Abstract
Background
To better understand the role of human rhinovirus‐associated wheeze as a risk factor for childhood recurrent wheezing, a cohort of young children experiencing their first wheezing episode was followed until school age.
Methods
All 111 hospitalized wheezing children (median age, 12 months) were initially participated in a randomized, double‐blind, placebo‐controlled, parallel trial on the efficacy of oral prednisolone. In this 7‐yr follow‐up, risk factors for recurrent wheezing were analysed, and then, the efficacy of prednisolone was evaluated overall and in pre‐specified subgroups post‐hoc. The main outcome was time to recurrent wheezing.
Results
The strongest independent risk factor for recurrent wheezing was rhinovirus detection (hazard ratio 3.54; 95% confidence interval 1.51–8.30) followed by sensitization (3.47; 1.55–8.30, respectively) age <1 yr (2.45; 1.29–4.65) and eczema (2.33; 1.11–4.90). Overall, prednisolone did not prevent recurrent wheezing. In subgroup analysis, prednisolone was associated with less recurrent wheezing in children affected by rhinovirus (0.32; 0.12–0.90, adjusted to sensitization, young age, viral aetiology and parental asthma) and/or with eczema (0.27; 0.08–0.87, adjusted respectively).
Conclusions
Our data strengthen the role of rhinovirus‐associated wheeze as an important risk factor for recurrent wheezing and asthma in young first‐time wheezing children. Prospective randomized trials on the efficacy of corticosteroids in rhinovirus‐associated early wheezing are warranted. (ClinicalTrials.gov number, NCT 00494624)
Keywords: bronchiolitis, corticosteroid, eczema, rhinovirus, wheezing
Short abstract


Abbreviations
- CI
confidence interval
- HR
hazard ratio
- HRV
human rhinovirus
- Ig
immunoglobulin
- OR
odds ratio
- PCR
polymerase chain reaction
- PIV
parainfluenza virus
- RSV
respiratory syncytial virus
Early identification of children with high risk of asthma is often based on atopic characteristics, especially aeroallergen sensitization, which however is rarely detectable from early infancy. Human rhinovirus (HRV)‐associated wheeze has been suggested as a new, significant and early risk factor for recurrent wheezing and later asthma 1, 2, 3. Of the long‐term follow‐up studies on HRV‐wheeze, only one study has focused on the first episode of lower airways infection 3, but it showed a positive link between HRV aetiology and recurrent wheezing in a 12‐month follow‐up. Moreover, susceptibility to more severe HRV infections has been linked to atopic characteristics and low interferon levels in vivo 4, 5, 6, 7, 8, 9 and epithelial damage in vitro 10.
The main treatment strategies of recurrent wheezing and exacerbations of asthma rely on inhaled and systemic corticosteroids, but these drugs have not been effective in early wheezing in most studies 11, 12. One problem has been the identification of high asthma risk children early. We have shown in a post‐hoc analysis of a controlled trial that oral prednisolone treatment of the first wheezing episode reduced recurrent wheezing in children with HRV aetiology and/or eczema in a 1‐yr follow‐up 13. Other efficacy studies have not investigated HRV aetiology. We have now completed a 7‐yr follow‐up of the same first‐time wheezing cohort. We aimed to evaluate the risk factors for recurrent wheezing and to assess the effect of prednisolone treatment on the development of recurrent wheezing overall and in pre‐specified subgroups according to viral aetiology and eczema status.
Methods
Study subjects
The Vinku study (vinku means wheeze in Finnish) was carried out in the Department of Paediatrics, Turku University Hospital (Turku, Finland) from September, 2000 to May, 2002 as previously described 13. Only those experiencing their first episode of wheezing and being aged <3 yr were included in the long‐term follow‐up (Fig. 1) 13. The study was approved by the Ethics Committee of the Turku University Hospital and was commenced only after obtaining written informed consent from the guardian.
Figure 1.
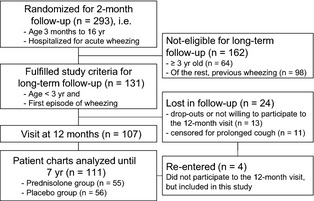
Study flow chart.
