Abstract
Numerous studies have demonstrated the role of the microbiota in supporting the physiological functions, owing to its metabolomic component. The presence of biocomponents generally leads to the correction of the microbial pattern correlated with the reduction of oxidative pressure. This study aims to present the main processes that correlate the bioavailability and bioactivity of some functional components through the action of the human microbiota. The use of probiotics and prebiotics is an innovative manner involving alternatives that increase the bioavailability of certain natural or metabolic components has been proposed. Probiotic strains (Saccharomyces cerevisiae or Lactobacillus (L.) plantarum) may represent an intermediary for increasing the antioxidant bioactivity, and they may be administered in the form of a biomass enriched with functional compounds, such as phenolic acids. The limiting effect of gastrointestinal transit is, in several cases, the key to the biopharmaceutical value of new products (or supplements). The identification of newer ways of formulating supplements also involves the compatibility of different types of products, the testing of bioaccessibility, and the elimination of biotransformations.
Keywords: probiotic, bioaccesibility, polyphenols, absorption, pattern
1. Introduction
Numerous studies on gut microbiota have confirmed its role in the appearance and evolution of some diseases like inflammatory bowel disease, obesity, diabetes, cardiovascular diseases, cancer, allergy, and neurological disorders [1]. Various factors are known to influence the microbiota of an individual, including diet, genetic factors, and the administration of certain drugs (e.g., antibiotics). These changes may persist for several days, weeks, or even permanently affect the microbiota, inducing dysbiosis. The health status of the microbiota directly influences the assimilation of some nutrients, compounds from food or compounds administered in order to maintain the health such as dietary supplements based on natural compounds, as well as their metabolism and bioavailability. Some of these compounds, which are derived from exogenous sources (e.g., polyphenols) may improve the status of the microbiota and may reduce its oxidative stress [2]. Defined as non-nutrients, secondary plant metabolites, antioxidants, or bioactive substances in plants [3], polyphenols represent a class of compounds that has been studied not only because of their biological properties (such as immunomodulatory and anti-inflammatory [4,5,6], cardioprotective [7,8], anti-diabetic and anti-obesity [9,10], anti-cancer [11,12,13], neuroprotective [14,15], anti-asthmatic [16,17], anti-hypertensive [18], anti-aging [8], hepatoprotective [19,20], antibacterial [21,22], anti-fungal, and antiviral [23,24]), but also because they are present in most types of diet. The main sources of polyphenols in the diet include fruits, vegetables, tea, and coffee, but they can also come from the administration of various food supplements.
The data on the role of the human microbiota in sustaining the general state of health are increasingly being used in the scientific community. The importance of the microbial pattern is determined by its involvement in the metabolism of different bioactive compounds resulting from the consumption of food and/or the administration of functional supplements. Together with probiotics and prebiotics, natural biocomponents (such as polyphenolic acids) are a target in the fight against chronic pathologies. These targets are determined by inflammatory progression, which is the true cause of most degenerative pathologies, and sustained by the constant pressure of oxidative stress [25].
The metabolism and process of absorption of polyphenols has been intensively studied considering their high therapeutic potential and the involved benefits to human health, also well known for some of polyphenols [26]. On ingestion, a part of them gets absorbed at the level of the small intestine, and most of the polyphenols are transformed by the microbiota in the colon. Seemingly, only a part of the bacteria strains can metabolize these compounds, and compositional changes of the bacterial strains or dysbiosis can affect the process. The use of dietary supplements containing beneficial bacteria, which are capable of modulating the microbiota for transforming these substrates, is an alternative with significant health implications. However, in the colon, the role of polyphenols is not only to act as a substrate for the enzyme apparatus of the microbiota or as a carbon source, in fact, they can also modulate the population of microorganisms due to the antimicrobial effect that many polyphenols have [27].
The antimicrobial effect is one of the most important properties from which the interest for these compounds starts. The antimicrobial effects of polyphenols are exerted by several mechanisms, such as: direct action on certain target bacterial strains, reducing the adhesion capacity of pathogenic strains, or disrupting ionic fluxes at the cell membrane [22,28]. One of the main mechanisms by which these compounds act is by stimulating the multiplication of favorable strains [29] for correcting the microbial and metabolomic patterns [1]. The bioavailability of the functional components represents the property that controls the bioactivity in vivo. For example, gallic acid and its derivatives are directly involved in the synthesis of short-chain fatty acids (SCFAs). Their biological action is mediated through the human microbiota, leading to an improvement of the physiological functions [30]. Thus, the clinical yield is controlled in such situations by the percent of bioavailability. In general, it is considered to be low [31]. In several cases (such as in the administration of functional products), the control of the clinical efficiency occurs through the products that result from the degradation of the majority compound, that determines the known general effect [32]. These data are usually considered based on the knowledge gained from traditional medicines.
2. Gut Microbiota and Polyphenols
The interaction between the polyphenols and the microbiota can be approached both from the perspective of how they are metabolized by the microbiota and also how they can modulate the microbiota to increase their impact in the prevention and improvement of some diseases [27,33].
Although in vivo studies provide the most valuable data about these interactions, in vitro studies involving the reproduction of gastrointestinal conditions provide extremely useful information for understanding the process of these interactions, which are not yet fully elucidated for their use in practice; for example, in obtaining a new type of dietary supplements.
Due to the chemical diversity of polyphenolic compounds, it is difficult to predict how the microbiota can respond to their action. They differently modulate the microbiota, favoring the development of some bacterial strains, and act like a prebiotic. A recent study revealed that the Curcuma longa extract with a high curcumin content (Table 1) modulates the microbiota of patients with hypertension by improving the ratio of SCFAs, which strongly influences the Enterobacteriaceae group and negatively influences the Bacteroides-Prevotella-Porphyromonas groups [29]. Polyphenols from the Lonicera caerulea L. berry reduced the Firmicutes/Bacteroidetes ratio in the fecal microbiota of a mouse model with an induced high-fat diet (HFD) and increased the relative abundance of Bacteroides and Parabacteroides. The results suggested the potential use of these extracts in microbiota modulation for the attenuation of inflammation present in non-alcoholic fatty liver disease [34]. The administration of a Concord grape extract stabilized with soy protein into mice on HFD showed a significant increase in Akkermansia muciniphila in parallel with the corresponding decrease in Firmicutes to Bacteroidetes. Grape extract modulates the gut microbiota and decreases the intestinal and systemic inflammation by improving the metabolic parameters [35].
Table 1.
