Abstract
The present study was conducted from July to August 2018 on milk samples taken at dairy farms in the Northern Province and Kigali District of Rwanda in order to identify Staphylococcus spp. associated with bovine intramammary infection. A total of 161 staphylococcal isolates originating from quarter milk samples of 112 crossbred dairy cattle were included in the study. Antimicrobial susceptibility testing was performed and isolates were examined for the presence of various resistance genes. Staphylococcus aureus isolates were also analyzed for the presence of virulence factors, genotyped by spa typing and further phenotypically subtyped for capsule expression using Fourier Transform Infrared (FTIR) spectroscopy. Selected S. aureus were characterized using DNA microarray technology, multi-locus sequence typing (MLST) and whole-genome sequencing. All mecA-positive staphylococci were further genotyped using dru typing. In total, 14 different staphylococcal species were detected, with S. aureus being most prevalent (26.7%), followed by S. xylosus (22.4%) and S. haemolyticus (14.9%). A high number of isolates was resistant to penicillin and tetracycline. Various antimicrobial and biocide resistance genes were detected. Among S. aureus, the Panton–Valentine leukocidin (PVL) genes, as well as bovine leukocidin (LukM/LukF-P83) genes, were detected in two and three isolates, respectively, of which two also carried the toxic shock syndrome toxin gene tsst-1 bovine variant. t1236 was the predominant spa type. FTIR-based capsule serotyping revealed a high prevalence of non-encapsulated S. aureus isolates (89.5%). The majority of the selected S. aureus isolates belonged to clonal complex (CC) 97 which was determined using DNA microarray based assignment. Three new MLST sequence types were detected.
Keywords: Staphylococcus species, Staphylococcus aureus, bovine mastitis, antibiotic resistance, spa typing, FTIR spectroscopy, capsule serotyping, MLST, whole-genome sequencing, dru typing
1. Introduction
Bovine mastitis is an important disease that affects the dairy sector and is one of the economically most important diseases worldwide [1]. In Rwanda, it has a significant relevance because livestock production is rapidly increasing [2]. One reason is that milk consumption and the demand for dairy products are increasing with the rapid growth of the human population, from 3 million to 12 million people [3] in the last 60 years.
Mastitis is an inflammation of the udder tissue and the mammary gland. It is usually caused by bacteria invading through the teat canal. There are two types of mastitis: clinical and subclinical. While cows with clinical mastitis show severe symptoms (e.g., fever, hot, painful and swollen udder) and have visible changes in their milk (e.g., change of colour and consistency), cows with subclinical mastitis produce less milk and have higher somatic cell counts in their milk [1]. The California Mastitis Test (CMT) is a useful onsite method to confirm a bovine intramammary infection [4].
Staphylococci are the leading cause of mastitis [5,6], with S. aureus considered to be a major pathogen associated with clinical mastitis and often-recurrent subclinical mastitis, even in well-managed dairy herds. The primary mode of transmission is from cow-to-cow [1]. Coagulase-negative Staphylococcus spp. (CoNS) are a heterogeneous group and are also known as common pathogens involved in bovine mastitis. CoNS are primarily derived from the environment and are usually associated with a moderate infection [1].
In Rwanda, udder infections are associated with contamination via hand-to-cow contact, clothing, and other materials because hand milking is common. Poor milking hygiene is a risk factor not only for mastitis, but also for teat-end damage [7]. Reduced milk production, high veterinary costs, as well as prolific bacterial and antimicrobial contamination are the consequences of mastitis which can result in significant economic losses for the farmers [8]. Recently, the Government of Rwanda launched a development program, called Rwanda Vision 2020, with the main goal of transforming the country into a knowledge-based middle-income country by modernizing its agriculture and livestock production [2]. Public veterinary services in Rwanda are provided by district and sector veterinary officers. They have a limited capacity to support dairy farmers. Often, veterinary service workers receive poor training in dairy management and are not equipped with adequate transportation to visit farms (approximately 3200 cattle/veterinary officers). Overall, access to veterinary services in rural areas is less developed compared to urban areas [9].
In 2015, the first private animal clinic was established in the district of Musanze, called the New Vision Veterinary Hospital (NVVH), to improve animal welfare and to provide veterinary services (clinical and laboratory) as well as education based on collaboration with local and foreign universities and organizations.
Nevertheless, the farmers’ access in Rwanda to veterinary drugs including antibiotics is possible through local pharmacies [9]. A recent report explained that in parts of the country, high usage of antibiotics in farm animals has become a common practice [9]. In a cross-sectional survey, the use of antibiotics in farm animals was declared by the majority of respondents (97.4%), mainly for disease prevention and growth promotion. More than half of the farmers (55.6%) were reported to use non-prescribed antibiotics in animals. Although policies and laws regulating the antibiotic use in humans and animals exist in Rwanda, antibiotics can be purchased without any medical or veterinary prescription [9]. The irrational use of antibiotics in humans and animals is highly related to the increase of antibiotic-resistant bacteria worldwide, including many classes of antimicrobial agents used in the veterinary field [10].
A recent study conducted in a hospital in Kigali, Rwanda assessing the antimicrobial susceptibility patterns of bacteria from human patients, showed a high prevalence of antimicrobial resistance, also among Staphylococcus spp. [11]. However, there is very limited information on the antimicrobial susceptibility pattern of bacteria isolated from milk samples obtained from cases of bovine mastitis in Rwanda. Recently, two studies showed a high prevalence of mastitis in the Northern Province and the peri-urban areas of Kigali [12,13], but characterization of causative agents and antimicrobial susceptibility testing, both phenotypic and genotypic, have not been performed. Thus, the present study aims to fill these gaps by fully characterizing a collection of bovine staphylococci associated with clinical and subclinical mastitis from the Northern Province and Kigali the District of Rwanda.
2. Results
From 303 CMT-positive milk samples collected from 112 crossbred milking cows, 161 non-repetitive staphylococcal isolates comprising 14 staphylococcal species were recovered: S. aureus (n = 43), S. xylosus (n = 36), S. haemolyticus (n = 24), S. sciuri (n = 14), S. chromogenes (n = 10), S. saprophyticus (n = 9), S. epidermidis (n = 8), S. succinus (n = 5), S. capitis (n = 3), S. hominis (n = 2), S. devriesei (n = 2), S. auricularis (n = 2), S. equorum (n = 2), and S. simulans (n = 1).
2.1. Antimicrobial Susceptibility Testing
All 161 isolates were susceptible to rifampicin, linezolid, and gentamicin. All but two were susceptible to cefoxitin and chloramphenicol. A high number of the isolates was resistant to penicillin (n = 73, 45.3%) and tetracycline (n = 63, 39.1%) (Table 1 and Table 2). Twenty-three isolates were resistant to clindamycin, ten to erythromycin, and six isolates were resistant to trimethoprim-sulfamethoxazole (Table 1 and Table 2).
Table 1.
Summarized molecular characterization, antimicrobial resistance and toxins profile of Coagulase-negative Staphylococcus isolates investigated.