Study design
Originally, the Vinku study was a randomized double‐blind placebo‐controlled paralleled trial investigating the efficacy of oral prednisolone in wheezing children overall and seeking patient characteristics which could explain a beneficial response to the drug 13. Viral aetiology was our special interest 14, 15. Study physicians interviewed their parents before inclusion in the study. The first wheezing episode ever was determined based on parental interview and revision of medical records. On the ward nasopharyngeal aspirate sample was taken, blood sample was drawn, and then all children were randomized to receive either oral prednisolone (first dose 2 mg/kg, then 2 mg/kg/day in three divided doses for 3 days, maximum dose 60 mg/day, Prednisolon® 5 mg tablets, Leiras, Helsinki, Finland) or a placebo. No stratified randomization was carried out for eligible participants because the long‐term follow‐up protocol was implemented during the efficacy trial.
Long‐term follow‐up visit was arranged at 12 months 13 (Fig. 1) and thereafter the hospital records of the 111 study children were reviewed for wheezing episodes and medications for 7 yr.
Outcome
The primary outcome was time to recurrent wheezing ever. It was defined as the earliest date during the 7‐yr follow‐up period when a child fulfilled one or more of the following criteria: three physician‐confirmed episodes of wheezing within the past 12 months; continuous lower respiratory symptoms (cough, wheezing) lasting >4 wk and relieved by recurrent use of bronchodilators; or moderate‐to‐severe wheezing episodes necessitating systemic corticosteroid use within 6 months 16.
Definitions
Wheezing was defined as high‐pitched whistling sound in expiratory breathing 16. The children were divided to three virus groups. The HRV group included 34 children (30%) [monoinfection (n = 16) or virus co‐infection (n = 18 where six cases with respiratory syncytial virus (RSV) co‐infection)], the RSV group 44 children (40%) [monoinfection (n = 27) or co‐infection excluding HRV (n = 17)] and the HRV‐/RSV‐negative group 33 children (30%) [no virus (n = 6), monoinfection (n = 21) or co‐infection (n = 6)]. The same grouping was used in our previous report 13 and based on the earlier hypothesis that HRV is more significant risk factor for recurrent wheezing than RSV 1, 2.
Allergic sensitization was defined as positive immunoglobulin (Ig) E antibodies to any of common allergen as previously described (cut‐off level 0.35 kU/l, fluoro‐enzyme immunoassay, CAP FEIA, Phadiatop Combi®, Phadia, Uppsala, Sweden) 6, 13. A cut‐off value for increased eosinophil count was ≥0.40 × 109/l.
Eczema was a physician‐made diagnosis according to typical symptoms that included pruritus, typical morphology and chronicity of disease. Eczema was defined as atopic eczema if a child was sensitized to any allergen.
Virus and questionnaire data
The nasopharyngeal aspirates were analysed for 18 viruses: adenovirus, coronaviruses (229E, OC43, NL63 and HKU1), enteroviruses, human bocavirus (HBoV), human metapneumovirus (hMPV), HRV, influenza A and B, parainfluenza virus (PIV) types 1–4, polyomaviruses WU and KI, and RSV using PCR or conventional methods for all viruses when applicable 14, 15. Blood eosinophil counts and allergen‐specific IgE antibodies were measured by the routine diagnostics of the Central Laboratory of Turku University Hospital 6. A standardized questionnaire on host and environment‐related risk factors for recurrent wheezing was used in parental interview (Data S1).
