Abstract
The objective of this study was to evaluate risk factors for persistent wheezing in a group of 2–4‐year‐old children after an index‐wheezing episode in infancy. Eighty infants who had been seen at the Emergency Department for an episode of acute wheezing were followed for 2 yr in this prospective study. Caregivers completed a questionnaire, and children underwent clinical evaluation and skin prick testing 2 yr following the index‐wheezing episode. Detection of respiratory viruses and analysis of exposure to major indoor allergens were carried out at enrollment. Immunoglobin E antibodies were measured at the beginning of the study and at the end of follow‐up, using the CAP system. Logistic regression analysis was performed to identify factors associated with persistent wheezing. Seventy‐three children (44 boys) completed the study. After 2 yr, 38 (52%) reported three or more wheezing episodes in the past 12 months (persistent wheezers). Independent risk factors for persistence of wheezing were allergic sensitization and exposure to cockroach allergen in the kitchen. Breast‐feeding for at least 1 month was a protective factor. A strong association between allergic sensitization and persistence of wheezing was found in a group of very young children living in a subtropical area.
Keywords: wheezing, asthma, cockroach, dust mites, allergic sensitization, breast‐feeding, respiratory virus, passive smoking, children
Asthma is a chronic inflammatory disease of the airways affecting up to one‐third of children living in industrialized countries (1). Prospective studies have shown that, in most cases of persistent asthma, the initial symptoms occur during the first 3 yr of life (2). A longitudinal study in New Zealand revealed that onset of wheezing at early age was associated with a greater risk for relapse among individuals who presented wheezing in childhood followed by remission during adolescence. Pulmonary function was found to be decreased in individuals with persistent or relapsing symptoms, as compared to those without persistent wheezing (3). In keeping with this, it has been shown that by age 3, children who presented at least one wheezing episode, and particularly those who had wheezed on more than two occasions, had significantly higher plethysmographic measurements of specific airway resistance, indicating poorer lung function, as compared to children who had never wheezed. In addition, children with physician‐confirmed wheeze have also been reported to have significantly lower lung function at age 3 than those who reported no wheeze (4). It has been suggested that the fast‐growing lung of the child at an early age may be particularly susceptible to the effects of chronic inflammation associated with persistent asthma, leading to loss of lung function in the first years of life (2).
In infants, wheezing episodes are frequently associated with respiratory viral infections, particularly those caused by respiratory syncytial virus (RSV) (5, 6). Studies have pointed to an association of RSV bronchiolitis and other early respiratory tract infections with recurrent wheezing or symptomatic asthma during the first 4–7 yr of life (7). Results of a long‐term study carried out in Tucson, Arizona, have also demonstrated an association of lower respiratory tract infection caused by RSV early in life with persistent wheezing at 3 and 6 yr of age, however, this effect was no longer observed by age 13 (8).
A major risk factor for wheezing after 2–3 yr of age is the development of specific sensitization to allergens, particularly those derived from house dust mites (HDM), cat, dog, cockroach, and fungi. Early sensitization has been associated with an increased risk for persistent asthma and bronchial hyper‐responsiveness and with a greater loss of lung function (9). More recently, studies have highlighted the synergistic effect of sensitization, allergen exposure, and concomitant viral infection in augmenting inflammatory responses in the airways and promoting acute symptoms of asthma in both children and adults (6, 10). In addition, sensitization to HDM and other aeroallergens was shown to be an important risk factor for hospital admissions for wheezing and adverse responses to viral infections, particularly those caused by rhinovirus, in 3–18‐year‐old children (11). On the other hand, factors including early exposure to microbial products (12) or to domestic pets may decrease the risk of subsequent development of asthma (13).
The effect of exclusive breast‐feeding in prevention of asthma has been controversial (14, 15). Recent reviews of available evidence point to a beneficial effect of diet in prevention of allergic diseases in high‐risk infants. The most effective regimen comprises recommendations of exclusive breast‐feeding for at least 4–6 months, or alternatively, extensively hydrolyzed cow's milk‐based formulas when breast‐feeding is not possible, combined with avoidance of solid foods and cow's milk, for at least 4 months (16, 17).
We have recently shown that respiratory viral infections, particularly those caused by RSV, were major risk factors for acute wheezing among children 0–2 yr of age who presented to an Emergency Department (ED) in a subtropical environment (18). Family history of allergy was also strongly associated with wheezing in this group of children. However, little is know about factors associated with persistence of wheezing in sub‐tropical areas of the world. The aim of the present study was to examine the role of respiratory viral infections, exposure to high levels of indoor allergens, allergic sensitization, and breast‐feeding as risk factors for persistent wheezing, in a group of children 0.5–2 yr of age followed prospectively for a period of 2 yr.
