Abstract
Insight into the hepatoprotective effects of medicinally important plants is important, both for physicians and researchers. Main reasons for the use of herbal medicine include their lesser cost compared with conventional drugs, lesser undesirable drug reactions and thus high safety, and reduced side effects. The present review focuses on the composition, pharmacology, and results of experimental trials of selected medicinal plants: Silybum marianum (L.) Gaertn., Glycyrrhiza glabra, Phyllanthus amarus Schumach. & Thonn., Salvia miltiorrhiza Bunge., Astragalus membranaceus (Fisch.) Bunge, Capparis spinosa (L.), Cichorium intybus (L.), Solanum nigrum (L.), Sapindus mukorossi Gaertn., Ginkgo biloba (L.), Woodfordia fruticosa (L.) Kurz, Vitex trifolia (L.), Schisandra chinensis (Turcz.) Baill., Cuscuta chinensis (Lam.), Lycium barbarum, Angelica sinensis (Oliv.) Diels, and Litsea coreana (H. Lev.). The probable modes of action of these plants include immunomodulation, stimulation of hepatic DNA synthesis, simulation of superoxide dismutase and glutathione reductase to inhibit oxidation in hepatocytes, reduction of intracellular reactive oxygen species by enhancing levels of antioxidants, suppression of ethanol‐induced lipid accumulation, inhibition of nucleic acid polymerases to downregulate viral mRNA transcription and translation, free radical scavenging and reduction of hepatic fibrosis by decreasing the levels of transforming growth factor beta‐1, and collagen synthesis in hepatic cells. However, further research is needed to identify, characterize, and standardize the active ingredients, useful compounds, and their preparations for the treatment of liver diseases.
Keywords: Hepatitis C, hepatoprotective activity, herbal, medicinal plants, treatment
Abbreviations
- ALT
alanine aminotransaminase
- ASP
Angelica sinensis polysaccharides
- AST
aspartate transaminase
- EGF
epidermal growth factor
- HBV
Hepatitis B virus
- LBPs
Lycium barbarum polysaccharides
- WF4
Woodfordia fruticosa flower extract.
1. INTRODUCTION
Liver disorders have been classified in the high priority areas of health care. According to an estimate by the World Health Organization, approximately 500 million people of the world are suffering from a severe form of liver disorders, that is, chronic hepatitis (Al‐Asmari et al., 2014). Medicine of herbal origin may serve as a feasible therapy for the prevailing liver problems because of their safety, easier availability, cost effectiveness, and environment friendliness (Izzo, Hoon‐Kim, Radhakrishnan, & Williamson, 2016). Medicinal plants have acquired importance in healthcare system throughout the world for their proven and effective therapeutic properties (Helmstädter & Staiger, 2014). An estimated 80% of the world's population is relying on medicines that contain compounds of herbal origin (Ekor, 2013). The International Union for Conservation of Nature has suggested that approximately 50,000 to 80,000 flowering plants are used for medicinal purposes (Chen, Li, Ren, & Hu, 2016). Many factors regarding these medicines are important. Herbal medicines are claimed to both treat and prevent diseases, which adds to a deep belief that these treatments are safe because they are “natural and gentle” and therefore, a harmless alternative to the conventional medicine. Moreover, the latter may sometimes cause disappointing results and undesirable side effects in patients (Izzo et al., 2016). In addition, the less expensive herbal products are often not subject to strict regulations and medication prescribed by a physician or other qualified practitioners (Hunter & Hegele, 2017). Although medicinal plants have been used globally, their wider usage is limited to a few countries like Japan, India, China, Pakistan, Thailand, Iran, and some African countries (Bahmani et al., 2014; Iwu, 2014; Li, 2016; Sivasankari, Anandharaj, & Gunasekaran, 2014). Other countries are also encouraging the use of plant‐based medicinal products in their healthcare systems. For example, Natural Health Product Regulations of Canada for the plant‐based product in healthcare encourages usage of modern technology and evidence‐based scientific support towards promoting medicinal plants and the associated products (Tomlinson & Akerele, 2015). A major concern of scientists investigating herbal treatments is that the chemical composition of the plants contributing to their biological effects is mostly undetermined (Ling et al., 2009).
Herbs and herbal medicines have been used for the treatment of liver diseases for a long time (Dhiman & Chawla, 2005). There are many herbs having ingredients that are potential sources of medicine for the treatment of liver diseases having various modes of actions and bioactivities (Babu, Bhuvaneswar, Sandeep, Ramaiah, & Rajendra, 2017; Gnanadesigan, Ravikumar, & Anand, 2017; Pereira, Barros, & Ferreira, 2016). However, several of them are well‐studied for their bioactive components and the mechanism of hepatoprotective activity. In the current review, we have selected some of these compounds for which elaborate detail about hepatoxicity is available in literature in the form of either in vivo studies, study into biochemical parameters and bioactive compounds. This article highlights the possible ways of inducing hepatoxicity in mice models and encompasses the mechanisms in which certain medicinally important plants perform their hepatoprotective activity. The article further aims to summarize studies conducted on the composition, pharmacology, and nature of the selected plants in the light of possible mechanism deduced from experimental trials.
2. METHODS
2.1. Sources and article selection criteria
A thorough search was conducted on the electronic literature databases, Google Scholar, PubMed, Scopus, and Web of Science. Literature was retrieved using the key words and phrases “Hepatitis C”, “Hepatoprotective activity”, “Mechanism of action”, “Medicinal plants”, “Herbal”, and “Treatment”. About 100 relevant articles were extracted after a narrow search for a combination of the keywords and subsequent analysis per the inclusion criteria. There were two sets of criteria applied to articles for inclusion in this manuscript. According to the first, “general criteria”, articles selected for this manuscript were those which (a) reported plants and their parts that were traditionally applied to hepatitis and liver disorders and any other type of hepatoprotective activity; (b) reported extract or pure compounds important for their hepatoprotective role; and (c) attempted to explain the mechanism of hepatoprotective action of these plants.
The 2nd criteria were used for selecting those plants that are discussed in detail (shown in Figure 3). For this purpose, seventeen plants were selected for which recent articles were available that (a) studied in vitro and in vivo hepatoprotective activities of herbal products, (b) reported active compounds from the plant, and (c) described the mechanism of action herbal hepatoprotective products. The plants described in detail were selected if literature available for them fulfilled at least two of the above 3‐point criteria.
Figure 3.
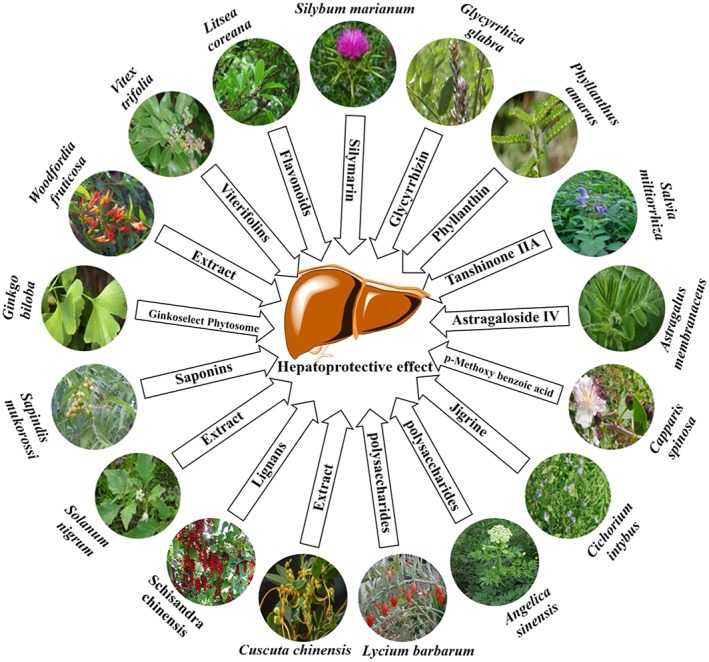
Important medicinal plants and their active ingredients associated with their hepatoprotective properties [Colour figure can be viewed at http://wileyonlinelibrary.com]
Each of the selected plants was discussed; mainly focusing its hepatoprotective activities, active compounds, and possible mechanism of action. In addition, featured hepatoprotective herbal combinations have been deliberated. Toxicity and quality control issues associated with these herbs/herbal products have been debated.
2.2. Full‐text review of the selected articles
Two of the authors independently reviewed all the full‐text articles obtained during the electronic search. Data from the eligible articles were extracted; all the disagreements were discussed and were referred to a third reviewer (one of the author) for a final decision. All the data were extracted in two tables (Tables 1 and 2), and the mechanisms of action were explained in respective subheadings and demonstrated through four different figures (Figures 1, 2, 3, and 4).
Table 1.
Some of the major hepatoprotective medicinal plants with potential bioactive compounds and their mechanism of actions
| Plant | Part used | Potential agents | Mechanism of action | References |
|---|---|---|---|---|
| Amaranthus spinosus L. | Whole plant | Flavonoids and phenolic compounds | Enzymatic levels of serum glutamate oxaloacetate transaminase (AST), serum glutamate pyruvate transaminase (ALT), serum alkaline phosphatase (SALP), and total bilirubin were reinstated to the normal level | (Takada, Takase, Takada, & Date, 1993) |
| Artemisia absinthium L. | Aerial parts | Sesquiterpene lactones, flavonoids, phenolic acids, tannins | Prevented chemically or immunologically induced increase in serum levels of hepatic enzymes in CCl4‐induced hepatic damaged rats. Reduced the lipid peroxidation in the liver and restored activities of defense antioxidant enzymes SOD and GPX towards normal levels | (Amat, Upur, & Blažeković, 2010) |
| Astragalus membranaceus (Fisch.) Bunge., and Salvia miltiorrhiza Bunge. | Whole plant | Astragalus; salvia crude extract | Exert antifibrosis effect in chronically injured liver by inhibiting tumor growth facto‐β/Smads signaling pathway in rats | (Dusheiko, 1996) |
| Calotropis procera (Aiton) Dryand. | Flowers | Crude hydro‐ethanol solution extract | Prevents of the depletion of GSH levels. C. procera contains flavonoids thus it also performs the antioxidant activity | (McOmish et al., 1994) |
| Clerodendrum abilioi R. Fern. | Leaves | Crude ethanol solution extract | Ethanol extract decreased the serum enzyme ALT, AST, ALP, TGL, and total cholesterol and considerably increased the glutathione level | (Chamberlain, Adams, Saeed, Simmonds, & Elliott, 1997) |
| Ficus carica L. | Leaves | Crude petroleum ether extract | Reduction in the levels of ALT and AST. The petroleum ether extract of Ficus leaves repair the damaged liver cell | (Gond & Khadabadi, 2008) |
| Glycyrrhiza uralensis | — | Glycyrrhizin | Glycyrrhizin administered in PLC/PRF/5 cells suppressed the secretion of HBsAg into the culture medium and concluded that glycyrrhizin modifies the intracellular transport and the surface nature of the hepatocytes | (Sato et al., 1996) |
| Momordica dioica Roxb. ex Willd. | Leaves | Alkaloids, phenolic compounds, glycosides, flavonoids | Oral administration of the extract significantly normalized and restored the elevated serum enzymatic levels of AST, ALT, SALP, and total bilirubin. Its hepatoprotective activity is due to the antioxidant and free radical scavenging activity. | (Surai, 2015) |
| Nelumbo nucifera Gaertn. | Leaves | Catechin glycoside, myricitrin‐3‐O‐glucoside, hyperin, isoquercitrin, quercetin‐3‐O‐rhamnoside, astragalin | Lotus leaf extract possess significant hepatoprotective and antioxidant activity in CCl4‐induced toxicity rat model. Free radical‐scavenging and antioxidant activity due to the presence of some flavonoids and phenolic compounds results in the hepatoprotective activity. | (Theplantlist, 2013) |
| Paeonia lactiflora Pall. and A. membranaceus (Fisch.) Bunge. | Roots | — | Progression of CCl4‐induced hepatic fibrosis was inhibited in rates by decreasing the level of tumor growth factor‐β1 and inhibit collagen synthesis | (Sun et al., 2007) |
| S. miltiorrhiza Bunge. | Roots | — | S. miltiorrhiza could reverse the CCl4‐induced fibrosis treatment by decreasing the levels of transforming growth factor‐β1, procollagens I and III, and metalloproteinase‐1 and decreasing the levels of metalloproteinase‐13 in liver of the affected rates | (Wasser et al., 1998) |
| S. miltiorrhiza Bunge. | Roots | S. miltiorrhiza polysaccharides | Protects liver against immunological injury by adjusting the levels of alanine aminotransferase, aspartate aminotransferase, nitric oxide, tumor necrosis factor and interleukin‐1 | (Zein et al., 1996) |
| Solanum nigrum L. | Total decoction | Crude aqueous extract | Inhibited thioacetamide‐induced collagen (α1) and transforming growth factor‐β1 mRNA levels in the liver of mice with thioacetamide‐induced liver fibrosis | (Hsieh et al., 2008) |
| S. nigrum L. and Cichorium intybus L. | Leaves | Crude plant extract |
Protect DNA against oxidative damage in the reaction mixture containing calf thymus DNA and free radical generating system |
(Sultana et al., 1995) |
| Tecomella undulata (Sm.) Seem. | Stem bark | Crude ethanol solution extracts | Hepatoprotective activity against thioacetamide‐induced hepatotoxicity | (Khatri, Garg, & Agrawal, 2009) |
| Tephrosia purpurea (L.) Pers. | Aerial parts | Crude aqueous–ethanol solution extract | Decreased serum aspartate aminotransaminase (35% and 31%), alanine aminotransaminase (50% and 42%), gamma glutamyl transpeptidase (56% and 49%), alkaline phosphatase (46% and 37%), total bilirubin (61% and 48%), and liver MDA levels (65% and 50%), and significant improvement in liver glutathione (73% and 68%) when compared with thioacetamide‐damaged rats. | (Hosseinzadeh & Nassiri‐Asl, 2015) |
| Vitex negundo L. | Leaves | Crude ethanol solution extract | Administration of ethanol solution extract of Vitex leaf caused a significant decrease in TB, AST, ALT, and ALP levels in rats. | (Abdulkarim et al., 1998) |
| Zanthoxylum armatum DC. | Bark | Berberine | Elevated serum enzymatic levels of serum transaminases, alkaline phosphatase. Total bilirubin was considerably restored to a normal level. | (Cha et al., 1991) |
Note. GPX = glutathione peroxidase; MDA = malondialdehyde; AST = aspartate transaminase; ALT = alanine aminotransaminase; CCl4 = carbon tetrachloride; SOD = superoxide dismutase; GSH = glutathione; TB = Total Bilirubin; ALP = alkaline phosphatase HBsAg = hepatitis B surface antigen; TGL = triglyceride lipase.