| Isolates | Species | Origin 1 | Antimicrobial Resistance Profile | Biocide and Metal Resistance Genes | ||
|---|---|---|---|---|---|---|
| Phenotype 2 | MIC 3 of streptomycin | Genes Detected | ||||
| 2FR | S. chromogenes | M 1 | 32 4 | str | ||
| 3RL | S. haemolyticus | M 1 | ERY, CLI | 32 | erm(C), str | |
| 4FR | S. epidermidis | M 1 | PEN, TET | 32 | blaZ, tet(K), tet(L), str | copB, qacAB, smr |
| 4RR1 | S. hominis | M 1 | BLA, FOX, ERY, TET, CIP | ‹4 | blaZ, mecA, msr(A), tet(K), tet(L), str | cadD, arsA, qacAB, smr |
| 4RR2 | S. capitis | M 1 | PEN | ‹4 | blaZ, str | cadD, arsA, qacAB, smr |
| 7FL | S. chromogenes | M 2 | ERY, CLI | ‹4 | erm(C), str | |
| 7RR | S. epidermidis | M 2 | PEN, ERY, CLI, TET | 32 | blaZ, erm(C), tet(K), tet(L), str | cadD, arsA, smr |
| 8RL | S. haemolyticus | M 2 | ERY, CLI | 32 | erm(C), str | |
| 13FLg | S. xylosus | M 3 | PEN | ‹4 | blaZ, str | cadD, copB |
| 13FLw | S. xylosus | M 3 | PEN, TET | 32 | blaZ, tet(K), tet(L), str | cadD, arsA, smr |
| 13FLw wh | S. xylosus | M 3 | ERY | ‹4 | msr(A), str | |
| 13RR | S. xylosus | M 3 | ERY, CLI, CHL | ‹4 | msr(A), fexA, str | |
| 14FL1 | S. equorum | M 3 | ‹4 | str | ||
| 17RR | S. equorum | M 4 | ‹4 | str | smr | |
| 18RLw1 | S. epidermidis | M 4 | PEN, TET | 32 | blaZ, tet(K), tet(L), str | cadD, arsA, qacAB, smr |
| 18RLw2 | S. haemolyticus | M 4 | PEN, TET | 32 | blaZ, tet(K), tet(L), str | cadD, arsA, qacAB, smr |
| 18RLg | S. haemolyticus | M 4 | ‹4 | str | cadD, copB, arsA, smr | |
| 18FL | S. auricularis | M 4 | 16 | str | copB | |
| 24RLw | S. xylosus | M 5 | ‹4 | str | cadD, smr | |
| 24RLg | S. haemolyticus | M 5 | 32 | str | ||
| 25FLw | S. hominis | M 5 | PEN | ‹4 | blaZ, str | cadD, arsA, qacAB, smr |
| 25FLg | S. xylosus | M 5 | PEN | ‹4 | blaZ, str | |
| 25FL3 | S. xylosus | M 5 | ‹4 | str | cadD | |
| 25RR | S. epidermidis | M 5 | PEN, TET | 32 | blaZ, tet(K), str | |
| 25RRg | S. sciuri | M 5 | CLI | ‹4 | sal(A), str | |
| 26RL1 | S. xylosus | M 6 | ‹4 | str | cadD | |
| 26RRw | S. xylosus | M 6 | ‹4 | str | ||
| 26RRg | S. xylosus | M 6 | ‹4 | str | ||
| 27RLg | S. xylosus | M 6 | ‹4 | str | ||
| 28FRg | S. xylosus | M 7 | ‹4 | str | ||
| 30FL | S. devriesei | M 8 | TET | 16 | tet(K), str | arsA |
| 30RL | S. devriesei | M 8 | PEN, TET | 32 | blaZ, tet(K), str | arsA |
| 30FR | S. chromogenes | M 8 | PEN, TET | ‹4 | blaZ, tet(K), str | |
| 32FR | S. chromogenes | M 8 | 32 | str | ||
| 33RL | S. chromogenes | M 8 | PEN, TET | 32 | blaZ, tet(K), str | |
| 33FR | S. haemolyticus | M 8 | 32 | str | ||
| 34RLw | S. haemolyticus | M 9 | 32 | str | cadD | |
| 35FR | S. haemolyticus | M 9 | 16 | str | arsA | |
| 35RRg | S. haemolyticus | M 9 | 16 | str | arsA | |
| 36FL | S. haemolyticus | M 9 | TET | 32 | tet(K), tet(L), str | |
| 38FL | S. auricularis | M 9 | ‹4 | str | cadD | |
| 42FR | S. haemolyticus | M 11 | TET | 32 | tet(K), tet(L), str | |
| 43FRw | S. xylosus | M 11 | TET | ‹4 | tet(K), str | copB |
| 44FL | S. xylosus | M 11 | ‹4 | str | ||
| 46FR | S. epidermidis | M 11 | PEN | 32 | blaZ, str | cadD |
| 47RRg | S. chromogenes | M 12 | 32 | str | qacAB, smr | |
| 50RL | S. sciuri | M 12 | CLI | ‹4 | erm(44), str | |
| 50RR | S. sciuri | M 12 | CLI | ‹4 | erm(44), sal(A) str | |
| 51RR | S. xylosus | M 12 | TET | ‹4 | tet(K), str | |
| 52FL | S. haemolyticus | K | PEN, CLI, TET | 32 | blaZ, erm(C), tet(K), tet(L), str | cadD, copB, qacAB, smr |
| 52FR | S. haemolyticus | K | ‹4 | str | cadD, copB, arsA | |
| 53FL | S. haemolyticus | K | PEN, CLI, TET | 32 | blaZ, tet(K), str | copB |
| 53RL | S. haemolyticus | K | CLI, TET | 32 | vga(A), sal(A), Inu(A), tet(K), tet(L), str | qacAB, smr |
| 53RR | S. haemolyticus | K | CLI, TET | 32 | vga(A), sal(A), Inu(A), tet(K), tet(L), str | qacAB, smr |
| 54FR | S. haemolyticus | K | CLI | 32 | vga(A), str | |
| 54RRw | S. haemolyticus | K | PEN, CLI, SXT, TET | 32 | blaZ, dfrA, dfrD, tet(K), str | |
| 54RRg | S. xylosus | K | 32 | str | smr | |
| 55RR1 | S. epidermidis | K | PEN, TET | ‹4 | blaZ, tet(K), str | copB, arsA, qacAB, smr |
| 55RR2 | S. capitis | K | PEN | ‹4 | blaZ, str | copB, arsA, smr |
| 56RL | S. sciuri | K | CLI | ‹4 | vga(A), sal(A), str | |
| 57FLw | S. capitis | K | PEN, TET | ‹4 | blaZ, tet(K), tet(L), str | cadD, smr |
| 57FRw | S. haemolyticus | K | CLI, TET | 32 | tet(K), tet(L), str | copB, smr |
| 58FL | S. haemolyticus | K | CLI, TET | 32 | erm(C), sal(A), tet(K), tet(L), str | smr |
| 58FR | S. haemolyticus | K | CLI, TET | 32 | vga(A), tet(K), tet(L), str | |
| 58RR | S. xylosus | K | ‹4 | str | ||
| 61RR | S. xylosus | K | SXT, TET | ‹4 | dfrA, dfrD, dfrG, tet(K), tet(L), str | smr |
| 61RL | S. xylosus | K | TET | 32 | tet(K), str | copB, smr |
| 62FR | S. xylosus | K | ‹4 | str | copB | |
| 62RR | S. haemolyticus | K | ‹4 | str | cadD | |
| 63RL | S. sciuri | K | PEN | ‹4 | blaZ, str | |
| 64RR | S. epidermidis | K | PEN, SXT, TET | 32 | blaZ, dfrA, dfrD, dfrG, tet(K), tet(L), tet(O), str | copB, arsA, smr |
| 65RL | S. haemolyticus | K | PEN, ERY, SXT, TET | 32 | blaZ, msr(A), dfrD, dfrG, tet(K), str | cadD, copB, arsA |
| 66RL | S. xylosus | K | PEN, TET | ‹4 | blaZ, tet(K), str | qacAB |
| 66RR | S. epidermidis | K | PEN, TET, TEC | 32 | blaZ, tet(K), str | cadD, smr |
| 67RL | S. chromogenes | K | 32 | str | ||
| 68RL | S. chromogenes | K | 32 | str | ||
| 68RR | S. xylosus | K | PEN | ‹4 | blaZ, str | |
| 70RLw | S. simulans | K | PEN | 32 | blaZ, str | copB |
| 70FR | S. sciuri | K | FOX | ‹4 | mecA, str | |
| 1stCowFL | S. chromogenes | M 13 | ‹4 | str | ||
| 2ndCowRL | S. xylosus | M 13 | TET | ‹4 | tet(K), str | |
| 73RL | S. sciuri | M 14 | PEN | ‹4 | blaZ, str | |
| 73RR | S. xylosus | M 14 | ‹4 | str | ||
| 78FR | S. xylosus | M 17 | ‹4 | str | ||
| 78RL | S. sciuri | M 17 | CLI | ‹4 | vga(A), sal(A), str | |
| 81 RR | S. haemolyticus | M 18 | PEN | ‹4 | blaZ, str | cadD |
| 82RL | S. sciuri | M 18 | CLI | ‹4 | erm(44), str | |
| 82RR | S. saprophyticus | M 18 | TET, CHL | 4 | tet(K), catpC221, str | |
| 84RR | S. saprophyticus | M 18 | TET | ‹4 | tet(K), str | copB |
| 85FR | S. xylosus | M 19 | TET | 8 | tet(K), str | |
| 85FL | S. saprophyticus | M 19 | TET | 8 | tet(K), str | copB, arsA, qacAB |
| 86FR | S. saprophyticus | M 19 | ‹4 | str | copB | |
| 87FL | S. saprophyticus | M 19 | TET | 4 | tet(K), str | copB |
| 89FR | S. sciuri | M 20 | ‹4 | str | ||
| 89RR | S. xylosus | M 20 | PEN | ‹4 | blaZ, str | |
| 94RR | S. succinus | M 21 | PEN | ‹4 | blaZ, str | copB |
| 94RL | S. sciuri | M 21 | PEN | ‹4 | blaZ, str | |
| 95FR | S. xylosus | M 21 | ‹4 | str | ||
| 95RR | S. xylosus | M 21 | ‹4 | str | ||
| 96FR | S. xylosus | M 21 | TET | ‹4 | tet(K), str | qacAB |
| 96RR | S. xylosus | M 21 | ‹4 | str | ||
| 97RL | S. sciuri | M 21 | ‹4 | str | ||
| 97RR | S. xylosus | M 21 | SXT | ‹4 | dfrD, dfrG, str | |
| 98RR | S. succinus | M 21 | PEN | ‹4 | blaZ, str | cadD |
| 99FR | S. xylosus | M 22 | ‹4 | str | ||
| 99RL | S. xylosus | M 22 | ‹4 | str | copB | |
| 103RR | S. chromogenes | M 22 | PEN | 32 | blaZ, str | |
| 104RR | S. succinus | M 23 | ‹4 | str | smr | |
| 104RL | S. succinus | M 23 | PEN | ‹4 | blaZ, str | cadD, arsA, smr |
| 105RL | S. succinus | M 24 | ‹4 | str | cadD, smr | |
| 106FL1 | S. saprophyticus | M 24 | ‹4 | str | copB | |
| 107RL | S. saprophyticus | M 25 | PEN | 16 | blaZ, str | copB |
| 108FL | S. saprophyticus | M 25 | SXT | ‹4 | dfrD, dfrG, str | arsA |
| 110RL | S. xylosus | M 26 | ‹4 | str | copB, smr | |
| 110RR1 | S. saprophyticus | M 26 | ‹4 | str | copB, arsA | |
| 110RR2 | S. xylosus | M 26 | ‹4 | str | copB | |
| 111RL | S. sciuri | M 26 | PEN, CLI | ‹4 | sal(A), blaZ, str | |
| 113RL | S. sciuri | M 26 | 16 | str | ||
1 Origin: M = Musanze Farm, K = Kigali Farm.2 Phenotype: PEN = penicillin; CIP = ciprofloxacin; CHL = chloramphenicol; CLI = clindamycin; ERY = erythromycin; SXT = trimethoprim-sulfamethoxazole; TET = tetracycline; FOX = cefoxitin, TEC = teicoplanin. 3 mg/L. 4 32 or higher (mg/L).
