Abstract
In the last years, consumers are paying much more attention to natural medicines and principles, mainly due to the general sense that natural compounds are safe. On the other hand, there is a growing demand by industry for plants used in traditional medicine that could be incorporated in foods, nutraceuticals, cosmetics, or even pharmaceuticals. Glycyrrhiza glabra Linn. belongs to the Fabaceae family and has been recognized since ancient times for its ethnopharmacological values. This plant contains different phytocompounds, such as glycyrrhizin, 18β‐glycyrrhetinic acid, glabrin A and B, and isoflavones, that have demonstrated various pharmacological activities. Pharmacological experiments have demonstrated that different extracts and pure compounds from this species exhibit a broad range of biological properties, including antibacterial, anti‐inflammatory, antiviral, antioxidant, and antidiabetic activities. A few toxicological studies have reported some concerns. This review addresses all those issues and focuses on the pharmacological activities reported for G. glabra. Therefore, an updated, critical, and extensive overview on the current knowledge of G. glabra composition and biological activities is provided here in order to explore its therapeutic potential and future challenges to be utilized for the formulation of new products that will contribute to human well‐being.
Keywords: Glycyrrhiza glabra, pharmacology, phytochemistry, toxicology, traditional use
1. INTRODUCTION
Since the beginning of human cultivation practices, the role of plants in medicine has been of huge importance. Glycyrrhiza glabra is one of the most popular medicinal plants belonging to the Fabaceae family (also known as Leguminosae), and its members are now commonly used as feed and food. The genus Glycyrrhiza is derived from the Greek words glykos (sweet) and rhiza (root). It is also called licorice, liquorice, glycyrrhiza, sweet wood, and Liquiritiae radix (in English); süssholz and lakritzenwurzel (in German); reglisse and bios doux (in French); shirin bayan and mak (in Persian); and liquirizia and regaliz (in Italian and Spanish, respectively). This species is a native of Mediterranean areas, but it is now also present in India, Russia, and China. The extracts are currently used in pharmaceutical and food industries, as well as in the manufacture of functional foods and food supplements (Hayashi & Sudo, 2009; Herrera, Herrera, & Ariño, 2009).
The use of liquorice predates the Greek and Roman empires, having a long history of traditional medicines and folk remedies. In fact, different geographical areas and periods are linked to different uses (Armanini, Fiore, Mattarello, Bielenberg, & Palermo, 2002). The first documents can be traced back to ancient Assyrian, Egyptian, Chinese, and Indian cultures. Theophrastus and Pedanius Dioscorides wrote about liquorice as a medicinal plant and described its therapeutic effects (Armanini et al., 2002). In traditional Chinese medicine, for example, the plant is recommended as a common remedy for gastrointestinal problems, cough, bronchitis, and arthritis. In particular, it is still widely used to treat gastritis, peptic ulcers, respiratory infections, and tremors in folk medicine. Commonly, G. glabra root is employed to prepare a tea that is an excellent thirst quencher. The dried root has been described as a tooth cleanser (Armanini et al., 2002). Actually, the most important industrial use of G. glabra is the production of food additives, such as flavours and sweetening agents (Mukhopadhyay & Panja, 2008). In particular, the root is used as a flavouring agent for American‐type tobacco, chewing gum, candies, baked goods, ice cream, and soft drinks (Rizzato, Scalabrin, Radaelli, Capodaglio, & Piccolo, 2017). In beers and fire extinguishers, the root extracts are used as foaming agents, whereas the root fibbers are used in insulation, wallboard, and boxboard materials, after removal of the medicinal and flavouring constituents. In the cosmetic field, G. glabra is described as a skin depigmentation agent and is being incorporated in topical products for that purpose.
With regard to government approval, liquorice extract and glycyrrhizin have been allowed for use in foods by the United States Food and Drug Administration, the Council of Europe, and the Joint FAO/WHO Expert Committee on Food Additives (FAO, 2005). Indeed, the U.S. Flavor and Extract Manufacturers Association has recognized it as generally safe.
To the best of our knowledge, a limited number of reviews have been published on this plant, particularly in what concerns to pharmacological aspects (Asl & Hosseinzadeh, 2008; Fiore, Eisenhut, Ragazzi, Zanchin, & Armanini, 2005). The objective of this review was to examine the bioactive compounds of G. glabra and the biological activities associated with these compounds.
2. BOTANICAL DESCRIPTIONS
G. glabra is a typical herbaceous perennial, growing to 1 m in height, presenting pinnate leaves with a length of 7 to 15 cm. The flowers are purple to pale whitish blue, being arranged in a hermaphrodite inflorescence, whereas the fruit is an oblong legume with 2 to 3 cm of length and containing several seeds.
The genus Glycyrrhiza (Fabaceae) consists of about 30 species, such as G. glabra, G. uralensis, G. inflata, G. aspera, G. korshinskyi, or G. eurycarpa. Like the other plants of Fabaceae, G. glabra is able to fix nitrogen, due to symbiosis with bacteria of the genus Rhizobium, at the root level, being suitable for sandy and clay soils, though preferring humid soils. Since the Egyptian age, the therapeutic properties of G. glabra are well documented (Fiore et al., 2005). The roots are the most used parts whereas leaves are considered an agrochemical waste. However, in the last years, different authors studied the phytochemical composition of G. glabra leaves, demonstrating that certain compounds present in the roots are also identified in leaves, although in smaller quantities (Hayashi & Sudo, 2009; Siracusa et al., 2011).
3. PHYTOCHEMISTRY AND BIOACTIVE COMPOUNDS
In the last years, the chemical constituents of liquorice have been extensively investigated by different authors (Hayashi et al., 2016; Siracusa et al., 2011). Nevertheless, few studies were carried out on the nutritional composition of G. glabra. Nutritionally, liquorice is a source of proteins, amino acids, polysaccharides and simple sugars, mineral salts (such as calcium, phosphorus, sodium, potassium, iron, magnesium, silicon, selenium, manganese, zinc, and copper), pectins, resins, starches, sterols, and gums (Q. Wang et al., 2015). Oestrogens, tannins, phytosterols (sitosterol and stigmasterol), coumarins, vitamins (B1, B2, B3, B5, E, and C), and glycosides have been reported (Q. Wang, Qian, et al., 2015). A large number of biological compounds have also been isolated, mostly triterpenes, saponins (responsible for the sweet taste), and flavonoids (Rizzato et al., 2017; Q. Wang, Qian, et al., 2015). The liquorice saponins are present as glucuronides, whereas the aglycones are present as oleananes. The triterpene saponins are the major characteristic constituents of liquorice, being responsible for the sweet taste. The contents of these compounds may vary significantly due to geographic sources, harvesting, and processing, affecting the therapeutic effects of liquorice.
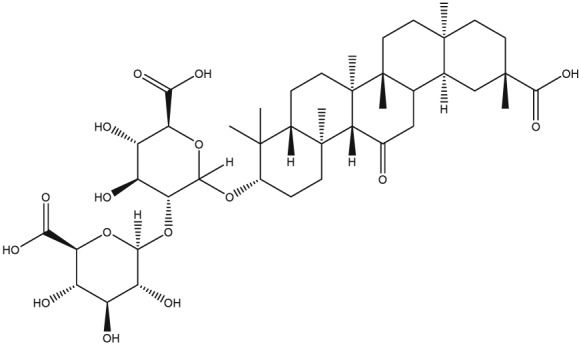
The main constituent of roots is glycyrrhizin, a triterpenoid saponin that is almost 50 times sweeter than sucrose, being the primary active ingredient (J. Y. Yu et al., 2015). Glycyrrhizin represents about 10% of the liquorice root dry weight, being a mixture of potassium, calcium, and magnesium salts of glycyrrhizic acid that varies between 2% and 25% (Rizzato et al., 2017). After oral administration, glycyrrhizin is metabolized to 18‐glycyrrhetic acid 3‐omonoglucuronide and glycyrrhetic acid by intestinal bacteria (Albermann, Musshoff, Hagemeier, & Madea, 2010).
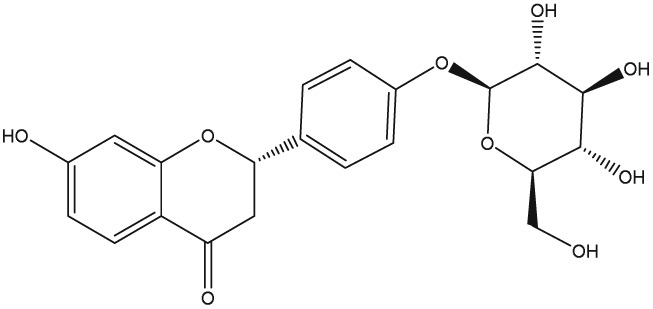
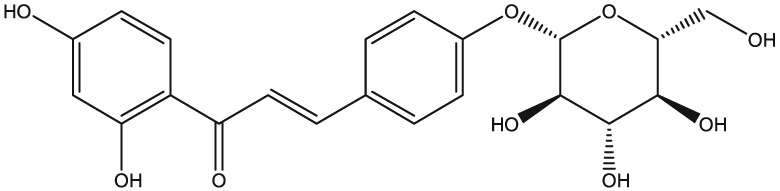
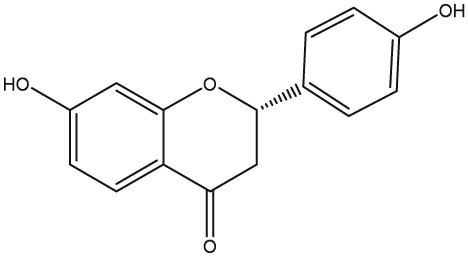
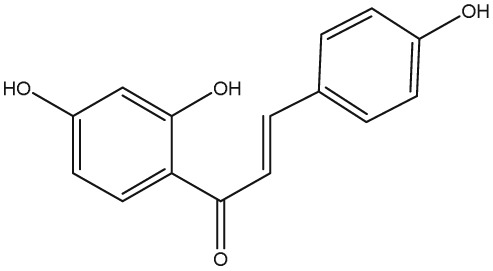
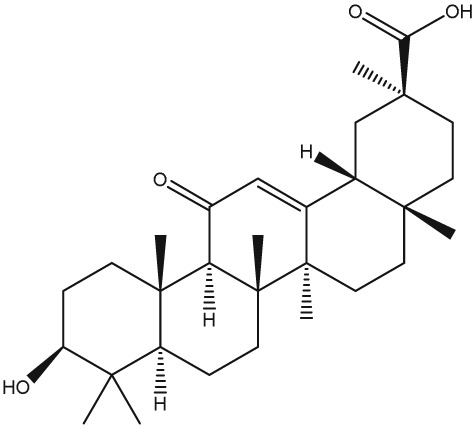
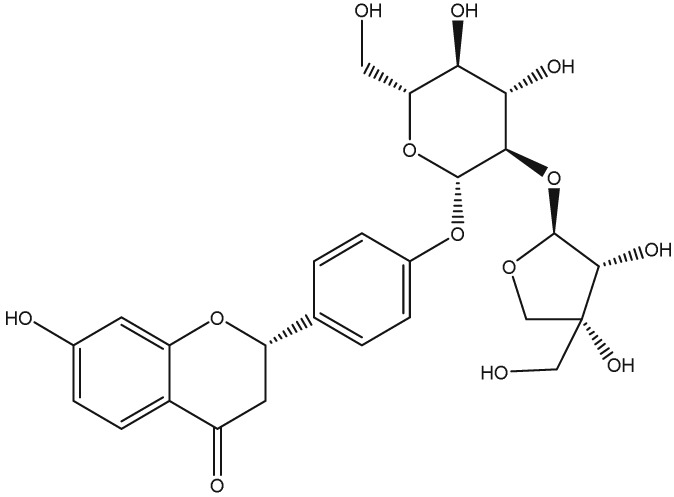
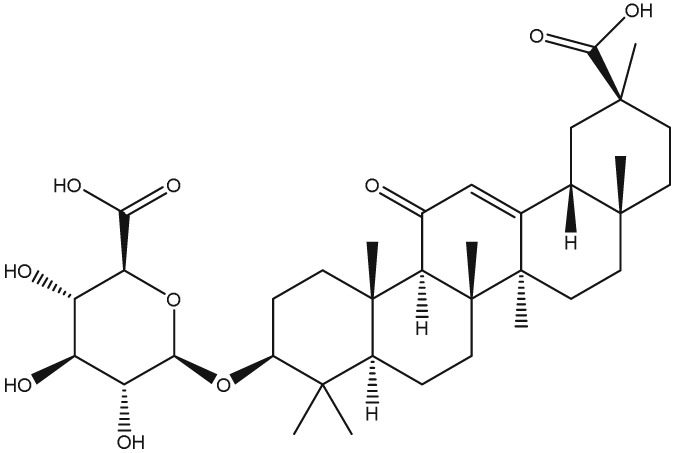
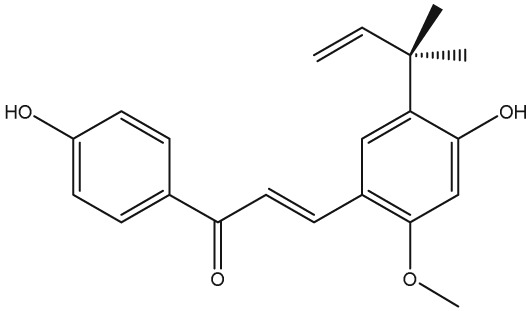
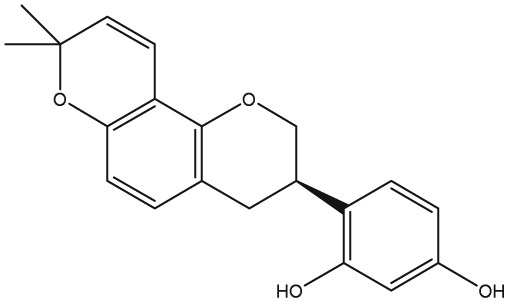
The yellow colour of liquorice is due to the flavonoid content. The flavonoids identified belong to different classes, including flavanones, flavones, flavanonols, chalcones, isoflavans, isoflavenes, isoflavones, and isoflavanones. The major flavonoids are glycosides of liquiritigenin (4′,7‐dihydroxyflavanone) and isoliquiritigenin (2′,4,4′‐trihydroxychalcone), such as liquiritin, isoliquiritin, liquiritin apioside, and licuraside (Rizzato et al., 2017). Five new flavonoids have been isolated from dried roots: glucoliquiritin apioside, shinflavanone, shinpterocarpin, prenyllicoflavone A, and 1‐methoxyphaseolin. Pinocembrin and licoflavanone were also isolated from the leaves (Fukui, Goto, & Tabata, 1988). Glabridin is the principal isoflavone identified, ranging between 0.08% and 0.35% of roots' dry weight (Simmler, Pauli, & Chen, 2013). The minor phenolic compounds are isoprenoid‐substituted flavonoids, chromenes, coumarins, dihydrostilbenes, coumestans, benzofurans, and dihydrophenanthrenes. Furthermore, many volatile components are present in roots, such as geraniol, pentanol, hexanol, terpinen‐4‐ol, and α‐terpineol, conferring the characteristic odour. The essential oil obtained from G. glabra is also rich in propionic acid, benzoic acid, furfuraldehyde, 2,3‐butanediol, furfuryl formate, maltol, 1‐methyl‐2‐formylpyrrole, and trimethylpyrazine (Chouitah, Meddah, Aoues, & Sonnet, 2011). Table 1 summarizes the chemical structure of the most important compounds identified in G. glabra.