Table 2.
Few important hepatoprotective herbal products alongside their source and possible mechanism of action in vivo and in‐vitro
| Herbal medicine | Botanical source | Potential target/mechanism of action | References |
|---|---|---|---|
| Crypto‐tanshinone | Salvia miltiorrhiza Bunge. | Protects hepatocytes from lipopolysaccharide‐ and ethanol‐induced cell death by inhibiting production and nuclear translocation of sterol regulatory element binding protein‐1, and the consequent transactivation of the target genes involved in fatty acid biosynthesis in dose‐dependent manner primary cultured rat hepatocytes | (Yin et al., 2009) |
| Glycyrrhizin | Licorice (Glycyrrhiza sp.) |
Glycyrrhizin administered in PLC/PRF/5 cells suppressed the secretion of HBsAg into the culture medium and concluded that glycyrrhizin modifies the intracellular transport and the surface nature of the hepatocytes Glycyrrhizin administered intraperitoneally inhibits the lipopolysaccharide‐ and D‐galactosamine‐induced liver injury by preventing inflammatory responses and IL‐18 production in mice Glycyrrhizin inhibited anti‐Fas antibody‐induced hepatitis in mice by acting upstream of CPP32‐like protease Administration of glycyrrhizin or glycyrrhetinic acid, significantly suppressed α2 (I) collagen gene promoter activation and progression of liver fibrosis induced by repeated CCl4 injections in transgenic mice |
(Liew, Erali, Page, Hillyard, & Wittwer, 2004; Martell et al., 1992; Ogata, Alter, Miller, & Purcell, 1991; Sato et al., 1996) |
| Phyllanthin | Phyllanthus amarus Schum. et Thonn. | Phyllanthin help in restoration of antioxidant potential of rat hepatocytes, level of GSH, and SOD and GR activities reduced by ethanol | (Chirdchupunseree & Pramyothin, 2010) |
| p‐Methoxy benzoic acid | Capparis spinosa L. | The compound alleviated the enzyme levels increased as result of administration of CCl4, and PCL | (Gadgoli & Mishra, 1999) |
|
Silymarin |
Silybum marianum (L.) Gaertn. | Silymarin attenuated the rifampicin‐ and/or pyrogallol‐induced hepatotoxicity by restoring the alterations in the expression and activity of CYP1A2 and CYP2E1, glutathione‐S‐transferase, glutathione reductase and glutathione peroxidase, and lipid peroxidation in male Swiss albino mice. Silymarin suppresses N‐nitrosodiethylamine induced hepatocarcinogenesis by modulating the antioxidant defense status of the animals | (Farghali et al., 2000; Upadhyay et al., 2007) |
Note. HBsAg = hepatitis B surface antigen; CPP32 = 32‐kDa putative cysteine protease; CCl4 = carbon tetrachloride; GSH = glutathione, SOD = superoxide dismutase; GR = glutathione reductase; CYP = Cytochrome P450; PCL = Paracetamol.
Figure 1.
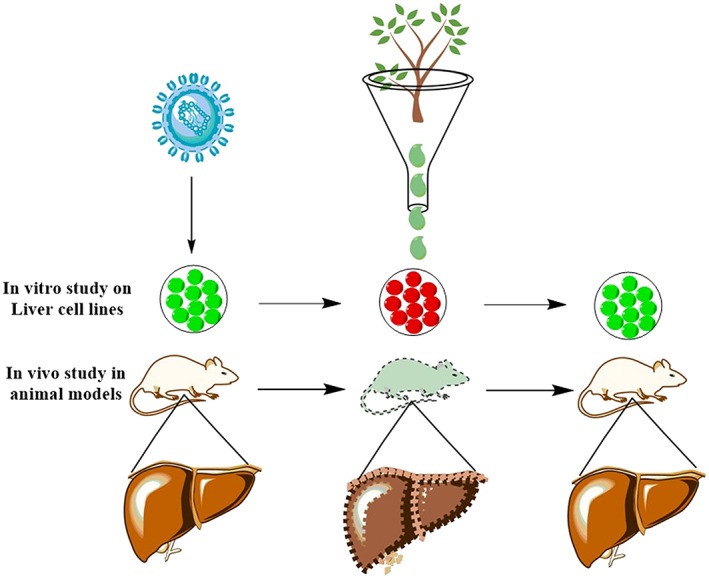
A general strategy to assay in vitro and in vivo hepatoprotective activity of plant extracts in mice. Coronavirus (mouse hepatitis virus) is mainly employed to induce inflammation of the liver in mice. These animal models have then treated with plant extracts to assay hepatoprotective effects of herbal products [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 2.
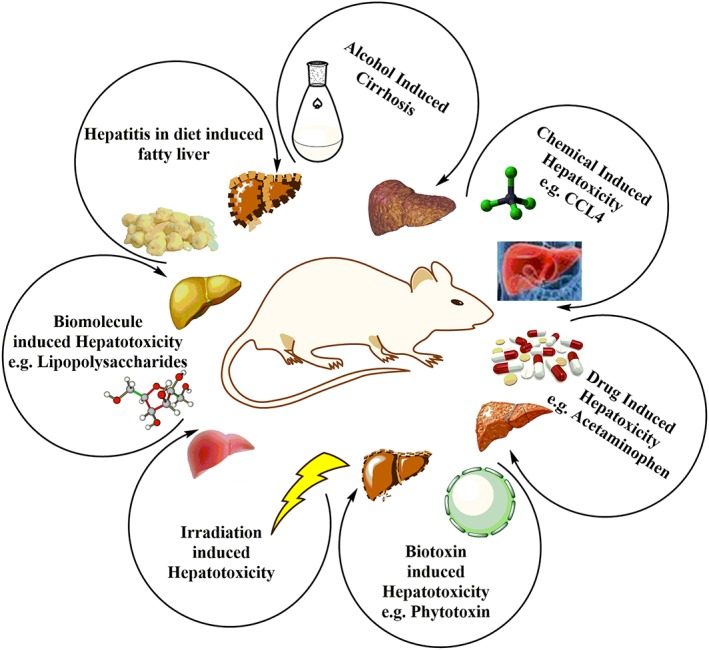
Illustration of several in vivo strategies (both physical and chemical) to induce hepatotoxicity in mice models [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
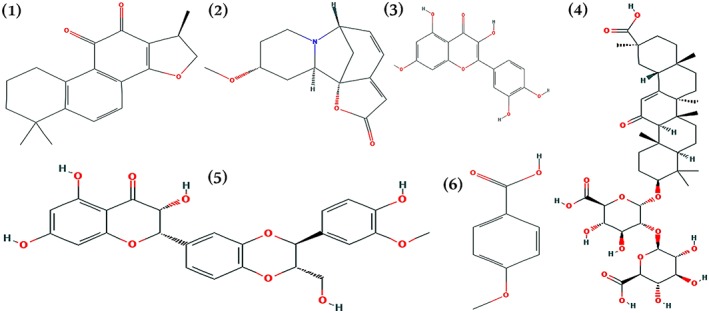
Chemical structures of (1) cryptotanshinone, (2) phyllanthin, (3) quercetin, (4) glycyrrhizin, (5) silymarin, and (6) p‐methoxybenzoic acid, also known as p‐Anisic acid. All the images were adopted from NCBI‐PubChem with the compound IDs; 160254, 358901, 5280343, 14982, 7073228, and 7478, respectively (Pubchem, 2017) [Colour figure can be viewed at http://wileyonlinelibrary.com]
2.3. Software and databases
The figures were constructed in ChemBioDraw Ultra (version 14.0) software package. Furthermore, the Plant Database “Plant list” was used for the taxonomic categorization of all the documented plant species (Theplantlist, 2013).
3. RESULTS AND DISCUSSION
3.1. The basis for in vivo studies of medicinal plants
The prerequisite to screen/study any medicinal compound for its hepatoprotective activity is to develop a model (animal model or cell culture model) in which hepatic injury is induced (Salehi, Karegar‐Borzi, Karimi, & Rahimi, 2016). Several studies have manipulated mice models to induce hepatotoxicity and then treat those induced liver diseases using herbs and herbal products (Figure 1). This approach provides insight into how hepatitis and other liver diseases are caused. However, for many plants, their mechanism of action against hepatotoxic agents is not well‐documented. The common prototype applied for hepatoprotective drug screening is the carbon tetrachloride (CCl4) induced hepatic injury (Rodrigues et al., 2016). As CCl4 have been reported for its damaging effects on the liver because on metabolism by P450, it produces free radicals (Johnston & Kroening, 1998). These free radicals cause lipid peroxidation by binding to DNA, proteins, or lipids (Yasuda, Izumi, Shimada, Kobayakawa, & Nakanishi, 1980). The degree of hepatic injury is evaluated by the higher level of biochemical parameters that is ascribed to the production of trichloromethyl free radicals which eventually causes lipids peroxidation present in cellular membrane (Chen, Yu et al., 2016). Figure 2 shows the different strategies applied for studying the in vivo effects of induced hepatotoxicity in mice models.
3.2. Selected medicinal plants and their possible mechanism of action
Hepatic disorders are usually caused by exposure to agents like drugs, viruses, parasites, and toxins. These substances through a different mechanism of action result in degeneration and inflammation of the liver, which may further result in fibrosis and cirrhosis. The main causes of liver cirrhosis are alcoholic liver disease, nonalcoholic fatty liver disease, and chronic viral hepatitis (B and C). Although generally, hepatic inflammation is caused by factors such as oxidative stress and reactive oxygen species (ROS) and DNA methylation (Ali et al., 2016; Lam et al., 2016).
There are many herbs explored for their hepatoprotective activity through different in vitro and in vivo tests. Some of them have, however, gained importance because of their enormous hepatoprotective potential, and thus attempts are made at a thorough understanding of their mechanism of action in the liver through in vivo experiments. Herbs and their products are generally thought to act as hepatoprotective agents through different mechanisms such as immunomodulation, which means they potentiate and modulate the immune system (Ilyas, Katare, Aeri, & Naseef, 2016). The following sections highlight and explain the hepatoprotective mechanism of action of the following important medicinal plants; Silybum marianum (L.) Gaertn., Glycyrrhiza glabra, Phyllanthus amarus Schumach. & Thonn., Salvia miltiorrhiza Bunge., Astragalus membranaceus (Fisch.) Bunge, Capparis spinosa L., Cichorium intybus L., Solanum nigrum L., Sapindus mukorossi Gaertn., Ginkgo biloba L., Woodfordia fruticosa (L.) Kurz, Vitex trifolia L., Schisandra chinensis (Turcz.) Baill., Cuscuta chinensis Lam., Lycium barbarum, Angelica sinensis, and Litsea coreana H. Lév. Furthermore, Tables 1 and 2 also summarize some of the other important herbs alongside their mode of application and mechanism of action.
3.3. S. marianum (L.) Gaertn.
S. marianum (L.) Gaertn. is an herbaceous stout thistle of the family Compositae, regarded generally as Milk Thistle. The plant grows up to 3 m in length in rocky soils bearing large purple flowering heads. The leaves are marked by distinct white “milky” veins that give the plant its common name (Theplantlist, 2013). Historically, S. marianum was used medicinally to treat disorders of the gallbladder, spleen, and liver, but the most important medicinal application of S. marianum is its use as a hepatoprotective herbal treatment and as supportive treatment for chronic inflammatory liver disorders such as hepatitis, cirrhosis, fatty infiltration, and some other forms of liver damages due to toxic chemicals, poisonous mushrooms, and alcohol (Freitag et al., 2015).