Table 2.
Summarized molecular characterization, antimicrobial resistance and toxin profile of the Staphylococcus aureus isolates investigated.
| Isolates | Origin 1 | CC 2 | ST 3 | spa | Antimicrobial Resistance Profile | Biocide and Metal Resistance Genes | Capsule Serotype 7 | cap gene (cap 8) | cap gene (cap 5) | Hemolysins | Leukocidin (luk) Components | Biofilm-Associated Genes | Adhesion Factors | Superantigens | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype 4 | MIC 5 of Streptomycin | Genes Detected | ||||||||||||||
| 1FR * | M 1 | ST97 | t1236 | PEN | 32 6 | blaZ, str | not tested | NEG 8 | POS 8 | hla, hlb, hld | NEG | icaC, icaD | clfA, fib, fnbA, fnbB, sasG | |||
| 6RR * | M 2 | CC152 | ST152 | t458 | ERY, CLI | 32 | erm(C), str | CP5 | NEG | POS | hla, hlb, hld | lukS-PV/lukF-PV | icaA, icaD | clfA, clfB, cna, fnbA, fnbB | ||
| 11RR * | M 3 | ST97 | t1236 | PEN | ‹4 | blaZ, str | smr | nt | NEG | POS | hla, hlb, hld | NEG | icaC, icaD | clfA, fib, fnbA, fnbB, sasG | ||
| 24RR * | M 5 | CC3666 | ST5477 | t1236 | PEN, TET | 32 | blaZ, tet(K), tet(L), str | nt | POS | NEG | hla, hld | lukD | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | tsst-1, sei, sem, sen, seo, seu | |
| 26FR | M 6 | t1236 | PEN | 32 | blaZ, str | not tested | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 26FL | M 6 | CC97 | t1236 | PEN | 16 | blaZ, str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 26RL2 | M 6 | nt7 | PEN | 32 | blaZ, str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 27FL | M 6 | t1236 | PEN | 32 | blaZ, str | nt | not tested | not tested | not tested | not tested | not tested | not tested | sec | |||
| 27RLw | M 6 | CC97 | t1236 | PEN | ‹4 | blaZ, str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 27RR | M 6 | t1398 | TET | 4 | tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 36RR | M 9 | CC97 | t1236 | PEN | 32 | blaZ, str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 37RR | M 9 | t9432 | PEN, TET | ‹4 | blaZ, tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 39FR | M 10 | CC97 | t2112 | PEN | 8 | blaZ, str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 40FL | M 10 | CC97 | t1236 | PEN, TET | 32 | blaZ, tet(K), str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 43RL | M 11 | CC97 | t18835 | PEN, TET | 32 | blaZ, tet(K), str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 44RR | M 11 | CC3591 | t458 | ‹4 | str | smr | CP8 | POS | NEG | hla, hlb, hld | lukM/lukF-PV (P83) | icaA, icaC, icaD | clfA, clfB, fib, cna, fnbA | |||
| 63FL | K | CC152 | ST152 | t355 | ERY, CLI | 32 | erm(C), str | not tested | NEG | POS | hla, hlb, hld | lukS-PV/lukF-PV | icaA, icaD | clfA, clfB, cna, fnbA, fnbB | ||
| 71FL | M 14 | CC3591 | ST5475 | t355 | TET | 32 | tet(K), str | nt | POS | NEG | hla, hlb, hld | NEG | icaA, icaC, icaD | clfA, clfB, fib, cna, fnbA | sem, seo | |
| 74FL | M 14 | t1236 | PEN, TET | 32 | blaZ, tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 75FR | M 15 | CC97 | t10103 | PEN, TET | 32 | blaZ, tet(K), str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 75FL | M 15 | t1236 | PEN, TET | 32 | blaZ, tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 76RR | M 16 | CC3591 | ST5476 | t458 | ‹4 | str | CP8 | POS | NEG | hla, hlb, hld | lukM/lukF-PV (P83) | icaA, icaC, icaD | clfA, clfB, fib, cna, fnbA | |||
| 77RR | M 17 | CC3591 | t458 | 16 | str | CP8 | POS | NEG | hla, hlb, hld | lukM/lukF-PV (P83) | icaA, icaC, icaD | clfA, clfB, fib, cna, fnbA | ||||
| 78FL | M 17 | CC97 | t1236 | PEN, TET | 32 | blaZ, tet(K), str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 80RL | M 18 | t380 | PEN, TET | 32 | blaZ, tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | sec | |||
| 82FL | M 18 | t380 | PEN, TET | ‹4 | blaZ, tet(K), str | not tested | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 83RL | M 18 | CC97 | t380 | PEN | 32 | blaZ, str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 84RL | M 18 | t380 | PEN, TET | 32 | blaZ, tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 85RR | M 19 | CC97 | t1236 | PEN, TET | 32 | blaZ, tet(K), str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 85RL | M 19 | t10103 | PEN, TET | 32 | blaZ, tet(K), str | qacAB | nt | not tested | not tested | not tested | not tested | not tested | not tested | |||
| 86FL | M 19 | t1236 | PEN, TET | 32 | blaZ, tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 87FR | M 19 | t10103 | PEN, TET | 32 | blaZ, tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 87RL | M 19 | t1236 | PEN, TET | 32 | blaZ, tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 90FR | M 20 | t9432 | PEN, TET | 32 | blaZ, tet(K), str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 90FL | M 20 | t9432 | PEN, TET | 32 | blaZ, tet(K), str | not tested | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 91FL | M 20 | CC97 | t9432 | PEN | 32 | blaZ, str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 100RR | M 22 | CC97 | t1236 | PEN, TET | ‹4 | blaZ, tet(K), str | qacAB | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | ||
| 100RL | M 22 | CC97 | t1236 | PEN, TET | ‹4 | blaZ, tet(K), str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 101RR | M 22 | CC97 | t10103 | PEN | 32 | blaZ, str | nt | NEG | POS | hla, hlb, hld | lukD, lukE | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | |||
| 101RL | M 22 | t10103 | PEN | 32 | blaZ, str | nt | not tested | not tested | not tested | not tested | not tested | not tested | ||||
| 103FR | M 22 | CC3666 | t18853 | PEN, TET | 32 | blaZ, tet(K), str | smr | nt | POS | NEG | hla, hlb, hld | lukD | icaA, icaC, icaD | clfA, clfB, fib, fnbA, fnbB, sasG | tsst-1, sei, sem, sen, seo, seu | |
| 104FR | M 23 | t1236 | PEN, TET | 32 | blaZ, tet(K), str | smr | nt | not tested | not tested | not tested | not tested | not tested | not tested | |||
| 106FL2 | M 24 | t18835 | PEN, TET | 32 | blaZ, tet(K), str | qacAB | nt | not tested | not tested | not tested | not tested | not tested | not tested | |||
1 Origin: M = Musanze Farm, K = Kigali Farm. 2 clonal complex. 3 sequence type. 4 Phenotype: PEN = penicillin; CIP = ciprofloxacin; CHL = chloramphenicol; CLI = clindamycin; ERY = erythromycin; SXT = trimethoprim-sulfamethoxazole; TET = tetracycline; FOX = cefoxitin, TEC = teicoplanin. 5 mg/L. 6 32 or higher (mg/L); 7 Capsule serotype: nt = non-typable; CP5 = Serotype 5; CP8 = Serotype 8. 8 NEG = negative, POS = positive. * analysed by whole-genome sequencing.
The detection of resistance genes confirmed the phenotypic resistance profiles of the respective isolates, detecting blaZ (n = 73, 45.3%), tet(K) (n = 45, 71.4%), both tet(K) and tet(L) (n = 17, 27.0%) and all three tet(K), tet(L) and tet(O) (n = 1, 1.6%). Clindamycin-resistant isolates carried the following resistance genes: erm(C) (n = 8, 34.8%), vga(A) (n = 2, 8.7%), erm(44) (n = 2, 8.7%), sal(A) (n = 2, 8.7%), both vga(A) and sal(A) (n = 2, 8.7%), both erm(C) and sal(A) (n = 1, 4.3%), both sal(A) and erm(44) (n = 1, 4.3%) and all three vga(A), sal(A) and lnu(A) (n = 2, 8.7%). In the erythromycin-resistant isolates, two macrolide resistance genes were present: erm(C) (n = 6), and msr(A) (n = 4), whereas the trimethoprim-sulfamethoxazole-resistant isolates carried both dfrA (also known as dfrS1) and dfrD genes (n = 1), both dfrD and dfrG genes (n = 3) and all three dfrA, dfrD and dfrG genes (n = 2). Two isolates were resistant to chloramphenicol, which was associated with the presence of fexA in a S. xylosus and catpC221 in a S. saprophyticus isolate. The streptomycin resistance gene str was detected in all 161 isolates, but its presence was not always associated with a higher MIC value (i.e., >8 mg/L) [14] (Table 1 and Table 2).
The mecA gene was detected in cefoxitin-resistant S. hominis and S. sciuri, whereas the mecC gene could not be identified. One dru type (dt10cz) was detected in a S. hominis isolate, but the other mecA-positive isolate was not dru-typeable.