Methods
Subjects
Eighty children 0.5–25 months of age who sought ED care for wheezing either at the Clinical Hospital of the University of São Paulo School of Medicine of Ribeirão Preto or Santa Lydia Community Hospital, between January 1999 and June 2000, were enrolled in this prospective study. All children 25 months of age and younger who participated in a previous case‐control study on risk factors for acute wheezing among children 0.5–12 yr of age (18) were invited to participate in the present study. Criteria for inclusion in the case‐control study have been previously described. Briefly, patients were selected if they presented wheezing that required therapy with inhaled β2‐agonists as judged by the attending physician. In the previous case‐control study, children with chronic respiratory illnesses (n = 2) (bronchopulmonary dysplasia), and those who reported therapy with corticosteroids within 4 wk prior to the ED visit (n = 8), were not included (18). During the time period of selection of patients, 3497 children 25‐month old or younger had been admitted to ED from both Hospitals. Of those, 126 were admitted for acute wheezing and 84 were residents in the city of Ribeirão Preto. Parents and/or caregivers of eighty of these children (95%) have agreed to participate in the study.
Study design
At the beginning of the study [time one – time of index episode of wheezing at age 0.5–25 months (T1)], parents or guardians completed a questionnaire that included questions on history of allergic diseases or asthma (in the patients and other family members) and housing conditions. A positive history of allergy in the child was defined as a report of physician‐diagnosed asthma, rhinitis, and/or atopic dermatitis (AR). A positive parental history for allergy was characterized by the report of asthma, rhinitis, and/or AR diagnosed by a physician in the mother and/or father. Current and retrospective information was obtained for some environmental factors including breast‐feeding, pre‐ and post‐natal exposure to tobacco smoke. Data on peripheral blood eosinophil counts; serum levels of total immunoglobulin E (IgE) and specific IgE antibodies to mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Blomia tropicalis), cockroach (Blattella germanica), cat, dog, and foods (cow's milk, egg, wheat, soy, peanut, and fish); detection of viruses in nasal washes and allergen levels in the homes were available from the initial study (18). Briefly, total and specific IgE were measured using the UniCAP system (Pharmacia, Uppsala, Sweden). Specific IgE levels ≥0.7 kU/l (CAP score ≥2) were considered positive. Nasal washings were collected and processed for detection of respiratory viruses as previously described (6). Respiratory syncytial virus antigen was detected using both a rapid enzyme immunoassay (Test‐Pack, Abbott) and indirect immunofluorescence (Chemicon, Temecula, CA, USA). Human rhinovirus and coronavirus RNA were detected by RT‐PCR, influenza, and parainfluenza viruses were detected by indirect immunofluorescence. Adenovirus was detected by culture; cytopathic effect was confirmed by immunofluorescence, and typing was carried out by PCR. Dust samples were collected from four sites in each patient's home: bedding, bedroom floor, TV room and kitchen, within 3 wk of the ED visit. Measurements of major allergens from mites (Groups 1 and 2), cockroach (Bla g 1), cat (Fel d 1), and dog (Can f 1) in house dust extracts were carried out using monoclonal antibody‐based enzyme‐linked immunosorbent assay (ELISA) (Indoor Biotechnologies Inc., Charlottesville, VA, USA). High‐level exposure to mites and cockroach was defined as Group 1 mite allergen levels ≥2 μg/g of dust in bedding, and cockroach allergen Bla g 1 ≥2 U/g of dust in the kitchen, respectively; high‐level exposure to cat and dog was characterized as at least one dust sample in the house containing concentrations of Fel d 1 ≥8 μg/g and Can f 1 ≥10 μg/g, respectively.
Children were evaluated in two subsequent visits: after 12 ± 3 months (T2) from the index episode of wheezing (T1), when the children were 12–37‐month old, and after 24 ± 3 months (T3) from the index‐wheezing episode, when the children were 24–52 months of age. The median ages of the children at T2 and T3 were 18‐ and 32‐month old, respectively. At T2, a questionnaire was used to record presence of wheezing, nasal symptoms, and skin disorders. At T3, patients were evaluated by clinical history and physical exam, and blood samples were collected for eosinophil quantitation and measurement of serum levels of total IgE and specific IgE to mites, cockroach, cat, and dog. All children underwent skin prick testing with extracts of D. pteronyssinus, D farinae, cat hair, dog dander, B. germanica, Periplaneta americana, and foods (cow's milk, egg, wheat, soy, peanut, and fish) (Bayer, Spokane, USA) and B. tropicalis extract (2 mg/ml, kindly provided by Dr Enrique Fernandez‐Caldas, CBF Leti, Spain). Skin prick tests (SPT) were carried out on the back or forearm, using a prick lancetter (Bayer, Spokane, USA). A reaction was considered positive when a wheal with a mean diameter of at least 3 mm, or at least 3 mm greater than the negative saline control, developed within 15 min after application of the extract. Presence of erythema was also an essential criterion for a positive reaction. Positive (10 mg/ml histamine dihydrochloride, Bayer) and negative (sterile albumin‐saline with phenol, Bayer) controls were used in all tests.
