Abstract
Transport in water is the most common method for achieving high survival rates when transporting cultured fish in China; yet, transport success relies on proper water quality and conditions. This research was designed to explore the effects of ascorbic acid and β-1,3-glucan on survival, physiological responses, and flesh quality of farmed tiger grouper (Epinephelus fuscoguttatus) during simulated transport. The transport water temperature for live tiger grouper was 15 °C, which had the highest survival rate, the lowest stress response, and metabolic rate, and this will reduce the susceptibility to diseases. It is stated that β-1,3-glucan influences the changes of cortisol content, heat shock protein 70, IL-1β, and IgM transcription levels during simulated transport. Rather than using ascorbic acid alone (the A-group), β-1,3-glucan (3.2 mg/L) in the presence of ascorbic acid (25 mg/L) can effectively reduce the increase of transport-induced serum cortisol content, heat shock protein 70, and IL-1β, but stimulated IgM. 25 mg/L ascorbic acid and 3.2 mg/L β-1,3-glucan had no obvious effect on the nutritional indexes and flavor of live tiger grouper; however, these can effectively reduce the stress response, improve the innate immune activity, and ensure a higher survival rate.
Keywords: Epinephelus fuscoguttatus; simulated transport in water; environment stress; β-1,3-glucan; physiological responses
1. Introduction
Aquaculture is a rapidly growing industry in the world, providing one of the most sustainable forms of edible protein and nutrient production [1]. According to a report by FAO in 2014, the global aquaculture production has doubled in the past decade and it now accounts for about 50% of fishery products. China produced 41.1 million tons of farmed food fish in 2012, contributing to approximately 61.7% of total world production [2]. High demand for fish is due to an increased consumer awareness of healthy food [3]. These increases have occurred in some commercially important species, such as grouper, seabass, rainbow trout, tilapia, large yellow croaker, and catfish. Live fish species are sold at a higher price than frozen ones. Therefore, research regarding the handling and transport of live fish to improve the viability of commercial fish is essential.
Currently, groupers are cultured on an industrial scale in Asian countries, because of their fast growth, efficient feed conversion, and good flesh quality [4,5]. Tiger grouper (Epinephelus fuscoguttatus) is known for its high protein, low fat, tender meat quality, and good taste, making it one of the most cultured grouper species in China, Japan, and Singapore [6,7].
Fish transport in China is not efficient in terms of technology and cost. It is required to develop the transport technology to produce a higher viability of commercial fish at a rational price. Transport is a strong stressor [8]. The stress intensity depends on transport conditions, such as package density and transport-water quality [9,10]. Environmental stressors could lead to a reduced immune system function, resulting in sickness and death [11,12,13,14]. Besides, fish were continuously stressed during fish transport, which resulted in quality deterioration [15,16]. Conversely, anesthesia could reduce the metabolic rate, oxygen demand, and response to stress [17,18], which enables fish to be more efficiently transferred in higher densities. Anesthesia has been used to handle and transport live fish for years to reduce stress on fish [19,20,21]. However, it is forbidden to use in the fish transport in China because of safety issues.
Low temperature dormancy, while using anti-stress and immunopotentiator agents, are common practices to relieve stress on fish transport [22,23]. Low temperature dormancy can reduce mechanical damage, energy consumption, and stress response during fish transport [24,25]. However, an unsuitable low temperature could lead to a low survival rate [26,27]. Anti-stress agents include bioactive polysaccharides, vitamins, amino acids, mineral elements, and electrolytes [28,29]. Ascorbic acid is not only a good anti-stress agent, but it is also an effective immunostimulant [30]. Cheng et al. [31] reported that ascorbic acid could protect against DNA damage, apoptosis, and proteolysis of pufferfish under low temperature stress. The use of immunostimulants from various sources, such as fungi, algae, and bacterial products, is a common practice in aquaculture to strengthen the immune system of the cultured fish [32]. β-1,3-glucan is a polysaccharide that is commonly used as feed additive in aquaculture. Feeding with β-1,3-glucan might enhance innate immune responses, which can reduce the inhibition of the immune system that is caused by glucocorticoids and steroidal corticosteroids that are secreted by fish under stress [33,34]. Lin et al. [35] showed that β-1,3-glucan, chitosan, and raffinose could enhance the immune responses of koi. Anti-stress agents and immunostimulants can improve the antioxidant capacity and immunity of fish during transport, thereby improving the survival rate [36,37]. Therefore, the objective of this study was to investigate the effects of ascorbic acid and β-1,3-glucan on survival, physiological responses, and flesh quality of cultured tiger grouper during fish transport.
2. Materials and Methods
2.1. Preparation of Tiger Grouper
The live cultured tiger grouper (500 ± 50 g) were purchased from a local market in Luchao Port town (Shanghai, China), and were then transported to the laboratory while using a truck that was equipped with an insulated tank. The fish were kept in a prepared polyethylene tank (2.4 × 1.7 × 0.6 m) for two days before the experiment, to allow them to adapt to the experimental environment, where the average water temperature was 27 °C, water tank salinity was 26‰, the mean pH was 7.0, and the average dissolved oxygen was 6.0 mg∙L−1.
2.2. Experimental Design
2.2.1. Experiment 1: Transport Temperature Determination
After the tiger grouper had acclimated for two days, the water temperature was adjusted at a rate of 2 °C/h from room temperature to 10, 13, 15, 18, 21, 24, 27, and 30 °C, respectively [38]. Subsequently, each fish was packed in a plastic bag with an equal weight of water, and filled with oxygen. Transport of fish was simulated in a vibration conveyor under 70 rpm (LX-100VTR, Shanghai Luxuan Instrument Equipment Factory, Shanghai, China). The survival rate was recorded, and fish were sampled at 0, 3, 10, 17, 24, 48, and 72 h after transport. Three fish were sampled in each group at each time. Survival time of each fish was also recorded, and mean values of these parameters were calculated to determine the transport temperature for the subsequent experiments. Each transport group had 20 fish and the total number of fish was 150.
2.2.2. Experiment 2: Anti-Stress Agent Exposure
β-1,3-glucan (2.4, 3.2, and 4.0 mg/L, respectively, Aladdin Biochemical Technology Co., Ltd., Shanghai, China), and 25 mg/L ascorbic acid (Aladdin Biochemical Technology Co., Ltd., Shanghai, China) were prepared. Afterwards, each fish was packed in a plastic bag with an equal weight of prepared water (fish-to-water ratio was 1:1). Moreover, oxygen was added to transport bags, and the content of oxygen reaches more than 80%. Table 1 shows the experimental treated samples. The transport of fish was simulated in a vibration conveyor under 70 rpm at 15 °C for 24 h. Three fish samples were randomly selected in each group at each time for analysis on 0 and 24 h during simulated transport and 48 h after recovery, respectively. The blood and liver of tiger grouper samples were used to determine the physiological and biochemical indicators, such as stress response, and muscle tissue was used to measure nutritional characteristics, taste, and flavor.
Table 1.
Experimental design for exposure to anti-stress agents of live tiger grouper during simulated transport in water.
| Samples | Anti-Stress Agent Addition |
|---|---|
| CK | Control |
| A | 25 mg/L ascorbic acid |
| A-G1 | 25 mg/L ascorbic acid + 2.4 mg/L β-1,3-glucan |
| A-G2 | 25 mg/L ascorbic acid + 3.2 mg/L β-1,3-glucan |
| A-G3 | 25 mg/L ascorbic acid + 4.0 mg/L β-1,3-glucan |
2.3. Serum Cortisol Assessment
Serum cortisol was measured by cortisol ELISA kits (Jiancheng Biological Engineering Institute, Nanjing, China), while following the manufacturer’s instructions.
2.4. Analysis of Enzymatic Activity
2.4.1. Metabolic and Antioxidant Enzyme Activities
Acid phosphatase (ACP-A060-2-2), alkaline phosphatase (AKP-A059-2-2), and glutathione reductase (GR-A062-1-1) of serum were analyzed by corresponding kits (Jiancheng Biological Engineering Institute, Nanjing, China), following the manufacturer’s instructions.
2.4.2. Immunological Enzyme Activity
Lysozyme (LZM-A050-1-1) was analyzed by using commercial analysis kits (Jiancheng Biological Engineering Institute, Nanjing, China), following the manufacturer’s instructions.
2.5. Real-Time PCR
Relative expression levels of HSP70, IgM, and IL-1β were determined by RT-PCR, as described by Lee et al. [39]. The total RNA from liver tissues was extracted with RNA rapid extraction kit (TaKaRa Biological Engineering Co., LTD., Dalian, China), quantified, and spectrophotometrically assessed for purity. RNA was then treated with DNase I (TaKaRa Biological Engineering Co., LTD., Dalian, China) to remove gDNA contamination, and complementary DNA (cDNA) was synthesized with M-MuLV reverse transcriptase. RT-PCR analyzed the expression levels of the selected immune-related genes. This was done in a 10 μm total volume while using SYBR Green I chimeric fluorescence, and with 500 nmol primers. PCR cycling conditions for all genes were, as follows: 94 °C for 10 min., 45 cycles at 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, followed by 10 min. at 72 °C. Relative expression levels of the target genes transcript (HSP70, IgM, and IL-1β), with GAPDH as an internal control, were calculated using a CFX manager software version 2.0 (Bio-Rad). Table 2 shows the primers used. The threshold cycle (Ct) values were obtained from each sample after finishing the program.