None of the tested isolates carried the genes erm(A), erm(B), erm(F), erm(T), erm(43), erm(33), Isa(B), vga(A)v vga(C), vga(E), vga(E)v, dfrK, tet(M), ant(6’)-la, cfr, catpC194, or catpC223.
2.2. Metal and Biocide Resistance Testing
Biocide resistance profiling revealed that 33 isolates carried the smr gene, most frequently the species S. haemolyticus (n = 7), S. epidermidis (n = 6), S. xylosus (n = 6) and S. aureus (n = 4). Seventeen isolates carried the qacAB gene, where the predominant species were S. haemolyticus (n = 4), S. epidermidis (n = 3), S. aureus (n = 3), S. xylosus (n = 2) and S. hominis (n = 2). Furthermore, the presence of the following metal resistance genes was confirmed: cadD (n = 25), copB (n = 27) and arsA (n = 21). The most prevalent species, which carried the cadD gene, was S. haemolyticus (n = 8), followed by S. xylosus (n = 5) and S. epidermidis (n = 4). A significant carriage rate of copB was shown by S. saprophyticus (n = 7) and S. xylosus (n = 7). The arsA gene was mostly detected in the species S. haemolyticus (n = 6), S. epidermidis (n = 4) and S. saprophyticus (n = 3). However, none of the isolates carried the czrC gene (Table 1 and Table 2) and all S. aureus isolates were negative for metal resistance genes.
2.3. Additional Characterization of S. aureus Isolates
Among S. aureus, the lukS-PV and lukF-PV genes coding for the Panton–Valentine leukocidin (PVL) were detected in two isolates, the bovine leukocidin gene lukM/lukF-P83 was present in three isolates. The tsst-1 gene was detected in two isolates and was solely found in combination with enterotoxin genes. The enterotoxin genes sei (n = 2), sem (n = 3), sen (n = 2), seo (n = 3) and seu (n = 2), that belonged to the egc cluster, and sec (n = 2) were detected. Staphylococcal enterotoxin genes sea, seb, sed, see, seg, seh, sej, sek, sel, seq, ser and the gene for the enterotoxin like protein CM14 could not be detected in the S. aureus isolates (Table 2).
Ten different spa types were identified among the tested isolates. The spa type t1236 (n = 18) was predominant, followed by t10103 (n = 5), t380 (n = 4) and t9432 (n = 4), t458 (n = 4), t355 (n = 2) and singletons t2112 and t1398. Two new spa types were detected: t18835 (n = 2, repeat order 26-23-34-34-34-34-33-34) and t18853 (n = 1, repeat order 04-20-17-24-17).
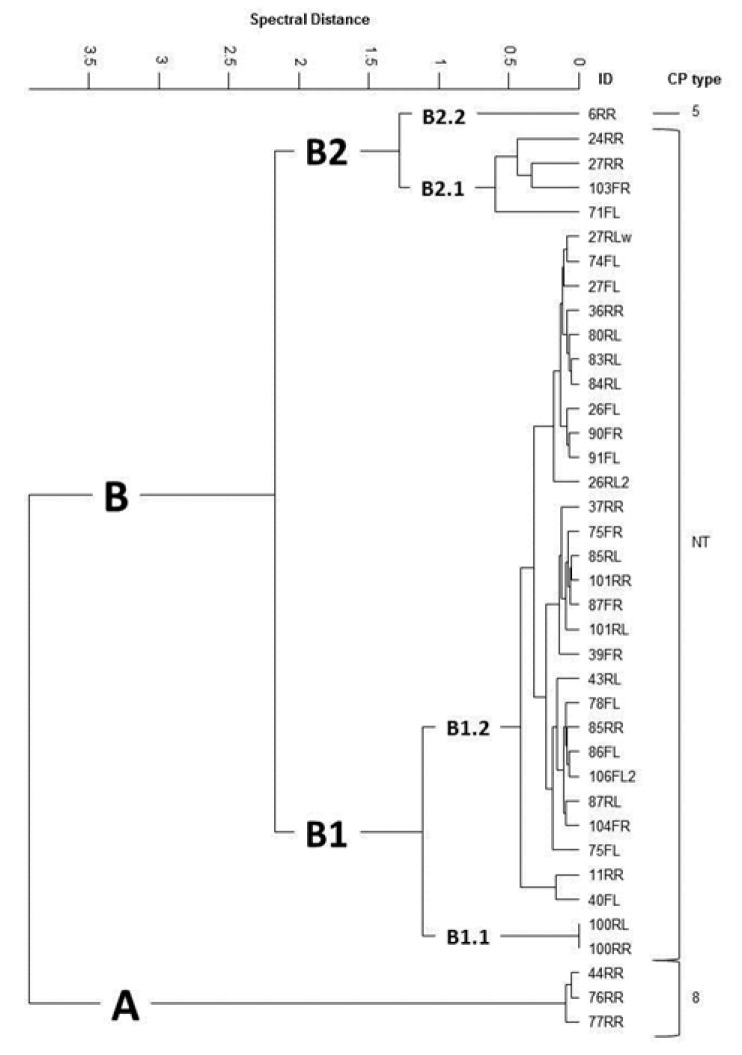
FTIR-based capsule serotyping revealed a high prevalence of non-encapsulated S. aureus isolates (n = 34; 89.5%) and the remaining isolates produced a capsule of either serotype 8 (CP8, n = 3) or 5 (CP5, n = 1). Hierarchical cluster analysis of spectral FTIR data grouped the S. aureus isolates into two main clusters (A; n = 3 and B; n = 35; Figure 1). Cluster A could be assigned to CP 8 while non-typeable (NT) isolates were grouped into the main cluster B, except one isolate assigned to CP5 (B2.2). All NT isolates were found to harbour either the cap8- (B2.1, n = 4) or cap5-specific allele (B1.1, n = 2 and B1.2, n = 28). No association between the origin of the samples and the FTIR cluster alignment was detectable.
Figure 1.
FTIR spectroscopy-based cluster of S. aureus isolated from quarter milk samples of cows with mastitis. CP = capsule type; NT = none typeable.
Among the selected S. aureus isolates examined using DNA microarray and whole-genome sequencing, different resistance genes (blaZ, erm(C), tet(K)) and virulence genes (hla, hlb, hld, lukD, lukE, lukM, lukF-P83, icaA, icaC, icaD, bap, clfA, clfB, fib, can, fnbA, fnbB, sasG) could be found (Table 2). Four different clonal complexes (CC) were identified. Here, the CC97 isolates (n = 14) clustered into FTIR cluster B1, the CC3591 isolates (n = 4) into clusters A and B2.1, the CC3666 isolates (n = 2) into cluster B2.1 and one isolate of CC152 into cluster B2.2. Three S. aureus that were selected for MLST revealed the new sequence types ST5475 (199-805-44-430-447-192-733), ST5476 (199-806-741-2-447-192-734) and ST5477 (6-55-45-2-109-14-741).
3. Discussion
Clinical and subclinical mastitis can be one of the serious consequences of poor milking hygiene [5,7]. Previous studies have shown that the prevalence of mastitis within the East African region is high and that CoNS are common pathogens in bovine mastitis [5,15,16,17]. This finding was also confirmed in this study.
In the present study, S. aureus was the predominant Staphylococcus spp., which is in accordance with studies from other countries in that region, such as Tanzania, and Kenya [16,18]. Another study from Uganda showed that the predominant Staphylococcus spp. were from the CoNS group, but they were not further characterized to the species level [15]. Among CoNS, S. chromogenes, S. haemolyticus, S. epidermidis, S. simulans and S. xylosus are usually the most common isolated species associated with bovine mastitis [19,20]. However, distribution of CoNS species has shown to be herd-specific and influenced by different management practices that can vary between countries [1,20].
Penicillin resistance is probably the best known antimicrobial resistance property of S. aureus and its frequency in the current study is in accordance with other studies that examined antibiotic susceptibility patterns of staphylococci isolated from cases of bovine mastitis in other parts of Africa as well as in Germany and Finland [16,21,22,23,24]. Penicillin is a routinely used antimicrobial agent for the prevention and treatment of mastitis in dairy cows in Rwanda [9] and the blaZ gene was present in all 73 penicillin-resistant Staphylococcus spp. isolates (100%) in the current study. This gene encodes a narrow-spectrum β-lactamase which confers penicillin resistance [10,25].
Tetracycline belongs to the broad-spectrum antimicrobial agents and is also an often-used antimicrobial agent in farm animals in Rwanda [9]. Resistance to tetracyclines is frequently mediated by the genes tet(K) and tet(L), which code for active efflux mechanisms, and occasionally by tet(M) and tet(O), which encode ribosome-protective proteins [10]. In the present study, tet(K) was found in all tetracycline-resistant staphylococci (100%), followed by tet(L) (28.6%) and tet(O) (1.6%), while tet(M) was not detected in any of the tetracycline-resistant isolates. In a study from Tunisia, 10.3% of the staphylococcal isolates (n = 68) showed resistance to tetracycline and this resistance was always encoded by the tet(K) gene [26]. In another study from Germany, the tet(M), tet(K) and tet(L) genes were investigated among resistant S. aureus sisolates, originating from cases of bovine clinical mastitis (n = 25) and from farm personnel (n = 2), and tet(M) was found in 100%, tet(K) in 92.6% and tet(L) in 40.7% of the isolates [23].