At the end of the follow‐up period (T3), children were identified as persistent wheezers if they had had three or more MD diagnosed wheezing episodes treated with a beta‐2 agonist during the past 12 months and as transient wheezers if they had had less than three wheezing episodes in the same period of time. Allergic sensitization was defined as the presence of a positive SPT to at least one allergen and/or serum specific IgE antibodies ≥0.7 kU/l (CAP score ≥2) to at least one inhalant allergen. Seventy‐three of the 80 children (91%) completed the 2‐year follow‐up. Five children moved to another city, and the mothers of two children did not agree to attend the follow‐up visits.
The study protocol was approved by the Ethics Committee of both hospitals, and the children's parents or guardians gave written informed consent to participate in the study.
Statistical analysis
Significant differences of variables compared between persistent and transient wheezers were assessed by the chi‐square test for proportions. The Fisher exact test was used when the observed frequency of any variable was <5. Data on total IgE levels were log transformed and analyzed by Student's t‐test. Peripheral blood eosinophils and allergen levels in house dust were analyzed by Mann–Whitney test. Univariate and multivariate analysis were carried out by logistic regression, using the software STATA Version 6.0. Values of p < 0.05 were considered as significant.
Results
Baseline characteristics of the children are shown on Table 1. Median age at enrollment was 6.5 months, varying from 0.5 to 25 months. Exclusive breast‐feeding for at least 1 month was reported for 70% of the children and most of them were already receiving cow's milk formula by the fourth month of age. The majority of children (55%) had already had a wheezing episode before enrollment. At the end of the follow‐up (T3), 38 children (52%) were identified as persistent wheezers (Fig. 1). Among those classified as transient wheezers (n = 35), two children had had one wheezing episode each, 8 and 10 months before the final evaluation. The remaining 32 transient wheezers did not report any wheezing episodes during the second year of follow‐up.
Table 1.
Characteristics at time 1 (T1 – time of index episode of wheezing at age 0.5–25 months) of infants subsequently identified, at time 3 (T3 – 24 ± 3 months after the index episode of wheezing), as persistent or transient wheezers (mean age 32 months)
| Variable | Persistent wheezers | Transient wheezers | ||
|---|---|---|---|---|
| n = 38 | % | n = 35 | % | |
| Age <12 months | 24 | 63.2 | 28 | 80 |
| Boys | 23 | 60.5 | 21 | 60 |
| Maternal smoking during pregnancy (n = 68) | 17/36 | 47.2 | 7/32 | 21.9 |
| Exposure to cigarette smoke in infancy | 23 | 60.5 | 24 | 68.6 |
| History of previous episode(s) of wheezing | 23 | 60.5 | 17 | 48.6 |
| Exclusive breast‐feeding * | 21 | 55.2 | 30 | 85.7 |
| Parental history of allergy † | 29 | 76.3 | 17 | 48.6 |
| Pets in the house (n = 66) | 18/34 | 52.9 | 13/32 | 40.6 |
| Virus identification (any virus) § | 32 | 84.2 | 24 | 68.6 |
| RSV | 18 | 47.4 | 10 | 28.6 |
| Total IgE >100 kU/l | 8 | 21.1 | 6 | 17.1 |
| Specific IgE ≥0.7 kUA/l | 7 | 18.4 | 3 | 8.6 |
| Exposure to high levels of mite allergen ¶ (n = 65) | 25/34 | 73.5 | 23/31 | 74.2 |
| Exposure to high levels of cockroach allergen ¶ (n = 65) | 17/34 | 50.0 | 7/31 | 22.6 |
RSV, respiratory syncycial virus.
*Exclusive breast‐feeding for at least 1 month.
†Physician‐diagnosed asthma, allergic rhinitis, and/or atopic dermatitis in mother and/or father.
§Detection of RSV antigen, rhinovirus RNA, adenovirus B, and/or coronavirus RNA in nasal washings.
¶High‐level exposure to mites and cockroach defined as Group 1 mite allergen levels ≥2 μg/g of dust in bedding, and cockroach allergen Bla g 1 ≥2 U/g of dust in the kitchen, respectively.
Figure 1.

Persistence of wheezing and development of allergic sensitization in children followed for 2 yr. T1, time of inclusion; T2, 12 ± 3 months after inclusion; T3, 24 ± 3 months after the index episode of wheezing. *Seven of the 73 children who were evaluated at the 2‐year follow‐up had missed the 1‐year follow‐up visit. †Median age in months (m).