Table 1.
Chemical structure of the most important compounds of Glycyrrhiza glabra
| Compounds | Chemical structures |
|---|---|
| Liquiritin |
|
| Isoliquiritin |
|
| Glycyrrhizin |
|
| Liquiritigenin |
|
| Isoliquiritigenin |
|
| 18β‐Glycyrrhetinic acid |
|
| Liquiritin apioside |
|
| Glycyrrhetic acid |
|
| Licochalcone A |
|
| Glabridin |
|
4. PHARMACOLOGICAL PROPERTIES
Liquorice is one of the oldest and most popular herbal medicines in the world. Many of the liquorice historical uses are still practised today. Table 2 summarizes the most important pharmacological activities reported for G. glabra as well as the individual compounds related to them.
Table 2.
Summary of the key studies conducted with liquorice‐derived compounds or extract
| Property | Compound | Concentration | Method | Major findings | Reference |
|---|---|---|---|---|---|
| Neuroprotective activity | Glabridin | 5–50 mg/kg | In vivo—oral administration to mice | Improvement of learning and memory in nondiabetic rats; it reversed learning and memory deficits of diabetic rats. Low‐dose glabridin did not alter cognitive function | (Hasanein, 2011) |
| Glycyrrhiza glabra extract | 75–300 mg/kg, 7 days | In vivo—oral administration to Swiss young male albino mice | Production of antidepressant‐like effect in mice in forced swim test and tail suspension test, probably by interaction with adrenergic and dopaminergic system | (Dhingra & Sharma, 2006) | |
| G. glabra aqueous extract | 75–300 mg/kg, 7 days | In vivo—oral administration to mice | Dose of 150 mg/kg significantly improved learning and memory of mice | (Parle, Dhingra, & Kulkarni, 2004) | |
| Sedative activity | Glabridin | 10−12–10−8 M | In vivo—acutely isolated dorsal raphe neurons of a rat | Sedative and hypnotic effects by potentiating GABAergic inhibition in dorsal raphe neurons by GABAA receptor | (Jin et al., 2013) |
| 30 μM | In vitro—Xenopus laevis oocytes expressing recombinant GABAA receptors | Strong potentiating effect on GABAA α1β(1−3)γ2 receptors | (Cho et al., 2010) | ||
| Antidepressive activity | G. glabra aqueous extract | 75–300 mg/kg | In vivo—forced swim test and tail suspension test applied to mice | Antidepressant‐like effect of liquorice extract seems to be mediated by increase of brain norepinephrine and dopamine, but not by increase of serotonin | (Dhingra & Sharma, 2006) |
| Oestrogenic activity | 18β‐Glycyrrhetinic acid | 0–200 μM | In vitro—human breast cancer cells (MCF‐7) | Induction of apoptosis in human breast carcinoma MCF‐7 cells via caspase activation and modulation of Akt/FOXO3a pathway | (Sharma, Kar, Palit, & Das, 2012) |
| Glabridin | 1 nM–10 μM | In vitro—endometrial cell line (Ishikawa cells) | Activation of ER‐α‐SRC‐1‐co‐activator complex, which displays a dose‐dependent increase in oestrogenic activity | (Su Wei Poh, Voon Chen Yong, Viseswaran, & Chia, 2015) | |
| 1 nM–25 μM | In vitro—human breast cancer cells (T‐47D, MCF‐7, and MDA‐MB‐468) | Inhibition of the growth of breast cancer cells | (Su Wei Poh et al., 2015) | ||
| 50 μg, 3–14 days | In vivo—daily feeding of prepubertal female Wistar rats | Stimulation of creatine kinase specific activity | (Tamir, Eizenberg, Somjen, Izrael, & Vaya, 2001) | ||
| Liquorice root extract | 25 μg/day, 2 weeks | In vivo—oral administration to female rats | Increase in creatine kinase activity | (Tamir et al., 2000) | |
| Liquiritigenin | 2–10 μg/ml | In vitro—MCF‐7 and T47D cells | Induction of oestrogen responsive alkaline phosphatase activity in endometrial cancer cells, oestrogen responsive element luciferase in MCF‐7 cells and Tff1 mRNA in T47D cells | (Somjen et al., 2004) | |
| Isoliquiritigenin | 0–0.04 mg/ml | In vivo—intraperitoneal injection of female ICR mice | Improvement of IVF rate | (Tung, Shoyama, Wada, & Tanaka, 2014) | |
| Skin effects | Glycyrrhizinic acid | 20%, 2 weeks | In vivo—double‐blind clinical trial in human patients | Reduction of erythema, oedema, and itching scores | (Halder & Richards, 2004) |
| — | In vitro—topical treatments in human patients during 4 weeks | Lighten hand solar lentigines | (Nerya et al., 2003) | ||
| Glycyrrhetinic acid; glabridin | 0–120 μM | In vitro—human keratinocyte culture | Prevention of oxidative DNA fragmentation and activation of apoptosis‐associated proteins in human keratinocyte | (Grippaudo & Di Russo, 2016) | |
| Glabridin; glabrene; isoliquiritigenin | 0.7 μM (glabridin), 7 μM (glabrene), and 26 μM (isoliquiritigenin) | In vitro—human melanocyte (G361) | Inhibition on tyrosinase‐dependent melanin biosynthesis | (Parvez, Kang, Chung, & Bae, 2007) | |
| Liquorice hydro‐alcoholic extract | 1–2% | In vivo—Wistar albino rats | Potentiation of hair growth activity | (Veratti et al., 2011) | |
| Antiviral activity | Glycyrrhizin | 10 mg/kg (compound) |
In vitro—Vero cells In vivo—ducks |
Stimulation of immune and antiviral effect against DHV | (Soufy et al., 2012) |
| 0.1 μg/ml (extract) | In vitro—human foreskin cell line | Protection of host cells against EV71 infection | (Kuo, Chang, Wang, & Chiang, 2009) | ||
| 316–625 mg/L (compound) | In vitro—Vero cells | Protection against coronavirus | (Cinatl et al., 2003) | ||
| 100 μg/ml (compound) | In vitro—peripheral blood mononuclear cells | Inhibition of nonsyncytium‐inducing variant of HIV replication | (Sasaki, Takei, Kobayashi, Pollard, & Suzuki, 2002) | ||
| 400–1,600 mg/day (compound) | In vitro—human immunodeficiency virus type 1 (HIV‐1) P24 antigen | Inhibition of HIV‐1 replication | (Hattori et al., 1989) | ||
| 80, 160, 240 mg 3× per week or 200 mg 6× per week | In vivo—human patients (intravenous) | Treatment of chronic hepatitis C infection | (van Rossum, Vulto, Hop, & Schalm, 1999) | ||
| 100 mg/day | In vivo—human patients (intravenous) | Prevention of autoimmune hepatitis progression | (Yasui et al., 2011) | ||
| 25–200 μg/ml | In vitro—lung epithelial A549 cells | Reduction of pathogenic H5N1 influenza A virus replication | (Michaelis et al., 2011) | ||
| Anticarcinogenic activity | Glabridin | 0–10 μM, 24, 48, and 72 hr | In vitro—cancer stem cells (CSCs) | Reduction of CSC‐like properties, enhancing the effectiveness of breast cancer therapy | (Jiang et al., 2016) |
| 0–20 mg/kg, 4 weeks | In vivo—BALB/c nude mice | ||||
| 0–100 μM (compound) | In vitro—human hepatic cell lines (Huh7, HepG2, Sk‐Hep‐1) | Induction of apoptosis in Huh7 cells | (Hsieh et al., 2016) | ||
| Licochalcone E | 12.5–50 μM (compound) | In vitro—human oral keratinocytes and human pharyngeal squamous carcinoma cell line | Induction of FaDu cell death | (Yu et al., 2017) | |
| Licochalcone A | 0–500 μM (compound) | In vitro—human gastric cancer cell lines (MKN‐28, AGS, MKN‐45) | Induction of apoptosis of gastric cancer cell via the caspase‐dependent mitochondrial pathway | (Xiao et al., 2011) | |
| Antimicrobial activity | Glabridin | 3.12–25 μg/ml | MIC | Inhibition of the growth of clinical isolates of multidrug‐resistant Staphylococcus aureus | (Fukai et al., 2002a; Singh, Pal, & Darokar, 2015) |
| 3.13–12.5 μg/ml | MIC | ||||
| 29.16 μg/ml | MIC | Decrease of Mycobacterium tuberculosis | (Gupta et al., 2008) | ||
| Glycyrrhetinic acid | 62.5–1.024 mg/L | MIC | Inhibition of the growth of clinical isolates of multidrug‐resistant S. aureus | (Oyama et al., 2016) | |
| 100–400 μg/ml | MIC, MBC | Decrease of Pseudomonas aeruginosa | (Chakotiya, Tanwar, Narula, & Sharma, 2016) | ||
| ≤50 mg/L | MIC | Decrease of Helicobacter pylori | (Krausse, Bielenberg, Blaschek, & Ullmann, 2004) | ||
| Antioxidant activity | Glabridin | 3.12–25 μg/ml | DPPH, FRAP, SOD | Protection of low‐density lipoprotein from oxidation | (Singh et al., 2015) |
| 60 mg | In vivo—oral administration to humans (LDL isolation) | (Carmeli & Fogelman, 2009) | |||
| Licochalcone | 2–20 μg/ml | DPPH, superoxide anion, lipid peroxidation, red blood cells | Inhibition of the microsomal lipid peroxidation | (Haraguchi, Ishikawa, Mizutani, Tamura, & Kinoshita, 1998) | |
| Hepatoprotective activity | Liquorice aqueous extract | 100–300 mg/kg 15 days | In vivo—oral administration to Wistar rats | Stimulation of the antioxidant enzymes and arrest of inflammatory cytokine production | (Huo, Wang, Liang, Bao, & Gu, 2011) |
| G. glabra aqueous root extract | 2 g/day, 2 months | In vivo—humans | ALT and AST decrease | (Hajiaghamohammadi, Ziaee, & Samimi, 2012) | |
| 10, 30, 100 mg/kg |
In vivo—BALB/c mice In vitro—RAW 264.7 macrophages |
Protection against LPS fulminant hepatic failure | (Yin et al., 2017) | ||
| Glycyrrhetinic acid | 0.5–20 μM | Metabolomics | Decrease of inflammation in RAW 264.7 cells | (Liu et al., 2017) | |
| Anti‐inflammatory activity | Glabridin | 75 mg/kg | In vivo—oral administration to mice | Decrease of MIP 1α expression | (Xiao et al., 2010) |
| Glycyrrhizin | 1–100 μM | DPPH, AAPH |
Protection against lipid peroxidation of liposomal membrane Inhibition of ROS |
(Rackova et al., 2007) | |
| 50–200 μg/ml | LPS inflammatory mediators production (TNF‐α, IL‐1β, COX‐2, PG2) | Decrease of endometriosis | (X. R. Wang, et al., 2017) | ||
| 10, 20, 100 mg/kg | In vivo—mice | Decrease of LPS inflammatory mediators | (Yin et al., 2017) |
Note. ALT: alanine aminotransferase; AST: aspartate aminotransferase; DHV: duck hepatitis virus; FRAP: ferric reducing antioxidant potential; ICR: Institute of Cancer Research; IVF: in vitro fertilization; LDL: low‐density lipoprotein; LPS: lipopolysaccharide; MBC: minimum bactericidal concentration; MIC: minimum inhibitory concentration; ROS; reactive oxygen species; SOD: superoxide dismutase.
4.1. Antioxidant activity
The antioxidant activity of G. glabra is one of the major reasons for its uses. The phenolic content is probably responsible for the powerful antioxidant activity observed (Rackova et al., 2007). Varsha and Sonam (2013) attributed this activity to flavonoids, whereas Singh et al. (2015) reported that mostly isoflavones, such as glabridin, hispaglabridin A, and 30‐hydroxy‐4‐O‐methylglabridin, are the responsible compounds. Biondi, Rocco, and Ruberto (2003) reported a huge antioxidant activity of the dihydrostilbene derivates present in G. glabra leaves. Also, licochalcones B and D are present in G. glabra, showing a strong scavenging activity on DPPH radical and the ability to inhibit the microsomal lipid peroxidation (Biondi et al., 2003; V. Sharma, Katiyar, & Agrawal, 2016). These phenolic compounds are effective in the protection of biological systems against oxidative stress, being able to inhibit the onset of skin damages (Haraguchi et al., 1998). According to Castangia et al. (2015), the topical application of liquorice extract formulations may be of value in innovative dermal and cosmetic products as it counteracts oxidative stress damage, maintaining the skin homeostasis due to its high antioxidant content. Table 1 summarizes the most important studies of antioxidant activity.