Table 2.
Sequences of primers for Real-time PCR.
| Target Gene | Primer Sequence (5′-3′) |
|---|---|
| HSP70 | F: GACAAGAAGGTTGGGTCTGAAAGG |
| R: GGTTGACCATGCGGTTGTCGAAATCT | |
| IgM | F: GCCTCAGCGTCCTTCAGTTT |
| R: TGGCGTCCCAGTCCTGTTTGC | |
| IL-1β | F: AGGATGCCTGAGGGACTG |
| R: GGTAATCGTCTCCAGATGTAA |
2.6. Biochemical Analysis
2.6.1. Chemical Composition of Muscle
Ash, fat, moisture content, and total protein were measured according to Ayanda et al. [40].
2.6.2. Serum Biochemical Testing
Creatine kinase (CK), uric acid (UA), total protein (TP), albumin (ALB), urea, and creatinine were assessed according to Jia et al. [41].
2.7. Nucleotides
The nucleotide extracts were prepared based on the method of Fang et al. [42]. ATP-related compounds, including inosine monophosphate (IMP), inosine (HxR), and hypoxanthine (Hx), were analyzed while using HPLC (Waters 2695, Milford, MA, USA), equipped with a VP-CDS C18 column (150 × 46 mm). 0.05 M phosphate buffer solution (pH 6.7) was used as the mobile phase. The flow rate was 1 mL/min., and the injection volume was 10 μL. The peak was detected at 254 nm.
The taste activity value (TAV) was calculated as the following equation:
in which C corresponds to the absolute concentration of taste substances, mg/100 g, and T reflects the taste threshold, mg/100 g, (IMP: 25 mg/100 g, AMP: 50 mg/100 g).
2.8. Free Amino Acids (FAAs) Assessment
5 g mashed tiger grouper muscle tissue sample and 15 mL of 15% cold trichloroacetic acid were mixed and homogenized at 10,000 rpm, for 5 min. After standing at 4 °C for 2 h, the homogenate was centrifuged at 5980× g for 15 min, at 4 °C. Next, 5 mL supernatant was immediately neutralized to pH 2.00 and then diluted to 10 mL with ultrapure water [43]. The mixture was then filtered through a 0.22 μm, and an amino acid analyzer determined the contents of FAAs (Hitachi L-8800, Tokyo, Japan).
2.9. Statistical Analysis
All of the assumptions were met prior to data analysis. Data were expressed as the mean ± SD and the one-way analysis of variance (ANOVA) procedure followed by Duncan’s multiple range tests was adopted to determine the significant difference (p < 0.05) between treatments.
3. Results and Discussion
3.1. Pre-Experiment: Selection of Tiger Grouper Transport Temperature and Ascorbic Acid Addition
Tiger groupers were transported at 10, 13, 15, 18, 21, 24, 27, and 30 °C. Survival rates that were recorded during simulated transport are presented in Table 3. Survival time of tiger grouper at 10 °C was less than 3 h. However, temperatures at 15, 18, 21, 24, and 27 °C could extend the survival time, and the survival rates were higher than 75%. Therefore, those transport temperatures were recommended for further experiments.
Table 3.
Survival rate of tiger grouper at different temperatures and survival time (%).
| Temperature/°C | Keeping Alive Time/h | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 3 | 10 | 17 | 24 | 48 | 72 | |
| 10 | 100 | - | - | - | - | - | - |
| 13 | 100 | 100 | 100 | 100 | 85 | 65 | - |
| 15 | 100 | 100 | 100 | 100 | 100 | 100 | 95 |
| 18 | 100 | 100 | 100 | 100 | 100 | 100 | 90 |
| 21 | 100 | 100 | 100 | 100 | 100 | 95 | 85 |
| 24 | 100 | 100 | 100 | 100 | 100 | 85 | 80 |
| 27 | 100 | 100 | 100 | 100 | 100 | 85 | 75 |
| 30 | 100 | 100 | 100 | 100 | 90 | 50 | - |
3.2. Effect of Temperature on Stress Responses of Tiger Grouper during Simulated Transport
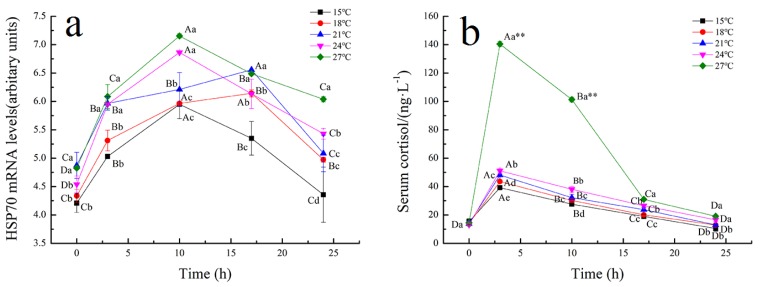
HSP is a protein that will respond to external stressful conditions [44]. It protects cells from extreme physiological, pathological, and environmental conditions, and plays a role in protein misfolding correction, preserving immature polypeptides from aggregation under stress [45,46]. Cortisol is the main glucocorticoid hormone in teleosts that are involved in the regulation of metabolic adjustments. Under stress conditions, the increase of plasma cortisol promotes protein, glucose, and lipids mobilization in the skeletal muscle, which provide energy to overcome the stress [47]. The transcriptional level of HSP70 in the liver of all tiger grouper samples, at different transport temperatures, increased to the maximum value at 10 h, and then decreased, as shown in Figure 1a. Besides, higher transport temperatures seem to correlate with a higher level of HSP70 during simulated transport. After 17 h transport, the HSP70 values gradually recovered, but did not return to the initial values, except at 15 °C. Cortisol showed a similar trend as HSP70, with significantly higher values at 27 °C than at other temperatures. At the end of transport, the cortisol concentration at 15, 18, and 21 °C recovered to the initial levels. HSP70 and cortisol increased with the transport temperature increase, which could illustrate that the tiger grouper had transport stress responses during simulated transport. A higher transport temperature could lead to unrecoverable stress response, resulting in death. Additionally, stress response was not significant, and fish could maintain the body balance through self-regulation [48].
Figure 1.
Effects of temperature on HSP70 (a) and serum cortisol (b) of tiger grouper during simulated transport. Among different temperature transport groups, different small and capital letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05). Different letters without * = p-value < 0.05, different letters with ** = p-value < 0.01.
3.3. Effect of Temperature on Antioxidant Enzyme of Tiger Grouper during Simulated Transport
Fish exposition to anoxia and hypoxia may result in oxidative changes, because oxygen consumption determines the levels of ROS generated, and also the antioxidant status [49]. Some clues could be given by an increase in activities of antioxidant enzymes under anoxic conditions [50]. Oxidative reactions are essential in normal metabolism of aerobic organisms, but ROS are produced during the oxidative metabolism, generating free radicals [51]. In a situation of oxidative stress, fish might show a typical reaction for ROS, involving lipoperoxidation (LPO), which can be quantified by an increase in TBARS levels. On the other hand, the deleterious effect of ROS can be balanced by the production of antioxidant defenses [52], such as CAT.
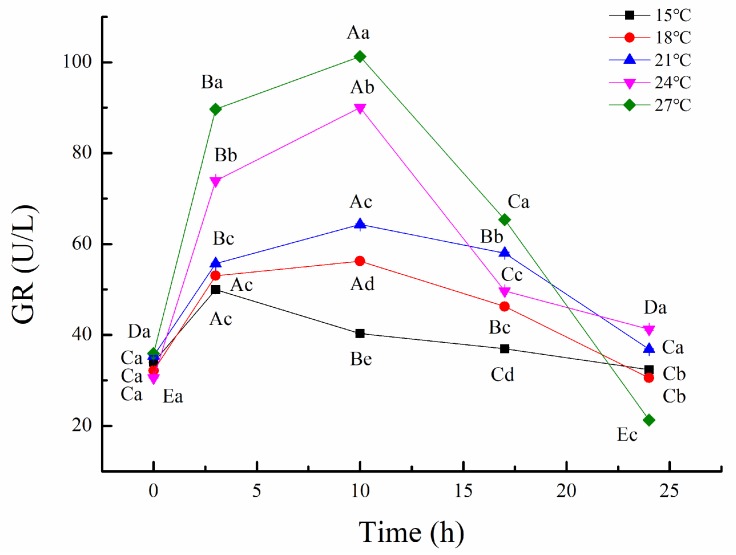
GR is an important indicator for evaluating the degree of oxidative stress [53]. Figure 2 showed that the GR levels increased first, and then decreased following transport time, reaching a peak level at 10 h, which was similar to cortisol and HSP70. It indicated that all tiger groupers were under different degrees of oxidative stress during early simulated transport. GR activity of tiger grouper transported at 27 °C was significantly higher than those at low temperatures, and the initial levels could not be recovered at the end of transport. In the 27 °C transport group, all of the tiger grouper suffered severe oxidation reaction, which caused irreversible damage and further affected the survival. However, GR activity recovered to the initial levels that were transported at 18 and 21 °C. Yan et al. [54] showed that fugu also had an oxidative stress reaction, due to the stressor of temperature. It led to HSP70 and GR activity increase. Therefore, a low temperature is suitable for tiger grouper transport, because of the decreased stress response and increased survival.