Statistical analysis
Host and environment‐related risk factors were analysed with Kruskal–Wallis anova, Mann–Whitney U test, or χ2 test when appropriate. Univariable Cox proportional‐hazards regression was used to analyse risk factors for recurrent wheezing, that is, parental asthma, any sensitization, eczema, day care, parental smoking, age at study entry, sex and viral aetiology. The analysis was repeated using multivariable backward stepwise model (exclusion criteria p > 0.10) including the same factors. Only the significant risk factors (p < 0.05) were included as covariates in the final model. Cox hazard ratio (HR) indicated the risk of recurrent wheezing. Interactions between treatment grouping (prednisolone vs. placebo) and pre‐specified risk factors (eczema status and HRV, RSV or HRV‐/RSV‐negative aetiology) were studied with Cox regression 13. Multivariable model was repeated with significant interactions 13. Each study subject was censored from the analysis after the initiation of the daily controller therapy with inhaled corticosteroid treatment. Analyses were made using pasw 18.0 software (SPSS Inc, Chicago, Ill, USA).
Results
Study population
The long‐term follow‐up criteria were fulfilled by 131 children (Fig. 1). Of these, nine children were lost to follow‐up, and 11 children were excluded from the analysis because inhaled corticosteroid was used for prolonged cough. Finally, 111 (85%) children were included. The prednisolone (n = 55) and placebo (n = 56) recipients were equally distributed overall and according to the risk factor characteristics (Fig. 1 and Table 1).
Table 1.
Distribution of risk factors at the study entry
| 7‐yr follow‐up | |||||||
|---|---|---|---|---|---|---|---|
| Children with recurrent wheezing | Children without recurrent wheezing | ||||||
| Risk factor | Entire cohort | All | Prednisolone | Placebo | All | Prednisolone | Placebo |
| 111 | 51 (46%) | 24 (47%) | 27 (53%) | 60 (54%) | 31 (52%) | 29 (48%) | |
| Age | |||||||
| 3–11 months | 51 (46%) | 27 (53%) | 12 (44%) | 15 (56%) | 24 (47%) | 13 (54%) | 11 (46%) |
| 12–35 months | 60 (54%) | 24 (40%) | 12 (50%) | 12 (50%) | 36 (60%) | 18 (50%) | 18 (50%) |
| Sex | |||||||
| Male | 75 (68%) | 37 (49%) | 18 (49%) | 19 (51%) | 38 (51%) | 16 (42%) | 22 (58%) |
| Female | 36 (32%) | 14 (39%) | 6 (43%) | 8 (57%) | 22 (61%) | 15 (68%) | 7 (32%) |
| Eczema | |||||||
| Yes | 36 (32%) | 20 (56%) | 7 (35%) | 13 (65%) | 16 (44%) | 8 (50%) | 8 (50%) |
| No | 75 (68%) | 31 (41%) | 17 (55%) | 14 (45%) | 44 (59%) | 23 (52%) | 21 (48%) |
| Sensitizationa | |||||||
| Any allergen | 16 (14%) | 11 (69%) | 6 (55%) | 5 (45%) | 5 (31%) | 2 (40%) | 3 (60%) |
| None | 95 (86%) | 40 (42%) | 18 (45%) | 22 (55%) | 55 (58%) | 29 (53%) | 26 (47%) |
| Food allergens | 16 (14%) | 11 (69%) | 7 (64%) | 4 (36%) | 5 (31%) | 2 (40%) | 3 (60%) |
| Aeroallergens | 3 (3%) | 3 (100%) | 1 (33%) | 2 (67%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Seasonal allergens | 3 (3%) | 3 (100%) | 1 (33%) | 2 (67%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Perennial allergens | 2 (2%) | 2 (100%) | 1 (50%) | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Blood eosinophil count | |||||||
| <0.4 × 109/l | 81 (73%) | 39 (48%) | 19 (49%) | 20 (51%) | 42 (52%) | 23 (55%) | 19 (45%) |
| ≥0.4 × 109/l | 30 (27%) | 12 (40%) | 5 (42%) | 7 (58%) | 18 (60%) | 8 (44%) | 10 (56%) |
| Parental asthma | 18 (16%) | 8 (44%) | 5 (63%) | 3 (37%) | 10 (56%) | 5 (50%) | 5 (50%) |
| Parental smoking | 48 (43%) | 23 (48%) | 10 (43%) | 13 (57%) | 25 (52%) | 11 (44%) | 14 (56%) |
| Day care | |||||||
| Home | 78 (70%) | 35 (45%) | 16 (46%) | 19 (54%) | 43 (55%) | 25 (58%) | 18 (42%) |
| Family day care | 17 (15%) | 6 (35%) | 4 (67%) | 2 (33%) | 11 (65%) | 4 (36%) | 7 (64%) |
| Group nursery | 16 (14%) | 10 (63%) | 4 (40%) | 6 (60%) | 6 (37%) | 2 (33%) | 4 (67%) |
| Viral aetiology | |||||||
| RSV | 44 (40%) | 12 (27%) | 7 (58%) | 5 (42%) | 32 (73%) | 18 (56%) | 14 (44%) |
| HRV | 34 (30%) | 19 (56%) | 7 (37%) | 12 (63%) | 15 (44%) | 10 (67%) | 5 (33%) |
| HRV‐/RSV‐negative | 33 (30%) | 20 (61%) | 10 (50%) | 10 (50%) | 13 (39%) | 3 (23%) | 10 (77%) |
Data presented as n (%). RSV, respiratory syncytial virus; HRV, human rhinovirus.
