Abstract
Background
The burden of respiratory syncytial virus (RSV) in neonates has not been clearly studied. The aims of this study were to determine the overall distribution of respiratory viruses in neonates hospitalized with acute lower respiratory tract infectiosns (ALRI) and to describe the clinical characteristics of RSV infections in these neonates.
Methods
From January 2009 through May 2010, neonates aged <1 month who were hospitalized with ALRI and did not have underlying disease were included in the study. Viruses were identified on multiplex reverse transcription polymerase chain reaction using nasal swab samples. Clinical variables were evaluated between the RSV and non‐RSV infection groups.
Results
Of the 108 infants included in the study, 46 (42.6%) had RSV; human rhinovirus (18.5%), human parainfluenza virus 3 (7.5%), and human metapneumovirus (3.7%) were the next most common infections. Codetections accounted for 8.3% of the cases. Crowding increased the risk of RSV infection compared to the non‐RSV group (OR, 16.5; P = 0.001). The RSV group had a greater incidence of dyspnea ( P = 0.027), pneumonia (P < 0.001), requirement for oxygen ( P < 0.001), and prolonged hospitalization ( P = 0.011) than the non‐RSV group.
Conclusions
RSV was the most common viral etiology in neonates without underlying diseases who were hospitalized with ALRI. The disease severity of RSV infection was worse than that of other detected viral infections. Strict prevention strategies should be considered in overcrowded situations.
Keywords: newborn infants, respiratory syncytial virus infections, respiratory tract infections
In recent years we have developed an understanding of respiratory infections through the detection of viruses using reverse transcription–polymerase chain reaction (RT‐PCR). In addition to respiratory syncytial virus (RSV), human rhinovirus (hRV), human parainfluenza virus (hPIV), and human metapneumovirus (hMPV) have been found to be major causes of acute lower respiratory tract infections (ALRI) in infants and young children.1, 2, 3 ALRI are leading causes of hospitalization and mortality among children worldwide, particularly young children.4, 5 Only a few studies, however, have reported on respiratory viruses in neonates with ALRI. Understanding the overall spectrum of respiratory viruses causing ALRI in hospitalized neonates is essential to establish preventive and therapeutic strategies in this population.
RSV is the most common etiological agent of ALRI in infants and young children.6, 7 To date, many reports have described the burden of RSV infections and its risk factors. Prematurity, low birthweight, and chronic lung disease (CLD) have been suggested as risk factors for RSV infections. Additionally, epidemiologic factors, such as crowding, having older siblings, day‐care center attendance and exposure to smoke are important risk factors.8, 9, 10, 11 Very few studies have focused on neonatal RSV‐associated ALRI among healthy neonates aged <1 month.5, 12
The primary objective of this study was to determine the distribution of respiratory viruses in otherwise healthy neonates hospitalized with ALRI. A secondary aim was to identify the clinical characteristics of RSV infections in this population.