The interaction between the microbiota and polyphenols.
| Polyphenols | Related Bacteria | Mechanisms | Effects | References |
|---|---|---|---|---|
| Curcumin |
Enterobacteriaceae group phylum Firmicutes |
Antimicrobial action | Increase antioxidant status/ Microbiota pattern modulation | [28] |
| Hesperetin/ Naringenin |
Helicobacter pylori, Escherichia coli, Salmonella aureus |
Antimicrobial action | Upper part of digestive tract Modulate gut microbiota fingerprint |
[36] |
| Vanillic acid and Gallic acid | Microbial diversity was altered | Changes in the bioavailability of phenolic acids | Induced dysbiosis | [37] |
| Catechin | Eubacterium sp. | Biotransformed by gut microbiota | Excretion in the feces, Xenobiotic |
[38] |
| Epicatechin | Bacteroides, Firmicutes, Bacteroidetes | Decrease serum levels of inflammatory cytokines including IL-2, IL-6, TNF-α, and MCP-1 | Bacterial ratio modulation | [34] |
| Concord grape polyphenols |
Akkermansia muciniphila
Firmicutes Bacteroidetes |
A lower intestinal expression of inflammatory markers (TNFα, IL-6, inducible nitric oxide synthase) and a gene for glucose absorption (Glut2) | Modify the structure of the gut microbiota | [35] |
| Epicatechin Catechin | Clostridium coccoides–Eubacterium rectale,Bifidobacterium spp. and Escherichia coli | Formation of 5- (3 ′, 4′-dihydroxyphenyl) -γ-valerolactone, 5-phenyl-γ-valerolactone and phenylpropionic acid | Bacterial ratio modulation | [39] |
| Cocoa flavanol |
Enterococcus spp. C. histolyticum |
Changes in C-reactive protein serum concentrations | Bacterial ratio modulation | [40] |
| Resveratrol | Coriobacteriaceae and Desulfovibrionaceae | Decreased serum interleukin-1 and tumor necrosis factor-alpha, | Prevents chronic inflammation, decreased oxidative stress, bacterial ratio modulation | [41] |
| Stilbenes | Bifidobacterium, Lactobacillus and Akkermansia | Alleviate symptoms of gut inflammation | prebiotic-like effect, modulation of pro-inflammatory cytokines | [42,43] |
| Caffeic acid, chlorogenic acid, ferulic acid, coumaric acid | Bifidobacterium, Lactobacillus | Microbiota pattern modulation | Increase butyrate production | [44,45] |
Determination of the flavanols (-) epicatechin and (+) -catechin monomers in a batch-culture fermentation system revealed that they are metabolized to 5- (3′,4′-dihydroxyphenyl) -γ-valerolactone, 5-phenyl-γ-valerolactone, and phenylpropionic acid via a transformation, that, for (+) catechin, first involves a conversion to (+) epicatechin. As a result, catechin significantly increased the growth of the Clostridium coccoides–Eubacterium rectale group, Bifidobacterium spp. and Escherichia coli, which, in total, had a significant inhibitory effect on the growth of the C. histolyticum group. The influence of epicatechin was less significant, leading to a more pronounced increase of the C. coccoides–E. rectale group [39]. The same monomeric flavanols that are present in a more complex matrix containing their oligomeric derivatives—cocoa drinks—were tested on human volunteers, as a daily dose (Table 1), resulting in an increase in the L. and Enterococcus spp. populations and a decrease in the C. histolyticum group. These results suggest that the two flavanols, which are present in a more complex matrix that brings an extra source of energy, can have far more beneficial effects on the microbiota [40].
After testing the individual effect of some polyphenols (such as naringenin, naringin, hesperetin, hesperidin, quercetin, rutin, and catechin) on the growth of bacterial strains representative of the human intestinal microbiota (such as Bacteroides galacturonicus and L. sp. Escherichia coli) revealed that their inhibition or activation seems to be in the function of the polyphenol structure. Catechin and rutin have no impact on bacterial strains. Aglycons of naringenin and hesperidin inhibit all bacteria. Quercetin had a strong impact, followed by naringenin and hesperidin [46]. Therefore, the manner in which polyphenols influence the microbiota, in general, and how each type of bacterial population in particular may be related to their structure, the matrix they are a part of, the dose of administration, and the type of diet, is a much more complex phenomenon. The administration of resveratrol—a low bioavailability polyphenol—along with a HFD to C57BL/6 mice has been shown to be beneficial in a dose-dependent manner for maintaining the microbiota’s compositional stability. The results showed that the administration of resveratrol does not reverse the changes induced by the HFD, but leads to a significant increase in Deferribacteraceae and a decrease in Desulfovibrionaceae [41].
3. Probiotics Strains and Bioavailability of Functional Compounds
The administration of probiotics, especially the strains of lactic bacteria, is currently an accepted method of controlling the microbial pattern at the microbiota level [47]. Although this is not a long-term solution, it can correct the possible temporary excesses of some drugs that intervene in the establishment of colonic dysbiosis [48]. The plasticity of the microbiota offers a high degree of acceptability of these strains, which eliminates the possible negative effects that may be caused by the introduction of new strains [49]. In the short-term, a correction can occur, but the high rate of rejection of the strains after cessation of administration is due to molecular incompatibility [50].
Thus, increasing the biopharmaceutical importance can be achieved in an innovative manner by enriching the biomass, especially yeasts (Saccharomyces sp.) with functional compounds such as polyphenolcarboxylics [51]. Normally, they are used as a carbon source by probiotic strains of the genus Lactobacillus and Bifidobacterium, but their role is much more complex. Their catabolism regulates the synthesis of SCFAs and corrects the microbial pattern in degenerative pathologies [2,29]. Partial in vitro studies conducted at the Faculty of Biotechnologies, Bucharest, Romania showed that the polyphenols present in matcha tea and green tea adopt a selective metabolization, the assimilation rate of which is about 20% (data unpublished as of yet). An increase, depending on the substrate, of the antioxidant status of the biomass was observed, with the effect of amplifying the bioactivity in vitro after their valorization.
It can thus be assumed, at least in vitro, that it has the capacity to adapt to stress as the exposure time increases, along with an intensification of the fermentative action. The claim is justified by increasing the amount of CO2 formed, which can support the use of the carbon source (data unpublished as of yet), by probiotic yeasts (S. cerevisiae and S. boulardii). This aspect has been demonstrated by previous studies that have shown the selective use, by certain strains, of bioactive components in extracts [29,52]. Increasing the number of favorable strains in the microbial pattern of the microbiota can thus have two causes, as detailed below:
The direct increase in the number of cells—a phenomenon that is much more difficult to prove—due to the lack of any direct scientific evidence;
Reduction (inhibition) of some pathogen strains and the release of ecological niches where Lactobacillus strains, for example, can proliferate [29].
On the other hand, the modulation of the microbial pattern does not have any proof of the persistence over time of the positive effect [53]. It is therefore assumed that the administered probiotics show this limiting effect. Thus, it can be considered that the limitation could be the result of the depletion of the carbon source that supports the rapid rate of multiplication. In contrast, if the effect is retained, it could be assumed that the elimination of a limiting factor (oxidative stress, [52]) may represent a new direction of valorization (research) that involves a multidisciplinary study [54].
