Pulmonol. 2017;52:820–826. © 2017 Wiley Periodicals, Inc.
Keywords: BMT, gram negative bacilli, gram positive cocci, fungi, virus, mold
INTRODUCTION
Lower respiratory tract infections (LRTIs) result in substantial morbidity and mortality for immunocompromised children.1, 2 Approximately 25% of children undergoing hematopoietic stem cell transplant (HSCT) will develop a LRTI.1, 3 Improving detection of pathogens from BAL specimens is critical to guide antimicrobial management, direct infection prevention and control strategies, understand potential changes in epidemiology, and avoid more invasive diagnostic interventions, for example, lung biopsy. Prior studies of BAL performed in immunocompromised children have reported detection of pathogens in 28–68% (mean 50%) of procedures using a variety of case definitions and patient populations.2, 4, 5, 6, 7, 8, 9, 10 However, few recent studies have examined the diagnostic yield of BAL in the immunocompromised pediatric population and compared the yield of different diagnostic assays.
Thus, the primary aim of this study was to determine the overall diagnostic yield of BALs in immunocompromised children over the past decade, and to compare the rates of isolation of bacterial, fungal, viral, and mycobacterial pathogens in the early (2001–2006) versus later (2007–2012) years of the study period. We hypothesized that the diagnostic yield of BALs performed in the earlier years of the study would be lower than BALs performed during the later years due to improvements in diagnostic assays. The secondary aim was to determine if BAL results were associated with changes in antimicrobial therapy.
METHODS
Study Design, Site, and Subjects
A retrospective case series of immunocompromised pediatric patients undergoing BAL from 2001 to 2012 was performed at NewYork‐Presbyterian Morgan Stanley Children's Hospital, Columbia University Medical Center, New York, New York. The decision to perform BAL was made by the patient's primary team in consultation with the pediatric pulmonology service. Eligible subjects were ≤18 years of age at the time of BAL and had multiple underlying diagnoses (Table 1). To identify cases, the BAL procedure records of the Pediatric Pulmonology division were reviewed. If a subject underwent more than one BAL during the study period, all procedures were included. Flexible bronchoscopy was performed to obtain BAL specimens in patients who were intubated endotracheally, or less commonly, via laryngeal mask airway (LMA). Sites for BAL were chosen based on chest imaging, usually CT scan, and by findings at the time of bronchoscopy. BAL was performed at multiple sites if there were multiple areas of involvement on imaging, or by bronchoscopic appearance. The institutional review board of Columbia University Medical Center approved the conduct of this study with a waiver of informed consent.
Table 1.
Demographic and Clinical Characteristics of Immunocompromised Children With and Without Potential Pathogens Detected by BAL, 2001–2012
| Characteristic | Patients (n = 47) with potential pathogen isolated from BAL N (%) | Patients (n = 76) without potential pathogen from BAL N (%) | P |
|---|---|---|---|
| BAL | 84 (48) | 90 (52) | |
| Mean age at 1st BAL (range) | 9.4 years (0.7–18.5) | 10.2 years (0.4–18.9) | 0.45 |
| Sex | |||
| M | 28 (59.6) | 50 (65.8) | 0.56 |
| Race/ethnicity | |||
| White non‐Hispanic | 13 (27.6) | 25 (64.8) | 0.68 |
| Black | 4 (8.5) | 7 (9.2) | 1.00 |
| Hispanic | 13 (27.6) | 19 (25.0) | 0.83 |
| Asian | 2 (4.3) | 4 (5.3) | 1.00 |
| Other | 1 (2.1) | 1 (13.2) | 1.00 |
| Unknown | 14 (29.8) | 20 (26.3) | 0.68 |