Methods
Patient and clinical variables
This study was a prospective investigation of neonates with ALRI. From January 2009 to May 2010, neonates (<1 month of age) admitted to the neonatal intensive care unit (NICU) at Gachon University Gil Hospital due to ALRI were enrolled. ALRI were defined as equivalent to clinical pneumonia or acute bronchiolitis, which is characterized by acute‐onset cough or difficulty breathing with increased respiratory rate (respiration rate ≥60 breaths/min). All neonates were isolated in a separate room in the NICU. After obtaining informed consent, nasal swab samples were obtained from neonates with ALRI within 1 h of hospitalization. Neonates with bacterial isolation from blood samples, or underlying medical conditions, such as premature birth (<35 weeks of gestational age), congenital heart disease, and CLD were excluded from this study. During the study period, one nurse, who was unaware of the present study, recorded the following variables: history of premature birth, the presence of underlying medical conditions, breastfeeding, number of siblings, siblings attending day‐care centers, number of residents at home, exposure to smoke, and residence in a neonatal day‐care center in the previous 48 h. In South Korea, neonatal day‐care centers are common places that provide caregivers for mothers and neonates after discharge from delivery hospitals. In these facilities, assistants take care of many neonates in a room, similar to a nursery. Respiratory infections were considered to be acquired at a neonatal day‐care center if the neonates became symptomatic within 48 h of discharge from such a center. The clinical features and clinical courses during the hospitalization were summarized by one of the attending physicians who were blinded to this study. Clinical variables, such as rhinorrhea and/or cough, fever (defined as axillary temperature ≥38°C), and requirement for oxygen therapy, were recorded. Apnea was defined as a lack of breathing activity for at least 20 s. Dyspnea referred to tachypnea (respiration rate ≥60 breaths/min) combined with the presence of pronounced chest wall retractions. Pneumonia was documented only if the clinical diagnosis was confirmed on chest radiography. On admission, a blood sample was taken to measure total white blood cell count and C‐reactive protein, and for blood cultures. Neutropenia was defined as an absolute neutrophil count <1000/mm3.
All included neonates were divided into the RSV group and non‐RSV group based on the results of multiplex RT‐PCR. Neonates with codetection or negative multiplex RT‐PCR were excluded from the grouping. Demographics, clinical variables, and laboratory data were compared between groups. Ethics approval was obtained from the Ethics Committee of Gachon University Gil Hospital.
Multiplex RT‐PCR
For the multiplex RT‐PCR, RNA was extracted from nasal swab samples using the Viral Gene‐spin TM Viral DNA/RNA Extraction Kit (iNtRON, Seoul, South Korea), and cDNAs were synthesized. RT was performed using the Revert Aid TM First Strand cDNA Synthesis Kit (Fermentas, Burlington, ON, Canada). PCR amplification was performed using 10 μL of Seeplex RV Master Mix 12 (Seegen, Seoul, South Korea), 4 μL of 5X multiplex primer sets, 3 μL of 8‐methoxypsoralen solution, and 3 μL of newly synthesized first‐strand cDNA. Seeplex RV contained A and B sets of primers designed from highly conserved regions of genetic sequences for 12 respiratory viruses, including human coronavirus (hCoV)‐229E/NL63, hCoV‐OC43, hMPV, hRV, RSV A/B, influenza A/B, hPIV‐1, 2, 3, and adenovirus. An initial pre‐PCR step of 94°C for 15 min was performed followed by a total of 40 PCR cycles under the following conditions: 95°C for 30 s, 60°C for 1.5 min, and 72°C for 1.5 min. The final cycle was followed by an extension step at 72°C for 10 min to complete any partial polymerization. The amplified PCR products were separated on 2% agarose gel and stained with ethidium bromide. The type of respiratory virus was identified by comparison with the reference band size provided by the manufacturer.
Statistical analysis
Statistical analysis was performed using SPSS version 14.0 (SPSS, Chicago, IL, USA). Significance was examined using the chi‐squared test for categorical variables, Fisher's exact test for binary variables, and the independent sample t‐test for continuous variables. Normally distributed data are expressed as mean ± SD. Non‐parametric data are expressed as median (25–75th percentiles). Differences were considered significant for P < 0.05.
Results
Subjects
In the study period from January 2009 to May 2010, a total of 140 neonates with ALRI were admitted to the NICU (Fig. 1). Table 1 lists the distribution of 108 viral agents isolated from the enrolled infants. Among the viruses, RSV was the predominant agent (42.6%), followed by hRV (18.5%), hPIV‐3 (7.5%) and hMPV (3.7%). In nine neonates (8.3%), two viruses were simultaneously present, while no virus was identified in 21 neonates (19.4%). Most RSV cases appeared in November and persisted until May, and then gradually diminished. Most hRV cases occurred in the Spring (March–April). Four hMPV cases newly appeared during the late Spring of 2010 (April–May; Fig. 2).