High levels of Group 1 mite allergens (≥2 μg/g dust) in bedding and Bla g 1 (≥2 Ug/g dust) in the kitchen were detected, respectively, in 74% and 37.5% of the houses visited at enrollment (Table 1). Mean levels of Bla g 1 allergen were significantly higher in kitchen dust samples collected from homes of persistent wheezers (Fig. 2).
Figure 2.
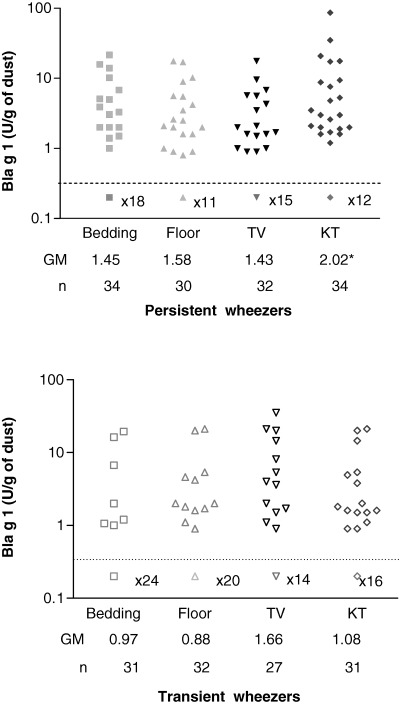
Levels of cockroach allergen Bla g 1 in dust samples from homes of children 0.5–2 yr of age, collected within 3 wk post‐time 1 (T1 – index episode of wheezing). Children were subsequently identified as persistent or transient wheezers at time 3 (24 ± 3 months after T1). Dust samples were collected from four sites of each patient's home: bedding, bedroom floor (floor), TV room (TV), and kitchen (KT), and levels of Bla g 1 were determined by enzyme‐linked immunosorbent assay (ELISA). Detection limit of the assay was 0.7 U/g of dust, and the number of samples with undetectable levels is indicated. * p = 0.02, when compared to dust samples collected from homes of transient wheezers.
Overall, 33 children (45%) were sensitized at the end of the follow‐up period (Fig. 1). Sensitization to mites and cockroach was observed in 88% and 57.5% of these children, respectively. The number of sensitized children was significantly higher among persistent wheezers as compared to transient wheezers (p < 0.001; Table 2). None of the children presented positive SPT to food extracts on evaluation at T3.
Table 2.
Univariate analysis of risk factors for persistence of wheezing among children 2–4‐year old
| Risk factor | Persistent wheezers (n = 38) | Transient wheezers (n = 35) | OR (95% CI) | p |
|---|---|---|---|---|
| Parental history of allergy * (%) | 76.3 | 48.6 | 3.4 (1.3–9.3) | 0.016 |
| High‐level exposure † at T1 to | ||||
| Mite (bed) (%) | 73.5 | 74.2 | 0.97 (0.3–2.9) | 0.95 |
| Cockroach (kitchen) (%) | 50.0 | 22.6 | 3.4 (1.2–10.0) | 0.025 |
| Maternal smoking during pregnancy (%) | 47.2 | 21.9 | 3.2 (1.1–9.3) | 0.03 |
| Exclusive breast‐feeding ‡(%) | 55.3 | 85.7 | 0.2 (0.07–0.65) | 0.007 |
| Viral identification at T1 (%) § | 84.2 | 68.6 | 2.4 (0.7–8.8) | 0.1 |
| IgE > 100 kU/l at T1 (%) | 21.1 | 17.1 | 1.3 (0.4–4.2) | 0.67 |
| IgE > 100 kU/l at T3 (%) | 68.4 | 20.1 | 8.7 (3.0–25.4) | <0.001 |
| Sensitization at T1 ¶ (%) | 18.4 | 8.6 | 2.4 (0.6–10.2) | 0.23 |
| Sensitization at T3 ** (%) | 71.1 | 17.1 | 11.8 (3.9–36.5) | <0.001 |
| Mite | 63.2 | 14.3 | 10.2 (3.3–32.6) | <0.001 |
| Cockroach | 44.7 | 5.7 | 13.4 (2.8–63.8) | 0.001 |
| Cat | 23.7 | 0 | – | |
| Dog | 10.5 | 0 | – | |
| Three or more allergens | 42.1 | 0 | – | |
| History of rhinitis in the child at T3 (%) | 79 | 14.3 | 22.5 (6.5–76.7) | <0.001 |
*Physician‐diagnosed asthma, allergic rhinitis and/or atopic dermatitis in mother and/or father.
†High‐level exposure to mites and cockroach defined as Group 1 mite allergen levels ≥2 μg/g of dust in bedding, and cockroach allergen Bla g 1 ≥2 U/g of dust in the kitchen, respectively.