Effect of Probiotics Strains on Bioavailability of Functional Compounds
In the recent years, the use of probiotics for microbiota modeling has proven to be an extremely promising alternative with beneficial health effects. To date, their contribution has been proven to enhance the nutrients’ bioavailability and reduce the risk of developing diseases. In cardiovascular diseases, a possible mechanism of action of probiotics is via the inhibition of hepatic lipogenesis and the lowering of blood glucose and insulinemia [55].
Increasing the synthesis of small-chain fatty acids due to the presence of probiotics seems to be a possible way of influencing the colon homeostasis by activating the free fatty acid receptors involved in regulating the immune system and secreting glucagon-like peptides-1 (GLP-1), which stimulates insulin secretion in pancreatic cells. In this way, autoimmune diseases such as type 1 diabetes can be managed [56].
The study of the microbiota for the promotion of functional food or dietary supplements is gaining new dimensions in the context of new research. The probiotic L. paracasei A221 (A221), for example, has proven to have a major influence on the functionality and bioavailability of kaempferol-3-o-sophroside (KP3S; a kaempferol-glucoside contained in kale). A221 strain can convert KP3S into aglycone by its unique beta-glucosidase activity; therefore, its administration plays an important role in the bioavailability of kaempferol and not least in increasing the anti-aging activity of KP3S in vivo [57]. The impact on the microbiota of a polyherbal formulation (TFLA) composed of equal quantities of Emblica officinalis, Terminalia chebula, and Terminalia belerica rich in phenolic, acids, flavonoids, and condensed and hydrolyzable tannins has increased considerably when it is administered with a probiotic consisting of L. plantarum, L. fermentum, and Bifidobacterium infantis. A polyphenolic prebiotic and probiotic formulation manifested synergistic effects, leading to an increase in the ratio of Bacteriodetes to Firmicutes, Lactobacillus spp., Ruminococcus spp. concentration and a decrease for Enterococcus, Staphylococcus, and E. coli [58].
A new approach to the probiotic–polyphenols relationship is the use of polyphenols to improve the physiological functionality of probiotics. This approach is extremely interesting in the context of developing new pharmaceutical and food probiotic and prebiotic formulas. The physiological functionalities of Lactobacillus strains with a probiotic potential have been tested at different concentrations of quercetin and resveratrol. The results show that the physiological functionality of bacterial strains can be enhanced in the presence of polyphenols. These improvements depend on the type and concentration of polyphenol and Lactobacillus strain tested. Quercetin showed better protective effects than resveratrol and among the tested strains, the best results were obtained for L. fermentum and L. plantarum strains [59].
4. Predictive Microbiota Response
The bioavailability of bioactive compounds is a predictive model of the effect that a functional product can determine. At present, different methods are used to improve the bioavailability and to increase the microbiota’s metabolic activity [60]. The whole process, which correlates with the physiological state of the target group, depends on the bioactive molecule and can influence the fermentative state if it has a concentration that determines a response in the microbial pattern [61].
The restoration of eubiosis has proven to be the key factor in clarifying the role of microbiota in the development of certain autoimmune pathologies. According to this study, the interaction of vital functions with the microbial pattern is the cause that leads to the appearance of pathologies that have no clear resolution in the medical field [48]. New types of drugs/supplements, with a clear direction toward pharmaceutical probiotics and prebiotic formulas, are seen as the solution to the physiological impairments that have arisen due to dysbiosis [62]. Increasing the number of strains is an alternative to the well known probiotics based on the species of the genera Lactobacillus, Bifidobacterium, and/or Streptococcus [48], although only the introduction of new strains cannot guarantee microbiota modulation over a period of time. The multitude of limiting factors (from genetic to behavioral) makes the success of such strategies a small one, in the current context [63].
The mechanisms that consider the bioavailability of some phytochemicals in the improvement of the metabolic syndrome are not well known. The biopharmaceutical effect depends on their chemical structure and their individual bioassimilation ability. A significant limiting factor is the lack of standardization, and the administration period is one of the two essential causes of reducing the efficacy in vivo [64].
An important example is the use of mushrooms with pharmaceutical potential (Table 1). This substrate is useful in combating cancer progression because the action of the bioactive molecules they contain expresses a low toxicity, with good tolerance in the human body [65]. Products that use components from wild mushrooms, and not only medicinal species, determine the restoration of eubiosis to target the population groups. By balancing the microbial pattern, it is possible to promote the synthesis of compounds that act as biomarkers towards improving health status. Such results can be considered to directly correlate with predictable metabolomic activity [48,52].
In fact, the diversity of molecules present in a functional compound (extract) cannot provide a predictable metabolomic pattern. This is an essential gap that could influence product bioactivity. This aspect may be significant in the consideration of nutraceuticals being clinically important for people suffering from diseases associated with oxidative stress [66]. The analysis of the metabolic differences determines the optimization of the potential biomarkers associated with the administration of compounds with a functional role. Thus, the studies characterizing the microbiota and the metabolomics pattern have led, through in vitro studies, to the better understanding of the physiological response, which should correlate with studies demonstrating bioavailability as an active support for the expression of bioactivity in vivo [67]. Metabolites produced by the microbiota play a significant role in modulating the physiological functions due to bioactivity. The new microbiota-generated compounds act as bioactivators [68].
5. The Causes of Disruption of the Microbiota-Mediated Response
Dysbiosis is the primary cause of the decreased efficiency of physiological response mediated by microbiota bioactivity. Changing the microbial balance is an effect that arises from different causes that can often act synergistically. If the temporary balancing by probiotics has a positive effect, over a period of time, this effect will be one of diminishing bioassimilation of molecules of pharmaceutical importance (e.g., antibiotics, flavonoids) [69]. The use of the main natural bioactive components as a carbon source [29] is a rarely accepted solution, but it does explain how the microbiota responds to the administration of some compounds that are considered to possess antibacterial effects.
Individual variability at the microbiota level is the key factor influencing the bioavailability of compounds in functional products [70]. Biotransformation of ingested components conditions the bioactivity, through the microbiota’s metabolizing capacity. Biotransformation is a process that is similar to the recognition process between substrate and receptor [71]. The process is conditioned by the classes of ingested compounds and the basic chemical structure. The bioavailability process will be influenced by the relationship between biotransformation and catabolization due to the chemical structure. The individual variation will balance the whole process, which indirectly controls the bioactivity. This equilibrium will determine which molecules may be active for certain target population groups, which increases the resultant biopharmaceutical value of the functional products [72].
The disruption of microbial eubiosis (the critical point for establishing dysbiosis) is the cause of physiological impairments associated with microbial infection. The process is correlated with a decrease in the number of strains of the genera Lactobacillus, Bifidobacterium, and Firmicutes and with an increase in pathogens, mainly E. coli [73]. This process is often associated with urinary tract infections, poor bioavailability of nutritional compounds and the disturbance of the ratio of SCFAs [2,29].