Figure 1.
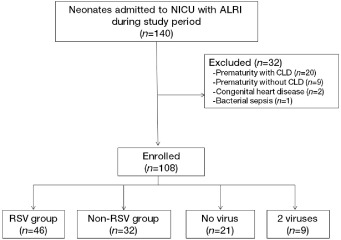
Flowchart of neonates enrolled in the study. A total of 140 neonates were assessed for eligibility; 108 neonates were enrolled. ALRI, acute lower respiratory infection; CLD, chronic lung disease; NICU, neonatal intensive care unit; RSV, respiratory syncytial virus.
Table 1.
Viral agents identified in enrolled neonates
| Viral agents | Patients n (%) |
|---|---|
| Single infections | 78 (72.2) |
| RSV A | 29 (26.9) |
| RSV B | 17 (15.7) |
| hRV | 20 (18.5) |
| hPIV‐ 3 | 8 (7.5) |
| hMPV | 4 (3.7) |
| Coinfections | 9 (8.3) |
| RSV A + hRV | 1 (0.9) |
| RSV B + hPIV‐3 | 1 (0.9) |
| RSV B + hCoV‐229E/NL63 | 1 (0.9) |
| hRV + hPIV‐3 | 3 (2.8) |
| hRV + hCoV‐OC43 | 1 (0.9) |
| hPIV‐3 + hMPV | 2 (1.9) |
| No virus detected | 21 (19.4) |
| Total | 108 (100.0) |
hCoV, human coronavirus; hMPV, human metapneumovirus; hPIV, human parainfluenza viruses; hRV, human rhinovirus; RSV, respiratory syncytial virus.
Figure 2.
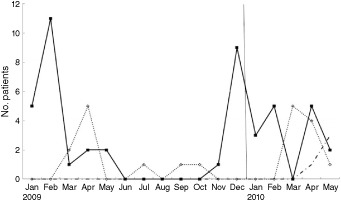
Monthly distribution of respiratory infections according to virus. hMPV, human metapneumovirus; hRV, human rhinovirus; RSV, respiratory syncytial virus.  , RSV;
, RSV;  , hRV;
, hRV;  , hMPV.
, hMPV.
Table 2 lists the neonates' demographic data. The median age at diagnosis in the RSV and non‐RSV groups was 22 days and 24 days, respectively, and the proportion of males was 69.6% and 65.6%, respectively. No significant difference was observed between the groups according to the presence of environmental risk factors, such as number of siblings and siblings attending day‐care center, number of residents at home, or exposure to smoke. A significantly higher proportion of infants in the RSV group had contracted infections from neonatal day‐care centers (34.8% vs 3.1%, P = 0.001, odds ratio, 16.5; 95% confidence interval: 2.06–132.6).
Table 2.
Demographic features
| Variables | RSV group (n = 46) n (%) | Non‐RSV group (n = 32) n (%) | P |
|---|---|---|---|
| Gestational age (weeks), mean ± SD | 39.2 ± 3.9 | 39.0 ± 3.7 | 0.807 |
| Birthweight (kg), mean ± SD | 3.31 ± 0.81 | 3.41 ± 0.92 | 0.612 |
| Age at diagnosis (days), median (25–75th percentile) | 22 (17–27) | 24 (11–28) | 0.252 |
| Male | 32 (69.6) | 21 (65.6) | 0.714 |
| Cesarean section | 19 (41.3) | 11 (34.4) | 0.536 |
| Breastfeeding | 32 (69.6) | 17 (53.1) | 0.139 |
| Infections at the neonatal day‐care center | 16 (34.8) | 1 (3.1) | 0.001 |
| ≥One sibling | 34 (73.9) | 25 (78.1) | 0.670 |
| Sibling attending day‐care center | 21 (45.7) | 21 (65.6) | 0.082 |
| Residents at home, mean ± SD | 3.94 ± 0.81 | 4.17 ± 0.62 | 0.303 |
| Exposure to smoke | 19 (41.3) | 11 (34.4) | 0.536 |
RSV, respiratory syncytial virus.
