Abstract
Background
Respiratory viral and mycoplasma infections are associated with childhood asthma exacerbations. Here, we explored epidemiologic profile of causative pathogens and possible factors for exacerbation in a single center over a three‐year period.
Methods
Hospitalized asthmatic children with attack aged 6 months‐17 years were recruited between 2012 and 2015 (n = 216). Nasopharyngeal mucosa cell samples were collected from the participants and examined by reverse transcription‐polymerase chain reaction to detect rhinovirus (RV), respiratory syncytial virus (RSV), enterovirus (EV), parainfluenza virus (PIV), Mycoplasma pneumoniae, and others. Clinical features, laboratory data, asthma exacerbation intensity, and asthma severity were compared among participants. Epidemiologic profile of causative pathogens and possible factors for exacerbation were explored.
Results
Viruses and/or Mycoplasma pneumoniae were detected in 75% of the participants. Rhinovirus (48%) was the most commonly detected virus in the participants with single infection, followed by RSV (6%). The median age at admission in the RV group was significantly higher than that in the RSV group. Insufficient asthma control and allergen sensitization were significantly related to RV‐associated asthma exacerbation. There was no seasonality of pathogen types associated with asthma exacerbation although a sporadic prevalence of EV‐D68 was observehinovirud. Rhinovirus were repeatedly detected in multiple admission cases.
Conclusion
Our three‐year analysis revealed that patients with RV infection were significantly prone to repeated RV infection in the subsequent exacerbation and good asthma control could prevent RV‐associated asthma development and exacerbation. Multiple‐year monitoring allowed us to comprehend the profile of virus‐ and/or mycoplasma‐induced asthma exacerbation.
Keywords: asthma, enterovirus D68, epidemiology, hospitalization, respiratory syncytial virus, rhinovirus
Key Message.
Asthma children (n = 216; 6 months‐17 years) hospitalized with exacerbation were enrolled consecutively for 3 years in a single center.
Multi‐season analyses allowed us to explore epidemiological profile of causative pathogens and possible factors for exacerbation.
Rhinovirus (RV) and respiratory syncytial virus were most frequently detected.
Although a sporadic epidemic of EV‐D68 was detected, no seasonality in pathogen types were observed.
Children with RV‐associated exacerbation were more likely infected with RV on the subsequent admission.
Multivariate analyses suggested that good asthma control and avoidance of allergy sensitization could prevent RV‐associated asthma.
1. INTRODUCTION
Respiratory viral infections are associated with the pathophysiology of childhood asthma exacerbation.1 The major pathogens involved are human rhinovirus (RV), respiratory syncytial virus (RSV), enterovirus (EV), influenza virus, and human metapneumovirus (hMPV).2 In previous studies, RSV has been associated with wheezing during infancy, whereas RV has been detected more frequently in school‐age children with wheezing.3 However, most studies on the association of viral infection with asthma exacerbation were conducted for only one season.4, 5, 6 One season analysis is insufficient to comprehend the prevalence of virus‐ and mycoplasma‐induced asthma exacerbation as viral or mycoplasma prevalence might show seasonal variation or some might be found temporary as an epidemic, suggesting that a longer‐term analysis is needed.
Therefore, this study aimed to clarify the association between asthma exacerbation and causative pathogens for three seasons. In addition, epidemiologic profile of causative pathogens and possible factors for exacerbation were explored.
2. PARTICIPANTS AND METHODS
2.1. Participants
Asthmatic children hospitalized with asthma attack (n = 226) were enrolled at the Department of Pediatrics, Yamaguchi University Hospital, between April 2012 and May 2015. Five subjects were excluded due to lack of the consents from the patients and parents, and three subjects were excluded due to sufficient clinical and laboratory data. Two subjects were excluded because the nasopharyngeal samples were not properly obtained. As a result, 216 participants were enrolled for the analysis (Figure S1).
2.2. Asthma definition
Bronchial asthma was diagnosed according to the Japanese Pediatric Guideline for the Treatment and Management of Bronchial Asthma 2012 (JPGL 2012),7 which includes recurrent respiratory symptoms such as whistling, wheezing, difficulty in breathing, and chest tightness appearing during the night or early morning, chest auscultation for wheezing, respiratory function tests, IgE tests, and family and patients' past histories of allergic diseases.
