Abstract
Psoralea corylifolia L. (Leguminosae) is a well‐known traditional medicinal plant used from ancient times for treatment of various ailments. It is widely distributed and an important part of therapeutics in Ayurveda and in Chinese medicines. The aim of this review is to present comprehensive and most up to date report on its ethnobotanical, ethnopharmacological, clinical, phytochemical, and side effects. Studies on the ethnobotanical, ethnopharmacological, clinical, phytochemical, and side effects of P. corylifolia were published until year 2017 and were searched using various scientific databases. The scientific literature searched revealed that these plant species has been extensively investigated in vivo and in vitro for various biological and phytochemical studies. It has cardiotonic, vasodilator, pigmentor, antitumor, antibacterial, cytotoxic, and anti‐helminthic properties and locally used for alopecia, inflammation, leukoderma, leprosy, psoriasis, and eczema. So far, about a hundred bioactive compounds have been isolated from seeds and fruits, and most important compounds identified belongs to coumarins, flavonoids, and meroterpenes groups. This review article summarized the most updated scientific literature on bioactive phytochemical and biological activities of P. corylifolia. This article will be a useful addition to providing information for future research, and more standard clinical trials are needed for the plant to be used as therapeutic agent.
Keywords: biological activities, coumarins, ethnopharmacology, flavonoids, phytoconstituents, Psoralea corylifolia
Abbreviations and Chemcials
- AD
Alzheimer's disease
- CYP
cytochrome P450
- E2
estradiol
- ER
estrogen receptors
- HPLC
high performance liquid chromatography
- IBC
isobavachalcone
- ICAM 1http://topics.sciencedirect.com/topics/page/Cell_adhesion_molecule
http://topics.sciencedirect.com/topics/page/Cell_adhesion_molecule
- I.multifiliis
Ichthyophthirius multifiliis
- LPS
http://topics.sciencedirect.com/topics/page/Lipopolysaccharide
- MAP
mitogen activated protein kinases
- MRSA
methicillin‐resistant Staphylococcus aureus
- MIC
minimum inhibitory concentration
- M. aurum
Mycobacterium aurum
- P. corylifolia
Psoralea corylifolia
- PC12
Pheochromocytoma
- PLpro
papain‐like protease
- PT
prothrombin time
- PTP‐1B
protein tyrosine phosphate 1B
- ROS
reactive oxygen species
- SA
Staphylococcus aureus
- SARS‐CoV
severe acute respiratory syndrome corona virus
- TLR4
- TR‐LE
temperature‐sensitive rat lymphatic endothelial
- TT
thrombin time
- ABTS, 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulphonic acid)
(PubChem CID: 9570474)
- Angelicin
(PubChem CID: 10658)
- Astraglin
(PubChem CID: 5282102)
- Bakuchalcone
(PubChem CID: 6476086)
- Bakuchicin
(PubChem CID: 3083848)
- Bakuchiol
(PubChem CID: 5468522)
- Bakuflavanone
(PubChem CID: 101888270)
- Bakuisoflavone
(PubChem CID: 102128916)
- Bavachalcone
(PubChem CID: 6450879)
- Bavachromene
(PubChem CID: 5321800)
- Bavachromanol
(PubChem CID: 5321790)
- Bavachin
(PubChem CID: 14236566)
- BCN, bavachinin
(PubChem CID: 10337211)
- Beta‐caryophyllene
(PubChem CID: 5281515)
- Biochanin A
(PubChem CID: 5280372)
- Corylifol
(PubChem CID: 25056407)
- D‐GalN, D‐Galactosamine
(PubChem CID: 24154)
- DPPH, 2,2‐diphenyl‐1‐picrylhydrazyl
(PubChem CID: 2735032)
- Genistein
(PubChem CID: 5280961)
- Isobavachalcone
(PubChem CID: 5281255)
- MTT, 3‐(4,5‐https://en.wikipedia.org/wiki/Di-https://en.wikipedia.org/wiki/Methylhttps://en.wikipedia.org/wiki/Thiazole‐2‐yl)‐2,5‐dihttps://en.wikipedia.org/wiki/Phenyltetrazolium bromide
(PubChem CID: 64965)
- Psoralen
(PubChem CID: 6199)
- (tBH), Tert‐butyl hydroperoxide
(PubChem CID: 6410)
- Wighteone
(PubChem CID: 5281814)
- Xanthanangelol
(PubChem CID: 643007).
1. INTRODUCTION
The family Leguminosae contains about 500 genera and is among the largest families of flowering plants. The plants of Leguminosae are widely distributed, and in terms of number of specie, it is one of the largest terrestrial family of plants after Orchidaceae and Asteraceae (Stevens, 2015). The genera that belong to this family are important medicinally and contain a variety of biologically important molecules; some of the examples are Psoralea, Indigofera Pongamia, and Alhegi. The plants of Psoralea had been given high value due to the medicinal properties they showed. The word Psoralea originates from the Greek psoraleos, which stands for “affected with the itch or with leprosy” (Chopra, Dhingra, & Dhar, 2013). The species of Psoralea are native to America, commonly found in the South and the Alleghenies in the west (Maisch, 1889). In India, it is found as a wild weed in cold weather of the Himalayas. It is found in Bombay, Bengal, Utter Pradesh, Rajasthan, in valley of Karnataka, Bihar, and Deccan (Sah, Agarwal, & Garg, 2006; Sharma, Yelne, Dennis, Joshi, & Billore, 2000). The plants are widely distributed in the Himalayan regions of China and Pakistan and there are reports that they have been seen in Southern Africa (Deshaprabhu, 1966; Srivastava, Singh Rawat, & Mehrotra, 2004; Xiong et al., 2009).
One of such important plant species is Psoralea corylifolia L. (Leguminosae). P. corylifolia grows annually and is an erect herb. The range of height to which this plant grows is between 30 and 180 cm, it does not grow in shade and requires warm location. The soil requirement for this plant is clay, sand, and loam types. The plant can survive in acid, basic, and neutral environment. The best sowing season for this plant is March to April. Seeds get mature in November. The plant may grow up to 5–7 years if proper care is given (Vijnan, 1986). The fruit of Psoralea is perennial. The fruit cannot survive in freezing weather. The fruit normally has no odor, but when chewed, it produces pungency. The taste of the fruit is bitter, acrid, and unpleasant. The size of the flowers are small and shaped like red clover (Krishnamurthi, 1969). The arrangement of the leaves is racemes. The simple leaves are broad, elliptic, having margins with dents. There are total of about five major nerves that originated from the leaf base. The leaves are pubescent with white hairs on upper and lower surfaces of the leaves. The color of the flowers when it blooms during rain are purple with blue tints. They are more crowded in the axils. The peduncles are long headed and a single raceme may carry 10–30 flowers. The color of the pods is black chocolate like. The pods are small sized (3.5–4.5 × 2.0–3.0 mm). The shape of pods is ovoid to oblong, may be flat. Psoralea contains a single seed. The shape of the seed is elongated with smooth surface. The seed is without hairs, compressed, and are pitted closely. The color of the seed is dark brown. The seeds have no starch, have oily texture, and have no endosperm (Krishnamurthi, 1969). The crop gets fully mature in 7–8 months. The seeds take time to mature, and therefore collection can be rendered 4–5 times from December to March (Sharma et al., 2000; Figure 1).
Figure 1.

File photo of Psoralea corylifolia [Colour figure can be viewed at http://wileyonlinelibrary.com]
Some vernacular names of this plant are Bemchi (Hindi), Aindavi, Chanderlekha, Kushthahantri, Sitavari, Vejani, Sugandhakantak, Krishnaphala, Kalameshi, Sasankarekha (Sanskrit), Babchi, Bawachi (Urdu), Babechi (Gujarati), Bavanchi (Marathi), Bavanchalu, Kala‐ginja (Telegu), Karpokarishi (Tamil), Somaraji (Kanad), Karkokil (Malyalam), Habucha (Assam), Bakuchi (Oriya), Kakuch, and Bakuchi (Bengal), others are Babchi seeds, Psoralea seeds, Malay tea, Scurf‐pea, Fountain bush, and West Indian Satinwood (English). In the other parts of world, Ku Tzu, Pu Ku Chih (China), Bawchan (German), Loelab el abid, Mahalep (Arabic), Bavanchi (Kanarese), Ravoli (Srilanka), Waghchi, Vabkuchi, Ba bakhi (Persian), Bakuchi (Nepalese), Buckidana (Bangladesh), and Bodi (Sinhalese; Agharkar, 1991; Brands, 1989–2005; Chopra, Chopra, Handa, & Kapur, 1958; Cseke et al., 2006; Cybulska et al., 2011; Deshaprabhu, 1966; Gupta, 2003; Joshi, 2000; Joy, Thomas, Mathew, & Skaria, 1998; Kapoor, 2000; Khare, 2004; Lin, Huang, Chien, Sheu, & Chen, 2007; Liu, Yang, Roberts, Elford, & Phillipson, 1992; Martinvalet, Dykxhoorn, Ferrini, & Lieberman, 2008; Nadkarni & Nadkarni, 1976; Okhale et al., 2010; Panda, 1999; Sharma et al., 2000; Warrier, Nambiar, & Ramankutty, 1995; Xiong et al., 2009). The other biological names of P. corylifolia are Cullen corylifolium, C. corylifolia, Psoralea patersoniae, Lotodes corylifolia, and Trifolium unifolium (Brands, 1989–2005).
2. METHODS
The ethnobotanical, ethnopharmacological, biological, and phytochemical data about P. corylifolia were searched and obtained using databases such as PubMed, Science Direct, Scopus, research gate, and Google Scholar. The plant's scientific name was validated using the plant name index database, http://plantlist.org.
3. ETHNOPHARMACOLOGY
P. corylifolia is an important part of therapeutics in Ayurveda and in Chinese medicines. The plant is cardio active and showed antimicrobial and cytotoxic properties. It is used as a pigmentor. The plant showed cytotoxicity against tumors and worms (Baquar, 1989; Rizvi, Saeed, & Zubairy, 2007; Sharma, 2004). In Chinese medicines, the Psoralea plant is considered warm by nature, and therefore shows many healing actions on kidney and spleen meridians (Daiquan, 2000–2006). The seeds of P. corylifolia are used in indigenous medicine systems for healing of various ailments. The seeds are diuretic, aphrodisiac, laxative, anti‐helminthic, and are used in febrile conditions. In Ayurveda, the seeds are used in the form of paste and as an ointment for external as well as internal use for treatment of different conditions such as alopecia, inflammation, leukoderma, leprosy, psoriasis, and eczema (Huang, 1998; Judd, Campbell, Kellogg, Stevens, & Donoghue, 1999; SJc, 2015). In India, the powder of seeds is mixed with haratalabhasma (yellow arsenic) and converted to paste with the urine of cow. This paste is used to treat leukoderma lesions. In another formulation, the mixture of powdered seeds with buttermilk have been used externally for treating ringworm and scabies. The seed oil is taken orally with betel nut leaf for treatment of leprosy. Another skin condition, dermatosis, has been treated with adjuvants therapy of bakuchi (P. corylifolia) with other local plants such as Amalaki and Khadira. Similarly, the oils of bakuchi and Karanja are mixed with Vaseline to treat chronic skin diseases (Khare, 2008). The seeds of the plant are also considered useful in bilious disorder, snakebite, and in scorpion sting. The seeds are also used in the production of perfumes (Deshaprabhu, 1966; Maisch, 1889; Sharma et al., 2000). The root of P. corylifolia has shown its effectiveness in dental caries. The fruits are aphrodisiac and have laxative effect, and the leaves are antidiarrheal (Baquar, 1989; SJc, 2015). The plant is also valuable in treating alopecia areata (Dua, Kumar, Pandey, & Kumar, 2013b). In combination with Haritka and Gokshura, this plant is used for urinary frequency, and with Ashwagandha and Bala, it is used for treatment of reproductive diseases and cough. In chronic diarrhea and for treating cold symptoms, this plant is used in combination with Nutmeg and Haritaki (Anderson & Voorhees, 1980). The seeds of Bakuchi in powder form mixed with the decoction of Bibhitaka (Terminalia bellirica bark) and Kaakodumbara (Ficushispida) was effective to treat vitiligo. The infection of ringworm is treated with a combination of Tila (sesame seeds) and Bakuchi (Gupta, Dhar, & Atal, 1978). A combination of bakuchi with Haratalabhasma provides relief against leukoderma when applied externally (Khatune et al., 2002).
In Japan, the plant extracted with alcohol has been used as an additive in processed foods and pickles (Nadkarni, 1976). When the fixed oils are removed, what is left after the seed cake are used as feed or manure due to the presence of nitrogen (6.7%) and the minerals (7.8%). Volatile oils obtained from fruits have an irritant effect on the skin and mucous membranes and stimulate the voluntary muscles in high concentrations (Chaudhuri, 2015; Gidwani et al., 2010; Huang, 1998). P. corylifolia in the form of extracts have been used in various herbal formulations when combined with other herbs and used in handling psoriasis and many other skin disorders (Kubo, Dohi, Odani, Tanaka, & Iwamura, 1989). This plant is also reported to be used in cardiac problems, asthma, and urinary discharge (KR, 1975). It also has anti‐leishmaniasis activity (Ali, Akhtar, Sultana, Baboota, & Ahuja, 2008). It is used to control watery stool, urinary frequency, and reproductive imbalances (Pole, 2006). In China, it is specially used against vitiligo disease (Anderson & Voorhees, 1980; Khare, 2004).
P. corylifolia is available in the form of various Ayurvedic marketed formulations in India and across the world, the most famous brands are Safuf Bars®, Zimad Kibrit®, Svitrakaravati®, Khadirarista®, Algushadi yoga®, Sarvangasundarigutika®, Bhallatakawaleha®, Maheshwaraghrita®, Ayorajodilepa®, Brihatsomarajitaila®, Somarajighrita®, Bawchichurna®, etc. (Ali et al., 2008; Pole, 2006; Qiao et al., 2007).
4. PHYTOCHEMICAL CONSTITUENTS
The investigation showed that P. corylifolia possessed a wide range of phytochemicals including flavones, coumarins, monoterpenes, chalcones, lipids, resins, stigmasteroids, and flavonoids. The volatile oils are also reported from this plant (Zhang, Zhao, Wang, Lu, & Chen, 2016). It is revealed that seasonal variation also effects the phytochemistry of P. corylifolia. Mostly, the bioactive compounds are found to be concentrated in the seeds. The phytochemistry of this plant is discussed as follows.
4.1. Whole plant
The whole plant of P. corylifolia was extracted with organic solvents such as petroleum ether and chloroform. The subsequent isolation methods lead to the purification of bioactive compounds, for example, psoralen, isopsoralen, corylifolin, corylin, and psoralidin (Gupta, Gupta, & Gupta, 2013). Peng and colleague obtained a new compound identified as Neo‐psoralen from the whole plant of P. corylifolia in 1996 and elucidated its structure on the grounds of chemical indications and spectroscopic analysis (Chaudhuri, 2015; Gupta et al., 2013; Table 1 and Figure 2).
Table 1.