Although probiotics are considered as products with guaranteed effect, which increases with the amount ingested, new theories on in vivo bioactivity contradict the known data [49,74]. The eubiosis considered to be established is a temporary one, in fact, molecular interactions lead to a microbiota imbalance and a different response over time. This does not mean that people will immediately experience a negative physiological effect, but that it would be a change that occurs over time as the microbial pattern is reshaped. Such an interpretation will develop new theories on functional supplements that are considered to have a known effect. The remodeling of the microbial pattern extends the research performed so far and opens up theories that will reconsider the introduction of new strains into the human microbial ecosystem by modifying/decreasing the bioavailability of some essential functional compounds [75]. This is a secondary explanation for the reduction of colonic bioavailability, microbial plasticity, and the incidence of degenerative diseases [76].
The overdose of pharmaceuticals (such as antibiotics) limits the plasticity of the metabolomic pattern in order to maintain a favorable health status. A much more useful solution for the long term is the use or administration of the fibers that lead to the synthesis of butyrate, while simulatenously preserving the integrity of the microbial pattern. Such products support the individual eubiotic state [33]. In this situation, no new molecular relationships is expected to emerge, and the intrinsic effects of this relationship can only be quantified in the long term [77].
The intestinal ecosystem has been shown to be responsible for xenobiotic biotransformation, and several recent studies have proven it to be an essential factor in the pharmacokinetics of orally administered drugs. This aspect has direct implications for bioavailability after oral administration [78]. The metabolism process of xenobiotics is an enzymatically controlled one and depends on the age of the individual. Enzyme synthesis varies with diet, and it also depends on the spatial distribution of the genera that compose the key pattern of the target-group microbiota [2,79]. From a clinical perspective, an increase in the number of enzymes with age has been noted, and a prolonged exposure to a compound implies a modulation of the metabolism capacity, with a direct effect on the bioavailability of drugs [80]. Such studies can explain the different roles that the microbiota play in the bioavailability of drugs in comparison to other functional components.
The absorption of different types of biomolecules depends on the molecular mass and their chemical structure. Polyphenolic compounds have been recognized to have a low bioavailability, and their effect is often mediated by intermediates [29]. A recent study assimilates these components after digestion in the upper segments as xenobiotics [81]. They are assimilated only after the enzymatic action of the microbial community in the colon and do not have any direct critical effect. If other xenobiotics cause a direct alteration of the microbial pattern, they have a different function [82], such as a modulatory one, with the clinical effect exerted by the expressed bioactivity [2].
6. Dietary Fiber and Their Role in the Bioavailability of Phenolic Compounds
The bioavailability of polyphenols in the gastrointestinal tract, as well as their catabolism at the microbiota level, may be influenced by the presence of fibers. Simultaneously, the presence of fibers stimulates the microbiota, as they are the main source for SCFAs synthesis. This type of interaction is not fully understood yet [83]. A recent study showed that the presence of highly fermented fibers can inhibit phenolic acid production from the catabolization of rutin by human fecal bacteria. In vitro experiments using the same highly fermented fibers showed that the presence of rutin or quercetin in the medium did not affect SCFAs synthesis [84]. A long-term diet with water-soluble dietary fibers (such as pectin, soybean fiber, and guar gum) and quercetin-3-O-glucosides mixture supplementation to rats led to increased SCFAs production and the improved bioavailability of quercetin glycosides. The increased levels of quercetin in the blood and urine for rats supplemented with soybean fiber and quercetin glycosides may be attributed to the use of fiber as a carbon source and the suppression of quercetin degradation by the microbiota [85].
Polyphenols interact with all types of fibers, and the nature of the interactions is defined by the chemical structure of that fiber and by the environmental conditions. These interactions may be hydrophobic, hydrogen bond, van der Waals, non-covalent, non-ionic, or weak electrostatic interaction [86]. As a result of these interactions, some polyphenols become less bioavailable in the small intestine and more in the large intestine. Therefore, fibers play an important role in the bioavailability of polyphenols in the colon, and polyphenol-binding fibers brings health benefits [87].
Mulberry leaf extracts are commonly used as a natural remedy in type 2 diabetes. Its efficacy in lowering the blood glucose levels has been demonstrated by numerous studies [88]. A recent study showed that the presence of fibers increased the efficiency of polyphenolic extract from mulberry leaf, and the polyphenol–fiber mixture influences the gut microbiota (Table 1). Their synergistic effect resulted in the reduction of Firmicutes abundance, the downstream of Clostridiales and Lachnospiraceae, and a high concentration of butyrate [89]. The effects of synergic polyphenols and dietary fibers has also been described by a study on gastrointestinal digestion of two grape pomace extracts (GPEs) [90]. The in vitro colonic digestion of GPEs administered in a single dose or continuously (14 days) resulted in the significant increase of SCFAs and ammonium ions production, as well as an increase in the count of Lactobacillus and Bacteroides groups. The presence of phenolic metabolites in the colon during the frequent administration of GPES indicate the bioavailability of polyphenols and has been assigned to the main GPE components, dietary fibers, and polyphenols.
7. Perspectives in Approaching Studies on Microbiota and Bioavailability of Functional Compounds
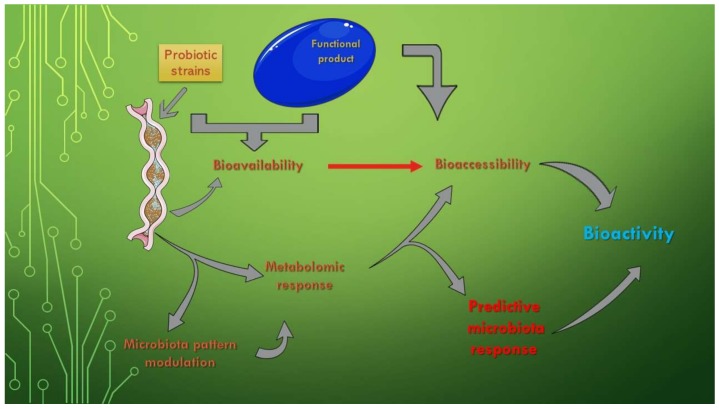
The existing data on the composition, properties, and role of the microbiota recommend the use of bacterial profiles as predictors of disease, as manipulating the gut microbiota is a promising alternative for the treatment of diseases. The question that arises in this case is whether the long-term use of functional foods is sufficient to restore the health of the microbiota or whether other interventions, such as fecal microbiota transplantation or genetic manipulation of the microbiota, are required to complete their actions [91] (Figure 1).
Figure 1.
The effects of microbiota bioactivity and bioavailability of functional compounds. This Figure was obtained in part by using images from Servier Medical Art, licensed under CC-BY 3.0.CC BY 3.0, and the PowerPoint program from the Microsoft Office 2016 software package (Microsoft Corporation, Redmond, WA, USA).
