Abstract
Aims
Ischaemia on single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is strongly associated with cardiovascular risk. Transient ischaemic dilation (TID) and post-stress wall motion abnormalities (WMA) are non-perfusion markers of ischaemia with incremental prognostic utility. Using a large, multicentre SPECT MPI registry, we assessed the degree to which these features increased the risk of major adverse cardiovascular events (MACE) in patients with less than moderate ischaemia.
Methods and results
Ischaemia was quantified with total perfusion deficit using semiautomated software and classified as: none (<1%), minimal (1 to <5%), mild (5 to <10%), moderate (10 to <15%), and severe (≥15%). Univariable and multivariable Cox proportional hazard analyses were used to assess associations between high-risk imaging features and MACE. We included 16 578 patients, mean age 64.2 and median follow-up 4.7 years. During follow-up, 1842 patients experienced at least one event. Patients with mild ischaemia and TID were more likely to experience MACE compared with patients without TID [adjusted hazard ratio (HR) 1.42, P = 0.023], with outcomes not significantly different from patients with moderate ischaemia without other high-risk features (unadjusted HR 1.15, P = 0.556). There were similar findings in patients with post-stress WMA. However, in multivariable analysis of patients with mild ischaemia, TID (adjusted HR 1.50, P = 0.037), but not WMA, was independently associated with increased MACE.
Conclusion
In patients with mild ischaemia, TID or post-stress WMA identify groups of patients with outcomes similar to patients with moderate ischaemia. Whether these combinations identify patients who may derive benefit from revascularization deserves further investigation.
Keywords: SPECT, myocardial perfusion imaging, transient ischaemic dilation
Introduction
Accurate risk estimation is a cornerstone of management in patients with known or suspected coronary artery disease (CAD), allowing physicians to target more aggressive interventions including revascularization for patients at the highest risk.1,2 Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) provides valuable non-invasive prognostic data for patients with CAD.1,3 The burden of ischaemia on SPECT MPI is a strong, independent predictor of cardiovascular risk.4,5 Recent studies have shown even minimal ischaemia is associated with increased cardiovascular events,6 while absence of ischaemia is associated with an excellent prognosis with respect to cardiac death.7 Observational studies have shown that patients with greater than 10% ischaemic myocardium may derive benefit from revascularization.1 The potential for moderate to severe ischaemia, using the 10% ischaemia threshold, to identify patients who benefit from revascularization forms the basis of the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial.8
In addition to assessing perfusion defects, SPECT MPI can identify high-risk non-perfusion features with close pathophysiologic links to ischaemia.9–12 Reduced resting left ventricular ejection fraction (LVEF) is strongly associated with an increase in cardiac death4 but was not associated with benefit from revascularization.4 Transient ischaemic dilation (TID) of the left ventricular (LV) post-stress regional wall motion abnormalities (WMA) are additional markers of cardiovascular risk.9–11 The degree to which these non-perfusion, high-risk features add risk to minimal or mild perfusion abnormalities has not been addressed in prior studies.
Using automated quantitative assessments, we sought to clarify the interaction between high-risk non-perfusion features and extent of ischaemia with respect to major adverse cardiovascular events (MACE). Additionally, we investigated whether high-risk non-perfusion features in patients with no, minimal, or mild ischaemia increased the risk of MACE to a level comparable to patients with moderate to severe ischaemia without these features.
Methods
Study population
The REgistry of Fast Myocardial Perfusion Imaging with NExt generation SPECT (REFINE SPECT) study is a large, multicentre, international registry of patients who have undergone MPI with dedicated cadmium zinc telluride solid-state detector SPECT scanners. The full details of the structure of the registry, image acquisition and analysis, and quality control have been outlined in detail previously.13 We analysed 20 418 consecutive patients enrolled in the REFINE SPECT registry between 2008 and 2014. Patients without stress and rest scans or who underwent dual-isotope scans (n = 3194) or supine data (n = 62) were excluded. Patients with early revascularization, defined as within 90 days (n = 674), were excluded as the decision for revascularization was likely influenced by the SPECT MPI.3
Clinical data
Demographic information included: age, gender, body mass index, family history of CAD, smoking status, history of previous myocardial infarction (MI), previous revascularization, hypertension, diabetes, and dyslipidaemia. Stress types were divided into pharmacologic or exercise stress. Patients were prospectively followed for development of MACE which includes all-cause mortality, non-fatal MI, and hospital admission for unstable angina (defined as recent onset or escalating cardiac chest pain with negative cardiac biomarkers), with all outcomes adjudicated by site leaders after considering all investigations.
Image acquisition and interpretation
Five centres participate in the prognostic arm of the REFINE SPECT registry. Three sites use a D-SPECT camera (Spectrum Dynamics, Caesarea, Israel), while the remaining sites used Discovery NM 530c or NM/CT570c systems (GE Healthcare, Haifa, Israel). Perfusion imaging was performed using either 99mTc-tetrofosmin or 99mTc-sestamibi radiotracers. One day rest-stress (67.4%), stress-rest (31.4%), or 2 day-stress-rest acquisitions (1.2%) were performed based on site-specific protocols. Weight-adjusted mean [± standard deviation (SD)] doses of 441 ± 296 MBq (12.0 ± 8.1 mCi) for rest and 726 ± 446 MBq (18 ± 12 mCi) for stress were used, equivalent to a total average effective dose of 7.6 mSv based on the latest dosimetry models and the International Commission on Radiological Protection Publication 103 definition of effective dose.14 Upright (D-SPECT) and supine (GE 530c) stress images were acquired 15–60 min after stress and lasted 4–6 min.13 Resting image acquisition was performed with 6 to 10-min acquisition times. Raw image datasets were de-identified and transferred to a single core laboratory (Cedars-Sinai Medical Center), where quality control is performed by experienced technologists.13 Myocardial contours are generated automatically with quantitative perfusion SPECT (QPS)/Quantitative Gated SPECT (QGS) software (Cedars-Sinai Medical Center, Los Angeles, CA, USA).
