Summary
Cough is the single most common reason for primary care physician visits and, when chronic, a frequent indication for specialist referrals. In children, a chronic cough (>4 weeks) is associated with increased morbidity and reduced quality of life. One common cause of childhood chronic cough is protracted bacterial bronchitis (PBB), especially in children aged <6 years. PBB is characterized by a chronic wet or productive cough without signs of an alternative cause and responds to 2 weeks of appropriate antibiotics, such as amoxicillin‐clavulanate. Most children with PBB are unable to expectorate sputum. If bronchoscopy and bronchoalveolar lavage are performed, evidence of bronchitis and purulent endobronchial secretions are seen. Bronchoalveolar lavage specimens typically reveal marked neutrophil infiltration and culture large numbers of respiratory bacterial pathogens, especially Haemophilus influenzae. Although regarded as having a good prognosis, recurrences are common and if these are frequent or do not respond to antibiotic treatments of up to 4‐weeks duration, the child should be investigated for other causes of chronic wet cough, such as bronchiectasis. The contribution of airway malacia and pathobiologic mechanisms of PBB remain uncertain and, other than reduced alveolar phagocytosis, evidence of systemic, or local immune deficiency is lacking. Instead, pulmonary defenses show activated innate immunity and increased gene expression of the interleukin‐1β signalling pathway. Whether these changes in local inflammatory responses are cause or effect remains to be determined. It is likely that PBB and bronchiectasis are at the opposite ends of the same disease spectrum, so children with chronic wet cough require close monitoring. Pediatr Pulmonol. 2016;51:225–242. © 2015 Wiley Periodicals, Inc.
Keywords: airways, bacterial bronchitis, Haemophilus influenzae, infection, inflammation, mechanism
Abbreviations
- AdV
adenovirus
- BAL
bronchoalveolar lavage
- BTS
British Thoracic Society
- CF
cystic fibrosis
- CFU
colony‐forming units
- CI
confidence interval
- CSLD
chronic suppurative lung disease
- CT
computed tomography
- hBD2
human β‐defensin 2
- IL
interleukin
- IQR
interquartile range
- IRAK
IL‐1 receptor associated kinase
- MBL
mannose‐binding lectin
- MMP
matrix metalloproteinase
- NNT‐B
number needed to treat for benefit
- NTHi
non typeable Haemophilus influenzae
- OR
odds ratio
- PBB
protracted bacterial bronchitis
- PCR
polymerase chain reaction
- QoL
quality of life
- RAGE
receptor for advanced glycation end‐products
- RCT
randomized controlled trial
- SP‐A
surfactant protein‐A
- sRAGE
soluble receptor for advanced glycation end‐products
- TLR
toll‐like receptor
- TNF
tumor necrosis factor
INTRODUCTION
Cough is the single most common reason given for patients seeking treatment in primary care.1, 2 In Australia, 7% of acute medical consultations (1.38 million/year) are for a coughing illness.1, 3 Among these presentations are children with a chronic cough >4‐weeks duration where in the previous 12 months as many as 80% are likely to have seen a doctor for their symptoms on more than five occasions.4 Chronic cough in childhood is also associated with considerable morbidity5 and decreased quality of life (QoL) scores,4, 6 which normalize once the cough resolves following appropriate therapy.7
Of the many possible etiologies, single8 and multi‐center studies7 recruiting primarily from pediatric referral centers have found that protracted bacterial bronchitis (PBB) is the most common cause of chronic cough in childhood, exceeding by two‐to‐threefold other underlying diagnoses, including asthma. This article summarizes current knowledge of PBB in children, presents further preliminary data on underlying disease mechanisms, and identifies research priorities to help further close the information gap for this common, but poorly understood condition.
DEFINITIONS
The term PBB was first defined a priori and was based on our clinical experience before being applied to a subgroup of children in our prospective study evaluating the etiology of chronic cough.8 The diagnostic criteria were: (i) history of chronic wet cough; (ii) positive bronchoalveolar lavage (BAL) fluid cultures for respiratory bacterial pathogens at densities ≥104 colony‐forming units (CFU)/ml of BAL fluid without serologic or polymerase chain reaction (PCR) assay evidence of infection by either Bordetella pertussis or Mycoplasma pneumoniae; and (iii) cough resolution after a 2‐week course of oral antibiotics (amoxicillin‐clavulanate).8
However, undertaking flexible bronchoscopy and BAL on every young child with a chronic wet cough is impractical and many older children are also unable to expectorate sputum of sufficient quality for reliable culture. Consequently, we altered our original definition of PBB to a more pragmatic one (Table 1). This acknowledges the multiple causes of chronic wet cough in children.7, 9
Table 1.
Diagnostic Criteria for Protracted Bacterial Bronchitis
| 1. Original microbiologic‐based case definition8(also termed PBB‐micro) |
| i. Presence of chronic wet cough (>4 weeks) |
| ii. Lower airway infection (recognized respiratory bacterial pathogens growing in sputum or at BAL at density of a single bacterial specifies ≥104 colony‐forming units/ml) |
| iii. Cough resolved following a 2‐week course of an appropriate oral antibiotic (usually amoxicillin‐clavulanate) |
| 2. Modified clinical‐based case definition45 (also termed PBB‐clinical) |
| i. Presence of chronic wet cough (>4 weeks) |
| ii. Absence of symptoms or signs of other causes of wet or productive cough 1 |
| iii. Cough resolved following a 2‐week course of an appropriate oral antibiotic (usually amoxicillin‐clavulanate) |
| 3. PBB‐extended = PBB‐clinical or PBB‐micro, but cough resolves only after 4 weeks of antibiotics |
| 4. Recurrent PBB = recurrent episodes (>3 per year) of PBB |
Specific cough pointers45, 94, 95 are: chest pain, history suggestive of inhaled foreign body, dyspnea, exertional dyspnea, hemoptysis, failure to thrive, feeding difficulties (including choking/vomiting), cardiac or neurodevelopmental abnormalities, recurrent sino‐pulmonary infections, immunodeficiency, epidemiological risk factors for exposure to tuberculosis, signs of respiratory distress, digital clubbing, chest wall deformity, auscultatory crackles, chest radiographic changes (other than perihilar changes), lung function abnormalities].
Diagnostic Criteria Validity
While the initial PBB criteria were derived from clinical observations, each of the initial PBB criteria have now been validated. For criterion‐(i), for example, chronic wet cough (>4 weeks), our urban‐based study of parental recognition of wet cough showed excellent agreement with pediatric pulmonologists (kappa (κ) = 0.75, 95% confidence interval (CI) 0.58–0.93) and bronchoscopic findings of endobronchial secretions (area under the receiver operating curve = 0.85, 95%CI 0.77–0.92).10 Also, prospective11, 12 and retrospective13 studies have demonstrated that BAL cultures from children with chronic wet cough have respiratory bacterial densities (≥104 CFU/ml) indicating lower airway infection. Further, increased amounts of secretions detected on bronchoscopy were associated with increased likelihood of infection and airway neutrophilia.11
The original criterion‐(ii) required BAL specimens to have positive respiratory bacterial cultures ≥104 CFU/ml,8 which is a threshold often used for diagnosing lower airway infection.14, 15, 16 The common pathogens (Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis) reported initially in PBB8 have been reaffirmed by several other groups.13, 17, 18, 19
Finally, a Cochrane review of two randomized controlled trial (RCTs) found that antibiotics (criterion‐iii) improved the odds of curing chronic wet cough in children odds ratio (OR) = 7.7 (95%CI 3.1–16.7).20 Although both RCTs were limited by small numbers (total n = 140), they nevertheless provided experimental data that supported clinical observations. These results have been confirmed recently by a double‐blind RCT.21
Diagnostic Criteria Limitations
Nonetheless, each criteria described above has their limitations. Recognizing wet cough is dependent upon clinical setting. The child's age, the accuracy of their parent/caregiver's history, and healthcare personnel training and experience can influence accurate identification. For example, unlike urban‐based parents,10 Australian Indigenous caregivers reporting of current cough is often inaccurate.22 It is also easier to recognize spontaneous wet cough in a young child. Agreement between clinicians and parents for cough quality (dry/wet) is better in children aged ≤2 years (κ = 0.85, 95%CI 0.57–1.0) than in older children (κ = 0.70, 95%CI 0.49–0.92).10 In our experience, older children may have an underlying productive cough despite it sounding dry when asked to cough in the clinic (“cough on request”). This may be partially related to the secretion volume relative to airway size, which has been described in animal studies where the amount of secretions alters sound characteristics.23
While lower airway infection is often defined as bacterial growth ≥104 CFU/ml in BAL,14, 15 the most robust cut‐off remains unknown.24 Thus, different studies employing BAL have used slightly different definitions of PBB (Tables 2, 3, 4).
Table 2.
Current National Pediatric Chronic Cough Guidelines
| First author, publication year | Country | Society | PBB mentioned or specified | Definition used 1 |
|---|---|---|---|---|
| Chang [2006]45 | Australia | Thoracic Society of Australia and New Zealand | Yes | PBB‐clinical ± BAL |
| Chang [2006]94 | USA | American College of Chest Physicians | Yes | Chronic wet cough and response to 2–4 wks of antibiotics |
| Gibson [2008]96 | Australia | Australian Lung Foundation | Yes (pediatric section) | PBB‐clinical ± BAL |
| Kohno [2006]97 | Japan | Japanese Respiratory Society | No | Not applicable |
| Leconte [2008]98 | Belgium | Primary care | Yes | Not defined |
| Lu [2014]99 | China | Multiple societies | Yes (based on translated article) | PBB‐clinical ± BAL |
| Shields [2008]48 | England | British Thoracic Society | Yes | Chronic wet cough and response to 4–6 wks of antibiotics |
| Zacharasiewicz [2014]100 | Austria | Austrian Society of Pediatrics, Austrian Society of Pneumology | Yes | PBB‐clinical ± BAL |
See Table 1.
Table 3.