The bioactive compounds isolated from P. corylifolia with reported activities
| No | Compound | Chemical nature | Part of the plant | Activity and reference |
|---|---|---|---|---|
| 1 | Angelicin | Furanocoumarin | Seeds | Antibacterial (Khatune et al., 2004) |
| 2 | Aryl coumarin | Coumarin | Seeds | Anticancer (Limper et al., 2013) |
| 3 | Astragalin | Flavonoid | Seeds | Antioxidant (Zhang et al., 2016) |
| 4 | Bakuchiol | Meroterpene |
Seeds/Fruit |
Anti‐acne (Iwamura et al., 1989) |
|
Antibacterial (Katsura et al., 2001; Newton et al., 2002) Antifungal (Newton et al., 2002), (Hosamani et al., 2012; Lau et al., 2010; Lau et al., 2014; Prasad et al., 2004; Savoia, 2012; Srinivasan & Sarada, 2012; Yang et al., 2006) | ||||
| Retinal regeneration (Seo et al., 2013) | ||||
| Anti‐aging (Seo et al., 2013) | ||||
|
Estrogen receptor agonist, Postmenopausal symptoms (Lim et al., 2011) | ||||
| Anti‐diabetic (Behloul & Wu, 2013) | ||||
| Lymphangiogenesis inhibition (Jeong et al., 2013) | ||||
| Anticancer (Chen et al., 2010; Li et al., 2016) | ||||
| 5 | Bavachinin | Flavone | Seeds |
Antibacterial (Khatune et al., 2004) Estrogen receptor agonist (Lim et al., 2011) Lymphangiogenesis inhibition (Jeong et al., 2013) osteoporosis (Liu et al., 2014) Anti‐Alzheimer (Chen et al., 2013) Carboxylesterase inhibitors (Li et al., 2015) |
| 6 | Bakuisoflavone | Flavone | Fruit | Antibacterial (Siva et al., 2015) |
| 7 | Bakuflavanone | Flavone | Fruit | Antibacterial (Siva et al., 2015) |
| 9 | Bavachin | Flavnonoid | Seeds/fruit | Osteoblast (Miura & Nishida, 1996) |
| 10 | Bakuchicin | Coumarin | Seeds | Topoisomerase inhibitor (Sun et al., 2003) |
| 11 | Bavachalcone | Chalcone | Seeds |
Anticancer (Shan et al., 2014) CVS protective effect (Dang et al., 2015) |
| 12 | Bavachinone A | Flavonoid | Fruit | Antibacterial (Won et al., 2015) |
| 13 | Bavachinone B | Flavonoid | Fruit | Antibacterial (Won et al., 2015) |
| 14 | Bavacoumestan C | Flavonoid | Fruit | Antibacterial (Won et al., 2015) |
| 15 | Corylifolinin | Chalcone | Seeds |
Antibacterial (Khatune et al., 2004) Carboxylesterase inhibitors (Sun et al., 2016) |
| 16 | Corylifols | Prenyl flavonoid | Seeds | Antibacterial (Yin et al., 2004) |
| 17 | Corylifol A | Flavonoid | Seeds/fruit | Carboxylesterase inhibitors (Li et al., 2015) |
| 18 | Corylifol B | Flavonoid | Seeds | Carboxylesterase inhibitors (Li et al., 2015) |
| 19 | Corylifol C | Flavonoid | Seeds | Protein kinase inhibition (Limper et al., 2013) |
| Anticancer (Limper et al., 2013) | ||||
| 20 | Corylifol D | Flavonoid | Seeds | Anticancer (stomach; Yang et al., 1996; Teschke et al., 2014) |
| 21 | Corylifol E | Flavonoid | Seeds | Anticancer (stomach; Yang et al., 1996; Teschke et al., 2014) |
| 22 | Coryfolin | Flavonoid | Whole plant | Antioxidant, anti‐diabetic (Behloul & Wu, 2013) |
| 23 | Corylin | Flavonoid | Whole plant |
Osteoblast (Miura & Nishida, 1996; Wang et al., 2001) Anticancer (Shan et al., 2014) Carboxylesterase inhibitors (Sun et al., 2016) |
| 24 | Coryaurone A | Flavonoid | Fruit | Antibacterial (Won et al., 2015) |
| 25 | Dadzin | Isoflavnoid | Fruit | Antioxidant (Shinde et al., 2010) |
| 26 | Dadzein | Isoflavnoid | Fruit |
Antioxidant (Shinde et al., 2010) Antidiabetic (Behloul & Wu, 2013) Topoisomerase inhibitor (Sun et al., 2003) |
| 27 | Dihydroxy coumestan | Essential oil component | Seeds | Insecticidal, genotoxic (Khatune et al., 2002; Dua et al., 2013b) |
| 28 | Genistein | Isoflavone | Fruit | Ani‐diabetic, anti‐obesity (Behloul & Wu, 2013), antioxidant (Shinde et al., 2010) |
| 29 | Hydroxy bukuchiol | Meroterpene | Seeds | Lymphangiogenisis inhibition (Jeong et al., 2013) |
| 30 | Hydroxypsoralenol A | Flavonoid | Fruit | Antibacterial (Won et al., 2015) |
| 31 | Hydroxypsoralenol B | Flavonoid | Fruit | Antibacterial (Won et al., 2015) |
| 32 | Isobavachalcone | Chalcone | Seeds |
Estrogen receptor agonist (osteoporosis; Lim et al., 2011) Neuroprotective (Lee et al., 2015) |
| Lymphangiogenesis inhibition (Jeong et al., 2013) | ||||
|
Anti‐Alzheimer (Chen et al., 2013) Carboxylesterase inhibitors (Li et al., 2015) |
||||
| 33 | Isobavachin | Flavonoid | Seed/fruit | Osteoblast (Li et al., 2014) |
| 34 | Isopsoralen | Furanocoumarin | Whole plant | Antiprotozoal (Song et al., 2015) |
| 35 | Neobavaisoflavone | Seeds | Antibacterial(Khatune et al., 2004) | |
| 36 | Psoralen | Furanocoumarin | Whole plant/root |
Leucoderma, psoriasis (Kim et al., 2013) Anticancer (Hao et al., 2014), antioxidant (Chen et al., 2011), anti‐Alzheimer (Somani et al., 2015), Collagengenesis (Xu et al., 2015) |
| 37 | Psoralidin | Coumarin | Whole plant/seed | Estrogen receptor modulator (Liu et al., 2014; Lim et al., 2011) |
| Antioxidant (Wang, Yin, Zhang, Peng, & Kang, 2013b), antibacterial (Khatune et al., 2004) | ||||
| Anti‐diabetic (Behloul & Wu, 2013), antiprotozoal (Song et al., 2015) | ||||
| Anticancer (Hao et al., 2014; Limper et al., 2013; Yang et al., 1996), anti‐depressent (Farahani et al., 2015) | ||||
| 38 | Psoracorylifol D | Flavonoid | Seed | Lymphangiogenesis inhibition (Jeong et al., 2013) |
| Psoracoumestan | Coumestans | Seeds essential oil | Anti‐ cancer (Limper et al., 2013) | |
| 39 | Xanthoangelol | Chalcone | Seeds | Anticancer (Limper et al., 2013) |
Figure 2.
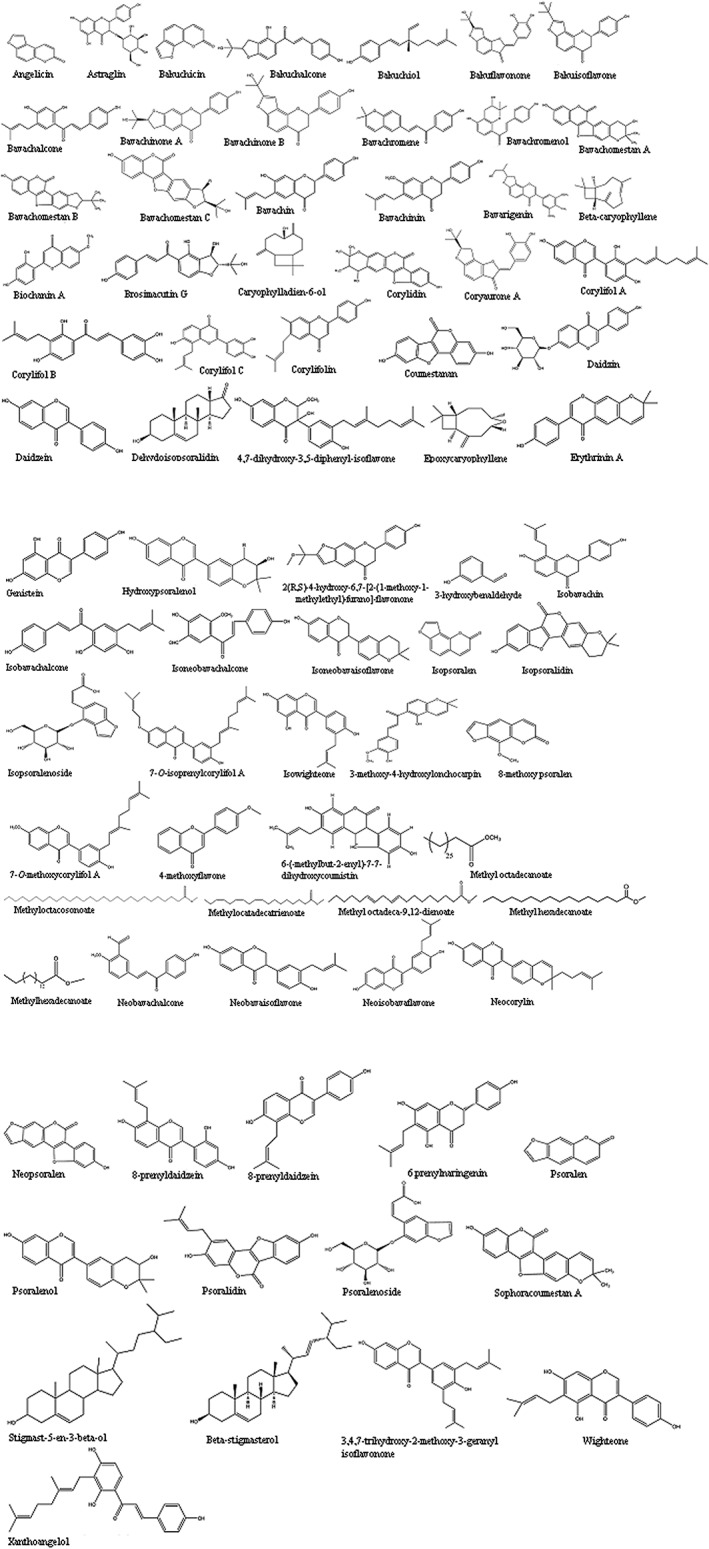
Structures of important compounds isolated from Psoralea corylifolia
4.2. Seeds
It was already discussed that most of the active constituents isolated so far from P. corylifolia are part of the seed. Sen and colleague extracted seed oil from P. corylifolia and found that the oil is unsaponifiable with boiling points 180–190 °C. During the experiment, another compound was also identified as methyl glucoside with melting points of 105–127 °C (Chopra & Chopra, 1933). Chopra and Chaterjee, in 1927, identified essential oil, a fixed oil and resin of dark brown color with some traces of alkaloids in P. corylifolia (Chopra et al., 2013). Dymock studied the sugar contents, extractive matter, albumin concentraton, and ash value, and also found some traces of manganese in seeds of P. corylifolia. In continuation of research for compounds from the seeds of P. corylifolia, three more important components were fractionated and identified as bakuchiol a monoterpene phenol and the two novels dimeric monoterpenoids, bisbakuchiols A and B (Panda, 1999).
A very important pharmacological compound known as bakuchiol has also been biosynthesized in 1983, and it was concluded that it is a derivative of phenylpropane pathway (Banerji & Chintalwar, 1983). Similarly, Bisbakuchiols A and B structures were evaluated, and it was noted that dimeric monoterpenoid skeleton contains two monoterpenes, which are connected through a dioxane bridge (Wu et al., 2007).
The low polarity ether extract of P. corylifolia seeds was investigated, and it revealed the presence of various ketones and aldehydes containing compounds such as Corylinal, C‐formylated chalcone, and Isoneobayachalcone. A new isoflavone compound known as Psorlenal was also identified in the seeds (Gupta et al., 1978). The same compounds, psoralen and isopsoralen, were also isolated by applying other chromatographic techniques such as high‐speed counter‐current chromatography (Liu, Li, Sun, & Kong, 2004).
The seed's sample has been the main focus for the search of bioactive compounds, and such an isolation procedure involving spectroscopic methods and crystal X‐ray diffraction resulted in isolation of five novel compounds. These compounds are named as psoracorylifols A–E and chalcone and bavachromanol (Yin, Fan, Dong, & Yue, 2006). Similarly, three new flavonoid compounds named as corylifols A, B, and C and bavachalcone were also fractionated from P. corylifolia seeds. Another compound known as Bakuchicin was also identified from the seeds (Yin, Fan, Wang, Dong, & Yue, 2004). The seeds are also reported to contain some other flavonoids such as bavachinin (BCN), bavachin, isobavachin, and isobavachalcone (IBC). The glycosides identified in seeds of P. corylifolia were psoralenoside and isopsoralenoside, which were of the benzofuran type.
P. corylifolia also reported to contain some polar compounds, namely, neobavachalcone, 7‐methyl bavachin, and bavachromene. These compounds were isolated and identified from the insoluble portion of the ethanolic extract. These compounds identified as Cyclobakuchiol C were isolated as a new molecule from the less‐polar part of the seeds of P. corylifolia. The defatted methanol soluble fraction of seeds of P. corylifolia also yielded new flavonol glycoside named as 3,5,3′, 4′‐tetrahydroxy‐7‐methoxyflavone‐3′‐O‐alpha‐L‐xylopyranosyl (1–N3)‐O‐alpha‐L‐arabinopyranosyl (1–N4)‐O‐beta‐D‐galacto pyranoside.
Types of esters were also studied in P. corylifolia, and psoralester and psorachromene were identified as two new metabolites. The psoralester is a 10‐membered lactone compound and the latter is an isomer of already known compound bayachromene (Tewari & Bhakuni, 2010). Khatune and colleague isolated new coumestan derivative 6‐(‐3‐methylbut‐2‐enyl)‐6 N‐7‐dihydroxycoumestan while working on the chloroform soluble portion of the seeds of P. corylifolia (Khatune et al., 2002). Coumestans‐3, 5′‐dihydroxy‐6′, 6′‐dimethyldihydropyrano (2′, 3′: 8,9) coumestan, 3‐hydroxy‐ 5′‐(1‐hydroxy‐1‐methylethyl) 4′, 5′‐dihydrofuro (2′, 3′: 8,9) coumestan, and sophoracoumestan A have been fractionated from the seeds of P. corylifolia. Lin and Kuo isolated two new benzofuran derivatives; corylifonol and isocorylifonol along with astragalin, p‐hydroxy benzoic acid from P. corylifolia seed extract (Lin & Kuo, 1992).
In 1989, Zaka and colleague investigated the lipid and fatty acid composition of P. corylifolia. The lipids recognized were triacylglycerols, diacylglycerols, free fatty acids, monoacylglycerols, hydrocarbons, wax esters, and polar lipids. The purified crude lipids of P. corylifolia seeds were analyzed by thin layer and gas chromatographic techniques. Among the components, the major polar lipid was C18:1. The mono and diacylglycerol portions contained huge amounts of C14:0 and C18:0 and the hydrocarbons. The wax ester portions were abundant in C22:0. Similarly, volatile oil contents were also investigated by GC–MS instrument, and main compounds identified were β‐caryophyllene, limonene, linalool, terpin‐4‐ol, and geranyl acetate (Zaka, Asghar, Raie, Khan, & Bhatty, 1989). A recent phytochemical analysis carried out on GC–MS of a low polar fraction of the methanolic seed extract of P. corylifolia revealed the presence of 14 compounds, which include aromatic, sesquiterpenes, furocoumarins, sterols, fatty acid, and their methyl esters. The most abundant compounds identified were epoxycaryophyllene, isopsoralen, psoralen, and bakuchiol. The same nonpolar fraction also exhibited substantial bactericidal activity against many bacterial strains (Ali et al., 2015).
Three new prenylflavones 2 (R,S)‐4′‐hydroxy‐6,7‐[2‐(1‐methoxy‐1‐methylethyl)‐furano]‐flavanone, 4′,7‐dihydroxy‐3′,5′‐diprenyl‐isoflavone and 3,4′,7‐trihydroxy‐2‐methoxy‐3′‐geranyl‐isoflavanone, were fractionated and purified from the seeds of P. corylifolia. Overall, 22 prenylated compounds, including flavanones, isoflavones, and chalcones analogues, were identified and evaluated on the PPAR‐γ agonist activity. The structures of important constituents are shown in Figure 2.