Clinical characteristics of RSV infections compared with other detected viral infections
Clinical data were compared between the RSV group (n = 46) and non‐RSV group (n = 32; Table 3). Dyspnea was documented significantly more often in the RSV group (34.8% vs 12.5%, P = 0.027). In contrast, fever ≥38°C was less frequent in the RSV group (15.2% vs 43.8%, P = 0.005). Pneumonia and particularly consolidation in the right upper lobe were more often found in the RSV group (54.3% vs 15.6%, P < 0.001; 45.7% vs 6.3%, P < 0.001, respectively). The RSV group more frequently required oxygen therapy (45.7% vs 6.3%, P < 0.001) and had longer hospital stay (9.41 ± 3.43 days vs 7.34 ± 3.51 days, P = 0.011). All nine neonates with viral codetections were diagnosed with pneumonia, and three of them (33.3%) needed supplemental oxygen therapy.
Table 3.
Clinical symptoms vs RSV infection
| Variables | RSV group (n = 46) n (%) | Non‐RSV group (n = 32) n (%) | P |
|---|---|---|---|
| Cough or rhinorrhea | 43 (93.5) | 30 (93.8) | 1.000 |
| Fever | 7 (15.2) | 14 (43.8) | 0.005 |
| Dyspnea | 16 (34.8) | 4 (12.5) | 0.027 |
| Apnea | 3 (6.5) | 0 | 0.265 |
| Neutropenia | 6 (13.0) | 1 (3.1) | 0.230 |
| C‐reactive protein (mg/dL), median (25–75th percentile) | 0.09 (0.02–0.48) | 0.07 (0.03–0.32) | 0.845 |
| Consolidation on right upper lobe | 21 (45.7) | 2 (6.3) | <0.001 |
| Diagnosis as pneumonia | 25 (54.3) | 5 (15.6) | <0.001 |
| Oxygen requirement | 21 (45.7) | 2 (6.3) | <0.001 |
| Duration of hospitalization (days), mean ± SD | 9.41 ± 3.43 | 7.34 ± 3.51 | 0.011 |
RSV, respiratory syncytial virus.
No neonates died during the study period. Two neonates from each group required mechanical ventilation. Both neonates presented apnea on admission and definite respiratory symptoms, such as cough and rhinorrhea, were subsequently followed.
Discussion
To the best of our knowledge, this is the first study to focus exclusively on previously healthy neonates with ALRI. To demonstrate the clinical impact of respiratory viral infection on neonates, we included only full‐term neonates without underlying disease. In the present study, 80.5% of the neonates who were tested on multiplex RT‐PCR had positive viral results. This detection rate is slightly higher than other similar reports for infants and small children.1, 13 One study based on hospitalized children with ALRI in Korea reported that RSV (33.2%) was the most common virus among 13 respiratory viruses and was followed by hRV (19.1%), influenza virus (16.9%), hMPV (15.4%), and hPIV (8.3%).14 This distribution was similar to the present results, although the proportion of RSV was greater in the present study. We found that most respiratory viral infections in neonates are acquired from the community. In the present study, hRV was the second most commonly detected virus, accounting for 18.5% of detected viruses. Previous studies reported that hRV was the second most commonly detected virus,2, 15 and could result in serious lower respiratory tract disease in early life.3, 16 Recently, several studies reported that most young infants with hRV detection had symptomatic illness, whereas half of the children in which hRV was detected were asymptomatic.17, 18 In young infants, including neonates, the chances of colonization may be lower than in older children. Regarding these findings, hRV should be considered the main virus in the present study.