Prospective Studies on Children With Chronic Cough Describing PBB Within Their Cohort (Adapted From Article101)
| First author, publication year, country | Setting | Inclusion criteria; exclusion | No. enrolled, no. completed, age | How cough was measured | Diagnoses, a priori defined; FU length | Period effect considered 1 | Prevalence of PBB in cohort (%) |
|---|---|---|---|---|---|---|---|
| Marchant [2006],8 Australia | Single center, Resp OPD | >3 wks cough, age <18 yrs, and newly referred; Exc: NR | 108, 103, median = 2.6 yrs, IQR 1.2–6.9 yrs | Cough diary102 | Yes; FU: max 12 mo for Dx, post‐Dx NR | Yes | 42 |
| Chang [2013],41 Australia 2 | Multicenter, Resp OPD | Aged <18 yrs, >4 wks cough, newly referred; Exc: chronic resp illness | 270, 253, mean = 4.5 yrs, SD = 3.7 yrs | At 6 wks: cough resolution by cough diary,102 PC‐QOL39 | Yes; FU: max 12 mo for Dx and 6 mo, post‐Dx | Yes, 2 wks of treatment | 42 |
| Asilsoy [2008],35 Turkey | Single center, pediatric OPD | >4 wks cough; Exc: NR | 108, 108, mean = 8.4 yrs, range 6–14 yrs | Cough‐unspecified | Partial; FU: NR | ND | 23 |
| Chang [2012],7 Australia 2 | Multicenter, Resp OPD | Aged <18 yrs, >4 wks cough and newly referred; Exc: chronic respir illness | 346, 346, mean = 4.5 yrs, SD 3 yrs | Cough resolution by cough diary,102 PC‐QOL39 | Yes; FU: max 12 mo for Dx and 6 mo, post‐Dx | Yes, 2 wks of treatment | 41 |
| Karabel [2014],43 Turkey | Single center, Resp OPD | >4 wks cough; Exc: NM, cardiac, syndromes, RTI in last 4 wks | 270, 270, mean = 6.5 yrs, range 7 mo–17 yrs | ND | Partial; FU: 12 mo | ND | 6 |
| Usta [2014],36 Turkey | Single center, pediatric allergy OPD | Inclusion: NRExc: see footnote 3 | 156, 156, Mean = 8.4 yrs SD 2.6 yrs | Cough‐unspecified | Partial; FU: max 18 mo for Dx, NR post‐Dx | ND | 12 |
Dx, diagnosis; Exc, exclusion criteria; FU, follow‐up; IQR, interquartile range; mo, months; ND, not described; NM, neuromuscular; No, number; NR, not reported; OPD, outpatients; PBB, protracted bacterial bronchitis; PC‐QOL, Parent Cough‐Specific Quality of Life; Resp, Respiratory; RTI, respiratory tract infection; SD, standard deviation; wks, weeks; yrs, years.
Period effect considered: temporal relationship between medication use and outcome was defined a priori.
Children in this RCT were from a subset of the same cohort.7
Preterm birth, neuromotor‐developmental delay, developmental‐growth retardation, chest wall deformity, tobacco smoke exposure, clubbing, cardiac disease, any known chronic disease and/or pulmonary disease, and those unable to undertake pulmonary function tests.36
Table 4.
Prospective Studies Involving Children With Protracted Bacterial Bronchitis
| 1st author, publication year; country | Setting; study design | Inclusion criteria; Exc or definitions | No. enrolled, no. completed; age | Main aim(s) | Key findings of the PBB component | Specimen; microbiology results | Other main findings |
|---|---|---|---|---|---|---|---|
| Marchant [2006],8 Australia | Single center, Resp OPD | >3 wks cough, age <18 yrs and newly referred Exc: NR | No. with PBB = 43; median age of whole cohort = 2.6 yrs (IQR 1.2–6.9) | In children with chronic cough, to; (a) evaluate the use of an adult‐based algorithmic approach in the management and (b) describe the etiology | BAL median total cell count and neutrophil% in PBB (350 × 106/L, 40%) was significantly higher than “natural resolution” group (228, 4%) | BAL; Hi = 47%, Spn = 35%; Mcat = 26% | |
| Marchant [2008],57 Australia | Single center, Resp OPD; cross‐ sectional | PBB = chronic wet cough (>3 wks), BAL bacterial culture (≥105 CFU/ml) and response to Abs (cough resolved 2 wks); other etiologies–= other causes of chronic cough in cohort,8 controls = children with stridor, but without chronic cough | PBB = 38, other Dx = 25, SR = 22, controls = 15; respective median age (IQR): 2.4 yrs (0.9–4.2), 2.6 yrs (1.1, 9.6), 3.8 yrs (0.9, 6.8), 2.8 yrs (0.6, 9.8) | To: (a) describe the clinical profile, airway cellularity and promoters of neutrophilic inflammation in BAL of children with PBB compared to children with other etiologies and controls without cough and (b) explore selected innate immunity signaling receptors, specifically TLR‐2,‐4 | BAL in PBB group had significantly elevated total cell counts, airway neutrophilia, IL‐8, and active MMP‐9 compared to other groupsTLR‐2 and −4 mRNA relative expression ratio in BAL of PBB group was significantly higher than controls | BAL; Hi = 45%, Spn = 32%; Mcat = 24% | IL‐8 levels significantly correlated with BAL neutrophil% and MMP‐9 |
| Chang [2012],73 Australia | Single center, Resp OPD; cross‐ sectional | PBB = chronic wet cough, response to ABs with resolution of cough within 2 wks and absence of signs or symptoms of other disease; PBB well = previous PBB, but no cough when FB done | Current PBB = 61,PBB well = 20, controls = 21; respective mean age (SD) = 2.5 yrs (2.3), 4.2 yrs (3.0), 2.2 yrs (2.8) | To determine whether BAL levels of hBD2, SP‐A, and MBL: (a) differed between children with current PBB, PBB well, and controls; and (b) related with airway neutrophilia and endobronchial infection | hBD2 and MBL levels in BAL were significantly higher in children with PBB compared with PBB well group and controls. hBD2 levels were associated with airway infection and are related to airway neutrophilia and MBL | BAL; microbiology NR | SP‐A levels in the BAL and cytokine production of stimulated BAL cells similar between groups |
| Marchant [2012],21 Australia | Single center, Pediatric and Resp OPD;RCT | Aged 0.5–18 yrs, doctor observed wet cough >3 wks;Exc: chronic lung, cardiac, or neuro‐developmental disorders, ABs in last 2 wks or if acutely unwell | 50, 3 lost to FU that were analyzed as failures. Mean = 1.8 yrs for treatment gp and 2.8 yrs for placebo group | Efficacy of 2 wks of oral amox‐clav (compared with placebo) in achieving cough resolution in children with chronic wet cough | Amox‐clav effective for wet cough‐ cough resolution rates (48%) in amox‐clav group versus placebo (16%), P = 0.016 | BAL; Hi = 38%, Spn = 24%; Mcat = 19%. All amox‐clav sensitive | BAL in subgroup (n = 37): results consistent with PBB |
| Wurzel [2014],33 Australia | Single center, Resp OPD; cross‐ sectional | PBB = clinical def; controls = chronic resp symptoms, but no PBB, CSLD | PBB = 104, controls = 21; respective median age (IQR) = 19 mo (12–30), and 20 mo (8–63) | To provide extensive clinical, laboratory, and BAL characterization of PBB | Previous parent‐reported wheeze (81%), but no increased propensity to atopy (IgE and RAST normal). Normal immunoglobulin levels and antibody responses (Hib and tetanus vaccines) | BAL; in PBB grp; Hi = 72%, Spn = 39%; Mcat = 43%; AdV = 23%; any virus = 38% | PBB and childcare attendance‐elevated serum NK‐cell levels; tracheo‐broncho‐malacia common, but similar rates in controls |
| Baines [2014],56 Australia | Single center, Resp OPD; exp and validation cohorts | PBB = clinical definition; resolved PBB = previous PBB, but no cough at FB | Exp: PBB = 21; controls = 33; respective mean ages = 2.3 and 9.7 yrs; validation: PBB = 36; controls = 11; respective mean ages = 2.0 and 0.7 yrs | To evaluate the IL‐1 and TNF‐α/NF‐κB pathways and mediators in 2 cohorts of PBB and control children | Increased expression of neutrophil‐related mediators in PBB, including IL‐1 pathway members, neutrophilα‐defensins, and the chemokine receptor CXCR2 | BAL: microbiology NR | IL‐1β protein levels correlated with BAL neutrophilia and duration and severity of cough symptoms |
| Wurzel [2014],67 Australia 1 | Single center, Resp OPD; cross‐ sectional | PBB = clinical def; BE = Dx on CT scan | PBB = 159, BE = 112; median (IQR) age: PBB with AdV = 17 mo (12–22), PBB without AdV = 26 mo (15–56) | To identify: (a) the prevalence of AdV;(b) diversity of genotypes and species; and (c) whether presence of AdV increased the odds of bacterial coinfection | AdV‐C (genotypes 1, 2) was the major AdV species in BAL; lower airway bacterial infection, (with Hi, Mcat and Spn, but not Sa) increased in those with AdV | BAL; in AdV+ grp; Hi = 68%, Spn = 39%; Mcat = 35%. In AdV‐ grp; Hi = 47%, Spn = 22%; Mcat = 19% | Younger age independent predictor of AdV with respiratory bacteria co‐infection |
| Van der Gast [2014],69 USA and Australia | Multi‐center, Resp OPD; cross‐ sectional | PBB = clinical def; BE = Dx on CT scan; CF = sweat test | PBB = 12, BE = 19; CF = 25; respective mean (SD) age: 8.9 yrs (4.7), 2.3 yrs (1.7), 12.5 yrs (3.5) | To compare: (a) the core and satellite microbiota in cohorts of children with different diseases and (b) the respiratory metacommunities in PBB and pediatric and adult CF and bronchiectasis cases | Similar resp sample core microbiota in the three diseases; microbiota from adults with CF and BE differed significantly from each other and from those of children with the same disease | BAL and sputum; traditional and non‐traditional organisms described | |
| Chang [2014],40 Australia | Multi‐center, Resp OPD | PBB = clinical definition | PBB = 138, asthma = 52, BE = 20, self‐ resolved = 40. Median age (IQR) PBB = 2.4 yrs (1.2, 4.8), BE = 3.9 (2.2, 6.4), self resolved = 5.2 (2.1, 8.2) | In children newly referred for chronic cough, to describe data relating specific cough pointers of the three most common etiologies | At presentation (preDx), cough score,102 PC‐QOL, PedQL scores, number of doctors' visits between groups were similar | Not applicable | Likelihood ratios, sensitivity, specificity of the different symptoms/signs presented |
| Hodge [2015],80 Australia | Single center, Resp OPD; cross‐ sectional | PBB‐micro; controls = no cough and FB undertaken for other reasons (e.g., stridor); BE = CT defined with clinical symptoms | PBB = 13, BE = 55, controls = 13. Median ages and IQR:PBB = 6.5 mo (1.6, 14), BE = 22 mo (14, 33), controls = 5.5 (4, 9.9) | (a) Quantify phagocytosis of airway apoptotic cells and NTHi by alveolar macrophages in children with PBB and BE and (b) determine if phagocytic capacity was associated with clinical or demographic variables, and differing patterns of airway inflammation | Macrophage phagocytic capacity significantly lower in PBB and BE c.f. controls (P = 0.003 and <0.001 for efferocytosis and 0.041 and 0.004 for phagocytosis of NTHi; PBB and BE, respectively); median phagocytosis of NTHi: BE = 13.7% (IQR 11–16%) PBB = 16% (11–16%), controls = 19.0% (13–21%); median efferocytosis: BE = 14.1% (10–16%), PBB = 16.2% (14–17%), controls = 18.1% (16–21%) | NR | IL‐1β levels significantly correlated BAL neutrophil% (r = 0.93, P = 0.0001). Negative, but non‐significant correlations between IL‐1β and phagocytosis of apoptotic cells or NTHi (r = −0.37, P = 0.080, −0.43,0.099, respectively) |
Although the response to 2 weeks of antibiotics is one of the criteria used to diagnose PBB, we9 and others18, 19 have observed that some children require a longer treatment of 4 weeks. The number needed to treat for benefit (NNT‐B) by 2 weeks found in the Cochrane review20 and the RCT21 was 3–4 (95%CI 2–27) and it is possible that using a longer course may reduce the NNT‐B (see below).