Two S. haemolyticus and one S. xylosus isolate exhibited phenotypic resistance to clindamycin although a corresponding resistance gene was not detected. Whole genome sequencing of these isolates in a future study will hopefully clarify the genetic basis for the observed lincosamide resistance. Another problem detected in this study was the phenotypic assessment of streptomycin resistance. All isolates carried the resistance gene str, but MICs to streptomycin varied between ≤4 and 32 mg/L. Neither CLSI, nor EUCAST provide clinical breakpoints for streptomycin and staphylococci. The sequenced str amplicons obtained from staphylococcal isolates with low streptomycin MICs as well as from those with high streptomycin MICs did not differ in their sequences (author’s own observation). Again, whole genome approaches may help to clarify the situation.
Quaternary ammonium compounds (QACs)-based antiseptics are frequently used worldwide and this prevailing usage can lead to bacterial resistance against these substances [27,28]. In the current study, the antiseptic resistance genes qacAB and smr were examined. The smr gene was found more frequently than the qacAB genes. These results were similar to those of a study from Norway assessing the resistance to QACs in bacteria from milk samples obtained from 127 dairy cattle herds and 70 goat herds, where the smr gene was present in 64.2% and the qacAB gene in 28.5% of the isolates (n = 42) [28]. Studies about the bacterial resistance to QACs in staphylococci originating from bovine milk in Africa are scarce. One study from three African countries (Angola, São Tomé and Príncipe, Cape Verde), where a total of 301 S. aureus isolates were investigated, reported an intermediate prevalence for the qacAB gene (40.5%) and a low prevalence for the smr gene (3.7%) [29].
Many other substances with antimicrobial effects, including metal-containing compounds, are used in food-animal production, where they can contribute to the selection of isolates among staphylococcal species [30]. According to a study from 2017 on cattle production in East Rwanda, only 3.6% (n = 13) of the farmers practiced supplementary feeding [2]. However, in the present study, conducted in Northern parts of Rwanda and Kigali, 51 (31.5%) of the bacterial isolates carried at least one heavy metal resistance gene. Heavy metal resistance genes occurred most frequently in S. haemolyticus (n = 12) followed by S. xylosus (n = 11) and S. saprophyticus (n = 8). In another study, S. haemolyticus and S. epidermidis carried the most heavy metal resistance genes [31], but the isolates in the current study did not show a high rate of heavy metal resistance genes, which is possibly explained by the different geographical collection sites.
The vast majority of the collected S. aureus mastitis isolates in this study were non-encapsulated as shown by spectroscopic capsule serotyping. This is in concordance with several previous reports showing a high prevalence of non-encapsulated mastitis isolates in Argentina, USA and Austria [32,33,34]. Moreover, non-encapsulation was associated with high within-herd prevalence of S. aureus-based persistent, contagious bovine intramammary infections [35]. Indeed, this study provides further evidence that loss of capsule expression is a key phenotypic feature associated with bovine mastitis, a primarily chronic infection [36]. Out of the 38 FTIR-typed isolates, 22 were selected for clonal complex (CCs) identification using DNA microarray-based technology and three of them (two CC3591 and one CC3666) were genotyped by MLST. The four CCs (CC97, CC3591, CC3666, CC152) identified were relatively distinctive for one of the FTIR clusters, also seen by Kümmel et al. in 2016 [34], though no connection to one particular farm could be found. Most isolates were assigned to the common bovine lineage CC97, indicating predominance of this cattle-adapted clone, which has already been reported from bovine mastitis cases worldwide including Europe, Japan, Algeria, and South Africa [37,38,39,40].
The most predominant spa type among S. aureus in the present study was t1236. This is a spa type within ST97 and associated with CC97 along with the other spa types t2112, t380, and t10103, commonly found among S. aureus from neighbouring Uganda [41]. The spa type t1236 has also been detected among S. aureus from bovine milk in Japan, reported as ST97 [38]. The spa type t458, which was found in four isolates in the current study, has been detected in S. aureus from a case of bovine mastitis in China [42] and from bovine milk in Japan [38]. Many African studies (Democratic Republic of the Congo, Gabon, Ghana, Kenya, Nigeria and Uganda) reported the presence of spa type t355 in S. aureus from humans [43,44,45,46,47,48], which was also identified in three isolates in the current study.
Five S. aureus isolates carried PVL genes, which is of interest due to the common association with soft tissue and skin infections and the reported human to cow transmission of S. aureus [49,50]. The PVL genes code for proteins which are responsible for cytotoxic activity, especially leukocytes are affected [51]. The lukS-PV and lukF-PV genes (PVL genes) were mainly detected in S. aureus of human origin [52], but have also been reported in isolates from bovine mastitis cases in Africa suggesting human to cow transmission of the respective isolates [41,50]. These human-associated genes were also detected in two S. aureus ST152 isolates obtained from two cows kept in two different farms in this study (Table 2). The LukM/LukF-PV(P83) protein only kills bovine neutrophils and is common in S. aureus isolated from bovine mastitis [51,52]. In a study from North-Western Ethiopia, however, this bovine-related leukocidin was detected in a low percentage (4%) and the isolates did not belong to the common ST97 [50]. This was in line with the results of the current study where this gene was only present in three of the further selected S. aureus isolates, which belonged to ST5476 and to CC3591. Previous reports demonstrated that isolates belonging to ST97 may also be negative for the bovine-related leukocidin [38,53].
In the present study, the tsst1 gene was detected in two isolates and further classified as bovine variant of tsst1 which has been described in previous studies dealing with S. aureus associated with bovine mastitis [39,50,51,52,53,54,55].
4. Materials and Methods
4.1. Isolation and Identification of Staphylococci
Isolation of Staphylococcus spp. was conducted from July to August 2018 from CMT-positive milk samples originating from 112 crossbred dairy cows kept on 28 farms in the Northern Province and the Kigali District of Rwanda. Farms were selected for sampling based on farmers’ reports on decreased milk production of multiple cows. Before sampling, a short clinical check was performed on each selected cow, including palpation of the udder, examination of the milk and measuring the body temperature. Afterwards, CMT was performed, which can indicate the presence of mastitis [4]. Collected milk samples were transported to the microbiological laboratory of NVVH, and bacteriological analyses were performed. Milk samples were cultivated on blood agar (Blood Agar Base, Rapid Labs, UK) supplemented with 5% of defibrinated sheep blood. After incubation at 37 °C for 24 h, each colony representing a distinct colony morphotype, but showing typical staphylococcal colony appearance, was regrown on the same medium. Pure staphylococcal cultures were stored at 4 °C until they were transported to the diagnostic laboratory of the Institute of Microbiology at the University of Veterinary Medicine, Vienna for further examination. All isolates were regrown on BD Columbia III agar plates with 5% Sheep Blood (Becton Dickinson, Heidelberg, Germany), and identified by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonik, Bremen, Germany). If MALDI-TOF MS yielded ambiguous results, rpoB gene sequencing was performed [56].
4.2. Antimicrobial Susceptibility Testing and Detection of Resistance Genes and SCCmec-Associated Direct Repeat Unit (dru) Typing
Antimicrobial susceptibility testing was performed by agar disk diffusion according to CLSI standards (CLSI, 2018) for the following antimicrobial agents (μg/disk): tetracycline (30), ciprofloxacin (5), erythromycin (15), clindamycin (2), penicillin (10 IU), cefoxitin (30), chloramphenicol (30), gentamicin (10), rifampicin (5), linezolid (30), and trimethoprim-sulfamethoxazole (1.25 + 23.75). In addition, minimum inhibitory concentrations (MICs) of streptomycin were established by the agar dilution method on Mueller–Hinton agar in serial twofold dilutions (4, 8, 16, and 32 μg/mL) in accordance with the CLSI document M7-A9 (CLSI, 2012).
Staphylococcal DNA was extracted as described previously [57]. PCR was used to detect the presence of the following antibiotic resistance genes: blaZ (confers resistance to penicillins except isoxazolyl-penicillins) [25]; mecA, mecC (confer resistance to all penicillins and cephalosporins approved for veterinary use) [58]; erm(A), erm(B), erm(C), erm(F), erm(T), erm(33), erm(43), and erm(44) (confer resistance to macrolides, lincosamides, and streptogramin B), vga(A), vga(A)v, vga(C), vga(E), vga(E)v and sal(A) (confer resistance to streptogramin A, lincosamides and pleuromutilins); Isa(B) and Inu(A) (confer elevated MICs or resistance to lincosamides) [23,59,60,61,62,63,64,65,66,67,68]; msr(A) (confers resistance to macrolides and streptogramin B) [57]; cfr (confers resistance to all phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A) [69]; fexA (confers resistance to all phenicols) [69]; catpC194, catpC221, and catpC223 (confer resistance to non-fluorinated phenicols, e.g. chloramphenicol) [70]; ant(6′)-Ia and str (confer resistance to the aminoglycoside streptomycin) [14]; dfrA, dfrD, dfrG, and dfrK (confer resistance to trimethoprim) [57,71]; tet(K) and tet(L) (confer resistance to tetracyclines except minocycline and glycylcyclines) [57]; tet(O) and tet(M) (confer resistance to tetracyclines, including minocycline, but excluding glycylcyclines) [72].
PCRs targeting qacAB (confers high-level resistance to antiseptics) and smr (confers low-level resistance to antiseptics) genes were performed as previously described [27]. Furthermore, PCRs were performed for detecting the presence of the following heavy metal resistance genes: cadD, copB, arsA and czrC [30,31].
The mecA-positive isolates were further examined by SCCmec-associated direct repeat unit (dru) typing [73].