Ischaemia was assessed using total perfusion deficit (TPD) which incorporates extent and severity of perfusion abnormalities and is more reproducible compared to visual ischaemia scoring.15,16 Ischaemic TPD was defined as the difference between stress and rest TPD. Ischaemia was categorized as none (<1% ischaemic TPD), minimal (1 to <5% ischaemic TPD), mild (5 to <10% ischaemic TPD), moderate (10 to <15% ischaemic TPD), or severe (≥15% ischaemic TPD).17 Supine resting studies were compared to supine stress studies. LVEF was assessed on the supine resting study, and <40% was classified as reduced. However, results were similar when reduced LVEF was classified as <50%, results in Supplementary data online, Table S1 and Figure S1. Large resting perfusion defect was defined as a resting TPD ≥10% of the myocardium.
TID was calculated as the ratio between LV volume at stress compared to rest on ungated acquisitions.11 TID was defined as 2 SD above the mean in low-risk patients with cut-offs for each combination of camera, stress protocol, and radiotracer [sestamibi (D-SPECT exercise 1.20; D-SPECT adenosine 1.27; D-SPECT regadenoson without walk 1.39; D-SPECT regadenoson with walk 1.28; Discovery exercise 1.18; Discovery dipyridamole 1.27), tetrofosmin (Discovery exercise 1.19; Discovery dipyridamole 1.22)] as previously described.18 Wall motion scores were automatically derived from the regional motion of the mid-myocardial surface and separated into coronary distributions based on five-score, the 17-segment model.19,20 Motion is compared to a normal database and is scored using a system similar to visual scoring.21 Post-stress, regional WMA was defined as an increase in wall motion score of ≥3 in a single coronary distribution on stress compared to rest imaging.10,19 End-diastolic eccentricity index (minimum diameter/maximum diameter at mid-ventricular level) and shape index (ratio between the maximum LV diameter in short axis and ventricular length in long axis × 100) were assessed on resting scans.22
Statistical analysis
Continuous variables were summarized as mean (SD) if normally distributed and compared using a Student’s t-test. Continuous variables which were not normally distributed were summarized as median [interquartile range (IQR)] and compared using a Wilcoxon rank-sum test. Categorical variables were summarized as number (proportion) and compared using a χ2 test or Fisher’s exact test as appropriate.
Significant interactions existed between ischaemia and TID [adjusted hazard ratio (HR) 0.96, P = 0.024] and post-stress regional WMA (adjusted HR 0.94, P = 0.001). Therefore, we assessed the significance of high-risk non-perfusion imaging variables stratified by extent of ischaemia. There were no interactions between stress LVEF (P = 0.159), eccentricity index (P = 0.139), or shape index (P = 0.076) and ischaemia. Kaplan–Meier survival curves, stratified by extent of ischaemia and high-risk non-perfusion variables, were used to assess the primary outcome of MACE and compared using the log-rank test. Additionally, the association with MACE was compared to patients with moderate to severe ischaemia without high-risk non-perfusion imaging findings, defined as reduced LVEF, TID, or post-stress WMA. Unadjusted analyses were used to allow direct comparison of event rates to patients with moderate to severe ischaemia. Patients with reduced resting LVEF [n = 850 (5.1%)] were excluded from unadjusted analyses given the association between reduced LVEF and high-risk non-perfusion markers as well as cardiovascular events.23
Univariable Cox proportional hazards analysis was used to determine associations between clinical and imaging factors and MACE. Results of the univariable analysis are shown in Supplementary data online, Table S2. Multivariable Cox proportional hazards analysis was performed including variables significantly associated with the primary outcome (P < 0.1) in univariable analyses. Backwards stepwise regression was used to create a more parsimonious final model and avoid overfitting in subgroups. As the variables of interest, TID and post-stress WMA were retained in the final models. The proportional hazard assumption was assessed with Schoenfeld residuals and was valid in all models.24 Collinearity between factors was assessed with a variance-covariance matrix with no significant collinearity identified. No significant interactions were identified in the final multivariable models. Multivariable analysis was not performed in patients with moderate or severe ischaemia due to lack of statistical power. No significant difference in results was found using a more stringent outcome of all-cause mortality or non-fatal MI, Supplementary data online, Table S3 and Figure S2. Additional analyses were performed to investigate associations with all-cause mortality alone (Supplementary data online, Table S4 and Figure S3) as well as the combined outcome of non-fatal MI or admission for unstable angina (Supplementary data online, Table S5 and Figure S4).
All statistical tests were two-sided, with a P-value <0.05 considered significant. All analyses were performed using Stata version 13 (StataCorp, College Station, TX, USA). The study was approved by the institutional review boards at each participating institution and the overall study was approved by the investigational review board at Cedars-Sinai Medical Center. All data were collected under the NIH sponsored REFINE SPECT registry.
Results
Patient population
Table 1 shows the overall population characteristics, as well as in patients with and without MACE. In total, 16 578 patients were included, with mean age 64.2 years and 59.4% male. The median follow-up duration was 4.7 years (IQR 3.6–6.0 years) during which 1842 (11.1%) patients experienced at least one MACE. The earliest event was non-fatal MI in 315 (17.1%) patients, admission for unstable angina in 221 (12.0%) patients, and all-cause mortality in 1306 (70.9%) patients.
Table 1.