The variation in definitions is similar to pediatric asthma where varying definitions depend upon age, clinical and/or research setting, and availability of tests for airway cellularity, lung function, and airway hyper‐responsiveness. Given these limitations, the definition of PBB may be refined further; particularly if a simple biomarker is identified that predicts the response to antibiotics.
Proposed PBB Definition
To advance the field for clarity, we propose the following definition:
PBB‐micro = original definition8
PBB‐clinical = the modified criteria where criterion‐ii (BAL component) is replaced by: “absence of other causes of wet/productive cough.”
PBB‐extended = PBB‐clinical or PBB‐micro, but cough resolves only after 4 weeks of antibiotics.
Recurrent PBB= recurrent episodes (>3 per year) of PBB.
HISTORICAL CONTEXT
When we first described PBB in 2006,8 its existence as a distinct diagnostic entity was controversial.25 However, it is becoming recognized increasingly and is now incorporated into pediatric chronic cough guidelines (Table 2) and into the European pediatric respiratory training curriculum.26
Astute clinicians alluded to PBB‐like conditions previously, but did not consolidate these observations into a clinical entity supported by laboratory‐based data. Various definitions used in the 1970–80s described “childhood chronic bronchitis” ranging from productive cough for 3 months/year to recurrent episodes of cough lasting >2 weeks with/without wheeze.27 During this period, a single center review of 20 children undergoing bronchoscopy and diagnosed with chronic bronchitis found all had evidence of bronchial wall inflammation and purulent endobronchial secretions, and most improved after a course of antibiotics.28 In the 1940s, a series of studies on childhood bronchiectasis raised the possibility of a pre‐bronchiectasis state,29 which was reversible by aggressive antibiotic treatment, while another study suggested a link between chronic bronchitis and bronchiectasis.30 A later report described chronic bacterial bronchitis in children with tracheo‐bronchomalacia,31 and more recently others have suggested that some children with so called “difficult asthma,” persistent wheeze32 or asthma‐bronchitis might have PBB (parents of children with PBB often describe wheezing illnesses).18, 33 Indeed, a retrospective French study reported one in eight children undergoing bronchoscopy for “severe chronic asthma” had lower airway infection (defined as >105 CFU/ml or >104 CFU/ml with activated neutrophils).34
DISEASE BURDEN
Several cohort studies of children with chronic cough have documented the disease burden, QoL scores, medication use and number of doctor visits for the subgroup with PBB.4, 7
Prevalence
The true prevalence of PBB cases presenting to community clinics is unknown. Studies from specialist clinics from Australia7, 8 and Turkey35, 36 found PBB to be among the top three diagnoses in children with chronic cough (Table 3). Using a standard management approach,37 a priori definitions and validated cough outcome measures, an Australian multicenter study7 found that 142/346 (41%) children newly referred for chronic cough had PBB, which predominated in younger children.7 A United Kingdom (UK)‐based retrospective study reported PBB was the most common diagnosis made in their clinic in children referred with difficult asthma or a chronic cough, and this disorder was increasing annually.18
Quality of Life
There are limited data on the QoL of children with PBB and that of their parents. In a multi‐center study, the generic health‐related (PedsQL38) and chronic cough‐specific (PC‐QoL) QoL scores of children with PBB were similar to other different diagnostic groups (asthma, bronchiectasis, those whose chronic cough resolved without treatment) presenting to pediatric pulmonologists.39, 40 Importantly, QoL scores normalize once the cough resolves.41
Treatment Burden
Typically, before diagnosis, children with PBB have received multiple medications and consulted several health physicians.4, 7 A Sheffield study18 reported that at the time of referral, 59% of 81 children with PBB were taking asthma treatments, while an Australian multi‐center study found 70% of 138 children with this diagnosis had received asthma medications and 76% had seen >5 doctors previously because of persistent cough.40 However, these findings were also similar to children with chronic cough from other causes.40
CLINICAL PHENOTYPE
There are relatively few clinical studies of children with PBB (Tables 4 and 5). By definition, they all have a chronic wet cough. Although children with PBB are typically young (mean or median age 1.8–4.8 years, Table 4), PBB also occurs in older (>12 years) children.7, 42, 43 Our first cohort of 43 children with PBB lacked systemic symptoms, including evidence of sinusitis and ear disease.8 This was confirmed in a subsequent prospective cohort of 104 children.33 Compared to “disease controls” undergoing bronchoscopy for non‐cough‐related indications (e.g., stridor or apnea), children with PBB were more likely to have attended childcare (OR = 8.4, 95%CI 2.3–30.5), but their tobacco smoke exposure (∼30%8, 33) was similar to that of the “controls.”33 The strong association with childcare attendance raises the possibility of respiratory viruses having a role in initiating PBB, at least in some children.
Table 5.
Retrospective Studies on Children Including Children With Protracted Bacterial Bronchitis
| First author, publication year; country | Setting; study design | Definition of PBB used | No. in study; age | Main aim(s) | Key finding(s) | Specimen; microbiology | Other main findings | Comment |
|---|---|---|---|---|---|---|---|---|
| Donnelly [2007],43 England | Resp OPD; review of clinic letters (random) | Persistent, wet cough present for 1 mo that resolved with “appropriate” AB treatment | 81; median = 3.8 yrs (range 0.4–14.8) | Review the outcomes in 81 randomly selected patients diagnosed with PBB | 95% cured with AB use; 48% reported “wheeze,” but had a “ruttle” instead | BAL (n = 19), cough swab (n = 51). Of infected specimens (∼50%): Hi = 81%, Spn = 37% | 59% symptomatic for >1 yr; bronchiectasis in 4/14 who had chest CT scan; 31% with concomitant “asthma” (BDR demonstrated or responsive to steroids) | Not all children had PBB based on original definition of PBB |
| Kompare [2011],19 USA | Resp and allergy OPD | Cough, wheeze or noisy breathing for >1 mo without other diagnoses, BAL (≥104 CFU/ml) and response to ≥2 wks AB treatment | 70; summary age NR | Review all BAL (≥104 CFU/ml) cultures of children aged <5 yrs with cough, wheeze, or noisy breathing for >1 mo without other diagnoses so as to determine if PBB was present | Tracheo‐ or broncho‐malacia present in 74% | BALHi = 56%, Spn = 37%; Mcat = 59% | Outcome data (available in 87%): symptoms resolved with AB treatment in all but one child | Bronchitis itself may cause malacia |
| Priftis [2013],42 Greece and England | Resp OPD | Children with chronic cough suspected of PBB who had FB to confirm diagnosis | Greece = 18, England = 39; median age = 4.8 yrs (range 0.9–14.4) | To (a) determine specific serotypes of Spn and NTHi in BAL samples and (b) compare Spn serotypes between the two countries and Spn vaccination | PCV‐13 Spn serotypes in all Greek BALs, but only in 72% of English BALs (significantly different b/w countries) | Greek BAL; Hi = 61%, Spn = 27.6%; Mcat = 32%; Sa = 6%; English BAL restricted to Spn+ specs | Vaccine Spn serotypes rarely found in immunized children (OR = 0.02; 95%CI 0.003–0.115); 26 NTHi isolates (English BAL) had unique genotypes | Recent or current AB use NR. Evidence of serotype replacement disease in Spn‐immunized children |
| Narang [2014],62 England | Pediatric and Resp OPD; 50 consecutive notes | Suspected PBB (ND) | 50; median age = 2.9 yrs (IQR 1.7, 4.4) | Review BAL and chest radiograph results, and assess the bacterial distribution across lung lobes | Bacterial distribution in PBB was heterogeneous | BAL; Hi = 50%, Spn = 16%, Mcat = 28%, Sa = 22% | Limiting sampling to 1–2 lobes will under‐estimate the microbiology of the lung (70% positive vs. 82%) | Positive culture undefined as quantitative culture was not done |
| Pritchard [2014],55 England | Pediatric and Resp OPD | AB responsive wet cough and positive BAL culture | 43; median = 2.7 yrs (IQR 1.5, 4) | Review of treatment outcomes for children with PBB | Cough resolved in 77% after initial AB course (6–8 wks) but only 24% remained cough free (i.e., 76% relapsed) | BAL; Hi = 63%, Spn = 23%, Mcat = 51%, Sa = 19% | Of the 10 whose cough did not resolve after 6–8 wks ABs, 7 required prolonged ABs and 3 had other resp conditions | BE more likely to be present when wet cough unresponsive to 4 wks of ABs.49 Thus, likely some in cohort did not have PBB |
ABs, antibiotics; AdV, human adenovirus; Amox‐clav, amoxicillin‐clavulanate; BAL, bronchoalveolar lavage; BE, bronchiectasis; BDR, bronchodilator responsive, btw, between, CF, cystic fibrosis, CFU, colony‐forming units, CSLD, chronic suppurative lung disease; CT, computed tomography, CXCR2, chemokine (C‐X‐C Motif) receptor 2, Dx, diagnosis, Exc, exclusion criteria, Exp, experimental; FB, flexible bronchoscopy; FU, follow‐up; hBD2, human β‐defensin‐2; Hi, Haemophilus influenzae, Hib, Haemophilus influenzae type b, IL, interleukin; IQR, interquartile range, MBL, mannose‐binding lectin; Mcat, Moraxella catarrhalis, MMP, matrix metalloproteinase, mo, months, mRNA, messenger RNA, ND, not described, NF‐κB, nuclear factor kappa‐light‐chain‐enhancer of activated B cells, NK, natural killer cell, No, number, NR, not reported, NTHi, nontypeable Haemophilus influenzae, OPD, outpatient department, OR, odds ratio, PBB, protracted bacterial bronchitis, PC‐QOL, parent cough‐specific quality of life, PCV‐13, 13 serotype pneumococcal conjugate vaccine; PedQL, pediatric quality of life, Pros, Prospective; RAST, radioallergosorbent test, RCT, randomized controlled trial, Resp, respiratory, Retro, retrospective; Sa, Staphylococcus aureus, SD, standard deviation, SP‐A, Surfactant protein‐A; Spn, Streptococcus pneumoniae, *SR, spontaneous resolution (defined below), TLR, toll‐like receptor; TNF, tumor necrosis factor, wk, weeks; yr, year.