4.2. Anti‐inflammatory activity
The anti‐inflammatory activity of G. glabra and its use in the treatment of inflammatory diseases have been documented since ancient times (R. Yang, Yuan, Ma, Zhou, & Liu, 2017). Shalaby, Ibrahim, Mahmoud, and Mahmoud (2004) evaluated the anti‐inflammatory activity of G. glabra in male rats after 4 weeks of food intake. The authors observed a significant decrease in the total cholesterol and triglyceride levels as well as in the levels of serum liver enzymes. Harwansh, Patra, Pareta, Singh, and Biswas (2011) reviewed the positive effects of G. glabra on the treatment of the upper respiratory tract and gastric system diseases. These pharmacological effects were due to an increase in the secretion of serotonin and prostaglandins in the stomach that led to a decrease of gastric inflammation (Bahmani et al., 2014). Different authors described that the anti‐inflammatory action is primary mediated by glycyrrhizin, which in vitro could inhibit factors responsible for inflammation as well as promote the healing of stomach and mouth ulcers (Rackova et al., 2007; Yin et al., 2017). In fact, the anti‐inflammatory effects of glycyrrhizin were described as similar to those of glucocorticoids and mineralocorticoids (Kageyama, Suzuki, & Saruta, 1994). Furthermore, G. glabra is used in renal and liver complications on the basis of its strong anti‐inflammatory effects (Y. Xiao et al., 2010). Y. Xiao et al. (2010) reported the inhibition of liver granuloma formation and the inflammatory cytokine production by glycyrrhizin, whereas X. R. Wang, Hao, and Chu (2017) described the anti‐inflammatory effects on endometriosis. Moreover, Liu et al. (2017) proved the anti‐inflammatory activity of glabridin on RAW cells.
4.3. Antitussic and expectorant activity
The antitussic and expectorant effects of liquorice have been reported by different authors, particularly its useful effects on the treatment of sore throat, cough, and bronchial catarrh (Damle, 2014; Fiore et al., 2005). These effects are associated with the presence of glycyrrhizin that helps to expel congestion in the upper respiratory tract and accelerates tracheal mucus secretion (V. Sharma et al., 2016). Likewise, liquiritin apioside, an active compound reported in the methanolic extract of liquorice, has the ability to inhibit capsaicin, a compound that induces cough (Kamei, Nakamura, Ichiki, & Kubo, 2003). The effect on sore throat has been compared with that of carbenoxolone, a glycyrrhetinic acid derivative with a steroid‐like structure, which stimulates gastric mucus secretion (Damle, 2014).
4.4. Antiulcerative activity
The use of G. glabra extract as antiulcerative is widely known. For the gastrointestinal system, it is used in gastric and duodenal ulcers (Bardhan, Cumberland, Dixon, & Holdsworth, 1978), whereas for the treatment of spasmodic pains of chronic gastritis, it is employed as an adjuvant (Armanini et al., 2002). The benefits of G. glabra in the treatment of duodenal and peptic ulcers have been reported since the 1970s, and this traditional use is related to the presence of anti‐inflammatory saponins (Krausse et al., 2004). The major compound responsible for this activity is glycyrrhizin, which can raise the concentration of prostaglandins in the digestive tract, promoting stomach mucus secretion (Jafarian & Ghazvini, 2007). In addition, liquorice prolongs the lifespan of stomach surface cells, demonstrating an antipepsin effect (Ram, Lachake, Kaushik, & Shreedhara, 2010). Furthermore, deglycyrrhizinated liquorice has shown some effects in the treatment of gastrointestinal ulcers, suggesting the presence of other active ingredients (Zadeh, Kor, & Goftar, 2013). Indeed, carbenoxolone, a glycyrrhetinic acid analogue, is reported to inhibit two important enzymes for the metabolism of prostaglandin, 15‐hydroxyprostaglandin dehydrogenase and Δ13 prostaglandin reductase, raising prostaglandin levels and leading to positive effects in clinical trials for gastric and duodenal ulcers (Damle, 2014). In fact, prostaglandins stimulate mucous secretion and cell proliferation leading to ulcer healing. Nevertheless, the glycyrrhetic acid derivative carbenoxolone presents secondary effects such as the potential development of pseudo aldosteronism, which limits its use. In a clinical trial, Bardhan et al. (1978) studied the effect of liquorice by oral administration in 96 patients with gastric ulcer. The patients were randomly allocated to the treatment either with deglycyrrhizinated liquorice or with placebo. However, after 4 weeks, no differences were observed between groups in the percentage of ulcer area reduction or clinical improvements (Bardhan et al., 1978).
4.5. Antimicrobial activity
Multidrug‐resistant microorganisms represent a serious problem in clinical medicine leading to the search of new active principles. Different authors reported the antimicrobial properties of G. glabra, particularly on Gram‐positive and Gram‐negative bacteria such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Bacillus subtilis (Gupta et al., 2008; L. Wang, Yang, Yuan, Liu, & Liu, 2015). The antibacterial activity observed is due to the presence of secondary metabolites, namely, saponins, alkaloids, and flavonoids (Fukui et al., 1988; L. Wang, Yang, et al., 2015). In particular, glabridin, glabrol, glabrene, hispaglabridin A, hispaglabridin B, 40‐methylglabridin, and 3‐hydroxyglabrol, isolated from G. glabra, are responsible for this activity (L. Wang, Yang, et al., 2015). The mechanism behind this could be the decrease of bacterial gene expression, the inhibition of bacterial growth, and the reduction of bacterial toxin production (Gupta et al., 2008; L. Wang, Yang, et al., 2015). In 2014, S.‐J. Ahn, Song, Mah, Cho, and Kook (2014) demonstrated that liquorice prevents bacterial caries caused by Streptococcus mutans and Streptococcus sobrinus. Likewise, in vitro studies proved that aqueous and ethanolic extracts of liquorice have an inhibitory activity on Streptococcus pyogenes (Fukai et al., 2002a, 2002b; L. Wang, Yang, et al., 2015). On the other hand, the ability to inactivate methicillin‐resistant S. aureus (MRSA) by decreasing the expression of SaeR and Hla, the key virulence genes of MRSA, have also been reported by different authors (Fukai et al., 2002a, 2002b; L. Wang, Yang, et al., 2015). It is also suggested that licochalcone E could be used for chemical synthesis of novel anti‐S. aureus compounds, reducing the production of α‐toxin in both methicillin‐sensitive S. aureus and MRSA (L. Wang, Yang, et al., 2015).
Besides, α‐haemolysin is an important exotoxin in the pathogenesis of S. aureus infections (Berube & Bubeck Wardenburg, 2013). Such infections are associated with a broad spectrum of diseases, ranging from endocarditis to minor skin infections, toxinoses, and lethal pneumonia. Liquiritigenin, one of the most active compounds of liquorice, demonstrated the capacity to prevent human lung cells (A549) from α‐haemolysin‐mediated injuries, by decreasing α‐haemolysin production (L. Wang, Yang, et al., 2015). Similarly, glabrin and glycyrrhetinic acid have shown antibacterial activity against S. aureus (Singh et al., 2015).
Different authors reported the antibacterial action of G. glabra against Mycobacterium tuberculosis (Gupta et al., 2008), demonstrating that glabridin is the responsible compound for this activity, instead of hispaglabridin B (Simmler et al., 2013). The antitubercular phenolic compounds were previously identified as licoisoflavone and licochalcone A (Chakotiya, Tanwar, Srivastava, Narula, & Sharma, 2017).
In a mice lung infection model, G. glabra was therapeutically active against multidrug‐resistant strain of P. aeruginosa (Chakotiya et al., 2016), and the hydro‐alcoholic extract led to a reduction of the microbial load in the blood, mainly due to the presence of stigmasterol, ergosterol, licochalcone, and glabridin (Chakotiya et al., 2017).
The activity of G. glabra against Helicobacter pylori has been also reported, as mentioned in the previous subsection (Krausse et al., 2004). According to Krausse et al. (2004), the compounds responsible for this activity are glabridin and glabrene. Cao et al. (2016) also reported that 18β‐glycyrrhetinic acid significantly attenuated the gastritis infection caused by H. pylori. Asha et al. (2013) found that the flavonoid glabridin exhibits activity against H. pylori whereas glycyrrhizin did not present activity even at a concentration of 250 μg/ml. These flavonoids also showed activity against H. pylori strains resistant to clarithromycin and amoxicillin (Fukai et al., 2002a, 2002b). The probable mechanism behind these action is the inhibition of the protein synthesis, DNA gyrase, and dihydrofolate reductase (Fukai et al., 2002a, 2002b). Moreover, the liquorice polysaccharides also present activity against Porphyromonas gingivalis adhesion, which is of huge importance because no specific adhesion inhibitors have been described (Chinsembu, 2016).
The antifungal activity of G. glabra is also detailed (Sato, Goto, Nanjo, Kawai, & Murata, 2000). Sato et al. (2000) reported that the methanolic extract of liquorice presents fungicidal activity against Arthrinium sacchari and Chaetomium funicola, whereas glabridin was found to be the active compound responsible for the observed effects (Sato et al., 2000). In fact, isoflavonoids, such as glabridin, glabrol, and their derivatives, are responsible for the in vivo inhibition of Mycobacterium smegmatis, Shigella, Salmonella, E. coli, S. mutans, and Lactobacillus acidophilus (Ajagannanavar et al., 2014). Recently, Chandra and Gunasekaran (2017) also proved the antifungal activity of crude methanolic extract of G. glabra against Aspergillus niger.
Different authors reported that C. albicans is susceptible to liquorice extracts due to their richness in liquiritigenin, liquiritin, licochalcone A, and glabridin (Chandra & Gunasekaran, 2017; J. Y. Lee et al., 2009; Singh et al., 2015). Nevertheless, according to Karahan, Avsar, Ozyigit, and Berber (2016), the antimicrobial activity could be influenced by the environmental conditions that may affect the chemical compound contents and the biological activity.
According to Messier et al., licochalcone A and glabridin present a therapeutic potential against C. albicans oral infections, whereas glycyrrhizic acid had no effect (Messier & Grenier, 2011; Moazeni et al., 2017). Fukui et al. (1988) isolated licoflavanone from the leaves of G. glabra, demonstrating its antimicrobial activity. Indeed, Chen et al. (1993) reported that licochalcone A is an antiparasitic compound with potential activity against human pathogenic protozoan Leishmania species.
4.6. Antiviral activity
The antiviral activity of G. glabra extracts against different viruses has been reported, including herpes simplex, Varicella zoster, Japanese encephalitis, influenza, and vesicular stomatitis virus (L. Wang, Yang, et al., 2015). Different studies have demonstrated that two triterpenoids are responsible for the antiviral activity reported: glycyrrhizin and 18β‐glycyrrhetinic acid (L. Wang, Yang, et al., 2015). These compounds have the ability to inhibit virus gene expression and replication, decreasing the adhesion force and stress and reducing HMGB1 binding to DNA (L. Wang, Yang, et al., 2015). Also, they can enhance host cell activities by blocking the degradation of IκB enzyme involved in the propagation of the cellular response to inflammation, activating T lymphocyte proliferation, and suppressing host cell apoptosis (L. Wang, Yang, et al., 2015). The antiviral mechanisms of both compounds are similar, inhibiting the adsorption and penetration of the virus in the early steps of the replicative cycle. Nevertheless, Cinatl et al. (2003) reported that these active principles are less effective if added during the adsorption period than after virus adsorption. On the other hand, Soufy et al. (2012) found that glycyrrhizin has excellent immunostimulant properties and induces a synergistic effect with duck hepatitis virus (DHV) vaccine by activating T lymphocyte proliferation. Thus, the treatment with glycyrrhizin alone or in combination with DHV vaccine could lead to an immune stimulation and antiviral effect against DHV (Soufy et al., 2012).
Herpes simplex virus (HSV) is one of the most common viruses infecting humans and animals. During HSV infection, the cellular adhesion is increased, playing a key role in inflammatory response. W. Huang et al. (2012) reported that the adhesion force and stress between the cerebral capillary vessel endothelial cells and the polymorphic nuclear leukocytes were amplified during HSV infection. Glycyrrhizin stimulates the mouse defence system against HSV‐1 infection (Sekizawa, Yanagi, & Itoyama, 2001). Furthermore, glycyrrhizic acid was found to have a distinctive effect against Kaposi sarcoma‐associated herpes virus (KSHV). It was proved that glycyrrhizic acid could terminate the latent infection of KSHV when all current drugs are ineffective (Damle, 2014). Also, glycyrrhizic acid down‐regulates the expression of latency‐associated nuclear antigen in B lymphocytes leading to natural cell death (apoptosis) of the KSHV‐infected cells (Damle, 2014). Recently, the antiviral activity of glycyrrhizin against severe acute respiratory syndrome virus was evaluated (Cinatl et al., 2003). Glycyrrhizin affects the cellular signalling pathways such as protein kinase C, casein kinase II, and transcription factors, namely, activator protein 1 and nuclear factor κB (Cinatl et al., 2003). Furthermore, glycyrrhizin and its aglycone, 18β‐glycyrrhetinic acid, up‐regulate the expression of inducible nitric oxide synthase and the production of nitric oxide in macrophages (Cinatl et al., 2003). Zhang et al. also reported that glycyrrhizin reduces the expression of proinflammatory cytokines affecting coxsackievirus B3‐induced myocarditis (L. Wang, Yang, et al., 2015; Zhang, Song, & Zhang, 2012). Also, the activity against human immunodeficiency virus (HIV) was evaluated (Sasaki et al., 2002). Glycyrrhizin has been used to treat patients with HIV‐1 (L. Wang, Yang, et al., 2015). The results revealed a low concentration of P24 antigen in patients, probably due to the up‐regulation of chemokines (Sabde et al., 2011).