Sensitization was defined as positive immunoglobulin (Ig) E antibodies to any of the common allergens (cut‐off level 0.35 kU/l for codfish, cow's milk, egg, peanut, soybean, wheat, cat, dog, horse, birch, mugwort, timothy, Cladosporium herbarum and Dermatophagoides pteronyssinus; fluoro‐enzyme immunoassay, CAP FEIA, Phadiatop Combi®, Phadia, Uppsala, Sweden). Aeroallergen sensitization was defined as IgE antibodies to any of the latter eight allergens, which were divided into perennial (dog, cat or D. pteronyssinus) and seasonal (birch, mugwort, timothy and C. herbarum).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Patient characteristics
Median age of the study children was 12 months (interquartile range 7, 18 months) at study entry and at the end of follow‐up 8.0 yr (7.3, 8.7 yr, respectively). At study entry, 75 of 111 (68%) children were boys, 36 of 111 (32%) had eczema, 16 of 109 (14%) were sensitized and 106 of 111 (95%) children were virus‐positive. The HRV group had a higher prevalence of allergic sensitization (29%) than the other groups (RSV 7%; HRV‐/RSV‐negative 9%, p = 0.010). The RSV group had a lower prevalence of blood eosinophil count ≥0.4 × 109/l (7%) than the other groups (HRV 44%; HRV‐/RSV‐negative 36%, p = 0.001). Sensitized children were older than non‐sensitized (medians 16 vs. 11 months, p = 0.003). Otherwise, no differences were found in descriptive statistics between risk factor groups stated in Table 1 or between prednisolone and placebo groups.
Risk factors for recurrent wheezing
During the 7‐yr follow‐up, 51 (46%) of the 111 study children suffered from recurrent wheezing: 90% (46/51) of children fulfilled the criterion of three physician‐confirmed wheezing episodes within the past 12 months; 12% (6/51) the criterion of continuous lower respiratory symptoms lasting >4 wk; and 2% (1/51) the criterion of ≥2 wheezing episodes necessitating systemic corticosteroid within 6 months. Two children had two positive criteria; either continuous symptoms or systemic corticosteroid use with concomitant recurrent wheezing.
The univariable analysis showed that sensitization at study entry was a risk factor for recurrent wheezing (HR 2.25, 95% CI 1.15–4.41). Sensitization was divided into aeroallergen (4.40, 1.35–14.3, respectively, n = 3) and to food‐allergen sensitizations (2.28, 1.17–4.46, respectively, n = 16). All three children with aeroallergen sensitization had recurrent wheezing. Otherwise, no significant risk factors were found in univariable model.
In the final model of backward stepwise multivariable analysis, the risk factors for recurrent wheezing were HRV (HR 3.54, 95% CI 1.51–8.30), sensitization (3.47, 1.55–8.30, respectively), age <1 yr (2.45, 1.29–4.65) and eczema at study entry (2.33, 1.11–4.90). The incidence of recurrent wheezing was 56% (19/34) in children with HRV wheezing, 69% (11/16) in children with sensitization, 53% (27/51) in children aged <1 yr and 56% (20/36) in children with eczema (Table 1).