Asthma exacerbation was defined as respiratory symptoms such as wheezing, cough, difficulty in breathing, chest tightness with chest auscultation for wheezing, and/or decrease in airway flow relieved by inhaled short‐acting beta‐2 adrenergic agonists (SABA), and the intensity of asthma exacerbation was classified into four stages: mild, moderate, severe exacerbation, and respiratory failure according to JPGL 2012.7 The level of asthma control was categorized in accordance with International Consensus on (ICON) Pediatric Asthma.8 The criteria for hospitalization were episodes of difficulty in sleeping at night, or oral ingestion difficulty, and/or oxygen saturation of peripheral artery (SpO2) < 95%. Patient demographic information was obtained from the hospital chart and questionnaires. Informed consent was obtained from the parents and/or the patients recruited in this study. This protocol was approved by the Institutional Review Board of Yamaguchi University Hospital (H24‐11).
2.3. Virus detection
Nasopharyngeal samples were collected from all patients. Sampling from the nasopharyngeal and transportation of the specimen to Yamaguchi Prefectural Institute of Public Health and Environment were performed by well‐experienced pediatricians. The samples were analyzed by reverse transcription‐polymerase chain reaction (RT‐PCR) to amplify specific genes from EV,9 RSV,10 PIV,11 hMPV,12 RV (subtypes A, B, and C),13 influenza viruses (subtypes A and B), adenoviruses (AdV),14 Coxsackie virus (Cox),9 human coronavirus (HCoV),15 and Mycoplasma pneumoniae 16 followed by nucleotide sequence determination and phylogenetic analysis. Virus detection and phylogenetic analysis for enterovirus D68 (EV‐D68) were performed as described previously.9, 17 In brief, nasopharyngeal swab specimens were collected with a sterile swab (Nippon Menbo). After collection, each swab was placed into 3 mL of Universal Viral Transport Medium (Becton, Dickinson and Company) for DNA extracts. Mycoplasma pneumonia DNA was detected by real‐time PCR targeting a conserved part of the gene encoding the P1 adhesin.18, 19
2.4. Statistical analysis
Pathogen‐specific clinical and laboratory features such as bronchial asthma severity, intensity of asthma exacerbation, the level of the asthma control, and hematologic and immunologic tests were analyzed both by univariate analyses (Mann‐Whitney U test or chi‐square test) and by multiple logistic regression analysis. Analyses and calculations were performed using BellCurve for Excel and StatFlex for Windows Ver 7.0 (Artech Inc).
3. RESULTS
3.1. Participants' characteristics
The characteristics of clinical and laboratorial data were summarized (Table 1). The median age of the participants for this study was 3.4 years old [range: 0.5‐17]. Exacerbation severity in 21 (10%), 146 (68%), and 49 patients (22%) was classified as mild, moderate, and severe, respectively. No participants with respiratory failure were observed. As for bronchial asthma control, 138 (64%), 28 (13%), and 50 patients (23%) were classified as mild, moderate, and severe, respectively. The median values of total IgE and house‐dust mite–specific IgE were 207 IU/mL [range: 4‐11 800 IU/mL] and 19.0 UA/mL [range: 0.1‐100 UA/mL], respectively.
Table 1.