Baseline population characteristics
| All Patients (n = 16 578) | MACE occurred (n = 1842) | No MACE (n = 14 736) | P-value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 64.2 ± 12.2 | 70.4 ± 12.0 | 63.4 ± 12.0 | <0.001 |
| Male, n (%) | 9846 (59.4) | 1184 (64.3) | 8662 (58.8) | <0.001 |
| Site, n (%) | ||||
| Assuta | 5137 (31.0) | 486 (26.4) | 4651 (31.6) | <0.001 |
| Brigham and Women’s | 2280 (13.8) | 353 (19.2) | 1927 (13.1) | <0.001 |
| Cedars-Sinai | 3297 (19.9) | 487 (26.4) | 2810 (19.1) | <0.001 |
| Oregon | 2477 (14.9) | 331 (18.0) | 2146 (14.6) | <0.001 |
| Ottawa | 3387 (20.4) | 185 (10.0) | 3202 (21.7) | <0.001 |
| Body mass index (kg/m2), median (IQR) | 27.3 (24.5–31.0) | 26.9 (23.9–30.8) | 27.4 (24.6–31.0) | <0.001 |
| Past medical history, n (%) | ||||
| Hypertension | 10 652 (64.3) | 1407 (76.4) | 9245 (62.7) | <0.001 |
| Diabetes | 4250 (25.6) | 704 (38.2) | 3546 (24.1) | <0.001 |
| Dyslipidaemia | 10 461 (63.1) | 1263 (68.6) | 9198 (62.4) | <0.001 |
| Current smoker | 3404 (20.5) | 361 (19.6) | 3043 (20.7) | 0.298 |
| PVD | 2204 (13.3) | 379 (20.6) | 1825 (12.4) | <0.001 |
| Prior MI | 2527 (15.2) | 441 (23.9) | 2086 (14.2) | <0.001 |
| Prior revascularization | 4521 (27.3) | 705 (38.3) | 3816 (25.9) | <0.001 |
| Family history of CAD, n (%) | 4655 (28.1) | 405 (22.0) | 4250 (28.8) | <0.001 |
| Typical angina, n (%) | 1019 (6.2) | 114 (6.2) | 905 (6.1) | 0.918 |
| Resting vital signs | ||||
| Systolic BP (mmHg), median (IQR) | 130 (120–146) | 134 (120–150) | 130 (120–145) | <0.001 |
| Diastolic BP (mmHg), median (IQR) | 80 (74–84) | 80 (70–81) | 80 (74–84) | <0.001 |
| Heart rate (bpm), median (IQR) | 68 (60–77) | 70 (61–80) | 68 (60–77) | <0.001 |
| Exercise stress, n (%) | 7489 (45.2) | 441 (23.9) | 7048 (47.8) | <0.001 |
| Pharmacologic stress, n (%) | 9089 (54.8) | 1401 (76.1) | 7688 (52.2) | <0.001 |
BP, blood pressure; bpm, beats per minute; CAD, coronary artery disease; IQR, interquartile range; MI, myocardial infarction; PVD, peripheral vascular disease; SD, standard deviation.
Imaging characteristics are outlined in Table 2. TID was an infrequent finding (4.3% of patients), while post-stress WMA was relatively frequent (13.8% of patients). Patients who experienced MACE were more likely to have post-stress WMA (20.3% vs. 13.0%, P < 0.001) or TID (5.9% vs. 4.1%, P < 0.001). Additionally, patients who experienced MACE were more likely to have reduced resting LVEF (13.0% vs. 4.2%, P < 0.001) and moderate or severe ischaemia (≥10% ischaemia, 10.2% vs. 4.5%, P < 0.001). Post-stress decreasing LVEF occurred in 2057 (12.4%) of patients. Of those patients, 1094 (53.2%) did not have a post-stress WMA. Similarly, 1327 (58.0%) patients with post-stress WMA did not have a drop in LVEF ≥5%.
Table 2.
Imaging characteristics
| All Patients (n = 16 578) | MACE occurred (n = 1842) | No MACE (n = 14 736) | P-value | |
|---|---|---|---|---|
| Resting LVEF, median (IQR) | 62.9 (55.1–70.4) | 59.9 (49.0–68.7) | 63.2 (55.7–70.6) | <0.001 |
| Low resting LVEF (<40%), n (%) | 850 (5.1) | 239 (13.0) | 611 (4.2) | <0.001 |
| Resting TPD ≥10%, n (%) | 787 (4.8) | 180 (9.8) | 607 (4.1) | <0.001 |
| Stress LVEF, median (IQR) | 63.0 (55.3–59.8) | 59.6 (48.7–67.1) | 63.3 (55.9–70.1) | <0.001 |
| Eccentricity index, median (IQR) | 0.84 (0.83–0.86) | 0.84 (0.82–0.86) | 0.84 (0.83–0.86) | <0.001 |
| Shape index, median (IQR) | 57.3 (55.6–63.7) | 57.3 (55.6–63.7) | 57.3 (53.2–60.6) | <0.001 |
| Transient ischaemic dilation, n (%) | 707 (4.3) | 109 (5.9) | 598 (4.1) | <0.001 |
| Post-stress WMA, n (%) | 2290 (13.8) | 374 (20.3) | 1916 (13.0) | <0.001 |
| Burden of ischaemia, n (%) | ||||
| None (<1% ischaemic TPD), n (%) | 5338 (32.2) | 397 (21.5) | 4941 (33.5) | <0.001 |
| Minimal (1 to <5% ischaemic TPD), n (%) | 7435 (44.9) | 756 (41.0) | 6679 (45.3) | <0.001 |
| Mild (5 to <10% ischaemic TPD), n (%) | 2961 (17.9) | 502 (27.3) | 2459 (16.7) | <0.001 |
| Moderate (10 to <15% ischaemic TPD), n (%) | 554 (3.3) | 129 (7.0) | 425 (2.9) | <0.001 |
| Severe (>= 15% ischaemic TPD), n (%) | 290 (1.8) | 58 (3.2) | 232 (1.6) | <0.001 |
IQR, interquartile range; LVEF, left ventricular ejection fraction; TPD, total perfusion deficit; WMA, wall motion abnormality.