Efficacy of prednisolone
Prednisolone showed no overall efficacy in reducing recurrent wheezing (Fig. 2, Table 1). Then the Cox regression analysis was performed in pre‐specified subgroups demonstrating an interaction between treatment grouping and viral aetiology (p = 0.029) or eczema (p = 0.033). Prednisolone treatment was associated with less recurrent wheezing in HRV‐affected children (non‐adjusted HR 0.37, 95% CI 0.15–0.95; HR adjusted for young age, sensitization, eczema and parental asthma 0.32, 95% CI 0.12–0.90), but not in RSV‐affected (0.74, 0.27–2.03; 0.73, 0.25–2.10, respectively), nor in the HRV‐/RSV‐negative group (1.73, 0.72–4.19; 1.74, 0.68–4.45; Fig. 2 and Table 1). Prednisolone treatment was associated with less recurrent wheezing in children with eczema (non‐adjusted HR 0.53, 95% CI 0.21–1.33; HR adjusted for sensitization, young age, viral aetiology and parental asthma 0.27, 95% CI 0.08–0.87), but not in those without eczema (1.12, 0.55–2.28; 1.45, 0.69–3.04, respectively) (Fig. 2). The incidence of recurrent wheezing in the prednisolone vs. placebo groups was 41% (7/17) vs. 71% (12/17) in children with HRV and 47% (7/15) vs. 62% (13/21) in children with eczema.
Figure 2.
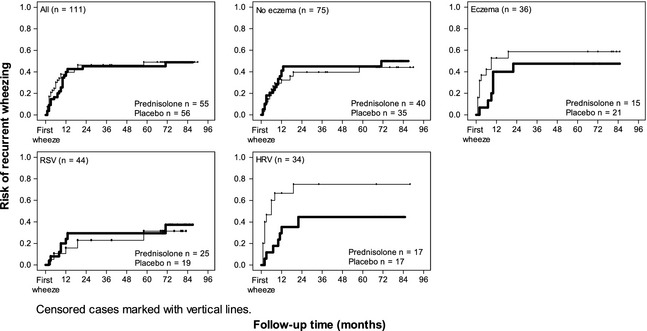
Risk of recurrent wheezing in prednisolone (bold line) and placebo recipients during a 7‐yr follow‐up. Prednisolone treatment was not associated with overall efficacy in preventing recurrent wheezing, but the treatment was associated with less recurrent wheezing in HRV and/or eczema group. In the HRV‐/RSV‐negative group, there were too few cases in prednisolone recipients with recurrent wheezing (n = 3) for statistical analysis. RSV, respiratory syncytial virus; HRV, human rhinovirus.
Discussion
This is the first study to investigate the long‐term (up to school age) prognosis of the first wheezing episode with the inclusion of full virology and atopic characteristics. Moreover, because the cohort was primarily randomized to receive prednisolone or placebo, we were able to assess the long‐term efficacy of oral prednisolone in this unique setting post‐hoc. We show that especially aeroallergen sensitization (HR4.4) and HRV aetiology (HR 3.5) are important predictive factors for recurrent wheezing already at the time of the first wheezing episode 2, 3, 6, 8, 9, 17. As a new and provocative finding, we found that oral prednisolone treatment decreased the risk of recurrent wheezing in children with HRV aetiology and/or eczema, and that the effect persisted the full 7 yr follow‐up period.
Our finding on HRV‐associated risk of recurrent wheezing agrees with the Italian cohort that also focused on the first episode of lower airways infection 3 and other early wheezing cohorts 1, 2, 9. The HRV‐associated risk has been explained by the increased susceptibility to lower airway HRV infections in individuals with pronounced atopic characteristics (allergen‐specific IgE sensitization, increased exhaled nitric oxide levels, increased blood eosinophil counts, atopic eczema, maternal atopic eczema and/or increased IL‐4, IL‐5, and IL‐13 responses in airway secretions), damaged airway epithelium, as well as decreased interferon α/β/γ/λ and IL‐10 responses in airway secretions or cells 1, 4, 5, 6, 7, 8, 9, 10, 18, 19. In line with these findings, our first‐time wheezing HRV‐affected children were more sensitized at study entry compared with other virus groups (29% vs. 7–9%). Moreover, compared with RSV, HRV‐induced wheezing has been connected to early decreased lung function, more severe illness and in high‐risk cohorts to earlier wheezing illness 20.