Clinical characteristics, asthma exacerbation intensity, and asthma severity in the total subjects and the asthmatic subjects with single infection of RV and RSV
| Median (range) | Total (n = 216) | RV (n = 87) | RSV (n = 10) |
P RV vs RSV |
|---|---|---|---|---|
| Age (mo: months, y: years) | 3.4 (6 mo‐17 y) | 4.5 (6 mo‐13 y) | 1.6 (8 mo‐6 y) | .008 |
| Body temperature (°C) | 37.5 (36.0‐40.0) | 37.5 (36.1‐40.4) | 37.3 (37.0‐39.1) | .916 |
| SpO2 (%) | 94 (84‐100) | 94 (84‐100) | 96 (94‐100) | .109 |
| WBC (×109/L) | 12.0 (3.4‐32.8) | 12.8 (4.7‐32.8) | 11.0 (5.1‐18.8) | .558 |
| CRP (mg/dL) | 0.97 (0.01‐14.86) | 0.82 (0.01‐12.16) | 0.62 (0.03‐14.86) | .309 |
| Total IgE (IU/mL) | 207 (4‐11,800) | 508 (4‐5,440) | 103 (10‐1,970) | .254 |
| Mite IgE (UA/mL) | 19.0 (0.1‐100) | 90.1 (0.1‐100) | 0.1 (0.1‐100) | .002 |
| Duration of hospitalization (days) | 10 (2‐75) | 9 (2‐56) | 10 (6‐14) | .999 |
| Exacerbation severity | ||||
| Mild | 21 (10%) | 5 (6%) | 1 (10%) | .824 |
| Moderate | 146 (68%) | 59 (68%) | 6 (60%) | |
| Severe | 49 (22%) | 23 (26%) | 3 (30%) | |
| BA control | ||||
| Good | 138 (64%) | 48 (55%) | 9 (90%) | .098 |
| Partial | 28 (13%) | 24 (28%) | 1 (10%) | |
| None | 50 (23%) | 15 (17%) | 0 (0%) | |
Abbreviations: BA, bronchial asthma; CRP, C‐reactive protein; IgE, immunoglobulin E; RSV, respiratory syncytial virus; RV, rhinovirus; SpO2, oxygen saturation of peripheral artery; WBC, white blood cell.
P < 0.05.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Prevalence of detected viruses in asthmatic patients with exacerbation
First, we evaluated the relationship of asthma exacerbation with viruses and/or M pneumoniae infection (s). Causative pathogens were observed in 171 patients (viruses: n = 199, M pneumoniae: n = 13). Because 35 patients had superinfection of two or three viruses, we summarized the results of 181 participants with a single virus infection and without detected pathogen (Figure 1). Rhinovirus (n = 87; 48%) was the most commonly detected virus in participants with asthma exacerbation followed by RSV (n = 10; 6%).
Figure 1.
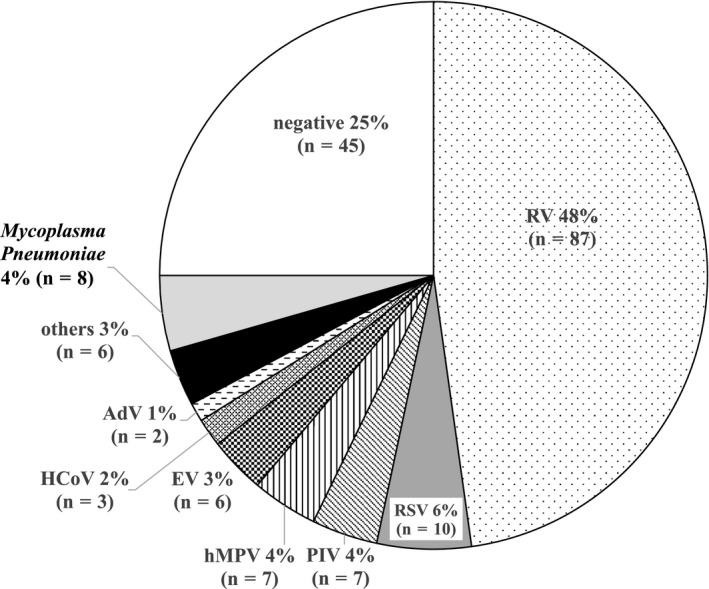
The relationship between causative viruses and/or Mycoplasma pneumoniae infection and asthma exacerbation
3.3. Comparison of clinical characteristics among RV and RSV groups
As RV and RSV were mostly detected in the participants with asthma exacerbation, we compared clinical characteristics among participants with single infection in whom we detected RV or RSV (Table 1). The median age and serum house‐dust mite IgE levels at admission were significantly higher in the RV group than those in the RSV groups (P < .01). There was no significant difference in body temperature, SpO2, duration of admission, the median number of peripheral white blood cells (WBCs), and serum levels of C‐reactive protein (CRP) between the two groups. No significant difference was observed in the intensity of asthma exacerbation on admission, bronchial asthma severity, and the level of asthma control between the groups as well.