Associations between non-perfusion imaging features and MACE
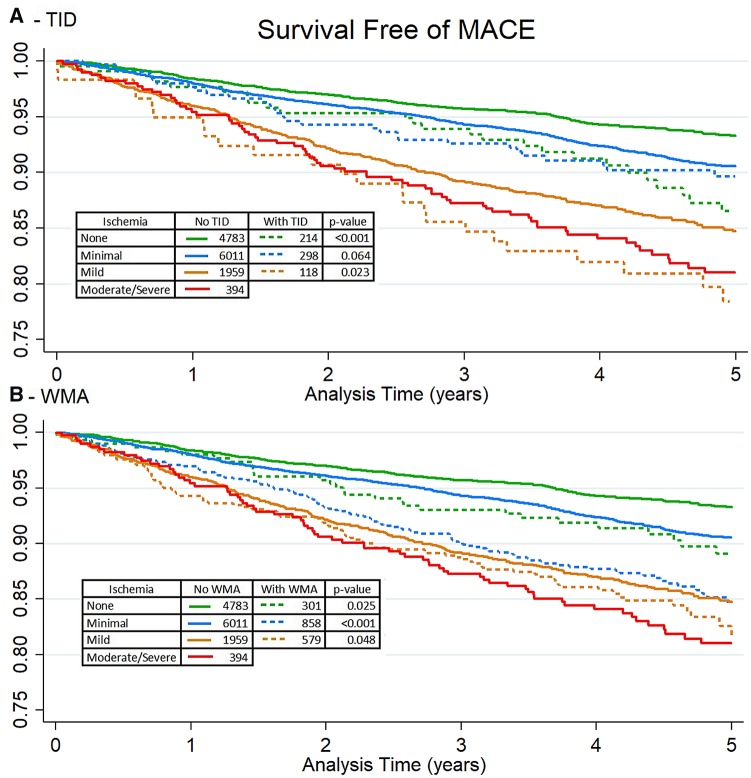
The population characteristics of patients included in the unadjusted analyses are shown in Table 3. Kaplan–Meier survival curves stratified by the presence of TID and extent of ischaemia are shown in Figure 1A. In patients with no ischaemia, those with TID were more likely to experience MACE compared to patients without other high-risk features (P = 0.001). In patients with minimal ischaemia there was no increase in MACE among those with TID (P = 0.064). In patients with mild ischaemia, TID was associated with increased MACE (unadjusted HR 1.42, P = 0.023). Kaplan–Meier survival curves stratified by the presence of post-stress WMA and burden of ischaemia are shown in Figure 1B. Patients with post-stress WMA and no, minimal, and mild ischaemia were more likely to experience MACE (P = 0.025, P < 0.001, and P = 0.048, respectively).
Table 3.
Characteristics of patients included in unadjusted analyses
| No ischaemia (<1% TPD), N = 5288 | Minimal ischaemia (1 to <5% TPD), N = 7132 | Mild ischaemia (5 to <10% TPD), N = 2627 | Moderate to severe ischaemia (≥10% TPD), N = 394 | |
|---|---|---|---|---|
| Age (years), mean ± SD | 61.9 ± 12.2 | 64.2 ± 12.1 | 66.9 ± 11.6 | 66.9 ± 11.9 |
| Male, n (%) | 2251 (42.6) | 4385 (61.5) | 1957 (74.5) | 325 (82.4) |
| Body mass index, median (IQR) | 27.3 (24.4–31.0) | 27.4 (24.7–31.1) | 27.2 (24.4–31.0) | 28.3 (25.0–31.6) |
| Past medical history, n (%) | ||||
| Hypertension | 3187 (60.3) | 4518 (63.4) | 1820 (69.3) | 308 (78.2) |
| Diabetes | 1082 (20.5) | 1815 (25.5) | 807 (30.7) | 132 (33.5) |
| Dyslipidaemia | 2989 (56.5) | 4454 (62.4) | 1893 (72.1) | 308 (78.2) |
| Current smoker | 1045 (19.8) | 1475 (20.7) | 527 (20.1) | 65 (16.5) |
| PVD | 479 (9.1) | 931 (13.1) | 423 (16.1) | 63 (16.0) |
| Prior MI | 311 (5.9) | 932 (13.1) | 637 (24.3) | 158 (40.1) |
| Prior revascularization | 723 (13.7) | 1914 (26.8) | 1077 (41.0) | 202 (51.3) |
| Family history of CAD | 1624 (30.7) | 1918 (26.9) | 682 (26.0) | 125 (31.7) |
| Typical angina | 287 (5.4) | 382 (5.4) | 192 (7.3) | 56 (14.2) |
| Resting vital signs | ||||
| Systolic BP, median (IQR) | 132 (120–148) | 130 (120–145) | 130 (120–144) | 130 (120–144) |
| Diastolic BP, median (IQR) | 80 (72–85) | 80 (74–83) | 80 (74–80) | 80 (70–82) |
| Heart rate, median (IQR) | 68 (60–77) | 68 (60–77) | 68 (60–78) | 66 (59–75) |
| Exercise stress, n (%) | 2730 (51.6) | 3207 (45.0) | 1053 (40.1) | 191 (48.5) |
| Imaging characteristics | ||||
| Resting LVEF, median (IQR) | 66.6 (59.6–73.5) | 63.1 (56.1–70.1) | 60.4 (53.4–67.9) | 58.6 (53.1–65.9) |
| Resting TPD ≥10%, n (%) | 15 (0.3) | 135 (1.9) | 199 (7.6) | 45 (11.4) |
| Stress LVEF, median (IQR) | 66.8 (60.6–73.2) | 62.9 (56.4–69.2) | 60.3 (53.2–66.7) | 56.9 (51.1–63.9) |
| Eccentricity index, median (IQR) | 0.84 (0.83–0.87) | 0.84 (0.82–0.87) | 0.84 (0.83–0.86) | 0.84 (0.83–0.85) |
| Shape index, median (IQR) | 57.3 (53.5–60.7) | 57.3 (53.0–61.1) | 57.3 (54.1–61.2) | 57.3 (55.1–61.4) |
| TID only, n (%) | 203 (3.8) | 263 (3.7) | 89 (3.4) | 0 (0.0) |
| Post-stress WMA only, n (%) | 291 (5.5) | 824 (11.6) | 550 (20.9) | 0 (0.0) |
| TID and post-stress WMA, n (%) | 11 (0.2) | 34 (0.5) | 29 (1.1) | 0 (0.0) |
BP, blood pressure; bpm, beats per minute; CAD, coronary artery disease; IQR, interquartile range; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PVD, peripheral vascular disease; SD, standard deviation; TPD, total perfusion deficit; WMA, wall motion abnormality.