Intravenous glycyrrhizin has been employed for more than 20 years in Japan for the treatment of chronic hepatitis (van Rossum et al., 1999). Glycyrrhizin, when compared with the placebo, presents clinical interest for the possible treatment of chronic hepatitis C, inducing a significant reduction of the serum aminotransferases and an improvement in the liver histology (Ploeger et al., 2001). Also, it prevents the development of hepatocellular carcinoma in chronic hepatitis C (van Rossum, Vulto, de Man, Brouwer, & Schalm, 1998). Intravenous glycyrrhizin can be also used for the treatment of acute‐onset autoimmune hepatitis (Yasui et al., 2011). Another study shows that glycyrrhizin interferes with highly pathogenic H5N1 influenza A virus replication (Michaelis et al., 2011).
Glycyrrhizin can also be used as a novel therapeutic method to control porcine epidemic diarrhoea virus (PEDV) infection, inhibiting the infection of Vero cells (namely, the entry and replication of PEDV) and decreasing the mRNA levels of proinflammatory cytokines (Huan et al., 2017).
4.7. Hepatoprotective activity
The hepatoprotective activity of glycyrrhizin and 18β‐glycyrrhetic acid by inhibition of free‐radical generation and lipid peroxidation has been extensively reported (Huo et al., 2011). One of these studies indicated that the hydromethanolic root extract of G. glabra exhibits a significant protection against hepatotoxicity induced by carbon tetrachloride in the liver tissue of mice (V. Sharma & Agrawal, 2017). The effects of liquorice on nonalcoholic fatty liver disease have also been investigated (Hajiaghamohammadi et al., 2012). According to Rizzato et al. (2017), glycyrrhizin and glycyrrhetinic acids prevent drug‐induced liver injury and ensure the disruption of bile acid metabolism in humans. Indeed glycyrrhetinic acid has been reported as anti‐inflammatory and hepatoprotective compound (Yin et al., 2017), whereas glycyrrhizin, when compared with the placebo, induced a significant reduction in the serum aminotransferases and improved the liver histology (van Rossum et al., 1998). It has also been reported that the long‐term use of glycyrrhizin prevents the development of hepatocellular carcinoma in chronic hepatitis C (van Rossum et al., 1998). In vitro studies have shown that glycyrrhizin modifies the intracellular transport and suppresses the hepatitis B virus surface antigen (Sato et al., 1996). In addition, it prevents the oxidative and hepatic damage caused by aflatoxins through increasing CYP1A1 and glutathione‐S‐transferase activity, contributing to the anticarcinogenic activity by metabolic deactivation of the hepatotoxin (Y. Yang et al., 2017). Mahmoud, Hussein, Hozayen, and Abd El‐Twab (2017) reported that the treatment with 18β‐glycyrrhetinic acid significantly reduced the serum enzymes, bilirubin, and proinflammatory cytokines in the liver, decreasing the expression of P450 E1.
4.8. Anticarcinogenic and antimutagenic activity
Different studies suggest that the extract of G. glabra may be a potential supplemental source for different cancer treatments (C. S. Lee, Kim, Lee, Han, & Lee, 2008; Ohtsuki, Oh‐Ishi, & Nagata, 1992). This activity is due to the 18β‐glycyrrhetinic and glycyrrhizic acids that induce mitochondrial permeability transition, leading to the apoptosis of tumour cells (C. S. Lee et al., 2008). C. S. Lee et al. (2008) demonstrated the toxic effect of G. glabra against the human cervix and uterus tumour cell line SiHa cells. Ohtsuki et al. (1992) reported the in vivo inhibition of Ehrlich ascites tumour cell growth by the aqueous and methanolic extracts of G. glabra, with the corresponding reduction in cell number, body weight, and ascites volume. The hydromethanolic root extract of G. glabra also exhibited antimutagenic potential by suppressing micronuclei formation and chromosomal aberration in bone marrow cells of albino mice (V. Sharma, Agrawal, & Shrivastava, 2014). Glycyrrhizin and glycyrrhetinic acids are effective compounds in gastric cancer treatment, whereas glycyrrhizin suppresses thromboxane A2 in lung cancer cell with low toxicity (Deng, Wang, Zeng, Chen, & Huang, 2017). According to S. Wang et al. (2017), 18β‐glycyrrhetinic acid has antitumour activities in breast and ovarian cancer, gastric tumours, and leukaemia. In liver cancer, the compound inhibits the proliferation of HepG2 cells without affecting the normal liver cell line. In particular, 18β‐glycyrrhetinic acid increases the formation of reactive oxygen species, nitric oxide production, and loss of the mitochondrial membrane potential (Hasan et al., 2016).
Glycyrrhetinic acid derivatives have also presented promising cytotoxicity on human breast cancer cell lines (MCF‐7, MDA‐MB‐231; Li, Feng, Song, Li, & Huai, 2016). Also, the anticancer activity in human leukaemia, by inducing the apoptosis of HL‐60 cells through the activation of extrinsic and intrinsic apoptotic pathways, was proved by Y. C. Huang et al. (2016). Recently, licochalcone E, when compared with well‐known antitumour agents, licochalcone A and isoliquiritigenin, exhibited the most potent cytotoxic effect (X. Y. Xiao et al., 2011; S. J. Yu et al., 2017). Xiao et al. explored the licochalcone A mechanism of action in MKN‐28, AGS, and MKN‐45 gastric cancer cells and human gastric epithelial immortalized cells (Park et al., 2015; X. Y. Xiao et al., 2011). The results indicated that licochalcone A inhibits gastric cancer cells growth in a dose‐dependent way, by blocking cell cycle progression at the G2/M transition, inducing apoptosis. In addition, licochalcone A induced apoptosis by its effects on the expression of PARP, caspase‐3, Bcl‐2, and Bax (X. Y. Xiao et al., 2011). Kanazawa et al. (2003) and Jung et al. (2006) showed that isoliquiritigenin inhibits the cell growth by G2/M cell cycle arrest in breast and prostate tumour cells.
Different studies demonstrated that isoliquiritigenin suppresses pulmonary metastasis in mice (Yamazaki et al., 2002) and human hepatoma cells (Hsu, Kuo, & Lin, 2005). Apoptosis was primarily mediated through mitochondrial death cascade, as shown by loss of mitochondrial membrane potential, release of cytochrome c, and activation of caspase‐9. A possible explanation is that cell growth was arrested through up‐regulation of p53 and p21 and down‐regulation of cdk2, cyclin E, and E2F‐1 while apoptosis was induced by increasing Bax protein expression and activating caspase‐7 (G. Sharma et al., 2012). Glabridin exhibited antitumour properties in various human cancer cells (Jiang et al., 2016). The results revealed that glabridin induced apoptosis in dose dependently in Huh7 cells through caspase‐3, caspase‐8, and caspase‐9 activation and PARP cleavage (Hsieh et al., 2016).
4.9. Neuroprotective activity
The effects of G. glabra on learning and memory were investigated in mice (Dhingra & Sharma, 2006; Parle et al., 2004). In 2004, Parle et al. (2004) administered the extract of G. glabra orally to mice during 7 days at different concentrations (75–300 mg/kg). Chakravarthi and Avadhani (2013) and Dhingra and Sharma (2006) studied the effects of G. glabra root aqueous extract on the learning and memory of 1‐month‐old male Wistar albino mice at doses between 75 and 300 mg/kg, orally administered during six successive weeks. Both studies demonstrated a significant improvement of learning and memory in mice, but the exact mechanism behind this action remains unknown (Chakravarthi & Avadhani, 2013; Dhingra & Sharma, 2006). These findings suggest a possible neuroprotective role of liquorice in the prevention of diseases such as Alzheimer. The basis of Alzheimer is the chronic inflammation of certain brain regions. Thus, the anti‐inflammatory activity of liquorice might contribute to the observed memory‐enhancing effects (Yokota, Nishio, Kubota, & Mizoguchi, 1998). Also, oxygen free radicals are implicated in the process of aging and could be responsible for the development of Alzheimer's disease in elderly persons. The protective role of liquorice extract may be attributed to its antioxidant properties, resulting in reduced brain damage and improvement of neuronal function and memory. The combination of anti‐inflammatory and antioxidant activities with neuroprotective role could lead to memory‐enhancing effects. Hasanein (2011) investigated the effect of chronic treatment with glabridin on the cognitive function of diabetic rats. The results showed an improvement of learning and memory in nondiabetic rats and a reversal of learning and memory deficits in diabetic rats. The effect was attributed to the combined antioxidant, neuroprotective, and anticholinesterase properties of glabridin, suggesting a potential use in the management of dementia diabetic patients (Hasanein, 2011).
4.10. Sedative activity
Gamma‐aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system, being GABAA receptors a target for anaesthetics and neuroleptic, anxiolytic, and anticonvulsant compounds (Simmler et al., 2013). G. glabra acts as a modulator of GABAA receptors (Hoffmann, Beltrán, Ziemba, Hatt, & Gisselmann, 2016), being able to induce sedative and anxiolytic effects. Glabridin was evaluated by examining GABA responses in acutely isolated dorsal raphe neurons of a rat (Jin et al., 2013). According to the authors, glabridin potentiated GABA‐induced responses by positive modulation of GABAA receptors, exhibiting sedative and hypnotic effects (Jin et al., 2013). Glabridin potentiation is not sensitive to flumazenil and uses a similar mechanism of the general anaesthetics involving the amino acids N265 and M286, which are located in the second and third transmembrane domains on the β‐subunit of GABAA receptors (Hanrahan, Chebib, & Johnston, 2011). Also, glabridin could contribute to the hypnotic effect, as it is able to cross the blood–brain barrier (Simmler et al., 2013).
4.11. Antidepressive activity
Liquorice extract may have potential therapeutic value for the treatment of depressive disorders. Recent studies have shown that liquorice extract produces significant antidepressant effects in mice during forced swim test (FST) and tail suspension test (TST; Dhingra & Sharma, 2006). In the FST model, mice were forced to swim in a restricted space and induced to a characteristic behaviour of immobility. This situation reflects a state of depression. The TST model also induces a state of immobility that is claimed to reproduce a condition similar to human depression. Both models are widely used to screen antidepressant drugs. The precise mechanisms by which liquorice extract produced this effect are not completely understood. However, it is suggested that the extract may interact with α1‐adrenoceptors and dopamine D2 receptors, increasing the levels of norepinephrine and dopamine in the mice brain (Dhingra & Sharma, 2006). Besides, p‐CPA (a serotonin synthesis inhibitor) did not attenuate the antidepressant effect of liquorice extract, suggesting that this is not mediated by the serotonergic system. On the other hand, p‐CPA reversed the antidepressant effect of fluoxetine, suggesting that fluoxetine acts in the serotonergic system. Reserpine produces a significant depression by depleting biogenic amines in the brain of rodents. As liquorice extract reversed reserpine‐induced depression, its antidepressant effect can be associated with the restoration of brain monoamines, such as norepinephrine and dopamine.
4.12. Oestrogenic and androgenic effects
Since ancient times, the influences of liquorice on the action of cortisol, reduction of testosterone synthesis, and the influence on oestrogen activity are well known (Armanini et al., 2002). S. H. Kim and Park (2012) reported that isoflavones can influence sexual development and impair oestrous cycling and ovarian and hypothalamus and pituitary glands function (S. H. Kim & Park, 2012). The oestrogenic effect of liquorice ethanolic extract could be explained by its agonist activity on MCF‐7 breast cancer cells, being this action mediated by 18β‐glycyrrhetinic acid (G. Sharma et al., 2012). Glabridin is a common component of herbal remedies used for the treatment of menopausal symptoms, resulting in favourable outcomes similar to those of 17β‐oestradiol (Su Wei Poh et al., 2015). In concentrations between 2.5 and 25 μg per animal, glabridin induces similar effects to the administration of oestradiol in a concentration of 5 μg per animal. Glabridin was found to be three to four times more active than 2′‐O‐methylglabridin and 4′‐O‐methylglabridin (Tamir et al., 2000). Moreover, according to Tamir et al. (2001), glabrene has a considerable oestrogenic activity. Glabridin and glabrene are similar to 17β‐oestradiol in the stimulation of the specific activity of creatine kinase, although at higher concentrations, differing in the extension rate of action as well as in the interaction with other drugs. In human premenopausal bone cells, the response to 17β‐oestradiol and glabridin (at a higher concentration) was higher than in postmenopausal cells, whereas glabrene (at a higher concentration) was more effective in postmenopausal cells (Somjen et al., 2004).
Isoliquiritigenin has a strong oestrogen‐like activity, suggesting that this compound may be cyclized to liquiritigenin, which is an active flavonoid under physiological conditions (Hajirahimkhan et al., 2013). In vivo, the stimulatory effects of glabrene are similar to those of oestradiol (Powers & Setzer, 2015). It is also interesting to observe that isoliquiritigenin and formononetin stimulate sperm during fertilization (Tung et al., 2014). This reveals that both phytoestrogens may be useful therapeutic agents for infertility treatments (Tung, Shoyama, Wada, & Tanaka, 2015). Zamansoltani, Nassiri‐Asl, Sarookhani, Jahani‐Hashemi, and Zangivand (2009) reported that the alcoholic extract of G. glabra has antiandrogenic effects probably by increasing the testosterone metabolism, down‐regulating androgen receptors, or activating oestrogenic receptors.