Eczema is classically the earliest manifestation of atopic march followed by food allergy, wheezing/asthma and allergic rhinitis. A recent meta‐analysis of 13 prospective cohort studies (four birth cohorts and nine eczema cohorts) studies showed OR 2.1 for the risk of asthma after eczema 21. Our finding on eczema‐associated risk of recurrent wheezing (HR 2.3) is in line with these earlier data. Eczema and asthma may involve mutual genetic mechanisms 22, but these were not investigated here.
Sensitization, especially to aeroallergens, is an important predictive factor for recurrent wheezing found by us and others (OR 3–16) 2, 6, 9, 17, 23 and showed stronger predictive value than HRV. Sensitization may be a predisposing factor for HRV illnesses in later life, possibly by suppressing antiviral responses 8, 19. In our study, however, there were only three aeroallergen‐sensitized children, but they all developed recurrent wheezing. Aeroallergen sensitization is highly specific, but not sensitive early risk marker for asthma 17, 23. The slow development of aeroallergen sensitization in general population (rarely before age 1 yr) 5, 23 decreases its value in asthma risk indices at early life and calls for better early risk factors such as HRV. Thus, our data suggest that early asthma risk indices could be improved if the HRV aetiology was included 24.
Our findings on the efficacy of prednisolone in HRV and/or eczema affected children are supported by others 1, 5, 6, 8, 9, suggesting that HRV‐wheeze and eczema are most likely linked to a pre‐existing, partly atopy‐related, inflammation in the airways 25 and that prednisolone may effectively down‐regulate this inflammation 26, 27 when administered early. Systemic dosing may particulary be important because it has recently been suggested that a local inflammation triggers a linkage to bone marrow from where cells potentially migrate to their different peripheral targets, for example lungs or skin 25. Although glucocorticoids preserve epithelial barrier and thereby could protect against respiratory viral infections 28, a more important mechanism may be their ability to repress the transcription of many inflammatory genes and/or their transcription factors and induce the expression of a number of anti‐inflammatory genes 26, 27. Moreover, glucocorticoids inhibit the HRV‐induced up‐regulation of its own receptor ICAM‐1 (intercellular adhesion molecule‐1, major HRV‐receptor) in pulmonary mucosa 29. Early administration, that is, for the first wheeze and early course of the acute illness may be a relevant factor for the long‐term clinical efficacy. The outcome of recurrent wheezing was almost completely fulfilled within the first 2 yr of follow‐up in our study.
Although the age range was rather broad (3–35 months, median 12 months), 86% were aged <2 yr which makes our study cohort and results overall very similar to another hospitalized population‐based study, Kuopio bronchiolitis study 1. However, the risk of recurrent wheeze in our study was inversely dependent on age, whereas other separate cohort studies on hospitalized children with lower respiratory symptoms have suggested that the risk increases by age: the OR was 3.3 for recurrent wheeze in children with bronchiolitis aged less <12 months (median age 2 months) 3, and OR was 4.1 for asthma in school age in wheezing children aged <2 yr 1. A potential explanation for the discrepancy could be that we studied infants with wheezing only (vs. bronchiolitis with or without wheezing in other studies). Infant wheezing may truly be an important risk factor for asthma, and studies on bronchiolitis should clearly define wheezing status of the subjects. In a high‐risk cohort, the risk of recurrent wheezing has been markedly higher in rhinovirus‐affected wheezing children than in other children and increased by age from the wheezing at first year of life (OR 10) to third year of life (OR 26) 2.