Although RV type A and type C were detected in the majority of participants infected with RV (type A, n = 33; type B, n = 1; type C, n = 52; unknown, n = 1), there were no significant differences in body temperature, SpO2, duration of admission, laboratory data, intensity of asthma exacerbation, bronchial asthma severity, and the level of asthma control between the two groups (data not shown).
To explore epidemiologic features of RV‐associated asthma exacerbation, multiple logistic regression analyses were performed by setting the status of RV vs. non‐RV‐associated exacerbation as objective variable (Table S1). It revealed that insufficient control status and higher serum house‐dust mite IgE levels are related to RV‐associated asthma exacerbation requiring hospitalization (P < .05 and P < .01, respectively).
3.4. Comparison of clinical characteristics between asthma exacerbation with single infection and superinfection
Next, clinical characteristics, intensity of asthma exacerbation, bronchial asthma severity, and the level of asthma control were compared in the asthmatic patients with single infection and superinfection (Table 2). The median age and house‐dust mite IgE levels at admission were significantly higher in the single infection group than those in the superinfection group (P < .001), whereas there were no significant differences in body temperature, SpO2, duration of hospitalization, serum levels of CRP, intensity of asthma exacerbation, and bronchial asthma severity between the two groups. Multiple logistic analyses revealed that elder age and higher house‐dust mite IgE levels at admission were related to single infection as well (P < .001 and P < .05, respectively, data not shown).
Table 2.
Clinical characteristics, asthma exacerbation intensity, and asthma severity in the asthmatic patients with single infection and superinfection
| Median (range) | Single infection (n = 136) | Superinfection (n = 35) | P |
|---|---|---|---|
| Age (mo: months, y: years) | 4.0 (6 mo‐14 y) | 1.4 (6 mo‐5.9 y) | <.001 |
| Body temperature (°C) | 37.5 (36.1‐40.4) | 37.7 (36.0‐40.0) | .372 |
| SpO2 (%) | 94 (84‐100) | 95 (84‐99) | .333 |
| WBC (×109/L) | 12.2 (3.6‐32.8) | 11.8 (5.6‐24.0) | .853 |
| CRP (mg/dL) | 0.93 (0.01‐14.86) | 1.14 (0.02‐7.28) | .772 |
| Total IgE (IU/mL) | 332 (4‐7,070) | 60 (4‐3,500) | .152 |
| Mite IgE (UA/mL) | 53.2 (0.1‐100) | 0.34 (0.1‐100) | <.001 |
| Duration of hospitalization (d) | 9.5 (2‐56) | 10 (2‐26) | .330 |
| Exacerbation severity | |||
| Mild | 10 (6%) | 5 (14%) | .413 |
| Moderate | 93 (51%) | 23 (66%) | |
| Sever | 33 (18%) | 7 (20%) | |
| BA control | |||
| Good | 85 (62%) | 25 (71%) | .163 |
| Partial | 31 (23%) | 9 (26%) | |
| None | 20 (15%) | 1 (3%) | |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.5. Seasonal pathogen distribution associated with asthma exacerbation
Seasonal pathogen distribution associated with asthma exacerbation has not been conducted so far because most studies on the association of pathogens with asthma exacerbation were conducted for only one season.4, 5, 6 Therefore, we just evaluated the relationship between asthma exacerbation and virus and/or M pneumoniae distribution based on the month of admission (Figure 2). There were no seasonal peaks in virus and/or M pneumoniae detections, although RV and PIV were detected in all seasons.
Figure 2.