The most important component extracted from S. marianum is silymarin (Lu, Lu, Chen, Zhang, & Wu, 2007; Wu, Wang, & Que, 2006), which is used to treat a variety of liver disorders, including chronic and acute viral or drug/toxin‐induced hepatitis, alcoholic liver disease, and liver cirrhosis (Lu et al., 2007). Silymarin is a combination of different ingredients with silibinin as the most active among them (Surai, 2015). Silymarin has been approved for clinical studies in treating the Hepatitis C virus infection (Ferenci et al., 2008).
There are many studies on the mechanism of hepatoprotective effects of silymarin. Recently, Tunca et al. (2009) showed that silymarin has a protective action on pyridine‐induced hepatic injury in Syrian hamsters. The study concluded that it decreases the metabolic activation of pyridine (by decreasing the cytochromes P450 1A1 protein concentration) and control the elevation of inducible nitric oxide synthase expression. All these factors play a protective role in liver injury. In another study, Farghali, Kamenikova, Hynie, and Kmonickova (2000) concluded that in addition to inhibition of lipid peroxidation, the inhibition of the increased intracellular Ca2 i plays a critical role in the hepatoprotective effect of Silymarin. Although, Upadhyay, Kumar, and Singh (2007) showed that silymarin restores the changes in the expression and activity of Cytochrome P450 (CYP) enzymes (CYP1A1, CYP1A2, and CYP2E1), glutathione‐S‐transferase, glutathione reductase and glutathione peroxidase, and lipid peroxidation in male Swiss albino mice.
3.4. G. glabra (licorice)
G. glabra is a member of the Glycyrrhiza genus (Isbrucker & Burdock, 2006), an ancient genus that contains the most commonly used herbs in Chinese traditional medicine (Hosseinzadeh & Nassiri‐Asl, 2015). Glycyrrhiza species are considered among the most important herbaceous plants for a diverse array of pharmacological activities (Hosseinzadeh & Nassiri‐Asl, 2015).
G. glabra was a prescriptive drug of Hippocrates in the treatment of chest diseases including a dry cough and asthma. In traditional Chinese medicine, Glycyrrhiza uralensis and Glycyrrhiza pallidiflora are called Chinese licorice and are among the oldest and most commonly used herbs in the treatment of numerous diseases (Isbrucker & Burdock, 2006).
Glycyrrhizin (a glycoside of glycyrrhizic acid) is a triterpene glycoside extracted from roots of licorice (Glycyrrhiza sp.; Kimura et al., 2008; Ram et al., 2006). Kimura et al. (2008) surgically removed about 70% of the total liver in rats and found that administering glycyrrhizin and epidermal growth factor (EGF) through intraperitoneal injections significantly stimulate both liver regeneration and recovery of liver functions possibly via stimulation of EGF receptor. The study by Kimura et al. (2008) demonstrated the glycyrrhizin and EGF‐induced stimulation of hepatic DNA synthesis and proliferation and decreased serum alanine aminotransaminase (ALT) and aspartate transaminase (AST) activity. This indicates rapid recovery of liver function from the partial surgical removal of liver and provides new dimensions to treat patients with acute or chronic hepatitis C or after living liver transplantation.
3.5. P. amarus Schumach. & Thonn.
P. amarus Schumach. & Thonn. is a small plant widely distributed in the tropical regions and highly valued by traditional practitioners for its healing properties (Patel, Tripathi, Sharma, Chauhan, & Dixit, 2011). P. amarus is used to treat various diseases in traditional medicine, and many studies, both preclinical and clinical, have confirmed most of the actions attributed to its use (Kumaran & Joel Karunakaran, 2007).
The species of the genus Phyllanthus have been linked to different important bioactivities in disorders such as digestive disease, jaundice, and renal calculus. These activities have been attributed to over 500 different medicinal compounds isolated from various species of the genus (Mao et al., 2016). One of the bioactive compounds from P. amarus is phyllanthin, which is a lignan compound and is traditionally applied in the treatment of many liver diseases (Hanh, Sinchaipanid, & Mitrevej, 2014). It was shown to have hepatoprotective effects on ethanol‐induced oxidative damage in primary culture of rat hepatocytes through its antioxidant activity especially the activities of superoxide dismutase (SOD) and glutathione reductase (Chirdchupunseree & Pramyothin, 2010). Previously, Naaz, Javed, and Abdin (2007) studied the hepatoprotective and antioxidant activities of the ethanol solution extract of P. amarus by histopathological analysis of liver samples. They observed that P. amarus possesses the strong capability to reduce the intracellular quantity of ROS by enhancing both enzymatic and non‐enzymatic antioxidants levels against aflatoxin B1‐induced hepatic damage. In another study, Venkateswaran, Millman, and Blumberg (1987) showed the in vitro effect of aqueous extract of Phyllanthus niruri and showed that it inhibits DNA polymerase of Hepatitis B virus (HBV) and also binds to the HBV surface antigen. These findings show the potential use of the P. amarus and other Phyllanthus species for the treatment of HBV infection and other liver diseases.
3.6. S. miltiorrhiza Bunge.
S. miltiorrhiza Bunge. belongs to the family Lamiaceae and has been widely used in Korea, China, Japan, and some other Asian countries to treat various diseases such as cerebrovascular disease, cardiovascular disease, diabetic vascular complications, liver dysfunction, renal deficiency (Han et al., 2008), and chronic hepatitis (Hong et al., 2017). According to Chinese Pharmacopoeia, S. miltiorrhiza is considered to possess slightly cold and bitter properties, enters the heart and liver channels and increases blood flow and scavenges free radicals that decreases cellular damage in patients with ischemic diseases (Sun et al., 2005).
Mao et al. (2009) evaluated lipophilic extract of S. miltiorrhiza, which stimulated extra cellular catecholamine secretion. This study showed that S. miltiorrhiza extract plays a key role in central nervous system's response to stress by activation of the catecholaminergic system. Wan, Sit, Lee, Fu, and Chan (2006) suggested that the antiinflammatory mechanism of actions of S. miltiorrhiza extracts includes modifying humoral and nonspecific immunity and alleviating hepatotoxicity. The standardized fraction of the root of S. miltiorrhiza (tanshinone I 11.5%; cryptotanshinone 19.1%; and tanshinone IIA 41.0%) protects hepatocytes from lipopolysaccharide and ethanol‐induced cell death and suppresses ethanol‐induced lipid accumulation. The mechanism involves inhibition of ethanol‐induced activation and nuclear translocation of sterol regulatory element binding protein‐1, which trans‐activate the target genes involved in fatty acid biosynthesis in a dose‐dependent manner. Moreover, cryptotanshinone blocks the hepatic cell death and fatty acid synthesis and hence has the potential to treat alcoholic liver diseases. These results show that standardized fraction of the roots of S. miltiorrhiza and one of its active ingredient, cryptotanshinone, decreases the risk of alcoholic steatosis and the progression to steatohepatitis (Yin et al., 2009).
Park, Zhao, Kim, and Sohn (2009) studied the purified extract of S. miltiorrhiza and its constituents, tanshinone I, cryptotanshinone, and tanshinone‐IIA and concluded that it protects the liver against CCl4‐induced acute and subacute damage. Their study assessed serum transaminase levels, antioxidant enzyme activities, reduced form of glutathione, and lipid peroxidation levels in the liver and observed that it protects against liver toxicity in vivo and in vitro due to its antioxidant effects. Wasser, Ho, Ang, and Tan (1998) demonstrated that the S. miltiorrhiza can reverse the fibrosis caused by CCl4 treatment in male Wistar rats. They examined that rats treated with the herb had reduced levels of procollagens I and III, transforming growth factor‐β1 and tissue inhibitor of metalloproteinase‐1 transcripts and an increased level of matrix metalloproteinase‐13 mRNA as compared with the disease control that significantly decreases CCl4‐induced hepatic fibrosis in rats.
3.7. A. membranaceus (Fisch.) Bunge.
A. membranaceus (Fisch.) Bunge, also known as Radix Astragali in Latin and Huangqi in Chinese, belongs to Fabaceae family (Zhang, Xie, Li, & Fu, 2009) and is commonly used in Chinese herbal medicine to treat liver diseases (Sun, Wei, Wu, Gui, & Wang, 2007). Its roots are amongst the most popular health‐promoting herbs in China and their use dates to more than 2,000 years. There is an evidence of the traditional use of A. membranaceus in Shen Nong's Materia Medica written in the Han dynasty (206 bc–220 ad). Scientific research in the last 20 years has exposed many of the pharmacological functions of different components of this plant. Pharmacological studies of 46 species of the genus Astragalus have reported more than 200 medicinal compounds, including saponins and flavonoids. Studies have reported that crude extracts of Astragalus and isolated constituents are potentially active as an immunostimulant, antiinflammatory, antioxidative, anticancer, antidiabetic, cardioprotective, hepatoprotective, and antiviral activities (Li et al., 2014). In traditional Chinese medicine, Huangqi is often combined with other herbs, such as angelica and ginseng, in various complex prescription formulas. Such herbal formulas have been used for centuries in Asia to treat hepatic inflammation, diabetes, cancers, strokes, kidney infections, and many other diseases (Cho & Leung, 2007).
Sun et al. (2007) induced liver fibrosis in male Sprague–Dawley rats by administering 50% CCl4 injections subcutaneously and studied the effect of root extract of Paeonia lactiflora and A. membranaceus and demonstrated excellent hepatoprotective activity. They observed approximately 30–60% decrease in CCl4‐induced elevation of hyaluronic acid, procollagen type‐III levels, laminin, hydroxyproline contents, and serum transaminase activities in liver tissue. It also restored the decrease in SOD, and glutathione peroxidase activities decreased the elevation of transforming growth factor‐beta‐1 and inhibited the formation of lipid peroxidative products during CCl4 treatment. This inhibited progression of hepatic fibrosis that might be associated with its ability to decrease the level of transforming growth factor beta 1, scavenge free radicals, and inhibit collagen synthesis and proliferation in hepatic stellate cells.
3.8. C. spinosa L.
C. spinosa L. (caper), a member of Capparaceae family (Lam & Ng, 2009) is a plant from the dry regions in the west and central Asia, used as a flavoring in cooking (Panico et al., 2005). C. spinosa is employed in traditional medicine since ancient times for the treatment of gout, rheumatism, and liver diseases (Ahmed, Alam, Varshney, & Khan, 2002). The first recorded use of C. spinosa dates back to Sumerians for medicinal purposes in 2000 bc (Romeo, Ziino, Giuffrida, Condurso, & Verzera, 2007). Research on different activities of the plant indicates its enormous antioxidant, anticancer, and antibacterial potential. Studies have shown the bioactivity of extracts from the plant that can be attributed to the polyphenolic compounds present in the plant (Nabavi et al., 2016). It is one of the main constituents of the formulation Liv‐52; an herbal treatment commonly used in Indian traditional herbal medicine for the treatment of various liver diseases (Huseini, Alavian, Heshmat, Heydari, & Abolmaali, 2005).
Gadgoli and Mishra (1999) found that p‐Methoxy benzoic acid, prepared from the aqueous extract of C. spinosa, possesses significant activity against paracetamol and CCl4‐induced hepatotoxicity in vivo and galactosamine and thioacetamide‐induced hepatotoxicity in isolated rat hepatocytes. They observed the maximum protective effect in concentrations of 1 and 100 mg/ml (milligram of p‐methoxy benzoic acid/milliliter) against galactosamine and thioacetamide‐induced hepatotoxicity, respectively. In another study on 36 cirrhotic patients treated with Liv‐52 (combination of C. spinosa with other plants) demonstrated decreased serum ALT and AST as well as decreased ascites. This protective effect of Liv‐52 can be attributed to the antiinflammatory, diuretic, antioxidative, and immunomodulating effects of the component herbs (Huseini et al., 2005).
3.9. C. intybus L.
C. intybus L., commonly known as chicory, belongs to the Lactuceae family and is typical Mediterranean plant indigenous to Western Asia, Europe, North America, and Egypt, which varies in perianth color from white, red to blue (Norbaek, Nielsen, & Kondo, 2002). C. intybus is known for being a famous coffee substitute. C. intybus has a long history of medicinal use and is especially its tonic effects upon the digestive tract and liver (Street, Sidana, & Prinsloo, 2013).
Increased levels of serum enzymes (AST and ALT) and bilirubin caused by CCl4‐induced hepatic damage in rats were very much reduced whereas the decreased levels of albumin and proteins were found to increase in rats treated with C. intybus root callus and natural root extracts. However, ingredients from cultured C. intybus cells are more effective antihepatotoxic as compared with that of natural root extract against CCl4‐induced hepatic damage (Elgengaihi, Mossa, Refaie, & Aboubaker, 2016).