The bioavailability of functional compounds can be used for enhancing the effects of one of the two clinical forms (Crohn’s Disease—CD and Ulcerative Colitis—UC) of Inflammatory Bowel Disease (IBD). This is a significant aspect because IBD is sustained by a chronic inflammatory process, which directly leads to disruption of the microbiota pattern [92]. The independent use of probiotics only temporarily ameliorates clinical manifestations because they do not intervene in the active reduction of the inflammatory process. The assimilation of functional components, especially of target compounds, is one of the complementary evolutionary solutions in this regard. These new product types, biomass-enriched with certain functional components, are addressed to clinical forms that have not shown a clear improvement after single biomass administration, such as UC [93]. Figure 1 also represents a schematic form of the physiological role that the increase of the bioavailability of some products (of the bioaccessibility of all the components of its composition) has on the modulation of the metabolic response. In addition, by knowing the effect of the entire functional fingerprint, a prediction of the physiological effect and a determination of the bioactivity after administration can be made.
Modulation of the metabolomic pattern for SCFA could represent a breakthrough in IBD researches. Butyric acid is the target of this response because the ratio of the three main acids varies with the target groups and decreases with the passage from one segment to another. The role of these acids in controlling inflammatory proliferation increases not only with the number of favorable strains, but also with the decrease of oxidative stress [29,94]. The prebiotic role of nutraceuticals is essential in the proliferation of strains synthesizing butyrate (microbial modulation), like food fibers. This aspect has a major influence in reducing the chronic inflammation that favors the appearance of tumors, a process relevant especially for the terminal segment of the human colon [94].
The use of favorable strains (as probiotic products) offers a new perspective through its association with certain molecules with a functional role, and directly influences the bioavailability process. Future research will need to evaluate the role that newly introduced strains can play in the microbiota–bioavailability relationship. Diversity and spatial spread play an important role in determining the optimal action in the interaction with the functional compound in the human colon. This process evolves gradually with administration, and results in an increase in the immune response [95]. The restoration of eubiosis, with increasing bioavailability, is partially associated with the administration of functional compounds [73,96], unless they are degraded by the interaction with the physico-chemical limiting factors of the gastrointestinal tract. Reducing the action of limiting factors that influence bioavailability is the main objective of research on the effect of functional compounds (such as phenolic compounds), by mitigating possible xenobiotic effects.
The prevention of oxidative stress through the consumption of phenolic compounds is a key solution for reducing the chronic pathologies associated with the establishment of dysbiosis. Although they are the main functional components in herbal products, phenolic compounds possess several characteristics that are not fully known. Bioaccessibility and biotransformation of phenolic compounds manifest, at the microbiota, a key point that affects their bioactivity and on which the physiological response depends [97].
Functional products are widely available in the present market. Research on improving the health status by administering these products should be undertaken by the biopharmaceutical industry toward developing newer products with the optimum efficiency in vivo. These should not be based solely on the combination of compounds with a known biological effect from the traditional phytomedicines [98].
8. Conclusions
The new evidences in microbiota research confirm the role played by nutrients in modulating it. The restoration of the gut microbiota commonly associated with the occurrence of diseases by increasing the bioavailability of some natural compounds is much easier than using pharmaceutical alternatives. We believe that studies on the interaction of microbiota with polyphenolic compounds and other factors may influence the restoration process and human well-being.
Acknowledgments
Many thanks to my wife (Albertina Vamanu) for her effort, critical comments, and partial English editing.
Author Contributions
E.V. and F.G., writing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This mini-review received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wang B., Yao M., Lv L., Ling Z., Li L. The Human Microbiota in Health and Disease. Engineering. 2017;3:71–82. doi: 10.1016/J.ENG.2017.01.008. [DOI] [Google Scholar]
- 2.Vamanu E. Polyphenolic Nutraceuticals to Combat Oxidative Stress Through Microbiota Modulation. Front. Pharmacol. 2019;10:492. doi: 10.3389/fphar.2019.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scalbert A., Williamson G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 4.Ganesan K., Xu B. A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients. 2017;9:455. doi: 10.3390/nu9050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph S.V., Edirisinghe I., Burton-Freeman B.M. Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Crit. Rev. Food Sci. Nutr. 2016;56:419–444. doi: 10.1080/10408398.2013.767221. [DOI] [PubMed] [Google Scholar]
- 6.Yahfoufi N., Alsadi N., Jambi M., Matar C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients. 2018;10:1618. doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishimoto Y., Tani M., Kondo K. Pleiotropic Preventive Effects of Dietary Polyphenols in Cardiovascular Diseases. Eur. J. Clin. Nutr. 2013;67:532–535. doi: 10.1038/ejcn.2013.29. [DOI] [PubMed] [Google Scholar]
- 8.Khurana S., Venkataraman K., Hollingsworth A., Piche M., Tai T.C. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients. 2013;5:3779–3827. doi: 10.3390/nu5103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekhon-Loodu S., Vasantha Rupasinghe H.P. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front. Nutr. 2019;25:53. doi: 10.3389/fnut.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwai K., Kim M.Y., Onodera A., Matsue H. α-Glucosidase Inhibitory and Antihyperglycemic Effects of Polyphenols in the Fruit of Viburnum dilatatum Thunb. J. Agric. Food Chem. 2006;54:4588–4592. doi: 10.1021/jf0606353. [DOI] [PubMed] [Google Scholar]
- 11.Lamoral-Theys D., Pottier L., Dufrasne F., Nève J., Dubois J., Kornienko A., Kiss R., Ingrassia L. Natural Polyphenols that Display Anticancer Properties through Inhibition of Kinase Activity. Curr. Med. Chem. 2010;17:812–825. doi: 10.2174/092986710790712183. [DOI] [PubMed] [Google Scholar]