Figure 2.
Effect of temperature on glutathione reductase (GR) of tiger grouper during simulated transport. Among different temperature transport groups, different small and capital letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05). Different letters without * = p-value < 0.05, different letters with ** = p-value < 0.01.
3.4. Effect of Temperature on Metabolic and Immune Enzyme Activity of Tiger Grouper during Simulated Transport
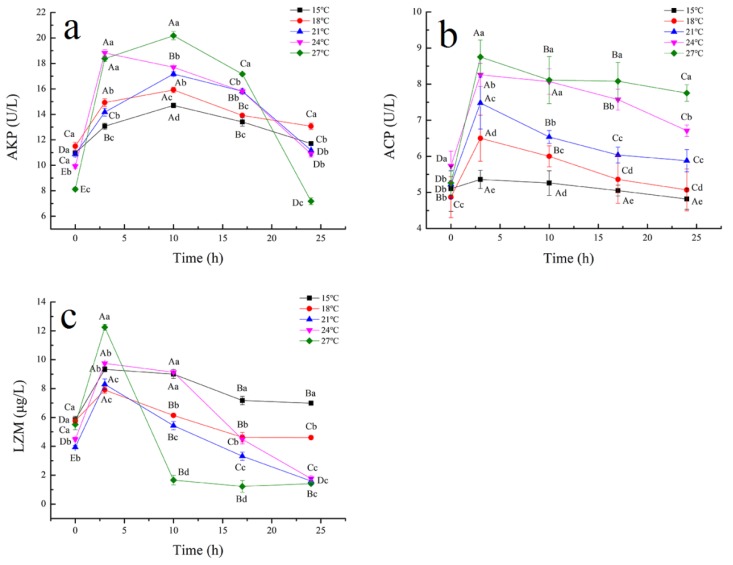
Changes in the transport temperature not only induced a stress response in tiger grouper, but also lead to immune suppression. The main evaluation indexes of humoral immunity in fish include LZM [55,56]. AKP and ACP play important roles in phosphate hydrolysis in metabolic process and are key compounds in lysosomal digestion of invading organisms in the immune system [57]. AKP values significantly increased and reached a peak level at 10 h, and then decreased, as shown in Figure 3a. The final AKP values did not recover to the initial values at 24 °C, which meant that high temperature simulated transport could affect fish metabolism. However, the final AKP values could recover to the initial values at 15, 18, and 21 °C. ACP activities had similar trends to AKP; however, it reached a peak at 3 h (Figure 3b). The results suggested that the low temperature induced immune suppression response at the early stage of transport. As the first barrier of immunity, fish skin contains a large number of innate immune factors, as LZM in skin mucus, which mediates the protection against exogenous pathogen infection [58]. When the body is attacked by pathogens, LZM is secreted in the blood and mucus to eliminate these by activating blood cells and complement, and phagocytes in the liver and pancreas [59]. LZM values in the skin mucus of all tiger grouper firstly increased, and then decreased during simulated transport, as shown in Figure 3c. The highest value of LZM activity reached at 3 h, and then LZM activity plummeted to lower levels than the initial ones, indicating that the response of the innate immune system to temperature was at least partially suppressed. LZM activity of tiger grouper transported at 15 and 18 °C for 24 h could recover to the initial values, which indicate that fish damage can be decreased to the minimum at these temperatures.
Figure 3.
Effect of temperature on alkaline phosphatase (AKP) (a), acid phosphatase (ACP) (b), and lysozyme (LZM) (c) of tiger grouper during simulated transport. Among different temperature transport groups, different small and capital letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05). Different letters without * = p-value < 0.05, different letters with ** = p-value < 0.01.
3.5. Effect of Temperature on the Relative Expression of Immune Indexes of Tiger Grouper during Simulated Transport
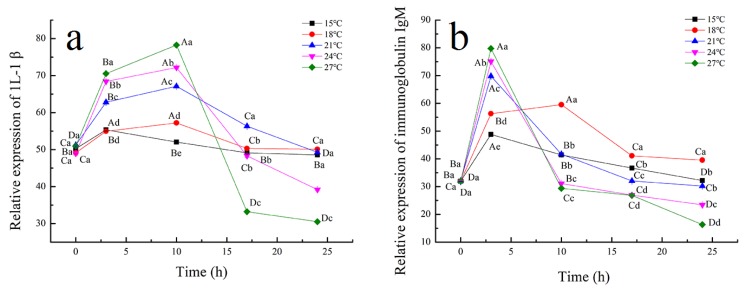
IgM and IL-1β are two important immune factors in fish immunity. IL-1β is one of the most important pro-inflammatory cytokines, which has a variety of immune response functions in viral infection, including the activation of innate immunity and regulation of adaptive immune response [60]. When affected by temperature stress, it can produce an acute-phase protein to activate innate immune regulation function [61]. IgM is one of the most important anti-pathogen antibodies, and the main immunoglobulin mediating humoral adaptive immunity of fish [62]. The expression levels of IgM and IL-1β significantly increased and came to a peak level at 3h and 10h, and then decreased, as shown in Figure 4. It was found that the expression of IgM in rainbow trout and Nile tilapia increased in a high-temperature environment, as compared to a low-temperature environment [63]. IgM expression in serum of tiger grouper transported at 27 °C was significantly higher than at other temperatures. The expression level at the end of transport was lower than the initial levels, which indicated that temperature stress made tiger grouper reach the threshold of innate immune ability, and unable to maintain the normal immune level through self-regulation. However, the tiger grouper transported at 15 °C could activate the innate immune system to maintain the immune balance and ensure the survival rate. This might be due to the dormancy induced in fish by this low-temperature.
Figure 4.
Effect of temperature on relative expression of 1L-1β (a) and IgM (b) of tiger grouper during simulated transport. Among different temperature transport groups, different small and capital letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05). Different letters without * = p-value < 0.05, different letters with ** = p-value < 0.01.
3.6. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Stress Responses of Tiger Grouper during Simulated Transport
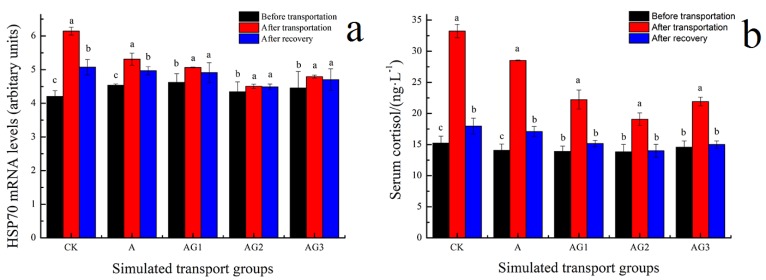
HSP70 and serum cortisol are indicators of transport stress response. The expression of HSP70 and the content of serum cortisol increased, and the treated samples were lower than that of CK, as recorded in Figure 5. The content of HSP70 and cortisol in A-G2 could recover to the initial level, which confirms the conclusion of Henrique et al. [64] that ascorbic acid addition can adjust the level of HSP70 and cortisol and, thus, regulate stress responses during fish transport. Therefore, ascorbic acid and β-1,3-glucan can be used to decrease transport stress responses for tiger grouper.
Figure 5.
Effect of ascorbic acid and β-1,3-glucan addition on HSP70 (a) and serum cortisol (b) of tiger grouper during simulated transport. Among different treatments transport groups, different small letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05). Different letters without * = p-value < 0.05, different letters with ** = p-value < 0.01.
3.7. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Relative Expression of Non-Specific Immune Indexes of Tiger Grouper during Simulated Transport
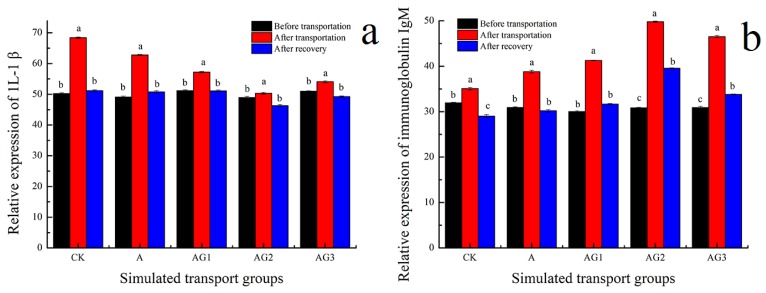
Simulated transport usually induces stress responses that could lead to increase susceptibility to diseases. Immunostimulants can reduce the outbreak of diseases by facilitating the function of phagocytic cells, improving resistance to bacterial challenges [65]. The expression levels of IL-1β significantly increased, and then decreased, as shown in Figure 6a. IL-1β expression of CK was significantly higher than that of other simulated transport groups. From Figure 6b, the expression of IgM had a similar trend as that of IL-1β, and the expression of IgM in the A-G2 group was significantly higher than that of the other groups, which indicated that ascorbic acid and β-1,3-glucan addition could stimulate non-specific immune factors. Moreover, A-G2 had the highest relative expression of IgM, thus signifying that this concentration of β-1,3-glucan was most effective in this study, and it is considered suitable for transport.