3.10. S. nigrum L.
S. nigrum L., commonly known as “Black Nightshade”, is a species in the family Solanaceae (Jimoh, Adedapo, & Afolayan, 2010). It is a common herb that grows abundantly in wild and open fields in temperate climate zones (Li, Li, Feng, & Li, 2008) and has been used as a potent hepatoprotective agent (Liu et al., 2016). The fruit of S. nigrum is used in the Mexican folk medicine as a nerve tonic (Perez, Perez, Garcia, & Sossa, 1998; Verma, Rai, Asthana, Jaiswal, & Jaiswal, 2010), and also believed to have antitumor properties (Son et al., 2003).
Increased free radical scavenging activity and inhibition of lipid peroxidation have been suggested as a possible mechanism of action for the hepatoprotective effects and other medicinal activities of this plant (Prashanth Kumar, Shashidhara, Kumar, & Sridhara, 2001; Sultana, Perwaiz, Iqbal, & Athar, 1995). Lin et al. (2008) observed that the treatment of aqueous extract of S. nigrum effectively lowered the CCl4‐induced serum levels of hepatic enzymes, alkaline phosphatase (ALP), serum glutamic‐pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), and total bilirubin, hydroxyl, and superoxide radicals in rats. The hepatic content of glutathione (GSH) and expressions of the antioxidative enzyme; SOD and decreased levels of detoxification enzymes; and glutathione S‐transferase were brought back to control levels by the administration of aqueous extract of S. nigrum. Liver histopathology showed that the extract reduced the incidence of liver lesions including lymphocytes infiltration, hepatic cells cloudy swelling, fibrous connective tissue proliferation, and CCl4‐induced hepatic necrosis. This hepatoprotective effect might be due to its adjustment of antioxidant activity, detoxification enzymes, and its free radical scavenger effects.
In another study, Hsieh, Fang, and Lina (2008) induced liver fibrosis by administering thioacetamide in mice and treated them with distilled water and S. nigrum extract via oral administration for 12 weeks. This treatment alleviated the hepatic hydroxyproline and α‐smooth muscle actin protein levels in mice and inhibited thioacetamide‐induced collagen and transforming growth factor‐β1 mRNA levels in the liver. Histological examination of liver also confirmed that this extract reduced the degree of fibrosis caused by thioacetamide treatment which is the probable reason for the reduction of hepatic fibrosis.
3.11. S. mukorossi Gaertn.
S. mukorossi Gaertn., commonly known as Ritha or Aritha, is abundantly found in India. Its fruit is reported to have expectorant, purgative, antidotal, and abortifacient effects. Additionally, it is used in epilepsy, extreme salivation, and chlorosis (Suhagia, Rathod, & Sindhu, 2011). The saponins extracted from this plant are spermicidal (in vitro) and due to this property, it has been used in contraceptive cream (Rastogi & MB, 1999).
Pharmacological studies of S. mukorossi have shown their potential effect as hepatoprotective agents (Upadhyay & Singh, 2012). To assess the hepatoprotective activity of the S. mukorossi, Wistar male rats were treated with CCl4. Administration of CCl4 to normal rats increased the serum levels of ALT, AST, ALP, and bilirubin. These enzymes eventually cause damage to the hepatic cells. The CCl4‐treated liver cells cultured on Petri plates were treated with the extracts of S. mukorossi and were reported to alleviate the levels of these enzymes. When histopathological studies of the CCl4‐treated rats were performed, they showed that it also causes the demolition of architectural configuration of target cells. However, rats that were treated with S. mukorossi presented normal lobular structural design, which shows its reparative properties and thus its hepatoprotective effects.
3.12. G. biloba L.
G. biloba L. belongs to family Ginkgoaceae (Theplantlist, 2013). It is one of the significant herbs of the Chinese traditional medicine. G. biloba leaf extract has been reported to have therapeutic activities against age‐related memory deficit problems, including Alzheimer's and dementia; cardioprotective, antiasthmatic, antidiabetic, hepatoprotective, photoprotective effects, DNA repair mechanism, antioxidant, and antiinflammatory activities (Mohanta, Tamboli, & Zubaidha, 2014).
G. biloba has been associated with a strong hepatoprotective activity through numerous studies (Parimoo et al., 2014). G. biloba amplifies cellular antioxidant protection system consisting of glutathione peroxidase, glutathione S‐transferase, glutathione reductase, nonprotein thiols, catalase, and antioxidant enzymes (SOD). The binding of an individual part of herbal tracks to that of phosphatidylcholine produces phytosome having better efficacy compared with traditional herbal extracts (Naik, Pilgaonkar, & Panda, 2006).
Rifampicin is an antibiotic widely used in tuberculosis chemotherapy. It has been reported to cause hepatoxicity, the reason of which is unknown as it is always given in combined form with other antibiotics such as isoniazid and ethambutol. Wistar albino rats were treated with rifampicin that caused hepatoxicity in them. Their blood samples were taken, and assays of their blood samples were performed to know the levels of SGPT, SGOT, and ALP. The elevated levels of SGOT, SGPT, and ALP show liver damage as these enzymes escape from the liver into the blood in case of liver damage. With parallel treatment through Ginkoselect Phytosome® and the standard drug Silymarin, the markers enzymes levels in serum were nearly at a normal level or marginally elevated. This suggests the hepatoprotective quality of G. biloba plant. It also elevates total protein levels and albumin, which shows its hepatoprotective effects. Synthesis of proteins speeds up the regeneration procedure of hepatic cells.
3.13. W. fruticosa (L.) Kurz
W. fruticosa (L.) Kurz, locally called Dhataki, is a member of family Lythraceae. It is a branched shrub of 1–3 m height and is found in the whole of North India. Flowers of Dhataki have stimulant properties and are dried and sprinkled over wounds and sores to inhibit discharge and promote granulation. The flowers also possess immunomodulatory and antitumor activities and are helpful in diarrhea, burning sensations, and headache. The plant through different studies has been associated with activities against chronic hepatic fibrosis (Nitha, Prabha, Ansil, & Latha, 2014). It also shows antiinflammatory, antibiotic, antileprosy, and antihelminthic properties (Arya et al., 2015; Shoaib et al., 2016; Syed & Khan, 2016).
In an experiment designed to check the hepatoprotective effect of W. fruticosa flower extract (WF4), albino Wistar rats were administered with CCl4 which resulted in the increased level of ALP, AST, ALT, and lactate dehydrogenase. These enzymes leak from serum into the blood. Thus, CCl4 damage causes loss of enzymes which are responsible for drug metabolism (Chandan et al., 2008). These rats were administered with WF4. The extract reversed the elevated lipid peroxidation and regulated the liver glucose‐6‐phosphate and GSH levels. These results are in line with former information for other hepatoprotective agents (Wu et al., 2007). WF4 significantly reversed the CCl4‐induced increase in triglycerides, indicating protective and curative effects against fatty liver disease. The impairment of the liver cells is shown by the increase in the level of bromsulphthalein (Chandan et al., 2008). Studies show that administering a variety of doses of WF4 reverts the elevated levels of bromsulphthalein. WF4 increases the choleretic activity indicating that it affects the activity of the liver. Histopathological activities performed under the light microscope showed the curative effect of WF4 against the CCl4‐induced liver damage as it decreases the severity of toxic effect (Suja, Latha, Pushpangadan, & Rajasekharan, 2004).
3.14. V. trifolia L.
V. trifolia L., known generally as chaste tree, is a high‐value medicinal plant that belongs to the family Verbenaceae. Its leaves are effective as plaster against pains, infections, and fever. Its fruits are used in curing amenorrhea, and the flowers are effective against fever (Chan, Baba, Chan, Kainuma, & Tangah, 2016). The active constituents of this plant are essential oil (Kvasnicka, Biba, Sevcik, Voldrich, & Kratka, 2003), viterifolins, and diterpenes. It also possesses some important pharmacological qualities, that is, antipyretic (Rani & Sharma, 2013), antibacterial (Lawitz et al., 2014), antiallergic, and antiasthmatic properties (Lawitz & Gane, 2013). Medical practitioners use this plant in the treatment of acute jaundice. However, literature study suggested that this plant is not well screened for its hepatoprotective activity. Nonetheless, the tribal groups of Western Ghats use this plant leaf extracts in treating jaundice, and these results give some scientific evidence of hepatoprotective activity.
The effect of ethanol and aqueous leaf extracts of V. trifolia was evaluated on rats whose livers were damaged. The experiment showed that ethanol solution extracts have proved protective against CCl4‐induced toxicity, and the reason behind this might be the effect of the hepatoprotective and antioxidant activity of its phytoconstituents. It also lowers the level of the raised serum enzymes and increases the total protein content up to the normal rate (Anandan et al., 2009; Manjunatha & Vidya, 2008). The results of screening showed that leaf extracts of V. trifolia have important hepatoprotective activity. Investigations revealed that leaf extracts also contain saponins, tannins, triterpenoids, steroids, glycosides, and flavonoids. The antioxidant and hepatoprotective potentials of flavonoids (Shehab, Abu‐Gharbieh, & Bayoumi, 2015) and triterpenoids are well identified.
3.15. L. coreana H. Lev.
L. coreana belongs to the family Lauraceae and is mainly found in the tropical and subtropical areas. About 74 plant species of the genus Litsea are found in China and have been used in traditional Chinese medicine for various diseases. L. coreana is used in traditional Chinese medicines against alcoholic hepatic injury. Different studies have reviewed the enormous pharmacological potential of the plant (Jia, Li, Wan, & He, 2017; Wang, Yuan et al., 2016).
The important flavonoids from L. coreana include kaempferol, kaempferol‐3‐O‐β‐D‐galactoside, kaempferol‐3‐O‐β‐D‐glucoside, quercetin‐3‐O‐β‐D‐galactoside, quercetin‐3‐O‐β‐D‐glucoside, (2R,3S)‐catechin, and (2R,3R)‐epicatechin (Chen et al., 2014). In rats fed with a high‐fat diet, flavonoids were assessed for their inhibition on steatohepatitis (also known as fatty liver disease; Jia et al., 2017). Oral administration, in a dose‐dependent manner, repressed the levels of total cholesterol, free fatty acid, triglyceride, and low‐density lipoprotein‐cholesterol in the liver and/or serum. Four hundred milligrams per kilogram total flavonoid content from L. coreana almost stopped the formation of steatosis. At the same time, peroxisome proliferator‐activated receptor α, a protein linked with amplified SOD, and reduced malondialdehyde levels were upregulated in rat liver treated with the flavonoids from L. coreana. Flavonoid treatment for 200 and 400 mg/kg alleviated the levels of ALP, ALT, AST, γ‐, triglyceride, γ‐glutamyltransferase, and total cholesterol in liver and serum. On the other hand, SOD and glutathione peroxidase were increased in fat suspension steatohepatitis model of rat. Thus, L. coreana is a potent hepatoprotective herb with flavonoids as their bioactive components (Petruzziello, Marigliano, Loquercio, & Cacciapuoti, 2016).
3.16. S. chinensis (Turcz.) Baill
S. chinensis (Turcz.) Baill is widely used in traditional and modern Chinese medicine for the treatment of many disorders including insomnia, respiratory failure, and weakness. Moreover, mental health improving ability along with fatigue reduction property is also validated for S. chinensis in Russian medicine (Szopa, Ekiert, & Ekiert, 2017).
In general, dibenzocyclooctadiene lignans found in S. chinensis are known to exhibit potent hepatoprotective activity (Zheng et al., 2017). In one of the study of individual lignin, Gomisin A was found responsible for the acceleration of hepatocytes proliferation and increase hepatic flow (Panossian & Wikman, 2008). Furthermore, elevation of mitochondrial glutathione concentration was found to be linked with γ‐schisandrin hepatoprotective mechanism. The increase in vitamin C concentration in the liver of test animals upon treatment with γ‐schisandrin also validates its hepatoprotective ability. Another individual lignin, Schisandrin B was also found to counter oxidative harm to liver tissues (Thandavarayan et al., 2015; Xin et al., 2017). In one scientific study, the hepatoprotective mechanism against acetaminophen‐induced liver injury of six Schisandra lignans (deoxyschisandrin, Schisantherin A, Schisandrin B, Gomisin A, Schisandrin C, and schisandrin) was elucidated. The hepatoprotective ability of these lignins was found to be associated with inhibition of cytochrome‐mediated bioactivation (Jiang et al., 2015). Furthermore, another mechanistic study investigated the hepatoprotective effect of Schisandra polysaccharide in nonalcoholic fatty liver disease mice models. The results demonstrate potential down regulation of hepatic lipogenesis genes and LXRα/SREBP‐1c/FAS/ACC and SREBP‐2/HMGCR signaling pathways in the liver (Wang, Song et al., 2016).
