Abstract
This study reports the characterization of protein elicitor PeBb1 derived from entomopathogenic fungus Beauveria bassiana ARSEF-2860 strain and its putative role in induced systemic resistance in Brassica rapa ssp. pekinensis against green peach aphid Myzus persicae. The sequence of purified elicitor protein was matched with the genomic sequence of a hypothetical protein BBA_10269 from B. bassiana ARSEF-2860 (GenBank Accession No. XP_008603588.1). The protein-encoding gene PeBb1 contained 534 bp cDNA encoding a polypeptide of 177 amino acids with a molecular mass of 19 kDa. The recombinant elicitor protein was expressed in Escherichia coli using pET-28a (+) expression vector and induced necrosis in the leaves of tobacco. The effects of elicitor protein on aphid M. persicae was determined by applying three different concentrations of PeBb1 (i.e., 26, 35, 53 μM) on B. rapa plants at 4-leaf stage and the treated plants were exposed to newly emerged (0–6 h old) apterous adult aphids. Bioassay results showed significant (p < 0.05) sub-lethal effects of the exogenous application of PeBb1 elicitor on M. persicae. Moreover, the RT-qPCR gene expression analyses showed a significant up-regulation of most of the key genes linked to ethylene (ET)- and jasmonic acid (JA)-associated plant defense pathways in elicitor-treated plants. These results not only recommend the putative utilization of PeBb1 elicitor protein in future biological pest control strategies against phloem-feeding insect pests such as M. persicae, but also help in better comprehension of the mechanisms through which beneficial fungi trigger the induced plant resistance.
Keywords: Beauveria bassiana, elicitor protein, induced systemic resistance, Myzus persicae, fecundity, jasmonic acid pathway, ethylene pathway
1. Introduction
Phytophagous insect pests are one of the major threats to agricultural production across the globe. These pests are estimated to cause more than 15% loss to global crop production annually [1,2]. Contemporary pest management programs against these insects primarily rely on the application of synthetic chemical insecticides that have always been an inevitable part of plant protection strategies [3]. Widespread and irrational use of these synthetic chemicals however has led to various health and environmental issues such as the eradication of non-target species including insect predators and parasitoids, contamination of soil, air and water resources, pest resistance and resurgence [4]. Increasing ill-effects being manifested by the conventional synthetic insecticides necessitate looking for the alternate biorational pest control tactics such as microbial biopesticides [5]. Many microbes including entomopathogenic fungi, bacteria, viruses, and nematodes have shown effectiveness against a large number of insect pest species [6,7]. With more than 750 species worldwide, entomopathogenic fungi provide a promising potential for biological control of insect pests. Particularly, fungal species belonging to genera Beauveria, Isaria, Lecanicillium, and Metarhizium exhibit an excellent pathogenicity against many insect pests [8,9,10].
Because of their low mammalian toxicity and residual activity and high host specificity [11], entomopathogenic fungi have been successfully employed against a number of agricultural, urban, and medically important insect pests [12,13,14]. However, the delayed pest mortality by entomopathogenic fungi as compared to synthetic insecticides can be improved either by increasing their toxicity through genetic modifications or by identifying and developing their bioactive metabolites exhibiting lethal and sublethal toxicity to insect pests [15,16,17,18]. Many entomopathogenic fungi have been reported to secrete different antifeedant, insecticidal, and toxic bioactive substances in the broth cultures [19,20,21].
Moreover, many entomopathogenic fungal strains can develop endophytically inside the plant tissues and induce systemic resistance against various abiotic and biotic stresses including herbivores and pathogens [22,23]. Recently, certain elicitor proteins derived from pathogenic fungi have been demonstrated to evoke induced systemic resistance and defense response in different plant species against various pathogenic organisms and phytophagous insect pests [23,24,25]. Plants develop resistance to their prospective attackers (pathogens and insect pests) at early stages through the induction of plant immune system [26,27,28]. In defense response, all signal molecules might be involved and could regulate and control the downstream signaling pathways through metabolic changes and gene expression [29]. In response to attack by pathogens and insect pests, plant defense systems are usually regulated by multiple signaling pathways including three key signaling molecules i.e., JA, SA, and ET [30,31,32].
This laboratory study was aimed to extract and purify an elicitor protein molecule from an entomopathogenic fungus Beauveria bassiana sensu lato, strain ARSEF 2860, followed by its molecular characterization and potential bioactivity against green peach aphid Myzus persicae Sulzer on Chinese cabbage (Brassica rapa ssp. pekinensis) plants. Moreover, the expression levels of important genes linked with ethylene (ET) and jasmonic acid (JA) plant defense pathways were measured by RT-qPCR in order to elucidate the mechanism of any local or systemic resistance induced by recombinant fungal protein in B. rapa plants against M. persicae. This research will elucidate the potential role of microbe-associated molecular patterns (MAMPs)-type protein elicitor derived from entomopathogenic fungi as novel microbial pest management tool and will enhance our understanding toward the possibility for the development of more virulent strains of entomopathogenic fungi through genetic improvements.
2. Results
2.1. Purification, Identification, and Characterization of PeBb1 Protein
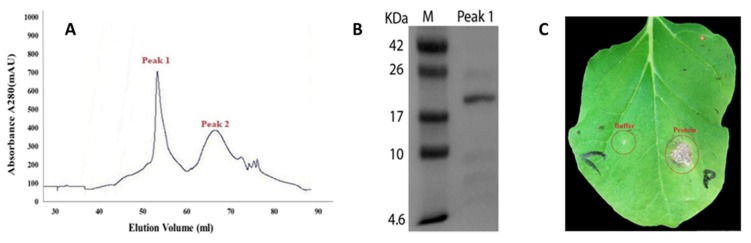
The crude protein from B. bassiana strain ARSEF 2860 (BB-72) was dialyzed and anion exchange chromatography was carried out and finally proteins were eluted with buffer B. Two protein peaks (Figure 1A) were collected, desalted, and injected into the leaves of tobacco (Nicotiana tabacum cv. Samsun-NN) to determine the activity of elicitor. Thereafter, we ran SDS-PAGE gel and the results revealed a single band of molecular weight of 19 kDa (Figure 1B). This protein was named as PeBb1. The band was recovered and bioassayed for necrosis-inducing activity (Figure 1C). SDS-PAGE was again subjected to mass spectrometry analysis. In brief, a single protein band was cut from SDS-PAGE gel for the recognition through liquid chromatography-mass spectrometry analysis. The outcomes were investigated by MASCOT software (Matrix Science Inc., London, UK) and we acquired the most matching protein (i.e., BBA_10269 hypothetical protein, GenBank: EJP60782.1).