Figure 6.
Effect of ascorbic acid and β-1,3-glucan addition on relative expression of 1L-1β (a) and IgM (b) of tiger grouper during simulated transport. Among different treatments transport groups, different small letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05). Different letters without * = p-value < 0.05, different letters with ** = p-value < 0.01.
3.8. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Serum Biochemical Parameters of Tiger Grouper during Simulated Transport
Creatine kinase activity can act as an indicator of live fish metabolism. Increased creatine kinase suggests that muscle and kidney of fish have been damaged [66]. Creatine kinase activity of serum increased in all samples during simulated transport, and then returned to the initial level after recovery, as shown in Table 4. However, there was no significant difference in creatine kinase activity of A-G2 throughout the simulated transport, which indicated that ascorbic acid and β-1,3-glucan addition can effectively reduce the damage to kidneys.
Table 4.
Effect of ascorbic acid and β-1,3-glucan addition on serum biochemical and physiological of tiger grouper during simulated transport.
| Transport | Samples | Creatine Kinase | Albumin | Total Protein | Uric Acid | Urea | Creatinine |
|---|---|---|---|---|---|---|---|
| Before transport | CK | 848.50 ± 0.25a | 11.00 ± 0.00a | 41.50 ± 0.00a | 13.00 ± 0.57b | 2.50 ± 0.12a | 17.00 ± 0.00a |
| A | 765.00 ± 0.13b | 7.00 ± 0.23c | 33.50 ± 0.71b | 13.50 ± 0.23b | 2.15 ± 0.16a | 18.00 ± 0.31a | |
| A-G1 | 227.00 ± 0.66c | 9.00 ± 0.16b | 32.00 ± 0.36b | 20.00 ± 0.06a | 2.35 ± 0.11a | 13.00 ± 0.06c | |
| A-G2 | 235.00 ± 0.57c | 7.50 ± 0.03c | 31.00 ± 0.00b | 21.00 ± 0.71a | 2.05 ± 0.08a | 13.00 ± 0.06c | |
| A-G3 | 221.00 ± 0.08c | 10.50 ± 0.00b | 32.00 ± 0.08b | 19.00 ± 0.35a | 2.10 ± 0.06a | 15.50 ± 0.24b | |
| After transport | CK | 1986.00 ± 0.58a | 26.00 ± 0.21a | 35.00 ± 0.32b | 14.00 ± 0.03c | 2.25 ± 0.00a | 18.50 ± 0.17a |
| A | 1181.50 ± 0.97b | 23.50 ± 0.00b | 31.00 ± 0.06c | 17.00 ± 0.21b | 2.05 ± 0.28a | 19.50 ± 0.00a | |
| A-G1 | 689.50 ± 0.69d | 12.00 ± 0.36c | 39.50 ± 0.00a | 21.50 ± 0.19a | 2.15 ± 0.14a | 14.00 ± 0.00c | |
| A-G2 | 391.50 ± 0.73e | 8.00 ± 0.42d | 33.00 ± 0.57c | 21.87 ± 0.09a | 1.94 ± 0.03a | 13.50 ± 0.25c | |
| A-G3 | 888.00 ± 0.29c | 12.50 ± 0.00c | 32.50 ± 0.69c | 22.05 ± 0.15a | 2.05 ± 0.00a | 17.00 ± 0.14b | |
| Recovery | CK | 273.00 ± 0.93c | 10.00 ± 0.32b | 38.50 ± 0.53c | 16.00 ± 0.32b | 2.35 ± 0.00a | 11.50 ± 0.27d |
| A | 769.00 ± 0.85a | 6.50 ± 0.31c | 46.00 ± 0.33a | 16.50 ± 0.31b | 2.40 ± 0.21a | 20.00 ± 0.32a | |
| A-G1 | 267.50 ± 0.23c | 13.00 ± 0.13a | 43.50 ± 0.00b | 17.00 ± 0.13b | 2.30 ± 0.18a | 18.00 ± 0.00b | |
| A-G2 | 225.00 ± 0.87d | 6.50 ± 0.19c | 32.50 ± 0.00d | 16.50 ± 0.19b | 2.30 ± 0.05a | 14.00 ± 0.00c | |
| A-G3 | 390.50 ± 0.25b | 11.00 ± 0.22b | 34.50 ± 0.22d | 22.00 ± 0.22a | 2.25 ± 0.17a | 18.50 ± 0.19b |
Note: Among different treatments transport groups, different small letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05).
Total protein (TP) and albumin (ALB) reflect the liver function. Albumin is synthesized by the liver, and plays a role as a carrier in the blood [67]. TP and ALB in the serum can accurately reflect the absorption and metabolism of the protein. The contents of TP and ALB in serum of CK were significantly higher than in other groups during simulated transport, as shown in Table 5. It suggests that the addition of ascorbic acid and β-1,3-glucan can improve the function of tiger grouper liver. The contents of TB and ALB in treated groups showed no significant difference throughout simulated transport. Moreover, TB and ALB of tiger grouper gradually returned to the initial level after transport and recovery.
Table 5.
Effect of ascorbic acid and β-1,3-glucan addition on the free amino acids of tiger grouper during simulated transport (mg/100 g).
| Transport | Samples | Free Amino Acids | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asp * | Thr # | Ser # | Glu * | Gly # | Ala # | Val | Met | Ile | ||
| Before transport | CK | 1.84 ± 0.25a | 7.12 ± 0.25c | 3.98 ± 0.17b | 4.06 ± 0.22b | 49.40 ± 0.49d | 38.22 ± 0.71a | 5.45 ± 0.31a | 2.96 ± 0.26a | 4.73 ± 0.34a |
| A | 1.76 ± 0.01a | 11.44 ± 0.06b | 4.87 ± 0.25a | 4.89 ± 0.71a | 86.09 ± 0.91a | 34.33 ± 0.59b | 4.30 ± 0.51b | 2.07 ± 0.21a | 3.01 ± 0.22b | |
| A-G1 | 1.65 ± 0.26a | 10.93 ± 0.18b | 3.77 ± 0.17b | 5.03 ± 0.14a | 75.24 ± 0.69b | 31.29 ± 0.06c | 5.98 ± 0.08a | 2.56 ± 0.18a | 3.97 ± 0.62a | |
| A-G2 | 1.79 ± 0.32a | 12.14 ± 0.21a | 4.05 ± 0.38b | 4.42 ± 0.61b | 71.25 ± 0.53b | 39.78 ± 0.71a | 5.09 ± 0.22a | 2.13 ± 0.11a | 4.17 ± 0.04a | |
| A-G3 | 1.84 ± 0.41a | 11.03 ± 0.01b | 4.97 ± 0.51a | 4.79 ± 0.25a | 66.42 ± 0.66c | 33.86 ± 0.81b | 4.97 ± 0.05a | 2.44 ± 0.01a | 3.57 ± 0.28b | |
| After transport | CK | 1.98 ± 0.03c | 5.87 ± 0.45c | 5.10 ± 0.05c | 8.57 ± 0.07a | 63.01 ± 0.21c | 42.56 ± 0.66b | 6.91 ± 0.06a | 3.32 ± 0.28a | 5.89 ± 0.41a |
| A | 3.37 ± 0.19a | 9.85 ± 0.06b | 7.62 ± 0.28a | 6.33 ± 0.21b | 124.35 ± 0.37a | 38.03 ± 0.08d | 5.13 ± 0.10b | 2.62 ± 0.01b | 2.50 ± 0.16c | |
| A-G1 | 2.38 ± 0.11b | 10.01 ± 0.32b | 7.99 ± 0.33a | 5.53 ± 0.03c | 75.44 ± 0.41b | 40.24 ± 0.19c | 6.02 ± 0.21a | 2.78 ± 0.06b | 3.11 ± 0.11b | |
| A-G2 | 1.93 ± 0.01c | 10.93 ± 0.22a | 6.46 ± 0.05b | 5.09 ± 0.10c | 78.03 ± 0.39b | 46.51 ± 0.73a | 5.67 ± 0.03b | 2.61 ± 0.01b | 3.55 ± 0.06b | |
| A-G3 | 1.70 ± 0.22c | 10.90 ± 0.06a | 5.48 ± 0.10c | 5.14 ± 0.19c | 74.29 ± 0.11b | 41.22 ± 0.37c | 5.13 ± 0.18b | 2.48 ± 0.12b | 3.71 ± 0.28b | |
| Recovery | CK | 1.82 ± 0.08a | 7.89 ± 0.18c | 4.77 ± 0.27a | 6.59 ± 0.47a | 58.98 ± 0.91c | 40.03 ± 0.57b | 6.84 ± 0.25a | 2.38 ± 0.18a | 4.84 ± 0.10a |
| A | 1.98 ± 0.10a | 11.19 ± 0.27a | 5.18 ± 0.27a | 5.94 ± 0.31b | 96.71 ± 0.43a | 35.28 ± 0.09d | 4.97 ± 0.33b | 2.46 ± 0.02a | 2.92 ± 0.16c | |