‡Exclusive breast‐feeding for at least 1 month.
§Detection of RSV antigen, rhinovirus RNA, adenovirus B and/or coronavirus RNA in nasal washings.
¶Levels of specific IgE antibodies ≥0.7 kUA/l (CAP class ≥2) to at least one ihalant allergen (mite, cockroach, cat, or dog) or food allergen (egg, milk, soy, wheat, fish, or peanut) detected at enrollment (T1).
**Levels of specific IgE antibodies ≥0.7 kUA/l (CAP class ≥2) to at least one inhalant allergen (mite, cockroach, cat, or dog) and/or positive skin prick test (SPT) to at least one inhalant or food allergen (egg, milk, soy, wheat, fish, or peanut) detected at the end of follow‐up (T3) – odds ratios for variables with a zero value could not be calculated with STATA 6.0.
T1, time of index episode of wheezing at age 0–25 months (median age 6.5 months).
T3, 24 ± 3 months after the index episode of wheezing.
At T1, geometric mean (GM) total IgE levels were 15 kU/l (range 2–441 kU/l) and 30 kU/l (range 2–1709 kU/l), for transient and persistent wheezers, respectively (p = 0.2), and total IgE levels >100 kU/l were found in 20% of the children. Specific IgE antibodies were present in 10/73 (13.6%) children: five were sensitized to foods (one to egg, three to egg and cow's milk, and one to egg and peanut) and five were sensitized to mite allergens.
At the end of follow‐up, GM total IgE serum levels were 176 kU/l (range 4–3488 kU/l) and 51 kU/l (range 6–1020 kU/l) for persistent and transient wheezers, respectively (p < 0.001). High levels (>100 kU/l) of total serum IgE at the end of follow‐up were significantly more frequent in persistent wheezers as compared to transient wheezers (p < 0.001) (Table 2). The number of peripheral blood eosinophils showed no significant differences in the two groups (GM = 304 and 274/mm3, respectively; p =0.52, data not shown).
Results of univariate analysis revealed that parental history of allergy, history of rhinitis in the child, high levels of total IgE and allergic sensitization at T3 were all strongly associated with persistence of wheezing. Maternal tobacco smoking during pregnancy and exposure to high levels of cockroach allergen Bla g 1 in the kitchen at T1 were also associated with persistent wheezing. Breast‐feeding for at least 1 month was a protective factor and identification of viruses during the index episode of wheezing showed no association with persistence of wheezing (Table 2).
Two models of multivariate analysis were constructed in order to evaluate exposure to indoor allergens and sensitization, since both could not be included in the same model due to the possibility of co‐linearity between the two risk factors. Allergic sensitization at 2–4 yr of age and exposure to cockroach allergen Bla g 1 in the first 2 yr of life remained as independent risk factors for persistence of wheezing. Exclusive breast‐feeding for 1 month was shown to be an independent protective factor. Parental history of allergy was not consistently associated with persistence of wheezing in the multivariate analysis (Table 3).
Table 3.
Multivariate analysis of risk factors for persistence of wheezing among children 2–4‐year old
| Risk factors | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.06 (0.95–1.2) | 0.31 | 0.96 (0.88–1.05) | 0.4 |
| Sex | 0.6 (0.14–2.8) | 0.5 | 0.9 (0.23–3.6) | 0.9 |
| Parental history of allergy * | 5.4 (1.0–28.3) | 0.044 | 1.3 (0.33–5.5) | 0.7 |
| Maternal smoking during pregnancy | 3.3 (0.7–13.3) | 0.14 | 3.5 (0.86–14.5) | 0.08 |
| Exclusive breast feeding † | 0.12 (0.02–0.63) | 0.013 | 0.14 (0.03–0.76) | 0.02 |
| High‐level exposure ‡ at T1 to | ||||
| Mite (bed) | 0.9 (0.2–4.7) | 0.9 | ||
| Cockroach (kitchen) | 7.6 (1.4–40.8) | 0.017 | ||
| Specific IgE to any allergen at T1 § | 1.57 (0.15–16.6) | 0.7 | ||
| Sensitization to any allergen at T3 ¶ | 14.7 (3.2–66.7) | <0.0001 | ||
*Physician‐diagnosed asthma, allergic rhinitis, and/or atopic dermatitis in mother and/or father.
†Exclusive breast‐feeding for at least 1 month.
‡High‐level exposure to mites and cockroach defined as Group 1 mite allergen levels ≥2 μg/g of dust in bedding, and cockroach allergen Bla g 1 ≥2 U/g of dust in the kitchen, respectively.
§Levels of specific IgE antibodies ≥0.7 kUA/l (CAP class ≥2) to at least one inhalant allergen (mite, cockroach, cat, or dog) or food allergen (egg, milk, soy, wheat, fish, or peanut) detected at enrollment (T1).