*Spontaneous resolution (SR) defined as resolution of cough without therapy or, if this was tried the cough resolved more than 2 weeks after ceasing treatment.
Children with PBB typically appear well. They have normal growth and development, and lack signs of underlying chronic suppurative lung disease (CSLD), such as digital clubbing, chest wall deformity, and adventitial auscultatory chest findings,33 although occasionally a “rattly chest” and crackles are heard. The chest radiograph is normal or near‐normal, showing only peri‐bronchial changes.9, 40, 44 When performed, both spirometry40 and respiratory system reactance and resistance measured by the forced oscillatory technique (unpublished) are also normal.
The prevalence of atopic features (eczema, systemic and airway eosinophilia, elevated IgE, or positive radioallergosorbent test) is similar to children without PBB.33 While many parents report previous “ever wheeze” (41–81%18, 33), auscultation‐confirmed wheeze by doctors is unusual. In the Iowa City BAL‐based retrospective cohort of children with PBB,19 17/70 (24%) children had “noisy breathing” (five also wheezed) and 27 (39%) parents reported wheezing. In the Sheffield cohort study,18 36/81 (45%) children were referred for “difficult asthma” and 35 (43%) reported “shortness of breath,” which was attributable to coughing bouts. While most children in this cohort18 had PBB, some did not based upon our definition since 11 (13%) required more than six courses of antibiotics for recurrent chronic wet cough and 4 (5%) had radiographic‐confirmed bronchiectasis.
In children, differentiation between acute bronchitis and PBB is based on the fact that acute bronchitis cough usually resolves within 2–4 weeks.45, 46 Nevertheless, difficulties arise when recurrent and acute bronchitis episodes overlap, especially during the “respiratory virus” season. Furthermore, PBB can co‐exist with other illnesses, including asthma, while recurrent episodes need to be differentiated from bronchiectasis where chronic wet cough and repeated respiratory exacerbations may not respond to oral antibiotics, physical signs of CSLD may develop and irreversible bronchial dilation and wall thickening are seen on plain radiographs or computed tomography (CT) scans of the chest.9, 47
TREATMENT
In PBB, the child's cough resolves only after a prolonged (2 weeks) course of appropriate antibiotics.8, 21 When a typical five to seven day course of antibiotics is prescribed the cough either relapses or does not resolve completely. However, some children require up to 4 weeks of treatment. In our RCT,21 many of the children whose cough was not cured by 2 weeks of antibiotics had underlying tracheo‐bronchomalacia and needed a longer course of antibiotics before their cough disappeared. We speculate that children in this RCT21 were also at the more severe end of the disease spectrum and more likely to require a longer antibiotic course. Hence the larger NNT‐B (4, 95%CI 2–27) compared to the Cochrane review (3, 95%CI 2–5).20
In contrast, the 2008 British Thoracic Society (BTS) cough guidelines48 suggest all children with PBB should receive 4–6 weeks of antibiotics. This recommendation, however, was based upon expert opinion as at the time no supportive high‐quality studies existed. Further studies are now needed to help identify whether those with PBB requiring a longer course of antibiotics are different (e.g., outcomes) from those responding to shorter courses.
While some children with PBB may need longer antibiotic treatment, we still advocate the shorter 2‐week course initially. This reflects the principles of good antimicrobial stewardship and should also reduce drug‐related adverse events. Furthermore, a recent study showed that children with a chronic wet cough failing to resolve after 4 weeks of appropriate oral antibiotics have increased likelihood of bronchiectasis on a chest CT scan.49 In this retrospective study, all chest multi‐detector CT scans performed over a 28 month period for assessment of chronic wet cough were reviewed.49 Among the 105 children with persistent cough despite at least 4 weeks of antibiotics, 88 (83.8%) had bronchiectasis, while of the 24 children whose cough resolved after antibiotics, only six (25.0%) received this diagnosis (adjusted OR 20.9; 95%CI 5.36–81.8).49
Chest airway clearance is now standard therapy in bronchiectasis management.47 The BTS guidelines48 also suggest physiotherapy for children with PBB. While this may be beneficial, the evidence to support this recommendation is limited. Furthermore, the time required for performing airway clearance techniques should not be underestimated.
Ideally, a lower airway specimen for microbiologic testing is obtained before treatment. Indeed, the BTS guidelines48 recommend that before making a diagnosis of PBB, sputum should be cultured first and other underlying conditions excluded. However, most children with chronic wet cough are young (Tables 4 and 5) and unable to expectorate, even following sputum induction. Thus, obtaining reliable lower respiratory secretions requires bronchoscopy, which will be impractical in most clinical settings. Nevertheless, if a bronchoscopy is performed, purulent secretions and evidence of bronchitis (Fig. 1) are usually present.
Figure 1.
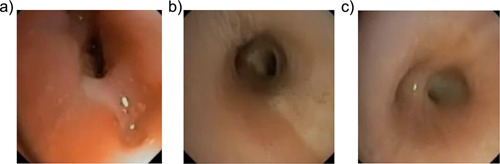
Bronchoscopic images from several children with protracted bacterial bronchitis. The bronchoscopy usually shows secretions in several bronchi and features of bronchitis (airway edema and inflammation Fig. 1a). In Fig. 1a, bronchomalacia and mucus (LB10) are also present. Sometimes secretions are seen in the trachea (Fig. 1b), but more often purulent secretions are seen in the bronchi (Fig. 1c—secretions seen at the right lower lobe bronchus).
Consequently, from a pragmatic perspective, in children with an “isolated” chronic wet cough, who have a normal or near normal chest radiograph and are without symptoms and signs of another diagnosis (e.g., aspiration lung disease, bronchiectasis, cystic fibrosis (CF), inhaled foreign body, tuberculosis, etc.) PBB should be suspected and we recommend they receive 2 weeks of an appropriate oral antibiotic. The most widely used first line empiric antibiotic is amoxicillin‐clavulanate,21 (as commonly associated pathogens, such as H. influenzae and especially M. catarrhalis, can be amoxicillin‐resistant) although depending upon local pathogen susceptibility patterns, alternatives such as an oral cephalosporin, trimethoprim‐sulfamethoxazole, or macrolide may be used when immediate hypersensitivity to penicillin exists. While antibiotics are generally well tolerated, caregivers should be counseled about possible adverse events (e.g., gastrointestinal complaints, rashes, hypersensitivity) occurring.50 When a child can expectorate, a sputum specimen should be obtained. In a minority of children, the cough will not respond to treatment and the antibiotic can be extended to a maximum of 4 weeks. However, if by this time the wet cough has not improved substantially, the child should be investigated for other causes of chronic wet cough.9, 49
PBB can also co‐exist with other conditions (e.g., asthma, tracheo‐bronchomalacia)7, 18 and these conditions should be diagnosed and managed concurrently. As with all children at risk of chronic lung disease, clinicians should also counsel on preventative care including avoidance of tobacco smoke and other inhaled environmental toxicants, timely vaccinations, ensuring good nutrition, and emphasizing the importance of physical activity.
PROGNOSIS
PBB often recurs at some point, particularly in the very young as recurrent respiratory infections are more common in this age group.51 The definition of recurrence (number of episodes per year) remains uncertain, but historically either at least three or more than three lower respiratory infections per year has been used.52, 53 Adopting the latter definition, a prospective BAL‐based study was undertaken in 106 children with PBB followed for a median 25 months (IQR 24–28) after this investigation.54 Their median age at bronchoscopy was 23 months (IQR 14–53). Compared with healthy controls (median age 27 months, IQR 26–29) children with PBB at baseline were significantly more likely to have had wet cough and to have visited their family doctor in the preceding 12 months. At the 24 month follow‐up, children with PBB were more likely to be coughing compared with controls (44% vs. 12% of respective cohort, P = 0.005) and to have had parent‐reported wheeze in the preceding 12 months (58% vs. 16%, P = 0.001). By the end of the study, 66 (62%) of those with PBB (and complete follow‐up) had experienced recurrent episodes (>3 per year) and 13 (12%) had bronchiectasis diagnosed by chest CT scans.54 The major independent risk factors for bronchiectasis were H. influenzae lower airway infection and having ≥2 siblings. H. influenzae infection conferred >6 times higher risk of bronchiectasis than a H. influenzae negative state (hazard ratio = 6.8, 95%CI 1.5–30.8).
A UK‐based retrospective study55 described 33 children whose wet cough resolved with antibiotics (“PBB”). During the median follow‐up period of 11.3 months (IQR 8.3–14.7) only eight (24%) remained cough free, while three (9%) had three or more recurrent episodes of persistent wet cough and nine were prescribed long‐term antibiotics. Another retrospective study19 reported that 43 of 70 children had recurrent symptoms (timeframe not provided). These studies suggest that physicians should warn parents about future recurrences and physicians themselves need to be aware that some children may progress to bronchiectasis.