| A-G1 | 1.73 ± 0.02a | 10.52 ± 0.04b | 4.85 ± 0.03a | 4.96 ± 0.80c | 74.04 ± 0.09b | 37.90 ± 0.18c | 4.99 ± 0.09b | 2.10 ± 0.11a | 4.06 ± 0.71a | |
| A-G2 | 1.58 ± 0.16a | 11.88 ± 0.11a | 5.16 ± 0.18a | 4.83 ± 0.33c | 77.13 ± 0.36b | 41.64 ± 0.78a | 4.96 ± 0.18b | 1.92 ± 0.02a | 3.49 ± 0.39b | |
| A-G3 | 1.69 ± 0.22a | 11.85 ± 0.65a | 4.86 ± 0.20a | 4.31 ± 0.57c | 73.04 ± 0.74b | 37.49 ± 0.07c | 4.67 ± 0.21b | 2.23 ± 0.18a | 3.39 ± 0.27b | |
| Leu | Tyr | Phe | Lys | His | Arg | Pro# | Total | |||
| Before transport | CK | 7.52 ± 0.37a | 3.22 ± 0.02a | 2.88 ± 0.02a | 28.54 ± 0.54a | 3.86 ± 0.25a | 7.86 ± 0.71a | 6.27 ± 0.68a | 177.91 | |
| A | 7.73 ± 0.41a | 1.45 ± 0.01c | 1.57 ± 0.11c | 21.89 ± 0.48c | 3.55 ± 0.34b | 5.38 ± 0.42c | 5.49 ± 0.71b | 199.82 | ||
| A-G1 | 7.88 ± 0.68a | 1.73 ± 0.22b | 2.05 ± 0.18b | 25.33 ± 0.71b | 3.18 ± 0.33b | 6.93 ± 0.74bc | 5.93 ± 0.01a | 193.45 | ||
| A-G2 | 7.03 ± 0.31a | 1.98 ± 0.17b | 2.47 ± 0.17a | 26.09 ± 0.31b | 4.09 ± 0.06a | 6.41 ± 0.11b | 6.09 ± 0.39a | 198.98 | ||
| A-G3 | 7.97 ± 0.45a | 2.06 ± 0.28b | 1.99 ± 0.01c | 23.45 ± 0.78c | 3.74 ± 0.28a | 7.07 ± 0.02b | 5.77 ± 0.81ab | 185.94 | ||
| Aftertransport | CK | 7.83 ± 0.31a | 3.76 ± 0.28a | 3.46 ± 0.15a | 31.35 ± 0.59a | 4.30 ± 0.45a | 10.74 ± 0.33a | 8.18 ± 0.39a | 212.83 | |
| A | 7.76 ± 0.28a | 1.34 ± 0.01c | 2.28 ± 0.25b | 24.39 ± 0.63c | 3.63 ± 0.19b | 4.99 ± 0.41d | 6.07 ± 0.08c | 250.26 | ||
| A-G1 | 8.74 ± 0.41b | 1.43 ± 0.12c | 2.46 ± 0.06b | 29.39 ± 0.71b | 4.12 ± 0.06a | 6.63 ± 0.71c | 7.74 ± 0.73b | 214.01 | ||
| A-G2 | 7.01 ± 0.37a | 1.55 ± 0.08c | 2.49 ± 0.03b | 27.83 ± 0.39b | 4.23 ± 0.28a | 6.69 ± 0.07c | 6.41 ± 0.36c | 216.99 | ||
| A-G3 | 7.76 ± 0.63a | 2.38 ± 0.19b | 2.48 ± 0.11b | 24.87 ± 0.23c | 3.93 ± 0.09a | 7.98 ± 0.20b | 5.71 ± 0.51d | 205.16 | ||
| Recovery | CK | 6.82 ± 0.10b | 2.95 ± 0.01a | 1.79 ± 0.02b | 22.19 ± 0.33c | 3.60 ± 0.17a | 6.59 ± 0.38a | 5.19 ± 0.62c | 183.27 | |
| A | 6.98 ± 0.12b | 1.52 ± 0.02b | 1.98 ± 0.10ab | 22.75 ± 0.62c | 3.42 ± 0.31a | 5.21 ± 0.67b | 5.94 ± 0.17b | 214.43 | ||
| A-G1 | 7.65 ± 0.41a | 1.67 ± 0.13b | 2.30 ± 0.21a | 26.91 ± 0.15a | 2.75 ± 0.07b | 6.87 ± 0.37a | 6.79 ± 0.57a | 200.09 | ||
| A-G2 | 7.83 ± 0.57a | 1.86 ± 0.03b | 2.19 ± 0.09a | 24.50 ± 0.31b | 2.98 ± 0.35b | 6.61 ± 0.65a | 5.81 ± 0.08b | 204.37 | ||
| A-G3 | 7.23 ± 0.69a | 1.44 ± 0.02b | 2.26 ± 0.11a | 19.59 ± 0.09d | 3.64 ± 0.41a | 6.55 ± 0.52a | 4.94 ± 0.72c | 189.18 | ||
Note: Among different treatments transport groups, different small letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05). * represents umami amino acids; # represents sweet taste amino acids.
Urea, creatinine, and uric acid (UA) reflect the renal function. Urea is the product of metabolism of nitrogen compounds, and also an important component in maintaining blood osmotic pressure [68]. At the end of transport, urea, creatinine and UA levels of treated samples decreased (Table 4), and these could not return to the initial level after recovery, which indicates damage that may have contributed to the fish majority during long-term transport.
3.9. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Nutritional Indexes of Tiger Grouper during Simulated Transport
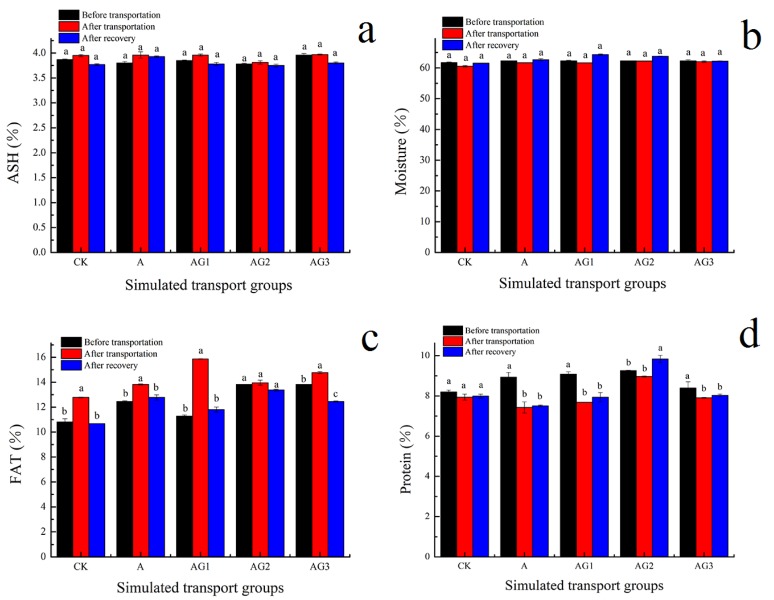
Figure 7 shows the changes in ash, moisture content, crude fat, and crude protein of tiger grouper during simulated transport. The contents of moisture and ash in all samples did not show obvious changes. Protein decreased, possibly because of stress related to transport and temperature change. Among all of the samples, the nutritional components of A-G2 showed no significant changes during simulated transport, which indicates that ascorbic acid and the G2 β-1,3-glucan concentration could effectively reduce the negative impact of transport and temperature changes on the nutritional indexes of tiger group.
Figure 7.
Effect of ascorbic acid and β-1,3-glucan addition on ash (a), moisture (b), fat (c) and protein (d) of muscle of tiger grouper during simulated transport. Among different treatments transport groups, different small letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05). Different letters without * = p-value < 0.05, different letters with ** = p-value < 0.01.
3.10. Effect of Ascorbic Acid and β-1,3-Glucan Addition on Free Amino Acids of Tiger Grouper during Simulated Transport
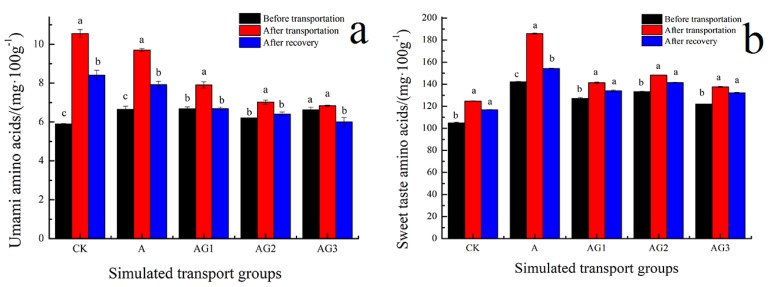
The total content of free amino acids of tiger grouper increased during simulated transport, and recovered to the initial level, as shown in Table 5. Transport stress could promote protein degradation, resulting in higher total free amino acids contents. It should be noted that there was no food for tiger grouper during simulated transport; therefore, transport stress could accelerate protein degradation and lead to nutrient content loss. However, the addition of ascorbic acid and β-1,3-glucan resulted in a reduction of free amino acid and thus probably slowed down the rate of protein degradation during simulated transport. This indicates moderation of the stress response. Free amino acids in the muscle tissue are usually related with different tastes, such as umami, sweetness, bitterness, and sourness. Umami amino acids include Asp and Glu, sweetness amino acids include Thr, Ser, Gly, Ala, and Pro [69]. Figure 8 shows the effect of ascorbic acid and β-1,3-glucan addition on umami and sweet taste amino acids of tiger grouper during simulated transport. In all samples, the amount of umami and sweet taste amino acids increased during simulated transport, due to transport stress response accelerated protein degradation. There was no significant difference in taste amino acids of A-G2 during simulated transport and recovery. The results indicate that ascorbic acid and the G2 β-1,3-glucan concentration can effectively reduce the changes of free amino acids during simulated transport.