4.3. Fruits
The sticky and oily pericarp constitute the fruit of P. corylifolia, and chemical investigation revealed some similar compounds as already isolated from seeds. A new isoflavone, corylinin, (7,4′‐dihydroxy‐3′‐[(E) ‐3,7‐dimethyl‐2,6‐octadienyl] isoflavone) along with six already identified compounds, isopsoralen, psoralen, sophoracoumestan A, neobavaisoflavone, daidzein, and uracil, have been stated from the dehydrated fruits of P. corylifolia (Ruan, Kong, Takaya, & Niwa, 2007). In another experiment, HPLC protocol was applied to identify some isoflavonoids such as daidzein, genistein, and biochanin A (Sehrawat, Sangwan, & Yadav, 2014). Further work on the fruit extracted with hexane showed the presence of a phenolic monoterpene known as Bakuchiol (Cui, Taniguchi, Kuroda, & Hatano, 2015). Dried P. corylifolia fruit powder extracted with methanol and analyzed on HPLC reverse column showed the presence of isoflavonoids known as genistein, daidzein, and biochanin A (Hsu, Wu, Chen, Yang, & Wang, 2001). Raun and colleagues have isolated seven compounds from the fruit of P. corylifolia and established the structure of compounds after the spectroscopic analysis. The components identified were corylinin (new compound), psoralen, neobavaisoflavone, sophoracoumestan A, uracil, and daidzin (Ruan et al., 2007). In 2015, two more new flavonoids, bakuisoflavone, and bakuflavanone were isolated from the fruit of P. corlylifolia with antimicrobial activities (Lee, Kim, Baik, Ryu, & Lee, 2015). In one study, six new flavonoid compounds and a meroterpenoid were isolated and identified by spectroscopic methods from P. corylifolia fruits and displayed medium activity against Staph. mutans (Won et al., 2015).
The dried fruits of the plant was investigated, and n‐hexane‐ and EtOAc‐soluble fractions have resulted in purification of two new isoflavones, 7‐O‐methylcorylifol A and 7‐O‐isoprenylcorylifol A, along with eight known compounds, including psoralen, angelicin, bavachalcone, 13‐dihydro‐12,13‐epoxybakuchiol, bakuchiol, 12, p‐hydroxybenzaldehyde, stigmasterol, and b‐sitosterol (Chen et al., 2011; Figure 2).
4.4. Roots
The roots of P. corylifolia has been investigated for bioactive compounds. It was found that furanocoumarins psoralen and isopsoralen isolated from a petroleum ether extract were responsible for the anti‐feedant activity against instar Spodoptera litura larvae (Sah et al., 2006; Figure 2).
The above discussions about phytochemical investigation of different parts of P. corylifolia clearly indicates that this plant is a very useful source of variety of bioactive constituents including flavonoids, terpenes, glycosides, alkaloids, coumarins, and others.
5. BIOLOGICAL ACTIVITIES
P. corylifolia is a widely used herb and have many diverse ethnopharmacological and medicinal applications. The numerous chemical and pharmacological research that have been carried out have resulted in the isolation of the diverse bioactive compounds that are summarized below.
5.1. Skin conditions
P. corylifolia proved a promising agent in anti‐acne formulations due to the presence of phenolic compounds Bakuchiol. It proved to be safe and non‐irritant and can be used for longer periods of the day because it showed no irritation and is non‐sensitized (Iwamura, Dohi, Tanaka, Odani, & Kubo, 1989). One of the bioactive isolated compound “soralen” found to have the ability to stimulate the development of melanin, and therefore it is employed for Leucoderma treatment (Kim, Shim, Ahn, & Jung, 2013). The plant is also used against the skin disease known as psoriasis (Chen, Yang, & Zhang, 2013). In one experiment, seeds of P. corylifolia was extracted with hexane and oil in water, cream was prepared with stearic acid as a base. In the next step, an open clinical trial was conducted on 30 patients suffering from eczema for a period of 30 days. After 2 weeks of cream application, symptoms score reduced. Final day observation revealed that the symptom scores for eczema reduced from 6.367 ± 1.098 to 0.333 ± 0.279 for length of the lesion, from 1.333 ± 0.994 to 0.165 ± 0.087 for exudation rate, and from 2.567 ± 0.504 to 0.165 ± 0.132 for the rate of itching. This formulation was compared with the placebo preparation, in which the formulated cream contained all the ingredients except for the hexane extract of P. corylifolia. This study concluded that this plant could be effectively used for the treatment of eczema (Gidwani et al., 2010).
5.2. Antibacterial activity
P. corylifolia has been tested for antibacterial activity. Wang et al. (2013) reported that two isolated compounds, Corylifolinin and http://europepmc.org.sci-hub.org/abstract/med/24199566/?whatizit_url_Chemicals=http://www.ebi.ac.uk.sci-hub.org/chebi/searchId.do?chebiId=CHEBI%3A66614, possessed significant antibacterial activity against Staphylococcus aureus (SA), Methicillin‐resistant Staphylococcus aureus (MRSA), and β‐lactamase positive Staphylococcus aureus (ESBLs‐SA). The minimum inhibitory concentration (MIC) for Corylifolinin and http://europepmc.org.sci-hub.org/abstract/med/24199566/?whatizit_url_Chemicals=http://www.ebi.ac.uk.sci-hub.org/chebi/searchId.do?chebiId=CHEBI%3A66614 against SA, MRSA, and ESBLs‐SA were (http://europepmc.org.sci-hub.org/abstract/med/24199566/?whatizit_url_gene_protein=http://www.uniprot.org.sci-hub.org/uniprot/?query=MIC&sort=score 0. 781, 3, 1.562, 5, 0.781 25 micro g x http://europepmc.org.sci-hub.org/abstract/med/24199566/?whatizit_url_go_term=http://www.ebi.ac.uk.sci-hub.org/ego/GTerm?id=GO:0031264 (−1) and 6.25, 6.25, 6.25 micro g x http://europepmc.org.sci-hub.org/abstract/med/24199566/?whatizit_url_go_term=http://www.ebi.ac.uk.sci-hub.org/ego/GTerm?id=GO:0031264 (−1), respectively. In another study, psoralidin and bakuchicin compounds were extracted from P. corylifolia (seeds) showed significant inhibition of Gram‐negative bacteria, including Shigella sonnei and Shigella flexneri, whereas psoralen and angelicin compounds showed promising activities against Gram‐positive bacteria, SA. The concentrations of various compounds used were in range of 200–400 μg/disc and the results were compared with the standard antibiotic Kanamycin at 30 μg/disc (Khatune, Islam, Haque, Khondkar, & Rahman, 2004). Yin and colleagues isolated 16 new compounds from P. corylifolia seeds including three new prenylflavonoids (corylifols). Nine compounds exhibited significant antibacterial activity against SA and S. epidermidis (Yin et al., 2004). In one experiment, bioassay guided isolation lead to the purification of antibacterial compound bakuchiol from crude methanol extracts of seeds of P. corylifolia. The compound found to be effective against Mycobacterium, M. aurum and M. smegmatis. But P. corylifolia extract was found to have significant antibacterial activity against M. aurum only (MIC = 62.5 μg/ml; Newton, Lau, Gurcha, Besra, & Wright, 2002).
In another study, bakuchiol was tested against some oral microorganisms in vitro. The growth of S. mutans was prevented in a concentration‐dependent manner, and growth of S. mutans was totally inhibited by 20 μg/ml of bakuchiol. Bakuchiol also showed antibacterial effects against other bacteria tested, including S. mutans, Strept. sanguis, Strep. salivarius, Strept. sobrinus, Enterococcus faecalis, Enterococcus faecium, Lactobacillus acidophilus, L. casei, L. plantarum, Actinomyces viscosus, and Porphyromonas gingivalis, with MICs ranging from 1 to 4 μg/ml. It was determined that bakuchiol would be an effective antibacterial agent against oral pathogens and has great potential for use in treatment of dental caries (Katsura, Tsukiyama, Suzuki, & Kobayashi, 2001). The seeds and aerial parts of P. corylifolia extracts with organic solvents showed activity against S. eidermidis and P. morganii. Dioxane extract of seeds showed maximum activity against the pathogens tested (Chanda, Kaneria, & Nair, 2011). Similarly, the compound bakuchiol (MIC 0.037 and 0.018 mM against S. areus and S. epidermdis, respectively (Liu et al., 2014; Park, Zhao, Kim, & Sohn, 2005; Seo et al., 2013), along with other related compounds that possess potent antibacterial activity (Pavela, 2009).
Acharya, Singh, and Patgiri (2015) formulated Bakuchi ointment, Bakuchi taila, and Bakuchi gel and tested for antimicrobial activity. The Bakuchi ointment displayed greater inhibitory activity against Gram +ve Bacillus subtilis and Gram −ve Escherichia coli, whereas Bakuchi taila showed greater inhibitory activity against both Gram +ve B. subtilis and S. aureus. Bakuchi gel displayed the highest inhibitory activity against Gram +ve S. aureus and Gram −ve Klebsiella pneumonia. Bakuchi siktha taila showed superior inhibitory activity against Gram −ve Klebsiella pneumonia. Although, inhibition was more against Gram +ve bacteria, the highest zone of inhibition 15 mm was of Bakuchi gel at only 20 μl drug concentration against S. aureus (Gram +ve). This activity was comparable with the Ampicillin (standard drug) that showed 11.5 mm zone of inhibition (Acharya et al., 2015). P. corylifolia in the form of the mouth rinsing solution has prevented the growth of S. mutans bacteria in a very low concentration. Along with mouth rinsing ability, the ethanol extract of P. corylifolia was also reported to inhibit the human gingival fibroblast. In the same series of experiments, it was concluded that the extract is safe to use and has no toxic effect in normal doses (Kim et al., 2015).
In 2015, two more new flavonoids, bakuisoflavone, and bakuflavanone were isolated from the fruit of P. corylifolia and were tested against the strains of MRSA (MRSA481 and MRSA 584) and it was found that two compounds showed some good antibacterial effects with MIC values of (>32 (>9.4) and >32 (>9.4)) and (>32 (>9.5) and >32 (>9.5 μg/ml)), respectively (Lee et al., 2015). Tissue culture studies showed that Jasmonic acid treated plants of P. corylifolia enhanced psoralen content in leaves and roots of P. corylifolia. The methanol extract of root sample displayed effective antimicrobial activity against tested bacterial and fungal pathogens in range 25, 50, and 75 μg/ml. Most effective activity 28 mm zone of inhibition was observed against P. aerugenosa at 50 μg/ml (Siva et al., 2015). P. corylifolia was also tested against the microbes SA, Pseudomonas aeruginosa, and Candida albicans, which are known to contaminate the cosmetics products. In the 96‐well microplate assay, it was found to have antimicrobial activity (Ryu et al., 2015).
5.3. Antiviral activity
The crude ethanol extract of the seeds of P. corylifolia was revealed to have high activity against the severe acute respiratory syndrome corona virus (SARS‐CoV) papain‐like protease (PLpro) with an IC50 of value of 15 μg/ml. SARS‐CoV‐PLpro is a main enzyme that have a vital role in SARS virus replication (Kim et al., 2014).
5.4. Antifungal
A phenolic compound bakuchiol extracted from P. corylifolia (seeds) exhibited antifungal activity against many strains of pathogenic fungi, including Microsporum gypseum, Epidermophyton floccosum, Trichophyton rubrum, and Trichophyton mentagrophytes in a dose range of about 250 μg/ml (Hosamani, Lakshman, & Sandeepkumar, 2012; Lau et al., 2010; Lau et al., 2014; Newton et al., 2002; Prasad, Anandi, Balasubramanian, & Pugalendi, 2004; Savoia, 2012; Srinivasan & Sarada, 2012; Yang et al., 2006). In another study, activity was found against other fungi such as Alternari brassicae, Aspergillus niger, Fusarium oxysporum, and Rhizoctonia cerealis, in which mycelial growth was inhibited (Satish, Raghavendra, & Raveesha, 2009; Vonshak et al., 2003; Yang et al., 2006).
In one study, P. corylifolia significantly reduced the incidents of seed‐borne fungi, for example, Fusarium verticillioides and Aspergillus flavus, which have the ability to cause many diseases in maize crop (Zeamays L.) and latterly released mycotoxins. These mycotoxins have very bad effect on human and animal health (Aiyaz, Divakara, Chandranayaka, & Niranjana, 2015).
5.5. Anthelmintic activity
The anti‐worm property of the seeds of P. corylifolia is clinically proven on roundworms and flatworms (Gidwani et al., 2010). The seeds and the leaves of P. corylifolia was extracted with water and alcohol, and were tested on the spontaneous movements of Setaria cervi whole worm and again on the isolated nerve muscle preparations. The survival of the microfilariae was tested in vitro. The dose required to inhibit the movements of whole worm and nerve muscle preparations for alcohol extracts of leaves were 160, 30, and for seeds were 50, 20 μg/ml (Maurya, Singh, & Seth, 2014; Mendam, Kavitha, & Naik, 2015; Qamaruddin, Parveen, Khan, & Singhal, 2002).
5.6. Insecticidal and genotoxic activity
Volatile oil extracted from the seeds of P. corylifolia displayed strong toxicity against both larvae and adult of the southern house mosquito, Culex quinquefasciatus (earlier known as Culex fatigans). The results were presented as both LC50, LC90 and LD50, LD90 values and respective values were 63.38 ± 6.30, 99.02 ± 16.63 ppm and 0.057 ± 0.007, 0.109 ± 0.014 mg/cm2. Genotoxicity of adults was determined at 0.034 and 0.069 mg/cm2. The mean comet tail length was 6.2548 ± 0.754 and 8.47 ± 0.931 μm. Eventually, DNA damage was also evaluated and found significant, that is, 6.713% and 8.864% of controls.
The bioactivity guided isolation from chloroform extract of seeds leads to the identification of pure compound, 6‐(‐3‐methylbut‐2‐enyl)‐6 N‐7‐dihydroxycoumestan and found active against larvae and adult Cx. Quinquefasciatus. The same compound has also showed activity against the red flour beetle, Tribolium casteneum Hebrst. In this detailed study, both the male and female adults were exposed to the compound for 24–72 hr period and a range of LD50 were calculated for dose starting from 400 to 1600 ppm and doses were used in five replications. It was concluded that the isolated coumestan compound can be a useful botanical insecticide (Dua, Kumar, Pandey, & Kumar, 2013a; Khatune et al., 2002).
5.7. Antiprotozoal activity
An external protozoan parasite Ichthyophthirius multifiliis (also called “ich”) has been reported to infest freshwater fish species. The P. corylifolia extracted with methanol showed excellent activity against I. multifiliis theronts in concentration of 1.25 mg/L or more when was exposed for a period of 4 hr. The P. corylifolia extract at 5.00 mg/L concentration has caused 100% mortality of protomonts and 88.9% of encysted tomonts. It was found that longer period (24 hr) and higher concentration (5.00 mg/L) caused the significant reduction of the survival rate and reproduction of tomont of I. multifiliis, which were exited from the fish after in‐bath handling in situ (Ling et al., 2013). P. corylifolia has been found to be an alternative to malachite green to control I. multifiliis, an external protozoan parasite. The screening showed that P. corylifolia extract have the maximum activity against I. multifiliis theronts. When the experiments were conducted in vivo, at 1.25 mg/L or more concentrations of methanol extract of P. corylifolia, it caused 00% mortality of theronts during the 4 hr of exposure (Ling et al., 2013). This study showed the damaging effect of P. corylifolia against I. multifiliis trophont in situ.
The same study leads to the evaluation of the activity of antiprotozoal compounds extracted form P. corylifolia against I. multifiliis. The two bioactivity guided isolated compounds, isopsoralen and psoralidin, were assessed. In comparison, study of psoralidin and isopsoralen, the inhibitory activity of psoralidin, was found more efficient. Further, when experimented in vivo, the compound psoralidin at 2.5 mg/L, efficiently reduced the theronts numbers in infected fish over an exposure period of 5 hr. This study showed that psoralidin could be a very good candidate for the formulation as a commercial drug against I. multifiliis (Song et al., 2015).