Nevertheless, the actual rate and risk factors of PBB recurrence are likely dependent on the sampling frame and definition. Factors in those severe enough to proceed to bronchoscopy are likely different from those enrolled from the community. Additional longer term studies are warranted as recurrent PBB is associated with more intense and possibly persistent activation of interleukin (IL)‐1 signalling pathways in the airways.56
PATHOBIOLOGY
Lower airway bacterial infection and neutrophilia are present in children with PBB.8, 19, 57 Protracted cough implies injurious chronic airway inflammation and mucus hypersecretion,58, 59 while on‐going endobronchial bacterial infection is harmful to the lungs.60, 61
Microbiology
Bacteria
In the first description of PBB,8 BAL cultures grew the common respiratory bacterial pathogens, S. pneumoniae, H. influenzae, and M. catarrhalis. Subsequently, several other groups confirmed these BAL findings.8, 18, 19, 21, 33, 55 One retrospective study also identified Staphylococcus aureus in 11/50 children with PBB,62 but quantitative bacteriology was not performed making interpretation difficult. This latter study62 also evaluated BAL sampling of one versus six lobes and reported sampling only one lobe in 41 children would have missed 17 potential bacterial pathogens in 15 patients. While it is generally accepted that more than one lobe should undergo BAL, additional prospective studies employing quantitative bacteriology with concomitant inflammation marker assays are needed. Data relating to current or recent antibiotic use and vaccination status should also be recorded as it is likely that antibiotics, vaccine types, and host immune response influence lower airway bacteriology in children suspected of PBB42 and CSLD.63, 64
H. influenzae (range 47–81%) is the most common bacteria reported in all but one19 of the published studies describing the lower airway bacteriology in children with PBB.8, 21, 33, 42, 55, 62 Most H. influenzae are non‐typeable H. influenzae (NTHi) strains representing diverse genotypes,42 a finding consistent with the increasing importance of NTHi in chronic pulmonary disorders in children and adults.65, 66
In some studies,8, 21 S. pneumoniae (24–39%) was the second most common organism detected in BAL cultures, but in others33, 42, 55, 62 M. catarrhalis (range 19–43%) was found more commonly. However, these BAL studies are difficult to compare as samples came from different sites and numbers of lobes, some accepted all positive culture results,55, 62 while others used quantitative bacteriology to diagnose infection.8, 19, 21, 33 S. pneumoniae serotypes were reported in one recent study involving immunized children in the UK and Greece undergoing BAL for suspected PBB.42 Although both vaccine and non‐vaccine‐related serotypes were isolated from BAL cultures, vaccine serotypes were found significantly less often in children who had received pneumococcal conjugate vaccines.42 Finally, culturing two or more bacterial pathogens was reported frequently (22–48%) in several studies,8, 42, 55, 62 but how this influenced clinical presentation or outcomes was not commented upon.
Viruses
Surprisingly, only one study has examined systematically for the presence of respiratory viruses in children with PBB.33 This study reported higher rates of virus detection by PCR in the BAL fluid from 104 PBB cases than from 49 other chronic respiratory disease controls (38% vs. 9%; OR = 6.3, 95%CI 2.1–19.1). The most common virus identified was adenovirus (AdV),33 which upon genotyping belonged predominantly to AdV species C.67 Children in the PBB AdV positive group were, however, significantly younger than those in the PBB AdV negative group (median 17 (IQR 12–22) vs. 26 (IQR 15–56) months; P = 0.001) and the positive association found between AdV detection and bacterial infection disappeared after adjusting for age.67 An extended panel for 17 viruses was undertaken in a subset of 27 children, which found rhinovirus in 11 (41%) and human bocavirus and human coronavirus in 1 (4%) each of the participants.33 The prevalence of these other viruses was similar in the control group.33 Other studies also report a similar prevalence of these viruses in asymptomatic children.68
Microbiota
The microbiota of the lungs of children with PBB has been examined in a single cross‐sectional study.69 Using bacterial 16S rRNA gene pyrosequencing, phylogenetic analysis and ecologic statistical tools, the core microbiota in the sputum and BAL specimens from 60 children (PBB n = 12, bronchiectasis = 19, CF = 25, controls = 4) and 68 adults (bronchiectasis = 38, CF =n30) with chronic pulmonary disorders were compared.69 One‐way analysis of variance showed the Shannon–Weiner index (a measure of species diversity) of the lower airway microbiota in children with PBB and bronchiectasis were similar and statistically higher (i.e., richer) than in CF (Fig. 2a).69 However, while all three pediatric cohorts shared remarkably similar core microbiota, with H. influenzae making the greatest contribution to the observed similarity, the lung microbiota in children were significantly different from that observed in adults with CF and bronchiectasis (Fig. 2b).69 These findings suggest that chronic airway infections begin similarly with defective airway clearance of otherwise normal airway microbiota, but over time with antibiotic treatment and perhaps the effects of the underlying disease the microbiota in these disease groups progressively diverge from one another.
Figure 2.
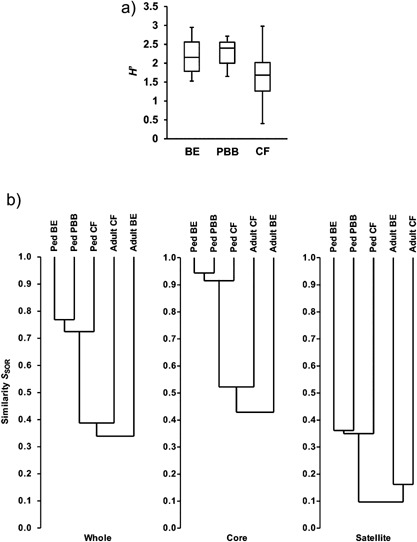
(Reprinted with permission of the American Thoracic Society. Copyright © 2015 American Thoracic Society. van der Gast CJ et al. Three Clinically Distinct Chronic Pediatric Airway Infections Share a Common Core Microbiota. Ann Am Thorac Soc 2014;11:1039–1048. The Annals of the American Thoracic Society is an official journal of the American Thoracic Society). 2a: Shannon–Weiner index (H′), which reflects the diversity of species present, depicting that protracted bacterial bronchitis (PBB) was similar to bronchiectasis (BE), but significantly different to cystic fibrosis (CF). Species diversity is richer in the PBB and BE groups. 2b: Dendrograms of community membership similarity between the pediatric (ped) BE, PBB, and CF bacterial metacommunities and compared with adult CF and BE metacommunities. Given are whole, core, and satellite microbiota. Metacommunity profiles were compared using the Sørensen index of similarity and unweighted pair‐group method using arithmetic mean (UPGMA).69 Sørensen index varies from 0 (no similarity) to 1 (entirely similar) and accounts for the number of species present in each community and those that are shared.69
Biofilm
Biofilms may be another speculative reason for the chronicity and the need for longer antibiotic courses for PBB treatment. Our group first documented biofilms in the BAL fluid of children with bronchiectasis,70 and our preliminary data show that biofilms are also present in the BAL fluid of children with PBB. None of these children had Pseudomonas aeruginosa.
Immunity, Inflammation, and Associated Signalling Pathways
A prospective study showed that children with PBB have higher and more prolonged “children's acute respiratory illness and flu” (CARIF) scores71 with respiratory illnesses than healthy controls and children with asthma.72 PBB is also characterized by airway neutrophilia and high densities of common respiratory pathogens in their lower airways,8, 33 suggesting that host immunological responses play a role in its pathogenesis.
Immunity
Both our initial8 and subsequent studies33 describing systemic immunity in children with PBB have confirmed the absence of an overt immunodeficiency. Serum levels of IgA, IgM, IgG, and IgE are normal and responses to protein (tetanus) and conjugated protein‐polysaccharide (H. influenzae type b) vaccines are robust.33 Humoral immunity, therefore, seems to be intact and no abnormalities in lymphocyte subsets have been found.33
Innate immunity among children with PBB also appears to be activated rather than deficient.57, 73 In one study,57 innate immunity was assessed by examining toll‐like receptors (TLRs). TLR‐2 and TLR‐4 are signaling pattern recognition receptors that play a key role in responding to invading bacteria, especially gram‐positive and gram‐negative bacteria, respectively.74 Compared to controls, BAL samples from children with PBB had significantly elevated TLR‐2 and TLR‐4 mRNA relative expression57 (Table 6). Whether the BAL of children with PBB had reduced TLR expressions levels prior to early infection is unknown. An opportunistic study of children undergoing gastroscopy for gastrointestinal symptoms, found that TLR‐4 mRNA relative expression was significantly lower in children with chronic cough and BAL evidence of bacterial infection (≥104 CFU/ml) than those without infection.75
Table 6.
| Median (IQR) | |||
|---|---|---|---|
| Variable | PBB | Controls | P‐value |
| TLR‐2 mRNA relative expression | 2.0 (1.1–2.5) | 0.9 (0.7–1.3) | 0.013 |
| TLR‐4 mRNA relative expression | 2.4 (2.2–3.7) | 2.0 (1.6–2.4) | 0.009 |
| Human β‐defensin 2, pg/ml | 164.4 (0–435.5) | 3.6 (0–126) | 0.018 |
| Mannose‐binding lectin, ng/ml | 1.7 (0.4–4) | 0.4 (0.02–79) | 0.013 |
IQR, interquartile range; mRNA, messenger RNA; PBB, protracted bacterial bronchitis; TLR, toll‐like receptor.
A second larger study evaluated pulmonary levels of three innate immunity components (human β‐defensin 2 [hBD2], mannose‐binding lectin [MBL], and surfactant protein‐A [SP‐A]) and the cytokine production of lipopolysaccharide‐stimulated BAL cells in 61 children with current PBB, 20 with previously treated PBB and 21 disease controls undergoing bronchoscopy for airway assessment of stridor.73 Median hBD2 and MBL levels were significantly higher in the current than previous PBB group and controls (Table 6). SP‐A levels and cytokine production of stimulated BAL cells were similar among all the groups.73
In a preliminary study, the systemic immune responses (cytokine production) against NTHi in 20 children with PBB were similar to 20 healthy control subjects,76 however, the caspase‐1 dependent, proinflammatory pathways were activated in PBB children.
Deficient efferocytosis (impaired clearance of apoptotic cells by alveolar macrophages)77 is another possible mechanism in PBB pathobiology. It is postulated that the apoptotic cells may undergo secondary necrosis with pro‐inflammatory effects contributing to the perpetuation of chronic inflammation, infection and tissue damage.78, 79 Support for this hypothesis comes from a small BAL‐based study showing significantly decreased ability of alveolar macrophages to phagocytose apoptotic bronchial cells and NTHi in children with bronchiectasis (n = 55) and PBB (n = 13) compared controls (n = 13).80 For both types of impaired phagocytosis, values in children with PBB were intermediate to those with BE and controls80 (Table 4).
Inflammation and Associated Signaling Pathways
Neutrophil influx is an important feature of the innate immune response. We and others have described intense airway neutrophilia (median 40–44%) in children with PBB,8, 19, 57 but whether this is a pathologically disproportionate response to infection is unknown.