| Underlying diagnosis | |||
| Acute myelogenous leukemia | 8 (17.0) | 14 (18.4) | 1.00 |
| Acute lymphocytic leukemia | 13 (27.6) | 17 (22.3) | 0.52 |
| Aplastic Anemia | 2 (4.3) | 11 (14.4) | 0.12 |
| Chronic myelogenous leukemia | 2 (4.3) | 0 | 0.14 |
| Hemophagocytic | |||
| lymphohistiocytosis | 4 (8.5) | 5 (6.6) | 0.73 |
| Hodgkin disease | 2 (4.3) | 2 (2.6) | 0.63 |
| Other malignant disorders | 5 (10.6) | 16 (21.0) | 0.70 |
| Other nonmalignant disorders 1 | 11 (23.4) | 11 (14.5) | 0.23 |
| HSCT Prior to BAL | 31 (70.0) | 44 (57.9) | 0.44 |
| Time from 1st HSCT to 1st BAL | |||
| ≤30 d | 8 (25.8) | 15 (34.1) | |
| >30 d | 23 (74.2) | 29 (65.9) | 0.61 |
| Degree of myeloablation | |||
| Myeloablative | 16 (51.6) | 25 (56.8) | 0.81 |
| Reduced toxicity | 7 (22.6) | 13 (29.5) | 0.60 |
| Reduced intensity | 8 (25.8) | 6 (13.6) | 0.23 |
| Received anti‐thymocyte globulin | 17 (54.8) | 21 (47.7) | 0.64 |
| Received alemtuzumab | 5 (16.1) | 10 (22.7) | 0.57 |
| Time period | |||
| 2001–2006 | 28 (59.6) | 30 (39.5) | |
| 2007–2012 | 19 (40.4) | 46 (60.5) | 0.041 |
HSCT, hematopoietic stem cell transplant.
Underwent HSCT prior to BAL. Other malignant and non‐malignant indications for HSCT included neuroblastoma, Langerhans cell histiocytosis, adrenoleukodystrophy, myelodysplastic syndrome, thalassemia, pansclerotic morphea and Wiskott–Aldrich syndrome.
BAL Assays
The results of all microbial studies performed on each BAL were reviewed, including bacterial cultures, fungal cultures, AFB smear and culture, and viral diagnostic studies (cultures, direct fluorescence assay [DFA], and/or enzyme‐linked immunoassays [EIAs], and polymerase chain reaction [PCR]). In May 2012, a multiplex reverse transcriptase (RT)‐PCR assay was introduced (FilmArray, Biofire Diagnostics, Inc., Salt Lake City, UT)11 comprising 20 viral and bacterial targets (adenovirus, four coronaviruses, human metapneumovirus, rhinovirus/enterovirus, five subtypes of influenza A/B, four subtypes of parainfluenza, respiratory syncytial virus, Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae). Results of cytologic staining for Pneumocystis jiroveci cysts, acid‐fast organisms, viral inclusions or evidence of cytologic transformation, and the Platelia Aspergillus galactomannan from BAL (Bio‐Rad, Hercules, CA)12 introduced in September 2012 were also reviewed. Legionella cultures were not routinely sent. Quantitative BAL was not introduced at our institution's microbiology lab until after the end of the study period.
Pathogen Case Definition
Potential pathogens were defined as any detection by PCR or growth in culture of respiratory viruses (e.g., RSV or parainfluenza), cytomegalovirus (CMV), herpes simplex virus (HSV), nontuberculous mycobacteria (NTM), pathogenic bacteria, or fungal organisms (e.g., Aspergillus, Pneumocystis, or Mucor spp.). Coagulase‐negative staphylococci (CoNS) or mixed commensal flora were not considered pathogens in this study. Quantitative BAL was not performed during the study.
Antimicrobial Management
Pharmacy records in the electronic medical record (EMR) were reviewed to assess whether BAL results were associated with changes in antimicrobial therapy. To assess changes in antimicrobial therapy, we recorded the treatment initiated during the 72 hr prior to BAL through the 7 days following BAL. For patients who grew mycobacteria, pharmacy records were reviewed for 60 days following bronchoscopy. Intravenous and inhaled antimicrobial agents and adjunctive agents, for example, CMV‐ or RSV‐specific immunoglobulin, were collected. Doses of antimicrobials were reviewed to differentiate prophylactic from treatment regimens.