Figure 1.
Purification of PeBb1 protein from B. bassiana strain ARSEF 2860 (BB-72). (A) Ion-exchange chromatography produced two peaks (1 and 2) of crude protein obtained from the ammonium sulfate precipitation and purified by ÄKTA Explorer 10 protein purification system; (B) PeBb1 (Peak 1) resolved on a SDS-PAGE gel; (C) necrosis induced by PeBb1 (53 µM) in tobacco leaves recorded at 24 h post infiltration. Control side of leaf lamina was treated with mock buffer (50 mM Tris-HCL, pH 8.0).
2.2. Gene Cloning
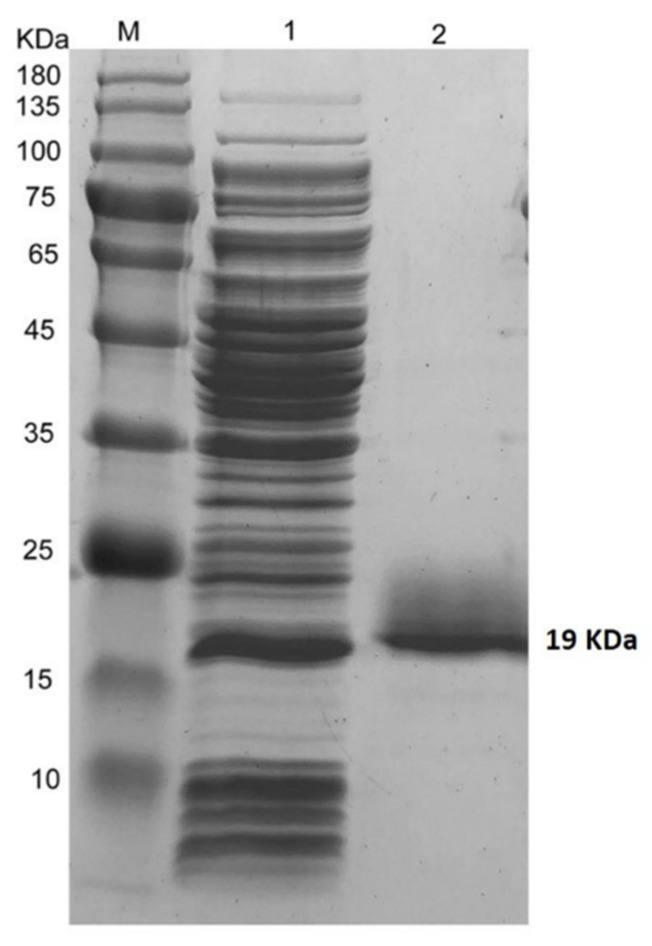
The purified protein was constituted of 177 amino acids and full length (534 bp) PeBb1 sequence was cloned and transformed into pET-28a (+) vector, expressed in E. coli BL21 (DE3) (Figure 2). By using column chromatography, the recombinant protein was purified and desalted. Thereafter, we ran SDS-PAGE gel of purified recombinant protein and the results showed a single band of 19 kDa molecular weight (Figure 3). This was consistent with the calculated size of the fusion elicitor.
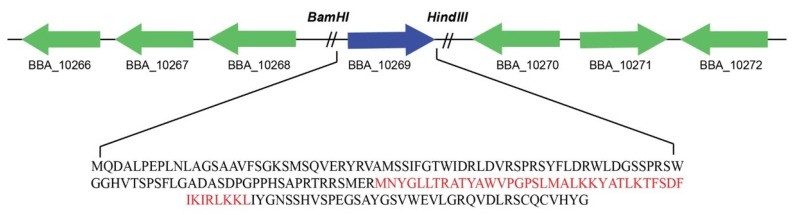
Figure 2.
Schematic representation showing the gene BBA_10269 location in the genome of Beauveria bassiana. The red portion in the protein sequence shows active binding domain inside protein.
Figure 3.
Purification of recombinant PeBb1 protein. 1 = total E. coli expressed proteins, 2 = purified His-tagged PeBb1 protein, M = protein molecular weight marker.
2.3. PeBb1-Induced Necrosis in Tobacco Leaves
Infiltration of PeBb1 into the leaves of tobacco ensued in quick macroscopic changes. There were conspicuous necrotic zones at the infiltration area recorded at 24 h post infiltration, whereas no necrosis was observed on the buffer infiltrated leaves (Figure 1C). It was observed that protein PeBb1 was stable and maintained its elicitor type activity of inducing necrotic lesions for 15 min at 4 °C or 25 °C, while it got denatured at 50, 75, and 100 °C (Supplementary Figure S1). Similarly, PeBb1 necrosis-inducing activity was observed only at a suitable pH range. At incubation for overnight in different pH solutions (pH 4, 6, 8, and 10) at 4 °C, necrotic reaction of PeBb1 was observed only at pH 6 or 8.
2.4. Effect of PeBb1 Elicitor on the Fecundity Rate of M. persicae
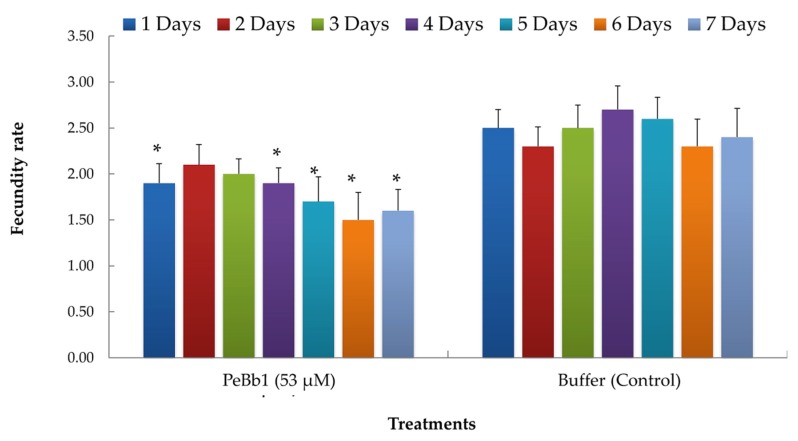
Results of bioassay with PeBb1 elicitor protein showed a significant reduction in mean aphid fecundity as compared to the control (F3,252 = 9.58, p < 0.001), while the interaction of fecundity with time and time alone exhibited no significant effect (Table 1). In the control treatments, mean fecundity rate of aphids was 2.5 nymph-1 day-1 female-1. Among protein concentrations, minimum mean fecundity rate (1.7 nymph-1 day-1 female-1) was recorded for the highest concentration (53 μM) and it was significantly different from the control treatment (Figure 4). However, other two concentrations (i.e., 26 and 35 μM) showed a mean fecundity rate of 2.1 nymph-1 day-1 female-1 without any significant difference from the control treatment.