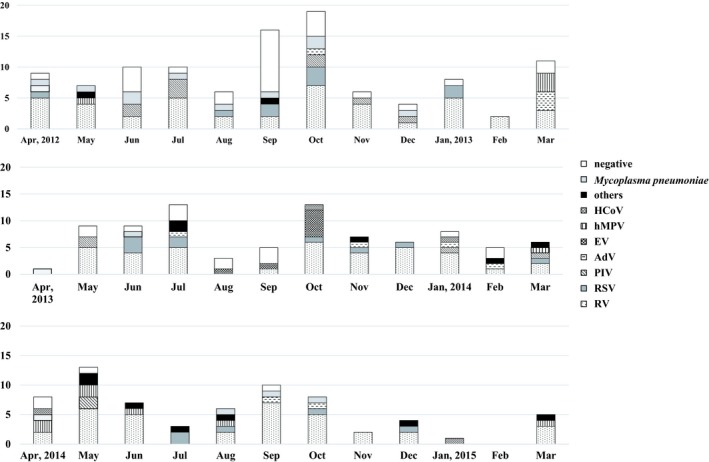
The relationship between asthma exacerbation and virus and/or Mycoplasma pneumoniae distribution based on the month of admission
3.6. Prevalence of detected viruses in the patients with asthma exacerbation on multiple admissions
Next, we evaluated the detected pathogens in the patients with asthma exacerbation on multiple admissions. Among the 31 patients on multiple admissions, RVs were repeatedly detected in 18 patients on subsequent admissions as well as on the initial admission (Table 3 ), which showed that those with initial RV infection associated asthma exacerbation were likely to be infected with RV on the subsequent admission as well (P < .01).
Table 3.
Causative pathogens in the hospitalized patients on multiple admissions
| Patient | Age (mo) | 1st admission | 2nd (mo) | 3rd (mo) | 4th (mo) | 5th (mo) |
|---|---|---|---|---|---|---|
| 1 | 8 | RSV + Cox | hMPV (12) | |||
| 2 | 8 | PIV | RSV (1) | |||
| 3 | 11 | RV type C + AdV | RV type C + EBV (6) | |||
| 4 | 12 | Negative | RV type A (5) | |||
| 5 | 12 | RV type C + PIV | RV type C + PIV (1) | |||
| 6 | 14 | RV type C | RV (9) | |||
| 7 | 16 | RV type C + RSV | RV type C (24) | |||
| 8 | 17 | EV‐D68 | RV type C (15) | |||
| 9 | 20 | Negative | AdV (17) | |||
| 10 | 24 | RV | RV type C + PIV (0.5) | |||
| 11 | 29 | RV type A | Others (1) | |||
| 12 | 29 | RV type C | RV type C (24) | |||
| 13 | 37 | RV type A | RV type C (12) | |||
| 14 | 45 | RV type A | RV type C (10) | |||
| 15 | 55 | RV type A | RV type B (19) | |||
| 16 | 72 | RV type C | RV type C (0.75) | |||
| 17 | 82 | RSV | RV type C (15) | |||
| 18 | 84 | RV type A | RV type A (16) | |||
| 19 | 90 | RV type A | RV type A (3) | |||
| 20 | 129 | RV type C | RV type C (4) | |||
| 21 | 202 | Negative | Negative (13) | |||
| 22 | 8 | hMPV + PIV | RSV (3) | RV type B + RSV+Cox (4) | ||
| 23 | 17 | Negative | RV type C (12) | RV type A (6) | ||
| 24 | 20 | RV type A + AdV +Echo | EV‐D68 (2) | RV type A (11) | ||
| 25 | 56 | Myco | RV type A (20) | HCoV (2) | ||
| 26 | 99 | RV type C | RV type A (9) | RV type A (8) | ||
| 27 | 11 | hMPV + Adeno | Negative (2) | Negative (2) | RV type C (6) | |
| 28 | 14 | RV type C | Flu type A (3) | RV type C (1) | hMPV (4) | |
| 29 | 24 | PIV + Myco | RV type C (4) | HCoV (14) | hMPV (2) | |
| 30 | 153 | Negative | Negative (6) | Negative (3) | HCoV (15) | |
| 31 | 8 | RV type A | Negative (1) | RV type C (3) | Myco (8) | RV type A(9) |
Months in 2nd, 3rd, 4th, and 5th represent the number of months showing the interval from the previous admission.