The EMR notes of the patient's primary team or intensive care team were reviewed to determine the rationale for changes in antimicrobial therapy. Antimicrobial therapy was categorized as targeted or non‐targeted. Targeted therapy was defined as antimicrobials that treated an identified bacterial pathogen based on susceptibility results. Non‐targeted therapy was defined as antimicrobials that did not treat an identified pathogen or that were continued for a negative BAL. Antimicrobial therapy was further categorized as follows:
narrowing therapy (discontinuation of ≥1 non‐targeted antimicrobial or discontinuation of second targeted antimicrobial, i.e., duplicate therapy);
continuing targeted therapy (continuing ≥1 targeted antimicrobial for >5 calendar days);
changing to targeted therapy (adding ≥1 targeted antimicrobial continued for ≥5 calendar days);
continuing non‐targeted therapy (continuing ≥1 nontargeted antimicrobial for ≥5 calendar days); or
no antimicrobial therapy recorded.
Treatment could reflect ≥1 category.
Data Collection
Demographic and clinical characteristics were collected from the EMR. In patients who underwent autologous HSCT followed by a planned allogeneic HSCT, the date and degree of myeloablation13 of the first HSCT were used in the analysis. Radiographic findings suggestive of LRTI, for example, nonspecific “opacities,” “nodules,” and “ground glass opacities” were abstracted from attending radiologists’ electronic reports of computed tomography (CT) performed within 10 days of BAL (when available) or chest radiographs (if a recent CT had not been performed). Radiographic findings reported for <5% of subjects (i.e., scarring, mass, reticulations, increased attenuation) were excluded from analysis, as the study was not powered to detect an association between such findings and potential pathogens.
Statistical Analysis
To analyze potential factors associated with isolation of pathogens from BAL, we assessed binary variables such as sex and transplantation status using chi‐square (or Fisher's exact test) or Student's t‐test, when applicable. Factors associated with positive BAL findings in the bivariate analysis (P‐value ≤0.2) were further assessed by multilogistic regression. P‐values ≤0.05 were considered significant.
The proportion of specimens sent for each test during the years 2001–2006 versus 2007–2012, as well as the overall yield of BALs performed during each period, was compared via test of proportions. The types of microorganisms, that is, bacterial, fungal, NTM, and/or viruses detected during these two time intervals were similarly analyzed. Additionally, the yield from the first versus second BAL performed within 30 days of the first BAL was compared for subjects in whom BAL was performed more than once. Radiographic findings associated with isolation of potential pathogens were compared via Fisher's exact test. Statistical analysis was performed using SAS version 9.3 (Cary, NC).
RESULTS
Study Subjects
During the study period, 123 subjects (mean age 9.9 years, range 0.4–18.9 years) underwent 174 BALs. The demographic and clinical characteristics of subjects, including their underlying diagnoses, are shown in Table 1. The majority of subjects (n = 75, 60.9%) had undergone HSCT prior to their BAL and the mean time from first HSCT to BAL was 123 days (range 1–625 days). No factors, including HSCT transplant status or degree of myeloablation, predicted detection of potential pathogens. Thirty‐one subjects had >1 BAL performed. Among those who had a second BAL within 1 year of the first, the mean time to second BAL was 163 days (range 9–316 days).
BAL Findings
Overall, 80 (45.9%) of the 174 BALs yielded 85 microorganisms (Fig. 1), of which 54/174 (31.0%) were positive for 58 potential pathogens and 26/174 (14.9%) were positive for non‐pathogenic organisms. The diagnostic yield of BALs performed from 2001 to 2006 versus 2007–2012 was similar (40.5% vs. 26.6%, respectively, P = 0.07), as shown in Table 2. In addition, the types of microorganisms detected in the earlier versus later era were similar. Overall, the most commonly isolated organisms were gram‐negative bacilli (GNB), CMV and Candida species. All cultures for anaerobic pathogens (n = 82), Legionella (n = 135), Actinomyces (n = 46), Nocardia (n = 49), and Mycoplasma (n = 80) were negative.
