Abstract
Aim
To assess the role of human bocavirus 1 (HBoV1) as a causative agent of non‐severe community‐acquired pneumonia (CAP) in children.
Methods
Patients aged 2‐59 months with non‐severe CAP (respiratory complaints and radiographic pulmonary infiltrate/consolidation) attending a University Hospital in Salvador, Brazil were enrolled in a prospective cohort. From 820 recruited children in a clinical trial (http://ClinicalTrials.gov NCT01200706), nasopharyngeal aspirate (NPA), and acute and convalescent serum samples were obtained from 759 (92.6%) patients. NPAs were tested for 16 respiratory viruses by PCR. Acute HBoV1 infection was confirmed by measuring specific IgM and IgG responses in paired serum samples.
Results
Respiratory viruses were detected in 693 (91.3%; 95%CI: 89.1‐93.2) CAP cases by PCR. HBoV1‐DNA was detected in 159 (20.9%; 95%CI: 18.2‐24.0) cases. Of these 159 PCR positive cases, acute HBoV1 infection was confirmed serologically in 38 cases (23.9%; 95%CI: 17.8‐31.0). Overall, acute HBoV1 infection was confirmed in 5.0% (38/759) of non‐severe CAP patients. HBoV1 was detected in 151 cases with at least one other virus making 31.7% of all multiple virus (n = 477) detections. Among all 759 cases, 216 had one respiratory virus detected, and sole HBoV1 was detected in only 8 (3.7%). Acute HBoV1 infection was serologically diagnosed in 34 (22.5%) HBoV1‐DNA‐positive cases with another virus, compared to 4 (50.0%) cases with sole virus detection (p = 0.09).
Conclusion
HBoV1 was detected by PCR in one fifth of the children with non‐severe CAP and acute HBoV1 infection was serologically confirmed in one quarter of these cases.
Keywords: acute respiratory infection, children, lower respiratory tract infection, respiratory virus, viral infection
1. INTRODUCTION
Community‐acquired pneumonia (CAP) remains a major cause of hospitalization and death among children under 5 years of age worldwide.1 Due to its significance in public health and social economy development,2 effective measures are needed to control this condition.3
The real role of respiratory viruses in the pathogenesis of CAP remains debatable. This concerns especially human bocavirus 1 (HBoV1) which is mostly detected with other respiratory viruses and is often detected in asymptomatic subjects.4 HBoV1 has, since its discovery in 2005,5 been found in 10‐20% of nasopharyngeal aspirate samples (NPA) of hospitalized children with lower respiratory illness.6, 7, 8 However, HBoV1‐DNA has been shown to persist for weeks or even months after an acute infection, detectable as low viral loads in the NPAs, questioning the clinical value of PCR‐based diagnosis.9 In 2008, it was shown that by measurement of HBoV1‐specific IgM and IgG antibodies, it was possible to diagnose acute HBoV1 infection in children hospitalized with acute severe respiratory diseases.9 In the current study, we used specific serology to assess the frequency of acute HBoV1 infections in children with both non‐severe CAP and with HBoV1‐DNA detected by PCR in the nasopharyngeal mucus.
2. MATERIALS AND METHODS
2.1. Study design
A prospective study was conducted at the Federal University of Bahia Hospital in Salvador, Northeast Brazil (clinical trial on the use of amoxicillin, http://ClinicalTrials.gov Identifier NCT01200706) from November 2006 to April 2011. Cases were community‐dwelling children aged between 2 and 59 months with non‐severe CAP, who came to the Pediatric Emergency Department. CAP was diagnosed by a pediatrician on duty. Diagnosis was based on respiratory complaints (cough or difficulty breathing) associated with lower respiratory pathologic findings plus presence of pulmonary infiltrate or consolidation on the chest radiograph (CXR) (frontal and lateral views) taken on admission and read by the pediatrician on duty. Non‐severe CAP cases were identified when CAP cases did not present any of the following findings: lower chest indrawing, inability to drink, seizure, somnolence, central cyanosis, grunting in a calm child, and nasal flaring (World Health Organization criteria for severity of CAP, 2000).10 The exclusion criteria were: chronic debilitating diseases (anatomic abnormalities of the respiratory tract, cancer, chronic pulmonary illness besides asthma, immunological defects, progressing neurological disorders, psychomotor retardation, heart disease with clinical repercussion, hemoglobinopathy, liver or kidney disease, severe malnutrition), other concurrent infection, HIV‐infected mother, hospitalization during the previous 7 days, amoxicillin or other antibiotics use during the last 48 h, allergy to amoxicillin, or history of aspiration. The primary results of this trial have been published.11 The assessment of respiratory virus detection was a secondary objective in the clinical trial.