4.3. Additional Characterization of S. aureus Isolates
All S. aureus isolates were examined by different PCRs targeting Panton–Valentine Leukocidin (PVL) genes, staphylococcal enterotoxins (SE), and the toxic shock syndrome toxin 1 (TSST1) as previously described [58]. Furthermore, S. aureus were genotyped by spa typing [57].
Using Fourier Transform Infrared (FTIR) spectroscopy, all isolates were further phenotypically subtyped based on their surface glyco structural composition that included the determination of the capsular polysaccharide (CP) expression [74,75]. On FTIR based clustering, 22 S. aureus isolates were selected and further analysed using DNA microarray-based technology to detect over 300 different target sequences including antimicrobial resistance and virulence-associated genes, species-specific genes, and SCCmec-associated genes [76]. Three isolates were genotyped using MLST as previously described [57]. In addition, whole-genome sequencing, as well as contig assembly and annotation, and comparative genomics were conducted as previously described using Seqsphere+ (Ridom, Münster, Germany) [77,78,79]. The same software was used for cgMLST [77]. The genomes of four S. aureus isolates were submitted under SUB6695668 in the NCBI BioProject database.
5. Conclusions
The present study is the first investigating not only the phenotypic but also the genotypic resistance to antimicrobial agents and biocides in Staphylococcus spp. isolated from cases of bovine mastitis in Rwanda. It improves our knowledge about the high diversity of Staphylococcus spp., their occurrence in the study area and about the presence of resistance genes.
Due to the rising importance of the dairy production system in Rwanda, improvements in the prevention and treatment of bovine mastitis are critical to prevent misuse of antimicrobial agents and the increase of resistance to antimicrobial agents and biocides, which is in accordance with the ‘one world, one health’ principle [80].
Acknowledgments
We are grateful to Barbara Tischler for technical assistance.
Author Contributions
Conceptualization, H.K., O.F., J.S. and I.L.; methodology, M.E.-S., A.T.F., S.S., S.M., R.E., T.G. and I.L.; validation, A.T.F., S.S. and I.L.; formal analysis, F.I.A., R.M., S.L., W.R., A.T.F., S.S., T.G. and I.L.; investigation, F.I.A., R.M., J.C.M., V.N., S.L., W.R., T.G., J.S. and I.L.; resources, O.F., M.E.-S. and J.S.; data curation, H.M., S.M., R.E. and I.L.; writing—original draft preparation, F.I.A., R.M., T.G. and I.L.; writing—review and editing, W.R., M.E.-S., A.T.F., S.S., S.M., R.E., R.E., T.G., J.S. and I.L.; visualization, I.L.; supervision, M.E.S., J.S. and I.L.; project administration, H.K., O.F. and I.L.; funding acquisition, T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Austrian Science Fund, FWF-P29304-B22 (TG).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bogni C., Odierno L., Raspanti C., Giraudo J., Larriestra A., Reinoso E., Lasagno M., Ferrari M., Ducrós E., Frigerio C., et al. War against mastitis: Current concepts on controlling bovine mastitis pathogens. In: Mendez-Vilas A., editor. Science against Microbial Pathogens: Communicafing Current Research and Technological Advances. World Scientific; Singapore: 2011. pp. 483–494. [Google Scholar]
- 2.Mazimpaka E., Mbuza F., Michael T., Gatari E.N., Bukenya E.M., James O.A. Current status of cattle production system in Nyagatare District-Rwanda. Trop. Anim. Health Prod. 2017;49:1645–1656. doi: 10.1007/s11250-017-1372-y. [DOI] [PubMed] [Google Scholar]
- 3.Laenderdaten Info. [(accessed on 11 October 2019)]; Available online: https://www.laenderdaten.info/Afrika/Ruanda/bevoelkerungswachstum.php.
- 4.Sargeant J.M., Leslie K.E., Shirley J.E., Pulkrabek B.J., Lim G.H. Sensitivity and specificity of somatic cell count and California Mastitis Test for identifying intramammary infection in early lactation. J. Dairy Sci. 2010;84:2018–2024. doi: 10.3168/jds.S0022-0302(01)74645-0. [DOI] [PubMed] [Google Scholar]
- 5.Mpatswenumugabo J.P., Bebora L.C., Gitao G.C., Mobegi V.A., Iraguha B., Kamana O., Shumbusho B. Prevalence of subclinical mastitis and distribution of pathogens in dairy farms of Rubavu and Nyabihu Districts, Rwanda. J. Vet. Med. 2017:1–8. doi: 10.1155/2017/8456713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervinkova D., Vlkova H., Borodacova I., Makovcova J., Babak V., Lorencova A., Vrtkova I., Marosevic D., Jaglic Z. Prevalence of mastitis pathogens in milk from clinically healthy cows. Vet. Med. 2013;58:567–575. doi: 10.17221/7138-VETMED. [DOI] [Google Scholar]
- 7.Iraguha B., Hamudikuwanda H., Mushonga B. Bovine mastitis prevalence and associated risk factors in dairy cows in Nyagatare District, Rwanda. J. S. Afr. Vet. Assoc. 2015;86:1–6. doi: 10.4102/jsava.v86i1.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaarst M., Enevoldsen C. Patterns of clinical mastitis manifestations in Danish organic dairy herds. J. Dairy Res. 1997;64:23–37. doi: 10.1017/S002202999600194X. [DOI] [PubMed] [Google Scholar]
- 9.Manishimwe R., Nishimwe K., Ojok L. Assessment of antibiotic use in farm animals in Rwanda. Trop. Anim. Health Prod. 2017;49:1101–1106. doi: 10.1007/s11250-017-1290-z. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz S., Feßler A.T., Loncaric I., Wu C., Kadlec K., Wang Y., Shen J. Antimicrobial resistance among staphylococci of animal origin. Microbiol. Spectr. 2018;6:1–29. doi: 10.1128/microbiolspec.ARBA-0010-2017. [DOI] [PubMed] [Google Scholar]
- 11.Ntirenganya C., Manzi O., Muvunyi C.M., Ogbuagu O. High prevalence of antimicrobial resistance among common bacterial isolates in a tertiary healthcare facility in Rwanda. Am. J. Trop. Med. Hyg. 2015;92:865–870. doi: 10.4269/ajtmh.14-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iraguha B., Hamudikuwanda H., Mushonga B., Kandiwa E., Mpatswenumugabo J.P. Comparison of cow-side diagnostic tests for subclinical mastitis of dairy cows in Musanze district, Rwanda. J. S. Afr. Vet. Assoc. 2017;88:1–6. doi: 10.4102/jsava.v88i0.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ndahetuye J.B., Persson Y., Nyman A.K., Tukei M., Ongol M.P., Båge R. Aetiology and prevalence of subclinical mastitis in dairy herds in peri-urban areas of Kigali in Rwanda. Trop. Anim. Health Prod. 2019:1–8. doi: 10.1007/s11250-019-01905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hauschild T., Vuković D., Dakić I., Ježek P., Djukić S., Dimitrijević V., Stepanović S., Schwarz S. Aminoglycoside resistance in members of the Staphylococcus sciuri group. Microb. Drug Resist. 2007;13:77–84. doi: 10.1089/mdr.2007.713. [DOI] [PubMed] [Google Scholar]
- 15.Abrahmsén M., Persson Y., Kanyima B.M., Båge R. Prevalence of subclinical mastitis in dairy farms in urban and peri-urban areas of Kampala, Uganda. Trop. Anim. Health Prod. 2014;46:99–105. doi: 10.1007/s11250-013-0455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suleiman T.S., Karimuribo E.D., Mdegela R.H. Prevalence of bovine subclinical mastitis and antibiotic susceptibility patterns of major mastitis pathogens isolated in Unguja island of Zanzibar, Tanzania. Trop. Anim. Health Prod. 2018;50:259–266. doi: 10.1007/s11250-017-1424-3. [DOI] [PubMed] [Google Scholar]
- 17.Pyörälä S., Taponen S. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet. Microbiol. 2009;134:3–8. doi: 10.1016/j.vetmic.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Gitau G.K., Bundi R.M., Vanleeuwen J., Mulei C.M. Mastitogenic bacteria isolated from dairy cows in Kenya and their antimicrobial sensitivity. J. S. Afr. Vet. Assoc. 2014;85:1–8. doi: 10.4102/jsava.v85i1.950. [DOI] [PubMed] [Google Scholar]
- 19.Vanderhaeghen W., Piepers S., Leroy F., Van Coillie E., Haesebrouck F., De Vliegher S. Identification, typing, ecology and epidemiology of coagulase negative staphylococci associated with ruminants. Vet. J. 2015;203:44–51. doi: 10.1016/j.tvjl.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Thorberg B.-M., Danielsson-Tham M.-L., Emanuelson U., Persson Waller K. Bovine subclinical mastitis caused by different types of coagulase-negative staphylococci. J. Dairy Sci. 2009;92:4962–4970. doi: 10.3168/jds.2009-2184. [DOI] [PubMed] [Google Scholar]
- 21.Kasozi K.I., Tingiira J.B., Vudriko P. High prevalence of subclinical mastitis and multidrug resistant Staphylococcus aureus are a threat to dairy cattle production in Kiboga District (Uganda) Open J. Vet. Med. 2014;4:35. doi: 10.4236/ojvm.2014.44005. [DOI] [Google Scholar]
- 22.Issa Ibrahim A., Duprez J.N., Bada-Alambedji R., Moula N., Mainil J., Bardiau M. Antibiotic resistance trend of Staphylococcus aureus isolated between 2010 and 2012 from mastitis cases in Azawak Zebu in Niger. Afr. J. Microbiol. Res. 2014;8:3271–3275. [Google Scholar]
- 23.Feßler A., Scott C., Kadlec K., Ehricht R., Monecke S., Schwarz S. Characterization of methicillin-resistant Staphylococcus aureus ST398 from cases of bovine mastitis. J. Antimicrob. Chemother. 2010;65:619–625. doi: 10.1093/jac/dkq021. [DOI] [PubMed] [Google Scholar]