Figure 8.
Effect of ascorbic acid and β-1,3-glucan addition on umami (a) and sweet (b) taste amino acids of tiger grouper during simulated transport. Among different treatments transport groups, different small letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05). Different letters without * = p-value < 0.05, different letters with ** = p-value < 0.01.
Nucleotides in aquatic products are of great significance for flavors [70]. IMP and AMP are the two main taste nucleotides in tiger grouper during simulated transport. IMP has a strong umami taste [71]. Table 6 shows the changes in IMP and AMP of tiger grouper during simulated transport. The results demonstrated that value of IMP was higher than 10. Therefore, IMP contributed most to the sweet and meaty flavor of tiger grouper. However, the content of AMP was obviously lower than IMP, and the TAV value of AMP was less than 1. IMP and AMP concentrations values were decreased during simulated transport and were able to recover to the initial levels. Different from the CK samples, the IMP and AMP concentrations in A-G2 samples were higher after recovery, and TAV value of IMP in A-G2 was 16.14, proving ascorbic acid and β-1,3-glucan addition could relieve flavor nucleotides degradation during simulated transport and recovery.
Table 6.
Effect of ascorbic acid and β-1,3-glucan addition on nucleotides of tiger grouper during simulated transport in water.
| Transport | Samples | IMP (mg/100 g) | TAV | AMP (mg/100 g) | TAV |
|---|---|---|---|---|---|
| Before transport | CK | 269.18 ± 0.78d | 10.77 | 13.24 ± 0.41ab | 0.26 |
| A | 273.40 ± 0.66c | 10.94 | 14.69 ± 0.59a | 0.29 | |
| A-G1 | 271.94 ± 0.96cd | 10.88 | 12.47 ± 0.65b | 0.25 | |
| A-G2 | 278.66 ± 0.55b | 11.15 | 13.93 ± 0.32ab | 0.28 | |
| A-G3 | 284.39 ± 0.47a | 11.38 | 13.54 ± 0.69ab | 0.27 | |
| Aftertransport | CK | 259.47 ± 0.84d | 10.38 | 9.55 ± 0.57b | 0.19 |
| A | 268.06 ± 0.28c | 10.72 | 11.23 ± 0.62ab | 0.22 | |
| A-G1 | 269.18 ± 0.71c | 10.77 | 11.37 ± 0.71ab | 0.23 | |
| A-G2 | 275.33 ± 0.49b | 11.01 | 12.99 ± 0.45a | 0.26 | |
| A-G3 | 280.91 ± 0.78a | 11.24 | 10.81 ± 0.66b | 0.22 | |
| Recovery | CK | 273.57 ± 0.67e | 10.94 | 14.43 ± 0.28a | 0.29 |
| A | 280.69 ± 0.71d | 11.23 | 10.98 ± 0.33c | 0.22 | |
| A-G1 | 301.42 ± 0.54c | 12.06 | 13.41 ± 0.19a | 0.27 | |
| A-G2 | 403.49 ± 0.66a | 16.14 | 12.79 ± 0.25b | 0.26 | |
| A-G3 | 390.24 ± 0.91b | 15.61 | 12.63 ± 0.36b | 0.25 |
Note: Among different treatments transport groups, different small letters indicate the results of Duncan’s test at different transport time. The same letters mean no significant difference (p > 0.05), while different letters mean significant difference (p < 0.05).
4. Conclusions
The minimum tolerable temperature of tiger grouper transported by water is 15 °C, and could induce dormancy, thus resulting in reducing life activities. The activities of metabolic enzymes, cortisol, HSP70 transcription level, GR enzyme activity, IL-1β, and IgM transcription levels in tiger grouper serum at 15 °C were significantly lower than in groups transported at other temperatures. Anti-stress agents, including ascorbic acid and β-1,3-glucan was added in the transport water for tiger grouper, during simulated transport and recovery. Cortisol content, HSP70 transcription level, and immune index of tiger grouper serum in A-G2 were lower than in other groups. However, there was no significant difference in nutritional content, taste amino acids, and nucleotides of muscle tissue in A-G2 before and after transport. The addition of ascorbic acid and β-1,3-glucan could effectively reduce the stress response of tiger grouper and improve their immunity and survival. Besides, it did not lead to loss of nutritional valued and flavor.
Acknowledgments
The authors would like to express their profound gratitude to Weiqiang Qiu from IAC of Shanghai Ocean University for his technical assistance.
Author Contributions
Conceptualization, B.W., J.M. and J.X.; Data curation, B.W., Q.W. and J.C.; Formal analysis, B.W., Q.W. and J.C.; Funding acquisition, J.X.; Investigation, B.W.; Methodology, Q.W. and J.M.; Project administration, J.X.; Software, B.W.; Validation, B.W. and J.X.; Writing—original draft, B.W.; Writing—review & editing, Q.W., J.C. and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
National Key R&D Program of China (2019YFD0901601), Shanghai Science and Technology Key Project on Agriculture from Shanghai Municipal Agricultural Commission (2019-02-08-00-10-F01143), China Agriculture Research System (CARS-47), and Shanghai Science and Technology Commission Platform Capacity Construction Project (19DZ2284000).
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
All animal experiments were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978). Shanghai Ocean University Ethics Committee approved this research protocol.
References
- 1.Hoseinifar S.H., Sun Y., Wang A., Zhou Z.J. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 2018;9:2429. doi: 10.3389/fmicb.2018.02429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X., Steele J.C., Meng X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017;223:161–169. doi: 10.1016/j.envpol.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 3.He X., Deng M., Wang Q., Yang Y., Yang Y., Nie X. Residues and health risk assessment of quinolones and sulfonamides in cultured fish from Pearl River Delta, China. Aquaculture. 2016;458:38–46. doi: 10.1016/j.aquaculture.2016.02.006. [DOI] [Google Scholar]
- 4.Lee M.K., Nam J. The determinants of live fish consumption frequency in South Korea. Food Res. Int. 2019;120:382–388. doi: 10.1016/j.foodres.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Purbosari N., Warsiki E., Syamsu K., Santoso J. Natural versus synthetic anesthetic for transport of live fish: A review. Aquac. Fish. 2019;4:129–133. doi: 10.1016/j.aaf.2019.03.002. [DOI] [Google Scholar]
- 6.Amar E.C., Apines-Amar M.J.S., Faisan J.P. Dietary onion or ginger modulates the stress response and susceptibility to Vibrio harveyi JML1 infection in brown-marbled grouper (Epinephelus fuscoguttatus) juveniles. J. Aquat. Anim. Health. 2017;30:39–49. doi: 10.1002/aah.10005. [DOI] [PubMed] [Google Scholar]
- 7.Afero F., Miao S., Perez A.A. Economic analysis of tiger grouper (Epinephelus fuscoguttatus) and humpback grouper Cromileptesaltivelis commercial cage culture in Indonesia. Aquacult. Int. 2010;18:725–739. doi: 10.1007/s10499-009-9295-x. [DOI] [Google Scholar]
- 8.Noor N.M., Defoirdt T., Alipiah N., Karim M., Daud H., Natrah I. Quorum sensing is required for full virulence of Vibrio campbellii towards tiger grouper (Epinephelus fuscoguttatus) larvae. J. Fish Dis. 2019;42:489–495. doi: 10.1111/jfd.12946. [DOI] [PubMed] [Google Scholar]
- 9.Fui C.F., Miura A., Nakagawa Y., Kato K., Sakamoto W., Takii K., Miyashita S., Senoo S. Aeration rate adjustment at night to prevent sinking syndrome-related death in the tiger grouper Epinephelus fuscoguttatus (Perciformes: Serranidae) larvae. Aquac. Res. 2016;47:165–175. doi: 10.1111/are.12479. [DOI] [Google Scholar]
- 10.Manuel R., Boerrigter J., Roques J., van der Heul J., van den Bos R., Flik G., van de Vis H. Stress in African catfish (Clarias gariepinus) following overland transportation. Fish Physiol. Biochem. 2014;40:33–44. doi: 10.1007/s10695-013-9821-7. [DOI] [PubMed] [Google Scholar]
- 11.Iversen M.H., Eliassen R.A. The effect of allostatic load on hypothalamic-pituitary-interrenal (HPI) axis before and after secondary vaccination in Atlantic salmon postsmolts (Salmo salar L.) Fish Physiol. Biochem. 2014;40:527–538. doi: 10.1007/s10695-013-9863-x. [DOI] [PubMed] [Google Scholar]