3.17. C. chinensis Lam.
C. chinensis Lam. also known as Chinese dodder is a parasitic plant having diverse traditional medicinal uses as a tonic, sex enhancer, and abortion preventer (Zheng, Dong, & She, 1998). Studies also have scientifically validated the hepatoprotective activity of C. chinensis (Donnapee et al., 2014). Yen, Wu, Lin, and Lin (2007) evaluated the hepatoprotective effect of C. chinensis ethanol solution extract in rats with the acetaminophen‐induced toxicity of liver. The elevated concentration of glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, and alkaline phosphatase was reduced significantly via treatment of rats with 125 and 250 mg/kg of C. chinensis ethanol extract orally. Furthermore, it was found that the ethanol solution extract prevented centribular hepatic necrosis and acetaminophen‐induced toxicity of liver. The ethanol solution extract was further found to also elevate the level of potential antioxidant enzymes like glutathione peroxidase, superoxide dismutase, and catalase. In another study by the same group, nanoparticles formulation of C. chinensis seeds ethanol solution extract was found to be more effective in rats with acetaminophen‐induced hepatotoxicity (Yen, Wu, Lin, Cham, & Lin, 2008). The mechanism of hepatoprotective potential as demonstrated by ethanol solution extract of C. chinensis is proposed to be the elevated activities of antioxidant enzymes.
3.18. L. barbarum L.
L. barbarum L. berries are very famous in traditional Chinese medicine for the treatment of inflammation, cancer, eye disorders, throat infection, and anemia. The use of these berries has been validated as food and also has gained great importance due to its significant antioxidant potential (Cheng et al., 2015).
The major active components of L. barbarum berries are L. barbarum polysaccharides (LBPs) which are reported widely to have diverse pharmacological properties. In a study, the hepatoprotective effect of LBPs in rats having alcohol‐induced liver injury has been validated. Liver injury model was made via treatment of ethanol, which exhibited elevated levels of liver enzymes and fatty liver. Upon treatment of L. barbarum polysaccharides for 30 days in a dosage of 300 mg/kg, the liver injury model revealed prevention of fatty liver and minimized liver injury (Cheng & Kong, 2011). Furthermore, Xiao et al. (2014) mechanistically proved that hepatic thioredoxin‐interacting protein inhibition via LBPs leads to a reduction in oxidative stress, inflammation, and cellular apoptosis.
3.19. A. sinensis (Oliv.) Diels
A. sinensis (Oliv.) Diels is reported in Chinese herbal medicine for the treatment of cardiovascular disease, anemia, and hepatic disorders (Bunel, Antoine, Nortier, Duez, & Stévigny, 2015). The A. sinensis polysaccharides (ASP) extracted from A. sinensis roots having the average molecular weight of 72,900 Da is regarded as a potential active component of A. sinensis that exhibits a wide range of pharmacognostic properties (Hsu, Tsai, & Tsai, 2014).
The hepatoprotective potential of ASP in CCl4‐induced liver injury and via using ischemia/reperfusion rat is widely established (Zhang et al., 2010). Wang, Wen, Li, Zhang, & Yang (2016) have investigated the hepatoprotective mechanism of ASP against Concanavalin A‐induced failure of the liver. It was found that 5 to 125 μg/mL of ASP has inhibited the Concanavalin A‐induced responses. Major reduction in the levels of serum transaminase was seen, whereas Hematoxylin and Eosin staining reported liver inflammation attenuation. The study concluded that pretreatment of ASP elicits antiinflammatory and antioxidant actions, which attenuates Concanavalin A‐induced liver injury. In another study, Zhao et al., investigated the role of A. sinensis derived Levistilide A against CCl4‐induced liver fibrosis. The results validated the potential role of Levistilide A in liver fibrosis inhibition via antiangiogenesis (Zhao et al., 2017).
3.20. Featured herbal products and their hepatoprotective potential
The number of hepatoprotective products from plants is ever increasing. In addition to the hepatoprotective role of a general class of phenolics and flavonoids, many studies have defined specific compounds for their preferable role in hepatitis and other liver disorders (Shehab et al., 2015). Some of the important plants and their products are highlighted in Figure 3. Among the many different compounds, few are distinguished for their promising role in liver inflammation. We have, therefore, selected six important compounds for their hepatoprotective role (Table 2).
Quercetin, for instance, a major flavonol commonly found most of the plants, is a potent hepatoprotective agent. The first basis of quercetin‐based potency has been attributed to the antioxidant activity of this compound. One of the specific mechanisms of Quercetin supplementation was established through ethanol‐induced cytotoxicity which affects the activity of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase. For instance, Quercetin supplementation restored the glutathione reductase activity which was affected by ethanol that in turn reduced the glutathione content of liver. Quercetin supplementation has also been attributed to hepatoprotection against metals, pesticides, drugs, toxins, and viruses (Miltonprabu et al., 2016).
Rhein (4, 5‐dihydroxyanthraquinone‐2‐carboxylic acid) is another important hepatoprotective compound extensively found in medicinal herbs, such as Rheum palmatum L., Cassia tora L., Polygonum multiflorum Thunb., and Aloe barbadensis Miller. The mechanism of action through which Rhein acts has been described as modulation of CYP enzymes in rat liver, attenuation of total cholesterol and triglyceride levels in serum, and amelioration of glucose and lipid metabolism. Similarly, Rhein downregulated the levels of serum ALT, hyaluronic acid, procollagen type III, and liver malondialdehyde and inhibited the expression of transforming growth factor beta 1 and alpha‐smooth muscle actin in tetrachloride/ethanol‐induced liver fibrosis rats (Zhou et al., 2015).
Similarly, flavonoids, lignans, terpenoids, and steroids from Vitex negundo L. have also been shown to demonstrate hepatoprotective activities (Zheng et al., 2015). Extracts containing these compounds have been shown to improve biochemical and functional parameters and thus alleviate CCl4‐induced damage in liver rats. Negundoside, for instance, which is a glycoside, has been demonstrated to reduce calcium‐mediated toxicity and CCl4‐induced oxidative stress through regulation of calcium homeostasis and decreasing the production of ROS and lipid peroxidation (Zheng et al., 2015).
Isoliquiritigenin, isolated from the roots of plants belonging to licorice, including Glycyrrhiza uralensis, Mongolian glycyrrhiza, and Glycyrrhiza glabra was shown to significantly increase liver toxicity biomarkers, serum glutamic‐pyruvic transaminase, serum glutamicoxaloacetic transaminase, triglyceride, lipid peroxidation, nitric oxide, and lactate dehydrogenase (Peng et al., 2015).
Another important compound, silymarin from S. marianum, has been regarded as one of the most important herbal product against hepatitis, especially Hepatitis C. Silymarin has proved very effective in improving the disease state. A study by Del Prete et al. (2012) suggested that a 6‐month course of 650 mg silymarin per day in patients with chronic Hepatitis C considerably decreased serum Hepatitis C virus RNA titer and improved level of serum aminotransferases. Similarly, Glycyrrhizin, structurally a saponin from G. glabra and other species of the genus, is a recognized hepatoprotective agent. Glycyrrhizin is shown to have an important role in the reduction of ROS formation, depletion of intracellular GSH, and inhibition of depolarization of the mitochondrial membrane (Hosseinzadeh & Nassiri‐Asl, 2015). The other important secondary metabolites from medicinal plants are detailed in Table 2 with their structures given in Figure 4.
3.21. Toxicity and quality control issues
The present literature is not sufficient to assess the safety of most of the hepatoprotective and liver regenerative herbs and herbal products, as most of the studies focus on their antihepatotoxic effects only. However, some previous experiments on rats show that no adverse effects were observed by administering intraperitoneal injection of the A. membranaceus extracts at 0.5 g/kg for 30 days, whereas large doses (1 g/kg) of A. membranaceus root extracts resulted in mutagenicity in mice when injected directly into the stomach lining (Zhang et al., 2009). In another study, Sato et al. (1996) observed no significant toxicity of various concentrations of glycyrrhizin on PLC/PRF/5 cells in vitro. Gadgoli and Mishra (1999) reported that p‐Methoxy benzoic acid extracted from C. spinosa was nontoxic at 1 mg/ml when applied to rat hepatocytes in vitro. This supports the claims made in the traditional system of medicine. Similarly, silymarin is shown to have a lack of toxicity and side effects even at high doses (Upadhyay et al., 2007). Isbrucker and Burdock (2006) reported that No‐Observed‐Effect Levels for purified glycyrrhizin are in the range of 15–229 mg/kg/day and concluded that current levels of consumption of licorice extract products and glycyrrhizinate are safe.
Although, several herbals show potential activity for the treatment of acute and chronic liver diseases premarketing drug‐testing, and pharmacovigilance is needed as with any other drug. So far, herbals to treat chronic liver diseases should not be recommended outside clinical trials as the evidence supporting its use is insufficient (Stickel, Patsenker, & Schuppan, 2005), and publications relevant to the cytotoxicity of medicinal plants should be encouraged (Mukhtar et al., 2008). Moreover, there are issues like approval of the plant products/extracts as a drug from regulatory agencies such as the Food and Drug Administration or any other equivalent agencies.
4. CONCLUSIONS
Extensive literature survey of hepatoprotective plants clearly indicates that herbal drugs have an enormous potential for the treatment of liver diseases. In this article, we reviewed the scientific merit of selected plants studied for their hepatoprotective mechanism of action. The major hepatoprotective mechanism identified by the majority of the studies is through combating the oxidative stress that damages the liver. We have summarized the effect of extracts and compounds from different herbs on liver injury considering changes in their biochemical parameters. We also presented the possible data available in the literature for different plants regarding their phytochemical constituents. We, therefore, conclude that herbs and herbal preparations are among the most important sources of hepatoprotective and liver regeneration medicines. However, further research is needed to identify, characterize, and standardize the active ingredients, useful compounds, and their preparations for the treatment of liver diseases. Moreover, a combination of the traditional herbal medicines with the modern and conventional medicine may be one of the best options for the treatment of liver disorders and other diseases and infections, soon.
4.1. Prospects and directions
The importance of medicinal plants can be determined from World Health Organization's estimates, which states that up to 80% of the world's population fulfill their healthcare needs from medicinal plants (Mukhtar et al., 2008). There has been a significant rise in using over‐the‐counter medicinal plant products containing powerful medicinal drugs and are believed to have to produce progressive effects with reduced side effects. However, therapeutic failures or adverse effects have been observed in many cases as pharmacological mechanisms of the herbal mixtures/preparations are not well‐studied. The most important concern involving the use of medicinal plants is to identify and standardize the exact method of preparation of an extract, identification of active ingredients and details of administration (Yip & Kwan, 2006). In this relationship, the screening and characterization of other undiscovered herbal products in traditional medicine is needed. The integration of the therapeutic use of traditional Chinese medicinal knowledge with the synthetic and traditional oriental medicinal knowledge is a key area of research (Cho & Leung, 2007).
However, medicinal plants cultivated in different geographical regions are believed to differ in therapeutic effects in different diseases and infections. For example, A. membranaceus used in Chinese traditional medicine, from certain locality contains more favorable trace elements and fewer harmful trace elements than those from other localities. In this context, the use of new therapeutic strategies based on natural plants may be useful to provide minimal toxicity, higher effectiveness, and a wider therapeutic background for effective manipulation than existing pharmaceutical products (Panico et al., 2005).
COMPLIANCE WITH ETHICAL STANDARDS
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST/COMPETING INTERESTS
Authors declare that they have no competing interests.
RESEARCH INVOLVING HUMAN PARTICIPANTS AND/OR ANIMALS
Not applicable.
ETHICAL APPROVAL
The current article is a review article and does not contain any studies with human participants performed by any of the authors.
AUTHORS' CONTRIBUTIONS
M. A. and M. I. conceived the idea. M. A. and T. K. drafted the manuscript. T. K. and M. O. constructed the figures. K. F., A. T. K., M. A., and Q. A. A. prepared the tables and reviewed information obtained from electronic search. I. U. and Z. K. S. contributed to botanical and taxonomic part of the manuscript. T. K., M. I., M. A., Z. K. S., and A. R. critically revised the manuscript.
Ali M, Khan T, Fatima K, et al. Selected hepatoprotective herbal medicines: Evidence from ethnomedicinal applications, animal models, and possible mechanism of actions. Phytotherapy Research. 2018;32:199–215. 10.1002/ptr.5957
Contributor Information
Muhammad Ali, Email: muhammad.ali@qau.edu.pk.
Tariq Khan, Email: tariqkhan@uom.edu.pk.