4.13. Skin effects
The main skin benefits reported for G. glabra are based on the antioxidant and anti‐inflammatory activities as well as on the ultraviolet (UV) protection (Halder & Richards, 2004). Saeedi, Morteza‐Semnani, and Ghoreishi (2003) reported the use of liquorice mainly for skin eruptions, including dermatitis, eczema, pruritus, and cysts. In particular, the G. glabra flavonoids present depigmenting capabilities and tyrosinase inhibition effects (Solano, Briganti, Picardo, & Ghanem, 2006). The presence of an α‐keto group in flavonoids is responsible for this activity (Y. J. Kim & Uyama, 2005; Parvez et al., 2007). Castangia et al. (2015) have reported the skin protective effects of liquorice against damage from oxidative stress. According to the authors, liquorice extract can scavenge DPPH free radicals with an inhibition of 80% and protect fibroblasts against oxidative stress (Castangia et al., 2015). Nevertheless, when evaluated in the isolated form, glycyrrhizin showed a poor antioxidant activity, being not able to efficiently counteract the oxidative effect (Castangia et al., 2015).
Tyrosinase is essential for skin pigmentation due to its role in melanin biosynthesis (Solano et al., 2006). The use of tyrosinase inhibitors is important in the cosmetic and medicinal industries, due to their preventive effect on pigmentation disorders such as melasma, age spots, and sites of actinic damage (Nerya et al., 2003). Alternatively, tyrosinase inhibitors may be targets for developing medicines to treat hypopigmentation‐related problems, such as albinism and piebaldism (Y. J. Kim & Uyama, 2005). In particular, glabridin, glabrene, isoliquiritigenin, licochalcone A, and liquiritin have been reported as G. glabra compounds able to inhibit the tyrosinase activity (Ebanks, Wickett, & Boissy, 2009; Nerya et al., 2003). Recently, Grippaudo and Di Russo (2016) described the effects of the topical application of glycyrrhetinic acid combined with fractional carbon dioxide laser for the benign treatment of hand hyperpigmentation during 4 weeks. Likewise, the treatment of human keratinocytes with 18β‐glycyrrhetinic acid and glabridin was documented to directly and indirectly prevent DNA damage, avoiding the apoptosis activation caused by UV B radiation (Veratti et al., 2011). Indeed, Yokota et al. (1998) described that glabridin inhibits tyrosinase activity, melanogenesis, and skin inflammation. Besides, glabrene acts as a tyrosinase inhibitor, preventing the formation of melanin in melanocytes, probably acting as skin‐lightening agent. Saeedi et al. (2003) exposed that liquorice extract could be considered as an effective agent in the treatment of atopic dermatitis. Finally, the hydro‐alcoholic extract of liquorice promotes hair growth, being safely used in herbal formulations for the treatment of various types of alopecia (Saumendu, Raj, Suvakanta, Jashabir, & Biswajit, 2014).
4.14. Other activities
Liquorice has been traditionally used as a sweetener due to its taste, (Tian, Liu, Zhen, & Tong, 2013; Tong, Xie, Rong, Zhou, & Meng, 2015). According to Bahmani et al. (2014), liquorice can reduce diabetes symptoms, such as polydipsia and frequent urination, but cannot reduce blood glucose. Takii et al. (2001) suggested that glycyrrhizin has an antidiabetic effect in noninsulin‐dependent diabetes mice model, reducing the postprandial blood glucose rise.
Glycyrrhizin is a thrombin inhibitor. An in vivo assay performed in rats demonstrated that the intravenous administration of glycyrrhizin causes a dose‐dependent reduction in thrombus size on a venous thrombosis model, combining stasis and hypercoagulability (Mendes‐Silva et al., 2003). The authors reported that doses between 180 and 360 mg/kg decreased the thrombus weight by 35% and 90%, respectively (Mendes‐Silva et al., 2003).
The immunomodulatory activity of an aqueous root extract of G. glabra was demonstrated in vitro to be linked to the presence of the phenolic compound glycyrrhizin (Mitra Mazumder, Pattnayak, Parvani, Sasmal, & Rathinavelusamy, 2012). Mitra Mazumder et al. (2012) reported the increased production of lymphocytes and macrophages from human granulocytes after contact with G. glabra root extract.
Different liquorice compounds, such as licochalcone A, 18β‐glycyrrhetinic acid, and glabridin, have antimalarial activity. Licochalcone A is the most promising one, inhibiting in vitro the growth of chloroquine‐susceptible and chloroquine‐resistant Plasmodium falciparum strains (Chen et al., 1994). Glabridin also showed in vitro activity against this parasite, probably by an induction of oxidative stress, mainly through the generation of reactive oxygen and nitrogen species that lead to apoptosis (Cheema et al., 2014).
It is well known that myocardial ischaemia is one of the principal diseases in the Western world. This disease occurs through occlusion or blockage of coronary arteries, resulting in myocardial cell death. However, the reperfusion produces the salvage of ischaemic tissue but also contributes to the myocardial cellular injury. The pretreatment with G. glabra significantly attenuates the ischaemic reperfusion, through an improvement of the heart antioxidant status, a positive modulation of the perturbed haemodynamic, and a recovery of left ventricular contractile function, along with histological salvage (Di Paola et al., 2009; Ojha, Golechha, Kumari, Bhatia, & Arya, 2013). In particular, glycyrrhizic acid induced protection against myocardial ischaemia in rats, probably due to its antioxidant potential (Ojha et al., 2013). Similarly, Nakagawa, Kishida, Arai, Nishiyama, and Mae (2004) demonstrated that G. glabra is safe for cardiomyocytes in a long‐term administration.
The extract of G. glabra has been used in the treatment of low bone mass, osteoporosis, fractures, bone defects, osteomalacia, osteogenesis imperfecta, bone disease, and periodontal diseases (Kumar et al., 2015). Rajesh (2004) described the inhibitory effect on bone reabsorption of G. glabra, whereas Choi (2011) reported that glabridin is responsible for this activity. Mitochondrial dysfunction, especially respiratory chain disruption, is responsible for aging‐related bone diseases. Hence, the target of glabridin is the reduction of mitochondrial dysfunction induced during aging and the prevention of osteoblast damage in osteoporotic patients (Choi, 2011).
Study on mice demonstrated that G. glabra, particularly glabridin, when integrated in a dietary supplement, could reduce the susceptibility of low‐density lipoprotein (LDL) to oxidation and the atherosclerotic lesion area (Fuhrman & Aviram, 2001; Grassi, Desideri, & Ferri, 2010). These results could be related to the absorption and binding of glabridin to the LDL and a subsequent protection of the LDL from oxidation (Fuhrman et al., 1997).
The methanolic extract of G. glabra rhizomes, at a dose of 150 mg/kg, has antiarthritic activity in male rats by inhibition of leukocyte migration, autoantigen production, and exhibition of antiproteinase activity (Choudhary, Kumar, Malhotra, & Singh, 2015). Also, Mishra, Bstia, Mishra, Chowdary, and Patra (2001) reported that a combined formulation of G. glabra and Boswellia serrata (1:1) had a significant synergistic action on arthritis.
Shin et al. (2007) studied the antiallergic effects, namely, the antiscratching behaviour and the IgE production inhibitory activity, of glycyrrhizin, 18β‐glycyrrhetinic acid, isoliquiritin, and liquiritigenin in dermatitis and asthma (Shin et al., 2007). In particular, 18β‐glycyrrhetinic acid attenuated the airway inflammation in an asthmatic mouse model, being classified as a novel therapeutic component for the treatment of allergic asthma (S. H. Kim, Hong, Lee, & Lee, 2017).
Finally, glabridin shows hypoglycaemic effects in an animal model with diabetes mellitus, significantly decreasing body weight (Wu, Jin, & Jin, 2013). J. Ahn, Lee, Jang, Kim, and Ha (2013) stated that G. glabra effectively inhibits the adipogenesis of 3T3‐L1 cells.
5. SIDE EFFECTS AND TOXICITY
Different adverse side effects were reported for high doses of G. glabra such as hypertension, hypokalaemia, or fluid retention (Omar et al., 2012). The exposure to high levels of glycyrrhizin can produce hypermineralocorticoid‐like effects. Glycyrrhetic acid and liquorice saponins can inhibit 11‐β‐hydroxysteroid dehydrogenase enzyme, leading to a cortisol‐induced mineralocorticoid effect and a consequent tendency to the elevation of sodium and reduction of potassium levels (Isbrucker & Burdock, 2006). For example, in 2010, a 34‐year‐old woman was suspected to have suffered a lethal acute intoxication from eating liquorice over a period of several months (Albermann et al., 2010). Albermann et al. associated the effects with the potential mineralocorticoid action of glycyrrhizin and its metabolite, glycyrrhetic acid, and quantified by liquid chromatography–tandem mass spectrometry these compounds in the blood. Nevertheless, only traces of glycyrrhetic acid had been found in the blood and stomach content of the deceased woman, which means that the possibility of acute lethal glycyrrhetic acid intoxication could be eliminated (Albermann et al., 2010).
Based on in vivo assays and clinical evidence, the amount of liquorice ingested daily by patients with mineralocorticoid excess syndromes appears to vary over a wide range (1.5–250 g/day; Isbrucker & Burdock, 2006). In addition to hypertension, patients may experience hypokalaemia and sodium retention, resulting in oedema. Studies on rodents and humans demonstrated that glycyrrhizin is poorly absorbed by the gastrointestinal tract but extensively metabolized by the intestinal microflora to glycyrrhetic acid and monoglucuronyl glycyrrhetic acid, which are both readily absorbed. Thus, an enterohepatic circulation of glycyrrhetic acid can occur, requiring several days for complete body elimination (Koga, Kawamura, Iwase, & Yoshikawa, 2013). Also, during premenstrual syndrome, the use of this plant may cause water retention and bloating.
6. CONCLUDING REMARKS
This review has presented a comprehensive view about the phytochemistry composition and pharmacology activities of G. glabra. This plant has been broadly used as a traditional medicine and food industry ingredient, particularly as a flavour and sweetening agent. The roots are used in the prevention and treatment of several complications, especially microbial/viral infection, cancer, and skin inflammation. Among the bioactive compounds, flavonoids are the most important, being responsible for most of the biological activities. Different phytochemicals, including glycyrrhizin, 18β‐glycyrrhetinic acid, glabrin A and B, or isoflavones, have been identified and associated with the biological activities reported, namely, antioxidant, antiviral, antimicrobial, anticancer, or anti‐inflammatory activities as well as hepatoprotection. These activities generally agree with traditional knowledge and folk medicine. Indeed, the side effects and toxicity associated with liquorice are few and are mainly linked with hypertension and fluid retention. Regarding this particular thematic, to date, few studies have been conducted, aside from some isolated ones. Thus, side effects remain an area of potential future study. This review not only details and explains the traditional use of this plant but also highlights its potential uses for other industries, such as cosmetic or pharmaceutical ones. More clinical trials should be performed to provide scientific basis for new uses.
In conclusion, although evidence has grown in the past decade, there is still a need to conduct further robust double‐blind randomized controlled trial about G. glabra. There is also an immense scope to explore different combinations of liquorice preparations in a wide range of disorders.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
Francisca Rodrigues is thankful for her postdoc research grant from the project Operação NORTE‐01‐0145‐FEDER‐000011. Giulia Pastorino was a recipient of a PhD scholarship from the Italian Ministry of Education, Universities and Research (MIUR). This work received financial support from the European Union (European Regional Development Fund [FEDER] funds POCI/01/0145/FEDER/007265) and national funds (FCT/MEC, Fundação para a Ciência e a Tecnologia, and Ministério da Educação e Ciência) under the Partnership Agreement PT2020 UID/QUI/50006/2013.
Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytotherapy Research. 2018;32:2323–2339. 10.1002/ptr.6178
REFERENCES
- Ahn, J. , Lee, H. , Jang, J. , Kim, S. , & Ha, T. (2013). Anti‐obesity effects of glabridin‐rich supercritical carbon dioxide extract of licorice in high‐fat‐fed obese mice. Food and Chemical Toxicology, 51, 439–445. [DOI] [PubMed] [Google Scholar]
- Ahn, S.‐J. , Song, Y.‐D. , Mah, S.‐J. , Cho, E.‐J. , & Kook, J.‐K. (2014). Determination of optimal concentration of deglycyrrhizinated licorice root extract for preventing dental caries using a bacterial model system. Journal of Dental Sciences, 9(3), 214–220. [Google Scholar]
- Ajagannanavar, S. L. , Battur, H. , Shamarao, S. , Sivakumar, V. , Patil, P. U. , & Shanavas, P. (2014). Effect of aqueous and alcoholic licorice (Glycyrrhiza glabra) root extract against Streptococcus mutans and Lactobacillus acidophilus in comparison to chlorhexidine: An in vitro study. Journal of International Oral Health, 6(4), 29–34. [PMC free article] [PubMed] [Google Scholar]
- Albermann, M. E. , Musshoff, F. , Hagemeier, L. , & Madea, B. (2010). Determination of glycyrrhetic acid after consumption of liquorice and application to a fatality. Forensic Science International, 197(1), 35–39. [DOI] [PubMed] [Google Scholar]
- Armanini, D. , Fiore, C. , Mattarello, M. J. , Bielenberg, J. , & Palermo, M. (2002). History of the endocrine effects of licorice. Experimental and Clinical Endocrinology & Diabetes, 110(06), 257–261. [DOI] [PubMed] [Google Scholar]
- Asha, M. K. , Debraj, D. , Prashanth, D. , Edwin, J. R. , Srikanth, H. S. , Muruganantham, N. , … Agarwal, A. (2013). In vitro anti‐Helicobacter pylori activity of a flavonoid rich extract of Glycyrrhiza glabra and its probable mechanisms of action. Journal of Ethnopharmacology, 145(2), 581–586. [DOI] [PubMed] [Google Scholar]
- Asl, M. N. , & Hosseinzadeh, H. (2008). Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytotherapy Research, 22(6), 709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani, M. , Rafieian‐Kopaei, M. , Jeloudari, M. , Eftekhari, Z. , Delfan, B. , Zargaran, A. , & Forouzan, S. (2014). A review of the health effects and uses of drugs of plant licorice (Glycyrrhiza glabra L.) in Iran. Asian Pacific Journal of Tropical Disease, 4, S847–S849. [Google Scholar]
- Bardhan, K. D. , Cumberland, D. C. , Dixon, R. A. , & Holdsworth, C. D. (1978). Clinical trial of deglycyrrhizinised liquorice in gastric ulcer. Gut, 19(9), 779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube, B. J. , & Bubeck Wardenburg, J. (2013). Staphylococcus aureus α‐toxin: Nearly a century of intrigue. Toxins, 5(6), 1140–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi, D. M. , Rocco, C. , & Ruberto, G. (2003). New dihydrostilbene derivatives from the leaves of Glycyrrhiza glabra and evaluation of their antioxidant activity. Journal of Natural Products, 66(4), 477–480. [DOI] [PubMed] [Google Scholar]
- Cao, D. , Jiang, J. , You, L. , Jia, Z. , Tsukamoto, T. , Cai, H. , … Cao, X. (2016). The protective effects of 18β‐glycyrrhetinic acid on Helicobacter pylori‐infected gastric mucosa in Mongolian gerbils. BioMed Research International, 4943793, 2016, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli, E. , & Fogelman, Y. (2009). Antioxidant effect of polyphenolic glabridin on LDL oxidation. Toxicol Ind Health, 25(4–5), 321–324. [DOI] [PubMed] [Google Scholar]
- Castangia, I. , Caddeo, C. , Manca, M. L. , Casu, L. , Latorre, A. C. , Diez‐Sales, O. , … Manconi, M. (2015). Delivery of liquorice extract by liposomes and hyalurosomes to protect the skin against oxidative stress injuries. Carbohydrate Polymers, 134, 657–663. [DOI] [PubMed] [Google Scholar]
- Chakotiya, A. S. , Tanwar, A. , Narula, A. , & Sharma, R. K. (2016). Alternative to antibiotics against Pseudomonas aeruginosa: Effects of Glycyrrhiza glabra on membrane permeability and inhibition of efflux activity and biofilm formation in Pseudomonas aeruginosa and its in vitro time‐kill activity. Microbial Pathogenesis, 98, 98–105. [DOI] [PubMed] [Google Scholar]
- Chakotiya, A. S. , Tanwar, A. , Srivastava, P. , Narula, A. , & Sharma, R. K. (2017). Effect of aquo‐alchoholic extract of Glycyrrhiza glabra against Pseudomonas aeruginosa in mice lung infection model. Biomedicine & Pharmacotherapy, 90, 171–178. [DOI] [PubMed] [Google Scholar]
- Chakravarthi, K. K. , & Avadhani, R. (2013). Beneficial effect of aqueous root extract of Glycyrrhiza glabra on learning and memory using different behavioral models: An experimental study. Journal of Natural Science, Biology, and Medicine, 4(2), 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, J. H. , & Gunasekaran, H. (2017). Screening of the phytochemical, antimicrobial and antioxidant activity of Glycyrrhiza glabra root extract. Journal of Environmental Biology, 38(1), 161–165. [Google Scholar]
- Cheema, H. S. , Prakash, O. , Pal, A. , Khan, F. , Bawankule, D. U. , & Darokar, M. P. (2014). Glabridin induces oxidative stress mediated apoptosis like cell death of malaria parasite Plasmodium falciparum . Parasitology International, 63(2), 349–358. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Christensen, S. B. , Blom, J. , Lemmich, E. , Nadelmann, L. , Fich, K. , … Kharazmi, A. (1993). Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania . Antimicrobial Agents and Chemotherapy, 37(12), 2550–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. , Theander, T. G. , Christensen, S. B. , Hviid, L. , Zhai, L. , & Kharazmi, A. (1994). Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. yoelii infection. Antimicrobial Agents and Chemotherapy, 38(7), 1470–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinsembu, K. C. (2016). Plants and other natural products used in the management of oral infections and improvement of oral health. Acta Tropica, 154, 6–18. [DOI] [PubMed] [Google Scholar]
- Cho, S.‐M. , Shimizu, M. , Lee, C. J. , Han, D. S. , Jung, C. K. , Jo, J. H. , & Kim, Y. M. (2010). Hypnotic effects and binding studies for GABAA and 5‐HT2C receptors of traditional medicinal plants used in Asia for insomnia. Journal of Ethnopharmacology, 132(1), 225–232. [DOI] [PubMed] [Google Scholar]
- Choi, E. M. (2011). Glabridin protects osteoblastic MC3T3‐E1 cells against antimycin A induced cytotoxicity. Chemico‐Biological Interactions, 193(1), 71–78. [DOI] [PubMed] [Google Scholar]
- Choudhary, M. , Kumar, V. , Malhotra, H. , & Singh, S. (2015). Medicinal plants with potential anti‐arthritic activity. Journal of Intercultural Ethnopharmacology, 4(2), 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouitah, O. , Meddah, B. , Aoues, A. , & Sonnet, P. (2011). Chemical composition and antimicrobial activities of the essential oil from Glycyrrhiza glabra leaves. Journal of Essential Oil‐Bearing Plants, 14(3), 284–288. [Google Scholar]
- Cinatl, J. , Morgenstern, B. , Bauer, G. , Chandra, P. , Rabenau, H. , & Doerr, H. W. (2003). Glycyrrhizin, an active component of liquorice roots, and replication of SARS‐associated coronavirus. The Lancet, 361(9374), 2045–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle, M. (2014). Glycyrrhiza glabra (liquorice)—A potent medicinal herb. International Journal of Herbal Medicine, 2(2), 132–136. [Google Scholar]
- Deng, Q. P. , Wang, M. J. , Zeng, X. , Chen, G. G. , & Huang, R. Y. (2017). Effects of glycyrrhizin in a mouse model of lung adenocarcinoma. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 41(4), 1383–1392. [DOI] [PubMed] [Google Scholar]
- Dhingra, D. , & Sharma, A. (2006). Antidepressant‐like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 30(3), 449–454. [DOI] [PubMed] [Google Scholar]
- Di Paola, R. , Menegazzi, M. , Mazzon, E. , Genovese, T. , Crisafulli, C. , Dal Bosco, M. , … Cuzzocrea, S. (2009). Protective effects of glycyrrhizin in a gut hypoxia (ischemia)–reoxygenation (reperfusion) model. Intensive Care Medicine, 35(4), 687–697. [DOI] [PubMed] [Google Scholar]
- Ebanks, J. P. , Wickett, R. R. , & Boissy, R. E. (2009). Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. International Journal of Molecular Sciences, 10(9), 4066–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2005). Evaluation of certain food additives In Sixty‐third report of the Joint FAO/WHO Expert Committee on Food Additives. Singapore: World Health Organization. [Google Scholar]
- Fiore, C. , Eisenhut, M. , Ragazzi, E. , Zanchin, G. , & Armanini, D. (2005). A history of the therapeutic use of liquorice in Europe. Journal of Ethnopharmacology, 99(3), 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman, B. , & Aviram, M. (2001). Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Current Opinion in Lipidology, 12(1), 41–48. [DOI] [PubMed] [Google Scholar]
- Fuhrman, B. , Buch, S. , Vaya, J. , Belinky, P. A. , Coleman, R. , Hayek, T. , & Aviram, M. (1997). Licorice extract and its major polyphenol glabridin protect low‐density lipoprotein against lipid peroxidation: In vitro and ex vivo studies in humans and in atherosclerotic apolipoprotein E‐deficient mice. The American Journal of Clinical Nutrition, 66(2), 267–275. [DOI] [PubMed] [Google Scholar]
- Fukai, T. , Marumo, A. , Kaitou, K. , Kanda, T. , Terada, S. , & Nomura, T. (2002a). Anti‐Helicobacter pylori flavonoids from licorice extract. Life Sciences, 71(12), 1449–1463. [DOI] [PubMed] [Google Scholar]
- Fukai, T. , Marumo, A. , Kaitou, K. , Kanda, T. , Terada, S. , & Nomura, T. (2002b). Antimicrobial activity of licorice flavonoids against methicillin‐resistant Staphylococcus aureus . Fitoterapia, 73(6), 536–539. [DOI] [PubMed] [Google Scholar]
- Fukui, H. , Goto, K. , & Tabata, M. (1988). Two antimicrobial flavanones from the leaves of Glycyrrhiza glabra . Chemical & Pharmaceutical Bulletin, 36(10), 4174–4176. [DOI] [PubMed] [Google Scholar]
- Grassi, D. , Desideri, G. , & Ferri, C. (2010). Flavonoids: Antioxidants against atherosclerosis. Nutrients, 2(8), 889–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippaudo, F. R. , & Di Russo, P. P. (2016). Effects of topical application of β‐resorcinol and glycyrrhetinic acid monotherapy and in combination with fractional CO2 laser treatment for benign hand hyperpigmentation treatment. Journal of Cosmetic Dermatology, 15(4), 413–419. [DOI] [PubMed] [Google Scholar]
- Gupta, V. K. , Fatima, A. , Faridi, U. , Negi, A. S. , Shanker, K. , Kumar, J. K. , … Khanuja, S. P. S. (2008). Antimicrobial potential of Glycyrrhiza glabra roots. Journal of Ethnopharmacology, 116(2), 377–380. [DOI] [PubMed] [Google Scholar]
- Hajiaghamohammadi, A. A. , Ziaee, A. , & Samimi, R. (2012). The efficacy of licorice root extract in decreasing transaminase activities in non‐alcoholic fatty liver disease: A randomized controlled clinical trial. Phytotherapy Research, 26(9), 1381–1384. [DOI] [PubMed] [Google Scholar]
- Hajirahimkhan, A. , Simmler, C. , Yuan, Y. , Anderson, J. R. , Chen, S. N. , Nikolic, D. , … Bolton, J. L. (2013). Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS One, 8(7), e67947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder, R. M. , & Richards, G. M. (2004). Topical agents used in the management of hyperpigmentation. Skin Therapy Letter, 9(6), 1–3. [PubMed] [Google Scholar]
- Hanrahan, J. R. , Chebib, M. , & Johnston, G. A. (2011). Flavonoid modulation of GABA (A) receptors. British Journal of Pharmacology, 163(2), 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi, H. , Ishikawa, H. , Mizutani, K. , Tamura, Y. , & Kinoshita, T. (1998). Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata . Bioorganic & Medicinal Chemistry, 6(3), 339–347. [DOI] [PubMed] [Google Scholar]
- Harwansh, R. K. , Patra, K. C. , Pareta, S. K. , Singh, J. , & Biswas, R. (2011). Pharmacological studies of Glycyrrhiza glabra: A review. Pharmacology, 2, 1032–1038. [Google Scholar]
- Hasan, S. K. , Siddiqi, A. , Nafees, S. , Ali, N. , Rashid, S. , Ali, R. , … Sultana, S. (2016). Chemopreventive effect of 18β‐glycyrrhetinic acid via modulation of inflammatory markers and induction of apoptosis in human hepatoma cell line (HepG2). Molecular and Cellular Biochemistry, 416(1–2), 169–177. [DOI] [PubMed] [Google Scholar]
- Hasanein, P. (2011). Glabridin as a major active isoflavan from Glycyrrhiza glabra (licorice) reverses learning and memory deficits in diabetic rats. Acta Physiologica Hungarica, 98(2), 221–230. [DOI] [PubMed] [Google Scholar]
- Hattori, T. , Ikematsu, S. , Koito, A. , Matsushita, S. , Maeda, Y. , Hada, M. , … Takatsuki, K. (1989). Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antiviral Research, 11(5), 255–261. [DOI] [PubMed] [Google Scholar]
- Hayashi, H. , & Sudo, H. (2009). Economic importance of licorice. Plant Biotechnology, 26(1), 101–104. [Google Scholar]