Abbreviations: AdV, adenovirus; Cox, Coxsackie virus; EBV, Epstein‐Barr virus; EV‐D68, enterovirus D68; Flu, influenza virus; HCoV, human coronavirus; hMPV, human metapneumovirus; Myco, mycoplasma; others, other virus; PIV, parainfluenza virus; RSV, respiratory syncytial virus; RV, rhinovirus.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.7. Prevalence of EV‐D68 during the study period
In this study, six asthmatic patients had EV infection, and EV‐D68 was detected in all six patients. We evaluated the relationship between EV‐D68 infection and hospital admission due to asthma exacerbation (Figure 3). A low prevalence was observed only in 2013, but not in 2012 or 2014, suggesting that EV‐D68 prevalence was not annual, unlike that of RV, RSV, or PIV.
Figure 3.
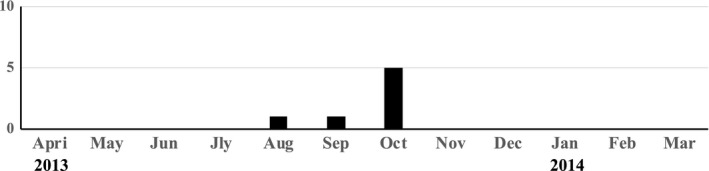
The relationship between hospital admission due to asthma exacerbation and infection with EV‐D68
4. DISCUSSION
In this study, RV (48%) was the most detected virus among subjects with asthma exacerbation followed by RSV (6%), and the age on admission was significantly higher in the RV group than in the RSV group. The detection frequency of RV and RSV in our study was consistent with that in previous reports on the relationship between causative viruses and exacerbation in asthmatic patients, suggesting that our study illustrates the actual virus prevalence associated with asthma exacerbation.2, 3 Heymann et al20 reported prevalence of rhinovirus infection was much less in the wheezing patients aged <3 years compared to those above the age. Thus, we compared RV prevalence between the patients with <2 years old (n = 67) and those above the age (n = 149). Pathogens were identically detected in 79% in both groups. However, RV was detected in only 38% of the patients <2 years of age, whereas the virus was detected in 57.6% of those ≥2 years. This difference in the RV detection rate was statistically significant (P = .002), which explains an overall lower prevalence of RV in our study (data not shown).
There are several studies showing that RV infection is more associated with wheezing in older children.3, 21 In our three‐season analysis, the median age of subjects with RV infection was significantly higher than that of participants with RSV infection, which was consistent with the results of previous reports based on one season analysis.
Allergic sensitization was reported to be related to RV‐associated wheeze.22 Interestingly, we observed a closer association of house‐dust mite–specific IgE with the RV group than with the RSV group. Multiple logistic regression analysis revealed that there is a closer association of insufficient asthma control and house‐dust mite–specific IgE with the RV group than with the non‐RV group. These suggest that RV‐associated asthma exacerbation is related to allergen sensitization and that good asthma control could prevent RV‐associated asthma exacerbation.
Rhinovirus type C is reported to cause more severe attacks than other RV types.23, 24 However, there was no significant difference in the intensity of asthma exacerbation between the RV type A and type C groups in our study. Zheng et al25 reported that RV type A infection with high viral load leads to severe asthma exacerbation; we therefore speculate that the difference in the viral load of subjects between RV type A and type C may reflect similar results in asthma exacerbation intensity and asthma severity in our study.
There was no significant difference in intensity of asthma exacerbation and bronchial asthma severity between the single infection group and the superinfection group, suggesting that superinfection does not exert additional effect on asthma exacerbation. Interestingly, we observed the association of elder age and higher house‐dust mite IgE levels with single infection in multiple logistic analyses. Allergic sensitization was suggested as leading to increased susceptibility to RV, and RV‐associated wheeze.22 Our results confirmed the reported finding of exacerbation of asthma associated with RV.
It is interesting that RVs were repeatedly detected in multiple admission cases based on our three‐season analysis; that is, patients with initial RV‐associated asthma exacerbation were likely to be infected with RV again on the subsequent admission. This observation has not been reported in previous studies that were all based on one season analysis. In our study, RV infections were not seen in six non‐attack asthmatic patients, mentioning the existence of RV in nasopharyngeal airway could be pathogenic for asthma exacerbation (data not shown).