On enrolment, after written informed consent had been obtained from parents/legal guardians, every patient was evaluated by trained medical students under the supervision of a senior pediatrician, all from the research team. A complete physical examination was performed. Data on demographics, clinical history, and physical examination were recorded in pre‐defined forms with the following variables: age, sex, disease duration, complaints (cough, fever, difficulty breathing, wheezing, vomiting), axillary temperature, respiratory rate (RR), weight, findings on physical examination (chest retraction, reduced pulmonary expansion, rhonchi, wheezing, crackles). A follow‐up visit was scheduled to occur 2‐4 weeks after recruitment. Fever was defined as axillary temperature >37.4°C12 and tachypnea as RR ≥ 50 breaths/min in children aged 2‐11 months or RR ≥40 breaths/min in children from 12 to 59 months of age.13 Nutritional evaluation was performed using the software Anthro, version 1.02 (Centre for Disease Control and Prevention and WHO) and malnutrition and severe malnutrition were defined as Z‐score for weight‐for‐age index under −2.00 or −3.00, respectively, using the National Centre for Health Statistics standard.14
2.2. Sampling and microbiological analyses
NPA and acute serum samples were collected from each patient upon admission and convalescent serum samples were collected on the follow‐up visit. The NPA was aspirated through a nostril with a disposable mucus extractor inserted into the nasopharynx. Each sample was immediately placed in an identified empty tube and frozen at −80°C at the Federal University of Bahia Hospital Laboratory pending shipment to the research laboratories. After data collection was finished, the NPA samples were sent to the University of Turku, Department of Clinical Virology, Turku, Finland, whereas the paired serum samples were sent to the University of Helsinki, Department of Virology, Helsinki, Finland, by airplane maintained at −80°C. After thawing and nucleic acid extraction, a multiplex real‐time PCR test kit (Anyplex [TM] II RV16, Seegene, Seoul, South Korea) was used to detect: human adenovirus (HAdV), influenza A (Flu A) and B (Flu B) viruses, parainfluenza virus types 1‐4 (PIV 1, PIV 2, PIV 3, PIV 4), rhinovirus (RV), respiratory syncytial viruses A (RSV A) and B (RSV B), HBoV1, coronaviruses 229E (HCoV 229E), NL63 (HCoV NL63), and OC43 (HCoV OC43), metapneumovirus (hMPV), and enteroviruses (EV) in NPA. The Anyplex (TM) II RV16 detection kit is a multiplex real‐time PCR assay based on tagging oligonucleotide cleavage extension. In addition, an in‐house PCR test was used to detect EV and RV in NPA samples, as previously described.15 Due to the known long persistence of HBoV1‐DNA in the airways, specific IgM and IgG were measured in 159 paired serum samples of HBoV1‐DNA‐positive patients. The diagnostic criteria of an acute primary HBoV1 infection was the presence of one of the following markers: presence of IgM, a fourfold or greater increase or conversion of IgG in paired sera. The HBoV IgG and the my‐capture IgM enzyme immunoassays (EIAs) have been described elsewhere.16, 17 Recombinant virus‐like particles of VP3 were used as antigen in the two EIAs and cross‐reacting antibodies for HBoV2 and 3 were blocked. The cutoff absorbance values for antibody presence (mean + 4 SDs) were 0.095 for our competition IgG and 0.131 for IgM.17 Borderline results in all EIAs were considered indeterminate.
2.3. Preventive measures for PCR contamination
The PCR methodology is highly vulnerable to possible false positive results due to contamination of reagents and specimens with PCR amplicons. The analyses were performed in an accredited clinical virus diagnostic laboratory. In the present work, rigorous measures were taken throughout the whole study to avoid amplicon as well as sample‐to‐sample contamination.