¶Levels of specific IgE antibodies ≥0.7 kUA/l (CAP class ≥2) to at least one inhalant allergen (mite, cockroach, cat, or dog) and/or positive skin prick test (SPT) to at least one inhalant or food allergen (egg, milk, soy, wheat, fish, or peanut) detected at the end of follow‐up (T3). T1, time of index episode of wheezing at age 0–25 months (median age 6.5 months). T3, 24 ± 3 months after the index episode of wheezing.
Discussion
This prospective 2‐year follow‐up study in infants treated in the ED for acute wheezing showed that 52% of these children continued to have repeated episodes of wheezing between 2 and 4 yr of age, and this outcome was highly associated with development of sensitization to indoor allergens.
Studies on the natural history of asthma in childhood revealed that over 60% of children who wheeze in the first 3 yr of life are transient wheezers and no longer report wheezing exacerbations by age 6. Major risk factors for wheezing in the first 2–3 yr of life are lower respiratory viral infections particularly those caused by RSV and lower pulmonary function at birth (2). By contrast, it has been consistently shown that early allergen sensitization becomes a major risk factor for wheezing exacerbations and hospitalizations for wheezing after age 3 (4, 5, 11). It is thought that IgE‐mediated inflammation found in most children with persistent symptoms of asthma is a key factor in causing lung function impairment and airway remodeling. However, identification of children at risk for development of persistent symptoms remains a challenge. A stringent clinical index has been defined for children with persistent wheezing during the first 3 yr of life that predicted subsequent asthma. This asthma predictive index included either a parental history of asthma, the presence of allergic eczema in the child, or two of three ‘minor’ criteria (eosinophilia, wheezing without colds, and allergic rhinitis) (19).
Although genetic factors have been shown to play a role in both allergic sensitization and asthma, evidence suggests that events taking place between 2 and 3 yr of age might be crucial determinants for the development of asthma. Treating children at this age with inhaled corticosteroids may alter the course of the disease and prevent loss of lung function, and this possibility is currently under investigation (20). Previously, results of the Childhood Asthma Management Program study (CAMP study) have failed to demonstrate an effect of prolonged treatment of 5–12‐year‐old children with asthma with low‐to‐moderate doses of inhaled budesonide in preventing decrease in lung function (21), however, in that study it is possible that treatment may have started too late. Alternative strategies have focused on manipulating the environment of high‐risk individuals as an attempt to reduce the prevalence of asthma in children. At present, six primary prevention controlled studies are in progress (22). The longest follow‐up reported has been from the Isle of Wight study. Stringent allergen avoidance measures instituted at birth, aimed at reducing exposure to HDM and preventing food sensitization, resulted in a significant decrease in asthma symptoms and sensitization to allergens at age 8. Likewise, outcomes of the Canadian Primary Prevention Study on high‐risk infants have been reported at age 2 yr, showing that intervention comprising avoidance of mite, pet allergens and environmental tobacco smoke, as well as dietary regimen resulted in reduction of asthma in the intervention group (23). In the present study, children were sensitized primarily to HDM and cockroach and this is in keeping with previous studies carried out in our area, evaluating children 2 yr and older with acute wheezing or those attending allergy specialty clinics (18, 24). A strong relationship between exposure and sensitization has been demonstrated for mites and cockroach (25, 26). Our results suggest that the high rates of sensitization to these allergens may be due to the fact that 74% and 37.5% of children in the present study were exposed to high levels of mite and cockroach allergens in their homes, respectively, similarly to other reports from Brazil (27, 28). Previous studies in our area have shown that daycare centers and schools are sources of significant exposure to mite and cockroach allergens (29), which might also contribute to sensitization.
Early exposure to cockroach allergen was an independent risk factor for persistence of wheezing among children in the present study. This observation is in keeping with previously reported data from children living in the metropolitan region of Boston (30) and also in a multi‐ethnic sample of low‐income infants younger than 2 living in Denver (31). The mechanisms by which cockroach allergens may cause airway inflammation are not fully understood. In the case of mites, it has been demonstrated that several mite allergens (Group 1, Der p 3, Der p 6, Der p 9) are biologically active proteolytic enzymes, and it is thought that this enzymatic activity may contribute to allergenicity (32). On the other hand, no cockroach allergens with enzymatic activity have been identified (33, 34). It is possible that high levels of cockroach allergen in the home function as surrogate marker for other environmental factors. In our study, the majority of children belonged to low‐income families and factors such as endotoxin exposure, not analyzed in the present study, could account for the observed association of exposure to cockroach with persistent wheezing.