REFERENCES
- Abdulkarim, A. S. , Zein, N. N. , Germer, J. J. , Kolbert, C. P. , Kabbani, L. , Krajnik, K. L. , … Persing, D. H. (1998). Hepatitis C virus genotypes and hepatitis G virus in hemodialysis patients from Syria: Identification of two novel hepatitis C virus subtypes. The American Journal of Tropical Medicine and Hygiene, 59(4), 571–576. [DOI] [PubMed] [Google Scholar]
- Ahmed, B. , Alam, T. , Varshney, M. , & Khan, S. A. (2002). Hepatoprotective activity of two plants belonging to the Apiaceae and the Euphorbiaceae family. Journal of Ethnopharmacology, 79(3), 313–316. [DOI] [PubMed] [Google Scholar]
- Al‐Asmari, A. K. , Al‐Elaiwi, A. M. , Athar, M. T. , Tariq, M. , Al Eid, A. , & Al‐Asmary, S. M. (2014). A review of hepatoprotective plants used in Saudi traditional medicine. Evidence‐based complementary and alternative medicine: eCAM, 2014, 890842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, M. , Afzal, S. , Zia, A. , Hassan, A. , Khalil, A. T. , Ovais, M. , … Idrees, M. (2016). A systematic review of treatment response rates in Pakistani hepatitis C virus patients; current prospects and future challenges. Medicine, 95(50), e5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat, N. , Upur, H. , & Blažeković, B. (2010). In vivo hepatoprotective activity of the aqueous extract of Artemisia absinthium L. against chemically and immunologically induced liver injuries in mice. Journal of Ethnopharmacology, 131(2), 478–484. [DOI] [PubMed] [Google Scholar]
- Anandan, R. , Jayakar, B. , Karar, B. , Babuji, S. , Manavalan, R. , & Kumar, R. S. (2009). Effect of ethanol extract of flowers of Vitex trifolia Linn. On CCL4 induced hepatic injury in rats. Pakistan Journal of Pharmaceutical Sciences, 22(4), 391–394. [PubMed] [Google Scholar]
- Arya, A. , Al‐Obaidi, M. M. J. , Karim, R. B. , Taha, H. , Khan, A. K. , Shahid, N. , … Ali, H. M. (2015). Extract of Woodfordia fruticosa flowers ameliorates hyperglycemia, oxidative stress and improves beta‐cell function in streptozotocin‐nicotinamide induced diabetic rats. Journal of Ethnopharmacology, 175, 229–240. [DOI] [PubMed] [Google Scholar]
- Babu, P. R. , Bhuvaneswar, C. , Sandeep, G. , Ramaiah, C. V. , & Rajendra, W. (2017). Hepatoprotective role of Ricinus communis leaf extract against d‐galactosamine induced acute hepatitis in albino rats. Biomedicine & Pharmacotherapy, 88, 658–666. [DOI] [PubMed] [Google Scholar]
- Bahmani, M. , Rafieian‐Kopaei, M. , Jeloudari, M. , Eftekhari, Z. , Delfan, B. , Zargaran, A. , & Forouzan, S. (2014). A review of the health effects and uses of drugs of plant licorice (Glycyrrhiza glabra L.) in Iran. Asian Pacific Journal of Tropical Disease, 4, S847–S849. [Google Scholar]
- Bunel, V. , Antoine, M.‐H. , Nortier, J. , Duez, P. , & Stévigny, C. (2015). Nephroprotective effects of ferulic acid, Z‐ligustilide and E‐ligustilide isolated from Angelica sinensis against cisplatin toxicity in vitro. Toxicology In Vitro, 29(3), 458–467. [DOI] [PubMed] [Google Scholar]
- Cha, T. A. , Kolberg, J. , Irvine, B. , Stempien, M. , Beall, E. , Yano, M. , … Han, J. H. (1991). Use of a signature nucleotide sequence of hepatitis C virus for detection of viral RNA in human serum and plasma. Journal of Clinical Microbiology, 29(11), 2528–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain, R. W. , Adams, N. , Saeed, A. A. , Simmonds, P. , & Elliott, R. M. (1997). Complete nucleotide sequence of a type 4 hepatitis C virus variant, the predominant genotype in the Middle East. The Journal of General Virology, 78(Pt 6), 1341–1347. [DOI] [PubMed] [Google Scholar]
- Chan, E. W. C. , Baba, S. , Chan, H. T. , Kainuma, M. , Tangah, J. (2016). Medicinal plants of sandy shores: A short review on Vitex trifolia L. and Ipomoea pes‐caprae (L.) R. Br.
- Chandan, B. K. , Saxena, A. K. , Shukla, S. , Sharma, N. , Gupta, D. K. , Singh, K. , … Qazi, G. N. (2008). Hepatoprotective activity of Woodfordia fruticosa Kurz flowers against carbon tetrachloride induced hepatotoxicity. Journal of Ethnopharmacology, 119(2), 218–224. [DOI] [PubMed] [Google Scholar]
- Chen, S.‐L. , Yu, H. , Luo, H.‐M. , Wu, Q. , Li, C.‐F. , & Steinmetz, A. (2016). Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chinese Medicine, 11(1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Ma, T. , Huang, C. , Zhang, L. , Zhong, J. , Han, J. , … Li, J. (2014). Efficiency of transcellular transport and efflux of flavonoids with different glycosidic units from flavonoids of Litsea coreana L. in a MDCK epithelial cell monolayer model. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences, 53, 69–76. [DOI] [PubMed] [Google Scholar]
- Chen, Z. W. , Li, H. , Ren, H. , & Hu, P. (2016). Global prevalence of pre‐existing HCV variants resistant to direct‐acting antiviral agents (DAAs): Mining the GenBank HCV genome data. Scientific Reports, 6, 20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, D. , & Kong, H. (2011). The effect of Lycium Barbarum polysaccharide on alcohol‐induced oxidative stress in rats. Molecules (Basel, Switzerland), 16(3), 2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, J. , Zhou, Z. W. , Sheng, H. P. , He, L. J. , Fan, X. W. , He, Z. X. , … Cao, C. (2015). An evidence‐based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Design, Development and Therapy, 9, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirdchupunseree, H. , & Pramyothin, P. (2010). Protective activity of phyllanthin in ethanol‐treated primary culture of rat hepatocytes. Journal of Ethnopharmacology, 128(1), 172–176. [DOI] [PubMed] [Google Scholar]
- Cho, W. C. , & Leung, K. N. (2007). In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. Journal of Ethnopharmacology, 113(1), 132–141. [DOI] [PubMed] [Google Scholar]
- Del Prete, A. , Scalera, A. , Iadevaia, M. D. , Miranda, A. , Zulli, C. , Gaeta, L. , … Loguercio, C. (2012). Herbal products: Benefits, limits, and applications in chronic liver disease. Evidence‐based Complementary and Alternative Medicine, 2012, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman, R. K. , & Chawla, Y. K. (2005). Herbal medicines for liver diseases. Digestive Diseases and Sciences, 50(10), 1807–1812. [DOI] [PubMed] [Google Scholar]
- Donnapee, S. , Li, J. , Yang, X. , Ge, A. H. , Donkor, P. O. , Gao, X. M. , & Chang, Y. X. (2014). Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. Journal of Ethnopharmacology, 157, 292–308. [DOI] [PubMed] [Google Scholar]
- Dusheiko, G. M. (1996). Summary: Antiviral treatment of hepatitis C virus. Antiviral Research, 29(1), 77–82. [DOI] [PubMed] [Google Scholar]
- Ekor, M. (2013). The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology, 4, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgengaihi, S. , Mossa, A.‐T. H. , Refaie, A. A. , & Aboubaker, D. (2016). Hepatoprotective efficacy of Cichorium intybus L. extract against carbon tetrachloride‐induced liver damage in rats. Journal of Dietary Supplements, 13(5), 570–584. [DOI] [PubMed] [Google Scholar]
- Farghali, H. , Kamenikova, L. , Hynie, S. , & Kmonickova, E. (2000). Silymarin effects on intracellular calcuim and cytotoxicity: A study in perfused rat hepatocytes after oxidative stress injury. Pharmacological Research, 41(2), 231–237. [DOI] [PubMed] [Google Scholar]
- Ferenci, P. , Scherzer, T. M. , Kerschner, H. , Rutter, K. , Beinhardt, S. , Hofer, H. , … Steindl–Munda, P. (2008). Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology, 135(5), 1561–1567. [DOI] [PubMed] [Google Scholar]
- Freitag, A. F. , Cardia, G. F. E. , da Rocha, B. A. , Aguiar, R. P. , Silva‐Comar, F. M. D. S. , Spironello, R. A. , … Cuman, R. K. N. (2015). Hepatoprotective effect of Silymarin (Silybum marianum) on hepatotoxicity induced by acetaminophen in spontaneously hypertensive rats. Evidence‐based complementary and alternative medicine : eCAM, 2015, 538317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgoli, C. , & Mishra, S. H. (1999). Antihepatotoxic activity of p‐methoxy benzoic acid from Capparis Spinosa. Journal of Ethnopharmacology, 66(2), 187–192. [DOI] [PubMed] [Google Scholar]
- Gnanadesigan, M. , Ravikumar, S. , & Anand, M. (2017). Hepatoprotective activity of Ceriops decandra (Griff.) Ding Hou mangrove plant against CCl4 induced liver damage. Journal of Taibah University for Science, 11(3), 450–457. [Google Scholar]
- Gond, N. Y. , & Khadabadi, S. S. (2008). Hepatoprotective activity of Ficus carica leaf extract on rifampicin‐induced hepatic damage in rats. Indian Journal of Pharmaceutical Sciences, 70(3), 364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. Y. , Fan, J. Y. , Horie, Y. , Miura, S. , Cui, D. H. , Ishii, H. , … Kimura, I . (2008). Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacology & Therapeutics, 117(2), 280–295. [DOI] [PubMed] [Google Scholar]
- Hanh, N. D. , Sinchaipanid, N. , & Mitrevej, A. (2014). Physicochemical characterization of phyllanthin from Phyllanthus amarus Schum. et Thonn. Drug Development and Industrial Pharmacy, 40(6), 793–802. [DOI] [PubMed] [Google Scholar]
- Helmstädter, A. , & Staiger, C. (2014). Traditional use of medicinal agents: A valid source of evidence. Drug Discovery Today, 19(1), 4–7. [DOI] [PubMed] [Google Scholar]
- Hong, M. , Li, S. , Wang, N. , Tan, H.‐Y. , Cheung, F. , & Feng, Y. (2017). A biomedical investigation of the hepatoprotective effect of Radix salviae miltiorrhizae and network pharmacology‐based prediction of the active compounds and molecular targets. International Journal of Molecular Sciences, 18(3), 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh, H. , & Nassiri‐Asl, M. (2015). Pharmacological effects of Glycyrrhiza spp. and its bioactive constituents: Update and review. Phytotherapy Research, 29(12), 1868–1886. [DOI] [PubMed] [Google Scholar]
- Hsieh, C. C. , Fang, H. L. , & Lina, W. C. (2008). Inhibitory effect of Solanum nigrum on thioacetamide‐induced liver fibrosis in mice. Journal of Ethnopharmacology, 119(1), 117–121. [DOI] [PubMed] [Google Scholar]
- Hsu, C.‐M. , Tsai, F.‐J. , & Tsai, Y. (2014). Inhibitory effect of Angelica sinensis extract in the presence of 2‐hydroxypropyl‐β‐cyclodextrin. Carbohydrate Polymers, 114, 115–122. [DOI] [PubMed] [Google Scholar]
- Hunter, P. M. , & Hegele, R. A. (2017). Functional foods and dietary supplements for the management of dyslipidaemia. Nature reviews . Endocrinology, 13(5), 278–288. [DOI] [PubMed] [Google Scholar]
- Huseini, H. F. , Alavian, S. M. , Heshmat, R. , Heydari, M. R. , & Abolmaali, K. (2005). The efficacy of Liv‐52 on liver cirrhotic patients: A randomized, double‐blind, placebo‐controlled first approach. Phytomedicin: international journal of phytotherapy and phytopharmacology, 12(9), 619–624. [DOI] [PubMed] [Google Scholar]
- Ilyas, U. , Katare, D. P. , Aeri, V. , & Naseef, P. P. (2016). A review on Hepatoprotective and immunomodulatory herbal plants. Pharmacognosy Reviews, 10(19), 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbrucker, R. A. , & Burdock, G. A. (2006). Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regulatory toxicology and pharmacology : RTP, 46(3), 167–192. [DOI] [PubMed] [Google Scholar]
- Iwu, M. M. (2014). Handbook of African Medicinal Plants (Second ed.). Taylor & Francis. [Google Scholar]
- Izzo, A. A. , Hoon‐Kim, S. , Radhakrishnan, R. , & Williamson, E. M. (2016). A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytotherapy research : PTR, 30(5), 691–700. [DOI] [PubMed] [Google Scholar]