2.4. Statistical analyses
Sample size was estimated as 73 cases with HBoV1 detected in NPA, considering an expected frequency of acute serologically diagnosed HBoV1 infection equal to 5%, total width of confidence interval (CI) of 10% and 95% CI. We performed a bivariate analysis: the frequency of each clinical characteristic of children with or without acute HBoV1 infection was compared by using chi‐square or Fisher's exact test as appropriate; continuous variables were presented as median (interquartile range [IQR]) and were assessed by using Mann‐Whitney U test due to non‐parametrical distribution. The tests were two‐tailed with significance at <0.05. As the frequency of missing information was very low, we chose to handle the absent data by excluding these cases. All laboratory procedures were performed by trained personnel blinded to clinical data. All analyses were performed using the Statistical Package for the Social Sciences (SPSS), Version 9.0.
2.5. Ethics approval
This study was conducted in accordance with the Declaration of Helsinki and national and institutional standards. It was approved by the Ethics Committee of the Federal University of Bahia (Approval reference number 24/2006).
3. RESULTS
A total of 820 children were enrolled in the clinical trial. Of these, 759 (92.6%) children had NPA and acute and convalescent serum samples collected and were included in this study. The median (IQR) interval between collection of acute and convalescent samples was 19 (16‐22) days. Table 1 presents the baseline characteristics of the 759 studied cases. All NPA samples were tested for 16 viruses. Viruses were detected in 693 samples (91.3%; 95% CI: 89.1‐93.2) and HBoV1 was found in 159 (20.9%; 95% CI: 18.2‐24.0) cases. Of 159 PCR‐positive cases, acute or recent HBoV1 infection was confirmed serologically in 38 cases (23.9 %; 95%CI: 17.8‐31.0). Overall, acute or recent HBoV1 infection was confirmed in 5.0% (38/759).
Table 1.
Baseline characteristics of children with non‐severe community‐acquired pneumonia and comparison between cases with or without acute human bocavirus 1 infection
| All cases | Acute HBoV1 infection d | ||
|---|---|---|---|
| Characteristics | (n = 759) n (%) a | Yes (n = 38) a | No (n = 721) a |
| Demographics | |||
| Age (median [IQR] months) | 25.5 (13.4‐40.1) | 21.7 (13.3‐26.9) | 25.8 (14.0‐40.5) |
| Male gender | 398 (52.4) | 16 (42.1) | 382 (53.0) |
| History | |||
| Disease duration(median [IQR] days) | 5 (4‐8) | 6.5 (4.0‐8.0) | 5.0 (4.0‐8.0) |
| Cough | 739/757 b (97.6) | 37 (97.4) | 702/719 c (97.6) |
| Fever | 702/758 b (92.6) | 38 (100.0) | 664/720 c (92.2) |
| Difficulty breathing | 472/757 b (62.4) | 27 (71.1) | 445/719 c (61.9) |
| Vomiting | 337/758 b (44.5) | 16 (42.1) | 321/720 c (44.6) |
| Physical examination | |||
| Rhonchi | 493/758 b (65.0) | 26 (68.4) | 467/720 c (64.9) |
| Crackles | 343/758 b (45.3) | 20 (52.6) | 323/720 c (44.9) |
| Tachypnea | 340/758 b (44.9) | 16 (42.1) | 324/720 c (45.0) |
| Wheezing | 225/758 b (29.7) | 12 (31.6) | 213/720 c (29.6) |
| Reduced pulmonary expansion | 64/757 b (8.5) | 3 (7.9) | 61/719 c (8.5) |
| Malnutrition | 29/758 b (3.8) | 1 (2.6) | 28/720 c (3.9) |
| Chest retraction | 28/758 b (3.7) | 1 (2.6) | 28/720 c (3.8) |
IQR, interquartile range.
Expressed as absolute number and percentage if not otherwise specified.
The denominator was not 759 because there was missing information.
The denominator was not 721 because there was missing information.
No statistically significant difference was found when cases with or without acute HBoV1 infection were compared.