Interestingly, in this study, influenza was not detected, although the study period coincided with the pandemic of influenza A (H1N1) virus. According to the Ko et al. Korean, nation‐wide data for 2009, infants <6 months old accounted for only 4.7% of hospitalized children with H1N1 influenza infection.19 It appears that younger infants have relatively lower risk for influenza because of lower exposure, but additional studies, including outpatient studies, are needed to support this conclusion.
Previous studies reported that viral codetections were found in 11–40% of subjects; in the present study only 8.3% presented codetection with two viruses. The relatively lower percentage of codetection in the present study may be due to lower colonization in neonates and lower chances of exposure to various viruses. An association between dual viral detection and more severe disease has not been properly established. Some authors consider that any dual viral infection increases severity of disease.20, 21 Other researchers, however, have found no difference when compared to single viral infections.13, 22 In the present study all neonates with codetection were diagnosed with lobar pneumonia. Based on these findings, we support an association of viral codetection with a more severe clinical course. Notably, RSV is the most common cause of ALRI‐associated mortality, especially in preterm infants. In the present study there were no cases of mortality. A high level of maternal RSV IgG antibodies was considered one reason for this finding because it may offer protection from severe illness in term infants during the first few months of life.23
In South Korea, the RSV season ranges from October to March,24 but it is reported that the season of RSV outbreaks varies considerably from year to year.9 The present findings were similar to that expected in temperate climate zones; the incidence of RSV infections peaked from January to February 2009. This pattern, however, changed in 2010; there was no significant variation in the monthly distribution of RSV throughout the period from January to May.
According to previous reports, male gender, presence of older siblings, tobacco smoke exposure, and siblings attending day‐care center were important risk factors for RSV infection.8, 25, 26, 27, 28, 29, 30 In the present study there were no significant differences in these factors between the RSV and non‐RSV groups. This implies that such risk factors could influence not only RSV infections but also other viral respiratory infections. In the present study a significantly higher proportion of the RSV group obtained infections from neonatal day‐care centers. Neonates from neonatal day‐care centers tended to be admitted as a group. Crowding situations in neonatal day‐care centers may affect the transmission of RSV infections.
In agreement with previous studies, we found that RSV infections had a more severe clinical course than other viral respiratory infections.3, 6 Fattouh et al. reported that compared with non‐RSV neonates, RSV neonates frequently had dyspnea and required supplemental oxygen with a higher rate of hospitalization.31 In the present study, 54.3% of neonates in the RSV group were diagnosed with pneumonia, whereas only 15.6% were diagnosed with pneumonia in the non‐RSV group. We performed chest radiography in all enrolled neonates and concluded that RSV infections were capable of causing pneumonia, especially in the right upper lobe.
To date, the majority of studies on RSV in neonates have been focused on neonates with underlying, high‐risk medical conditions, such as premature infants, especially those with CLD or congenital heart disease. Immunoprophylaxis, which is effective at reducing RSV hospitalization, is recommended only for the highest risk infants because of cost‐effectiveness.32 Most infants who are hospitalized with RSV‐related disease, however, have no underlying medical conditions.9 Therefore, until a safe and effective vaccine is developed, prevention is essential to reduce RSV infections. In the present study, overcrowded environments, such as neonatal day‐care centers, was a strong risk factor for RSV infection. Education regarding transmission‐based precautions, such as hand hygiene and standard contact precautions, should be considered.
There were several limitations in this study. Because we focused only on neonates with infections severe enough to require hospitalization, we could not estimate the burden of viral respiratory infections not requiring hospitalization. Although our regional center is the largest one in the study area, it is unknown whether these data represent regionwide demographics. In the present study, only eight cases of codetection were confirmed. We could not determine the severity of codetection due to the small sample size. Further studies with larger sample sizes, including asymptomatic control groups and detailed subgroup analyses, are necessary.
Conclusion
The overall distribution of respiratory viruses in neonates hospitalized with ALRI has been described in the present study. RSV was the most common viral ALRI etiology and hRV was the second most common. RSV had a more severe course than other detected viruses. Crowding situations increased the risk of RSV detection. Strict prevention strategies should be considered in overcrowded situations.