Figure 1.
Kaplan–Meier survival estimates stratified by extent of ischaemia and presence of TID (A) and post-stress WMA (B). Tables contain number of patients at risk at baseline and log-rank P-value comparing survival curves. No ischaemia (<1% ischaemic TPD), minimal ischaemia (1 to <5% ischaemic TPD), mild (5 to <10% ischaemic TPD), moderate/severe (≥10% ischaemic TPD). Patients with reduced LVEF were excluded. TID, transient ischaemic dilation; WMA, wall motion abnormality.
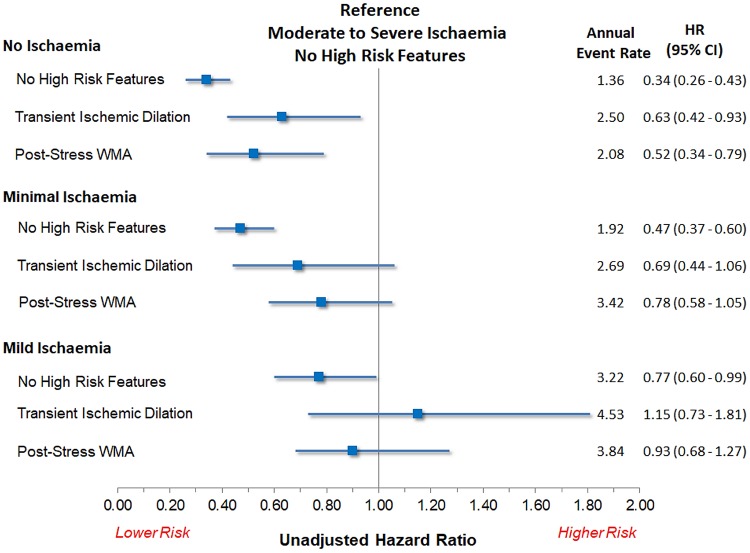
The associations between high-risk imaging features and MACE stratified by burden of ischaemia and compared to a reference standard of moderate to severe ischaemia are shown in Figure 2. Patients with TID and mild ischaemia had outcomes not significantly different from patients with moderate to severe ischaemia without other high-risk features [unadjusted HR 1.15, 95% confidence interval (CI) 0.73–1.81; P = 0.556]. Patients with post-stress WMA and mild ischaemia also had outcomes that were not significantly different (unadjusted HR 0.93, 95% CI 0.68–1.27; P = 0.629). Patients with post-stress WMA and minimal ischaemia had outcomes that were not significantly better than those of patients with moderate to severe ischaemia (unadjusted HR 0.78, 95% CI 0.58–1.05; P = 0.107). However, their outcomes were essentially the same as patients with mild ischaemia (unadjusted HR 0.97, 95% CI 0.78–1.20; P = 0.779). Findings were similar in patients with TID and minimal ischaemia (unadjusted HR 0.69, P = 0.089 compared to moderate ischaemia; unadjusted HR 0.86, P = 0.457 compared to mild ischaemia).
Figure 2.
Associations with all-cause mortality between combinations of ischaemia and high-risk non-perfusion imaging features compared to patients with moderate to severe ischaemia without high-risk features. No ischaemia (<1% ischaemic TPD), minimal ischaemia (1 to <5% ischaemic TPD), mild (5 to <10% ischaemic TPD), and moderate to severe (≥10% ischaemic TPD). Patients with reduced LVEF were excluded. CI, confidence interval; HR, hazard ratio; TID, transient ischaemic dilation; WMA, wall motion abnormality.
Multivariable analysis
Results of the multivariable analysis of predictors of MACE are shown by category of ischaemia in Table 4. In patients without ischaemia, TID was independently associated with increased MACE (adjusted HR 1.80, 95% CI 1.23–2.63; P = 0.002). In patients with minimal ischaemia, reduced LVEF (adjusted HR 1.37, 95% CI 1.04–1.82; P = 0.028) and post-stress regional WMA (adjusted HR 1.24, 95% CI 1.03–1.50; P = 0.024) were independently associated with increased MACE, but TID was not (adjusted HR 1.23, 95% CI 0.88–1.70; P = 0.223). In patients with mild ischaemia, TID was independently associated with an increase in MACE (adjusted HR 1.50, 95% CI 1.02–2.21; P = 0.037), but post-stress WMA was not (adjusted HR 0.92, 95% CI 0.75–1.13; P = 0.440).