Out of 693 cases with virus detection, 477 (68.8%: 95%CI: 65.3‐72.2) had multiple viruses detected, among which HBoV1 was found in 151 (31.7%). Conversely, out of 216 cases with sole virus detection, HBoV1 was detected in 8 (3.7%) (P < 0.001). Acute HBoV1 infection was serologically diagnosed in 34 (22.5%) of the HBoV1 cases with at least one other virus, compared to 4 (50.0%) cases with sole virus detection (P = 0.09). Among those 34 cases with acute HBoV1 infection and multiple virus detection, 11 (32.4%) cases had 1 virus detected, 12 (35.3%) cases had 2 viruses detected, 6 (17.6%) cases had 3 viruses detected, 3 (8.8%) cases had 4 viruses detected, and 2 (5.9%) cases had 5 viruses detected alongside HBoV1, being up to 6 respiratory viruses concomitantly found. Figure 1 demonstrates the frequency of each respiratory virus found among these cases. Rhinovirus was the most frequently found virus being detected in 50% of these cases whereas neither Flu A nor PIV 2 were detected. Table 1 also shows the comparison between cases with or without acute HBoV1 infection. No significant differences were found when children with acute HBoV1 sole infection were compared with children with acute HBoV1 and multiple virus detections (Table 2). Figure 2 presents the monthly distribution of cases with acute HBoV1 infection diagnosed by serology, of cases with HBoV1 detected in NPA, and of the recruited CAP cases in each month of the study period.
Figure 1.
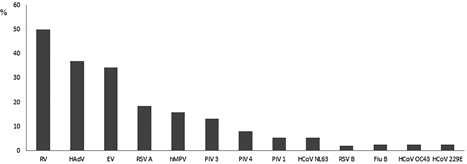
Frequency (%) of respiratory viruses detected among 34 children with acute human bocavirus 1 infection alongside multiple other viruses detected
Table 2.
Comparison between cases with acute HBoV1 sole infection and cases with acute human bocavirus 1 infection and multiple viruses detection
| Acute HBoV1 infection | |||
|---|---|---|---|
| Characteristics | Sole infection (n = 4) a | Multiple viruses detection (n = 34) a | P |
| Demographics | |||
| Age (median [IQR] months) | 29.0 (15.3‐44.8) | 21.1 (13.3‐26.7) | 0.2 |
| Male gender | 1 (25.0) | 15 (44.1) | 0.6 |
| History | |||
| Disease duration(median [IQR] days) | 5.5 (4.0‐13.0) | 6.5 (3.8‐8.0) | 0.9 |
| Cough | 4 (100.0) | 33 (97.1) | 1.0 |
| Fever | 4 (100.0) | 34 (100.0) | ‐ |
| Difficulty breathing | 2 (50.0) | 25 (73.5) | 0.6 |
| Vomiting | 3 (75.0) | 13 (38.2) | 0.3 |
| Physical examination | |||
| Rhonchi | 3 (75.0) | 23 (67.6) | 1.0 |
| Crackles | 3 (75.0) | 17 (50.0) | 0.6 |
| Tachypnea | 2 (50.0) | 14 (41.2) | 1.0 |
| Wheezing | 1 (25.0) | 11 (32.4) | 1.0 |
| Reduced pulmonary expansion | 0 (0.0) | 3 (8.8) | 1.0 |
| Malnutrition | 0 (0.0) | 1 (2.9) | 1.0 |
| Chest retraction | 0 (0.0) | 1 (2.9) | 1.0 |
IQR, interquartile range.
Expressed as absolute number and percentage if not otherwise specified.
Figure 2.
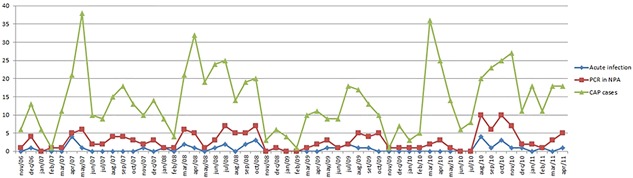
Monthly distribution (n) of cases with acute human bocavirus 1 infection diagnosed by serology, of cases with human bocavirus 1 detected in nasopharyngeal aspirate samples, and of the recruited community‐acquired pneumonia cases in each month of the study period
4. DISCUSSION
In this study, we found that 21% of children with non‐severe CAP had HBoV1‐DNA in their nasopharynx but only one quarter of them showed serologic evidence of acute or recent HBoV1 infection. Three quarters of PCR‐positive HBoV1 detections in children with CAP were not associated with the current infection being most probably due to prolonged shedding after a previous HBoV1 infection. In agreement with many earlier studies we detected HBoV1 in the majority of cases together with other respiratory viruses. Importantly, one fifth of the children with multiple viral detections had acute HBoV1 infection showing that HBoV1 in coinfections is not always just an innocent bystander.