Table 1.
Factorial analysis of variance for the effect of elicitor protein PeBb1 extracted from B. bassiana ARSEF 2860 strain on the fecundity rate of M. persicae.
| SOV | DF | SS | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Concentration | 3 | 18.67 | 6.224 | 9.58 | <0.001 |
| Time | 6 | 3.94 | 0.656 | 1.01 | 0.4198 |
| Concentration × Time | 18 | 3.18 | 0.177 | 0.27 | 0.9989 |
| Error | 252 | 163.80 | 0.650 | ||
| Total | 279 | 189.586 | |||
| GM/CV | 2.19/36.77 | ||||
p < 0.05 = significant and p < 0.001 = highly significant; one-way factorial ANOVA at α = 0.05; MS = mean sum of squares; SS = sum of squares; DF = degree of freedom; F = F-statistic; CV = coefficient of variation; GM = grand mean.
Figure 4.
Mean fecundity of M. persicae recorded for the highest concentration of elicitor PeBb1 and for the control treatment. The columns represent mean fecundity rate ± SE (n = 10). Asterisk symbols indicate significant difference among the control and protein treatments (Student’s t-test at p ≤ 0.05).
2.5. Expression of Plant Defense-Related Genes in Response to PeBb1 Elicitor
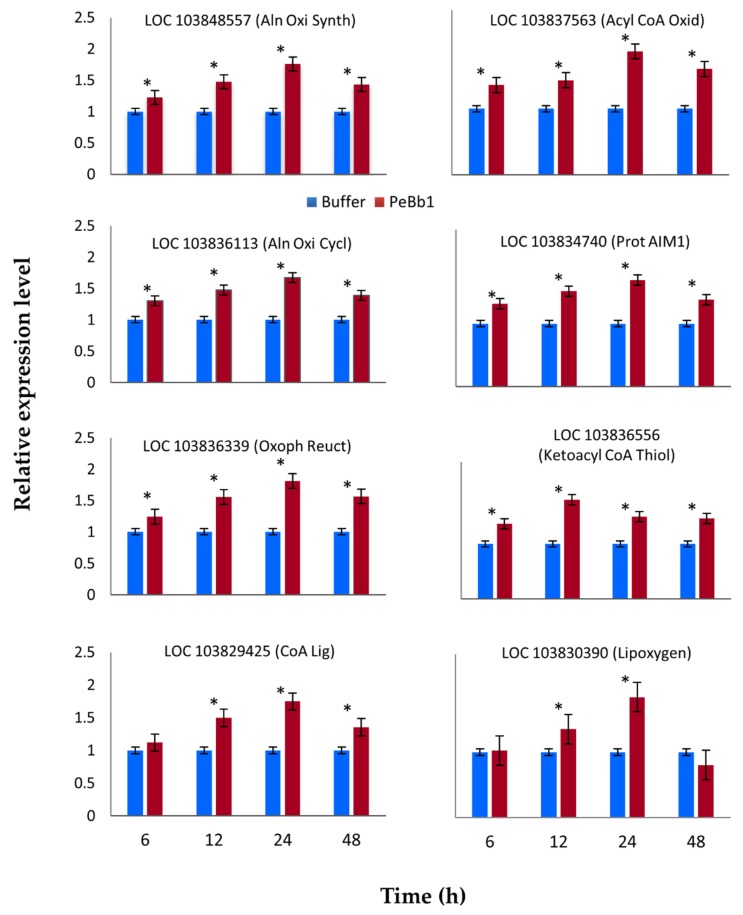
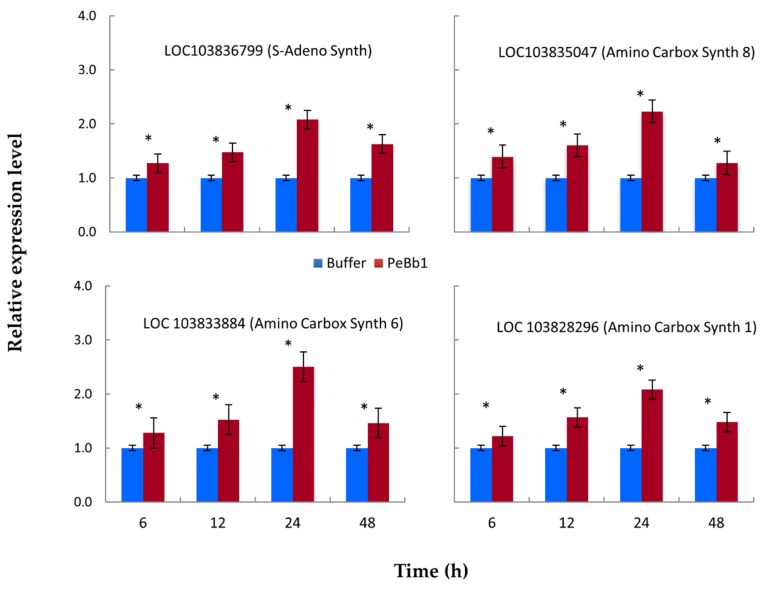
To clarify one of the putative modes of PeBb1 action resulting in plant resistance, we evaluated the expression profiles of ET and JA pathway related marker genes inducing plant defense mechanisms against aphids up to 48 h after treatment with PeBb1 (53 µM) or buffer. RT-qPCR analyses showed that JA pathway-associated genes were moderately elevated, while ET pathway-associated genes exhibited highest levels of expression. Almost all the JA pathway associated key genes (i.e., LOC103848557, LOC103836113, LOC103836339, LOC103829425, LOC103837563, LOC103834740 and LOC103836556) were up-regulated except one gene LOC103830390 that exhibited no considerable difference between the protein- and buffer-treated plants after 6 h of aphid attack and was down-regulated after 24 h of aphid attack (Figure 5). Maximum gene expression level was observed at 12 and 24 h post aphid infestation. On the other hand, all ethylene pathway linked genes (i.e., LOC103836799, LOC103835047, LOC103833884, and LOC103828296) were up-regulated at each time after the application of elicitor and aphid attack (Figure 6).