5.8. Protective Effect
The compounds from P. corylifolia were found to have a protective effect when tested against retinal damage caused by oxidative stress. To evaluate the effects of bakuchiol on the cell viability of differentiated RGC‐5 cells, various concentrations of bakuchiol for protective effects were tested against BSO and glutamate‐induced, staurosporine‐mediated differentiation of RGC‐5 cells. It was found that 0.01 μM of bakuchiol protected against the effect of BSO and glutamate on staurosporine‐mediated differentiation of RGC‐5 cells. It was concluded that bakuchiol could be a good candidate to prevent the retinal damage by reducing the ganglion cell death (Kim et al., 2013). The same compound bakuchiol inhibited the formation of reactive oxygen species and mitochondrial dysfunction induced by oxidative stress in hepatocytes HepG2 so this role has been considered to help in slowing the aging process. In this study, HepG2 cells were treated with (200 μg/mL) P. corylifolia extract for 24 hr and 1 mM H2O2 was added for the last 3 hr. Resveratrol (50 μM) was used as a positive control (Seo et al., 2013). In that respect, studies available that showed the protective action against tert‐butyl hydroperoxide (tBH), carbon tetrachloride (CCl4), or D‐Galactosamine (D‐GalN) induced hepatotoxicity in vivo and in vitro (Park et al., 2005).
A recent and very useful research on the same subject of hepatotoxicity shows that the isolated compound bakuchiol when administered at a dose of 52.5 and 262.5 mg/kg for 6 weeks in rats, many abnormalities were observed in the bakuchiol‐treated groups including suppression of weight gain and food intake, change of some parameters in serum biochemistry, and increased weight of liver. The mRNA expression of CYP7A1, HMG‐CoA reductase, PPARα, and SREBP‐2 decreased in bakuchiol‐treated group, the expression of BSEP increased in bakuchiol‐treated low dosage, and the expression of BSEP decreased in bakuchiol‐treated high dosage. It was concluded that bakuchiol could induce cholestatic hepatotoxicity, suggesting potential hepatotoxicity. The mechanism may be related to effects on liver lipid metabolism (Li et al., 2017).
5.9. Estrogen Receptor agonist
In one study, six compounds isolated from P. corylifolia were studied for their binding affinities for estrogen receptors, ERα and ERβ, using the yeast transactivation test. Among the compounds, bakuchiol was found to be the key compound and exhibited the maximum ER‐binding affinity (IC50 for ERα = 1.01 × 10−6 M, IC50for ERβ = 1.20 × 10−6 M), and BCN showed the lowest ER‐binding affinity. Likewise, another compound, IBC, had shown two fold more selective for ERβ than for ERα (RBAERα = 0.03 and RBAERβ = 0.07), but displayed a feeble estrogenic activity. These activities of compound can be touched on to diverse estrogenic activities inside the human body, such as ERβ inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle check. On the other hand, ERα agonists might facilitate bone protecting properties. These outcomes of experiments supported the traditional use of P. corylifolia, L. in the treatment of osteoporosis or postmenopausal conditions (Liu et al., 2014). A bioactivity guided isolation study on P. corylifolia showed that methanol extract and subsequent isolated compounds, including monoterpenes, isoflavones, and coumestan have estrogenic activities, and bakuchiol being the most effective ER agonist. The compound Psoralidin isolated from the ethyl acetate fraction of P. corylifolia seeds proved as novel ER modulator with 92.3% of (estradiol) E2‐induced ERE‐luciferase activity (Lim, Ha, Ahn, & Kim, 2011).
In another study, psoralidin activity as ER (α and β) agonist was evaluated in endometrial and human breast cell lines. Psoralidin at 10 μM was able to get the highest reporter gene expression conforming to that of E2‐treated cells and such an activation of the ERE‐reporter gene by psoralidin was totally stopped by the treatment of a pure ER antagonist, indicating that the biological actions of psoralidin are intermediated by ER. Psoralidin was also able to stimulate the endogenous estrogen‐responsive gene (pS2) in MCF‐7, the human breast cancer cells. It was observed that activation of the classical ER‐signaling pathway by psoralidin is mediated via induction of ER conformation by psoralidin and direct binding of the psoralidin–ER complex of the EREs present in the promoter region of estrogen‐responsive genes. Lastly, molecular docking of psoralidin to the ligand‐binding pocket of the ERα exposed that psoralidin is able to mimic the binding interactions of E2, and therefore, in the cellular environment it could act as an ER agonist (Liu et al., 2014).
5.10. Chemo preventive effects/Protein kinase inhibition activity
P. corylifolia reported to have chemo preventive effect and found to have the ability to inhibit the mitogen activated protein kinases (MAP) pathways. The isolated compounds showed high toxicity in mammalian tumor cell lines by inducing the apoptosis. A compound named as corylifol C inhibited the protein kinase at very low concentration (Limper et al., 2013).
The compounds, particularly newly defined 7,2,4‐trihydroxy‐3‐arylcoumarin and psoracoumestan revealed to have an effective impact on cellular pathways and are of pharmacological interest.
5.11. Anti‐Alzheimer's
Two compounds isolated from commonly used in clinical practices of Traditional Chinese Medicine P. corylifolia named as IBC and BCN modulate amyloid β (Aβ) peptides, especially the peptides with 40 (Aβ40) or 42 (Aβ42) residues, which are believed to be responsible for the development of amyloid plaques in Alzheimer's disease. The peptides were prepared in the lab in dried form in DMSO; Aβ42 5 mg/ml was used and was diluted in PBS to 50 μM. Both the compounds acted in a different way. IBC significantly inhibits both oligomerization and fibrillarization of Aβ42, whereas BCN converts Aβ42 into large unstructured aggregates in neuroblastoma cells. Both compounds were quite effective in Alzheimer's (Chen et al., 2013).
Psoralen isolated from P. corylifolia fruits were investigated as an inhibitor of AChE enzyme in an attempt to explore its potential for the management of Alzheimer's disease. The concentration of psoralen used was 25–400 μg/ml. It inhibited the AchE in a dose‐dependent way in animal models. Adult male Wistar rats, weighing 180–250 g, were used in the study. While a molecular docking study was also carried out, which showed that psoralen binds well within the binding site of the enzyme showing interactions such as π‐π stacking and hydrogen bonding (Somani et al., 2015). Although the activity measured in this study was moderate when compared with the standard compound used, despite that, the compound could serve as lead for synthetic analog preparation to improve the inhibitory activity.
5.12. Antidepressant activity
P. corylifoia also found to possess antidepressant activity. Marzieh Sarbandi Farahani and colleague mentioned the mechanism of action of the plants with antidepressant action and the chemical components isolated from them. They mentioned that psoralidin isolated from seeds of P. corylifolia modify the hypothalamic–pituitary–adrenal axis (Farahani, Bahramsoltani, Farzaei, Abdollahi, & Rahimi, 2015).
A similar study was conducted on psoralidin by Yi and colleague on the ICR strain of male mice. The dose was administered orally in forced swimming test and they observed the increased levels of 5‐hydroxytryptamine and 5‐hydroxyindoleacetic acid in the brain and an altered dopamine level. The mechanism for antidepressant activity was proposed to be through involvement of monoamine neurotransmitter and the hypothalamic pituitary adrenal axis systems (Yi et al., 2008).
Some previous studies are also available, for example, one study was conducted on mice models and it was concluded that furocoumarins were actually responsible for antidepressant activity. In this study, the well‐established antidepressants were used as standards for comparison with the seed extract of P. corylifolia. The dose range used was 7.5 to 100 mg/kg in comparison with amitriptyline (10 and 20 mg/kg) and fluoxetine (13 mg/kg). This study was well designed and results indicate the potential of the seed extract as competitive antidepressant when compared to the conventional therapeutic agents (Chopra et al., 2013).
5.13. Antioxidant
http://europepmc.org/abstract/med/24199566/?whatizit_url_Species=http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=429560&lvl=0 also has a wide range of antioxidant activity. Different compounds isolated from http://europepmc.org/abstract/med/24199566/?whatizit_url_Scorylifolia://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=429560&lvl=0 were tested for their antioxidant potential. A compound Psoralidin proved to be a better scavenger of DPPH free radical with IC50 values of 43.85 mg/L. Tested for ABTS free radical scavenging activity, the compounds showed different antioxidant activities, for example, http://europepmc.org/abstract/med/24199566/?whatizit_url_Chemicals=http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI%3A8616 (IC50 1.32 mg/L), coryfolin (IC50 4.97 mg/L), http://europepmc.org/abstract/med/24199566/?whatizit_url_Chemicals=http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI%3A42202 (IC50 10.47 mg/L), http://europepmc.org/abstract/med/24199566/?whatizit_url_Chemicals=http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI%3A77764 (IC50 34.22 mg/L), and http://europepmc.org/abstract/med/24199566/?whatizit_url_Chemicals=http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI%3A30200 (IC50 31.27 mg/L; Wang, Yin, Zhang, Peng, & Kang, 2013a).
In the understanding of the reputation of this plant species in medicines now, attention has been given to produce callus culture. In one study, relationship between isoflavone and antioxidant activity of P. corylifolia cultures were experimented, and it was found that root‐derived callus cultures produced more daidzein, whereas leaf‐dervied callus produced more genistein, and this enhanced production was related to enhanced antioxidant activities (Shinde, Malpathak, & Fulzele, 2010).
One of the isolated compound, psoralen showed the promising antioxidant activity (IC50 value = 1.10 ± 0.60 μg/ml) against the superoxide anion production by human neutrophils in response to formyl‐L‐methionyl‐L‐leucyl‐L‐phenylalanine/cytochalasin B (fMLP/CB; Chen et al., 2011).
5.14. Anti‐diabetic activity
Various components from P. corylifolia exhibited a variety of activities against the enzymes involved in different forms of diabetes. One of such enzyme is Protein tyrosine phosphate 1B (PTP‐1B), which caused a negative regulation of insulin signaling. Two compounds, psoralidin and bakuchiol, were isolated from Ethyl‐acetate fraction P. corylifolia seeds showed protein tyrosine phosphatase 1B inhibitory activity. One compound, corylin, was found inactive. The first two compounds repressed PTP‐1B activity in a concentration‐dependent method, with IC50 values of 9.4 and 20.8 μM, respectively. Similarly, the compounds isolated from http://europepmc.org/abstract/med/24199566/?whatizit_url_Species=http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=429560&lvl=0 were tested in vitro for http://europepmc.org.sci-hub.org/abstract/med/24199566/?whatizit_url_gene_protein=http://www.uniprot.org.sci-hub.org/uniprot/?query=alpha-glucosidase&sort=score inhibitory activity, among the compounds, psoralidin showed more potency with IC50 values of 40.74 mg/L, coryfolin inhibited the enzyme with IC50 values of 45.73 mg/L, and daidzein showed IC50 values of 49.44 mg/L. It was concluded that these compounds have the potential to be used against type 2 diabetes (Wang et al., 2013a). Genistein, from P. corylifolia extract possesses anti‐diabetic activity by its action as the protective effects on pancreatic β cells (Behloul & Wu, 2013).
A more detailed biochemical study was conducted on the aqueous extract of seed of P. corylifolia that caused a significant recovery in the activities of hexokinase, glucose‐6‐phosphatase, and glucose‐6‐phosphate dehydrogenase and antioxidant enzymes such as peroxidase, catalase, and superoxide dismutase, along with the lipid peroxidation level in liver tissue and serum transaminase, and corrected the fasting blood glucose level in streptozotocin‐induced diabetic rats at a dose of 20 mg/0.5 ml water/100gm body weight (Ghosh, Bera, Chatterjee, Ali, & Debasis, 2009).
5.15. Neuroprotective
P. corylifolia has been the part of many Ayurvedic formulation that are used for the treatment of various central nervous system conditions such as for neurotropic activity and as central nervous system protective agent (Goel & Ojha, 2015). Based on such report, a study was conducted on the extract P. corylifolia L. seeds. The results displayed a significant protective effect against 3‐nitropropionic acid (3‐NP) induced cytotoxicity. The seed extract of P. corylifolia L. stimulated mitochondrial respiration with uncoupling and induced an increased bioenergetic reserve capacity. Moreover, cultured rat pheochromocytoma (PC12) cells pretreated with the extract of P. corylifolia L. seed significantly attenuated 3‐NP induced cell death, reduced ATP levels, and lowered the mitochondrial membrane potential. This study was conducted using MTS assay on PC12 cells with a dose range of 10 μM to 1 mM. A cell viability of 54.1% was observed with a dose of 25 μM 3‐NP for 3‐hr exposure, while the control showed 100% viability with the same dose. This study showed that P. corylifolia seed extracts may have potential usefulness as therapeutic agents against neurodegenerative diseases (Im, Chae, & jun Zhang, G, Lee, M‐Y., 2014).
In another study, it was revealed that IBC, a flavonoid from http://topics.sciencedirect.com/topics/page/Psoralea_corylifolia, has the ability to ameliorate the neuronal injury in brain diseases related to inflammation, and this was accomplished through inhibition of http://topics.sciencedirect.com/topics/page/Lipopolysaccharide induced http://topics.sciencedirect.com/topics/page/Cell_adhesion_molecule expression and leukocyte adhesion to brain endothelial cell by blocking http://topics.sciencedirect.com/topics/page/TLR_4 signaling (Lee et al., 2015).
5.16. Anti‐obesity
Various studies on animals showed that genistein has the ability to decrease body weight by decreasing food intake. It also reduced the fat pad weight and enhanced the apoptosis of adipose tissues. For example, one such study was conducted on ovariectomised mice. This well‐known trihydroxyflavone, Genistein, has also been isolated from P. corylifolia, exhibited a potential anti‐obesity and obesity related low grade inflammation activities through multiple mechanisms and cell signaling pathways. P. corylifolia extract possesses anti‐obesity and ant‐diabetic activity by its action on adipocyte life cycle, obesity‐related low‐grade inflammation, and oxidative stress (Behloul & Wu, 2013).
5.17. Effect on osteoblast
The well‐known Chinese herb P. corylifolia L. (Scurfpea fruit) has been employed for the treatment of bone fractures and also for joint diseases for thousands of years. P. corylifolia also improved the pathological bone condition, Hyperosteoidosis, by increasing the serum inorganic phosphate level at a dose of 30 mg/kg. It was observed previously that the extract markedly decreased osteoid volume and there has been improvement in bone calcification (Miura, Nishida, & Linuma, 1996). The flavonoids of corylin and bavachin has the osteoblastic proliferation stimulating activity in UMR106 cell line cultured in vitro (Cho et al., 2001; Miura et al., 1996; Wang, Li, & Jiang, 2001). P. corylifolia extract when administered orally to OVX rats, it was noted that there was a decrease in urinary calcium excretion and serum osteocalcin at a dose of 25–50 mg/kg body weight. The experiments in this study showed that the extract also increased the bone mineral density and bone formation at 50 mg/kg bw (Tsai et al., 2007). Such experiment, which were extended to 3 months, it can be concluded that P. corylifolia extract can be used at postmenopause state to prevent the osteoporosis. A further in depth study is still needed to make a therapeutic candidate. This study leads to the isolation of the compound responsible for the mentioned activity by Weng and colleagues. The isolated compounds from P. corylifolia, known as bakuchiol and bavachin, showed to prevent the estrogen deficiency by inducing the upregulation in primary human osteoblast differentiation (Weng et al., 2015).
An advanced herbal formula containing Psoraleae Fructus has previously showed promising bone protecting effect when tested in rats, and later on showed excellent results in women with osteoporosis. This herbal formula could efficiently have promoted the osteogenesis and suppress the adipogenesis in mesenchymal stem cells (Siu et al., 2013).
An HPLC analysis was carried out to study the important constituents in scurfpea fruit. In the method employed, the compounds were identified by comparing their retention indexes with standard substances and it resulted in the identification of 11 compounds. The biological methods such as MTT and ALP were employed to study the osteoblasts proliferation and differentiation activity. Bavachin and isobavachin showed significant cell proliferation by stimulation, the compound bakuchiol displayed higher effect to boost osteoblasts differentiation. From the results, it was hypothesized that prenyl group as a side chain might be responsible for the activity mentioned, because this structural component was found as a common entity among the compounds tested for activity (Li et al., 2014).