There are also marked pro‐inflammatory mediator responses (IL‐8, matrix metalloproteinase (MMP)‐9 active, IL‐1β) that correlate with BAL neutrophil percentages.56, 81 Median BAL levels of IL‐8 and MMP‐9 in children with PBB were 5.4‐ to 19.1‐fold higher than in controls and in those whose cough resolved without specific treatment.57 Levels of IL‐8 and MMP‐9 were also significantly correlated (r = 0.61, P < 0.0001).57
We have shown in experimental and validation cohorts that children with PBB have significantly higher BAL fluid levels of IL‐1β, α‐defensin, IL‐1 pathway members, and CXCR2 gene and protein expression than non‐PBB disease controls.56 IL‐1β levels correlated with duration and severity of cough,56 and with elevated expression of α‐defensins 1–3 in PBB cases. In those with recurrent PBB (>3 in the next 12 months), the baseline (at bronchoscopy) gene expression of the IL‐1β signaling molecules pellino‐1 and IL‐1 receptor associated kinase (IRAK)‐2 were significantly higher than those without recurrent PBB, suggesting this pathway's involvement in recurrence.56
Other innate immune factors related to wet cough and airway neutrophilia, but not specifically studied in children with PBB include the pattern recognition receptor for advanced glycation end‐products (RAGE), which plays an important, but complex role in many inflammatory responses within the lungs.82 Soluble RAGE (sRAGE) levels within the circulation and BAL fluid were associated with age and airway inflammatory cell profiles (lower sRAGE in airway neutrophilia), highlighting a potentially important role for sRAGE in pediatric lung disease.82
Association With Large Airway Lesions
Older studies have showed an association among airway lesions, chronic cough,83, 84 and chronic bronchitis.31 While some clinicians believe tracheo‐bronchomalacia causes chronic cough, it is more likely that infection predisposes the individual to prolonged, inefficient airway clearance. Since the cough resolves once the underlying infection is treated, this suggests malacia has a limited causative role.8 Nevertheless, tracheo‐bronchomalacia impairs airway clearance,85 increasing the likelihood of persistent lower airway infection and inflammation. Tracheo‐bronchomalacia is also found commonly in children with PBB.8, 19 This association may be primary (airway malacia predisposes to PBB through reduced efficiency in airway clearance) or secondary (malacia developing because of intense airway inflammation86, 87). One retrospective study reported tracheo‐bronchomalacia was present in 52/74 (74%) children with PBB.19 However, a prospective study involving 104 children with PBB found that these airway abnormalities were no more common in children with PBB than in those undergoing bronchoscopy for other respiratory indications at a tertiary pediatric hospital (68% vs. 53%, respectively).33 Nevertheless, these proportions are higher than estimates for the general population (1 in 2,100)31 and there is little doubt that children with tracheo‐bronchomalacia have a higher frequency of respiratory infections and symptoms.88, 89
THE OVERLAP BETWEEN PBB, CSLD, AND BRONCHIECTASIS
In 2008, we proposed a paradigm (Fig. 3) where PBB, CSLD, and bronchiectasis shared common underlying pathobiologic mechanisms and progressed variably along an increasing spectrum of severity.9 The current evidence suggests that this paradigm still remains clinically useful, ensuring that children with bronchiectasis are not missed. PBB overlaps with CSLD, which in turn overlaps with radiographic‐confirmed bronchiectasis.9 Children with established bronchiectasis would have had CSLD, at some stage earlier in the disease process, while those with CSLD would have had PBB previously.
Figure 3.
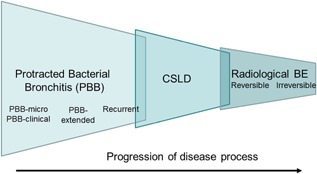
(Modified with permission from Chang AB, Redding GJ, and Everard ML. (2008), Chronic wet cough: protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr Pulmonol;43:519–531; doi: 10.1002/ppul.20821) Using the pathobiologic model, protracted bacterial bronchitis (PBB), chronic suppurative lung disease (CSLD), and radiographic‐confirmed bronchiectasis likely represents different ends of a spectrum with similar underlying mechanisms of airway neutrophilia, endobronchial bacterial infection, and impaired mucociliary clearance. Untreated it is likely some (but not all) children with PBB will progress to develop CSLD and some will ultimately develop bronchiectasis, initially reversible and subsequently irreversible if left to progress. There is a degree of overlap between each of the entities.
The potential for injury with persistent infection and inflammation is probably more important in the growing and developing lungs of young children than in the mature lung. This is supported by animal and human studies where early lung insults are most likely to have adverse long‐term effects.90, 91, 92 As children with PBB are typically very young,8, 9 it is plausible that recurrent PBB episodes are a risk factor for developing CSLD or bronchiectasis.
The similarities between these three conditions include chronic wet cough, ±rattling breathing, or “ruttles,” as well as impaired regional muco‐ciliary clearance, endobronchial bacterial infection and neutrophilic airway inflammation. The core microbiota69 and cultured classical respiratory bacterial pathogens are also similar in PBB33 and pediatric CSLD and bronchiectasis.16, 67 The key differences between these three entities lie in the severity of symptoms and signs, the response to 2–4 weeks of oral antibiotics, chest high‐resolution CT scan findings, and consequent management. CSLD is differentiated from bronchiectasis only by lacking the radiographic signs of bronchiectasis on chest high‐resolution CT scans.9, 93
SUMMARY
PBB is recognized increasingly as a common cause of chronic wet cough in children. In most PBB cases, 2 weeks of oral antibiotics are sufficient, but some will require a 4‐week course. The pathobiology of PBB involves lower airway bacterial infection with intense airway neutrophilia accompanied by increased inflammatory markers involving IL‐1β signaling. The pulmonary innate immune system is upregulated, but so far no systemic or local immune dysfunction has been identified, other than of decreased efferocytosis and phagocytosis of NTHi.80 Recurrent episodes (>3 per year) occur in some, and these children have increased gene expression of the IL‐1β signaling molecules pellino‐1 and IRAK‐2, and are more likely to have bronchiectasis diagnosed within the next 2 years. Hence, those with PBB should be monitored closely.
The Road Ahead
In addition to consolidating the preliminary data presented above, other important research questions remain to be answered in this surprisingly common, but poorly recognized pulmonary disorder. Some of these epidemiology, pathobiologic, and management research goals are listed in Table 7.
Table 7.
Research Priorities for PBB
| Epidemiology |
| •Determining the burden of disease (e.g., incidence, prevalence, QoL, economic cost) in the general community |
| •Establishing modifiable risk factors |
| •Ascertaining the long‐term outcomes of children with recurrent and non‐recurrent episodes |
| •Clarifying whether children with PBB‐extended have different long term outcomes |
| Pathobiology |
| •Identifying the underlying developmental and pathobiologic mechanisms |
| •Uncovering host biological susceptibility factors, including the role of airway malacia |
| •Describing the frequency and mechanisms of virus‐induced PBB episodes |
| Management |
| •Detecting a biomarker that can predict response to antibiotics and risk of recurrence |
| •Determining if longer courses of antibiotics of up to 4 weeks duration reduce recurrences |
| •Performing multicenter intervention trials to help identify those requiring longer antibiotic courses of up to 4 weeks evaluating the role of prophylactic antibiotics in patients with frequent (>3 annually) recurrences, but still lacking disease pointers and evidence of bronchiectasis |
ACKNOWLEDGEMENTS
None of the authors have any fiscal conflict of interest and none received an honorarium or other form of payment to produce the manuscript.
Conflict of interest: None.
REFERENCES
- 1. Britt H, Miller GC, Charles J, Henderson J, Bayram C, Valenti L, Pan Y, Harrison C, Fahridin S, O'Halloran J, et al. 2009. General practice activity in Australia 1999–00 to 2008–09: 10 year data tables. General practice series no. 26. Cat. no. GEP 26. Canberra: AIHW.
- 2. Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, et al. Diagnosis and management of cough executive summary: accp evidence‐based clinical practice guidelines. Chest 2006; 129:1S–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Britt H, Miller GC, Knox S, Charles J, Valenti L, Pan Y, Henderson J, Bayram C, O'Halloran J, Ng A. General practice activity in Australia 2003–2004. Australian Institute of Health and Welfare 2004; AIHW Cat. No. GE P 16.
- 4. Marchant JM, Newcombe PA, Juniper EF, Sheffield JK, Stathis SL, Chang AB. What is the burden of chronic cough for families? Chest 2008; 134:303–309. [DOI] [PubMed] [Google Scholar]
- 5. Faniran AO, Peat JK, Woolcock AJ. Persistent cough: is it asthma? Arch Dis Child 1998; 79:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newcombe PA, Sheffield JK, Chang AB. Minimally important change in a parent‐proxy quality of life questionnaire for pediatric chronic cough (PC‐QOL). Chest 2010; 139:576–580. [DOI] [PubMed] [Google Scholar]
- 7. Chang AB, Robertson CF, van Asperen PP, Glasgow NJ, Mellis CM, Masters IB, Teoh L, Tjhung I, Morris PS, Petsky HL, et al. A multi‐centre study on chronic cough in children: burden and etiologies based on a standardized management pathway. Chest 2012; 142:943–950. [DOI] [PubMed] [Google Scholar]
- 8. Marchant JM, Masters IB, Taylor SM, Cox NC, Seymour GJ, Chang AB. Evaluation and outcome of young children with chronic cough. Chest 2006; 129:1132–1141. [DOI] [PubMed] [Google Scholar]
- 9. Chang AB, Redding GJ, Everard ML. State of the Art – chronic wet cough: protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr Pulmonol 2008; 43:519–531. [DOI] [PubMed] [Google Scholar]