Figure 1.
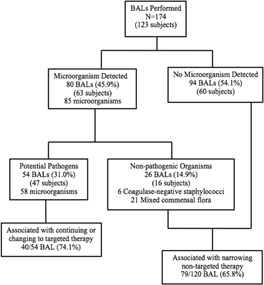
Diagnostic yield of BAL.
Table 2.
Yield of BAL for Potential Pathogens, 2001–2006 Versus 2007–2012
| Diagnostic assays obtained | 2001–2006, N = 84 | 2007–2012, N = 90 | P |
|---|---|---|---|
| Mean antimicrobial duration prior to BAL (range) | 4.6 d (1–15) | 5.6 d (0–24) | 0.42 |
| Negative BAL | 40 (47.6%) | 54 (60.0%) | 0.13 |
| CoNS or mixed commensal flora | 12 (14.3%) | 15 (16.7%) | 0.68 |
| Potential pathogens isolated | 34 (40.5%) | 24 (26.6%) | 0.07 |
| Bacteria (all) | 11 (13.1%) | 6 (6.7%) | 0.20 |
| Gram‐negative bacilli | 6 | 5 | |
| Gram‐positive cocci | 5 | 1 | |
| Viruses (all) | 9 (10.7%) | 7 (7.8%) | 0.60 |
| CMV | 5 | 3 | |
| Adenovirus | 2 | 2 | |
| RSV | 1 | 1 | |
| Other 1 | 1 | 1 | |
| Fungi or molds (all) | 11 (13.1%) | 10 (11.1%) | 0.81 |
| Candida spp. | 6 | 7 | |
| Pneumocystis | 3 | 1 | |
| Aspergillus spp. | 1 | 1 | |
| Other 2 | 1 | 1 | |
| Mycobacterium avium complex | 3 (3.6%) | 1 (1.1%) | 0.36 |
CoNS, coagulase‐negative staphylococci; CMV, cytomegalovirus; RSV, respiratory syncytial virus.
Parainfluenza type 3 and HSV.
Trichosporon and Mucor spp.
There was a significant increase in obtaining fungal and CMV cultures during the years 2007–2012, when compared to the earlier years of the study (P < 0.01). Additionally, in the later years of the study, there was a decrease in viral cultures (P = 0.05) and an increase in viral comprehensive panels (P = 0.012) as the former was supplanted by the latter at our institution. The proportion of BALs sent for bacterial culture, AFB culture, and cytologic examination was similar over time.
Twelve subjects had a second BAL performed within 30 days of their first BAL. The yield of the first BAL was 50% (6/12) while the diagnostic yield for the second BAL was 33% (4/12). Three of the second BALs detected new microorganisms including Candida krusei, Aspergillus niger, and Pseudomonas aeruginosa.
Radiographic Findings
The majority of BALs (166, 95.4%) were preceded by abnormal findings on CT scan (n = 134, 77%) or chest radiography. Nonspecific opacities (n = 32), nodules (n = 30), and ground glass opacities (n = 21) were significantly associated with the detection of potential pathogens (Table 3).
Table 3.
Radiographic Findings Associated With BAL
| Radiographic findings | Potential pathogen isolated from BAL, n = 58 (%) | Negative or non‐pathogen (CoNS or mixed commensal flora), n = 116 (%) | P |
|---|---|---|---|
| Nonspecific opacity | 32 (55.2) | 32 (27.6) | <0.001 |
| Nodule | 30 (51.7) | 26 (22.4) | <0.001 |
| Atelectasis | 16 (27.6) | 33 (28.4) | 1.00 |
| Ground glass opacity | 21 (36.2) | 22 (19.0) | 0.016 |
| Effusion | 12 (29.7) | 29 (25.0) | 0.575 |
| Consolidation | 15 (25.9) | 15 (12.9) | 0.054 |
CoNS, coagulase‐negative staphylococci.