Previous surveys revealed bakuchiol and bavachin, the two important components of P. corylifolia L., showed osteoblastic property. Both the compounds displayed the prevention of bone loss caused by deficiency of estrogen in overiectomized animal models such as rats. One of in vitro study proposed that bavachin and bakuchiol caused the induction of primary human osteoblast differentiation by up regulating the http://topics.sciencedirect.com/topics/page/Wnt_signaling_pathway. This research proposes that bone‐protective role makes these two compounds a promising and safe estrogen supplement for the Estrogen replacement therapy (Weng et al., 2015).
5.18. Effect on cartilage
In one investigation, the compound psoralen with different concentrations (1, 10, and 100 μM) was tested on chondrocytes isolated from rats at 3‐ and 9‐day intervals. It was found that at the low dose concentration psoralen was safe toward chondrocytes; however, at higher dose suppression of chondrocytes, proliferation was observed. The compound psoralen also increased the synthesis of type II collagen at 100 μM, by 0.48‐fold on day 3 and 0.56‐fold on day 9. This was a detailed study, including MTS assay, Alcian blue colorimetry, western blotting, and qRT‐PCR. The compound psoralen was also tested for cytotoxicity and exhibited low cytotoxicity toward chondrocytes at a dose range of 1–10 μM. A much higher dose of 100 μM suppression of chondrocytes proliferation was observed. The compound psoralen caused the inhibition of the type I collagen in gene expression and also in protein synthesis. It was concluded that psoralen could be an important biological compound for triggering the cartilaginous cellular functions of chondrocytes (Xu et al., 2015).
5.19. Lymphangiogenisis inhibition
The extract of Psoraleae and the bioactivity guided fractioned compounds, including psoralen, p‐hydroxybenzaldehyde, psoracorylifol D, angelicin, BCN, isobavachalone, and bakuchiol hydroxybakuchiol, caused significant inhibition (in vitro) of the proliferation of temperature‐sensitive rat lymphatic endothelial (TR‐LE) cells. Among isolated compounds, psoracorylifol D, isobavachalone, BCN, hydroxybakuchiol, and bakuchiol inhibited proliferation and the formation of the capillary‐like tube of TR‐LE cells. In the Tube formation assay to analyze the cell cycle of the TR‐LE cells, the concentration of 10 μm of bakuchiol was used and incubated for 6–48 hr. After harvesting, the propidium iodide was used for staining. Other compounds tested showed selective activity. The compounds tested might be good candidates for development of anti‐neoplastic and anti‐metastatic agents against lymphangiogenesis (Jeong et al., 2013). This was the first kind of study on P. corylifolia.
5.20. Anti‐coagulant effect against snake venom
The plant P. corylifolia extract neutralized the coagulation of caused by Naja naja karachiensis snakebite when compared with the antidote used as a standard. The snake venom was experimented on human plasma (citrated) to evaluate its effect on activated partial thromboplastin time (aPTT), prothrombin time (PT), and thrombin time (TT). Snake venom (200 μg/ml) was found to delay PT (13 ± 0.57 to 23 ± 0.57 sec), aPTT (35 ± 1.52 to 48 ± 2.0 sec), and TT (13 ± 0.57 to 33 ± 0.57 sec). PT and TT were prolonged, and it suggested the occurrence of thrombin‐like or plasminogen activating enzymes (Asad et al., 2013; Asad et al., 2014). A further in depth study of this activity is still underway in the author's laboratory.
5.21. Immunomodulatory activity
The extract of seeds of P. corylifolia have been reported to have stimulant activity against natural killer cells when tested in mice. This study report that the extract also modulates the antibody dependent cellular toxicity. During tumor development, the seed extract also inhibited the antibody complement mediated cytotoxicity. The study was conducted on Balb/c male mice. The dose of 100 mg/kg was administered intraperitoneally. Blood collected from punctured heart and serum was separated to study the antibody complement‐mediated cytotoxicity. The natural killer cells were removed from spleen, and antibody‐dependent cellular cytotoxicity was assessed (Latha, Evans, Panikkar, & Jayavardhanan, 2000).
5.22. Anticancer activity
The isolated compounds from P. corylifolia including arylcoumarin and psoracoumestan showed strong anticancer potential by strongly inhibiting enzyme system of MAPK/ERK kinase phosphorylation. The mechanism underlying was apoptosis. Other compounds, including corylifol C and xanthoangelol, has been proved to be a strong inhibitor of protein kinase (inhibitory concentration 50% values for epidermal growth factor receptor: 1.1 and 4.4 × 10−6 μg/ml, respectively; Limper et al., 2013). This was a very important study from a pharmacological point of view. Psoralidin is an ER agonist also have revealed its activity in MCF‐7 cancer cells (isolated from human breast) by induction of gene pS2 activity. EC50 values of ERE‐reporter gene transcription activities by psoralidin in cell lines MCF‐7 was 1.85 μM (Liu et al., 2014). Psoralen also showed to invade the breast cancer cells MDA‐MB‐231BO in another in vitro study. It also stimulates osteoblast differentiation in an in vivo study. Psolaren when tested in Human Hepatocarcinoma cells, it showed its inhibitory activity by inducing the mechanism of Apoptosis (Guo, Liu, Ye, & Han, 2011; Jiang & Xiong, 2014; Khan, Iqbal, Ahmed, & Jamil, 2015; Mohammadparast, Rustaiee, Rasouli, Zardari, & Agrawal, 2014; Nehybova, Smarda, & Benes, 2014; Rajan, Tripathi, Variyar, & Pandey, 2014; Tang et al., 2011; Wong & Rabie, 2011; Yang et al., 2012). Similarly, two more compounds from the same specie identified as IBC and BCN attenuate Aβ42‐induced cell toxicity. The investigation was carried out on yeast two‐hybrid system (Chen et al., 2013).
During this study, eight compounds were tested, and among them IBC (3 μM) and BCN (30 μM) were proved to be active at non‐toxic concentrations. The results were confirmed by a standard ThT fluorescence method. The compound IBC also exhibited strong inhibitory effect on ThT fluorescence, and BCN showed more efficient activity, which was comparable with the reference. Moreover, the determination of IC50 of IBC and BCN in the ThT assay were about 25 and 45 μM, respectively.
Psoralidin in another study, caused the generation of reactive oxygen and it also thought to cause the inhibition of A549 cell proliferation. The method adopted was MTT assay. This study was designed to study the relationship of time and concentration used. The IC50 values obtained after 24‐, 48‐ and 72‐hr treatment were 19.2, 15.4, and 11.8 μM, respectively. (Hao, Zhang, Zhao, & Chen, 2014). Another evidence of anticancer activity came from the compound Bakuchiol that suppressed the testosterone induced cell proliferation and gene expression in LNCaP cells. This study was designed to explore the bakuchiol action in the androgen‐dependent PCa cell line (LNCaP). MTT assay and real‐time PCR method were employed. The IC50 of bakuchiol to androgen receptor was 8.87 × 104, which was similar to the standard flutamide (10.00 × 104; MIAO et al., 2013). These experiments showed that bakuchiol is a useful agent for drug development for androgen dependent PCa. The same compound, bakuchiol, has also showed a strong anticancer action against human lung adenocarcinoma cell line A549 and showed much better results than its analogue resveratrol. IC50 of bakuchiol at 72 hr was 9.58 ± 1.12 μmol/L, much lower than that of resveratrol (33.02 ± 2.35 μmol/L). Bakuchiol triggered the process of apoptosis to a higher level, compared with resveratrol. It was also noted that oxygen species related apoptosis also contribute the cytotoxic properties of bakuchiol, and therefore, it also supports the use of bakuchiol against non‐small‐cell lung cancer. (Chen et al., 2010).
Bakuchiol also showed very selective clearance activity in hepatic stellate cells by a mechanism involving apoptosis. This study showed that P. corylifolia also has anticancer activity in liver cancer (Chen et al., 2010; Yang, Paik, Cho, Cho, & Kim, 2008). In search of cytotoxic compounds from P. corylifolia, two isoflavnoids were isolated, named as corylifols D and E from the ethyl acetate extract. Similarly, psoralidin was also found active against stomach carcinoma cell lines (Teschke, Wolff, Frenzel, & Schulze, 2014; Yang et al., 1996). Bakuchiol is also an active ingredient of the dried ripe fruit of P. corylifolia that caused the cell proliferation and ERα expression in MCF‐7 cells in low doses, which suggest the in vitro estrogenic activity of bakuchiol. While in higher doses, bakuchiol caused the inhibition of growth of breast cancer cell, with a maximum anti‐proliferative action. Bakuchiol also induced ERβ expression and suppressed the ERα expression in MCF‐7 cells. It also caused the arrest of S phase arrest in MCF‐7 and MDA‐MB 231 cells. Additionally, bakuchiol caused the apoptotic cell induction and interrupted membrane potential in mitochondria of MCF‐7 cells via an intrinsic apoptotic pathway. The same compound when tested for in vivo anti‐breast cancer effect in zebrafish xenografts the reduction in MCF‐7 cell mass was significant Bavachalcone and Corylin from P. corylifolia has been investigated against UDP‐Glucuronosyltransferases and showed strong inhibition (Shan et al., 2014). It also inhibits lipopolysaccharide‐induced endothelial‐mesenchymal transition via down regulation of the NF‐κB‐SNAIL signaling pathway (Jung et al., 2015). In one investigation against DNA polymerase enzyme, P. corylifolia extract showed strong inhibitory activity.
Importance of isolated components from P. corylifolia is obvious from the fact that compounds such as psoralen is being produced from callus derived from various plant portions. In one experiment, it was found that in the in vitro conditions, cinnamic acid proved a very strong precursor of psoralen pathway that induced a maximum amount of psoralen (Mohammadparast, Rustaiee, Rasouli, Zardari, & Agrawal, 2014). The dried and ripe fruit of Psoralea Fructus showed an anticancer potential against human colorectal cancer. Psoralea suppressed the proliferation of human colorectal cancer cell lines, such as SW480 (IC50: 37.9 ± 1.6 μg/ml), HCT116 (IC50: 45.3 ± 1.2 μg/ ml), LoVo (IC50: 23.3 ± 1.9 μg/ml), and HT‐29 (IC50 value: 40.7 ± 1.5 μg/ml), by decreasing in the protein expression of cyclin D1 and CDK4. Succeeding experiments with many kinase inhibitors propose that PF‐mediated degradation of cyclin D1 and CDK4 is dependent on ERK1/2 and/or GSK3β (Park, Sung, Song, & Jeong, 2016).
5.23. DNA polymerase and topoisomerase II inhibitors
The crude extract of P. corylifolia with solvent ethanol was found a strong DNA polymerase inhibitor of DNA replication enzyme in an activity directed isolation assay that resulted in the purification of novel compound corylifolin, bakuchiol, neobavaisoflavone, and resveratrol. In a similar enzyme assay, some topoisomerase II inhibitors were also isolated, namely, daidzein and bakuchicin (Sun, Woo, Cassady, & Snapka, 2003).
5.24. Carboxylesterase Inhibitors
Many studies have been designed so far to study the behavior of P. corylifolia extract and isolated compounds on important enzyme. One of the investigations revealed that crude ethanol extract of P. corylifolia showed inhibitory activity on human carboxylesterase 2 (hCE2). Using LC‐DAD‐ESI‐MS/MS technique, five major hCE2 inhibitors were identified, including IBC, neobavaisoflavone, corylifol A, BCN, and bakuchiol (Li et al., 2015). The major ingredients from P. corylifolia including neobavaisoflavone, corylifolinin, coryfolin, psoralidin, corylin and BCN showed concentration‐dependent inhibitory effect on the human carboxylesterase 1 (hCE1) enzyme. The catalytic activity of hCE2 could be inhibited near completely at the final concentration of 12 μg/ml by the crude extract, and in same dose, the extract showed weak activity against hCE1. After that, activity was tested using different concentrations, and IC50 values were calculated for different compounds tested. The different inhibitory activity of compounds against hEC2 were Neobavaisoflavone (IC50 (μM) 6.39), IBC (IC50 (μM) 2.85), BCN (IC50 (μM) 4.31), Corylifol A (IC50 (μM) 0.87), and Bakuchiol (IC50 (μM) 7.28). These results revealed that the ethanol extract of P. corylifolia have inhibited the enzymatic activity of hCE2, suggesting to contain strong hEC2 inhibitory components. The compound corylifol A was more potent among the compounds tested with least IC50 values.
Additionally, the inhibition kinetics were calculated by Dixon and Lineweaver–Burk plots for the inhibitory activities toward CES1. The inhibition kinetic parameters (K i) were calculated to be 5.3, 9.4, 1.9, 0.7, and 0.5 μM for neobavaisoflavone, corylifolinin, coryfolin, corylin and BCN, respectively. It was concluded that this inhibition by the constituents might be responsible for the possible adverse effects of FP through the disrupting CES1‐catalyzed metabolism of endogenous substances and xenobiotics (Sun et al., 2016).
5.25. Inhibitory activity on CYP isoforms
Cytochrome P450 (CYP) is an assembly of heme‐containing enzymes fixed essentially in the lipid bilayer of the endoplasmic reticulum, and helps metabolize several drugs and carcinogens. The regulations of CYP activities are the main cause of drug–drug or herb–drug interactions. The compound Bakuchicin isolated from P. corylifolia has been studied for its modulation potency of CYP isoforms by using a mixture of probe substrates in pooled human liver microsomes (HLMs) and human recombinant cDNA‐expressed CYP. Bakuchicin powerfully inhibited CYP1A‐mediated phenacetin O‐deethylation with an IC50 value of 0.43 μM in HLMs. It was confirmed by human recombinant cDNA‐expressed CYP1A1 and CYP1A2 with a value of 0.11 and 0.32 μM, respectively. This study showed that bakuchicin was a strong and a selective competitive inhibitor of CYP1A1 and CYP1A2 in HLMs (Kim, Oh, Kim, Jeong, & Lee, 2016).
5.26. CVS effect
The plant P. corylifolia has been reported to be used in cardiac problems in traditional medicine (KR, 1975). Bavachalcone, a compound from P. corylifolia, has reported to have increased the luciferase activity of the Manganese superoxide dismutase (MnSOD) promoter and enhanced MnSOD mRNA and protein expressions. Further, it was found that bavachalcone suppressed the mitochondrial superoxide production in endothelial cells. On the other hand, bavachalcone (in concentration range of 1, 2.5, and 5 μmol/l for 24 hr) stimulated liver kinase B1 and AMPKα phosphorylation in a converse manner. mRNA interfering by using short hairpin RNA (shRNA) of AMP‐activated protein kinase (AMPK) inhibited bavachalcone‐induced MnSOD expression. Moreover, AMPK reduced by shRNA–AMPK reversed the inhibition of bavachalcone on mitochondrial superoxide production in endothelial cells. These results showed that bavachalcone could shield the endothelial function by enhancing the AMPK activity and MnSOD expression and lowering the mitochondrial oxidative stress, which is considered to be the key etiological reason in cardiovascular diseases (Dang et al., 2015).
5.27. Photosensitization and toxicity
Many herbal remedies, including essential oils, contain psoralen, and methoxy psoralen are reported to cause photosensitivity in the form of erythema and blisters (Koh & Ong, 1999). P. corylifolia has been the part of many herbal remedy used to treat vitiligo and patients using such treatments in the form of creams are also reported to experienced erythema when exposed to sunlight (Maurice & Cream, 1989). P. corylifolia is frequently indicated for vitiligo in India, and it contains psoralen, isopsoralen, and psoralidin. Its extracts when tested on guinea pig skin have been stated to possess potent sensitizing action (Pathak, Daniels, & Fitzpatrick, 1963). Another study reported the gonadal toxicity. Although the ethanol extract of P. corylifolia seeds are proposed to use in processed food preservation, but it showed toxicity when tested on male and female rats. The period of the experiment was 90 days, and various concentrations were administered were 0%, 0.375%, 0.75%, 1.5% or 3.0%. Histopathological investigation showed atrophy of seminiferous tubules, leydig cells, seminal vesicles, and prostate cells were observed in male rats administered with the 1.5% and 3.0%. With the same concentrations, the female rats showed a reduced number of corpora lutea in the ovaries and less frequent endometrial glands in the uterus. It was suggested that the extract caused the hypothalamus‐pituitary‐gonadal axis (Takizawa et al., 2002).