- 12.Tacchi L., Lowrey L., Musharrafieh R., Crossey K., Larragoite E.T., Salinas I. Effects of transportation stress and addition of salt to transport water on the skin mucosal homeostasis of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2015;435:120–127. doi: 10.1016/j.aquaculture.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bricknell I., Dalmo R.A. The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immun. 2005;19:457–472. doi: 10.1016/j.fsi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Salbego J., Becker A.G., Gonçalves J.F., Menezes C.C., Heldwein C.G., Spanevello R.M., Loro V.L., Schetinger M.R.C., Morsch V.M., Heinzmann B.M. The essential oil from Lippia alba induces biochemical stress in the silver catfish (Rhamdia quelen) after transportation. Neotrop. Ichthyol. 2014;12:811–818. doi: 10.1590/1982-0224-20130178. [DOI] [Google Scholar]
- 15.Souza D.M., Martins Á.C., Jensen L., Monserrat J.M., Wasielesky W., Jr., Garcia L. Effects of water temperature on oxidative stress parameters in the pink shrimp Farfantepenaeus brasiliensis during transport. Aquaculture. 2013;416:310–314. doi: 10.1016/j.aquaculture.2013.09.032. [DOI] [Google Scholar]
- 16.Wu S.M., Tseng Y.J., Lin J.J., Pan B.S. Mitigation of stress and water deterioration with a root extract of Glycine tomentella during simulated transport of orange-spotted grouper (Epinephelus coioides) Aquaculture. 2020;514:734485. doi: 10.1016/j.aquaculture.2019.734485. [DOI] [Google Scholar]
- 17.Taheri Mirghaed A., Ghelichpour M. Effects of anesthesia and salt treatment on stress responses, and immunological and hydromineral characteristics of common carp (Cyprinus carpio, Linnaeus, 1758) subjected to transportation. Aquaculture. 2019;501:1–6. doi: 10.1016/j.aquaculture.2018.11.008. [DOI] [Google Scholar]
- 18.Vilhena C.S., do Nascimento L.A.S., de Aguiar Andrade E.H., da Silva J.K.d.R., Hamoy M., Torres M.F., Barbas L.A.L. Essential oil of Piper divaricatum induces a general anaesthesia-like state and loss of skeletal muscle tonus in juvenile tambaqui, Colosso mamacropomum. Aquaculture. 2019;510:169–175. doi: 10.1016/j.aquaculture.2019.05.057. [DOI] [Google Scholar]
- 19.De Oliveira C.P.B., Lemos C.H.d.P., Felix e Silva A., de Souza S.A., Albinati A.C.L., Lima A.O., Copatti C.E. Use of eugenol for the anaesthesia and transportation of freshwater angelfish (Pterophyllum scalare) Aquaculture. 2019;513:734409. doi: 10.1016/j.aquaculture.2019.734409. [DOI] [Google Scholar]
- 20.De Oliveira C.P.B., Lemos C.H.d.P., Vidal L.V.O., Couto R.D., Pereira D.S.P., Copatti C.E. Anaesthesia with eugenol in hybrid Amazon catfish (Pseudoplatystoma reticulatum × Leiarius marmoratus) handling: Biochemical and haematological responses. Aquaculture. 2019;501:255–259. doi: 10.1016/j.aquaculture.2018.11.046. [DOI] [Google Scholar]
- 21.Pounder K.C., Mitchell J.L., Thomson J.S., Pottinger T.G., Sneddon L.U. Physiological and behavioural evaluation of common anaesthesia practices in the rainbow trout. Appl. Anim. Behav. Sci. 2018;199:94–102. doi: 10.1016/j.applanim.2017.10.014. [DOI] [Google Scholar]
- 22.Zhang Y., Xiao X., Yan L., Thi Tuyet Nga M., Zhang X. Survival prediction system for waterless live Chinese Sturgeon transportation based on temperature related glucose changes. J. Food Process Eng. 2018;41:e12646. doi: 10.1111/jfpe.12646. [DOI] [Google Scholar]
- 23.Wang M., Zhu Z.J.B., Equipment B. Nrf2 is involved in osmoregulation, antioxidation and immunopotentiation in Coilia nasus under salinity stress. Biotechnol. Biotechnol. Equip. 2019;33:1453–1463. doi: 10.1080/13102818.2019.1673671. [DOI] [Google Scholar]
- 24.Lu D.L., Ma Q., Sun S.X., Zhang H., Chen L.Q., Zhang M.L., Du Z.Y.J. Reduced oxidative stress increases acute cold stress tolerance in zebrafish. Comp. Biochem. Phys. 2019;235:166–173. doi: 10.1016/j.cbpa.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Zeng P., Chen T., Shen J. Effects of cold acclimation and storage temperature on crucian carp (Carassius auratusgi belio) in a waterless preservation. Fish Physiol. Biochem. 2014;40:973–982. doi: 10.1007/s10695-013-9898-z. [DOI] [PubMed] [Google Scholar]
- 26.Cheng C.H., Ye C.X., Guo Z.X., Wang A.L. Immune and physiological responses of pufferfish (Takifugu obscurus) under cold stress. Fish Shellfish Immunol. 2017;64:137–145. doi: 10.1016/j.fsi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Wang W., Yan L., Glamuzina B., Zhang X. Development and evaluation of an intelligent traceability system for waterless live fish transportation. Food Control. 2019;95:283–297. doi: 10.1016/j.foodcont.2018.08.018. [DOI] [Google Scholar]
- 28.Peng S., Shi Z., Fei Y., Gao Q., Sun P., Wang J.J. Effect of high-dose vitamin C supplementation on growth, tissue ascorbic acid concentrations and physiological response to transportation stress in juvenile silver pomfret, Pampus argenteus. J. Appl. Ichthyol. 2013;29:1337–1341. doi: 10.1111/jai.12250. [DOI] [Google Scholar]
- 29.Balamurugan J., Kumar T.T.A., Prakash S., Meenakumari B., Balasundaram C., Harikrishnan R.J. Clove extract: A potential source for stress free transport of fish. Aquaculture. 2016;454:171–175. doi: 10.1016/j.aquaculture.2015.12.020. [DOI] [Google Scholar]
- 30.Roosta Z., Hajimoradloo A., Ghorbani R., Hoseinifar S.H. The effects of dietary vitamin C on mucosal immune responses and growth performance in Caspian roach (Rutilus rutilus caspicus) fry. Fish Physiol. Biochem. 2014;40:1601–1607. doi: 10.1007/s10695-014-9951-6. [DOI] [PubMed] [Google Scholar]
- 31.Wan J., Ge X., Liu B., Xie J., Cui S., Zhou M., Xia S., Chen R.J. Effect of dietary vitamin C on non-specific immunity and mRNA expression of three heat shock proteins (HSPs) in juvenile Megalobrama amblycephala under pH stress. Aquaculture. 2014;434:325–333. doi: 10.1016/j.aquaculture.2014.08.043. [DOI] [Google Scholar]
- 32.Chettri J.K., Kania P.W., Buchmann K. Immunomodulation of rainbow trout (Oncorhynchus mykiss) fry by bath exposure to a β-glucan from Euglena gracilis. Aquac. Res. 2013;44:1407–1415. doi: 10.1111/j.1365-2109.2012.03145.x. [DOI] [Google Scholar]
- 33.Gopalakannan A., Arul V. Enhancement of the innate immune system and disease-resistant activity in Cyprinus carpio by oral administration of β-glucan and whole cell yeast. Aquac. Res. 2010;41:662–670. doi: 10.1111/j.1365-2109.2009.02368.x. [DOI] [Google Scholar]
- 34.Kumari J., Sahoo P.K.J. Dietary β-1,3 glucan potentiates innate immunity and disease resistance of Asian cat fish, Clariasbatrachus (L.) J. Fish Dis. 2006;29:95–101. doi: 10.1111/j.1365-2761.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 35.Lin S., Yu P., Lin L., Li L. Effects of dietary β-1,3-glucan, chitosan or raffinose on the growth, innate immunity and resistance of koi (Cyprinus carpio koi) Fish Shellfish Immunol. 2011;31:794. doi: 10.1016/j.fsi.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Q.C., Buentello J.A., Lii D.M.G. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum (Sciaenops ocellatus) Aquaculture. 2010;309:253–257. doi: 10.1016/j.aquaculture.2010.09.003. [DOI] [Google Scholar]
- 37.Dong C., Wang J. Immunostimulatory effects of dietary fructooligosaccharides on red swamp crayfish, Procambarusclarkii (Girard) Aquaculture. 2013;44:1416–1424. doi: 10.1111/j.1365-2109.2012.03146.x. [DOI] [Google Scholar]
- 38.Fan X., Qin X., Zhang C., Zhu Q., Chen J., Chen P.J. Metabolic and anti-oxidative stress responses to low temperatures during the waterless preservation of the hybrid grouper (Epinephelus fuscogutatus♀ × Epinephelus lanceolatus♂) Aquaculture. 2019;508:10–18. doi: 10.1016/j.aquaculture.2019.04.054. [DOI] [Google Scholar]
- 39.Seunghan L., Kumar K., Ali H., Jeongwhui H., Dae-Jung K., Bai S.C. Synergistic effects of dietary supplementation of Bacillus subtilis WB60 and mannanoligosaccharide (MOS) on growth performance, immunity and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 2018;83:283–291. doi: 10.1016/j.fsi.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 40.Ayanda I.O., Ekhator U.I., Bello O.A. Determination of selected heavy metal and analysis of proximate composition in some fish species from Ogun River, Southwestern Nigeria. Heliyon. 2019;5:e02512. doi: 10.1016/j.heliyon.2019.e02512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Y., Gao Y., Chen X., Huang B. Determination of optimal fasting time before blood sampling to get baseline data on serum biochemical characteristics in juvenile turbot (Scophthalmus maximus) Aquaculture. 2018;487:83–88. doi: 10.1016/j.aquaculture.2018.01.009. [DOI] [Google Scholar]