- 24.Pitkälä A., Haveri M., Pyörälä S., Myllys V., Honkanen-Buzalski T. Bovine mastitis in Finland 2001—prevalence, distribution of bacteria, and antimicrobial resistance. J. Dairy Sci. 2010;87:2433–2441. doi: 10.3168/jds.S0022-0302(04)73366-4. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira A.M., Martins K.B., Silva V.R.D., Mondelli A.L., Cunha M.D.L.R.D. Correlation of phenotypic tests with the presence of the blaZ gene for detection of beta-lactamase. Braz. J. Microbiol. 2017;48:159–166. doi: 10.1016/j.bjm.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klibi A., Maaroufi A., Torres C., Jouini A. Detection and characterization of methicillin-resistant and susceptible coagulase-negative staphylococci in milk from cows with clinical mastitis in Tunisia. Int. J. Antimicrob. Agents. 2018;52:930–935. doi: 10.1016/j.ijantimicag.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Sidhu M.S., Heir E., Leegaard T., Wiger K., Holck A. Frequency of disinfectant resistance genes and genetic linkage with β-lactamase transposon Tn552 among clinical staphylococci. Antimicrob. Agents Chemother. 2002;46:2797–2803. doi: 10.1128/AAC.46.9.2797-2803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjorland J., Steinum T., Kvitle B., Waage S., Sunde M., Heir E. Widespread distribution of disinfectant resistance genes among staphylococci of bovine and caprine origin in Norway. J. Clin. Microbiol. 2005;43:4363–4368. doi: 10.1128/JCM.43.9.4363-4368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conceição T., Coelho C., De Lencastre H., Aires-De-Sousa M. High prevalence of biocide resistance determinants in Staphylococcus aureus isolates from three African countries. Antimicrob. Agents Chemother. 2016;60:678–681. doi: 10.1128/AAC.02140-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Argudín M.A., Lauzat B., Kraushaar B., Alba P., Agerso Y., Cavaco L., Butaye P., Porrero M.C., Battisti A., Tenhagen B.A., et al. Heavy metal and disinfectant resistance genes among livestock-associated methicillin-resistant Staphylococcus aureus isolates. Vet. Microbiol. 2016;191:88–95. doi: 10.1016/j.vetmic.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Argudín M.A., Butaye P. Dissemination of metal resistance genes among animal methicillin-resistant coagulase-negative staphylococci. Res. Vet. Sci. 2016;105:192–194. doi: 10.1016/j.rvsc.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Guidry A., Fattom A., Patel A., O’Brien C. Prevalence of capsular serotypes among Staphylococcus aureus isolates from cows with mastitis in the United States. Vet. Microbiol. 1997;51:53–58. doi: 10.1016/S0378-1135(97)00172-7. [DOI] [PubMed] [Google Scholar]
- 33.Sordelli D.O., Buzzola F.R., Gomez M.I., Steele-Moore L., Berg D., Gentilini E., Catalano M., Reitz A.J., Tollersrud T., Denamiel G., et al. Capsule expression by bovine isolates of Staphylococcus aureus from Argentina: Genetic and epidemiologic analyses. J. Clin. Microbiol. 2000;38:846–850. doi: 10.1128/jcm.38.2.846-850.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kümmel J., Stessl B., Gonano M., Walcher G., Bereuter O., Fricker M., Grunert T., Wagner M., Ehling-Schulz M. Staphylococcus aureus entrance into the dairy chain: Tracking S. aureus from dairy cow to cheese. Front. Microbiol. 2016:1603. doi: 10.3389/fmicb.2016.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grunert T., Stessl B., Wolf F., Sordelli D.O., Buzzola F.R., Ehling-Schulz M. Distinct phenotypic traits of Staphylococcus aureus are associated with persistent, contagious bovine intramammary infections. Sci. Rep. 2018;8:15968. doi: 10.1038/s41598-018-34371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuchscherr L., Lffler B., Buzzola F.R., Sordelli D.O. Staphylococcus aureus adaptation to the host and persistence: Role of loss of capsular polysaccharide expression. Future Microbiol. 2010;5:1823–1832. doi: 10.2217/fmb.10.147. [DOI] [PubMed] [Google Scholar]
- 37.Boss R., Cosandey A., Luini M., Artursson K., Bardiau M., Breitenwieser F., Hehenberger E., Lam T., Mansfeld M., Michel A., et al. Bovine Staphylococcus aureus: Subtyping, evolution, and zoonotic transfer. J. Dairy Sci. 2016;99:512–528. doi: 10.3168/jds.2015-9589. [DOI] [PubMed] [Google Scholar]
- 38.Hata E., Katsuda K., Kobayashi H., Uchida I., Tanaka K., Eguchi M. Genetic variation among Staphylococcus aureus strains from bovine milk and their relevance to methicillin-resistant isolates from humans. J. Clin. Microbiol. 2010;48:2130–2139. doi: 10.1128/JCM.01940-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt T., Kock M.M., Ehlers M.M. Molecular characterization of Staphylococcus aureus isolated from bovine mastitis and close human contacts in South African dairy herds: Genetic diversity and inter-species host transmission. Front. Microbiol. 2017;8:511. doi: 10.3389/fmicb.2017.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akkou M., Bouchiat C., Antri K., Bes M., Tristan A., Dauwalder O., Martins-Simoes P., Rasigade J.P., Etienne J., Vandenesch F., et al. New host shift from human to cows within Staphylococcus aureus involved in bovine mastitis and nasal carriage of animal’s caretakers. Vet. Microbiol. 2018;223:173–180. doi: 10.1016/j.vetmic.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Asiimwe B.B., Baldan R., Trovato A., Cirillo D.M. Prevalence and molecular characteristics of Staphylococcus aureus, including methicillin resistant strains, isolated from bulk can milk and raw milk products in pastoral communities of South-West Uganda. BMC Infect. Dis. 2017;17:422. doi: 10.1186/s12879-017-2524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li T., Lu H., Wang X., Gao Q., Dai Y., Shang J., Li M. Molecular characteristics of Staphylococcus aureus causing bovine mastitis between 2014 and 2015. Front. Cell. Infect. Microbiol. 2017;7:127. doi: 10.3389/fcimb.2017.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandendriessche S., De Boeck H., Deplano A., Phoba M.F., Lunguya O., Falay D., Dauly N., Verhaegen J., Denis O., Jacobs J. Characterisation of Staphylococcus aureus isolates from bloodstream infections, Democratic Republic of the Congo. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:1163–1171. doi: 10.1007/s10096-017-2904-0. [DOI] [PubMed] [Google Scholar]
- 44.Schaumburg F., Ngoa U.A., Kösters K., Köck R., Adegnika A.A., Kremsner P.G., Lell B., Peters G., Mellmann A., Becker K. Virulence factors and genotypes of Staphylococcus aureus from infection and carriage in Gabon. Clin. Microbiol. Infect. 2011;17:1507–1513. doi: 10.1111/j.1469-0691.2011.03534.x. [DOI] [PubMed] [Google Scholar]
- 45.Egyir B., Guardabassi L., Nielsen S.S., Larsen J., Addo K.K., Newman M.J., Larsen A.R. Prevalence of nasal carriage and diversity of Staphylococcus aureus among inpatients and hospital staff at Korle Bu Teaching Hospital, Ghana. J. Glob. Antimicrob. Resist. 2013;1:189–193. doi: 10.1016/j.jgar.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Omuse G., Zyl K.N., Hoek K., Abdulgader S., Kariuki S., Whitelaw A., Revathi G. Molecular characterization of Staphylococcus aureus isolates from various healthcare institutions in Nairobi, Kenya: A cross sectional study. Ann. Clin. Microbiol. Antimicrob. 2016;15:51. doi: 10.1186/s12941-016-0171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shittu A.O., Oyedara O., Okon K., Raji A., Peters G., von Müller L., Schaumburg F., Herrmann M., Ruffing U. An assessment on DNA microarray and sequence-based methods for the characterization of methicillin-susceptible Staphylococcus aureus from Nigeria. Front. Microbiol. 2015;6:1160. doi: 10.3389/fmicb.2015.01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seni J., Bwanga F., Najjuka C.F., Makobore P., Okee M., Mshana S.E., Kidenya B.R., Joloba M.L., Kateete D.P. Molecular Characterization of Staphylococcus aureus from patients with surgical site infections at Mulago Hospital in Kampala, Uganda. PLoS ONE. 2013;8:e66153. doi: 10.1371/journal.pone.0066153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaumburg F., Alabi A.S., Peters G., Becker K. New epidemiology of Staphylococcus aureus infection in Africa. Clin. Microbiol. Infect. 2014;20:589–596. doi: 10.1111/1469-0691.12690. [DOI] [PubMed] [Google Scholar]