Studies evaluating possible associations of breast‐feeding with persistence of wheezing have produced conflicting results (14, 15, 16, 17). A meta‐analysis revealed that children who were breast‐fed for at least 3 months had a 20% decrease in the risk of asthma (14). In contrast, data from a general population cohort showed an increased risk for asthma associated with breastfeeding for 4 wk or more (15). Likewise, data from the Tucson cohort suggested that breast‐feeding might lead to higher prevalence of asthma in allergic children by the age of 6 yr (35). It is possible that this result may reflect a reverse effect due to the fact that families with an allergic predisposition may have been advised to breast fed their children for a longer time. We have found that exclusive breast‐feeding during the first month of life was an independent protective factor against the persistence of wheezing at 2–4 yr of age. Reassessment of these children as they reach school age will show whether this protective influence is still present at that time.
In the present study, we have not found an association of respiratory viral infection early in life with persistent wheezing. However, monitoring viral infections prospectively would have been a more appropriate way to assess the role of respiratory viruses in development of asthma. The small number of patients in our cohort may have caused the estimates of incident asthma and risk factors, including respiratory viral infections, to be underpowered. Classification of children as persistent wheezers in the present study was based on criteria described in the Tucson Children's Respiratory Study (2). Although our evaluation has been carried out at an earlier age as compared to the Tucson study, results of this long‐term prospective investigation (2) have revealed that children who presented frequent wheezing in their first 3 yr of life were more likely to develop persistent symptoms throughout childhood and to have lower pulmonary function by ages 6 and 13, as compared to those who had not wheezed in the first 3 yr of life.
In conclusion, we have found that a high proportion of children who wheeze in the first 2 yr of life continue to wheeze after 2 yr. Allergen sensitization, to HDM and cockroach in particular, and early exposure to cockroach allergen were strong and independent risk factors for persistence of wheezing among these children living in a subtropical environment. Our results highlight the importance of evaluating young children who wheeze for presence of allergen sensitization in order to identify those with a likelihood of developing persistent symptoms over time.
Acknowledgments
The authors wish to thank Gustavo F. Pacca MD and the Pediatric residents of Santa Lydia hospital for help with enrolling of the patients and Maria Lucia da Silva BSc, for technical assistance. V.P.L.F., E.A. and L.K.A are recipients of CNPq scholarships. Supported by FAPESP Grant numbers 1995/9690‐0 and 1995/9692‐2; CNPq‐Instituto de Investigação em Imunologia – iii and National Institutes of Health Grant AI‐20565.
References
- 1. ISAAC Steering Committee . Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 1998: 351: 1225–32. [PubMed] [Google Scholar]
- 2. Martinez FD. Development of wheezing disorders and asthma in preschool children. Pediatrics 2002: 109: 362–7. [PubMed] [Google Scholar]
- 3. Sears MR, Greene JM, Willan AR, et al. A longitudinal, population‐based, cohort study of childhood asthma followed to adulthood. N Engl J Medicine 2003: 349: 1414–22. [DOI] [PubMed] [Google Scholar]
- 4. Lowe L, Murray CS, Martin L, et al. Reported versus confirmed wheeze and lung function in early life. Arch Dis Child 2004: 89: 540–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duff AL, Pomeranz ES, Gelber LE, et al. Risk factors for acute wheezing in infants and children: viruses, passive smoke and IgE antibodies to inhalants allergens. Pediatrics 1993: 92: 535–40. [PubMed] [Google Scholar]
- 6. Rakes GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. Am J Respir Crit Care Med 1999: 159: 785–90. [DOI] [PubMed] [Google Scholar]
- 7. Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000: 161: 1501–7. [DOI] [PubMed] [Google Scholar]
- 8. Stein RT, Sherrill D, Morgan WJ, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999: 354: 541–5. [DOI] [PubMed] [Google Scholar]
- 9. Platts‐Mills TAE, Rakes GP, Heymann PW. The relevance of allergen exposure to the development of asthma in childhood. J Allergy Clin Immunol 2000: 105: S503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case‐control study. BMJ 2002: 324: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol 2004: 114: 239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Mutius E. Environmental factors influencing the development and progression of pediatric asthma. J Allergy Clin Immunol 2002: 109 (Suppl. 6): S525–32. [DOI] [PubMed] [Google Scholar]