- Hayashi, H. , Tamura, S. , Chiba, R. , Fujii, I. , Yoshikawa, N. , Fattokhov, I. , & Saidov, M. (2016). Field survey of Glycyrrhiza plants in Central Asia (4). Characterization of G. glabra and G. bucharica collected in Tajikistan. Biological & Pharmaceutical Bulletin, 39(11), 1781–1786. [DOI] [PubMed] [Google Scholar]
- Herrera, M. , Herrera, A. , & Ariño, A. (2009). Estimation of dietary intake of ochratoxin A from liquorice confectionery. Food and Chemical Toxicology, 47(8), 2002–2006. [DOI] [PubMed] [Google Scholar]
- Hoffmann, K. M. , Beltrán, L. , Ziemba, P. M. , Hatt, H. , & Gisselmann, G. (2016). Potentiating effect of glabridin from Glycyrrhiza glabra on GABAA receptors. Biochemistry and Biophysics Reports, 6, 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, M. J. , Chen, M. K. , Chen, C. J. , Hsieh, M. C. , Lo, Y. S. , Chuang, Y. C. , … Yang, S. F. (2016). Glabridin induces apoptosis and autophagy through JNK1/2 pathway in human hepatoma cells. Phytomedicine, 23(4), 359–366. [DOI] [PubMed] [Google Scholar]
- Hsu, Y.‐L. , Kuo, P.‐L. , & Lin, C.‐C. (2005). Isoliquiritigenin induces apoptosis and cell cycle arrest through p53‐dependent pathway in Hep G2 cells. Life Sciences, 77(3), 279–292. [DOI] [PubMed] [Google Scholar]
- Huan, C. C. , Wang, H. X. , Sheng, X. X. , Wang, R. , Wang, X. , & Mao, X. (2017). Glycyrrhizin inhibits porcine epidemic diarrhea virus infection and attenuates the proinflammatory responses by inhibition of high mobility group box‐1 protein. Archives of Virology, 162(6), 1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, W. , Chen, X. , Li, Q. , Li, P. , Zhao, G. , Xu, M. , & Xie, P. (2012). Inhibition of intercellular adhesion in herpes simplex virus infection by glycyrrhizin. Cell Biochemistry and Biophysics, 62(1), 137–140. [DOI] [PubMed] [Google Scholar]
- Huang, Y. C. , Kuo, C. L. , Lu, K. W. , Lin, J. J. , Yang, J. L. , Wu, R. S. , … Chung, J. G. (2016). 18α‐Glycyrrhetinic acid induces apoptosis of HL‐60 human leukemia cells through caspases‐ and mitochondria‐dependent signaling pathways. Molecules, 21(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo, H. Z. , Wang, B. , Liang, Y. K. , Bao, Y. Y. , & Gu, Y. (2011). Hepatoprotective and antioxidant effects of licorice extract against CCl(4)‐induced oxidative damage in rats. International Journal of Molecular Sciences, 12(10), 6529–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbrucker, R. A. , & Burdock, G. A. (2006). Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regulatory Toxicology and Pharmacology, 46(3), 167–192. [DOI] [PubMed] [Google Scholar]
- Jafarian, M. M. , & Ghazvini, K. (2007). In vitro susceptibility of Helicobacter pylori to licorice extract. Iranian Journal of Pharmaceutical Research, 6(1), 69–72. [Google Scholar]
- Jiang, F. , Li, Y. , Mu, J. , Hu, C. , Zhou, M. , Wang, X. , … Li, Z. (2016). Glabridin inhibits cancer stem cell‐like properties of human breast cancer cells: An epigenetic regulation of miR‐148a/SMAd2 signaling. Molecular Carcinogenesis, 55(5), 929–940. [DOI] [PubMed] [Google Scholar]
- Jin, Z. , Kim, S. , Cho, S. , Kim, I. H. , Han, D. , & Jin, Y. H. (2013). Potentiating effect of glabridin on GABAA receptor‐mediated responses in dorsal raphe neurons. Planta Medica, 79(15), 1408–1412. [DOI] [PubMed] [Google Scholar]
- Jung, J. I. , Lim, S. S. , Choi, H. J. , Cho, H. J. , Shin, H.‐K. , Kim, E. J. , … Park, J. H. (2006). Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. The Journal of Nutritional Biochemistry, 17(10), 689–696. [DOI] [PubMed] [Google Scholar]
- Kageyama, Y. , Suzuki, H. , & Saruta, T. (1994). Role of glucocorticoid in the development of glycyrrhizin‐induced hypertension. Clinical and Experimental Hypertension, 16(6), 761–778. [DOI] [PubMed] [Google Scholar]
- Kamei, J. , Nakamura, R. , Ichiki, H. , & Kubo, M. (2003). Antitussive principles of Glycyrrhizae radix, a main component of the Kampo preparations Bakumondo‐to (Mai‐men‐dong‐tang). European Journal of Pharmacology, 469(1–3), 159–163. [DOI] [PubMed] [Google Scholar]
- Kanazawa, M. , Satomi, Y. , Mizutani, Y. , Ukimura, O. , Kawauchi, A. , Sakai, T. , … Miki, T. (2003). Isoliquiritigenin inhibits the growth of prostate cancer. European Urology, 43(5), 580–586. [DOI] [PubMed] [Google Scholar]
- Karahan, F. , Avsar, C. , Ozyigit, I. I. , & Berber, I. (2016). Antimicrobial and antioxidant activities of medicinal plant Glycyrrhiza glabra var. glandulifera from different habitats. Biotechnology & Biotechnological Equipment, 30(4), 797–804. [Google Scholar]
- Kim, S. H. , Hong, J. H. , Lee, J. E. , & Lee, Y. C. (2017). 18β‐Glycyrrhetinic acid, the major bioactive component of Glycyrrhizae radix, attenuates airway inflammation by modulating Th2 cytokines, GATA‐3, STAT6, and Foxp3 transcription factors in an asthmatic mouse model. Environmental Toxicology and Pharmacology, 52, 99–113. [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , & Park, M. J. (2012). Effects of phytoestrogen on sexual development. Korean Journal of Pediatrics, 55(8), 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. J. , & Uyama, H. (2005). Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cellular and Molecular Life Sciences, 62(15), 1707–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga, K. , Kawamura, M. , Iwase, H. , & Yoshikawa, N. (2013). Intestinal absorption and biliary elimination of glycyrrhizic acid diethyl ester in rats. Drug Design, Development and Therapy, 7, 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausse, R. , Bielenberg, J. , Blaschek, W. , & Ullmann, U. (2004). In vitro anti‐Helicobacter pylori activity of extractum liquiritiae, glycyrrhizin and its metabolites. Journal of Antimicrobial Chemotherapy, 54(1), 243–246. [DOI] [PubMed] [Google Scholar]
- Kumar, B. S. , Hemalatha, T. , Deepachitra, R. , Raghavan, R. N. , Prabu, P. , & Sastry, T. P. (2015). Biphasic calcium phosphate‐casein bone graft fortified with Cassia occidentalis for bone tissue engineering and regeneration. Indian Academy of Sciences, 38. [Google Scholar]
- Kuo, K. K. , Chang, J. S. , Wang, K. C. , & Chiang, L. C. (2009). Water extract of Glycyrrhiza uralensis inhibited enterovirus 71 in a human foreskin fibroblast cell line. American Journal Chinese Medicine, 37(2), 383–394. [DOI] [PubMed] [Google Scholar]
- Lee, C. S. , Kim, Y. J. , Lee, M. S. , Han, E. S. , & Lee, S. J. (2008). 18β‐Glycyrrhetinic acid induces apoptotic cell death in SiHa cells and exhibits a synergistic effect against antibiotic anti‐cancer drug toxicity. Life Sciences, 83(13–14), 481–489. [DOI] [PubMed] [Google Scholar]
- Lee, J. Y. , Lee, J. H. , Park, J. H. , Kim, S. Y. , Choi, J. Y. , Lee, S. H. , … Han, Y. (2009). Liquiritigenin, a licorice flavonoid, helps mice resist disseminated candidiasis due to Candida albicans by Th1 immune response, whereas liquiritin, its glycoside form, does not. International Immunopharmacology, 9(5), 632–638. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Feng, L. , Song, Z. F. , Li, H. B. , & Huai, Q. Y. (2016). Synthesis and anticancer activities of glycyrrhetinic acid derivatives. Molecules, 21(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Pi, F. , Zhang, H. , Ji, J. , Xia, S. , Cui, F. , … Sun, X. (2017). Metabolomics analysis to evaluate the anti‐inflammatory effects of polyphenols: Glabridin reversed metabolism change caused by LPS in RAW 264.7 cells. Journal of Agricultural and Food Chemistry, 65(29), 6070–6079. [DOI] [PubMed] [Google Scholar]
- Mahmoud, A. M. , Hussein, O. E. , Hozayen, W. G. , & Abd El‐Twab, S. M. (2017). Methotrexate hepatotoxicity is associated with oxidative stress, and down‐regulation of PPARgamma and Nrf2: Protective effect of 18β‐glycyrrhetinic acid. Chemico‐Biological Interactions, 270, 59–72. [DOI] [PubMed] [Google Scholar]
- Mendes‐Silva, W. , Assafim, M. , Ruta, B. , Monteiro, R. Q. , Guimaraes, J. A. , & Zingali, R. B. (2003). Antithrombotic effect of glycyrrhizin, a plant‐derived thrombin inhibitor. Thrombosis Research, 112(1–2), 93–98. [DOI] [PubMed] [Google Scholar]
- Messier, C. , & Grenier, D. (2011). Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans . Mycoses, 54(6), e801–e806. [DOI] [PubMed] [Google Scholar]
- Michaelis, M. , Geiler, J. , Naczk, P. , Sithisarn, P. , Leutz, A. , Doerr, H. W. , & Cinatl, J. (2011). Glycyrrhizin exerts antioxidative effects in H5N1 influenza a virus‐infected cells and inhibits virus replication and pro‐inflammatory gene expression. PLoS One, 6(5), e19705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, N. K. , Bstia, S. , Mishra, G. , Chowdary, K. A. , & Patra, S. (2001). Anti‐arthritic activity of Glycyrrhiza glabra, Boswellia serrata and their synergistic activity in combined formulation studied in Freund's adjuvant induced arthritic rats. Journal of Pharmaceutical Education and Research, 2, 92–98. [Google Scholar]
- Mitra Mazumder, P. , Pattnayak, S. , Parvani, H. , Sasmal, D. , & Rathinavelusamy, P. (2012). Evaluation of immunomodulatory activity of Glycyrhiza glabra L. roots in combination with zing. Asian Pacific Journal of Tropical Biomedicine, 2(1), S15–S20. [Google Scholar]
- Moazeni, M. , Hedayati, M. T. , Nabili, M. , Mousavi, S. J. , Abdollahi Gohar, A. , & Gholami, S. (2017). Glabridin triggers over‐expression of MCA1 and NUC1 genes in Candida glabrata: Is it an apoptosis inducer? Journal de Mycologie Medicale, 27(3), 369–375. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, M. , & Panja, P. (2008). A novel process for extraction of natural sweetener from licorice (Glycyrrhiza glabra) roots. Separation and Purification Technology, 63(3), 539–545. [Google Scholar]
- Nakagawa, K. , Kishida, H. , Arai, N. , Nishiyama, T. , & Mae, T. (2004). Licorice flavonoids suppress abdominal fat accumulation and increase in blood glucose level in obese diabetic KK‐A(y) mice. Biological & Pharmaceutical Bulletin, 27(11), 1775–1778. [DOI] [PubMed] [Google Scholar]
- Nerya, O. , Vaya, J. , Musa, R. , Izrael, S. , Ben‐Arie, R. , & Tamir, S. (2003). Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. Journal of Agricultural and Food Chemistry, 51(5), 1201–1207. [DOI] [PubMed] [Google Scholar]
- Ohtsuki, K. , Oh‐Ishi, M. , & Nagata, N. (1992). The stimulatory and inhibitory effects of glycyrrhizin and a glycyrrhetinic acid derivative on phosphorylation of lipocortin I by A‐kinase in vitro. Biochemistry International, 28(6), 1045–1053. [PubMed] [Google Scholar]
- Ojha, S. , Golechha, M. , Kumari, S. , Bhatia, J. , & Arya, D. S. (2013). Glycyrrhiza glabra protects from myocardial ischemia–reperfusion injury by improving hemodynamic, biochemical, histopathological and ventricular function. Experimental and Toxicologic Pathology, 65(1–2), 219–227. [DOI] [PubMed] [Google Scholar]
- Omar, H. R. , Komarova, I. , El‐Ghonemi, M. , Fathy, A. , Rashad, R. , Abdelmalak, H. D. , … Camporesi, E. M. (2012). Licorice abuse: Time to send a warning message. Therapeutic Advances in Endocrinology and Metabolism, 3(4), 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama, K. , Kawada‐Matsuo, M. , Oogai, Y. , Hayashi, T. , Nakamura, N. , & Komatsuzawa, H. (2016). Antibacterial Effects of Glycyrrhetinic Acid and Its Derivatives on Staphylococcus aureus. PLoS One, 11(11), e0165831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M. R. , Kim, S. G. , Cho, I. A. , Oh, D. , Kang, K. R. , Lee, S. Y. , … Kim, J. S. (2015). Licochalcone‐A induces intrinsic and extrinsic apoptosis via ERK1/2 and p38 phosphorylation‐mediated TRAIL expression in head and neck squamous carcinoma FaDu cells. Food and Chemical Toxicology, 77, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parle, M. , Dhingra, D. , & Kulkarni, S. K. (2004). Memory‐strengthening activity of Glycyrrhiza glabra in exteroceptive and interoceptive behavioral models. Journal of Medicinal Food, 7(4), 462–466. [DOI] [PubMed] [Google Scholar]
- Parvez, S. , Kang, M. , Chung, H. S. , & Bae, H. (2007). Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytotherapy Research, 21(9), 805–816. [DOI] [PubMed] [Google Scholar]
- Ploeger, B. , Mensinga, T. , Sips, A. , Seinen, W. , Meulenbelt, J. , & DeJongh, J. (2001). The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metabolism Reviews, 33(2), 125–147. [DOI] [PubMed] [Google Scholar]
- Powers, C. N. , & Setzer, W. N. (2015). A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements. In Silico Pharmacology, 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackova, L. , Jancinova, V. , Petrikova, M. , Drabikova, K. , Nosal, R. , Stefek, M. , … Kovácová, M. (2007). Mechanism of anti‐inflammatory action of liquorice extract and glycyrrhizin. Natural Product Research, 21(14), 1234–1241. [DOI] [PubMed] [Google Scholar]
- Rajesh, L. (2004). Protective activity of Glycyrrhiza glabra Linn. on carbon tetrachloride‐induced peroxidative damage. Indian Journal of Pharmacology, 36, 284–287. [Google Scholar]