Table 4.
Multivariable models of associations with mace stratified by ischaemia categories
| No ischaemia (ischaemic TPD <1%) |
Minimal (< 5% ischaemic TPD) |
Mild (5 to <10% ischaemic TPD) |
||||
|---|---|---|---|---|---|---|
| Variables | Adjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value |
| Age (years) | 1.04 (1.03–1.05) | <0.001 | 1.04 (1.03–1.05) | <0.001 | 1.03 (1.02–1.04) | <0.001 |
| Male | 1.89 (1.55–2.31) | <0.001 | – | – | – | – |
| Body mass index | 0.96 (0.94–0.98) | <0.001 | 0.98 (0.97–0.99) | 0.002 | – | – |
| Hypertension | 1.42 (1.12–1.80) | 0.004 | – | – | – | – |
| Diabetes | 1.46 (1.17–1.83) | 0.001 | 1.80 (1.55–2.09) | <0.001 | 1.33 (1.11–1.59) | 0.002 |
| Prior MI | 1.49 (1.08–2.07) | 0.016 | 1.29 (1.07–1.56) | 0.008 | 1.23 (1.02–1.50) | 0.034 |
| Family history | – | – | 0.76 (0.64–0.92) | 0.004 | – | – |
| Typical angina | – | – | 0.66 (0.46–0.95) | 0.026 | – | – |
| Exercise stress | 0.43 (0.34–0.55) | <0.001 | 0.54 (0.45–0.65) | <0.001 | 0.53 (0.42–0.67) | <0.001 |
| Reduced LVEF | – | – | 1.37 (1.04–1.82) | 0.028 | 1.75 (1.37–2.23) | <0.001 |
| Shape index | – | – | 1.03 (1.02–1.04) | <0.001 | 1.03 (1.01–1.04) | <0.001 |
| TID | 1.80 (1.23–2.63) | 0.002 | 1.23 (0.88–1.70) | 0.223 | 1.50 (1.02–2.21) | 0.037 |
| Post-stress WMA | 1.11 (0.77–1.61) | 0.582 | 1.24 (1.03–1.50) | 0.024 | 0.92 (0.75–1.13) | 0.440 |
– indicates variable not included in model.
CAD, coronary artery disease; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; TID, transient ischaemic dilation; WMA, wall motion abnormality.
To investigate this latter finding further, we performed an exploratory analysis accounting only for age, diabetes, and reduced LVEF given that patients with post-stress WMA were older (mean age 66.6 vs. 63.8 years, P < 0.001) and more likely to have diabetes (33.5% vs. 24.4%, P < 0.001) or reduced LVEF (13.2% vs. 3.8%, P < 0.001). In this simpler analysis post-stress WMA was not associated with an increase in MACE (adjusted HR 1.04, 95% CI 0.85–1.27; P = 0.695).
Discussion
We performed a retrospective cohort study of the interactions between ischaemia and high-risk non-perfusion SPECT MPI findings in patients with known or suspected CAD. In patients with mild ischaemia, TID and post-stress WMA were associated with an increase in MACE. Furthermore, the association with MACE in these patients was not significantly different from patients with moderate to severe ischaemia.
Prognostic significance of TID with various levels of myocardial ischaemia
TID has been established as an independent predictor of cardiac events with virtually all forms of SPECT MPI.25 In patients with mild ischaemia, TID was associated with an increase in MACE after correcting for demographics and resting LVEF. In patients with mild ischaemia and TID, the incidence of MACE was similar to that of patients with moderate to severe ischaemia. TID in patients without ischaemia (ischaemic TPD <1%) was also independently associated with an increase in MACE (adjusted HR 1.80, P = 0.002). Several recent studies have questioned the significance of TID in patients with normal perfusion without a history of diabetes or known CAD.25–27 Importantly, our analysis accounted for reduced LVEF, presence of diabetes, and prior MI without any significant interactions identified. While this difference was statistically significant, it may not be clinically significant since these patients remained at lower risk compared to patients with moderate to severe ischaemia. Overall this suggests that physicians should integrate TID with the burden of ischaemia when estimating cardiovascular risk.
Prognostic significance of post-stress WMA
Post-stress WMA was associated with an increase in MACE in patients with mild ischaemia, raising the risk of MACE to a level similar to that in patients with moderate to severe ischaemia. However, stress-induced WMA was not independently associated with an increase in MACE in multivariable modelling after correcting for comorbidities, resting LVEF, and TID. Patients with post-stress WMA were older and more likely to have diabetes, which have known prognostic significance.21,28 In an exploratory analysis, accounting only for age, diabetes, and reduced LVEF, post-stress WMA was no longer associated with an increase in MACE suggesting that they may account for the excess risk seen in univariable analyses. Earlier post-stress imaging may better delineate post-stress WMA and may impact the prognostic significance.29 Nevertheless, when imaging features are interpreted in isolation the combination of mild ischaemia and post-stress WMA identifies a higher risk cohort who may warrant consideration of invasive management.
Pathophysiologic basis of high-risk non-perfusion markers
The presence of TID and post-stress WMA are believed to be markers of ischaemia. Post-stress WMA is due to myocardial stunning, which is a well-known marker of severe ischaemia.12 There are two-postulated mechanisms of TID. Weiss et al.30 defined TID as a post-stress increase in the epicardial boundary, suggesting that true ventricular dilation occurs, which may be due to extensive ischaemia causing global myocardial stunning. Alternatively, TID may be related to diffuse sub-endocardial ischaemia. This mechanism is supported by a study of 59 patients showing an increase in TID ratio is driven primarily by changes in the endocardial border.31 While some combination of both mechanisms may be involved, both are closely related to ischaemia, suggesting that they should be interpreted in conjunction with relative perfusion deficits.