In one investigation, P. corylifolia and its natural compounds (bavachin, corylifol A, neobavaisoflavone, IBC, and BCN) were evaluated for its potential toxicity, and results showed it had a potent inhibitory effect against human UDP‐glucuronosyltransferase 1A1 (UGT1A1), and it is considered as main stimulant for P. corylifolia related toxicity, including hepatic injury and raised bilirubin levels (Wang et al., 2015). In another study P. corylifolia extract and fractionated compounds such as psoralen and isopsoralen were incubated with the recombinant CYP3A4 enzyme or differentiated HuH‐7 and HepaRG cells. P. corylifolia extract, psoralen, and isopsoralen caused the inhibition of concentration CYP3A4 activity in a dose dependent manner with different potency in vitro. It was also noted that none of the sample tested showed any toxicity (Liu & Flynn, 2015).
5.28. Industrial application of isolated compounds
Several compounds isolated from P. corylifolia have many industrial applications and are available commercially. Some of the examples are the following: the compound Angelicin (CAS 523‐50‐2) is available as antifungal compound (Sardari, Amin, Sekhon, Micetich, & Daneshtalab, 2000). The compound Psoralen has been used as photochemical probe in studies of DNA mutation and repair mechanisms (Zarmouh, Eyunni, & Soliman, 2017). Methoxsalen (8‐Methoxypsoralen; CAS 298‐81‐7) has been known as a potent suicide inhibitor of cytochrome P‐450 (Toyooka & Ibuki, 2009). Bakuchiol (CAS 10309‐37‐2) is available as a PTP1B and DNA polymerase inhibitor (Choi et al., 2015). Bavachin (CAS 19879‐32‐4) is available as a weak antioxidant that stimulates bone formation (Jing et al., 2017). Corylifol C and, to a lesser extent, xanthoangelol are potent protein kinase inhibitors (inhibitory concentration 50% values for epidermal growth factor receptor; Limper et al., 2013). A well‐known compound Daidzin (CAS 552‐66‐9) isolated from many plant species is a potent inhibitor of human mitochondrial aldehye dehydrogenase that demonstrates chemopreventitive activities (Keung & Vallee, 1993). The compound Genistein (CAS 446‐72‐0) is available as a highly specific inhibitor of protein tyrosine kinase (Zarmouh, Messeha, Elshami, & Soliman, 2016). These examples clearly show the importance of bioactive compounds isolated from P. corylifolia, an important traditional medicinal plant.
6. CONCLUSION
The above‐mentioned summary about P. corylifolia clearly showed that it is a very important plant from ethnobotanical, pharmacological, and chemical point of view. To date, more or less hundreds compounds have been separated from P. corylifolia, which was also mentioned by Zhang et al., (2016). We here presented the latest version of scientific literature on P. corylifolia, including the research work carried out in the recent years. P. corylifolia contains a wide variety of chemical constituents belonging to various groups, including flavonoids, coumarins, and meroterpenes, which are more dominant. P. corylifolia L. (Fabacese) is a fortified source of biological active compounds, which gives the plant with great value for its use in pharmaceuticals, health, and body‐care products. Sehrawat and colleagues reported that P. corylifolia is a rare and endangered herbaceous medicinal plant. Because most of the plant materials are collected from naturally occurring stands and are therefore being depleted rapidly and there is a possibility of extinction. Therefore, it is suggested that there is a need for in vitro propagation of this species (Sehrawat et al., 2014).
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
AUTHORS CONTRIBUTIONS
Fiaz Alam contributed in the Correspondence, Conception, design of the work, and Data collection of the study. Gul Nawaz Khan contributed in the Data collection, Data analysis and interpretation, and Chemical structure design of the study. Mohammad Hasham Hussan Bin Asad contributed in Drafting the article, Critical revision of the article, and Final approval of the version to be published
Alam F, Khan GN, Asad MHHB. Psoralea corylifolia L: Ethnobotanical, biological, and chemical aspects: A review. Phytotherapy Research. 2018;32:597–615. 10.1002/ptr.6006
REFERENCES
- Acharya, M. , Singh, T. , & Patgiri, B. (2015). Anti microbial activity of different dosage forms of Bakuchi (Psoralea corylifolia Linn.) taila, an Ayurvedic formulation. International Journal of Ayurvedic Medicine, 6(3). [Google Scholar]
- Agharkar, S. (1991). Medicinal plants of Bombay presidency. Medicinal plants of Bombay Presidency. [Google Scholar]
- Aiyaz, M. , Divakara, S. T. , Chandranayaka, S. , & Niranjana, S. R. (2015). Efficacy of seed hydropriming with phytoextracts on plant growth promotion and antifungal activity in maize. International Journal of Pest Management, 61(2), 153–160. [Google Scholar]
- Ali, J. , Akhtar, N. , Sultana, Y. , Baboota, S. , & Ahuja, A. (2008). Antipsoriatic microemulsion gel formulations for topical drug delivery of babchi oil (Psoralea corylifolia). Methods and Findings in Experimental and Clinical Pharmacology, 30(4), 277–285. [DOI] [PubMed] [Google Scholar]
- Ali, S. T. , Anwar, H. , Ali, S. K. , Perwaiz, S. , Mujahid, T. Y. , Wahab, A. , & Subhan, S. A. (2015). Phytochemical studies and antimicrobial screening of non/less‐polar fraction of Psoralea corylifolia by using GC‐MS. Journal of Basic & Applied Sciences, 11, 159. [Google Scholar]
- Anderson, T. F. , & Voorhees, J. J. (1980). Psoralen photochemotherapy of cutaneous disorders. Annual Review of Pharmacology and Toxicology, 20(1), 235–257. [DOI] [PubMed] [Google Scholar]
- Asad, B. , Hassan, M. H. , Choudary, B. A. , Asad, A. F. , Muratza, G. , & Hussain, I . (2014). Compensatory effects of medicinal plants of Pakistan upon prolongation of coagulation assays induced by Naja Naja Karachiensis bite. Current Science (00113891) 106(6). [Google Scholar]
- Asad, M. , Razi, M. , Sabih, D. , Saqib, Q. , Nasim, S. , Murtaza, G. , & Hussain, I. (2013). Anti‐venom potential of Pakistani medicinal plants: Inhibition of anticoagulation activity of Naja naja karachiensis toxin. Cur. Sci, 105(10), 1419–1424. [Google Scholar]
- Banerji, A. , & Chintalwar, G. J. (1983). Biosynthesis of bakuchiol, a meroterpene from Psoralea corylifolia. Phytochemistry, 22(9), 1945–1947. [PubMed] [Google Scholar]
- Baquar, S. R. (1989). Medicinal and poisonous plants of Pakistan. Karachi: Printas Karachi 506p.‐illus.. En Icones. Geog, 6. [Google Scholar]
- Behloul, N. , & Wu, G. (2013). Genistein: A promising therapeutic agent for obesity and diabetes treatment. European Journal of Pharmacology, 698(1), 31–38. [DOI] [PubMed] [Google Scholar]
- Brands, S. J. (1989. –2005). Systema Naturae. The Netherlands, Amsterdam. [Google Scholar]
- Chanda, S. , Kaneria, M. , & Nair, R. (2011). Antibacterial activity of Psoralea corylifolia L. seed and aerial parts with various extraction methods. Research Journal of Microbiology, 6, 124–131. [Google Scholar]
- Chaudhuri, R. K. (2015). Bakuchiol: A Retinol‐Like Functional Compound, Modulating Multiple Retinol and Non‐Retinol Targets. Boca Raton: Taylor and Francis. [Google Scholar]
- Chen, J. , Chen, C. , Lai, R. , Chen, H. , Kuo, W. , & Liao, T. (2011). New isoflavones and bioactive constituents from the fruits of Psoralea corylifolia. Planta Medica, 77(12), PG39. [Google Scholar]
- Chen, X. , Yang, Y. , & Zhang, Y. (2013). Isobavachalcone and bavachinin from Psoraleae Fructus modulate Aβ42 aggregation process through different mechanisms in vitro. FEBS Letters, 587(18), 2930–2935. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , Jin, K. , Gao, L. , Lou, G. , Jin, Y. , Yu, Y. , & Lou, Y. (2010). Anti‐tumor effects of bakuchiol, an analogue of resveratrol, on human lung adenocarcinoma A549 cell line. European Journal of Pharmacology, 643(2), 170–179. [DOI] [PubMed] [Google Scholar]
- Cho, H. , Jun, J. Y. , Song, E. K. , Kang, K. H. , Baek, H. Y. , Ko, Y. S. , & Kim, Y. C. (2001). Bakuchiol: A hepatoprotective compound of Psoralea corylifolia on tacrine‐induced cytotoxicity in Hep G2 cells. Planta Medica, 67(8), 750–751. [DOI] [PubMed] [Google Scholar]
- Choi, J. M. , Oh, S. J. , Lee, J.‐Y. , Jeon, J. S. , Ryu, C. S. , Kim, Y.‐M. , … Kim, S. K. (2015). Prediction of drug‐induced liver injury in HepG2 cells cultured with human liver microsomes. Chemical Research in Toxicology, 28(5), 872–885. [DOI] [PubMed] [Google Scholar]
- Chopra, B. , Dhingra, A. K. , & Dhar, K. L. (2013). Psoralea corylifolia L. (Buguchi)—Folklore to modern evidence: Review. Fitoterapia, 90, 44–56. [DOI] [PubMed] [Google Scholar]
- Chopra, R. , Chopra, I. , Handa, K. , & Kapur, L. (1958). Indigenous drugs of India (2nd Edn.) UN Dhar and sons. Calcutta, India, 426. [Google Scholar]
- Chopra, R. N. , & Chopra, I. (1933). Indigenous drugs of India. Academic publishers. [Google Scholar]
- Cseke, L. J. , Kirakosyan, A. , Kaufman, P. B. , Warber, S. , Duke, J. A. , & Brielmann, H. L. (2006). Natural products from plants. CRC Press. [Google Scholar]
- Cui, Y. , Taniguchi, S. , Kuroda, T. , & Hatano, T. (2015). Constituents of Psoralea corylifolia fruits and their effects on methicillin‐resistant Staphylococcus aureus. Molecules, 20(7), 12500–12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulska, P. , Thakur, S. D. , Foster, B. C. , Scott, I. M. , Leduc, R. I. , Arnason, J. T. , & Dillon, J. A. (2011). Extracts of Canadian first nations medicinal plants, used as natural products, inhibit neisseria gonorrhoeae isolates with different antibiotic resistance profiles. Sexually Transmitted Diseases, 38(7), 667–671. [DOI] [PubMed] [Google Scholar]
- Daiquan, L. (2000. –2006). Chinese Medicine. China: Shanghai Scientific and Technical Publishers. [Google Scholar]
- Dang, Y. , Ling, S. , Duan, J. , Ma, J. , Ni, R. , & Xu, J.‐W. (2015). Bavachalcone‐induced manganese superoxide dismutase expression through the AMP‐activated protein kinase pathway in human endothelial cells. Pharmacology, 95(3–4), 105–110. [DOI] [PubMed] [Google Scholar]
- Deshaprabhu, S. (1966). The Wealth of India: Raw Materials, Vol. VII; N‐Pe. The Wealth of India: Raw Materials, Vol. VII; N‐Pe. [Google Scholar]
- Dua, V. K. , Kumar, A. , Pandey, A. C. , & Kumar, S. (2013a). Insecticidal and genotoxic activity of Psoralea corylifolia Linn.(Fabaceae) against Culex quinquefasciatus say, 1823. Parasites & Vectors, 6, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua, V. K. , Kumar, A. , Pandey, A. C. , & Kumar, S. (2013b). Insecticidal and genotoxic activity of Psoralea corylifolia Linn.(Fabaceae) against Culex quinquefasciatus say, 1823. Parasites & Vectors, 6(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani, M. S. , Bahramsoltani, R. , Farzaei, M. H. , Abdollahi, M. , & Rahimi, R. (2015). Plant‐derived natural medicines for the management of depression: An overview of mechanisms of action. Reviews in the Neurosciences, 26(3), 305–321. [DOI] [PubMed] [Google Scholar]
- Ghosh, D. , Bera, T. , Chatterjee, K. , Ali, K. , & Debasis, D. (2009). Antidiabetic and antioxidative effects of aqueous extract of seed of Psoralea corylifolia (somraji) and seed of Trigonella foenum‐graecum L.,(methi) in separate and composite manner in streptozotocin‐induced diabetic male albino rat. Intl J Pharm Res Dev, 7(9), 1–10. [Google Scholar]
- Gidwani, B. , Alaspure, R. , Duragkar, N. , Singh, V. , Rao, S. P. , & Shukla, S. (2010). Evaluation of a novel herbal formulation in the treatment of eczema with Psoralea corylifolia. Iranian Journal of Dermatology, 13, 122–127. [Google Scholar]
- Goel, S. , & Ojha, N. K. (2015). Ashtang Ghrita: A noble Ayurveda drug for central nervous system. Journal of Ayurveda and Holistic Medicine (JAHM), 3(2), 18–24. [Google Scholar]
- Guo, B. , Liu, S. , Ye, Y. , & Han, X. (2011). Inhibitory effects of osthole, psoralen and aconitine on invasive activities of breast cancer MDA‐MB‐231BO cell line and the mechanisms. Zhong xi yi jie he xue bao= Journal of Chinese Integrative Medicine, 9(10), 1110–1117. [DOI] [PubMed] [Google Scholar]
- Gupta, A. K . (2003). Quality standards of Indian medicinal plants. Volume 1. Quality standards of Indian medicinal plants. Volume 1.
- Gupta, G. , Dhar, K. , & Atal, C. (1978). Corylinal: A new isoflavone from seeds of Psoralea corylifolia. Phytochemistry, 17(1), 164. [Google Scholar]
- Gupta, M. , Gupta, A. , & Gupta, S . (2013). Phytochemical analysis of methanol extracts of Psoralea Corylifolia.