Figure 5.
Relative expression levels of jasmonic acid (JA) pathway-related key genes determined at different time intervals post PeBb1 elicitor application. Blue and red columns show the result of buffer-treated (control) and elicitor-treated plants, respectively. For each gene, asterisk symbols show the significant difference among treatments (Student’s t-test at p ≤ 0.05).
Figure 6.
Relative expression levels of ethylene (ET) pathway-related key genes determined at different time intervals post PeBb1 elicitor application. Blue and red columns show the result of buffer-treated (control) and elicitor-treated plants, respectively. For each gene, asterisk symbols show the significant difference among treatments (Student’s t-test at p ≤ 0.05).
3. Discussion
Elicitor proteins perform an important function in signaling plant defense pathways and are being considered as novel biological pest management strategies. Many biotrophic and necrotrophic microorganisms including pathogenic fungi are the main sources of different microbial elicitors (PAMPs or MAMPs) [33]. This study comprised of purification, molecular characterization, and in vitro evaluation of a yet uncharacterized elicitor protein PeBb1 derived from B. bassiana for its putative role against M. persicae (green peach aphids). We purified and cloned a 19-kDa protein (PeBb1) from B. bassiana ARSEF 2860 strain, having the ability to induce necrosis in tobacco plants and to reduce the fecundity of green peach aphid M. persicae on Chinese cabbage B. rapa plants. Moreover, this PeBb1 protein was able to up-regulate the transitory or local expression of ET and JA pathways associated plant defense genes in aphid infested elicitor-treated plants.
Our results demonstrated that aphid individuals developed significantly slower on the elicitor-treated B. rapa plants as compared to control plants because the exogenous application of PeBb1 elicitor protein considerably reduced the fecundity rate of M. persicae on B. rapa plants as compared to control ones, suggesting the putative role of PeBb1 in induced systemic resistance in B. rapa against M. persicae. These results are in accordance to some previous works indicating the detrimental effects of exogenous applications of different elicitor molecules such as benzothiadiazole (BTH), JA, and methyl jasmonate (MJ) on the fitness traits and population growth of aphids [34,35]. Nevertheless, some studies have shown how elicitors induce resistance against different sucking and chewing insect pests by the use of co-expression of different proteinase inhibitors [33,36,37]. Likewise, the exogenous treatment of different plant defense related proteins such as polyphenol oxidase and proteinase inhibitors have been shown to significantly reduce the incidence of insect pests on tomato plants [34,38].
JA, SA, and ET pathways play a significant role in inducing plant resistance against insects. Results of this study are in line with a recent work by Basit et al. [39] who revealed that different concentrations of an elicitor protein PeBC1, derived from a necrotrophic fungus Botrytis cinerea, reduced the fecundity rate of M. persicae concomitantly with a significant up-regulation of the expression of different SA and JA pathways-linked genes in common beans (Phaseolus vulgaris). These plant defense pathways are involved in the signaling transduction and regulation of downstream plant defense genes, thereby instigating a more effective plant defense response against insect pests [39,40,41]. Feeding by aphids induce both local and systemic defense reactions in plants [42]. Our findings showed the local expression of ET and JA responsive genes in B. rapa leaves, though no systemic change in the expression levels of these genes has been observed. PeBb1 induced a significant and strong up-regulation of the expression of all ET and JA pathway related genes.
4. Materials and Methods
4.1. Rearing of Aphids
Individual clones of M. persicae (green peach aphid) were collected from the young seedlings of Chinese cabbage (Brassica rapa ssp. pekinensis) maintained in the greenhouse facility of Chinese Academy of Agricultural Sciences (CAAS), Beijing, China. Aphids were reared on the same plant species at 25 ± 2 °C temperature and 50–60% relative humidity under 16:8 h light: dark photoperiod. During the entire study period, plants were changed every week 33.
4.2. Plant, Pathogen and Bacterial Culture
Seeds of B. rapa ssp. pekinensis were allowed to germinate in a 9-cm Petri dish for 72 h at room temperature (27 °C) and then were transplanted to pots containing sterilized soil mixture in a growth chamber at 25 ± 2 °C temperature and 60% relative humidity under 16:8 h light:dark photoperiod. Similarly, plants of tobacco (Nicotiana tabacum cv. Samsun-NN) were raised in a growth chamber at 25 ± 2°C under a 16:8 h light:dark photoperiod. Fungal isolate of Beauveria bassiana strain ARSEF 2860 (BB-72) was maintained on solid media of potato dextrose agar (PDA: 20 g L−1 agar, 20 g L−1 dextrose and 200 g L−1 potato) and LBA agar (5 g L−1 yeast extract, 10 g L−1 tryptone and NaCl each and 15 g L−1 agar). Liquid medium used for bacterial culture was Luria-Bertani (LB) broth.
4.3. Isolation of Crude Protein
For primary culture of B. bassiana strain ARSEF 2860 (BB-72), 1 mL of conidial suspension (1.0 × 108 conidia mL−1) was added in 25 mL of Adámek’s liquid medium (40 g yeast extract (Difco, Detroit, MI, USA), 40 g dextrose and 30 g corn steep liquor (Sigma-Aldrich, Saint Louis, MO, USA)). Incubation of primary culture was done in a rotary shaker at 200 rpm at 25 °C for three days. Thereafter, secondary culture was made by accumulation of 10 mL primary culture into 1 L of Adámek’s liquid medium for six days at 140 rpm at 25 °C. The sample was centrifuged for 20 min at 12000 rpm at 4 °C, and the supernatant was filtered with a 0.45 μm pore size filter (Millipore Corp., Billerica, MA, USA) to acquire the filtrate. Ammonium sulfate (NH)4SO4 was added to fungal filtrate to attain 80% (w/v) relative saturation at 4 °C overnight and the complex was centrifuged for 15 min at 12000 rpm at 4 °C. The precipitate was dissolved in 30 mL buffer A (50 mM Tris-HCL, pH 8.0) and was dialyzed against the buffer A for 48 h to remove (NH)4SO4. The insoluble debris was removed from the dialysate by centrifugation for 15 min at 12000 rpm at 44 °C, and then the crude protein was filtered with a 0.22 μm pore size filter (Millipore, Corp., Billerica, MA, USA). The extracted crude protein was stored at -80 °C till further purification. The crude protein (50 μL) was analyzed for its potential to elicitate necrosis in tobacco plants.