- Jia, X. , Li, P. , Wan, J. , & He, C. (2017). A review on phytochemical and pharmacological properties of Litsea coreana. Pharmaceutical Biology, 55(1), 1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. , Fan, X. , Wang, Y. , Tan, H. , Chen, P. , Zeng, H. , … Bi, H. (2015). Hepato‐protective effects of six schisandra lignans on acetaminophen‐induced liver injury are partially associated with the inhibition of CYP‐mediated bioactivation. Chemico‐Biological Interactions, 231, 83–89. [DOI] [PubMed] [Google Scholar]
- Jimoh, F. O. , Adedapo, A. A. , & Afolayan, A. J. (2010). Comparison of the nutritional value and biological activities of the acetone, methanol and water extracts of the leaves of Solanum nigrum and Leonotis leonorus. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 48(3), 964–971. [DOI] [PubMed] [Google Scholar]
- Johnston, D. E. , & Kroening, C. (1998). Mechanism of early carbon tetrachloride toxicity in cultured rat hepatocytes. Pharmacology & Toxicology, 83(6), 231–239. [DOI] [PubMed] [Google Scholar]
- Khatri, A. , Garg, A. , & Agrawal, S. S. (2009). Evaluation of hepatoprotective activity of aerial parts of Tephrosia purpurea L. and stem bark of Tecomella undulata. Journal of Ethnopharmacology, 122(1), 1–5. [DOI] [PubMed] [Google Scholar]
- Kimura, M. , Moro, T. , Motegi, H. , Maruyama, H. , Sekine, M. , Okamoto, H. , … Ogihara, M. (2008). In vivo glycyrrhizin accelerates liver regeneration and rapidly lowers serum transaminase activities in 70% partially hepatectomized rats. European Journal of Pharmacology, 579(1–3), 357–364. [DOI] [PubMed] [Google Scholar]
- Kumaran, A. , & Joel Karunakaran, R. (2007). In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT ‐ Food Science and Technology, 40(2), 344–352. [Google Scholar]
- Kvasnicka, F. , Biba, B. , Sevcik, R. , Voldrich, M. , & Kratka, J. (2003). Analysis of the active components of silymarin. Journal of Chromatography. A, 990(1–2), 239–245. [DOI] [PubMed] [Google Scholar]
- Lam, P. , Cheung, F. , Tan, H. Y. , Wang, N. , Yuen, M. F. , & Feng, Y. (2016). Hepatoprotective effects of Chinese medicinal herbs: A focus on anti‐inflammatory and anti‐oxidative activities. International Journal of Molecular Sciences, 17(4), 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, S. K. , & Ng, T. B. (2009). A protein with antiproliferative, antifungal and HIV‐1 reverse transcriptase inhibitory activities from caper (Capparis spinosa) seeds. Phytomedicine : international journal of phytotherapy and phytopharmacology, 16(5), 444–450. [DOI] [PubMed] [Google Scholar]
- Lawitz, E. , & Gane, E. J. (2013). Sofosbuvir for previously untreated chronic hepatitis C infection. The New England Journal of Medicine, 369(7), 678–679. [DOI] [PubMed] [Google Scholar]
- Lawitz, E. , Sulkowski, M. S. , Ghalib, R. , Rodriguez‐Torres, M. , Younossi, Z. M. , Corregidor, A. , … Lim, J. K. (2014). Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non‐responders to pegylated interferon and ribavirin and treatment‐naive patients: The COSMOS randomised study. Lancet (London, England), 384(9956), 1756–1765. [DOI] [PubMed] [Google Scholar]
- Li, J. , Li, Q. , Feng, T. , & Li, K. (2008). Aqueous extract of Solanum nigrum inhibit growth of cervical carcinoma (U14) via modulating immune response of tumor bearing mice and inducing apoptosis of tumor cells. Fitoterapia, 79(7–8), 548–556. [DOI] [PubMed] [Google Scholar]
- Li, T. S. C. (2016). Chinese & related north american herbs: Phytopharmacology & therapeutic values (Second ed.). CRC Press. [Google Scholar]
- Li, X. , Qu, L. , Dong, Y. , Han, L. , Liu, E. , Fang, S. , … Wang, T. (2014). A review of recent research progress on the astragalus genus. Molecules (Basel, Switzerland), 19(11), 18850–18880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew, M. , Erali, M. , Page, S. , Hillyard, D. , & Wittwer, C. (2004). Hepatitis C genotyping by denaturing high‐performance liquid chromatography. Journal of Clinical Microbiology, 42(1), 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H. M. , Tseng, H. C. , Wang, C. J. , Lin, J. J. , Lo, C. W. , & Chou, F. P. (2008). Hepatoprotective effects of Solanum nigrum Linn extract against CCl(4)‐induced oxidative damage in rats. Chemico‐Biological Interactions, 171(3), 283–293. [DOI] [PubMed] [Google Scholar]
- Ling, S. , Luo, R. , Dai, A. , Guo, Z. , Guo, R. , & Komesaroff, P. A. (2009). A pharmaceutical preparation of Salvia miltiorrhiza protects cardiac myocytes from tumor necrosis factor‐induced apoptosis and reduces angiotensin II‐stimulated collagen synthesis in fibroblasts. Phytomedicine: international journal of phytotherapy and phytopharmacology, 16(1), 56–64. [DOI] [PubMed] [Google Scholar]
- Liu, F. P. , Ma, X. , Li, M. M. , Li, Z. , Han, Q. , Li, R. , … Lin, Y. X. (2016). Hepatoprotective effects of Solanum nigrum against ethanol‐induced injury in primary hepatocytes and mice with analysis of glutathione S‐transferase A1. Journal of the Chinese Medical Association, 79(2), 65–71. [DOI] [PubMed] [Google Scholar]
- Lu, C. , Lu, Y. , Chen, J. , Zhang, W. , & Wu, W. (2007). Synchronized and sustained release of multiple components in silymarin from erodible glyceryl monostearate matrix system. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 66(2), 210–219. [DOI] [PubMed] [Google Scholar]
- Manjunatha, B. K. , & Vidya, S. M. (2008). Hepatoprotective activity of Vitex trifolia against carbon tetrachloride‐induced hepatic damage. Indian Journal of Pharmaceutical Sciences, 70(2), 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, H. , Zhang, H. , Wang, H. , Wang, Y. , Zhao, F. , Hu, L. , … Gao, X. (2009). Dual effects of lipophilic extract of Salvia miltiorrhiza (Danshen) on catecholamine secretion in cultured bovine adrenal medullary cells. Journal of Ethnopharmacology, 125(1), 59–67. [DOI] [PubMed] [Google Scholar]
- Mao, X. , Wu, L. F. , Guo, H. L. , Chen, W. J. , Cui, Y. P. , Qi, Q. , … Zhu, D. (2016). The genus Phyllanthus: An Ethnopharmacological, phytochemical, and pharmacological review. Evidence‐based Complementary and Alternative Medicine, 2016, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell, M. , Esteban, J. I. , Quer, J. , Genesca, J. , Weiner, A. , Esteban, R. , … Gomez, J. (1992). Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: Quasispecies nature of HCV genome distribution. Journal of Virology, 66(5), 3225–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOmish, F. , Yap, P. L. , Dow, B. C. , Follett, E. A. , Seed, C. , Keller, A. J. , … Naukkarinen, R. (1994). Geographical distribution of hepatitis C virus genotypes in blood donors: An international collaborative survey. Journal of Clinical Microbiology, 32(4), 884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltonprabu, S. , Tomczyk, M. , Skalicka‐Woźniak, K. , Rastrelli, L. , Daglia, M. , Nabavi, S. F. , … Nabavi, S. M. (2016). Hepatoprotective effect of quercetin: From chemistry to medicine. Food and Chemical Toxicology. [DOI] [PubMed]
- Mohanta, T. K. , Tamboli, Y. , & Zubaidha, P. K. (2014). Phytochemical and medicinal importance of Ginkgo biloba L. Natural Product Research, 28(10), 746–752. [DOI] [PubMed] [Google Scholar]
- Mukhtar, M. , Arshad, M. , Ahmad, M. , Pomerantz, R. J. , Wigdahl, B. , & Parveen, Z. (2008). Antiviral potentials of medicinal plants. Virus Research, 131(2), 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaz, F. , Javed, S. , & Abdin, M. Z. (2007). Hepatoprotective effect of ethanolic extract of Phyllanthus amarus Schum. et Thonn. On aflatoxin B1‐induced liver damage in mice. Journal of Ethnopharmacology, 113(3), 503–509. [DOI] [PubMed] [Google Scholar]
- Nabavi, S. F. , Maggi, F. , Daglia, M. , Habtemariam, S. , Rastrelli, L. , & Nabavi, S. M. (2016). Pharmacological effects of Capparis spinosa L. Phytotherapy research : PTR, 30(11), 1733–1744. [DOI] [PubMed] [Google Scholar]
- Naik, S. R. , Pilgaonkar, V. W. , & Panda, V. S. (2006). Neuropharmacological evaluation of Ginkgo biloba phytosomes in rodents. Phytotherapy research : PTR, 20(10), 901–905. [DOI] [PubMed] [Google Scholar]
- Nitha, A. , Prabha, S. P. , Ansil, P. N. , & Latha, M. S. (2014). Methanolic extract of Woodfordia fruticosa Kurz flowers ameliorates carbon tetrachloride‐induced chronic hepatic fibrosis in rats. Toxicology and Industrial Health, 32(7), 1224–1236. [DOI] [PubMed] [Google Scholar]
- Norbaek, R. , Nielsen, K. , & Kondo, T. (2002). Anthocyanins from flowers of Cichorium intybus. Phytochemistry, 60(4), 357–359. [DOI] [PubMed] [Google Scholar]
- Ogata, N. , Alter, H. J. , Miller, R. H. , & Purcell, R. H. (1991). Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proceedings of the National Academy of Sciences of the United States of America, 88(8), 3392–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panico, A. M. , Cardile, V. , Garufi, F. , Puglia, C. , Bonina, F. , & Ronsisvalle, G. (2005). Protective effect of Capparis spinosa on chondrocytes. Life Sciences, 77(20), 2479–2488. [DOI] [PubMed] [Google Scholar]
- Panossian, A. , & Wikman, G. (2008). Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. Journal of Ethnopharmacology, 118(2), 183–212. [DOI] [PubMed] [Google Scholar]
- Parimoo, H. A. , Sharma, R. , Patil, R. D. , Sharma, O. P. , Kumar, P. , & Kumar, N. (2014). Hepatoprotective effect of Ginkgo biloba leaf extract on lantadenes‐induced hepatotoxicity in guinea pigs. Toxicon, 81, 1–12. [DOI] [PubMed] [Google Scholar]
- Park, E. J. , Zhao, Y. Z. , Kim, Y. C. , & Sohn, D. H. (2009). Preventive effects of a purified extract isolated from Salvia miltiorrhiza enriched with tanshinone I, tanshinone IIA and cryptotanshinone on hepatocyte injury in vitro and in vivo. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 47(11), 2742–2748. [DOI] [PubMed] [Google Scholar]
- Patel, J. R. , Tripathi, P. , Sharma, V. , Chauhan, N. S. , & Dixit, V. K. (2011). Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. Journal of Ethnopharmacology, 138(2), 286–313. [DOI] [PubMed] [Google Scholar]
- Peng, F. , Du, Q. , Peng, C. , Wang, N. , Tang, H. , Xie, X. , … Chen, J. (2015). A review: The pharmacology of Isoliquiritigenin. Phytotherapy research : PTR, 29(7), 969–977. [DOI] [PubMed] [Google Scholar]
- Pereira, C. , Barros, L. , & Ferreira, I. C. F. R. (2016). Extraction, identification, fractionation and isolation of phenolic compounds in plants with hepatoprotective effects. Journal of the Science of Food and Agriculture, 96(4), 1068–1084. [DOI] [PubMed] [Google Scholar]
- Perez, R. M. , Perez, J. A. , Garcia, L. M. , & Sossa, H. (1998). Neuropharmacological activity of Solanum nigrum fruit. Journal of Ethnopharmacology, 62(1), 43–48. [DOI] [PubMed] [Google Scholar]
- Petruzziello, A. , Marigliano, S. , Loquercio, G. , & Cacciapuoti, C. (2016). Hepatitis C virus (HCV) genotypes distribution: An epidemiological up‐date in Europe. Infectious agents and cancer, 11, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prashanth Kumar, V. , Shashidhara, S. , Kumar, M. M. , & Sridhara, B. Y. (2001). Cytoprotective role of Solanum nigrum against gentamicin‐induced kidney cell (Vero cells) damage in vitro. Fitoterapia, 72(5), 481–486. [DOI] [PubMed] [Google Scholar]
- Pubchem , (2017). National center for biotechnology information. PubChem Compound Database;.