- 12.Rosa L.S., Silva N.J.A., Soares N.C.P., Monteiro M.C., Teodoro A.J. Anticancer Properties of Phenolic Acids in Colon Cancer – A Review. J. Nutr. Food Sci. 2016;6:2. [Google Scholar]
- 13.Jeong H., Phan A.N., Choi J.W. Anti-cancer Effects of Polyphenolic Compounds in Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor-Resistant Non-Small Cell Lung Cancer. Phcog. Mag. 2017;13:595–599. doi: 10.4103/pm.pm_535_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Sarrías A., Núñez-Sánchez M.Á., Tomás-Barberán F.A., Espín J.C. Neuroprotective Effects of Bioavailable Polyphenol-Derived Metabolites against Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. J. Agric. Food Chem. 2017;65:752–758. doi: 10.1021/acs.jafc.6b04538. [DOI] [PubMed] [Google Scholar]
- 15.Szwajgier D., Borowiec K., Pustelniak K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients. 2017;9:477. doi: 10.3390/nu9050477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magrone T., Jirillo E. Influence of Polyphenols on Allergic Immunereactions: Mechanisms of Action. Proc. Nutr. Soc. 2012;71:316–321. doi: 10.1017/S0029665112000109. [DOI] [PubMed] [Google Scholar]
- 17.Kandhare A.D., Bodhankar S.L., Singh V., Mohan V., Thakurdesai P.A. Anti-asthmatic Effects of Type-A Procyanidine Polyphenols from Cinnamon Bark in Ovalbumin-Induced Airway Hyperresponsiveness in Laboratory Animals. Biomed. Aging Pathol. 2013;3:23–30. doi: 10.1016/j.biomag.2013.01.003. [DOI] [Google Scholar]
- 18.Ikarashi N., Toda T., Hatakeyama Y., Kusunoki Y., Kon R., Mizukami N., Kaneko M., Ogawa S., Sugiyama K. Anti-Hypertensive Effects of Acacia Polyphenol in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2018;19:700. doi: 10.3390/ijms19030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H., Zhang G., Huang L., Pang H., Zhang N., Chen Y., Wang G. Hepatoprotective Effect of Polyphenol-Enriched Fraction fromFolium Microcos on Oxidative Stress and Apoptosis in Acetaminophen-Induced Liver Injury in Mice. Oxid. Med. Cell. Longev. 2017;3631565 doi: 10.1155/2017/3631565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odeyemi S., Dewar J. Repression of Acetaminophen-Induced Hepatotoxicity in HepG2 Cells by Polyphenolic Compounds from Lauridia tetragona. Molecules. 2019;24:2118. doi: 10.3390/molecules24112118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouarab-Chibane L., Forquet V., Lantéri P., Clément Y., Léonard-Akkari L., Oulahal N., Degraeve P., Bordes C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019;10:829. doi: 10.3389/fmicb.2019.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y., Chen J., Xiao A., Liu L. Antibacterial Activity of Polyphenols: Structure-Activity Relationship and Influence of Hyperglycemic Condition. Molecules. 2017;22:1913. doi: 10.3390/molecules22111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirasawa M., Takada K. Multiple Effects of Green Tea Catechin on the Antifungal Activity of Antimycotics against Candida albicans. J. Antimicrob. Chemother. 2004;53:225–229. doi: 10.1093/jac/dkh046. [DOI] [PubMed] [Google Scholar]
- 24.Jasso-Miranda C., Herrera-Camacho I., Flores-Mendoza L.K., Dominguez F., Vallejo-Ruiz V., Sanchez-Burgos G.G., Pando-Robles V., Santos-Lopez G., Reyes-Leyva J. Antiviral and Immunomodulatory Effects of Polyphenols on Macrophages Infected with Dengue Virus Serotypes 2 and 3 Enhanced or not with Antibodies. Infect. Drug Resist. 2019;12:1833–1852. doi: 10.2147/IDR.S210890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mileo A.M., Nisticò P., Miccadei S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019;10:729. doi: 10.3389/fimmu.2019.00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Rio D., Rodriguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (Poly)phenolics in Human Health:Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases, Antioxid. Redox Signal. 2013;18:14. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selma M.V., Espín J.C., Tomás-Barberán F.A. Interaction Between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 28.Mattio L.M., Dallavalle S., Musso L., Filardi R., Franzetti L., Pellegrino L., D’Incecco P., Mora D., Pinto A., Arioli S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci Rep. 2019;9:19525. doi: 10.1038/s41598-019-55975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vamanu E., Gatea F., Sârbu I., Pelinescu D. An In Vitro Study of the Influence of Curcuma longa Extracts on the Microbiota Modulation Process, In Patients with Hypertension. Pharmaceutics. 2019;11:191. doi: 10.3390/pharmaceutics11040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawabata K., Yoshioka Y., Terao J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J.B., Zhao Z.X., Peng R., Pan L.B., Fu J., Ma S.R., Han P., Cong L., Zhang Z.W., Sun L.X., et al. Gut Microbiota-Based Pharmacokinetics and the Antidepressant Mechanism of Paeoniflorin. Front. Pharmacol. 2019;10:268. doi: 10.3389/fphar.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vamanu E. Complementary Functional Strategy for Modulation of Human Gut Microbiota. Curr. Pharm. Des. 2018;24:35. doi: 10.2174/1381612824666181001154242. [DOI] [PubMed] [Google Scholar]
- 33.Carrera-Quintanar L., López Roa R.I., Quintero-Fabián S., Sánchez-Sánchez M.A., Vizmanos B., Ortuño-Sahagún D. Phytochemicals That Influence Gut Microbiota as Prophylactics and for the Treatment of Obesity and Inflammatory Diseases. Mediat. Inflamm. 2018;2018:9734845. doi: 10.1155/2018/9734845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu S., Hu R., Nakano H., Chen K., Liu M., He X., Zhang H., He J., Hou D.X. Modulation of Gut Microbiota by Lonicera caerulea L. Berry Polyphenols in a Mouse Model of Fatty Liver Induced by High Fat Diet. Molecules. 2018;23:3213. doi: 10.3390/molecules23123213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roopchand D.E., Carmody R.N., Kuhn P., Moskal K., Rojas-Silva P., Turnbaugh P.J., Raskin I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet–Induced Metabolic Syndrome. Diabetes. 2015;64:2847–2858. doi: 10.2337/db14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duda-Chodak A., Tarko T., Satora P., Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015;54:325–341. doi: 10.1007/s00394-015-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frolinger T., Sims S., Smith C., Wang J., Cheng H., Faith J., Ho L., Hao K., Pasinetti G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019;9:3546. doi: 10.1038/s41598-019-39994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozdal T., Sela D.A., Xiao J., Boyacioglu D., Chen F., Capanoglu E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients. 2016;8:78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzounis X., Vulevic J., Kuhnle G.G.C., George T., Leonczak J., Gibson G.R., Kwik-Uribe C., Spencer J.P.E. Flavanol Monomer-Induced Changes to the Human Faecal Microflora. Brit. J. Nutr. 2008;99:782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 40.Tzounis X., Rodriguez-Mateos A., Vulevic J., Gibson G.R., Kwik-Uribe C., Spencer J.P.E. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. Am. J. Clin. Nutr. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 41.Campbell C.L., Yu R., Li F., Zhou Q., Chen D., Qi C., Yin Y., Sun J. Modulation of Fat Metabolism and Gut Microbiota by Resveratrol on High-Fat Diet-Induced Obese Mice Diabetes. Metab. Syndr. Obes. Targ. Ther. 2019;2019:97–107. doi: 10.2147/DMSO.S192228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavefve L., Howard L.R., Carbonero F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020;11:45–65. doi: 10.1039/C9FO01634A. [DOI] [PubMed] [Google Scholar]