Antimicrobial Therapy
The majority of study subjects (106 subjects, 86.2%) were receiving ≥1 antimicrobial agent at the time of BAL; 13.2%, 64.2%, and 22.6% were receiving one, 2 or >3 agents, respectively. The mean duration of antimicrobial therapy prior to BAL was 5.3 days (range, 0–24 days).
Therapy was narrowed (i.e., at least one non‐targeted agent was discontinued) following 79 (65.8%) of 120 negative BALs. However, a non‐targeted agent was also continued following 68 (56.7%) negative BALs. Following the 54 positive BALs, therapy was continued for 13 (32.5%) or changed to targeted therapy for 27 (67.5%). Of the 13 positive BALs resulting in continuation of targeted therapy, 10 (76.9%) had a bacterial or fungal organism identified. Of the 27 positive BALs resulting in changing to targeted therapy, 17 (63.0%) had a viral or mycobacterial organism identified.
DISCUSSION
In this study, we describe a large cohort of immunocompromised children who underwent BAL over a 12‐year period and found that the infectious diagnostic yield of BAL was 31%. We did not identify demographic or clinical risk factors associated with detection of a potential pathogen. Subjects who had undergone HSCT and/or who had received more myeloablative conditioning regimens were not more likely to have a detectable pathogen. However, radiographic findings of nonspecific opacities, ground glass opacities and nodules were more likely to be associated with isolation of a potential pathogen; the presence of such findings may therefore have clinical utility in the decision to perform BAL in immunocompromised children.
The rate of positive BALs in previous studies has ranged from 28% to 68%, as shown in Table 4,4, 5, 7, 8, 9, 10, 14, 15, 16 and this variability likely reflects institutional differences in patient populations, BAL testing modalities, and local epidemiology of pathogens.4, 14 Perhaps more importantly, the case definitions for pathogenic microorganisms has varied; in contrast to the current study, some studies have included CoNS and mixed commensal flora as pathogenic.4, 5 Yet others have variously excluded isolates of CMV, Candida spp., NTM and Aspergillus spp. as airway contaminants.4, 6, 9
Table 4.
Case Series of BAL Findings in Immunosuppressed Children
| Ref. | Study period | Subject n/BAL N | Underlying diagnoses | BAL yield n/N 1 (%) | Bacteria (%) | Viruses (%) | Fungi 2 (%) | NTM |
|---|---|---|---|---|---|---|---|---|
| Current study | 2001–2012 | 123/174 | Hematologic malignancies, HLH, aplastic anemia, cancer, HSCT | 54/174 (31) | 29.3 | 27.5 | 36.2 | 6.9% |
| 4 | 2000–2009 | 33/44 | Hematologic malignancies, HSCT | 22/44 (55) | 32.3 3 | 35.3 | 32.3 | 0 |
| 7 | 2000–2004 | 58/62 | Primary immunodeficiency, cancer, HSCT | 33/62 (53) | 59.6 3 | 26.9 | 13.4 | 0 |
| 9 | 1995–2003 | 89/92 | Cancer, HSCT | 41/92 (45) | 24.4 | 24.4 | 51.2 | 0 |
| 10 | 1990–2002 | 78/78 | HSCT | 53/78 (68) | 52.3 | 13.6 | 6.8 | 0 |
| 5 | 1993–2003 | 54/68 | Leukemia, HSCT, AIDS, OHT, cancer, SLE | 25/36 (37) | 56.7 3 | 16.2 | 27.0 | 0 |
| 8 | 1988–1998 | 53/53 | Cancer | 15/53 (28) | 20.0 | 26.7 | 46.7 | 6.7% |
| 14 | 1995–1999 | 52/86 | Cancer, HSCT, SCID | 27/86 (31) | 24.9 | 24.9 | 41.2 | 0 |
| 15 | 1990–1996 | 28/31 | HSCT | 16/31 (52) | 37.5 | 50.0 | 12.5 | 0 |
| 19 | 1985–1990 | 27/29 | HSCT | 15/29 3 (52) 4 | 0 | 75.0 | 25.0 | 0 |
HLH, hemophagocytic lymphohistiocytosis; HSCT, hematopoietic stem cell transplantation; AIDS, acquired immunodeficiency syndrome; OHT, orthotopic heart transplant; SLE, systemic lupus erythematosus; SCID, severe combined immunodeficiency.