- 50.Mekonnen S.A., Lam T.J.G.M., Hoekstra J., Rutten V.P.M.G., Tessema T.S., Broens E.M., Riesebos A.E., Spaninks M.P., Koop G. Characterization of Staphylococcus aureus isolated from milk samples of dairy cows in small holder farms of North-Western Ethiopia. BMC Vet. Res. 2018;14:1–8. doi: 10.1186/s12917-018-1558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaneko J., Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: Structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 2004;68:981–1103. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- 52.Vrieling M., Boerhout E.M., van Wigcheren G.F., Koymans K.J., Mols-Vorstermans T.G., de Haas C.J.C., Aerts P.C., Daemen I.J.J.M., van Kessel K.P.M., Koets A.P., et al. LukMF′ is the major secreted leukocidin of bovine Staphylococcus aureus and is produced in vivo during bovine mastitis. Sci. Rep. 2016;6:37759. doi: 10.1038/srep37759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlotter K., Ehricht R., Hotzel H., Monecke S., Pfeffer M., Donat K. Leukocidin genes lukF-P83 and lukM are associated with Staphylococcus aureus clonal complexes 151, 479 and 133 isolated from bovine udder infections in Thuringia, Germany. Vet. Res. 2012;43:42. doi: 10.1186/1297-9716-43-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jans C., Merz A., Johler S., Younan M., Tanner S.A., Kaindi D.W.M., Wangoh J., Bonfoh B., Meile L., Tasara T. East and West African milk products are reservoirs for human and livestock-associated Staphylococcus aureus. Food Microbiol. 2017;65:64–73. doi: 10.1016/j.fm.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Snel G.G.M., Malvisi M., Pilla R., Piccinini R. Evaluation of biofilm formation using milk in a flow cell model and microarray characterization of Staphylococcus aureus strains from bovine mastitis. Vet. Microbiol. 2014;174:489–495. doi: 10.1016/j.vetmic.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 56.Mellmann A., Becker K., Von Eiff C., Keckevoet U., Schumann P., Harmsen D. Sequencing and staphylococci identification. Emerg. Infect. Dis. 2006;12:33. doi: 10.3201/eid1202.050962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loncaric I., Künzel F., Licka T., Simhofer H., Spergser J., Rosengarten R. Identification and characterization of methicillin-resistant Staphylococcus aureus (MRSA) from Austrian companion animals and horses. Vet. Microbiol. 2014;168:381–387. doi: 10.1016/j.vetmic.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt T., Kock M.M., Ehlers M.M. Diversity and antimicrobial susceptibility profiling of staphylococci isolated from bovine mastitis cases and close human contacts. J. Dairy Sci. 2015;98:6256–6269. doi: 10.3168/jds.2015-9715. [DOI] [PubMed] [Google Scholar]
- 59.Martineau F., Picard F.J., Lansac N., Ménard C., Roy P.H., Ouellette M., Bergeron M.G. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2000;44:231–238. doi: 10.1128/AAC.44.2.231-238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung W.O., Werckenthin C., Schwarz S., Roberts M.C. Host range of the ermF rRNA methylase gene in bacteria of human and animal origin. J. Antimicrob. Chemother. 1999;43:5–14. doi: 10.1093/jac/43.1.5. [DOI] [PubMed] [Google Scholar]
- 61.Liu X., Deng S., Huang J., Huang Y., Zhang Y., Yan Q., Wang Y., Li Y., Sun C., Jia X. Dissemination of macrolides, fusidic acid and mupirocin resistance among Staphylococcus aureus clinical isolates. Oncotarget. 2017;8:58086. doi: 10.18632/oncotarget.19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwendener S., Perreten V. New MLS B resistance gene erm(43) in Staphylococcus lentus. Antimicrob. Agents Chemother. 2012;56:4746–4752. doi: 10.1128/AAC.00627-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wipf J.R.K., Schwendener S., Perreten V. The novel macrolide-lincosamide-streptogramin B resistance gene erm(44) is associated with a prophage in Staphylococcus xylosus. Antimicrob. Agents Chemother. 2014;58:6133–6138. doi: 10.1128/AAC.02949-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wendlandt S., Kadlec K., Feßler A.T., Schwarz S. Identification of ABC transporter genes conferring combined pleuromutilin-lincosamide-streptogramin A resistance in bovine methicillin-resistant Staphylococcus aureus and coagulase-negative staphylococci. Vet. Microbiol. 2015;177:353–358. doi: 10.1016/j.vetmic.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 65.Li B., Wendlandt S., Yao J., Liu Y., Zhang Q., Shi Z., Wei J., Shao D., Schwarz S., Wang S., et al. Detection and new genetic environment of the pleuromutilin-lincosamide-streptogramin a resistance gene lsa(e) in methicillin resistant Staphylococcus aureus of swine origin. J. Antimicrob. Chemother. 2013;68:1251–1255. doi: 10.1093/jac/dkt015. [DOI] [PubMed] [Google Scholar]
- 66.Lina G., Quaglia A., Reverdy M.E., Leclercq R., Vandenesch F., Etienne J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 1999;43:1062–1066. doi: 10.1128/AAC.43.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li J., Li B., Wendlandt S., Schwarz S., Wang Y., Wu C., Ma Z., Shen J. Identification of a novel vga(E) gene variant that confers resistance to pleuromutilins, lincosamides and streptogramin A antibiotics in staphylococci of porcine origin. J. Antimicrob. Chemother. 2014;69:919–923. doi: 10.1093/jac/dkt482. [DOI] [PubMed] [Google Scholar]
- 68.Kehrenberg C., Ojo K.K., Schwarz S. Nucleotide sequence and organization of the multiresistance plasmid pSCFS1 from Staphylococcus sciuri. J. Antimicrob. Chemother. 2004;54:936–939. doi: 10.1093/jac/dkh457. [DOI] [PubMed] [Google Scholar]
- 69.Kehrenberg C., Schwarz S. Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol-resistant Staphylococcus isolates. Antimicrob. Agents Chemother. 2006;50:1156–1163. doi: 10.1128/AAC.50.4.1156-1163.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schnellmann C., Gerber V., Rossano A., Jaquier V., Panchaud Y., Doherr M.G., Thomann A., Straub R., Perreten V. Presence of new mecA and mph(C) variants conferring antibiotic resistance in Staphylococcus spp. isolated from the skin of horses before and after clinic admission. J. Clin. Microbiol. 2006;44:4444–4454. doi: 10.1128/JCM.00868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Argudín M.A., Tenhagen B.-A., Fetsch A., Sachsenröder J., Käsbohrer A., Schroeter A., Hammerl J.A., Hertwig S., Helmuth R., Bräunig J., et al. Virulence and resistance determinants of German Staphylococcus aureus ST398 isolates from nonhuman sources. Appl. Environ. Microbiol. 2011;77:3052–3060. doi: 10.1128/AEM.02260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwarz S., Roberts M.C., Werckenthin C., Pang Y., Lange C. Tetracycline resistance in Staphylococcus spp. from domestic animals. Vet. Microbiol. 1998;63:217–227. doi: 10.1016/S0378-1135(98)00234-X. [DOI] [PubMed] [Google Scholar]
- 73.Goering R.V., Morrison D., Al-doori Z., Edwards G.F.S., Gemmell C.G. Usefulness of mec-associated direct repeat unit (dru) typing in the epidemiological analysis of highly clonal methicillin-resistant Staphylococcus aureus in Scotland. Clin. Microbiol. Infect. 2008;14:964–969. doi: 10.1111/j.1469-0691.2008.02073.x. [DOI] [PubMed] [Google Scholar]
- 74.Grunert T., Wenning M., Barbagelata M.S., Fricker M., Sordelli D.O., Buzzola F.R., Ehling-Schulz M. Rapid and reliable identification of Staphylococcus aureus capsular serotypes by means of artificial neural network-assisted Fourier-transform infrared spectroscopy. J. Clin. Microbiol. 2013;51:2261–2266. doi: 10.1128/JCM.00581-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johler S., Stephan R., Althaus D., Ehling-Schulz M., Grunert T. High-resolution subtyping of Staphylococcus aureus strains by means of Fourier-transform infrared spectroscopy. Syst. Appl. Microbiol. 2016;39:189–194. doi: 10.1016/j.syapm.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Monecke S., Jatzwauk L., Weber S., Slickers P., Ehricht R. DNA microarray-based genotyping of methicillin-resistant Staphylococcus aureus strains from Eastern Saxony. Clin. Microbiol. Infect. 2008;14:534–545. doi: 10.1111/j.1469-0691.2008.01986.x. [DOI] [PubMed] [Google Scholar]
- 77.Leopold S.R., Goering R.V., Witten A., Harmsen D., Mellmann A. Bacterial whole-genome sequencing revisited: Portable, scalable, and standardized analysis for typing and detection of virulence and antibiotic resistance genes. J. Clin. Microbiol. 2014;52:2365–2370. doi: 10.1128/JCM.00262-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lepuschitz S., Mach R., Springer B., Allerberger F., Ruppitsch W. Draft genome sequence of a community-acquired methicillin-resistant Staphylococcus aureus USA300 isolate from a river sample. Genome Announc. 2017;5:e01166-17. doi: 10.1128/genomeA.01166-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lepuschitz S., Huhulescu S., Hyden P., Springer B., Rattei T., Allerberger F., Mach R.L., Ruppitsch W. Characterization of a community-acquired-MRSA USA300 isolate from a river sample in Austria and whole genome sequence based comparison to a diverse collection of USA300 isolates. Sci. Rep. 2018;8:9467. doi: 10.1038/s41598-018-27781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Helden P.D., Van Helden L.S., Hoal E.G. One world, one health: Humans, animals and the environment are inextricably linked—A fact that needs to be remembered and exploited in our modern approach to health. EMBO Rep. 2013;6:497–501. doi: 10.1038/embor.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]