- 10. Chang AB, Eastburn MM, Gaffney J, Faoagali J, Cox NC, Masters IB. Cough quality in children: a comparison of subjective vs. bronchoscopic findings. Respir Res 2005; 6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang AB, Faoagali J, Cox NC, Marchant JM, Dean B, Petsky HL, Masters IB. A bronchoscopic scoring system for airway secretions‐airway cellularity and microbiological validation. Pediatr Pulmonol 2006; 41:887–892. [DOI] [PubMed] [Google Scholar]
- 12. Wurzel D, Marchant JM, Clark JE, Masters IB, Yerkovich ST, Upham JW, Chang AB. Wet cough in children: infective and inflammatory characteristics in broncho‐alveolar fluid. Pediatr Pulmonol 2014; 49:561–568. [DOI] [PubMed] [Google Scholar]
- 13. Douros K, Alexopoulou E, Nicopoulou A, Anthracopoulos MB, Fretzayas A, Yiallouros P, Nicolaidou P, Priftis KN. Bronchoscopic and high resolution CT findings in children with chronic wet cough. Chest 2011; 140:317–323. [DOI] [PubMed] [Google Scholar]
- 14. Pizzutto SJ, Grimwood K, Bauert P, Schutz KL, Upham JW, Yerkovich ST, Chang AB. Bronchoscopy contributes to the clinical management of Indigenous children newly doagnosed with non‐cystic fibrosis bronchiectasis. Pediatr Pulmonol 2013; 48:67–73. [DOI] [PubMed] [Google Scholar]
- 15. DeSchutter I, DeWachter E, Crokaert F, Verhaegen J, Soetens O, Pierard D, Malfroot A. Microbiology of bronchoalveolar lavage fluid in children with acute nonresponding or recurrent community‐acquired pneumonia: identification of nontypeable Haemophilus influenzae as a major pathogen. Clin Infect Dis 2011; 52:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kapur N, Grimwood K, Masters IB, Morris PS, Chang AB. Lower airway microbiology and cellularity in children with newly diagnosed non‐CF bronchiectasis. Pediatr Pulmonol 2012; 47:300–307. [DOI] [PubMed] [Google Scholar]
- 17. Zgherea D, Mendiratta M, Marcus MG, Shelov SP, Pagala S, Kazachkov M. Bronchoscopic findings in children with chronic wet cough. Pediatrics 2012; 129:e363–e369. [DOI] [PubMed] [Google Scholar]
- 18. Donnelly DE, Critchlow A, Everard ML. Outcomes in children treated for persistent bacterial bronchitis. Thorax 2007; 62:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kompare M, Weinberger M. Protracted bacterial bronchitis in young children: association with airway malacia. J Pediatr 2012; 160:88–92. [DOI] [PubMed] [Google Scholar]
- 20. Marchant JM, Morris P, Gaffney J, Chang AB. Antibiotics for prolonged moist cough in children. Cochrane Database Syst Rev 2005. [DOI] [PubMed] [Google Scholar]
- 21. Marchant JM, Masters IB, Champion A, Petsky HL, Chang AB. Randomised controlled trial of amoxycillin‐clavulanate in children with chronic wet cough. Thorax 2012; 67:689–693. [DOI] [PubMed] [Google Scholar]
- 22. Morey MJ, Cheng AC, McCallum GB, Chang AB. Accuracy of cough reporting by carers of Indigenous children. J Paediatr Child Health 2013; 49:E199–E203. [DOI] [PubMed] [Google Scholar]
- 23. Korpas J, Widdicombe JG, Vrabec M. Influence of simulated mucus on cough sounds in cats. Resp Med 1993; 87:49–54. [DOI] [PubMed] [Google Scholar]
- 24. Hare KM, Marsh RL, Smith‐Vaughan HC, Bauert PA, Chang AB. Respiratory bacterial culture from two sequential bronchoalveolar lavages of the same lobe in children with chronic cough. J Med Microbiol 2015; 64:1353–1360. [DOI] [PubMed] [Google Scholar]
- 25. Rubin BK. Pediatricians are not just small internists. Chest 2006; 129:1118–1121. [DOI] [PubMed] [Google Scholar]
- 26. Pohunek P, Svobodova T. In: Eber E, Midulla F, editors. ERS Handbook of Paediatric Medicine. Sheffield: European Resiratory Society; 2013. [Google Scholar]
- 27. Taussig LM, Smith SM, Blumenfeld R. Chronic bronchitis in childhood: what is it? Pediatrics 1981; 67:1–5. [PubMed] [Google Scholar]
- 28. Smith TF, Ireland TA, Zaatari GS, Gay BB, Zwiren GT, Andrews HG. Characteristics of children with endoscopically proved chronic bronchitis. Am J Dis Child 1985; 139:1039–1044. [DOI] [PubMed] [Google Scholar]
- 29. Field CE. Bronchiectasis in childhood; III. Prophylaxis, treatment and progress with a follow‐up study of 202 cases of established bronchiectasis. Pediatrics 1949; 4:355–372. [PubMed] [Google Scholar]
- 30. Finke W. Prospects for prevention of chronic bronchitis and bronchiectasis; rational management of bronchopulmonary infections by penicillin aerosol therapy. J Pediatr 1948; 33:29–42. [DOI] [PubMed] [Google Scholar]
- 31. Boogaard R, Huijsmans SH, Pijnenburg MW, Tiddens HA, De Jongste JC, Merkus PJ. Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest 2005; 128:3391–3397. [DOI] [PubMed] [Google Scholar]
- 32. De Schutter, I , Dreesman A, Soetens O, De WM, Crokaert F, Verhaegen J, Pierard D, Malfroot A. In young children, persistent wheezing is associated with bronchial bacterial infection: a retrospective analysis. BMC Pediatr 2012; 12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wurzel D, Marchant JM, Yerkovich ST, Upham JW, Mackay IM, Masters IB, Chang AB. Prospective characterisation of protracted bacterial bronchitis in children. Chest 2014; 145:1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fayon M, Just J, Thien HV, Chiba T, Pascual L, Sandouk G, Grimfeld A. Bacterial flora of the lower respiratory tract in children with bronchial asthma. Acta Paediatr 1999; 88:1216–1222. [DOI] [PubMed] [Google Scholar]
- 35. Asilsoy S, Bayram E, Agin H, Apa H, Can D, Gulle S, Altinoz S. Evaluation of chronic cough in children. Chest 2008; 134:1122–1128. [DOI] [PubMed] [Google Scholar]
- 36. Usta GB, Asilsoy S, Durmaz C. The assessment and management of chronic cough in children according to the British Thoracic Society guidelines: descriptive, prospective, clinical trial. Clin Respir J 2014; 8:330–337. [DOI] [PubMed] [Google Scholar]
- 37. Chang AB, Robertson CF, van Asperen PP, Glasgow NJ, Masters IB, Mellis CM, Landau LI, Teoh L, Morris PS. Can a management pathway for chronic cough in children improve clinical outcomes: protocol for a multicentre evaluation. Trials 2010; 11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003; 3:329–341. [DOI] [PubMed] [Google Scholar]
- 39. Newcombe PA, Sheffield JK, Juniper EF, Halstead RA, Masters IB, Chang AB. Development of a parent‐proxy quality‐of‐life chronic cough‐specific questionnaire: clinical impact vs psychometric evaluations. Chest 2008; 133:386–395. [DOI] [PubMed] [Google Scholar]
- 40. Chang AB, Robertson CF, van Asperen PP, Glasgow NJ, Mellis CM, Masters IB, Teoh L, Mellis CM, Landau LI, Marchant JM, et al. Children with chronic cough: when is watchful waiting appropriate? Development of likelihood ratios for assessing children with chronic cough. Chest 2015; 147:745–753. [DOI] [PubMed] [Google Scholar]
- 41. Chang AB, Robertson CF, van Asperen PP, Glasgow NJ, Mellis CM, Masters IB, Teoh L, Mellis CM, Landau LI, Marchant JM, et al. A cough algorithm for chronic cough in children: a multicentre, randomized controlled study. Pediatrics 2013; 131:e1576–e1583. [DOI] [PubMed] [Google Scholar]
- 42. Priftis KN, Litt D, Manglani S, Anthracopoulos MB, Thickett K, Tzanakaki G, Fenton P, Syrogiannopoulos GA, Vogiatzi A, Douros K, et al. Bacterial bronchitis caused by Streptococcus pneumoniae and nontypable Haemophilus influenzae in children: the impact of vaccination. Chest 2013; 143:152–157. [DOI] [PubMed] [Google Scholar]
- 43. Karabel M, Kelekci S, Karabel D, Gurkan MF. The evaluation of children with prolonged cough accompanied by American College of Chest Physicians guidelines. Clin Respir J 2014; 8:152–159. [DOI] [PubMed] [Google Scholar]
- 44. Marchant JM, Masters IB, Taylor SM, Chang AB. Utility of signs and symptoms of chronic cough in predicting specific cause in children. Thorax 2006; 61:694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang AB, Landau LI, van Asperen PP, Glasgow NJ, Robertson CF, Marchant JM, Mellis CM. The Thoracic Society of Australia and New Zealand. Position statement. Cough in children: definitions and clinical evaluation. Med J Aust 2006; 184:398–403. [DOI] [PubMed] [Google Scholar]
- 46. Thompson M, Vodicka TA, Blair PS, Buckley DI, Heneghan C, Hay AD. Duration of symptoms of respiratory tract infections in children: systematic review. BMJ 2013; 347:f7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang AB, Bell SC, Torzillo PJ, King PT, Maguire GP, Byrnes CA, Holland AE, O'Mara P, Grimwood K. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand Thoracic Society of Australia and New Zealand guidelines. Med J Aust 2015; 202:130. [DOI] [PubMed] [Google Scholar]
- 48. Shields MD, Bush A, Everard ML, McKenzie SA, Primhak R. British Thoracic Society Guidelines Recommendations for the assessment and management of cough in children. Thorax 2008; 63:iii1–iii15. [DOI] [PubMed] [Google Scholar]
- 49. Goyal V, Grimwood K, Marchant JM, Masters IB, Chang AB. Does failed chronic wet cough response to antibiotics predict bronchiectasis? Arch Dis Child 2014; 99:522–525. [DOI] [PubMed] [Google Scholar]
- 50. Ferwerda A, Moll HA, Hop WC, Kouwenberg JM, Tjon Pian Gi CV, Robben SG, de GR. Efficacy, safety and tolerability of 3 Dayazithromycin versus 10 Dayco‐amoxiclav in the treatment of children with acute lower respiratory tract infections. J Antimicrob Chemother 2001; 47:441–446. [DOI] [PubMed] [Google Scholar]
- 51. Leder K, Sinclair MI, Mitakakis TZ, Hellard ME, Forbes A. A community‐based study of respiratory episodes in Melbourne, Australia. Aust NZ J Public Health 2003; 27:399–404. [DOI] [PubMed] [Google Scholar]
- 52. Schaad UB. OM‐85 BV, an immunostimulant in pediatric recurrent respiratory tract infections: a systematic review. World J Pediatr 2010; 6:5–12. [DOI] [PubMed] [Google Scholar]
- 53. Alsan MM, Westerhaus M, Herce M, Akashima K, Armer PE. Poverty, global health and infectious disease: lessons from Haiti and Rwanda. Infect Dis Clin North Am 2011; 25:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wurzel D, Marchant JM, Yerkovich ST, Upham JW, Petsky H, Chang AB. Protracted Bacterial Bronchitis (PBB) in children: natural history and risk for bronchiectasis. Respirology 2015; 20(Suppl. 2):20. A25–Abstract TO 032. [DOI] [PubMed] [Google Scholar]