Several lines of evidence indicate that RV‐induced wheezing illnesses in the first few years of life are closely associated with the risk of subsequent asthma.1, 3 We postulated the possibility that this close association could be ascribed to not only RV infection in itself but also repetitive RV infection inducing wheezing illnesses or asthma exacerbations.
The unprecedented outbreak of EV‐D68 infection in the autumn of 2014 that led to an upsurge in hospitalization and admission to intensive care units has raised a concern about a potential uncontrollable epidemic of severe respiratory disease26; we have therefore evaluated the relationship between hospital admission due to asthma exacerbation and infection with EV‐D68. EV‐D68 was only detected in 2013 during the three‐season study, which is in agreement with the outbreak that occurred in Japan during the study period.27 We previously reported EV‐D68 outbreak in the same area in 2010.28 There has never been an outbreak of EV‐D68 since autumn 2010 to spring 2015 (data not shown). This suggests that EV‐D68 outbreak is not an annual but a time‐limited event.
There are some limitations in this study. First, this is a pilot study conducted in a single center. Further multi‐center studies involving different regions are warranted to confirm the relationship between causative pathogens and asthma exacerbation. Second, the patients with asthma exacerbations requiring hospitalization were recruited in our study, which may have resulted in a selection bias; that is, children presenting with mild exacerbation in relatively good control were not included. Therefore, there can be some limitation in analyzing the impact of pathogens for the entire range of asthma exacerbation.
In conclusion, our three‐season analysis on the prevalence of causative pathogens in hospitalized children with asthma exacerbation revealed that RV and RSV were most frequently detected. Insufficient asthma control and allergen sensitization were clearly related to RV‐associated asthma exacerbation. Rhinovirus were repeatedly detected in multiple admission cases in our three‐season analysis, suggesting that repetitive RV infections could contribute to subsequent aggravation of asthma. Putting these findings together, good asthma control could prevent RV‐associated asthma development and exacerbation.
Additionally, epidemic of EV‐D68 infection was observed during a few months, suggesting the requirement for multiple‐year monitoring to comprehend the prevalence of asthma exacerbation with viral infections including temporary small epidemic.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
AUTHOR CONTRIBUTION
NA and HY contributed equally to this work. HY, KS, KO, SO, and SH were the principal investigators taking primary responsibility for the manuscript. RF and SO performed the clinical management with helpful discussion for the completion of the study. NA, TN, HW, FO, ST, and RO took responsibility for the diagnosis and data collection.
MATERIAL SUBMITTED TO ELECTRONIC REPOSITORIES
The nucleotide sequences determined in this study were deposited in the DDBJ under the accession numbers AB618506‐AB618528.
Supporting information
ACKNOWLEDGMENTS
We are grateful to Prof. Kiyoshi Ichihara of our university, a biostatistician with medical background, for his dedicated support for the statistical analyses and writing to improve the scientific quality of our manuscript.