Limitations
Our study has a few important limitations. TID and post-stress WMA were assessed as binary variables. While this was chosen due to difficulties comparing values between camera systems, stress protocols, and radiotracers it is also more reflective of their routine clinical use. However, greater degrees of abnormality are likely to be associated with greater effect on outcomes. Earlier post-stress imaging may have altered the prognostic significance of TID or post-stress WMA by identifying subtler abnormalities.32 However, our results reflect current imaging guidelines suggesting up to a 2-h delay is acceptable.33 While only two camera types were utilized, several combinations of SPECT camera and stress protocols were included in our study, which may have limited the precision of our estimates. However, this would also tend to increase the generalizability of our findings. Low-dose imaging protocols were not routinely used during the study period and may also influence TID and post-stress WMA assessments. We only used supine assessments of TID and post-stress WMA, so the application to labs which only perform supine combined with upright imaging or prone imaging is not addressed. In this multicentre study, only all-cause mortality and not cardiac mortality were collected. Finally, we performed several sub-group analyses, some of which lacked statistical power. This may be particularly relevant to the lack of association between TID and MACE in patients with minimal ischaemia given the lack of a clear pathophysiologic basis for this finding. Therefore, while we cannot exclude small differences in these analyses, it is unlikely that clinically significant differences were missed.
Conclusions
In patients with mild ischaemia, the presence of TID or post-stress WMA identifies a group of patients with outcomes similar to patients with moderate to severe ischaemia without other high-risk features. Whether these combinations of findings identify a group of patients who derive benefit from revascularization deserves further investigation.
Funding
This research was supported in part by grant R01HL089765 from the National Heart, Lung, and Blood Institute/National Institutes of Health (NHLBI/NIH) (PI: P.J.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work was also supported in part by the Dr. Miriam and Sheldon Adelson Medical Research Foundation. R.J.H.M. receives funding support from the Arthur J E Child Fellowship grant.
Conflict of interest: D.S.B. and P.J.S. participate in software royalties for QPS software at Cedars-Sinai Medical Center. P.J.S. has received research grant support from Siemens Medical Systems. D.S.B., S.D., A.J.E., and E.J.M. have served as consultants for GE Healthcare. S.D. has served as a consultant to Bracco Diagnostics; her institution has received grant support from Astellas. M.D.C. has received research grant support from Spectrum Dynamics and consulting honoraria from Sanofi and GE Healthcare. T.D.R. has received research grant support from GE Healthcare and Advanced Accelerator Applications Dr. Einstein and his institution has received research support from GE Healthcare, Philips Healthcare, and Toshiba America Medical Systems. E.J.M. has served as a consultant for Bracco Inc.; and he and his institution has received grant support from Bracco Inc. D.S.B.’s institution has received grant support from HeartFlow. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Supplementary Material
References
- 1. Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS.. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900–7. [DOI] [PubMed] [Google Scholar]
- 2. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R. et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines . Circulation 2014;129:S49–73. [DOI] [PubMed] [Google Scholar]
- 3. Berman DS, Hachamovitch R, Kiat H, Cohen I, Cabico JA, Wang FP. et al. Incremental value of prognostic testing in patients with known or suspected ischemic heart disease: a basis for optimal utilization of exercise technetium-99m sestamibi myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol 1995;26:639–47. [DOI] [PubMed] [Google Scholar]
- 4. Hachamovitch R, Rozanski A, Hayes SW, Thomson LE, Germano G, Friedman JD. et al. Predicting therapeutic benefit from myocardial revascularization procedures: are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? J Nucl Cardiol 2006;13:768–78. [DOI] [PubMed] [Google Scholar]
- 5. Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA. et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998;97:535–43. [DOI] [PubMed] [Google Scholar]
- 6. Otaki Y, Betancur J, Sharir T, Hu L-H, Nejatbakhsh Azadani P, Fish M. et al. Prognostic value of quantitative high-speed myocardial perfusion imaging in multi-center study. J Nucl Med 2018;59:505. [Google Scholar]
- 7. Romero-Farina G, Candell-Riera J, Aguadé-Bruix S, Ferreira-González I, Cuberas-Borrós G, Pizzi N. et al. Warranty periods for normal myocardial perfusion stress SPECT. J Nucl Cardiol 2015;22:44–54. [DOI] [PubMed] [Google Scholar]
- 8. Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW. et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: rationale and design. Am Heart J 2018;201:124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson LL, Verdesca SA, Aude WY, Xavier RC, Nott LT, Campanella MW. et al. Postischemic stunning can affect left ventricular ejection fraction and regional wall motion on post-stress gated sestamibi tomograms. J Am Coll Cardiol 1997;30:1641–8. [DOI] [PubMed] [Google Scholar]
- 10. Sharir T, Bacher-Stier C, Dhar S, Lewin HC, Miranda R, Friedman JD. et al. Identification of severe and extensive coronary artery disease by postexercise regional wall motion abnormalities in Tc-99m sestamibi gated single-photon emission computed tomography. Am J Cardiol 2000;86:1171–5. [DOI] [PubMed] [Google Scholar]