4.4. Protein Purification and Mass Spectrometry
Purification was carried out with the ÄKTA Explorer 10 protein purification system (GE Healthcare, Piscataway, NJ, USA). The crude protein was loaded on an anion exchange chromatography column (HP Q HiTrapTM 5 mL, GE Healthcare, Uppsala, Sweden), previously equilibrated with buffer A (50 mM Tris-HCL, pH 8.0). The bounded proteins were eluted with buffer B (50 mM Tris-HCL, 1.0 M NaCl, pH 8.0) at a flow rate of 2 mL min−1 and then all fractions were collected. Each fraction was applied to a desalting column (GE Healthcare, Uppsala, Sweden) and was observed for its anti-insect activity against M. persicae. Moreover, purified B. bassiana-derived protein was also tested for its ability to induce necrosis in the leaves of tobacco. The fraction that showed maximum necrosis induction was further purified using 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and was tested for necrosis-inducing potential of the purified protein fraction. The fractions that showed activity were collected and concentrated by ultrafiltration and were washed 3 times with buffer A (50 mM Tris-HCL, pH 8.0) and were stored at -80 °C. Protein sample isolated on SDS-PAGE gel was further characterized by mass spectrometry (MS) analysis (Beijing Protein Innovation Co. Ltd., Beijing, China). Using MASCOT search engine (Matrix Science Inc., London, UK; http://www.matrixscience.com), the tandem MS (MS-MS) data were analyzed automatically.
4.5. Gene Cloning
Using E.Z.N.A.® fungal RNA Kit (Omega Bio-Tek Inc., Norcross, GA, USA), total RNA was extracted from the fungal cells. First-strand cDNA was synthesized using TransScript® One-Step gDNA Removal and cDNA Synthesis Super Mix Kit (TransGen Biotech, Beijing, China). On the basis of peptide sequence retrieved from the National Center for Biotechnology Information (NCBI) database and the de novo sequencing acquired by MS analysis, a pair of gene-specific primers (forward primer: 5′-ATGCAAGATGCGTTGCCAGAG-3′ and reverse primer: 5′-TCAGCCATAATGGACACATTGAC-3′) was designed to amplify the entire coding sequence of elicitor protein-encoding gene of B. bassiana. Primer pairs were designed based on the sequence of XM_008605366 gene encoding protein of XP_008603588.1.
The amplified gene was cloned into the pET-28a(+) vectors (Novagen, USA) and was transformed into the competent cells of Escherichia coli BL21 (DE3) (TransGen Biotech, Beijing, China), and then was cultured in LB broth in a shaker for 14 h. The cells were harvested by centrifugation and the plasmids were extracted from these harvested cells. The full-length cDNA of PeBb1 gene was ratified by DNA sequencing (Beijing Genomics Institution, Beijing, China).
4.6. Expression and Purification of Recombinant Protein
In order to express the elicitor as a C-terminally His6-tagged protein, the PeBb1 gene was inserted into the BamHI/HindIII sites of His-tagging pET-28a(+) vector (Novagen, San Diego, CA, USA) and was then transformed into the competent cells of E. coli strain BL21 (DE3) (TransGen Biotech, Beijing, China). The primers designed included the BamH1/HindIII restriction sites and the 5′ and 3′ ends of the PeBb1 gene (forward primer: 5′-GGATCCATGCAAGATGCGTTGCCAGAG-3′ and reverse primer: 5′-AAGCTTTCAGCCATAATGGACACATTGAC-3′ (restriction sites are underlined). The clones of PeBb1 gene insertion were identified by PCR. The PCR thermal protocol was as follows; 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 62 °C for 30 s, and 72 °C for 30 s and a final extension at 72 °C for 10 min. Amplified DNA was isolated on a 1% agarose gel via gel electrophoresis and was detected visually by staining with Gold View (SBS Genetech, Beijing, China) using Trans2K® Plus II DNA Marker (TransGen Biotech, Beijing, China).
For the expression of recombinant PeBb1 protein, bacteria were cultured for 4 h at 37 °C, and then 200 μM isopropyl β-D-1-thiogalactopyranoside (Sigma, St. Louis, MO, USA) was added to the culture when OD600 was 0.6–0.8, to subsequently induce the recombinant protein for 14–16 h at 200 rpm and at 16 °C. Bacterial cells were obtained by centrifugation and then were re-suspended in buffer C (50 mM Tris-HCl, 200 mM NaCl, pH 8.0) and the cells were disrupted by ultrasonic disruptor for three times. Supernatant having the recombinant protein was collected by centrifugation for 30 min at 12000 rpm. PeBb1 protein was further purified by affinity chromatography through a His-Trap HP column (GE Healthcare, Waukesha, WI, USA) using loading buffer C. It was directly eluted with buffer D (50 mM Tris-HCl, 200 mM NaCl, 500 mM imidazole, pH 8.3) and was subsequently desalted with buffer E (50 mM Tris-HCl, pH 8.3) in a HiTrapTM desalting column (GE Healthcare, Waukesha, WI, USA). The molecular weight was examined in a 12% SDS-PAGE gel and a protein marker (Thermo Scientific, Rockford, IL, USA) was used to assess the apparent molecular weight of the purified recombinant elicitor protein.
4.7. Characterization of PeBb1 Elicitor Protein
Following the protein purification, the necrosis-inducing activity of protein was assayed in 8-week old plants of tobacco (N. tabacum cv. Samsun-NN). For this purpose, mesophyll tissues of completely developed leaves were infiltrated using mock buffer (50 µL of 50 mM Tris-HCL, pH 8.0) with the help of a syringe (without needle) covering an area of 1 cm2. The necrosis symptoms were precisely examined after 24 h according to the method described by D’Silva and Heath [43]. To check the heat stability and pH of the elicitor, the recombinant PeBb1 was treated at different temperatures (i.e., 4, 25, 50, 75 and 100 ℃) for 15 min and also at different pH values (i.e., 4, 6, 8 and 10) overnight. The necrosis induction by the elicitor protein was observed after 24 h.