Abe N, Yasudo H, Fukano R, et al. Multi‐season analyses of causative pathogens in children hospitalized with asthma exacerbation. Pediatr Allergy Immunol. 2019;30:724–731. 10.1111/pai.13102
REFERENCES
- 1. Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140:895‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng XY, Xu YJ, Guan WJ, Lin LF. Regional, age and respiratory‐secretion‐specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol. 2018;163:845‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rake GP, Arruda E, Ingram JM, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785‐790. [DOI] [PubMed] [Google Scholar]
- 4. Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khetsuriani N, Kazerouni NN, Erdman DD, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119:314‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matthew J, Pinto Pereira LM, Pappas TE, et al. Distribution and seasonality of rhinovirus and other respiratory viruses in a cross‐section of asthmatic children in Trinidad, West Indies. Ital J Pediatr. 2009;35:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamasaki Y, Kohno Y, Ebisawa M, et al. Japanese pediatric guideline for the treatment and management of bronchial asthma 2012. Pediatr Int. 2014;56:441‐450. [DOI] [PubMed] [Google Scholar]
- 8. Papadopoulos NG, Arakawa H, Carlsen KH, et al. International consensus on (ICON) pediatric asthma. Allergy. 2012;67:976‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishiko H, Shimada Y, Yonaha M, et al. Molecular diagnosis of human enteroviruses by phylogeny‐based classification using the VP4 sequence. J Infect Dis. 2002;185:744‐754. [DOI] [PubMed] [Google Scholar]
- 10. Abels S, Nadal D, Stroehle A, Bossart W. Reliable detection of respiratory syncytial virus infection in children for adequate hospital infection control management. J Clin Microbiol. 2001;39:3135‐3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aguilar JC, Perez‐Brena MP, Garcia ML, Cruz N, Erdman DD, Echevarria JE. Detection and identification of human parainfluenza viruses 1, 2, 3, and 4 in clinical samples of pediatric patients by multiplex reverse transcription‐PCR. J Clin Microbiol. 2000;38:1191‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hara M, Takao S, Fukuda S, Shimazu Y, Miyazaki K. Human metapneumovirus infection in febrile children with lower respiratory diseases in primary care settings in Hiroshima, Japan. Jpn J Infect Dis. 2008;61:500‐502. [PubMed] [Google Scholar]
- 13. Ishiko H, Miura R, Shimada Y, et al. Human rhinovirus 87 identified as human enterovirus 68 by VP4‐based molecular diagnosis. Intervirology. 2002;45:136‐141. [DOI] [PubMed] [Google Scholar]
- 14. Heim A, Ebnet C, Harste G, Pring‐Akerblom P. Rapid and quantitative detection of human adenovirus DNA by real‐time PCR. Med Virol. 2003;70:228‐239. [DOI] [PubMed] [Google Scholar]
- 15. van Elden LJ, van Loon AM, van Alphen F, et al. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real‐time reverse‐transcriptase polymerase chain reaction. J Infect Dis. 2004;189:652‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Specjalski K, Jassem E. Chlamydophila pneumoniae, Mycoplasma pneumoniae infections, and asthma control. Allergy Asthma Proc. 2011;32:9‐17. [DOI] [PubMed] [Google Scholar]
- 17. Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698‐2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao F, Liu G, Wu J, et al. Surveillance of macrolide‐resistant Mycoplasma pneumonia in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother. 2008;2013(57):1521‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka T, Oishi T, Miyata I, et al. Macrolide‐resistant Mycoplasma pneumoniae infection, Japan, 2008–2015. Emerg Infect Dis. 2017;23:1703‐1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwon JM, Shim JW, Kim DS, Jung HL, Park MS, Shim JY. Prevalence of respiratory viral infection in children hospitalized for acute lower respiratory tract diseases, and association of rhinovirus and influenza virus with asthma exacerbations. Korean J Pediatr. 2014;57:29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stenberg‐Hammar K, Hedlin G, Söderhäll C. Rhinovirus and preschool wheeze. Pediatr Allergy Immunol. 2017;28:513‐520. [DOI] [PubMed] [Google Scholar]
- 23. Lauinger IL, Bible JM, Halligan EP, et al. Patient characteristics and severity of human rhinovirus infections in children. J Clin Virol. 2013;58:216‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Linder JE, Kraft DC, Mohamed Y, et al. Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol. 2013;131:69‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng SY, Wang LL, Ren L, Luo J, Liao W, Liu EM. Epidemiological analysis and follow‐up of human rhinovirus infection in children with asthma exacerbation. J Med Virol. 2018;90:219‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holm‐Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: a systematic review. Lancet Infect Dis. 2016;16:e64‐e75. [DOI] [PubMed] [Google Scholar]
- 27. Korematsu S, Nagashima K, Sato Y, et al. “Spike” in acute asthma exacerbations during enterovirus D68 epidemic in Japan: a nation‐wide survey. Allergol Int. 2018;67:55‐60. [DOI] [PubMed] [Google Scholar]
- 28. Hasegawa S, Hirano R, Okamoto‐Nakagawa R, Ichiyama T, Shirabe K. Enterovirus 68 infection in children with asthma attacks: virus‐induced asthma in Japanese children. Allergy. 2011;66:1618‐1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