References
- 1. Cilla G, Onate E, Perez‐Yarza EG, Montes M, Vicente D, Perez‐Trallero E. Viruses in community‐acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J. Med. Virol. 2008; 80: 1843–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calvo C, Garcia‐Garcia ML, Blanco C, Pozo F, Flecha IC, Perez‐Brena P. Role of rhinovirus in hospitalized infants with respiratory tract infections in Spain. Pediatr. Infect. Dis. J. 2007; 26: 904–908. [DOI] [PubMed] [Google Scholar]
- 3. Regamey N, Kaiser L, Roiha HL et al. Viral etiology of acute respiratory infections with cough in infancy: A community‐based birth cohort study. Pediatr. Infect. Dis. J. 2008; 27: 100–105. [DOI] [PubMed] [Google Scholar]
- 4. Williams BG, Gouws E, Boschi‐Pinto C, Bryce J, Dye C. Estimates of world‐wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 2002; 2: 25–32. [DOI] [PubMed] [Google Scholar]
- 5. Nair H, Nokes DJ, Gessner BD et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta‐analysis. Lancet 2010; 375: 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia CG, Bhore R, Soriano‐Fallas A et al. Risk factors in children hospitalized with RSV bronchiolitis versus non‐RSV bronchiolitis. Pediatrics 2010; 126: 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stockman LJ, Curns AT, Anderson LJ, Fischer‐Langley G. Respiratory syncytial virus‐associated hospitalizations among infants and young children in the United States, 1997‐2006. Pediatr. Infect. Dis. J. 2012; 31: 5–9. [DOI] [PubMed] [Google Scholar]
- 8. Rossi GA, Medici MC, Arcangeletti MC et al. Risk factors for severe RSV‐induced lower respiratory tract infection over four consecutive epidemics. Eur. J. Pediatr. 2007; 166: 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr. Infect. Dis. J. 2011; 30: 510–517. [DOI] [PubMed] [Google Scholar]
- 10. Carbonell‐Estrany X, Quero J. Hospitalization rates for respiratory syncytial virus infection in premature infants born during two consecutive seasons. Pediatr. Infect. Dis. J. 2001; 20: 874–879. [DOI] [PubMed] [Google Scholar]
- 11. Figueras‐Aloy J, Carbonell‐Estrany X, Quero‐Jimenez J et al. FLIP‐2 Study: Risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr. Infect. Dis. J. 2008; 27: 788–793. [DOI] [PubMed] [Google Scholar]
- 12. Houben ML, Bont L, Wilbrink B et al. Clinical prediction rule for RSV bronchiolitis in healthy newborns: Prognostic birth cohort study. Pediatrics 2011; 127: 35–41. [DOI] [PubMed] [Google Scholar]
- 13. Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer‐Krantz S, Adams O. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real‐time polymerase chain reaction. Pediatr. Infect. Dis. J. 2008; 27: 589–594. [DOI] [PubMed] [Google Scholar]
- 14. Lim JS, Woo SI, Kwon HI, Baek YH, Choi YK, Hahn YS. Clinical characteristics of acute lower respiratory tract infection due to 13 respiratory viruses detected by multiplex PCR in children. Korean J. Pediatr. 2010; 53: 373–379. [Google Scholar]
- 15. Khadadah M, Essa S, Higazi Z, Behbehani N, Al‐Nakib W. Respiratory syncytial virus and human rhinoviruses are the major causes of severe lower respiratory tract infections in Kuwait. J. Med. Virol. 2010; 82: 1462–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louie JK, Roy‐Burman A, Guardia‐Labar L et al. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr. Infect. Dis. J. 2009; 28: 337–339. [DOI] [PubMed] [Google Scholar]