4.8. Bioassay of Elicitor Activity against M. Persicae
The laboratory bioassay was carried out to assess the activity of elicitor PeBb1 against M. persicae (green peach aphid). Treatments included three concentrations of the elicitor protein (i.e., 26, 35, and 53 μM) and one control (buffer A; 50 mM Tris-HCl; pH 8.0). All protein concentrations were measured by BCA Protein Assay Kit (Pierce, Rockford, IL, USA). For bioassay, potted Chinese cabbage (B. rapa) plants at 4-leaf stage were used. Each plant was sprayed by approximately 4 mL of the elicitor PeBb1 solution with the help of an aerosol spray bottle, and was placed for 24 h to dry. Ten freshly moulted (0 to 6 h old) adult apterous aphids were confined for 12 h on each treated cabbage plant, and then all aphid individuals were removed, while leaving 10 active and healthy aphid nymphs each plant. In order to determine the fecundity, one survived adult aphid per leaf was confined using a small leaf cage. Data regarding number of offspring per adult aphid was noted for one week. Ten replications were maintained for each treatment. All treatments were maintained at 50 to 60% relative humidity and 25 ± 2 °C temperature.
4.9. Expression Analysis of Plant Defense-Related Genes Using RT-qPCR
To assess the plant defense mechanisms induced by the exogenous foliar application of PeBb1 elicitor on B. rapa plants, relative expression of key genes associated with B. rapa’s ET and JA pathways (Table 2) were examined by real-time quantitative PCR (RT-qPCR). Using the EasyPure® Plant RNA Kit (TransGen Biotech, Beijing, China), total RNA was extracted from the leaves of aphid infested protein-treated and buffer-treated (control) plants of B. rapa. TransScript® All-in-One SuperMix for qPCR Kit (TransGen Biotech, Beijing, China) was used to synthesize first-strand cDNA. The relative expression levels of both type of defense-related genes were determined by RT-qPCR performed on ABI 7500 Real-Time PCR System thermocycler (Applied Biosystems, Foster City, CA, USA) using TransStart® Green qPCR SuperMix UDG (TransGen Biotech, Beijing, China). Three independent biological and three technical replicates were performed for each sample. Actin was used as a quantitative control. Relative expression levels were calculated using the 2–ΔΔCT method [44]. Primer pairs used for the amplification of these plant defense associated genes are detailed in Table 3.
Table 2.
Key genes associated with the ethylene (ET) and jasmonic (JA) pathways of B. rapa ssp. pekinensis.
| Sr. No. | Gene ID | Gene Biochemical Name (Abbreviated) | Gene Biochemical Name (Detailed) |
|---|---|---|---|
| Jasmonic Pathway | |||
| 1 | LOC103848557 | Aln Oxi Synth | Allene oxide synthase, chloroplastic |
| 2 | LOC103836113 | Aln Oxi Cycl | Allene oxide cyclase 4, chloroplastic |
| 3 | LOC103836339 | Oxoph Reuct | Putative 12-oxophytodienoate reductase-like protein 1 |
| 4 | LOC103829425 | CoA Lig | 4-coumarate--CoA ligase-like 4 |
| 5 | LOC103837563 | Acyl CoA Oxid | peroxisomal-like Acyl-coenzyme A oxidase 2 |
| 6 | LOC103834740 | Prot AIM1 | Peroxisomal fatty acid beta-oxidation multifunctional protein AIM1-like |
| 7 | LOC103836556 | Ketoacyl CoA Thiol | Peroxisomal-like 3-ketoacyl-CoA thiolase |
| 8 | LOC103830390 | Lipoxygen | Lipoxygenase 2, chloroplastic-like |
| Ethylene Pathway | |||
| 1 | LOC103836799 | S-Adeno Synth | S-adenosylmethionine synthase-like |
| 2 | LOC103835047 | Amino Carbox Synth 8 | 1-aminocyclopropane-1-carboxylate synthase 8 |
| 3 | LOC103833884 | Amino Carbox Synth 6 | 1-aminocyclopropane-1-carboxylate synthase 6 |
| 4 | LOC103828296 | Amino Carbox Synth 1 | 1-aminocyclopropane-1-carboxylate oxidase 1 |
Table 3.
Primer sequences used for RT-qPCR amplifications of the defense-related and quantitative control genes of B. rapa spp. pekinensis.
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Actin | TATGCTCTTCCACATGCTATTC | CCTTACGATTTCACGCTCTG |
| 103848557 | TTACTTCCACAAGCAAAAACCC | TTATCAACATCGAACAAAACCG |
| 103836113 | CGACCTCGTCCCTTTCACTA | AGCGTTCGCCTTTCTTCTCA |
| 103836339 | TCAGAACACTCTATTGCCACAT | CCTTCAAACGCCTTCCTCAT |
| 103829425 | CTATGGGCTACTCTGCTTCACT | CTCCGTCACCTCCTTACTCAA |
| 103837563 | CCAACGCACGACACCAAAGG | GAACGGAACCGCAAAGCCCC |
| 103834740 | CCTCTTTCGGCTTGCCATTA | TCCCATTTCTCCCGCTTTTA |
| 103836556 | GCTTCATCATCTTCAACCTC | CTTCTCTATCACCGCTCTCA |
| 103830390 | GGTCTTCACGCCAGGTTATG | ATTGTCTGTTTGCCGCTATT |
| 103836799 | GCAAAGTCCTCGTCAACATC | TCATCAGTAGCGTACCCAAA |
| 103835047 | CCTGGAGATGCTTTCTTGCT | TTAGTTCGGTTCGGGTTGTT |
| 103833884 | CATCCGCAAGAGCAAACTAC | CCATCCATATGAACAAACCG |
| 103828296 | GTGAAAATCTTGGTCTCCCTCG | CAGTATGTTCTCTCAGCCCTCT |
4.10. Statistical Analysis
All experiments were conducted in three independent replications and the data are presented as the means along with standard errors. Using SAS® Statistical Software Program (SAS Institute Inc., Cary, NC, USA), significant differences between the treatments (elicitor concentrations) were calculated by one-way factorial analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) test was used for pairwise comparisons at the probability level of 0.05. Comparative CT (2–ΔΔCT) method was used to determine the RT-qPCR expression levels. Moreover, data of elicitor-treated and buffer-treated (control) plants were compared using the Student’s t-test at the probability level of 0.05.