n = pathogenic microorganism(s) detected; N = number of BALs.
Fungi includes: yeast, mould, and Pneumocystis.
Included coagulase‐negative staphylococci and/or viridans‐group streptococci as pathogenic microorganisms.
Toxoplasma isolated from one BAL.
Contrary to our hypothesis, the rate of isolation of potential pathogens was higher in the earlier years of the study than in later years, although not statistically significant, despite the fact that significantly more samples were submitted for fungal and CMV cultures in the later study period. Our results indicate that the majority of patients were on multiple antimicrobials at the time of BAL, and this may have affected culture results. Additionally, during the last two decades, isolation of viral microorganisms from BAL has decreased overall,5, 15 which may reflect improving antiviral prophylaxis in patients who are CMV‐seropositive. The decreasing incidence of Pneumocystis pneumonia due to prophylaxis against this organism is also well documented.17 Furthermore, negative BALs may have reflected non‐infectious causes of lower tract disease such as graft‐versus‐host disease or pulmonary fibrosis, as has been previously described.2, 10, 16, 18, 19 Recent comparison studies have suggested that lung biopsy may elucidate noninfectious diagnoses in a manner superior to that of BAL,20, 21, 22 though this procedure is associated with higher morbidity and mortality than BAL.
Our study supports the observations made in prior series that a positive result from BAL impacts treatment decisions.4, 8, 20 We found that most negative BALs were associated with discontinuing at least one non‐targeted antimicrobial, although often another non‐targeted agent was continued. In addition, the majority of positive BALs were associated with changing to targeted antimicrobials for viral or mycobacterial pathogens. Thus, prescribing was improved following 119 (68.4%) of 174 BALs.
Limitations
This single center retrospective study had limitations. Not all diagnostic tests were performed for every BAL and we were unable to consistently determine the reason for this. We also did not examine whether indication for bronchoscopy (e.g., fever, worsening respiratory status, radiographic findings) was associated with detection of potential pathogens. Furthermore, we could not assess the usefulness of RT‐PCR for respiratory viral pathogens as our institution introduced this technology in May 2012; nor could we assess the usefulness of Aspergillus galactomannan as so few tests were sent. Furthermore, quantitative BAL analysis was unavailable during the study period. The study was not sufficiently powered to detect the association of relapse, refractory disease, type of chemotherapy, or different conditioning regimens, on the yield of BALs. We did not analyze the association of neutropenia with the yield of BAL or with radiographic findings. Finally, we could not conclusively determine providers’ rationale for continuing non‐targeted therapy or discontinuing targeted therapy.
CONCLUSIONS
BAL contributes to treatment decision‐making in immunocompromised patients with presumed LRTI, and continues to have a role in the care of this population. A multicenter study should be performed to validate a case definition for positive BALs, to examine the relative contribution of newer diagnostic modalities, and to further assess the impact of BALs on antimicrobial management.
ACKNOWLEDGMENTS
The authors wish to acknowledge Philip Zachariah, MD, Division of Pediatric Infectious Disease, for assistance with statistical analysis. We also wish to thank the divisions of Pediatric Hematology/Oncology/Bone Marrow Transplant and Pediatric Pulmonology, as well as the Clinical Microbiology Laboratory of Columbia University Medical Center. Some of the results of this study were presented at the Infectious Diseases Society of America meeting in October of 2013 (#1157).