With the use of HBoV1 serodiagnosis it has been clearly shown that by 6 years of age all children have been infected with HBoV1.18 In the original serodiagnosis study of Kantola et al, 59% of 49 HBoV1‐DNA‐positive children with acute wheezing exhibited IgM antibodies and/or an increase in IgG antibody levels.9 Don et al19 found that 12% of 101 children with CAP had serological evidence of acute HBoV1 infection. Seven of 12 patients were hospitalized. Some members of this research team have previously studied serologically HBoV1 infection in 273 children hospitalized due to CAP20; with strict criteria 8% of these CAP patients had evidence of acute HBoV1 infection. Our observations and those of others strongly suggest that HBoV1 is a causative agent of childhood CAP. Furthermore, the observations suggest that in half of HBoV1 acute infection CAP cases, the illness is clinically non‐severe thus not necessitating hospitalization.
The widely used multiplex PCR tests have greatly increased our understanding of the role of respiratory viruses in pneumonia. In many studies in children with CAP, respiratory viruses have been detected in 50‐70% of the cases.4 However, many viruses including HBoV1 have been detected at similar or higher rates in asymptomatic controls which has questioned whether these viruses are causative agents of CAP.21, 22, 23 This concept is debatable, because respiratory viruses may also cause asymptomatic infections which explains the detection of viruses in asymptomatic subjects.15, 24, 25 Recently, serology has made an important contribution to this question. In the study of Zhang et al, in children with CAP, serology and RT‐PCR mostly agreed but serology for RSV, hMPV, PIV 1‐3, and HAdV increased the diagnostic yields, ranging from 12% to 49%.26 Interestingly, after an acute infection HBoV1 is the only respiratory virus with prolonged viral shedding lasting longer than 1‐3 weeks,27 which is why serology markedly decreases the yield of HBoV1 diagnoses compared with PCR in the nasopharyngeal mucus. It is of note that HBoV1‐asymptomatic acute infections have not been reported. In one study, none of 28 asymptomatic HBoV1‐DNA‐positive children had spliced HBoV1 mRNA in their nasopharyngeal mucus suggesting that the viruses detected were not replicating.28
The clinical role of multiple respiratory virus detection in the pathogenesis of childhood CAP is not fully understood. Recently published reports have concluded that there is no association between multiple respiratory virus detection and disease severity.29, 30 In our study, multiple viruses were detected in approximately 70% of the cases with virus‐positive non‐severe CAP. Our study suggests that HBoV1 might have a pathogenic role in children with CAP, also with multiple virus detection.
Cases were randomly found among all months of the year in this study (Figure 2). Among Lebanese children hospitalized either with bronchiolitis or with CAP, HBoV1‐DNA was found in airways samples in all seasons, except spring.31 This may be explained by the difference in the weather conditions during the year in each of these countries: while Lebanon has mild weather allowing the occurrence of significant temperature and climate changes, Brazil is a tropical country having steady climate conditions throughout the year.
Our study has certain limitations. The study was a single center study limiting wider generalization of the observations. Due to economical restrictions, HBoV1 serology was studied only from HBoV1‐DNA‐positive patients. It is probable that also studying HBoV1‐DNA‐negative CAP children would have increased our diagnostic yield. In the study on acute wheezing 13% of children who tested negative for HBoV1‐DNA in the nasopharynx showed serologic evidence of acute HBoV1 infection.9 Furthermore, we did not study HBoV1 copy numbers nor HBoV1 DNA in the serum. However, it has been previously shown in a Finnish study that 38% of the children with low‐load PCR positivity have acute infection diagnosed by serology whereas 96% of the high‐load positive PCR have the same serological diagnosis.16 Therefore, serology should be enough to show reliably the actual acute infections. Moreover, it was very recently published that when compared with serology, DNA PCR had high clinical sensitivity (100%) but, because of viral persistence, low specificity (76%).32 An additional limitation is that no virus‐bacterial co‐infections were analyzed. The strength of our study includes a long recruitment period lasting almost four and half years, reducing the risk of bias regarding viral seasonality, and a high number of CAP patients. In addition, the serologic studies were carried out in a well‐established laboratory of HBoV1 virology. It has been recognized that serology is the most accurate method to diagnose acute HBoV1 infection, even when it was compared to detection of HBoV1 messenger RNA which implies viral replication.32
In conclusion, our observations provide further support for the causal role of HBoV1 in the pathogenesis of childhood CAP, both as a sole agent or with other respiratory viruses.