5. Conclusions
This study reports the purification, characterization, and evaluation of a yet uncharacterized protein elicitor PeBb1 from B. bassiana strain ARSEF 2860 as putative microbial pest control tool against green peach aphid M. persicae. The bioassays with recombinant PeBb1 protein showed a significant reduction in mean aphid fecundity and a significant up-regulation of the expression levels of ET and JA pathway related genes in the protein-treated B. rapa ssp. pekinensis plants. These findings suggest that PeBb1 can be a prospective candidate molecule to enhance the defense mechanism in B. rapa ssp. pekinensis. Hence, activating plant defense responses with PeBb1 elicitor protein, an alternative method like biopesticides and transgenic crops, can be exploited to control the most destructive pest M. persicae and to reduce the risk of environmental contamination with synthetic chemical insecticides. Our research presents the potential of a yet uncharacterized elicitor protein from fungus B. bassiana to fortify host plant resistance against pests. However, additional studies are needed to better understand the processes by which the changes are induced in B. rapa by the elicitor PeBb1 and to find out how exactly these responses influence the fitness traits such as fecundity of aphids M. persicae, and to see if this protein is produced by other strains of B. bassiana and elicits similar defense response in other plant species. Moreover, assessing sublethal effects of PeBb1 elicitor on other life-history traits of aphids, such as nymphal development time, adult longevity, mortality, intrinsic rate of population increase etc., constitutes the future perspective of the study.
Acknowledgments
Authors thank Lihua Guo and Xiufen Yang of the Institute of Plant Protection, Chinese Academy of Agricultural Sciences for their valued help in the improvement of experimental protocols and for their technical assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/2/111/s1, Figure S1: The hypersensitive response induced by elicitor protein PeBb1 (53 µM L−1) in leaves of tobacco (Nicotiana tabacum cv. Samsun-NN) at different temperature regimes captured 24 h post infiltration.
Author Contributions
Conceptualization, T.N. and D.Q.; data curation, T.N.; formal analysis, T.N. and M.Z.M.; funding acquisition, D.Q.; investigation, T.N.; methodology, T.N., A.H., and A.B.; project administration, D.Q.; resources, D.Q.; software, M.Z.M., I.N., and T.N.; supervision, D.Q.; writing—original draft, T.N. and T.A.; writing—review and editing, D.Q. and M.Z.M. All authors have read and agree to the published version of the manuscript.
Funding
This research work was financially aided by the Graduate School of Chinese Academy of Agricultural Sciences (GSCAAS) and by the National Key Research and Development Program of China (2017YFD0200900).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Oerke E.-C. Crop losses to pests. J. Agric. Sci. 2006;144:31–43. doi: 10.1017/S0021859605005708. [DOI] [Google Scholar]
- 2.Deutsch C.A., Tewksbury J.J., Tigchelaar M., Battisti D.S., Merrill S.C., Huey R.B., Naylor R.L. Increase in crop losses to insect pests in a warming climate. Science. 2018;361:916–919. doi: 10.1126/science.aat3466. [DOI] [PubMed] [Google Scholar]
- 3.Chowański S., Kudlewska M., Marciniak P., Rosiński G. Synthetic Insecticides—Is There an Alternative? Polish J. Environ. Stud. 2014;23:291–302. [Google Scholar]
- 4.Mesnage R., Séralini G.-E. Editorial: Toxicity of Pesticides on Health and Environment. Front. Public Health. 2018;6:268. doi: 10.3389/fpubh.2018.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Copping L.G., Menn J.J. Biopesticides: A review of their action, applications and efficacy. Pest Manag. Sci. Former. Pestic. Sci. 2000;56:651–676. doi: 10.1002/1526-4998(200008)56:8<651::AID-PS201>3.0.CO;2-U. [DOI] [Google Scholar]
- 6.Burges H.D. Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments. Springer; Berlin/Heidelberg, Germany: 2012. [Google Scholar]
- 7.Ruiu L. Microbial biopesticides in agroecosystems. Agronomy. 2018;8:235. doi: 10.3390/agronomy8110235. [DOI] [Google Scholar]
- 8.Wraight S.P., Carruthers R.I. Biopesticides: Use and Delivery. Springer; Berlin/Heidelberg, Germany: 1999. Production, delivery, and use of mycoinsecticides for control of insect pests on field crops; pp. 233–269. [Google Scholar]
- 9.Vega F.E., Meyling N.V., Luangsa-ard J.J., Blackwell M. In: Chapter 6-Fungal Entomopathogens. Vega F.E., Kaya H.K., editors. Academic Press; San Diego, CA, USA: 2012. pp. 171–220. [Google Scholar]
- 10.Ortiz-Urquiza A., Keyhani N.O., Quesada-Moraga E. Culture conditions affect virulence and production of insect toxic proteins in the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci. Technol. 2013;23:1199–1212. doi: 10.1080/09583157.2013.822474. [DOI] [Google Scholar]
- 11.Zimmermann G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 2007;17:553–596. doi: 10.1080/09583150701309006. [DOI] [Google Scholar]
- 12.Butt T.M. Agricultural Applications. Springer; Berlin/Heidelberg, Germany: 2002. Use of entomogenous fungi for the control of insect pests; pp. 111–134. [Google Scholar]
- 13.Fan Y., Fang W., Guo S., Pei X., Zhang Y., Xiao Y., Li D., Jin K., Bidochka M.J., Pei Y. Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl. Environ. Microbiol. 2007;73:295–302. doi: 10.1128/AEM.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas M.B., Read A.F. Can fungal biopesticides control malaria? Nat. Rev. Microbiol. 2007;5:377. doi: 10.1038/nrmicro1638. [DOI] [PubMed] [Google Scholar]
- 15.Hegedus D.D., Khachatourians G.G. The impact of biotechnology on hyphomycetous fungal insect biocontrol agents. Biotechnol. Adv. 1995;13:455–490. doi: 10.1016/0734-9750(95)02006-O. [DOI] [PubMed] [Google Scholar]
- 16.St Leger R., Screen S., Butt T., Jackson C., Magan N. Fungi as Biocontrol Agents Progress, Probl. Potential. CABI; Wallingford, UK: 2001. Prospects for strain improvement of fungal pathogens of insects and weeds; pp. 219–237. [Google Scholar]