- Hao, W. , Zhang, X. , Zhao, W. , & Chen, X. (2014). Psoralidin induces autophagy through ROS generation which inhibits the proliferation of human lung cancer A549 cells. PeerJ, 2, e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosamani, P. , Lakshman, H. , & Sandeepkumar, K. (2012). Antimicrobial activity of leaf extract of Psoralea Corylifolia l. Life Science Leaflets. [Google Scholar]
- Hsu, Y.‐T. , Wu, C.‐J. , Chen, J.‐M. , Yang, Y.‐C. , & Wang, S.‐Y. (2001). The presence of three isoflavonoid compounds in Psoralea corylifolia. Journal of Chromatographic Science, 39(10), 441–444. [DOI] [PubMed] [Google Scholar]
- Huang, K. C. (1998). The pharmacology of Chinese herbs. CRC press. [Google Scholar]
- Im, A.‐R. , Chae, S.‐W. , jun Zhang, G. , & Lee, M.‐Y. (2014). Neuroprotective effects of Psoralea corylifolia Linn seed extracts on mitochondrial dysfunction induced by 3‐nitropropionic acid. BMC Complementary and Alternative Medicine, 14(1), 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura, J. , Dohi, T. , Tanaka, H. , Odani, T. , & Kubo, M. (1989). Cytotoxicity of corylifoliae fructus. II. Cytotoxicity of bakuchiol and the analogues. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan, 109(12), 962–965. [DOI] [PubMed] [Google Scholar]
- Jeong, D. , Watari, K. , Shirouzu, T. , Ono, M. , Koizumi, K. , Saiki, I. , … Miyamoto, T. (2013). Studies on lymphangiogenesis inhibitors from Korean and Japanese crude drugs. Biological and Pharmaceutical Bulletin, 36(1), 152–157. [DOI] [PubMed] [Google Scholar]
- Jiang, Z. , & Xiong, J. (2014). Induction of apoptosis in human Hepatocarcinoma SMMC‐7721 cells in vitro by Psoralen from Psoralea corylifolia. Cell Biochemistry and Biophysics, 70(2), 1075–1081. [DOI] [PubMed] [Google Scholar]
- Jing, H. , Wang, S. , Wang, M. , Fu, W. , Zhang, C. , & Xu, D. (2017). Isobavachalcone attenuates MPTP‐induced Parkinson's disease in mice by inhibition of microglial activation through NF‐κB pathway. PLoS One, 12(1), e0169560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, S. G. (2000). Medicinal plants. Oxford and IBH publishing. [Google Scholar]
- Joy, P. , Thomas, J. , Mathew, S. , & Skaria, B. P. (1998). Medicinal plants. Tropical horticulture, 2, 449–632. [Google Scholar]
- Judd, W. S. , Campbell, C. S. , Kellogg, E. A. , Stevens, P. F. , & Donoghue, M. J. (1999). Plant systematics: A phylogenetic approach. Ecologia Mediterranea, 25(2), 215. [Google Scholar]
- Jung, B. , Jang, E. H. , Hong, D. , Cho, I. H. , Park, M.‐J. , & Kim, J.‐H. (2015). Aqueous extract of Psoralea corylifolia L. inhibits lipopolysaccharide‐induced endothelial‐mesenchymal transition via downregulation of the NF‐κB‐SNAIL signaling pathway. Oncology Reports, 34(4), 2040–2046. [DOI] [PubMed] [Google Scholar]
- Kapoor, L. (2000). Handbook of Ayurvedic medicinal plants: Herbal reference library. CRC press. [Google Scholar]
- Katsura, H. , Tsukiyama, R.‐I. , Suzuki, A. , & Kobayashi, M. (2001). In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrobial Agents and Chemotherapy, 45(11), 3009–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung, W.‐M. , & Vallee, B. L. (1993). Daidzin: A potent, selective inhibitor of human mitochondrial aldehyde dehydrogenase. Proceedings of the National Academy of Sciences, 90(4), 1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, S. , Iqbal, T. , Ahmed, N. , & Jamil, A. (2015). Antioxidant, hemolytic and mutagenic potential of Psoralea corylifolia. JAPS, Journal of Animal and Plant Sciences, 25(5), 1451–1456. [Google Scholar]
- Khare, C. (2004). Encyclopedia of Indian medicinal plants. New York: Springes‐Verlag Berlin Heidelberg. [Google Scholar]
- Khare, C. P. (2008). Indian medicinal plants: an illustrated dictionary. Springer Science & Business Media. [Google Scholar]
- Khatune, N. , Islam, M. , Rahman, M. , Baki, M. , Sadik, G. , & Haque, M. (2002). Pesticidal activity of a novel coumestan derivative isolated from Psoralea corylifolia Linn. against Tribolium casteneum herbst. adults and larvae (Coleptera: Tenebrionidae). Journal of Agronomy, 1, 112–115. [Google Scholar]
- Khatune, N. A. , Islam, M. E. , Haque, M. E. , Khondkar, P. , & Rahman, M. M. (2004). Antibacterial compounds from the seeds of Psoralea corylifolia. Fitoterapia, 75(2), 228–230. [DOI] [PubMed] [Google Scholar]
- Kim, D. W. , Seo, K. H. , Curtis‐Long, M. J. , Oh, K. Y. , Oh, J.‐W. , Cho, J. K. , … Park, K. H. (2014). Phenolic phytochemical displaying SARS‐CoV papain‐like protease inhibition from the seeds of Psoralea corylifolia. Journal of Enzyme Inhibition and Medicinal Chemistry, 29(1), 59–63. [DOI] [PubMed] [Google Scholar]
- Kim, K.‐A. , Shim, S. H. , Ahn, H. R. , & Jung, S. H. (2013). Protective effects of the compounds isolated from the seed of Psoralea corylifolia on oxidative stress‐induced retinal damage. Toxicology and Applied Pharmacology, 269(2), 109–120. [DOI] [PubMed] [Google Scholar]
- Kim, S.‐I. , Jeong, M.‐J. , Ahn, Y.‐S. , Kim, A. , Kim, M.‐N. , & Lim, D.‐S. (2015). Antimicrobial effect of commercially available mouth rinsing solutions and natural herbal extracts on Streptococcus mutans. Journal of Dental Hygiene Science, 15(3), 308–317. [Google Scholar]
- Kim, S. J. , Oh, H. C. , Kim, Y.‐C. , Jeong, G.‐S. , & Lee, S . (2016). Selective Inhibition of Bakuchicin Isolated from Psoralea corylifolia on CYP1A in Human Liver Microsomes. Evidence‐Based Complementary and Alternative Medicine 2016. [DOI] [PMC free article] [PubMed]
- Koh, D. , & Ong, C. (1999). Phytophotodermatitis due to the application of Citrus hystrix as a folk remedy. British Journal of Dermatology, 140, 737–738. [DOI] [PubMed] [Google Scholar]
- KR, K. (1975). Indian Medicinal Plants (2nd ed.). Dehradun, India: International Book Distributors. [Google Scholar]
- Krishnamurthi, A. (1969). The wealth of India: Raw materials: Vol. VIII. Ph‐Re. The Wealth of India: Raw Materials: Vol. VIII. Ph‐Re.
- Kubo, M. , Dohi, T. , Odani, T. , Tanaka, H. , & Iwamura, J. (1989). Cytotoxicity of Corylifoliae fructus. I. Isolation of the effective compound and the cytotoxicity. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan, 109(12), 926–931. [DOI] [PubMed] [Google Scholar]
- Latha, P. G. , Evans, D. A. , Panikkar, K. R. , & Jayavardhanan, K. K. (2000). Immunomodulatory and antitumour properties of Psoralea corylifolia seeds. Fitoterapia, 71(3), 223–231. [DOI] [PubMed] [Google Scholar]
- Lau, K.‐M. , Fu, L.‐H. , Cheng, L. , Wong, C.‐W. , Wong, Y.‐L. , Lau, C.‐P. , … Leung, P. C. (2010). Two antifungal components isolated from Fructus Psoraleae and folium eucalypti Globuli by bioassay‐guided purification. The American Journal of Chinese Medicine, 38(05), 1005–1014. [DOI] [PubMed] [Google Scholar]
- Lau, K.‐M. , Wong, J. H. , Wu, Y.‐O. , Cheng, L. , Wong, C.‐W. , To, M‐H , … Lau, C. B. (2014). Anti‐dermatophytic activity of bakuchiol: In vitro mechanistic studies and in vivo tinea pedis‐inhibiting activity in a guinea pig model. Phytomedicine, 21(7), 942–945. [DOI] [PubMed] [Google Scholar]
- Lee, K. M. , Kim, J. M. , Baik, E. J. , Ryu, J. H. , & Lee, S. H. (2015). Isobavachalcone attenuates lipopolysaccharide‐induced ICAM‐1 expression in brain endothelial cells through blockade of toll‐like receptor 4 signaling pathways. European Journal of Pharmacology, 754, 11–18. [DOI] [PubMed] [Google Scholar]
- Li, L. , Chen, X. , Liu, C. C. , Lee, L. S. , Man, C. , & Cheng, S. H. (2016). Phytoestrogen Bakuchiol exhibits in vitro and in vivo anti‐breast cancer effects by inducing S phase arrest and apoptosis. Frontiers in Pharmacology, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. D. , Yan, C. P. , Wu, Y. , Weng, Z. B. , Yin, F. Z. , Yang, G. M. , … Chen, Z. P. (2014). Osteoblasts proliferation and differentiation stimulating activities of the main components of Fructus Psoraleae corylifoliae. Phytomedicine, 21(4), 400–405. [DOI] [PubMed] [Google Scholar]
- Li, Y.‐G. , Hou, J. , Li, S.‐Y. , Lv, X. , Ning, J. , Wang, P. , … Yang, L. (2015). Fructus Psoraleae contains natural compounds with potent inhibitory effects towards human carboxylesterase 2. Fitoterapia, 101, 99–106. [DOI] [PubMed] [Google Scholar]
- Li, Z. J. , Abulizi, A. , Zhao, G. L. , Wang, T. , Zhou, F. , Jiang, Z. Z. , … Zhang, L. Y. (2017). Bakuchiol contributes to the hepatotoxicity of Psoralea corylifolia in rats. Phytotherapy Research, 31(8), 1265–1272. [DOI] [PubMed] [Google Scholar]
- Lim, S.‐H. , Ha, T.‐Y. , Ahn, J. , & Kim, S. (2011). Estrogenic activities of Psoralea corylifolia L. seed extracts and main constituents. Phytomedicine, 18(5), 425–430. [DOI] [PubMed] [Google Scholar]
- Limper, C. , Wang, Y. , Ruhl, S. , Wang, Z. , Lou, Y. , Totzke, F. , … Wätjen, W. (2013). Compounds isolated from Psoralea corylifolia seeds inhibit protein kinase activity and induce apoptotic cell death in mammalian cells. Journal of Pharmacy and Pharmacology, 65(9), 1393–1408. [DOI] [PubMed] [Google Scholar]
- Lin, C.‐F. , Huang, Y.‐L. , Chien, M.‐Y. , Sheu, S.‐J. , & Chen, C.‐C. (2007). Analysis of bakuchiol, psoralen and angelicin in crude drugs and commercial concentrated products of Fructus Psoraleae. 藥物食品分析, 15(4), 433–437. [Google Scholar]
- Lin, Y.‐L. , & Kuo, Y.‐H. (1992). Two new benzofuran derivatives, corylifonol and isocorylifonol from the seeds of Psoralea corylifolia. Heterocycles, 34(8), 1555–1564. [Google Scholar]
- Ling, F. , Lu, C. , Tu, X. , Yi, Y. , Huang, A. , Zhang, Q. , & Wang, G. (2013). Antiprotozoal screening of traditional medicinal plants: Evaluation of crude extract of Psoralea corylifolia against Ichthyophthirius multifiliis in goldfish. Parasitology Research, 112(6), 2331–2340. [DOI] [PubMed] [Google Scholar]
- Liu, K. C.‐S. C. , Yang, S.‐L. , Roberts, M. , Elford, B. , & Phillipson, J. (1992). Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Reports, 11(12), 637–640. [DOI] [PubMed] [Google Scholar]
- Liu, R. , Li, A. , Sun, A. , & Kong, L. (2004). Preparative isolation and purification of psoralen and isopsoralen from Psoralea corylifolia by high‐speed counter‐current chromatography. Journal of Chromatography A, 1057(1), 225–228. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Nam, J.‐W. , Song, Y. S. , Viswanath, A. N. I. , Pae, A. N. , Kil, Y.‐S. , … Chang, M. (2014). Psoralidin, a coumestan analogue, as a novel potent estrogen receptor signaling molecule isolated from Psoralea corylifolia. Bioorganic & Medicinal Chemistry Letters, 24(5), 1403–1406. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , & Flynn, T. J. (2015). CYP3A4 inhibition by Psoralea corylifolia and its major components in human recombinant enzyme, differentiated human hepatoma HuH‐7 and HepaRG cells. Toxicology Reports, 2, 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch, J. M. (1889). Useful plants of the genus Psoralea. The American Journal of Pharmacy, 61(7). [Google Scholar]
- Martinvalet, D. , Dykxhoorn, D. M. , Ferrini, R. , & Lieberman, J. (2008). Granzyme a cleaves a mitochondrial complex I protein to initiate caspase‐independent cell death. Cell, 133(4), 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice, P. , & Cream, J. (1989). The dangers of herbalism. BMJ: British Medical Journal, 299(6709), 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya, S. K. , Singh, A. K. , & Seth, A. (2014). Potential medicinal plants for lymphatic FILARIASIS: A review. Journal of Critical Reviews, 2(1). [Google Scholar]
- Mendam, K. , Kavitha, B. , & Naik, S. J. K. (2015). Natural sources used for treatment and prevention of FILARIASIS.
- Miao, L. , Ma, S.‐w. , Fan, G.‐w. , Wang, H. , Wang, Y.‐f. , & Chai, L.‐j. (2013). Bakuchiol inhibits the androgen induced‐proliferation of prostate cancer cell line LNCaP through suppression of AR transcription activity. Tianjin Journal of Traditional Chinese Medicine, 5, 012. [Google Scholar]
- Miura, H. , & Nishida, H. (1996). Effect of crude fractions of Psoralea corylifolia seed extract on bone calcification. Planta Medica, 62(02), 150–153. [DOI] [PubMed] [Google Scholar]
- Miura, H. , Nishida, H. , & Linuma, M. (1996). Effect of crude fractions of psoralea corylifolia seed extract on bone calcification. Planta Medica, 62(2), 150–153. [DOI] [PubMed] [Google Scholar]
- Mohammadparast, B. , Rustaiee, A. R. , Rasouli, M. , Zardari, S. , & Agrawal, V. (2014). In vitro enhancement of psoralen as an important anticancer compound in Psoralea corylifolia through precursor feeding. Pharmaceutical Biology, 53(5), 735–738. [DOI] [PubMed] [Google Scholar]
- Nadkarni, K. (1976). Indian Materia Medica with Ayurvedic, Unani products and home remedies. Vol.‐I. Popular Prakashan, Mumbai.
- Nadkarni, K. , & Nadkarni, A . (1976). Indian Materia Medica, Vol 1, Mumbai, M/S Popular Prakasan Pvt. Ltd.