Conflict of interest: None
REFERENCES
- 1. Collaco J, Gower W, Mogayzel P. Pulmonary dysfunction in pediatric hematopoietic stem cell transplant patients: overview, diagnostic considerations, and infectious complications. Pediatr Blood Cancer 2007; 49:117–126. [DOI] [PubMed] [Google Scholar]
- 2. Griese M, Rampf U, Hofmann D, Fuhrer M, Reinhardt D, Bender‐Gotze C. Pulmonary complications after bone marrow transplantation in children: twenty‐four years of experience in a single pediatric center. Pediatr Pulmonol 2005; 30:393–401. [DOI] [PubMed] [Google Scholar]
- 3. Eikenberry M, Bartakova H, Defor T, Haddad I, Ramsay N, Blazar B, Milla C, Cornfeld D. Natural history of pulmonary complications in children after bone marrow transplantation. Biol Blood Marrow Transplant 2005; 11:56–64. [DOI] [PubMed] [Google Scholar]
- 4. Rao U, Piccin A, Malone A, O'Hanlon K, Breatnach F, O'Meara A, McDermott M, Butler K, O'Sullivan N, Russell J, et al. Utility of bronchoalveolar lavage in the diagnosis of pulmonary infection in children with haematological malignancies. Ir J Med Sci 2013; 182:177–183. [DOI] [PubMed] [Google Scholar]
- 5. Vega‐Briceño LE, Holmgren NL, Bertrand P, Rodriguez JI, Barriga F, Contreras I, Sanchez I. Utility of bronchoalveolar lavage in immunocompromised children: diagnostic yield and complications. Arch Bronconeumol 2004; 40:570–574. [DOI] [PubMed] [Google Scholar]
- 6. Efrati O, Gonik U, Bielorai B, Barak A, Vardi A, Paret G, Mishaly D, Toren A. Fiberoptic bronchoscopy and bronchoalveolar lavage for the evaluation of pulmonary disease in children with primary immunodeficiency and cancer. Pediatr Blood Cancer 2007; 48:324–329. [DOI] [PubMed] [Google Scholar]
- 7. Efrati O, Gonik U, Modan‐Moses D, Barak A, Szeinberg A, Vardi A, Paret G, Toren A, Vilozni D, Yahav Y. Flexible bronchoscopy and bronchoalveolar lavage in pediatric patients with lung disease. Pediatr Crit Care Med 2009; 10:80 –84. [DOI] [PubMed] [Google Scholar]
- 8. Park JR, Fogarty S, Brogan TV. Clinical utility of bronchoalveolar lavage in pediatric cancer patients. Med Pediatr Oncol 2002; 39:175 –180. [DOI] [PubMed] [Google Scholar]
- 9. Armenian SH, Siegel RJ, LaVia WV, Mascarenhas L. Evaluation of persistent pulmonary infiltrates in pediatric oncology patients. Pediatr Blood Cancer 2007; 48:165 –172. [DOI] [PubMed] [Google Scholar]
- 10. Kasow KA, King E, Rochester R, Tong X, Srivastava DK, Horwitz EM, Leung W, Woodard P, Handgretinger R, Hale GA. Diagnostic yield of bronchoalveolar lavage is low in allogeneic hematopoietic stem cell recipients receiving immunosuppressive therapy or with acute graft‐versus‐host disease: the St. Jude Experience, 1990–2002. Biol Blood Marrow Transplant 2007; 13:831 –837. [DOI] [PubMed] [Google Scholar]
- 11. Poritz M, Blaschke A, Byington C, Meyers L, Nilsson K, Jones DE, Thatcher SA, Robbins T, Lingenfelter B, Amiott E, et al. FilmArray, an automated nested multiplex PCR system for multi‐pathogen detection: development and application to respiratory tract infection. PLoS ONE 2011; 6:e26047. http://doi.org.laneproxy.stanford.edu/10.1371/journal.pone.0026047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Mol M, de Jongste JC, van Westreenen M, Merkus P, de Vries A, Hop W, Warris A, Janssens H. Diagnosis of invasive pulmonary aspergillosis in children with bronchoalveolar lavage galactomannan. Pediatr Pulmonol 2012; 48:789 –796. [DOI] [PubMed] [Google Scholar]