CONFLICTS OF INTEREST
None to be declared by all authors.
ACKNOWLEDGMENTS
The authors are in debt to the pediatricians and nurses of the Federal University of Bahia Hospital, Salvador, Brazil, for their cooperation in recruiting the patients. This work was supported by a grant from the Bahia State Agency for Research Funding (FAPESB) (Grant No. PPSUS No. SUS0023/2013) as well as by the Helsinki University 375‐year grant, the Sigrid Jusélius Foundation and the Chinese Scholarship Council. CMN‐C is senior investigator at the Brazilian Council for Scientific and Technological Development (CNPq).
Nascimento‐Carvalho AC, Vilas‐Boas A‐L, Fontoura MH, et al. Serologically diagnosed acute human bocavirus 1 infection in childhood community‐acquired pneumonia. Pediatric Pulmonology. 2018;53: 88–94. 10.1002/ppul.23891
The abstract of this manuscript was orally presented during the 35th Annual Meeting of the European Society for Paediatric Infectious Diseases Research Masterclass, held in Madrid, Spain, on May 27, 2017.
PNEUMOPAC‐Efficacy Study Group Phase I (in alphabetical order):
Adriana R. Matutino, Bruna B. Barreto, Carolina C. Silva, Daniel A. Braga, Gabriel Xavier‐Souza, Giorgio V. Nogueira, Ícaro S. Oliveira, Lais B. Neiva, Milena C. Santana, Monalisa Nobre‐Bastos, Sérgio F. Câmara, Uri R. Sirmos, Vital F. Araújo: Department of Pediatrics, Federal University of Bahia School of Medicine, Salvador, Bahia, Brazil; Felipe Oliveira, Igor Lorgetto, Itana N. Costa, Jamile Araripe, Júlia R. Vieira, Bahiana School of Medicine, Bahiana Foundation for Science Development, Salvador, Bahia, Brazil; Lúcia Noblat, Pablo M. Santos, Solange Carneiro, Pharmacy Unit, Federal University of Bahia Hospital, Salvador, Bahia, Brazil.
PNEUMOPAC‐Efficacy Study Group Phase II (in alphabetical order):
Caroline Vilas‐Boas, Fausto Azevedo, José‐Raimundo Maia Jr., Larissa Pirajá, Priscila S. Jesus, Taiane Fonseca, Ticiana Vilar: Department of Pediatrics, Federal University of Bahia School of Medicine, Salvador, Bahia, Brazil; Carolina C. Silva, Denise Gantois: Bahiana School of Medicine, Bahiana Foundation for Science Development, Salvador, Bahia, Brazil.
REFERENCES
- 1. Walker Cl, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013; 381:1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polverino E, Torres Marti A. Community acquired pneumonia. Minerva Anestesiol. 2011; 77:196–211. [PubMed] [Google Scholar]
- 3. Lu G, Li J, Xie Z, et al. Human metapneumovirus associated with community‐acquired pneumonia in children in Beijin, China. J Med Virol. 2013; 85:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011; 377:1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allander T, Tammi M, Eriksson M, Bjerkner A, Tiveljung‐Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci. 2005; 102:12801–12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allander T, Jatti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007; 44:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foulongne V, Rodiere M, Segondy M. Human bocavirus in children. Emerg Infect Dis. 2006; 12:862–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu X, Chittaganpitch M, Olsen SJ, et al. Real‐time PCR assays for detection of bocavirus in human specimens. J Clin Microbiol. 2006; 44:3231–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kantola K, Hedman L, Allander T, et al. Serodiagnosis of human bocavirus infection. Clin Infect Dis. 2008; 46:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Management of the child with a serious infection or severe malnutrition: guidelines for care at the first‐referral level in developing countries. 2000. http://apps.who.int/iris/bitstream/10665/42335/1/WHO_FCH_CAH_00.1.pdf. Page 20. Accessed 2 May 2003.