- 13. Platts‐Mills TAE, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitization, asthma, and a modified Th2 response in children exposed to cat allergen: a population‐based cross‐sectional study. Lancet 2001: 357: 752–56. [DOI] [PubMed] [Google Scholar]
- 14. Peat JK, Li J. Reversing the trend: reducing the prevalence of asthma. J Allergy Clin Immunol 1999: 103: 1–10. [DOI] [PubMed] [Google Scholar]
- 15. Sears MR, Greene JM, Willan AR, et al. Long‐term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal study. Lancet 2002: 360: 901–7. [DOI] [PubMed] [Google Scholar]
- 16. Muraro A, Dreborg S, Halken S, et al. Dietary prevention of allergic diseases in infants and small children. Part III: Critical review of published peer‐reviewed observational and interventional studies and final recommendations. Pediatr Allergy Immunol 2004: 15: 291–307. [DOI] [PubMed] [Google Scholar]
- 17. van Odijk J, Kull I, Borres MP, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966–2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 2003: 58: 833–84. [DOI] [PubMed] [Google Scholar]
- 18. Camara AA, Silva JM, Ferriani VPL, et al. Risk factors for acute wheezing among children in a subtropical environment: role of respiratory viruses, IgE antibodies and allergen exposure. J Allergy Clin Immunol 2004: 113: 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castro‐Rodrigues JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000: 162: 1403–6. [DOI] [PubMed] [Google Scholar]
- 20. Guilbert TW, Morgan WJ, Krawiek M, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials 2004: 25: 286–310. [DOI] [PubMed] [Google Scholar]
- 21. The Childhood Asthma Management Program Research Group . Long‐term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000: 343: 1054–63. [DOI] [PubMed] [Google Scholar]
- 22. Simpson A, Custovic A. Allergen avoidance in the primary prevention of asthma. Curr Opin Allergy Clin Immunol 2004: 4: 45–51. [DOI] [PubMed] [Google Scholar]
- 23. Becker A, Watson W, Ferguson A, Dimich‐Ward H, Chan‐Yeung M. The Canadian asthma primary prevention study: outcomes at 2 years of age. J Allergy Clin Immunol 2004: 113: 650–6. [DOI] [PubMed] [Google Scholar]
- 24. Santos ABR, Chapman MD, Aalberse RC, et al. Cockroach allergens and asthma in Brazil: Identification of Tropomyosin as a major allergen with potential cross‐reactivity with mite and shrimp allergens. J Allergy Clin Immunol 1999: 104: 329–37. [DOI] [PubMed] [Google Scholar]
- 25. Sporik R, Holgate ST, Platts‐Mills TA, Cogswell JJ. Exposure to house‐dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med 1990: 323: 502–7. [DOI] [PubMed] [Google Scholar]
- 26. Eggleston PA, Rosenstreich D, Lynn H, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner‐city children with asthma. J Allergy Clin Immunol 1998: 102: 563–70. [DOI] [PubMed] [Google Scholar]
- 27. Tobias KR, Ferriani VP, Chapman MD, Arruda LK. Exposure to indoor allergens in homes of patients with asthma and/or rhinitis in southeast Brazil: effect of mattress and pillow covers on mite allergen levels. Int Arch Allergy Immunol 2004: 133: 365–70. [DOI] [PubMed] [Google Scholar]
- 28. Sopelete MC, Silva DA, Arruda LK, Chapman MD, Taketomi EA. Dermatophagoides farinae (Der f 1) and Dermatophagoides pteronyssinus (Der p 1) allergen exposure among subjects living in Uberlandia, Brazil. Int Arch Allergy Immunol 2000: 122: 257–63. [DOI] [PubMed] [Google Scholar]
- 29. Rullo VE, Rizzo MC, Arruda LK, Sole D, Naspitz CK. Daycare centers and schools as sources of exposure to mites, cockroach, and endotoxin in the city of São Paulo, Brazil. J Allergy Clin Immunol 2002: 110: 582–8. [DOI] [PubMed] [Google Scholar]
- 30. Gold DR, Burge HA, Carey V, Milton DK, Platts‐Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med 1999: 160: 227–36. [DOI] [PubMed] [Google Scholar]
- 31. Klinnert MD, Price MR, Liu AH, Robinson JAL. Morbidity patterns among low‐income wheezing infants. Pediatrics 2003: 112: 49–57. [DOI] [PubMed] [Google Scholar]
- 32. Stewart GA, Robinson C. The immunobiology of allergenic peptidases. Clin Exp Allergy 2003: 33: 3–6. [DOI] [PubMed] [Google Scholar]
- 33. Arruda LK, Vailes LD, Ferriani VPL, Santos AB, Pomes A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol 2001: 107: 419–28. [DOI] [PubMed] [Google Scholar]
- 34. Pomes A, Chapman MD, Vailes LD, Blundell TL, Dhanaraj V. Cockroach allergen Bla g 2: structure, function, and implications for allergic sensitization. Am J Respir Crit Care Med 2002: 165: 391–7. [DOI] [PubMed] [Google Scholar]
- 35. Wright AL, Holberg CJ, Taussig LM, Martinez FD. Factors influencing the relation of infant feeding to asthma recurrent wheeze in childhood. Thorax 2001: 56: 192–97. [DOI] [PMC free article] [PubMed] [Google Scholar]