The strengths of our study are comprehensive assessments of virus aetiology and atopic characteristics, no delay in study drug initiation, long‐term follow‐up, verification of wheezing episodes from hospital/health care records and the use of the non‐selected population. The verification of wheeze was important because the highest odds ratios for recurrent wheeze have been reported in wheezing children 1, 2, 9, 17. Ninety per cent of the primary outcomes were fulfilled by the definition of episodic wheeze which was defined slightly different from National Asthma Education and Prevention Program (NAEPP) 16. Here and in our previous report, we used three physician‐confirmed wheezing episodes within a past year according to the Finnish practice 13. Of other limitations, the study population was rather small, and we focused only on new diagnoses during the follow‐up according to our previous report instead of current asthma 13. Although the study design was randomized and controlled, the subgroup analyses were carried out post‐hoc. The risk of bias of multiple comparisons was avoided by using pre‐specified subgroups. Prospective intervention studies on HRV‐induced wheezing are challenging because virus PCR detection usually is an over‐night diagnosis. Upper airway samples for virus PCR analysis are considered to adequately reflect the infection status in the lower airways 30.
In conclusion, our data from first‐time wheezing children support the link between atopic characteristics and HRV aetiology. It also strengthens the role of HRV aetiology as an early risk marker for recurrent wheezing 3, 13. The reduction in recurrent wheezing by prednisolone in children with HRV and/or eczema suggests that pre‐existing, atopy‐related, inflammation in the airways increases susceptibility to HRV‐wheeze. Prospective randomized trials are warranted to test the hypothesis that early anti‐inflammatory treatment reduces recurrent wheezing in high asthma risk children.
Author contributions
OR, TV and TJ were involved in the conception and design of the study. TJ, PL and ML were involved in the acquisition of data. HL and ML conducted the statistical analyses, ML and TJ drafted the manuscript. All authors read and approved the final form of the manuscript.
Supporting information
Data S1. Parental questionnaire on admission for Vinku study.
Acknowledgments
Supported by the Academy of Finland (TJ, grants 114034 and 132595), Helsinki; the Finnish Medical Foundation, Helsinki (TJ, PL); the Foundation for Paediatric Research (TJ, ML), Helsinki; the TYKS Foundation (ML), Turku; Research Funds from Specified Government Transfers (TJ, ML), Turku; the Maud Kuistila Memorial Foundation (ML), Helsinki; Tampere Tuberculosis Foundation (ML), Tampere; the Paulo Foundation (ML), Helsinki; the Ida Montin Foundation (ML), Espoo, and the Allergy Research Foundation (TJ, ML), Helsinki, all in Finland.
Lukkarinen M, Lukkarinen H, Lehtinen P, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7‐year follow‐up. Pediatr Allergy Immunol 2013: 00.
References
- 1. Kotaniemi‐Syrjänen A, Vainionpää R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus‐induced wheezing in infancy–the first sign of childhood asthma? J Allergy Clin Immunol 2003: 111: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. Am J Respir Crit Care Med 2008: 178: 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Midulla F, Pierangeli A, Cangiano G, et al. Rhinovirus bronchiolitis and recurrent wheezing: one year follow‐up. Eur Respir J 2012: 39: 396–402. [DOI] [PubMed] [Google Scholar]
- 4. Contoli M, Message SD, Laza‐Stanca V, et al. Role of deficient type III interferon‐lambda production in asthma exacerbations. Nat Med 2006: 12: 1023–1026. [DOI] [PubMed] [Google Scholar]
- 5. Jartti T, Lehtinen P, Vuorinen T, Ruuskanen O. Bronchiolitis: age and previous wheezing episodes are linked to viral etiology and atopic characteristics. Pediatr Infect Dis J 2009: 28: 311–317. [DOI] [PubMed] [Google Scholar]