- 11. Vilas‐Boas AL, Fontoura MS, Xavier‐Souza G, et al. Comparison of oral amoxicillin given thrice or twice daily to children between 2 and 59 months old with non‐severe pneumonia: a randomized controlled trial. J Antimicrob Chemother. 2014; 69:1954–1959. [DOI] [PubMed] [Google Scholar]
- 12. El‐Radhi AS, Barry W. Thermometry in paediatric practice. Arch Dis Child. 2006; 91:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Integrated Management of Childhood Illness chart booklet (WC 503.2). 2008. http://www.whqlibdoc.who.int/publications/2008/9789241597289_eng.pdf. Accessed 15 January 2009.
- 14.World Health Organization. Training Course on Child Growth Assessment. 2008. http://www.whqlibdoc.who.int/publications/2008/9789241595070_A_eng.pdf. Accessed 13 July 2009.
- 15. Peltola V, Waris M, Österback R, Susi P, Ruuskanen O, Hyypiä T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008; 197:382–389. [DOI] [PubMed] [Google Scholar]
- 16. Söderlund‐Venermo M, Lahtinen A, Jartti T, et al. Clinical assessment and improved diagnosis of bocavirus‐induced wheezing in children, Finland. Emerg Infect Dis. 2009; 15:1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kantola K, Hedman L, Tanner L, et al. B‐cell responses to human Bocaviruses 1‐4: new insights from a childhood follow‐up study. PLoS ONE. 2015; 10:e0139096. 10.1371/journal.pone.0139096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meriluoto M, Hedman L, Tanner L, et al. Association of human bocavirus 1 infection with respiratory disease in childhood follow‐up study, Finland. Emerg Infect Dis. 2012; 18:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Don M, Söderlund‐Venermo M, Valent F, et al. Serologically verified human bocavirus pneumonia in children. Pediatr Pulmonol. 2010; 45:120–126. [DOI] [PubMed] [Google Scholar]
- 20. Nascimento‐Carvalho CM, Cardoso M‐RA, Meriluoto M, et al. Human bocavirus infection diagnosed serologically among children admitted to hospital with community‐acquired pneumonia in a tropical region. J Med Virol. 2012; 84:253–258. [DOI] [PubMed] [Google Scholar]
- 21. Rhendin S, Lindstrand A, Hjelmgren A, et al. Respiratory viruses associated with community‐acquired pneumonia in children: matched case‐control study. Thorax. 2015; 70:847–853. [DOI] [PubMed] [Google Scholar]
- 22. Martin ET, Kuypers J, McRoberts JP, Englund JA, Zerr DM. Human bocavirus 1 primary infection and shedding in infants. J Infect Dis. 2015; 212:516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korppi M. Polymerase chain reaction in respiratory samples alone is not a reliable marker of bocavirus infection. Pediatr Pulmonol. 2014; 49:515–516. [DOI] [PubMed] [Google Scholar]
- 24. Hayward AC, Fragaszy EB, Bermingham A, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014; 2:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luoto R, Jartti T, Ruuskanen O, Waris M, Lehtonen L, Heikkinen T. Review of the clinical significance of respiratory virus infections in newborn infants. Acta Paediatr. 2016; 105:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Sakthivel SK, Bramley A, et al. Serology enhances molecular diagnosis of respiratory virus infections other than influenza in children and adults hospitalized with community‐acquired pneumonia. J Clin Microbiol. 2016; 55:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peltola V, Söderlund‐Venermo M, Jartti T. Human bocavirus infections. Pediatr Infect Dis J. 2013; 32:178–179. [DOI] [PubMed] [Google Scholar]
- 28. Christensen A, Dollner H, Skanke LH, Krokstad S, Moe N, Nordbo SA. Detection of spliced mRNA from human bocavirus 1 in clinical samples from children with respiratory tract infections. Emerg Infect Dis. 2013; 19:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goka EA, Valley PJ, Mutton KJ, Klapper PE. Single and multiple respiratory virus infections and severity of disease: a systematic review. Paediatr Respir Rev. 2014; 15:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi SH, Chung JW, Kim HR. Clinical relevance of multiple respiratory virus detection in adult patients with acute respiratory illness. J Clin Microbiol. 2015; 53:1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Finianos M, Issa R, Curran MD, et al. Etiology, seasonality, and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon. J Med Virol. 2016; 88:1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu M, Arku B, Jartti T, et al. Comparative diagnosis of human bocavirus 1 respiratory infection by mRNA RT‐PCR, DNA quantitative PCR and serology. J Infect Dis. 2017; 215:1551–1557. [DOI] [PubMed] [Google Scholar]