- 17.St Leger R.J., Wang C., Stock S., Vandenberg J., Glazer I. Entomopathonic fungi and the genomics era. Insect Pathog. Mol. Approaches Tech. CABI. 2009:365–400. [Google Scholar]
- 18.Ortiz-Urquiza A., Garrido-Jurado I., Borrego A., Quesada-Moraga E. Effects of cultural conditions on fungal biomass, blastospore yields and toxicity of fungal secreted proteins in batch cultures of Metarhizium anisopliae (Ascomycota: Hypocreales) Pest Manag. Sci. 2010;66:725–735. doi: 10.1002/ps.1934. [DOI] [PubMed] [Google Scholar]
- 19.Quesada-Moraga E., Carrasco-Díaz J., Santiago-Álvarez C. Insecticidal and antifeedant activities of proteins secreted by entomopathogenic fungi against Spodoptera littoralis (Lep. Noctuidae) J. Appl. Entomol. 2006;130:442–452. doi: 10.1111/j.1439-0418.2006.01079.x. [DOI] [Google Scholar]
- 20.Ortiz-Urquiza A., Vergara-Ortiz A., Santiago-Álvarez C., Quesada-Moraga E. Insecticidal and sublethal reproductive effects of Metarhizium anisopliae culture supernatant protein extract on the Mediterranean fruit fly. J. Appl. Entomol. 2010;134:581–591. doi: 10.1111/j.1439-0418.2010.01533.x. [DOI] [Google Scholar]
- 21.Ortiz-Urquiza A., Keyhani N. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects. 2013;4:357–374. doi: 10.3390/insects4030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaber L.R., Ownley B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control. 2018;116:36–45. doi: 10.1016/j.biocontrol.2017.01.018. [DOI] [Google Scholar]
- 23.Basit A., Hanan A., Nazir T., Majeed M.Z., Qiu D. Molecular and Functional Characterization of Elicitor PeBC1 Extracted from Botrytis cinerea Involved in the Induction of Resistance against Green Peach Aphid (Myzus persicae) in Common Beans (Phaseolus vulgaris L.) Insects. 2019;10:35. doi: 10.3390/insects10020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu D., Mao J., Yang X., Zeng H. Expression of an elicitor-encoding gene from Magnaporthe grisea enhances resistance against blast disease in transgenic rice. Plant Cell Rep. 2009;28:925–933. doi: 10.1007/s00299-009-0698-y. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W., Yang X., Qiu D., Guo L., Zeng H., Mao J., Gao Q. PeaT1-induced systemic acquired resistance in tobacco follows salicylic acid-dependent pathway. Mol. Biol. Rep. 2011;38:2549–2556. doi: 10.1007/s11033-010-0393-7. [DOI] [PubMed] [Google Scholar]
- 26.Thomma B.P., Nürnberger T., Joosten M.H. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell. 2011;23:4–15. doi: 10.1105/tpc.110.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dangl J.L., Jones J.D.G. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- 28.Chisholm S.T., Coaker G., Day B., Staskawicz B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Brugger A., Lamotte O., Vandelle E., Bourque S., Lecourieux D., Poinssot B., Wendehenne D., Pugin A. Early signaling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 2006;19:711–724. doi: 10.1094/MPMI-19-0711. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel B.N., Brooks D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002;5:325–331. doi: 10.1016/S1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 31.De Ilarduya O.M., Xie Q., Kaloshian I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant-Microbe Interact. 2003;16:699–708. doi: 10.1094/MPMI.2003.16.8.699. [DOI] [PubMed] [Google Scholar]
- 32.Koornneef A., Pieterse C.M.J. Cross talk in defense signaling. Plant Physiol. 2008;146:839–844. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maffei M.E., Arimura G.-I., Mithöfer A. Natural elicitors, effectors and modulators of plant responses. Nat. Prod. Rep. 2012;29:1288–1303. doi: 10.1039/c2np20053h. [DOI] [PubMed] [Google Scholar]
- 34.Boughton A.J., Hoover K., Felton G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2006;120:175–188. doi: 10.1111/j.1570-7458.2006.00443.x. [DOI] [Google Scholar]
- 35.Cooper W.R., Goggin F.L. Effects of jasmonate-induced defenses in tomato on the potato aphid, Macrosiphum euphorbiae. Entomol. Exp. Appl. 2005;115:107–115. doi: 10.1111/j.1570-7458.2005.00289.x. [DOI] [Google Scholar]
- 36.Bostock R.M., Karban R., Thaler J.S., Weyman P.D., Gilchrist D. Signal interactions in induced resistance to pathogens and insect herbivores. Eur. J. Plant Pathol. 2001;107:103–111. doi: 10.1023/A:1008703904253. [DOI] [Google Scholar]
- 37.Hamza R., Pérez-Hedo M., Urbaneja A., Rambla J.L., Granell A., Gaddour K., Beltrán J.P., Cañas L.A. Expression of two barley proteinase inhibitors in tomato promotes endogenous defensive response and enhances resistance to Tuta absoluta. BMC Plant Biol. 2018;18:24. doi: 10.1186/s12870-018-1240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thaler J.S., Stout M.J., Karban R., Duffey S.S. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 1996;22:1767–1781. doi: 10.1007/BF02028503. [DOI] [PubMed] [Google Scholar]
- 39.Thaler J.S., Humphrey P.T., Whiteman N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012;17:260–270. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Schaller F., Schaller A., Stintzi A. Biosynthesis and metabolism of jasmonates. J. Plant Growth Regul. 2004;23:179–199. doi: 10.1007/s00344-004-0047-x. [DOI] [Google Scholar]
- 41.Moran P.J., Thompson G.A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001;125:1074–1085. doi: 10.1104/pp.125.2.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Vos M., Van Oosten V.R., Van Poecke R.M.P., Van Pelt J.A., Pozo M.J., Mueller M.J., Buchala A.J., Métraux J.-P., Van Loon L.C., Dicke M. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant-Microbe Interact. 2005;18:923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- 43.D’Silva I., Heath M.C. Purification and characterization of two novel hypersensitive response-inducing specific elicitors produced by the cowpea rust fungus. J. Biol. Chem. 1997;272:3924–3927. doi: 10.1074/jbc.272.7.3924. [DOI] [PubMed] [Google Scholar]
- 44.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.