- Ram, A. , Mabalirajan, U. , Das, M. , Bhattacharya, I. , Dinda, A. K. , Gangal, S. V. , & Ghosh, B. (2006). Glycyrrhizin alleviates experimental allergic asthma in mice. International Immunopharmacology, 6(9), 1468–1477. [DOI] [PubMed] [Google Scholar]
- Rani, A. , & Sharma, A. (2013). The genus Vitex: A review. Pharmacognosy Reviews, 7(14), 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi, R. P. , & MB (1999). Compendium of Indian medicinal plants. CDRI Publication: New Delhi, 2, 609–610. [Google Scholar]
- Rodrigues, N. , Almeida, A. , Silva, H. , Pinto, D. , Seca, A. , Pereira, M. (2016). Potential anti‐inflammatory effects of Artemisia gorgonum on rat liver injury induced by CCl4–ERRATUM. Microscopy and microanalysis: The official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada: 1–2. [DOI] [PubMed]
- Romeo, V. , Ziino, M. , Giuffrida, D. , Condurso, C. , & Verzera, A. (2007). Flavour profile of capers (Capparis spinosa L.) from the Eolian archipelago by HS‐SPME/GC–MS. Food Chemistry, 101(3), 1272–1278. [Google Scholar]
- Salehi, M. , Karegar‐Borzi, H. , Karimi, M. , & Rahimi, R. (2016). Medicinal plants for management of gastroesophageal reflux disease: A review of animal and human studies. The Journal of Alternative and Complementary Medicine, 23(2), 82–95. [DOI] [PubMed] [Google Scholar]
- Sato, H. , Goto, W. , Yamamura, J. , Kurokawa, M. , Kageyama, S. , Takahara, T. , … Shiraki, K. (1996). Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Research, 30(2–3), 171–177. [DOI] [PubMed] [Google Scholar]
- Shehab, N. G. , Abu‐Gharbieh, E. , & Bayoumi, F. A. (2015). Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Complementary and Alternative Medicine, 15, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib, M. , Shah, S. W. , Ali, N. , Shah, I. , Ullah, S. , Ghias, M. , … Ullah, A. (2016). Scientific investigation of crude alkaloids from medicinal plants for the management of pain. BMC Complementary and Alternative Medicine, 16, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankari, B. , Anandharaj, M. , & Gunasekaran, P. (2014). An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. Journal of Ethnopharmacology, 153(2), 408–423. [DOI] [PubMed] [Google Scholar]
- Son, Y. O. , Kim, J. , Lim, J. C. , Chung, Y. , Chung, G. H. , & Lee, J. C. (2003). Ripe fruit of Solanum nigrum L. inhibits cell growth and induces apoptosis in MCF‐7 cells. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 41(10), 1421–1428. [DOI] [PubMed] [Google Scholar]
- Stickel, F. , Patsenker, E. , & Schuppan, D. (2005). Herbal hepatotoxicity. Journal of Hepatology, 43(5), 901–910. [DOI] [PubMed] [Google Scholar]
- Street, R. , Sidana, J. , & Prinsloo, G. (2013). Cichorium intybus: Traditional uses, Phytochemistry, pharmacology, and toxicology. Evidence‐based Complementary and Alternative Medicine, 2013, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhagia, B. , Rathod, I. , & Sindhu, S. (2011). Sapindus mukorossi (Areetha): an overview. International Journal of Pharmaceutical Sciences and Research, 2(8), 1905. [Google Scholar]
- Suja, S. R. , Latha, P. G. , Pushpangadan, P. , & Rajasekharan, S. (2004). Evaluation of hepatoprotective effects of Helminthostachys zeylanica (L.) Hook against carbon tetrachloride‐induced liver damage in Wistar rats. Journal of Ethnopharmacology, 92(1), 61–66. [DOI] [PubMed] [Google Scholar]
- Sultana, S. , Perwaiz, S. , Iqbal, M. , & Athar, M. (1995). Crude extracts of hepatoprotective plants, Solanum nigrum and Cichorium intybus inhibit free radical‐mediated DNA damage. Journal of Ethnopharmacology, 45(3), 189–192. [DOI] [PubMed] [Google Scholar]
- Sun, J. , Huang, S. H. , Tan, B. K. , Whiteman, M. , Zhu, Y. C. , Wu, Y. J. , … Zhu, Y. Z. (2005). Effects of purified herbal extract of Salvia miltiorrhiza on ischemic rat myocardium after acute myocardial infarction. Life Sciences, 76(24), 2849–2860. [DOI] [PubMed] [Google Scholar]
- Sun, W. Y. , Wei, W. , Wu, L. , Gui, S. Y. , & Wang, H. (2007). Effects and mechanisms of extract from Paeonia Lactiflora and Astragalus membranaceus on liver fibrosis induced by carbon tetrachloride in rats. Journal of Ethnopharmacology, 112(3), 514–523. [DOI] [PubMed] [Google Scholar]
- Surai, P. F. (2015). Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants (Basel, Switzerland), 4(1), 204–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed, Y. H. , & Khan, M. (2016). Chromatographic profiling of Ellagic acid in Woodfordia fruticosa flowers and their gastroprotective potential in ethanol‐induced ulcers in rats. Pharmacognosy research, 8(Suppl 1), S5–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szopa, A. , Ekiert, R. , & Ekiert, H. (2017). Current knowledge of Schisandra chinensis (Turcz.) Baill.(Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochemistry Reviews, 16(2), 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada, N. , Takase, S. , Takada, A. , & Date, T. (1993). Differences in the hepatitis C virus genotypes in different countries. Journal of Hepatology, 17(3), 277–283. [DOI] [PubMed] [Google Scholar]
- Thandavarayan, R. A. , Giridharan, V. V. , Arumugam, S. , Suzuki, K. , Ko, K. M. , Krishnamurthy, P. , … Konishi, T. (2015). Schisandrin B prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of MAPK/p53 signaling. PLoS One, 10(3), e0119214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theplantlist , 2013. The Plant list Version 1.1. Published on the Internet.
- Tomlinson, T. R. , & Akerele, O. (2015). Medicinal plants: Their role in health and biodiversity. Incorporated: University of Pennsylvania Press. [Google Scholar]
- Tunca, R. , Sozmen, M. , Citil, M. , Karapehlivan, M. , Erginsoy, S. , & Yapar, K. (2009). Pyridine induction of cytochrome P450 1A1, iNOS and metallothionein in Syrian hamsters and protective effects of silymarin. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie, 61(3), 243–255. [DOI] [PubMed] [Google Scholar]
- Upadhyay, A. , & Singh, D. K. (2012). Pharmacological effects of Sapindus mukorossi. Revista do Instituto de Medicina Tropical de São Paulo, 54, 273–280. [DOI] [PubMed] [Google Scholar]
- Upadhyay, G. , Kumar, A. , & Singh, M. P. (2007). Effect of silymarin on pyrogallol‐ and rifampicin‐induced hepatotoxicity in mouse. European Journal of Pharmacology, 565(1–3), 190–201. [DOI] [PubMed] [Google Scholar]
- Venkateswaran, P. S. , Millman, I. , & Blumberg, B. S. (1987). Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses: In vitro and in vivo studies. Proceedings of the National Academy of Sciences of the United States of America, 84(1), 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, S. K. , Rai, M. K. , Asthana, P. , Jaiswal, V. S. , & Jaiswal, U. (2010). In vitro plantlets from alginate‐encapsulated shoot tips of Solanum nigrum L. Scientia Horticulturae, 124(4), 517–521. [Google Scholar]
- Wan, J. M. , Sit, W. H. , Lee, C. L. , Fu, K. H. , & Chan, D. K. (2006). Protection of lethal toxicity of endotoxin by Salvia miltiorrhiza BUNGE is via reduction in tumor necrosis factor alpha release and liver injury. International Immunopharmacology, 6(5), 750–758. [DOI] [PubMed] [Google Scholar]
- Wang, K. , Song, Z. , Wang, H. , Li, Q. , Cui, Z. , & Zhang, Y. (2016). Angelica sinensis polysaccharide attenuates concanavalin A‐induced liver injury in mice. International Immunopharmacology, 31, 140–148. [DOI] [PubMed] [Google Scholar]
- Wang, Y.‐S. , Wen, Z.‐Q. , Li, B.‐T. , Zhang, H.‐B. , & Yang, J.‐H. (2016). Ethnobotany, phytochemistry, and pharmacology of the genus Litsea: An update. Journal of Ethnopharmacology, 181, 66–107. [DOI] [PubMed] [Google Scholar]
- Wang, C. M. , Yuan, R. S. , Zhuang, W.‐Y. , Sun, J.‐H. , Wu, J. Y. , Li, H. , & Chen, J. G. (2016). Schisandra polysaccharide inhibits hepatic lipid accumulation by downregulating expression of SREBPs in NAFLD mice. Lipids in Health and Disease, 15(1), 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser, S. , Ho, J. M. , Ang, H. K. , & Tan, C. E. (1998). Salvia miltiorrhiza reduces experimentally‐induced hepatic fibrosis in rats. Journal of Hepatology, 29(5), 760–771. [DOI] [PubMed] [Google Scholar]
- Wu, W. , Wang, Y. , & Que, L. (2006). Enhanced bioavailability of silymarin by self‐microemulsifying drug delivery system. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 63(3), 288–294. [DOI] [PubMed] [Google Scholar]
- Wu, Z. M. , Wen, T. , Tan, Y. F. , Liu, Y. , Ren, F. , & Wu, H. (2007). Effects of salvianolic acid a on oxidative stress and liver injury induced by carbon tetrachloride in rats. Basic & Clinical Pharmacology & Toxicology, 100(2), 115–120. [DOI] [PubMed] [Google Scholar]
- Xiao, J. , Zhu, Y. , Liu, Y. , Tipoe, G. L. , Xing, F. , & So, K.‐F. (2014). Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP‐NLRP3 inflammasome pathway. International Journal of Biological Macromolecules, 69, 73–78. [DOI] [PubMed] [Google Scholar]
- Xin, D. Q. , Hu, Z. M. , Huo, H. J. , Yang, X. J. , Han, D. , Xing, W. H. , … Qiu, Q. H. (2017). Schisandrin B attenuates the inflammatory response, oxidative stress and apoptosis induced by traumatic spinal cord injury via inhibition of p53 signaling in adult rats. Molecular Medicine Reports. [DOI] [PMC free article] [PubMed]
- Yasuda, H. , Izumi, N. , Shimada, O. , Kobayakawa, T. , & Nakanishi, M. (1980). The protective effect of tinoridine against carbon tetrachloride hepatotoxicity. Toxicology and Applied Pharmacology, 52(3), 407–413. [DOI] [PubMed] [Google Scholar]
- Yen, F.‐L. , Wu, T.‐H. , Lin, L.‐T. , Cham, T.‐M. , & Lin, C.‐C. (2008). Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen‐induced hepatotoxicity in rats. Food and Chemical Toxicology, 46(5), 1771–1777. [DOI] [PubMed] [Google Scholar]
- Yen, F.‐L. , Wu, T.‐H. , Lin, L.‐T. , & Lin, C.‐C. (2007). Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen‐induced hepatotoxicity in rats. Journal of Ethnopharmacology, 111(1), 123–128. [DOI] [PubMed] [Google Scholar]
- Yin, H. Q. , Choi, Y. J. , Kim, Y. C. , Sohn, D. H. , Ryu, S. Y. , & Lee, B. H. (2009). Salvia miltiorrhiza Bunge and its active component cryptotanshinone protects primary cultured rat hepatocytes from acute ethanol‐induced cytotoxicity and fatty infiltration. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 47(1), 98–103. [DOI] [PubMed] [Google Scholar]
- Yip, P. Y. , & Kwan, H. S. (2006). Molecular identification of Astragalus membranaceus at the species and locality levels. Journal of Ethnopharmacology, 106(2), 222–229. [DOI] [PubMed] [Google Scholar]
- Zein, N. N. , Rakela, J. , Krawitt, E. L. , Reddy, K. R. , Tominaga, T. , & Persing, D. H. (1996). Hepatitis C virus genotypes in the United States: Epidemiology, pathogenicity, and response to interferon therapy. Collaborative study group. Annals of Internal Medicine, 125(8), 634–639. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Xie, X. , Li, C. , & Fu, P. (2009). Systematic review of the renal protective effect of Astragalus membranaceus (root) on diabetic nephropathy in animal models. Journal of Ethnopharmacology, 126(2), 189–196. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , He, B. , Ge, J. , Li, H. , Luo, X. , Zhang, H. , … Fei, X. (2010). Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia–reperfusion rats. International Journal of Biological Macromolecules, 47(4), 546–550. [DOI] [PubMed] [Google Scholar]
- Zhao, Z. M. , Liu, H. L. , Sun, X. , Guo, T. , Shen, L. , Tao, Y. Y. , & Liu, C. H. (2017). Levistilide A inhibits angiogenesis in liver fibrosis via vascular endothelial growth factor signaling pathway. Experimental Biology and Medicine, 242(9), 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, C. J. , Li, H. Q. , Ren, S. C. , Xu, C. L. , Rahman, K. , Qin, L. P. , & Sun, Y. H. (2015). Phytochemical and pharmacological profile of Vitex negundo. Phytotherapy research : PTR, 29(5), 633–647. [DOI] [PubMed] [Google Scholar]
- Zheng, H. , Dong, Z. , & She, J. (1998). Modern study of traditional Chinese medicine. Xue Yuan Press Beijing China, 3, 2057. [Google Scholar]
- Zheng, N. , Liu, F. , Lu, H. , Zhan, Y. , Zhang, M. , Guo, W. , & Ding, G. (2017). Schisantherin A protects against liver ischemia‐reperfusion injury via inhibition of mitogen‐activated protein kinase pathway. International Immunopharmacology, 47, 28–37. [DOI] [PubMed] [Google Scholar]
- Zhou, Y.‐X. , Xia, W. , Yue, W. , Peng, C. , Rahman, K. , & Zhang, H. (2015). Rhein: A review of pharmacological activities. Evidence‐based Complementary and Alternative Medicine, 2015, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]