- 13. Satwani P, Jin Z, Duffy D, Morris E, Bhatia M, Garvin J, George D, Bradley MB, Harrison L, Petrillo K, et al. Transplantation‐related mortality, graft failure, and survival after reduced‐toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transpl 2013; 19:552 –561. [DOI] [PubMed] [Google Scholar]
- 14. Ben‐Ari J, Yaniv I, Nahum E, Stein J, Samra Z, Schonfeld T. Yield of bronchoalveolar lavage in ventilated and non‐ventilated children after bone marrow transplantation. Bone Marrow Transplant 2001; 27:191 –194. [DOI] [PubMed] [Google Scholar]
- 15. Lanino E, Sacco O, Kotitsa Z, Rabagliati A, Castagnola E, Garaventa A, Dallorso S, Gandolfo A, Manfredini L, Venzano P, et al. Fiberoptic bronchoscopy and bronchoalveolar lavage for the evaluation of pulmonary infiltrates after BMT in children. Bone Marrow Transplant 1996; 18:1 –5. [PubMed] [Google Scholar]
- 16. Dishop MK. Paediatric interstitial lung disease: classification and definitions. Paediatr Respir Rev 2011; 12:230 –237. [DOI] [PubMed] [Google Scholar]
- 17. Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of pneumocystis pneumonia in immunocompromised non‐HIV‐infected patients: systematic review and meta‐analysis of randomized controlled trials. Mayo Clin Proc 2007; 82:1052–1059. [DOI] [PubMed] [Google Scholar]
- 18. Joos L, Chhajed PN, Wallner J, Battegay M, Steiner J, Gratwohl A, Tamm M. Pulmonary infections diagnosed by BAL: a 12‐year experience in 1066 immunocompromised patients. Respir Med 2007; 101:93 –97. [DOI] [PubMed] [Google Scholar]
- 19. McCubbin MM, Trigg ME, Hendricker CM, Wagener JS. Bronchoscopy with bronchoalveolar lavage in the evaluation of pulmonary complications of bone marrow transplantation in children. Pediatr Pulmonol 1992; 12:43 –47. [DOI] [PubMed] [Google Scholar]
- 20. Batra S, Li B, Underhill N, Maloney R, Katz B, Hijiya N. Clinical utility of bronchoalveolar lavage and respiratory tract biopsies in diagnosis and management of suspected invasive respiratory fungal infections in children. Pediatr Blood Cancer 2015; 62:1579 –1586. [DOI] [PubMed] [Google Scholar]
- 21. Chellapandian D, Lehrnbecher T, Phillips B, Fisher BT, Zaoutis TE, Steinbach WJ, Beyene J, Sung L. Bronchoalveolar lavage and lung biopsy in patients with cancer and hematopoietic stem‐cell transplantation recipients: a systematic review and meta‐analysis. J Clin Oncol 2015; 33:501 –509. [DOI] [PubMed] [Google Scholar]
- 22. Qualter E, Satwani P, Ricci A, Geyer MB, Alobeid B, Radhakrishnan K, Bye M, Middlesworth W, Della‐Latta P, Behr G, et al. A comparison of bronchoalveolar lavage versus lung biopsy in pediatric recipients after stem cell transplantation Biol Blood Marrow Transplantation 2014; 20:1229 –1237. [DOI] [PMC free article] [PubMed] [Google Scholar]