- 17. van der Zalm MM, van Ewijk BE, Wilbrink B, Uiterwaal CS, van der Wolfs TF, Ent CK. Respiratory pathogens in children with and without respiratory symptoms. J. Pediatr. 2009; 154: 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peltola V, Waris M, Osterback R, Susi P, Ruuskanen O, Hyypia T. Rhinovirus transmission within families with children: Incidence of symptomatic and asymptomatic infections. J. Infect. Dis. 2008; 197: 382–389. [DOI] [PubMed] [Google Scholar]
- 19. Ko JH, Kim JH, Kang JH et al. Characteristics of hospitalized children with 2009 pandemic influenza A (H1N1): A multicenter study in Korea. J. Korean Med. Sci. 2012; 27: 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richard N, Komurian‐Pradel F, Javouhey E et al. The impact of dual viral infection in infants admitted to a pediatric intensive care unit associated with severe bronchiolitis. Pediatr. Infect. Dis. J. 2008; 27: 213–217. [DOI] [PubMed] [Google Scholar]
- 21. Paranhos‐Baccala G, Komurian‐Pradel F, Richard N, Vernet G, Lina B, Floret D. Mixed respiratory virus infections. J. Clin. Virol. 2008; 43: 407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi EH, Lee HJ, Kim SJ et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000‐2005. Clin. Infect. Dis. 2006; 43: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fryzek JP, Martone WJ, Groothuis JR. Trends in chronologic age and infant respiratory syncytial virus hospitalization: An 8‐year cohort study. Adv. Ther. 2011; 28: 195–201. [DOI] [PubMed] [Google Scholar]
- 24. Kim CK, Choi J, Callaway Z et al. Clinical and epidemiological comparison of human metapneumovirus and respiratory syncytial virus in seoul, Korea, 2003‐2008. J. Korean Med. Sci. 2010; 25: 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simon A, Muller A, Khurana K et al. Nosocomial infection: A risk factor for a complicated course in children with respiratory syncytial virus infection: Results from a prospective multicenter German surveillance study. Int. J. Hyg. Environ. Health 2008; 211: 241–250. [DOI] [PubMed] [Google Scholar]
- 26. Simon A, Ammann RA, Wilkesmann A et al. Respiratory syncytial virus infection in 406 hospitalized premature infants: Results from a prospective German multicentre database. Eur. J. Pediatr. 2007; 166: 1273–1283. [DOI] [PubMed] [Google Scholar]
- 27. Carbonell‐Estrany X, Bont L, Doering G, Gouyon JB, Lanari M. Clinical relevance of prevention of respiratory syncytial virus lower respiratory tract infection in preterm infants born between 33 and 35 weeks gestational age. Eur. J. Clin. Microbiol. Infect. Dis. 2008; 27: 891–899. [DOI] [PubMed] [Google Scholar]
- 28. Fodha I, Vabret A, Ghedira L et al. Respiratory syncytial virus infections in hospitalized infants: Association between viral load, virus subgroup, and disease severity. J. Med. Virol. 2007; 79: 1951–1958. [DOI] [PubMed] [Google Scholar]
- 29. Law BJ, Langley JM, Allen U et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr. Infect. Dis. J. 2004; 23: 806–814. [DOI] [PubMed] [Google Scholar]
- 30. Horn SD, Smout RJ. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J. Pediatr. 2003; 143: S133–141. [DOI] [PubMed] [Google Scholar]
- 31. Fattouh AM, Mansi YA, El‐Anany MG, El‐Kholy AA, El‐Karaksy HM. Acute lower respiratory tract infection due to respiratory syncytial virus in a group of Egyptian children under 5 years of age. Ital. J. Pediatr. 2011; 37: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Groothuis JR, Hoopes JM, Hemming VG. Prevention of serious respiratory syncytial virus‐related illness. II: Immunoprophylaxis. Adv. Ther. 2011; 28: 110–125. [DOI] [PubMed] [Google Scholar]