- 6. Jartti T, Kuusipalo H, Vuorinen T, et al. Allergic sensitization is associated with rhinovirus‐, but not other virus‐, induced wheezing in children. Pediatr Allergy Immunol 2010: 21: 1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carroll KN, Gebretsadik T, Minton P, et al. Influence of maternal asthma on the cause and severity of infant acute respiratory tract infections. J Allergy Clin Immunol 2012: 129: 1236–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jackson DJ, Evans MD, Gangnon RE, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med 2012: 185: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kusel MM, Kebadze T, Johnston SL, Holt PG, Sly PD. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J 2012: 39: 876–882. [DOI] [PubMed] [Google Scholar]
- 10. Jakiela B, Brockman‐Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol 2008: 38: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. N Engl J Med 2009: 360: 329–338. [DOI] [PubMed] [Google Scholar]
- 12. Plint AC, Johnson DW, Patel H, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med 2009: 360: 2079–2089. [DOI] [PubMed] [Google Scholar]
- 13. Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol 2007: 119: 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007: 44: 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Söderlund‐Venermo M, Lahtinen A, Jartti T, et al. Clinical assessment and improved diagnosis of bocavirus‐induced wheezing in children. Emerg Infect Dis 2009: 15: 1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Asthma Education and Prevention Program . Expert panel report 3 (epr‐3): guidelines for the diagnosis and management of asthma‐summary report 2007. J Allergy Clin Immunol 2007: 120: S94–138. [DOI] [PubMed] [Google Scholar]
- 17. Matricardi PM, Illi S, Gruber C, et al. Wheezing in childhood: incidence, longitudinal patterns and factors predicting persistence. Eur Respir J 2008: 32: 585–592. [DOI] [PubMed] [Google Scholar]
- 18. Jartti T, Korppi M. Rhinovirus‐induced bronchiolitis and asthma development. Pediatr Allergy Immunol 2011: 22: 350–355. [DOI] [PubMed] [Google Scholar]
- 19. Sykes A, Edwards MR, Macintyre J, et al. Rhinovirus 16‐induced IFN‐alpha and IFN‐beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol 2012: 129(1506–1514): e1506. [DOI] [PubMed] [Google Scholar]
- 20. Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF Jr, Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J 2008: 32: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol 2007: 120: 565–569. [DOI] [PubMed] [Google Scholar]
- 22. Schuttelaar ML, Kerkhof M, Jonkman MF, et al. Filaggrin mutations in the onset of eczema, sensitization, asthma, hay fever and the interaction with cat exposure. Allergy 2009: 64: 1758–1765. [DOI] [PubMed] [Google Scholar]
- 23. Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 2006: 368: 763–770. [DOI] [PubMed] [Google Scholar]
- 24. Jackson DJ, Guilbert TW, Evans MD, et al. Inclusion of Rhinovirus Wheezing History in Early Life Improves the Sensitivity of the Modified Asthma Predictive Index (mAPI). J Allergy Clin Immunol 2009: 123: S82. [Google Scholar]
- 25. Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med 2012: 18: 726–735. [DOI] [PubMed] [Google Scholar]
- 26. Stellato C. Glucocorticoid actions on airway epithelial responses in immunity: functional outcomes and molecular targets. J Allergy Clin Immunol 2007: 120: 1247–1263; quiz 1264‐1245. [DOI] [PubMed] [Google Scholar]
- 27. de Benedictis FM, Bush A. Corticosteroids in respiratory diseases in children. Am J Respir Crit Care Med 2012: 185: 12–23. [DOI] [PubMed] [Google Scholar]
- 28. Hermanns MI, Unger RE, Kehe K, Peters K, Kirkpatrick CJ. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo‐capillary barrier in vitro . Lab Invest 2004: 84: 736–752. [DOI] [PubMed] [Google Scholar]
- 29. Papi A, Papadopoulos NG, Degitz K, Holgate ST, Johnston SL. Corticosteroids inhibit rhinovirus‐induced intercellular adhesion molecule‐1 up‐regulation and promoter activation on respiratory epithelial cells. J Allergy Clin Immunol 2000: 105: 318–326. [DOI] [PubMed] [Google Scholar]
- 30. Jartti T, Söderlund‐Venermo M, Hedman K, Ruuskanen O, Mäkelä M. New molecular virus detection methods and their clinical value in lower respiratory tract infections in children. Paediatr Respir Rev 2013: 1: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Parental questionnaire on admission for Vinku study.