- Ram, H. N. A. , Lachake, P. , Kaushik, U. , & Shreedhara, C. S. (2010). Formulation and evaluation of floating tablets of liquorice extract. Pharmacognosy Research, 2(5), 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzato, G. , Scalabrin, E. , Radaelli, M. , Capodaglio, G. , & Piccolo, O. (2017). A new exploration of licorice metabolome. Food Chemistry, 221, 959–968. [DOI] [PubMed] [Google Scholar]
- Sabde, S. , Bodiwala, H. S. , Karmase, A. , Deshpande, P. J. , Kaur, A. , Ahmed, N. , … Singh, I. P. (2011). Anti‐HIV activity of Indian medicinal plants. Journal of Natural Medicines, 65(3), 662–669. [DOI] [PubMed] [Google Scholar]
- Saeedi, M. , Morteza‐Semnani, K. , & Ghoreishi, M. R. (2003). The treatment of atopic dermatitis with licorice gel. The Journal of Dermatological Treatment, 14(3), 153–157. [DOI] [PubMed] [Google Scholar]
- Sasaki, H. , Takei, M. , Kobayashi, M. , Pollard, R. B. , & Suzuki, F. (2002). Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV‐seropositive patients. Pathobiology: Journal of Immunopathology, Molecular and Cellular Biology, 70(4), 229–236. [DOI] [PubMed] [Google Scholar]
- Sato, H. , Goto, W. , Yamamura, J. , Kurokawa, M. , Kageyama, S. , Takahara, T. , … Shiraki, K. (1996). Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Research, 30(2–3), 171–177. [DOI] [PubMed] [Google Scholar]
- Sato, J. , Goto, K. , Nanjo, F. , Kawai, S. , & Murata, K. (2000). Antifungal activity of plant extracts against Arthrinium sacchari and Chaetomium funicola . Journal of Bioscience and Bioengineering, 90(4), 442–446. [PubMed] [Google Scholar]
- Saumendu, D. R. , Raj, K. P. , Suvakanta, D. , Jashabir, C. , & Biswajit, D. (2014). Hair growth stimulating effect and phytochemical evaluation of hydro‐alcoholic extract of Glycyrrhiza glabra . Global Journal of Research on Medicinal Plants & Indigenous Medicine, 3(2), 40–47. [Google Scholar]
- Sekizawa, T. , Yanagi, K. , & Itoyama, Y. (2001). Glycyrrhizin increases survival of mice with herpes simplex encephalitis. Acta Virologica, 45(1), 51–54. [PubMed] [Google Scholar]
- Shalaby, A. M. , Ibrahim, H. S. , Mahmoud, E. M. , & Mahmoud, A. F. (2004). Some effects of Glycyrrhiza glabra (liquorice) roots extract on male rats. Egyptian Journal of Natural Toxins, 1, 83–94. [Google Scholar]
- Sharma, G. , Kar, S. , Palit, S. , & Das, P. K. (2012). 18β‐Glycyrrhetinic acid induces apoptosis through modulation of Akt/FOXO3a/Bim pathway in human breast cancer MCF‐7 cells. Journal of Cellular Physiology, 227(5), 1923–1931. [DOI] [PubMed] [Google Scholar]
- Sharma, V. , & Agrawal, R. C. (2017). In vivo antioxidant and hepatoprotective potential of Glycyrrhiza glabra extract on carbon tetra chloride (CCl4) induced oxidative‐stress mediated hepatotoxicity. International Journal of Research in Medical Sciences, 2(1), 7. [Google Scholar]
- Sharma, V. , Agrawal, R. C. , & Shrivastava, V. K. (2014). Assessment of median lethal dose and anti‐mutagenic effects of Glycyrrhiza glabra root extract against chemically induced micronucleus formation in Swiss albino mice. International Journal of Basic & Clinical Pharmacology, 3(2), 292–297. [Google Scholar]
- Sharma, V. , Katiyar, A. , & Agrawal, R. C. (2016). Glycyrrhiza glabra: Chemistry and pharmacological activity In Merillon J.‐M., & Ramawat K. G. (Eds.), Sweeteners: Pharmacology, biotechnology, and applications (pp. 1–14). Cham: Springer International Publishing. [Google Scholar]
- Shin, Y. W. , Bae, E. A. , Lee, B. , Lee, S. H. , Kim, J. A. , Kim, Y. S. , & Kim, D. H. (2007). In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta Medica, 73(3), 257–261. [DOI] [PubMed] [Google Scholar]
- Simmler, C. , Pauli, G. F. , & Chen, S.‐N. (2013). Phytochemistry and biological properties of glabridin. Fitoterapia, 90, 160–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, V. , Pal, A. , & Darokar, M. P. (2015). A polyphenolic flavonoid glabridin: Oxidative stress response in multidrug‐resistant Staphylococcus aureus . Free Radical Biology and Medicine, 87, 48–57. [DOI] [PubMed] [Google Scholar]
- Siracusa, L. , Saija, A. , Cristani, M. , Cimino, F. , D'Arrigo, M. , Trombetta, D. , … Ruberto, G. (2011). Phytocomplexes from liquorice (Glycyrrhiza glabra L.) leaves—Chemical characterization and evaluation of their antioxidant, anti‐genotoxic and anti‐inflammatory activity. Fitoterapia, 82(4), 546–556. [DOI] [PubMed] [Google Scholar]
- Solano, F. , Briganti, S. , Picardo, M. , & Ghanem, G. (2006). Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Research, 19(6), 550–571. [DOI] [PubMed] [Google Scholar]
- Somjen, D. , Katzburg, S. , Vaya, J. , Kaye, A. M. , Hendel, D. , Posner, G. H. , & Tamir, S. (2004). Estrogenic activity of glabridin and glabrene from licorice roots on human osteoblasts and prepubertal rat skeletal tissues. Journal of Steroid Biochemistry and Molecular Biology, 91(4–5), 241–246. [DOI] [PubMed] [Google Scholar]
- Soufy, H. , Yassein, S. , Ahmed, A. R. , Khodier, M. H. , Kutkat, M. A. , Nasr, S. M. , & Okda, F. A. (2012). Antiviral and immune stimulant activities of glycyrrhizin against duck hepatitis virus. African Journal of Traditional, Complementary, and Alternative Medicines, 9(3), 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Wei Poh, M. , Voon Chen Yong, P. , Viseswaran, N. , & Chia, Y. Y. (2015). Estrogenicity of glabridin in Ishikawa cells. PLoS One, 10(3), e0121382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takii, H. , Kometani, T. , Nishimura, T. , Nakae, T. , Okada, S. , & Fushiki, T. (2001). Antidiabetic effect of glycyrrhizin in genetically diabetic KK‐Ay mice. Biological & Pharmaceutical Bulletin, 24(5), 484–487. [DOI] [PubMed] [Google Scholar]
- Tamir, S. , Eizenberg, M. , Somjen, D. , Izrael, S. , & Vaya, J. (2001). Estrogen‐like activity of glabrene and other constituents isolated from licorice root. Journal of Steroid Biochemistry and Molecular Biology, 78(3), 291–298. [DOI] [PubMed] [Google Scholar]
- Tamir, S. , Eizenberg, M. , Somjen, D. , Stern, N. , Shelach, R. , Kaye, A. , & Vaya, J. (2000). Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Research, 60(20), 5704–5709. [PubMed] [Google Scholar]
- Tian, J. , Liu, W. , Zhen, Z. , & Tong, X. (2013). Successful treatment of latent autoimmune diabetes in adults with traditional Chinese medicine: A case report. Journal of Traditional Chinese Medicine, 33(6), 766–769. [DOI] [PubMed] [Google Scholar]
- Tong, X. , Xie, Q. , Rong, G. , Zhou, S. , & Meng, Q. (2015). Detection of consensuses and treatment principles of diabetic nephropathy in traditional Chinese medicine: A new approach. Journal of Traditional Chinese Medical Sciences, 2(4), 270–283. [Google Scholar]
- Tung, N. H. , Shoyama, Y. , Wada, M. , & Tanaka, H. (2014). Improved in vitro fertilization ability of mouse sperm caused by the addition of licorice extract to the preincubation medium. The Open Reproductive Science Journal, 6, 1–7. [Google Scholar]
- Tung, N. H. , Shoyama, Y. , Wada, M. , & Tanaka, H. (2015). Two activators of in vitro fertilization in mice from licorice. Biochemical and Biophysical Research Communications, 467(2), 447–450. [DOI] [PubMed] [Google Scholar]
- van Rossum, T. G. , Vulto, A. G. , de Man, R. A. , Brouwer, J. T. , & Schalm, S. W. (1998). Review article: Glycyrrhizin as a potential treatment for chronic hepatitis C. Alimentary Pharmacology & Therapeutics, 12(3), 199–205. [DOI] [PubMed] [Google Scholar]
- van Rossum, T. G. , Vulto, A. G. , Hop, W. C. , & Schalm, S. W. (1999). Pharmacokinetics of intravenous glycyrrhizin after single and multiple doses in patients with chronic hepatitis C infection. Clinical Therapeutics, 21(12), 2080–2090. [DOI] [PubMed] [Google Scholar]
- Varsha, A. R. , & Sonam, P. (2013). Phytochemical screening and determination of anti‐bacterial and anti‐oxidant potential of Glycyrrhiza glabra root extracts. Journal of Environmental Research and Development, 7(4A), 1552–1558. [Google Scholar]
- Veratti, E. , Rossi, T. , Giudice, S. , Benassi, L. , Bertazzoni, G. , Morini, D. , … Magnoni, C. (2011). 18β‐Glycyrrhetinic acid and glabridin prevent oxidative DNA fragmentation in UVB‐irradiated human keratinocyte cultures. Anticancer Research, 31(6), 2209–2215. [PubMed] [Google Scholar]
- Wang, L. , Yang, R. , Yuan, B. , Liu, Y. , & Liu, C. (2015). The antiviral and antimicrobial activities of licorice, a widely‐used Chinese herb. Acta Pharmaceutica Sinica B, 5(4), 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Qian, Y. , Wang, Q. , Yang, Y.‐f. , Ji, S. , Song, W. , … Ye, M. (2015). Metabolites identification of bioactive licorice compounds in rats. Journal of Pharmaceutical and Biomedical Analysis, 115, 515–522. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Shen, Y. , Qiu, R. , Chen, Z. , Chen, Z. , & Chen, W. (2017). 18β‐Glycyrrhetinic acid exhibits potent antitumor effects against colorectal cancer via inhibition of cell proliferation and migration. International Journal of Oncology, 51(2), 615–624. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Zhang, H. , Chen, L. , Shan, L. , Fan, G. , & Gao, X. (2013). Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. Journal of Ethnopharmacology, 150(3), 781–790. [DOI] [PubMed] [Google Scholar]
- Wang, X. R. , Hao, H. G. , & Chu, L. (2017). Glycyrrhizin inhibits LPS‐induced inflammatory mediator production in endometrial epithelial cells. Microbial Pathogenesis, 109, 110–113. [DOI] [PubMed] [Google Scholar]
- Wu, F. , Jin, Z. , & Jin, J. (2013). Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Molecular Medicine Reports, 7(4), 1278–1282. [DOI] [PubMed] [Google Scholar]
- Xiao, X. Y. , Hao, M. , Yang, X. Y. , Ba, Q. , Li, M. , Ni, S. J. , … du, X. (2011). Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Letters, 302(1), 69–75. [DOI] [PubMed] [Google Scholar]
- Xiao, Y. , Xu, J. , Mao, C. , Jin, M. , Wu, Q. , Zou, J. , … Zhang, Y. (2010). 18β‐Glycyrrhetinic acid ameliorates acute Propionibacterium acnes‐induced liver injury through inhibition of macrophage inflammatory protein‐1α. The Journal of Biological Chemistry, 285(2), 1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki, S. , Morita, T. , Endo, H. , Hamamoto, T. , Baba, M. , Joichi, Y. , … Tokue, A. (2002). Isoliquiritigenin suppresses pulmonary metastasis of mouse renal cell carcinoma. Cancer Letters, 183(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Yang, R. , Yuan, B. C. , Ma, Y. S. , Zhou, S. , & Liu, Y. (2017). The anti‐inflammatory activity of licorice, a widely used Chinese herb. Pharmaceutical Biology, 55(1), 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Yang, L. , Han, Y. , Wu, Z. , Chen, P. , Zhang, H. , & Zhou, J. (2017). Protective effects of hepatocyte‐specific glycyrrhetic derivatives against carbon tetrachloride‐induced liver damage in mice. Bioorganic Chemistry, 72, 42–50. [DOI] [PubMed] [Google Scholar]
- Yasui, S. , Fujiwara, K. , Tawada, A. , Fukuda, Y. , Nakano, M. , & Yokosuka, O. (2011). Efficacy of intravenous glycyrrhizin in the early stage of acute onset autoimmune hepatitis. Digestive Diseases and Sciences, 56(12), 3638–3647. [DOI] [PubMed] [Google Scholar]
- Yin, X. , Gong, X. , Zhang, L. , Jiang, R. , Kuang, G. , Wang, B. , … Wan, J. (2017). Glycyrrhetinic acid attenuates lipopolysaccharide‐induced fulminant hepatic failure in d‐galactosamine‐sensitized mice by up‐regulating expression of interleukin‐1 receptor‐associated kinase‐M. Toxicology and Applied Pharmacology, 320, 8–16. [DOI] [PubMed] [Google Scholar]
- Yokota, T. , Nishio, H. , Kubota, Y. , & Mizoguchi, M. (1998). The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Research, 11(6), 355–361. [DOI] [PubMed] [Google Scholar]
- Yu, J. Y. , Ha, J. Y. , Kim, K. M. , Jung, Y. S. , Jung, J. C. , & Oh, S. (2015). Anti‐inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules, 20(7), 13041–13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S. J. , Cho, I. A. , Kang, K. R. , Jung, Y. R. , Cho, S. S. , Yoon, G. , … Kim, J. S. (2017). Licochalcone‐E induces caspase‐dependent death of human pharyngeal squamous carcinoma cells through the extrinsic and intrinsic apoptotic signaling pathways. Oncology Letters, 13(5), 3662–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadeh, J. B. , Kor, Z. M. , & Goftar, M. K. (2013). Licorice (Glycyrrhiza glabra Linn) as a valuable medicinal plant. International Journal of Advanced Biological and Biomedical Research, 1(10), 1281–1288. [Google Scholar]
- Zamansoltani, F. , Nassiri‐Asl, M. , Sarookhani, M. R. , Jahani‐Hashemi, H. , & Zangivand, A. A. (2009). Antiandrogenic activities of Glycyrrhiza glabra in male rats. International Journal of Andrology, 32(4), 417–422. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Song, Y. , & Zhang, Z. (2012). Glycyrrhizin administration ameliorates coxsackievirus B3‐induced myocarditis in mice. The American Journal of the Medical Sciences, 344(3), 206–210. [DOI] [PubMed] [Google Scholar]