- 43.Bensalem J., Dal-Pan A., Gillard E., Calon F., Pallet V. Protective effects of berry polyphenols against age-related cognitive impairment. Nutr. Ag. 2015;3:89–106. doi: 10.3233/NUA-150051. [DOI] [Google Scholar]
- 44.Cardona F., Andrés-Lacueva C., Tulipani S., Tinahones F.J., Queipo-Ortuño M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Aprotosoaie A.C., Miron A., Trifan A., Luca V.S., Costache I.I. The Cardiovascular Effects of Cocoa Polyphenols-An Overview. Diseases. 2016;4:39. doi: 10.3390/diseases4040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duda-Chodak A. The Inhibitory Effect of Polyphenols on Human Gut Microbiota. J. Physiol. Pharmacol. 2012;63:497–503. [PubMed] [Google Scholar]
- 47.Piqué N., Berlanga M., Miñana-Galbis D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019;20:2534. doi: 10.3390/ijms20102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeGruttola A.K., Low D., Mizoguchi A., Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016;22:1137–1150. doi: 10.1097/MIB.0000000000000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markowiak P., Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:E1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuchs E.J. HLA-Haploidentical Blood or Marrow Transplantation with High-Dose, Post-Transplantation Cyclophosphamide. Bone Marrow Transplant. 2015;50:S31–S36. doi: 10.1038/bmt.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmermann A., Hofer S., Pendl T., Kainz K., Madeo F., Carmona-Gutierrez D. Yeast as a tool to identify anti-aging compounds. FEMS Yeast Res. 2018;18 doi: 10.1093/femsyr/foy020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vamanu E., Gatea F., Sârbu I. In Vitro Ecological Response of the Human Gut Microbiome to Bioactive Extracts from Edible Wild Mushrooms. Molecules. 2018;23:2128. doi: 10.3390/molecules23092128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conlon M.A., Bird A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients. 2014;7:17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saini R., Sain S., Sharma S. Potential of Probiotics in Controlling Cardiovascular Diseases. J. Cardiovasc. Dis. Res. 2010;1:213–214. doi: 10.4103/0975-3583.74267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mishra S., Wang S., Nagpal R., Miller B., Singh R., Taraphder S., Yadav H. Probiotics and Prebiotics for the Amelioration of Type 1 Diabetes: Present and Future Perspectives. Microorganisms. 2019;7:67. doi: 10.3390/microorganisms7030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimojo Y., Ozawa Y., Toda T., Igami K., Shimizu T. Probiotic Lactobacillus paracaseiA221 Improves the Functionality and Bioavailability of Kaempferol Glucoside in Kale by its Glucosidase Activity. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-27532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westfall S., Lomis N., Prakash S. A Novel Polyphenolicprebiotic and Probiotic Formulation have Synergistic Effects on the Gut Microbiota Influencing Drosophila melanogaster Physiology. Artif. Cells, Nanomed. Biotechnol. 2018;46:441–455. doi: 10.1080/21691401.2018.1458731. [DOI] [PubMed] [Google Scholar]
- 59.dos Santos A.S., de Albuquerque T.M.R., de Brito J.L., de Souza A.E.L. Effects of Quercetin and Resveratrol on In Vitro Properties Related to the Functionality of Potentially Probiotic Lactobacillus Strains. Front. Microbiol. 2019;10:2229. doi: 10.3389/fmicb.2019.02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paradaa J., Pérez-Correab J.R., Pérez-Jiménez J. Design of Low Glycemic Response Foods Using Polyphenols from Seaweed. J. Funct. Foods. 2019;56:33–39. doi: 10.1016/j.jff.2019.03.004. [DOI] [Google Scholar]
- 61.Diether N.E., Willing B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet-Microbe-Host Interaction. Microorganisms. 2019;13:19. doi: 10.3390/microorganisms7010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balakrishnan B., Taneja V. Microbial Modulation of the Gut Microbiome for Treating Autoimmune Diseases. Exp. Rev. Gastroenterol. Hepatol. 2018;10:985–996. doi: 10.1080/17474124.2018.1517044. [DOI] [PubMed] [Google Scholar]
- 63.Wong B.B.M., Candolin U. Behavioral Responses to Changing Environments. Behav. Ecol. 2015;26:665–673. doi: 10.1093/beheco/aru183. [DOI] [Google Scholar]
- 64.Betoret N., Hinestroza L.I., Seguí L., Barrera C. Probiotics and Other Bioactive Compounds with Proven Effect against Obesity and Hypertension: Food Design Opportunities from Lulo Fruit (Solanum quitoense) IntechOpen. 2019 doi: 10.5772/intechopen.85482. [DOI] [Google Scholar]
- 65.Kornienko A., Evidente A., Vurro M., Mathieu V., Cimmino A., Evidente M., van Otterlo W.A.L., Dasari R., Lefranc F., Kiss R. Toward a Cancer Drug of Fungal Origin. Med. Res. Rev. 2015;35:937–967. doi: 10.1002/med.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan B.L., Norhaizan M.E., Liew W.P., Sulaiman Rahman H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018;9:1162. doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mallick H., Franzosa E.A., Mclver L.J., Banerjee S., Sirota-Madi A., Kostic A.D., Clish C.B., Vlamakis H., Xavier R.J., Huttenhower C. Predictive Metabolomic Profiling of Microbial Communities Using Amplicon or Metagenomic Sequences. Nat. Commun. 2019;17:3136. doi: 10.1038/s41467-019-10927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sridharan G.V., Choi K., Klemashevich C., Wu C., Prabakaran D., Pan L.B., Steinmeyer S., Mueller C., Yousofshahi M., Alaniz R.C., et al. Prediction and Quantification of Bioactive Microbiota Metabolites in the Mouse Gut. Nat. Commun. 2014;5:5492. doi: 10.1038/ncomms6492. [DOI] [PubMed] [Google Scholar]