- Nehybova, T. , Smarda, J. , & Benes, P. (2014). Plant Coumestans: Recent advances and future perspectives in cancer therapy. Anti‐Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry‐Anti‐Cancer Agents), 14(10), 1351–1362. [DOI] [PubMed] [Google Scholar]
- Newton, S. M. , Lau, C. , Gurcha, S. S. , Besra, G. S. , & Wright, C. W. (2002). The evaluation of forty‐three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. Journal of Ethnopharmacology, 79(1), 57–67. [DOI] [PubMed] [Google Scholar]
- Okhale, S. E. , Amanabo, M. O. , Jegede, I. A. , Egharevba, H. O. , Muazzam, I. W. , & Kunle, O. F. (2010). Phytochemical and Pharmacognostic investigation of Antidiabetic Scoparia dulcis Linn Scrophulariaceae whole plant grown in Nigeria. Research, 2(6), 7–16. [Google Scholar]
- Panda, H. (1999). Herbs cultivation and medicinal uses. National Institute of Industrial Re. [Google Scholar]
- Park, E.‐J. , Zhao, Y.‐Z. , Kim, Y.‐C. , & Sohn, D. H. (2005). Protective effect of (S)‐bakuchiol from Psoralea corylifolia on rat liver injury in vitro and in vivo. Planta Medica, 71(06), 508–513. [DOI] [PubMed] [Google Scholar]
- Park, G. H. , Sung, J. H. , Song, H. M. , & Jeong, J. B. (2016). Anti‐cancer activity of Psoralea fructus through the downregulation of cyclin D1 and CDK4 in human colorectal cancer cells. BMC Complementary and Alternative Medicine, 16(1), 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak, M. , Daniels, F. Jr. , & Fitzpatrick, T. (1963). The presently known distribution of Furocoumarins (Psoralens) in plants. Journal of Occupational and Environmental Medicine, 5(2), 111. [Google Scholar]
- Pavela, R. (2009). Larvicidal property of essential oils against Culex quinquefasciatus say (Diptera: Culicidae). Industrial Crops and Products, 30(2), 311–315. [Google Scholar]
- Pole, S. (2006). Ayurvedic medicine: the principles of traditional practice. Elsevier Health Sciences. [Google Scholar]
- Prasad, N. R. , Anandi, C. , Balasubramanian, S. , & Pugalendi, K. (2004). Antidermatophytic activity of extracts from Psoralea corylifolia (Fabaceae) correlated with the presence of a flavonoid compound. Journal of Ethnopharmacology, 91(1), 21–24. [DOI] [PubMed] [Google Scholar]
- Qamaruddin, A. , Parveen, N. , Khan, N. U. , & Singhal, K. C. (2002). Potential antifilarial activity of the leaves and seeds extracts of Psoralea corylifolia on cattle filarial parasite Setaria cervi. Journal of Ethnopharmacology, 82(1), 23–28. [DOI] [PubMed] [Google Scholar]
- Qiao, C. F. , Han, Q. B. , Song, J. Z. , Mo, S. F. , Kong, L. D. , Kung, H. F. , & Xu, H.‐X. (2007). Chemical fingerprint and quantitative analysis of Fructus Psoraleae by high‐performance liquid chromatography. Journal of Separation Science, 30(6), 813–818. [DOI] [PubMed] [Google Scholar]
- Rajan, V. , Tripathi, J. , Variyar, P. , & Pandey, B. N. (2014). Mechanism of cytotoxicity by Psoralea corylifolia extract in human breast carcinoma cells. Journal of Environmental Pathology, Toxicology and Oncology, 33(3). [DOI] [PubMed] [Google Scholar]
- Rizvi, M. , Saeed, A. , & Zubairy, N. (2007). Medicinal plants history, cultivation and uses. Karachi: Hamdard Institute of Advance Studies and Research, 85–87. [Google Scholar]
- Ruan, B. , Kong, L.‐Y. , Takaya, Y. , & Niwa, M. (2007). Studies on the chemical constituents of Psoralea corylifolia L. Journal of Asian Natural Products Research, 9(1), 41–44. [DOI] [PubMed] [Google Scholar]
- Ryu, Y. H. , Kim, D. G. , Yeon, I. K. , Huh, C. S. , Ryu, J. A. , Jo, W. S. , … Lee, Y. S. (2015). Screening for inhibition activity of plant extracts on microorganism contaminating in cosmetics. Korean Journal of Medicinal Crop Science, 23(1), 57–76. [Google Scholar]
- Sah, P. , Agarwal, D. , & Garg, S. (2006). Isolation and identification of furocoumarins from the seeds of Psoralea corylifolia linn. Indian Journal of Pharmaceutical Sciences, 68(6), 768. [Google Scholar]
- Sardari, S. , Amin, G. , Sekhon, A. , Micetich, R. , & Daneshtalab, M. (2000). Antifungal activity of Diplotaenia damavandica. Pharmacy and Pharmacology Communications, 6(10), 455–458. [Google Scholar]
- Satish, S. , Raghavendra, M. , & Raveesha, K. (2009). Antifungal potentiality of some plant extracts against Fusarium sp. Archives of Phytopathology and Plant Protection, 42(7), 618–625. [Google Scholar]
- Savoia, D. (2012). Plant‐derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiology, 7(8), 979–990. [DOI] [PubMed] [Google Scholar]
- Sehrawat, N. , Sangwan, A. , & Yadav, M. (2014). Psoralea corylifolia l. an endangered medicinal plant with broad spectrum properties. Medicinal Plants‐International Journal of Phytomedicines and Related Industries, 6(1), 13–20. [Google Scholar]
- Seo, E. , Oh, Y. S. , Kim, D. , Lee, M.‐Y. , Chae, S. , & Jun, H.‐S . (2013). Protective role of Psoralea Corylifolia L. seed extract against hepatic mitochondrial dysfunction induced by oxidative stress or aging. Evidence‐Based Complementary and Alternative Medicine 2013. [DOI] [PMC free article] [PubMed]
- Shan, L. , Yang, S. , Zhang, G. , Zhou, D. , Qiu, Z. , Tian, L. , … Shi, X. (2014). Comparison of the inhibitory potential of Bavachalcone and Corylin against UDP‐Glucuronosyltransferases. Evidence‐based Complementary and Alternative Medicine, 2014, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, P. , Yelne, M. , Dennis, T. , Joshi, A. , & Billore, K . (2000). Database on medicinal plants used in Ayurveda.
- Sharma, R. (2004). Agro‐techniques of medicinal plants. Daya Publishing House. [Google Scholar]
- Shinde, A. N. , Malpathak, N. , & Fulzele, D. P. (2010). Determination of isoflavone content and antioxidant activity in Psoralea corylifolia L. callus cultures. Food Chemistry, 118(1), 128–132. [Google Scholar]
- Siu, W. S. , Wong, H. L. , Lau, C. P. , Shum, W. T. , Wong, C. W. , Gao, S. , … Leung, P.‐C. (2013). The effects of an Antiosteoporosis herbal formula containing Epimedii Herba, Ligustri Lucidi Fructus and Psoraleae Fructus on density and structure of rat long bones under tail‐suspension, and its mechanisms of action. Phytotherapy Research, 27(4), 484–492. [DOI] [PubMed] [Google Scholar]
- Siva, G. , Sivakumar, S. , Kumar, G. P. , Vigneswaran, M. , Vinoth, S. , Selvan, A. M. , … Jayabalana, N. (2015). Optimization of elicitation condition with Jasmonic acid, characterization and antimicrobial activity of Psoralen from direct regenerated plants of Psoralea corylifolia L. Biocatalysis and Agricultural Biotechnology, 4(4), 624–631. [Google Scholar]
- SJc, B , November (2015). Systema Naturae The Netherlands, Amsterdam1989‐2005 Amsterdam.
- Somani, G. , Kulkarni, C. , Shinde, P. , Shelke, R. , Laddha, K. , & Sathaye, S. (2015). In vitro acetylcholinesterase inhibition by psoralen using molecular docking and enzymatic studies. Journal of Pharmacy & Bioallied Sciences, 7(1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, K. , Ling, F. , Huang, A. , Dong, W. , Liu, G. , Jiang, C. , … Wang, G. (2015). In vitro and in vivo assessment of the effect of antiprotozoal compounds isolated from Psoralea corylifolia against Ichthyophthirius multifiliis in fish. International Journal for Parasitology: Drugs and Drug Resistance, 5(2), 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, S. , & Sarada, D. V. L. (2012). Antifungal activity of phenyl derivative of Pyranocoumarin from Psoralea corylifolia L. seeds by inhibition of acetylation activity of Trichothecene 3‐O‐Acetyltransferase (Tri101). Journal of Biomedicine and Biotechnology, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, S. K. , Singh Rawat, A. K. , & Mehrotra, S. (2004). Pharmacognostic evaluation of the root of Berberis asiatica. Pharmaceutical Biology, 42(6), 467–473. [Google Scholar]
- Stevens, P. F. (2015). “ Fabaceae” Angiosperm Phylogeny Website, 13 ed. Missouri Botanical Garden and University of Missouri, USA. [Google Scholar]
- Sun, D.‐X. , Ge, G.‐B. , Dong, P.‐P. , Cao, Y.‐F. , Fu, Z.‐W. , Ran, R.‐X. , … Zhang, Z.‐Z. (2016). Inhibition behavior of fructus psoraleae's ingredients towards human carboxylesterase 1 (hCES1). Xenobiotica, 46(6), 503–510. [DOI] [PubMed] [Google Scholar]
- Sun, N. J. , Woo, S. H. , Cassady, J. M. , & Snapka, R. M. (2003). DNA polymerase and topoisomerase II inhibitors from Psoralea c orylifolia. Journal of Natural Products, 66(5), 734–734. [DOI] [PubMed] [Google Scholar]
- Takizawa, T. , Mitsumori, K. , Takagi, H. , Onodera, H. , Yasuhara, K. , Tamura, T. , … Hirose, M. (2002). Gonadal toxicity of an ethanol extract of Psoralea corylifolia in a rat 90‐day repeated dose study. The Journal of Toxicological Sciences, 27(2), 97–105. [DOI] [PubMed] [Google Scholar]
- Tang, D.‐Z. , Yang, F. , Yang, Z. , Huang, J. , Shi, Q. , Chen, D. , & Wang, Y.‐J. (2011). Psoralen stimulates osteoblast differentiation through activation of BMP signaling. Biochemical and Biophysical Research Communications, 405(2), 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschke, R. , Wolff, A. , Frenzel, C. , & Schulze, J. (2014). Review article: Herbal hepatotoxicity–an update on traditional Chinese medicine preparations. Alimentary Pharmacology & Therapeutics, 40(1), 32–50. [DOI] [PubMed] [Google Scholar]
- Tewari, A. , & Bhakuni, R. (2010). New constituents from Psoralea corylifolia. Indian Journal Of Chemistry. Section B, Organic Including Medicinal, 49(2), 256. [Google Scholar]
- Toyooka, T. , & Ibuki, Y. (2009). Histone deacetylase inhibitor sodium butyrate enhances the cell killing effect of psoralen plus UVA by attenuating nucleotide excision repair. Cancer Research, 69(8), 3492–3500. [DOI] [PubMed] [Google Scholar]
- Tsai, M.‐H. , Huang, G. S. , Hung, Y.‐C. , Bin, L. , Liao, L.‐T. , & Lin, L.‐W. (2007). Psoralea corylifolia extract ameliorates experimental osteoporosis in ovariectomized rats. The American Journal of Chinese Medicine, 35(04), 669–680. [DOI] [PubMed] [Google Scholar]
- Vijnan, SPD . (1986). (1st edn): India Varanasi.
- Vonshak, A. , Barazani, O. , Sathiyamoorthy, P. , Shalev, R. , Vardy, D. , & Golan‐Goldhirsh, A. (2003). Screening south Indian medicinal plants for antifungal activity against cutaneous pathogens. Phytotherapy Research, 17(9), 1123–1125. [DOI] [PubMed] [Google Scholar]
- Wang, D. , Li, F. , & Jiang, Z. (2001). Osteoblastic proliferation stimulating activity of Psoralea corylifolia extracts and two of its flavonoids. Planta Medica, 67(08), 748–749. [DOI] [PubMed] [Google Scholar]
- Wang, T. , Yin, Z. , Zhang, W. , Peng, T. , & Kang, W. (2013b). Chemical constituents from Psoralea corylifolia and their antioxidant alpha‐glucosidase inhibitory and antimicrobial activities. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China journal of Chinese Materia Medica, 38(14), 2328–2333. [PubMed] [Google Scholar]
- Wang, T.‐X. , Yin, Z.‐H. , Zhang, W. , Peng, T. , & Kang, W.‐Y. (2013a). Chemical constituents from Psoralea corylifolia and their antioxidant alpha‐glucosidase inhibitory and antimicrobial activities. Zhongguo Zhong Yao Za Zhi, 38(14), 2328–2333. [PubMed] [Google Scholar]
- Wang, X.‐X. , Lv, X. , Li, S.‐Y. , Hou, J. , Ning, J. , Wang, J.‐Y. , … Yang, L. (2015). Identification and characterization of naturally occurring inhibitors against UDP‐glucuronosyltransferase 1A1 in Fructus Psoraleae (Bu‐gu‐zhi). Toxicology and Applied Pharmacology, 289(1), 70–78. [DOI] [PubMed] [Google Scholar]
- Warrier, P. , Nambiar, V. , & Ramankutty, C . (1995). Indian medicinal plants. A compendium of 500 species, vol. 4. Arya Vaidya Sala, Orient Longman, Kottakal.
- Weng, Z.‐B. , Gao, Q.‐Q. , Wang, F. , Zhao, G.‐H. , Yin, F.‐Z. , Cai, B.‐C. , … Li, W. D. (2015). Positive skeletal effect of two ingredients of Psoralea corylifolia L. on estrogen deficiency‐induced osteoporosis and the possible mechanisms of action. Molecular and Cellular Endocrinology, 417, 103–113. [DOI] [PubMed] [Google Scholar]
- Won, T. H. , Song, I.‐H. , Kim, K.‐H. , Yang, W.‐Y. , Lee, S. K. , Oh, D.‐C. , … Shin, J. (2015). Bioactive metabolites from the fruits of Psoralea corylifolia. Journal of Natural Products, 78(4), 666–673. [DOI] [PubMed] [Google Scholar]
- Wong, R. W. , & Rabie, A. B. M. (2011). Effect of psoralen on bone formation. Journal of Orthopaedic Research, 29(2), 158–164. [DOI] [PubMed] [Google Scholar]
- Wu, C.‐Z. , Cai, X. F. , Dat, N. T. , Hong, S. S. , Han, A.‐R. , Seo, E.‐K. , … Lee, J. J. (2007). Bisbakuchiols A and B, novel dimeric meroterpenoids from Psoralea corylifolia. Tetrahedron Letters, 48(50), 8861–8864. [Google Scholar]
- Xiong, Y. , Xiao, B. , Ma, X. , Li, C. , Zheng, J. , & Ye, J. (2009). Effects of Gaultheria yunnanensis on adjuvant arthritis in rats. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China journal of Chinese materia medica, 34(19), 2516–2519. [PubMed] [Google Scholar]
- Xu, K. , Pan, X. , Sun, Y. , Xu, W. , Njunge, L. , & Yang, L. (2015). Psoralen activates cartilaginous cellular functions of rat chondrocytes in vitro. Pharmaceutical Biology, 53(7), 1010–1015. [DOI] [PubMed] [Google Scholar]
- Yang, X. , Li, J. , Wang, X. , Fang, W. , Bidochka, M. J. , She, R. , … Pei, Y. (2006). Psc‐AFP, an antifungal protein with trypsin inhibitor activity from Psoralea corylifolia seeds. Peptides, 27(7), 1726–1731. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Paik, J. H. , Cho, D. , Cho, J.‐A. , & Kim, C.‐W. (2008). Resveratrol induces the suppression of tumor‐derived CD4+ CD25+ regulatory T cells. International Immunopharmacology, 8(4), 542–547. [DOI] [PubMed] [Google Scholar]
- Yang, Y.‐M. , Hyun, J.‐W. , Sung, M.‐S. , Chung, H.‐S. , Kim, B.‐K. , Paik, W.‐H. , … Park, J.‐G. (1996). The cytotoxicity of psoralidin from Psoralea corylifolia. Planta Medica, 62(04), 353–354. [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Huang, J.‐h. , Liu, S.‐f. , Zhao, Y.‐j. , Shen, Z.‐y. , Wang, Y.‐j. , & Bian, Q. (2012). The osteoprotective effect of psoralen in ovariectomy‐induced osteoporotic rats via stimulating the osteoblastic differentiation from bone mesenchymal stem cells. Menopause, 19(10), 1156–1164. [DOI] [PubMed] [Google Scholar]
- Yi, L.‐T. , Li, Y.‐C. , Pan, Y. , Li, J.‐M. , Xu, Q. , Mo, S.‐F. , … Kung, H.‐F. (2008). Antidepressant‐like effects of psoralidin isolated from the seeds of Psoralea Corylifolia in the forced swimming test in mice. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 32(2), 510–519. [DOI] [PubMed] [Google Scholar]
- Yin, S. , Fan, C.‐Q. , Dong, L. , & Yue, J.‐M. (2006). Psoracorylifols A–E, five novel compounds with activity against helicobacter pylori from seeds of Psoralea corylifolia. Tetrahedron, 62(11), 2569–2575. [Google Scholar]
- Yin, S. , Fan, C.‐Q. , Wang, Y. , Dong, L. , & Yue, J.‐M. (2004). Antibacterial prenylflavone derivatives from Psoralea corylifolia, and their structure–activity relationship study. Bioorganic & Medicinal Chemistry, 12(16), 4387–4392. [DOI] [PubMed] [Google Scholar]
- Zaka, S. , Asghar, B. , Raie, M. , Khan, S. , & Bhatty, M. (1989). Lipid class and fatty acid composition of Psoralia corylifolia seed. Lipid/Fett, 91(5), 205–207. [Google Scholar]
- Zarmouh, N. O. , Eyunni, S. K. , & Soliman, K. F. (2017). The Benzopyrone Biochanin‐A as a reversible, competitive, and selective monoamine oxidase B inhibitor. BMC Complementary and Alternative Medicine, 17(1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarmouh, N. O. , Messeha, S. S. , Elshami, F. M. , & Soliman, K. F. (2016). Evaluation of the isoflavone genistein as reversible human monoamine oxidase‐a and‐b inhibitor. Evidence‐based Complementary and Alternative Medicine, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhao, W. , Wang, Y. , Lu, J. , & Chen, X. (2016). The chemical constituents and bioactivities of Psoralea corylifolia Linn.: A review. The American Journal of Chinese Medicine, 44(01), 35–60. [DOI] [PubMed] [Google Scholar]