- 11. Abidov A, Bax JJ, Hayes SW, Hachamovitch R, Cohen I, Gerlach J. et al. Transient ischemic dilation ratio of the left ventricle is a significant predictor of future cardiac events in patients with otherwise normal myocardial perfusion SPECT. J Am Coll Cardiol 2003;42:1818–25. [DOI] [PubMed] [Google Scholar]
- 12. Hida S, Chikamori T, Tanaka H, Usui Y, Igarashi Y, Nagao T. et al. Diagnostic value of left ventricular function after stress and at rest in the detection of multivessel coronary artery disease as assessed by electrocardiogram-gated SPECT. J Nucl Cardiol 2007;14:68–74. [DOI] [PubMed] [Google Scholar]
- 13. Slomka PJ, Betancur J, Liang JX, Otaki Y, Hu L-H, Sharir T. et al. (June 19, 2018) Rationale and design of the REgistry of Fast Myocardial Perfusion Imaging with NExt generation SPECT (REFINE SPECT). J Nucl Cardiol, 10.1007/s12350-018-1326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andersson M, Johansson L, Minarik D, Leide-Svegborn S, Mattsson S.. Effective dose to adult patients from 338 radiopharmaceuticals estimated using ICRP biokinetic data, ICRP/ICRU computational reference phantoms and ICRP 2007 tissue weighting factors. EJNMMI Phys 2014;1:9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berman DS, Kang X, Gransar H, Gerlach J, Friedman JD, Hayes SW. et al. Quantitative assessment of myocardial perfusion abnormality on SPECT myocardial perfusion imaging is more reproducible than expert visual analysis. J Nucl Cardiol 2009;16:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu Y, Fish M, Gerlach J, Lemley M, Berman D, Germano G. et al. Combined quantitative analysis of attenuation corrected and non-corrected myocardial perfusion SPECT: method development and clinical validation. J Nucl Cardiol 2010;17:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LEJ, Friedman JD. et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012–24. [DOI] [PubMed] [Google Scholar]
- 18. Hu L, Sharir T, Miller RJH, Einstein AJ, Fish MB, Ruddy TR. et al. (May 13, 2019) The normal limits of transient ischemic dilatation ratio for different protocols on CZT cameras: a report from the international multicenter prognostic refine SPECT registry. J Nucl Cardiol, 10.1007/s12350-019-01730-y. [Google Scholar]
- 19. Slomka PJ, Berman DS, Xu Y, Kavanagh P, Hayes SW, Dorbala S. et al. Fully automated wall motion and thickening scoring system for myocardial perfusion SPECT: method development and validation in large population. J Nucl Cardiol 2012;19:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK. et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- 21. Vaccaro O, Eberly LE, Neaton JD, Yang L, Riccardi G, Stamler J; Multiple Risk Factor Intervention Trial Research Group. Impact of diabetes and previous myocardial infarction on long-term survival: 25-year mortality follow-up of primary screenees of the Multiple Risk Factor Intervention Trial. Arch Intern Med 2004;164:1438. [DOI] [PubMed] [Google Scholar]
- 22. Abidov A, Slomka PJ, Nishina H, Hayes SW, Kang X, Yoda S. et al. Left ventricular shape index assessed by gated stress myocardial perfusion SPECT: initial description of a new variable. J Nucl Cardiol 2006;13:652–9. [DOI] [PubMed] [Google Scholar]
- 23. Hung GU, Lee KW, Chen CP, Lin WY, Yang KT.. Relationship of transient ischemic dilation in dipyridamole myocardial perfusion imaging and stress-induced changes of functional parameters evaluated by Tl-201 gated SPECT. J Nucl Cardiol 2005;12:268–75. [DOI] [PubMed] [Google Scholar]
- 24. Hiller L, Marshall A, Dunn J.. Assessing violations of the proportional hazards assumption in Cox regression: does the chosen method matter? Trials 2015;16:P134. [Google Scholar]
- 25. Alama M, Labos C, Emery H, Iwanochko RM, Freeman M, Husain M. et al. Diagnostic and prognostic significance of transient ischemic dilation (TID) in myocardial perfusion imaging: a systematic review and meta-analysis. J Nucl Cardiol 2018;25:724–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lester D, El-Hajj S, Farag AA, Bhambhvani P, Tauxe L, Heo J. et al. Prognostic value of transient ischemic dilation with regadenoson myocardial perfusion imaging. J Nucl Cardiol 2016;23:1147–55. [DOI] [PubMed] [Google Scholar]
- 27. Doukky R, Frogge N, Bayissa YA, Balakrishnan G, Skelton JM, Confer K. et al. The prognostic value of transient ischemic dilatation with otherwise normal SPECT myocardial perfusion imaging: a cautionary note in patients with diabetes and coronary artery disease. J Nucl Cardiol 2013;20:774–84. [DOI] [PubMed] [Google Scholar]
- 28. Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T. et al. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol 2013;61:1736–43. [DOI] [PubMed] [Google Scholar]
- 29. Yoda S, Sato Y, Matsumoto N, Tani S, Takayama T, Nishina H. et al. Incremental value of regional wall motion analysis immediately after exercise for the detection of single-vessel coronary artery disease: study by separate acquisition, dual-isotope ECG-gated single-photon emission computed tomography. Circ J 2005;69:301–5. [DOI] [PubMed] [Google Scholar]
- 30. Weiss AT, Berman DS, Lew AS, Nielsen J, Potkin B, Swan HJ. et al. Transient ischemic dilation of the left ventricle on stress thallium-201 scintigraphy: a marker of severe and extensive coronary artery disease. J Am Coll Cardiol 1987;9:752–9. [DOI] [PubMed] [Google Scholar]
- 31. Iskandrian AS, Heo J, Nguyen T, Lyons E, Paugh E.. Left ventricular dilatation and pulmonary thallium uptake after single-photon emission computer tomography using thallium-201 during adenosine-induced coronary hyperemia. Am J Cardiol 1990;66:807–11. [DOI] [PubMed] [Google Scholar]
- 32. Brodov Y, Fish M, Rubeaux M, Otaki Y, Gransar H, Lemley M. et al. Quantitation of left ventricular ejection fraction reserve from early gated regadenoson stress Tc-99m high-efficiency SPECT. J Nucl Cardiol 2016;23:1251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S. et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 2016;23:1187–226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.