- 69.Choi M.S., Kim J.-K., Kim D.-H., Yoo H.H. Effects of Gut Microbiota on the Bioavailability of Bioactive Compounds from Ginkgo Leaf Extracts. Metabolites. 2019;9:132. doi: 10.3390/metabo9070132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut Microbiota Functions: Metabolism of Nutrients and other Food Components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Omiecinski C.J., Vanden Heuvel J.P., Perdew G.H., Peters J.M. Xenobiotic Metabolism, Disposition, and Regulation by Receptors: From Biochemical Phenomenon to Predictors of Major Toxicities. Toxicol. Sci. Off. J. Soc. Toxicol. 2011;120:S49–S75. doi: 10.1093/toxsci/kfq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marín L., Miguélez E.M., Villar C.J., Lombó F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015;2015:905215. doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abaidullah M., Peng S., Kamran M., Song X., Yin Z. Current Findings on Gut Microbiota Mediated Immune Modulation against Viral Diseases in Chicken. Viruses. 2019;25:681. doi: 10.3390/v11080681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoffmann D.E., Fraser C.M., Palumbo F., Ravel J., Rowthorn V., Schwartz J. Probiotics: Achieving a better regulatory fit. Food Drug Law J. 2014;69:237–272. [PMC free article] [PubMed] [Google Scholar]
- 75.Derrien M., van Hylckama Vlieg J.E.T. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 76.Li Z., Zhu H., Zhang L., Qin C. The Intestinal Microbiome and Alzheimer’s Disease: A review. Animal Model. Exp. Med. 2018;1:180–188. doi: 10.1002/ame2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kho Z.Y., Lal S.K. The Human Gut Microbiome – A Potential Controller of Wellness and Disease. Front. Microbiol. 2018;9:1835. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.George F., Daniel C., Thomas M., Singer E., Guilbaud A., Tessier F.J., Revol-Junelles A.M., Borges F., Foligné B. Occurrence and Dynamism of Lactic Acid Bacteria in Distinct Ecological Niches: A Multifaceted Functional Health Perspective. Front. Microbiol. 2018;9:2899. doi: 10.3389/fmicb.2018.02899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jourova L., Anzenbacher P., Anzenbacherova E. Human Gut Microbiota Plays a Role in the Metabolism of Drugs. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2016;160:317–326. doi: 10.5507/bp.2016.039. [DOI] [PubMed] [Google Scholar]
- 80.Das A., Srinivasan M., Ghosh T.S., Mande S.S. Xenobiotic Metabolism and Gut Microbiomes. PLoS ONE. 2016;11:e0163099. doi: 10.1371/journal.pone.0163099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Filosa S., Di Meo F., Crispi S. Polyphenols-Gut Microbiota Interplay and Brain Neuromodulation. Neural Regen. Res. 2018;13:2055–2059. doi: 10.4103/1673-5374.241429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vamanu E., Pelinescu D., Gatea F., Sârbu I. Altered in Vitro Metabolomic Response of the Human Microbiota to Sweeteners. Genes. 2019;10:535. doi: 10.3390/genes10070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edwards C.A., Havlik J., Cong W., Mullen W., Preston T., Morrison D.J., Combe E. Polyphenols and Health: Interactions Between Fibre, Plant Polyphenols and the Gut Microbiota. Nutr. Bull. 2017;42:356–360. doi: 10.1111/nbu.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mansoorian B., Combet E., Alkhaldy A., Garcia A.L., Edwards C.A. Impact of Fermentable Fibres on the Colonic Microbiota Metabolism of Dietary Polyphenols Rutin and Quercetin. Int. J. Environ. Res. Public Health. 2019;16:292. doi: 10.3390/ijerph16020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trakooncharoenvit A., Tanaka S., Mizuta E., Hira T., Hara H. Water-Soluble Dietary Fibers Enhance Bioavailability of Quercetin and a Fiber Derived from Soybean is Most Effective after long-term Feeding in Rats. Eur. J. Nutr. 2019;11 doi: 10.1007/s00394-019-01992-9. [DOI] [PubMed] [Google Scholar]
- 86.Jakobek L., Matić P. Non-covalent Dietary Fiber - Polyphenol Interactions and their Influence on Polyphenol Bioaccessibility. Trends Food Sci. Technol. 2019;83:235–247. doi: 10.1016/j.tifs.2018.11.024. [DOI] [Google Scholar]
- 87.Gong L., Cao W., Chi H., Wang J., Zhang H., Liu J., Sun B. Whole Cereal Grains and Potential Health Effects: Involvement of the Gut Microbiota. Food Res. Int. 2018;103:84–102. doi: 10.1016/j.foodres.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 88.Mudra M., Ercan-Fang N., Zhong L., Furne J., Levitt M. Influence of Mulberry Leaf Extract on the Blood Glucose and Breath Hydrogen Response to Ingestion of 75 g Sucrose by Type 2 Diabetic and Control Subjects. Diabet. Care. 2007;30:1272–1274. doi: 10.2337/dc06-2120. [DOI] [PubMed] [Google Scholar]
- 89.Li Q., Liu F., Liu J., Liao S., Zou Y. Mulberry Leaf Polyphenols and Fiber Induce Synergistic Antiobesity and Display a Modulation Effect on Gut Microbiota and Metabolites. Nutrients. 2019;11:1017. doi: 10.3390/nu11051017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gil-Sánchez I., Cueva C., Sanz-Buenhombre M., Guadarrama A., Moreno-Arribas M.V., Bartolomé B. Dynamic Gastrointestinal Digestion of Grape Pomace Extracts: Bioaccessible Phenolic Metabolites and Impact on Human Gut Microbiota. J. Food Compos. Anal. 2018;68:41–52. doi: 10.1016/j.jfca.2017.05.005. [DOI] [Google Scholar]
- 91.Shang Q. From Correlation to Causation: The Missing Point in the Study of Functional Foods and Gut Microbiota. J. Func. Foods. 2019;61:103466. doi: 10.1016/j.jff.2019.103466. [DOI] [Google Scholar]
- 92.Sinagra E., Tomasello G., Cappello F., Leone A., Cottone M., Bellavia M., Rossi F., Facella T., Damiani P., Zeenny M.N., et al. Probiotics, Prebiotics and Symbiotics in Inflammatory Bowel Diseases: State-of-the-Art and New Insights. J. Biol. Reg. Homeos. Ag. 2013;27:919–933. [PubMed] [Google Scholar]
- 93.Tralongo P., Tomasello G., Sinagra E., Damiani P., Leone A., Palumbo V.D., Giammanco M., Di Majo D., Abruzzo A., Bruno A., et al. The role of butyric acid as a protective agent against inflammatory bowel disease. Euromediterr. Biomed. J. 2014;9:24–35. [Google Scholar]
- 94.Sinagra E., Tomasello G., Raimondo D., Rossi F., Facella T., Damiani P., Abruzzo A., Bruno A., Palumbo V.D., Cosentino L., et al. Nutrition, malnutrition and dietary interventions in inflammatory bowel disease. Progr. Nutr. 2014;16:79–89. [Google Scholar]
- 95.Belkaid Y., Hand T.W. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gagliardi A., Totino V., Cacciotti F., Iebba V., Neroni B., Bonfiglio G., Trancassini M., Passariello C., Pantanella F., Schippa S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health. 2018;15:1679. doi: 10.3390/ijerph15081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Espín J.C., González-Sarrías A., Tomás-Barberán F.A. The Gut Microbiota: A Key Factor in the Therapeutic Effects of (Poly)phenols. Biochem. Pharmacol. 2017;1:82–93. doi: 10.1016/j.bcp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 98.Hussain M.B., Hassan S., Waheed M., Javed A., Farooq M.A., Tahir A. Bioavailability and Metabolic Pathway of Phenolic Compounds. In: Soto-Hernández M., García-Mateos R., Palma-Tenango M., editors. Plant Physiological Aspects of Phenolic Compounds. IntechOpen; London, UK: 2019. [DOI] [Google Scholar]