- 42.Fang S., Zhou Q., Hu Y., Liu F., Mei J., Xie J. Antimicrobial carvacrol incorporated in flaxseed gum-sodium alginate active films to improve the quality attributes of Chinese sea bass (Lateolabrax maculatus) during cold storage. Molecules. 2019;24:3292. doi: 10.3390/molecules24183292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q., Li P., Fang S., Liu W., Mei J., Xie J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (−0.9 °C) storage. Coatings. 2019;9:489. doi: 10.3390/coatings9080489. [DOI] [Google Scholar]
- 44.Kraitavin W., Yoshitake K., Igarashi Y., Mitsuyama S., Kinoshita S., Kambayashi D., Watabe S., Asakawa S. Transcriptome analysis of yamame (Oncorhynchus masou) in normal conditions after heat stress. Biology. 2019;8:21. doi: 10.3390/biology8020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eid I.I., Bhassu S., Goh Z.H., Khoo L.T., Tan G.Y.A. Molecular characterization and gene evolution of the heat shock protein 70 gene in snakehead fish with different tolerances to temperature. Biochem. Syst. Ecol. 2016;66:137–144. doi: 10.1016/j.bse.2016.02.011. [DOI] [Google Scholar]
- 46.Zhou C., Liu B., Ge X., Xie J., Xu P.J. Effect of dietary carbohydrate on the growth performance, immune response, hepatic antioxidant abilities and heat shock protein 70 expression of Wuchang bream, Megalobrama amblycephala. J. Appl. Ichthyol. 2013;29:1348–1356. doi: 10.1111/jai.12264. [DOI] [Google Scholar]
- 47.Aedo J.E., Zuloaga R., Boltaña S., Molina A., Valdés J.A. Membrane-initiated cortisol action modulates early pyruvate dehydrogenase kinase 2 (pdk2) expression in fish skeletal muscle. Comp. Biochem. Phys. 2019;233:24–29. doi: 10.1016/j.cbpa.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 48.Song J., Brill R.W., McDowell J.R. Plasticity in standard and maximum aerobic metabolic rates in two populations of an estuarine dependent teleost, spotted seatrout (Cynoscion nebulosus) Biology. 2019;8:46. doi: 10.3390/biology8020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azambuja C.R., Mattiazzi J., Riffel A.P.K., Finamor I.A., de Oliveira Garcia L., Heldwein C.G., Heinzmann B.M., Baldisserotto B., Pavanato M.A., Llesuy S.F. Effect of the essential oil of Lippia alba on oxidative stress parameters in silver catfish (Rhamdia quelen) subjected to transport. Aquaculture. 2011;319:156–161. doi: 10.1016/j.aquaculture.2011.06.002. [DOI] [Google Scholar]
- 50.Lushchak V.I., Bagnyukova T.V. Effects of different environmental oxygen levels on free radical processes in fish. Comp. Biochem. Phys. B. 2006;144:283–289. doi: 10.1016/j.cbpb.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Valavanidis A., Vlahogianni T., Dassenakis M., Scoullos M.J. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006;64:178–189. doi: 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Paital B., Chainy G.B.N. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Phys. 2010;151:142–151. doi: 10.1016/j.cbpc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M., Li M., Wang R., Qian Y. Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish Shellfish Immunol. 2018;79:313–320. doi: 10.1016/j.fsi.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 54.Yan J., Liang X., Zhang Y., Li Y., Cao X., Gao J. Cloning of three heat shock protein genes (HSP70, HSP90α and HSP90β) and their expressions in response to thermal stress in loach (Misgurnus anguillicaudatus) fed with different levels of vitamin C. Fish Shellfish Immunol. 2017;66:103–111. doi: 10.1016/j.fsi.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 55.Dong J., Cheng R., Yang Y., Zhao Y., Wu G., Zhang R., Zhu X., Li L., Li X. Effects of dietary taurine on growth, non-specific immunity, anti-oxidative properties and gut immunity in the Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol. 2018;82:212–219. doi: 10.1016/j.fsi.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 56.Chen Q.X., Zheng W.Z., Lin J.Y., Shi Y., Xie W.Z., Zhou H.M. Effect of metal ions on the activity of green crab (Scylla serrata) alkaline phosphatase. Int. J. Biochem. Cell B. 2000;32:879–885. doi: 10.1016/S1357-2725(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y., Zhang W., Xu W., Mai K., Zhang Y., Liufu Z. Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2012;32:750–755. doi: 10.1016/j.fsi.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 58.Jia R., Liu B.L., Feng W.R., Han C., Huang B., Lei J.L. Stress and immune responses in skin of turbot (Scophthalmus maximus) under different stocking densities. Fish Shellfish Immunol. 2016;55:131–139. doi: 10.1016/j.fsi.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 59.Ma J., Bu Y., Li X.J. Immunological and histopathological responses of the kidney of common carp (Cyprinus carpio L.) sublethally exposed to glyphosate. Environ. Toxicol. Pharm. 2015;39:1–8. doi: 10.1016/j.etap.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Chen X.X., Guo Z., Jin Q., Qiao S., Li R., Li X., Deng R., Feng W.H., Zhang G.P.J. Porcine reproductive and respiratory syndrome virus induces interleukin-1β through MyD88/ERK/AP-1 and NLRP3 inflammasome in microglia. Vet. Microbiol. 2018;227:82–89. doi: 10.1016/j.vetmic.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 61.Klaper R., Arndt D., Setyowati K., Chen J., Goetz F.J. Functionalization impacts the effects of carbon nanotubes on the immune system of rainbow trout, Oncorhynchus mykiss. Aqua. Toxicol. 2010;100:211–217. doi: 10.1016/j.aquatox.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 62.Lauriano E., Pergolizzi S., Capillo G., Kuciel M., Alesci A., Faggio C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 2016;59:250–255. doi: 10.1016/j.fsi.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Dominguez M., Takemura A., Tsuchiya M., Nakamura S. Impact of different environmental factors on the circulating immunoglobulin levels in the Nile tilapia, Oreochromis niloticus. Aquaculture. 2004;241:491–500. doi: 10.1016/j.aquaculture.2004.06.027. [DOI] [Google Scholar]
- 64.Henrique M.M.F., Gomes E.F., Gouillou-Coustans M.F., Oliva-Teles A., Davies S.J. Influence of supplementation of practical diets with vitamin C on growth and response to hypoxic stress of seabream, Sparus aurata. Aquaculture. 2013;161:415–426. doi: 10.1016/S0044-8486(97)00289-5. [DOI] [Google Scholar]
- 65.Barros M.M., Falcon D.R., Ricardo D.O.O. Non-specific immune parameters and physiological response of Nile tilapia fed β-glucan and vitamin C for different periods and submitted to stress and bacterial challenge. Fish Shellfish Immunol. 2014;39:188–195. doi: 10.1016/j.fsi.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Steinbach C., Burkina V., Schmidt-Posthaus H., Stara A., Kolarova J. Effect of the human therapeutic drug diltiazem on the haematological parameters, histology and selected enzymatic activities of rainbow trout Oncorhynchus mykiss. Chemosphere. 2016;157:57–64. doi: 10.1016/j.chemosphere.2016.04.137. [DOI] [PubMed] [Google Scholar]
- 67.Andreeva A.M. The strategies of organization of the fish plasma proteome: With and without albumin. Russ. J. Mar. Biol. 2019;45:263–274. doi: 10.1134/S1063074019040023. [DOI] [Google Scholar]
- 68.Yancey P.H. Nitrogen compounds as osmolytes. Fish Physiol. 2001;20:309–341. [Google Scholar]
- 69.Osako K., Fujii A., Ruttanapornvareesakul Y., Nagano N., Kuwahara K., Okamoto A. Differences in free amino acid composition between testis and ovary of sea urchin Anthocidarisc rassispina during gonadal development. Fish. Sci. 2007;73:660–667. doi: 10.1111/j.1444-2906.2007.01379.x. [DOI] [Google Scholar]
- 70.Hong H., Regenstein J.M., Luo Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. 2017;57:1787–1798. doi: 10.1080/10408398.2014.1001489. [DOI] [PubMed] [Google Scholar]
- 71.Chen D.W., Zhang M.J. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis) Food Chem. 2007;104:1200–1205. doi: 10.1016/j.foodchem.2007.01.042. [DOI] [Google Scholar]