- 55. Pritchard MG, Lenney W, Gilchrist FJ. Outcomes in children with protracted bacterial bronchitis confirmed by bronchoscopy. Arch Dis Child 2015; 100:112. [DOI] [PubMed] [Google Scholar]
- 56. Baines KJ, Upham JW, Yerkovich ST, Chang AB, Marchant JM, Carroll M, Simpson JL, Gibson PG. Mediators of neutrophil function in children with protracted bacterial bronchitis. Chest 2014; 146:1013–1020. [DOI] [PubMed] [Google Scholar]
- 57. Marchant JM, Gibson PG, Grissell TV, Timmins NL, Masters IB, Chang AB. Prospective assessment of protracted bacterial bronchitis: airway inflammation and innate immune activation. Pediatr Pulmonol 2008; 43:1092–1099. [DOI] [PubMed] [Google Scholar]
- 58. Sabroe I, Whyte MK. Toll‐like receptor (TLR)‐based networks regulate neutrophilic inflammation in respiratory disease. Biochem Soc Trans 2007; 35:1492–1495. [DOI] [PubMed] [Google Scholar]
- 59. Chaudhuri N, Whyte MK, Sabroe I. Reducing the toll of inflammatory lung disease. Chest 2007; 131:1550–1556. [DOI] [PubMed] [Google Scholar]
- 60. Stockley RA. Role of bacteria in the pathogenesis and progression of acute and chronic lung infection. Thorax 1998; 53:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cole PJ. Inflammation: a two edged sword. The model of bronchiectasis. Eur J Respir Dis Suppl 1986; 147:6–15. [PubMed] [Google Scholar]
- 62. Narang R, Bakewell K, Peach J, Clayton S, Samuels M, Alexander J, Lenney W, Gilchrist FJ. Bacterial distribution in the lungs of children with protracted bacterial bronchitis. PLoS ONE 2014; 9:e108523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pizzutto SJ, Yerkovich ST, Upham JW, Hales BJ, Thomas WR, Chang AB. Improving immunity to Haemophilus influenzae in children with chronic suppurative lung disease. Vaccine 2015; 33:321–326. [DOI] [PubMed] [Google Scholar]
- 64. Hare KM, Grimwood K, Leach AJ, Smith‐Vaughan HC, Torzillo PJ, Morris P, Chang AB. Respiratory bacterial pathogens in the nasopharynx and lower airways of Australian Indigenous children with bronchiectasis. J Pediatr 2010; 157:1001–1005. [DOI] [PubMed] [Google Scholar]
- 65. Van Eldere J, Slack MP, Ladhani S, Cripps AW. Non‐typeable Haemophilus influenzae, an under‐recognised pathogen. Lancet Infect Dis 2014; 14:1281–1292. [DOI] [PubMed] [Google Scholar]
- 66. Pizzutto SJ, Yerkovich ST, Upham JW, Hales BJ, Thomas WR, Chang AB. Children with chronic suppurative lung disease have a reduced capacity to synthesize interferon‐gamma in vitro in response to non‐typeable Haemophilus influenzae . PLoS ONE 2014; 9:e104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wurzel DF, Mackay IM, Marchant JM, Wang CY, Yerkovich ST, Upham JW, Smith‐Vaughan HC, Petsky HL, Chang AB. Adenovirus species C is associated with chronic suppurative lung diseases in children. Clin Infect Dis 2014; 59:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Advani S, Sengupta A, Forman M, Valsamakis A, Milstone AM. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J 2012; 31:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. van der Gast CJ, Cuthbertson L, Rogers GB, Pope C, Marsh RL, Redding GJ, Bruce KD, Chang AB, Hoffman LR. Three clinically distinct chronic pediatric airway infections share a common core microbiota. Ann Am Thorac Soc 2014; 11:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marsh RL, Thornton RB, Smith‐Vaughan HC, Richmond P, Pizzutto SJ, Chang AB. Identification of biofilm in bronchoalveolar lavage from children with bronchiectasis without Pseudomonas aeruginosa infection. Pediatr Pulmonol 2014; doi: 10.1002/ppul.23031 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 71. Jacobs B, Young NL, Dick PT, Ipp MM, Dutkowski R, Davies HD, Langley JM, Greenberg S, Stephens D, Wang EE, et al. Canadian Acute Respiratory Illness and Flu Scale (CARIFS): development of a valid measure for childhood respiratory infections. J Clin Epidemiol 2000; 53:793–799. [DOI] [PubMed] [Google Scholar]
- 72. Petsky HL, Acworth JP, Clark R, Winter D, Masters IB, Chang AB. Asthma and protracted bronchitis: who fares better during an acute respiratory infection? J Paediatr Child Health 2009; 45:42–47. [DOI] [PubMed] [Google Scholar]
- 73. Chang AB, Yerkovich ST, Gibson PG, Anderson‐James S, Petsky HL, Caroll M, Masters IB, Marchant JM, Wurzel D, Upham JW, et al. Pulmonary innate immunity in children with protracted bacterial bronchitis. J Pediatr 2012; 161:621–625. [DOI] [PubMed] [Google Scholar]
- 74. Schnare M, Rollinghoff M, Qureshi S. Toll‐like receptors: sentinels of host defence against bacterial infection. Int Arch Allergy Immunol 2006; 139:75–85. [DOI] [PubMed] [Google Scholar]
- 75. Grissell T, Chang AB, Gibson PG. Impaired toll‐like receptor 4 and substance P gene expression is linked to airway bacterial colonisation in children. Pediatr Pulmonol 2007; 42:380–385. [DOI] [PubMed] [Google Scholar]
- 76. Upham JW, Chen A, Carroll M, Petsky H, Pizzutto SJ, Yerkovich ST, Baines KJ, Simpson JL, Gibson P, Chang A, et al. Immnue responses to non typebale Haemophilus influenzae in protracted bacterial bronchitis. Respirology 2015; 20:A34. [Google Scholar]
- 77. Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 2003; 81:289–296. [DOI] [PubMed] [Google Scholar]
- 78. Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 2006; 129:1673–1682. [DOI] [PubMed] [Google Scholar]
- 79. Hodge S, Hodge G, Holmes M, Reynolds PN. Increased airway epithelial and T‐cell apoptosis in COPD remains despite smoking cessation. Eur Respir J 2005; 25:447–454. [DOI] [PubMed] [Google Scholar]
- 80. Hodge S, Upham JW, Pizzutto SJ, Petsky H, Yerkovich ST, Baines KJ, Gibson P, et al. Is alveolar macrophage phagocytc dysfunction in children with protracted bacterial bronchitis a forerunner to bronchiectasis? Chest 2015; in press. [DOI] [PubMed] [Google Scholar]
- 81. Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 2009; 64:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yerkovich ST, Chang AB, Caroll M, Petsky HL, Upham JW. Soluble receptor for advanced glycation end products in the lungs of children. Respirology 2012; 17:841–846. [DOI] [PubMed] [Google Scholar]
- 83. Wood RE. Localised tracheomalacia or bronchomalacia in children with intractable cough. J Pediatr 1990; 116:404–406. [DOI] [PubMed] [Google Scholar]
- 84. Thomson F, Masters IB, Chang AB. Persistent cough in children – overuse of medications. J Paediatr Child Health 2002; 38:578–581. [DOI] [PubMed] [Google Scholar]
- 85. Finder JD. Primary bronchomalacia in infants and children. J Pediatr 1997; 130:59–66. [DOI] [PubMed] [Google Scholar]
- 86. Chang AB, Boyce NC, Masters IB, Torzillo PJ, Masel JP. Bronchoscopic findings in children with non‐cystic fibrosis chronic suppurative lung disease. Thorax 2002; 57:935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Feist JH, Johnson TH, Wilson RJ. Acquired tracheomalacia: etiology and differential diagnosis. Chest 1975; 68:340–345. [DOI] [PubMed] [Google Scholar]
- 88. Masters IB, Zimmerman PV, Pandeya N, Petsky HL, Wilson SB, Chang AB. Quantified tracheobronchomalacia disorders and their clinical profiles in children. Chest 2007; 133:461–467. [DOI] [PubMed] [Google Scholar]
- 89. Masters IB, Zimmerman PV, Chang AB. Longitudinal quantification of growth and changes in primary tracheobronchomalacia sites in children. Pediatr Pulmonol 2007; 42:906–913. [DOI] [PubMed] [Google Scholar]
- 90. Tennant PW, Gibson GJ, Parker L, Pearce MS. Childhood respiratory illness and lung function at ages 14 and 50 years. Chest 2010; 137:146–155. [DOI] [PubMed] [Google Scholar]
- 91. Maritz G, Probyn M, De MR, Snibson K, Harding R. Lung parenchyma at maturity is influenced by postnatal growth but not by moderate preterm birth in sheep. Neonatology 2008; 93:28–35. [DOI] [PubMed] [Google Scholar]
- 92. Snibson K, Harding R. Postnatal growth rate, but not mild preterm birth, influences airway structure in adult sheep challenged with house dust mite. Exp Lung Res 2008; 34:69–84. [DOI] [PubMed] [Google Scholar]
- 93. Chang AB, Grimwood K, Macguire G, King PT, Morris PS, Torzillo PJ. Management of bronchiectasis and chronic suppurative lung disease (CSLD) in Indigenous children and adults from rural and remote Australian communities. Med J Aust 2008; 189:386–393. [DOI] [PubMed] [Google Scholar]
- 94. Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP Evidence‐Based Clinical Practice Guidelines. Chest 2006; 129:260S–283S. [DOI] [PubMed] [Google Scholar]
- 95. Chang AB. State of the Art: cough, cough receptors, and asthma in children. Pediatr Pulmonol 1999; 28:59–70. [DOI] [PubMed] [Google Scholar]
- 96. Gibson PG, Chang AB, Glasgow NJ, Holmes PW, Katelaris P, Kemp AS, Landau LI, Mazzone S, Newcombe P, Van Asperen P, et al. CICADA: Cough in Children and Adults: Diagnosis and Assessment. Australian Cough Guidelines summary statement. Med J Aust 2010; 192:265–271. [DOI] [PubMed] [Google Scholar]
- 97. Kohno S, Ishida T, Uchida Y, Kishimoto H, Sasaki H, Shioya T, Tokuyama K, Niimi A, Nishi K, Fujimura M, et al. The Japanese Respiratory Society guidelines for management of cough. Respirology 2006; 11:S135–S186. [DOI] [PubMed] [Google Scholar]
- 98. Leconte S, Paulus D, Degryse J. Prolonged cough in children: a summary of the Belgian primary care clinical guideline. Prim Care Respir J 2008; 17:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lu Q. [Guideline for diagnosis and treatment of chronic cough in Chinese children]. Zhonghua Er Ke Za Zhi 2014; 52:184–188. [PubMed] [Google Scholar]
- 100. Zacharasiewicz A, Eber E, Riedler J, Frischer T. [Consensus statement on the evaluation and therapy of chronic cough in children]. Wien Klin Wochenschr 2014; 126:439–450. [DOI] [PubMed] [Google Scholar]
- 101. Chang AB, Oppenheimer JJ, Irwin RS, Weir KA, Rubin BK, Weinberger MM. Use of management pathways or algorithms in children with chronic cough: systematic reviews. Chest 2015; in press. doi: 10.1378/chest.15-1403. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 102. Chang AB, Newman RG, Carlin J, Phelan PD, Robertson CF. Subjective scoring of cough in children: parent‐completed vs child‐completed diary cards vs an objective method. Eur Respir J 1998; 11:462–466. [DOI] [PubMed] [Google Scholar]


