Abstract
Enveloped animal viruses such as human immunodeficiency virus (HIV), hepatitis B virus, hepatitis C virus, human papillomavirus, Marburg, and influenza are major public health concerns around the world. The prohibitive cost of antiretroviral (ARV) drugs for most HIV‐infected patients in sub‐Saharan Africa and the serious side effects in those who have access to ARV drugs make a compelling case for the study of complementary and alternative therapies. Such therapies should have scientifically proved antiviral activity and minimal toxic effects. A plant extract, Secomet‐V, with an anecdotal indication in humans for promise as an anti‐HIV treatment, was investigated. Using a previously described attenuated vaccinia virus vGK5, we established the antiviral activity of Secomet‐V. Chemical analysis showed that it has an acidic pH, nontoxic traces of iron (<10 ppm), and almost undetectable levels of arsenic (<1.0 ppm). The color varies from colorless to pale yellow to dark brown. The active agent is heat stable at least up to sterilizing temperature of 121°C. The crude plant extract is a mixture of several small molecules separable by high‐pressure liquid chromatography. The HIV viral loads were significantly reduced over several months in a few patients monitored after treatment with Secomet‐V. Secomet‐V was also found to have antiviral activity against the SARS virus but not against the West Nile virus. Secomet‐V, therefore, is a broad‐spectrum antiviral, which possibly works by neutralizing viral infectivity, resulting in the prevention of viral attachment.
Keywords: anti‐HIV, anti‐poxvirus, Secomet‐V, anti‐SARS, broad‐spectrum antiviral, acidic plant extract, nontoxic, enveloped viruses
INTRODUCTION
Enveloped viruses such as the human immunodeficiency virus (HIV), hepatitis B virus, and influenza virus remain a major threat to human health worldwide, infecting over 1 billion people. Together, these three viruses affect at least 5–10 million individuals, or roughly 25% of the South African population, causing considerable morbidity and mortality. In 2004, HIV resulted in more than 3 million total deaths worldwide, with an estimated 5.3 million people infected in South Africa alone (data provided by UNAIDS). To combat diseases caused by HIV and other envelopedviruses, a sustained multipronged approach is needed such as the one described previously.1 One of the most powerful weapons against HIV has been the life‐prolonging drug cocktails for AIDS treatment. Unfortunately, the availability of such drug cocktails is limited to a very small fraction of those infected because of the high cost of treatment. To make matters worse, there are also severe side effects seen in people taking the drugs. Therefore, there is an urgent need to continue to search for new therapies, which may be just as effective as new antiviral drugs. Other enveloped viruses such as variola virus, the causative agent of smallpox, are considered to be prime candidates for bioweapons in the aftermath of terrorist attacks. In addition, the emergence of monkeypox as a disease‐causing agent in the Democratic Republic of Congo (DRC) in central Africa once again emphasizes a need to continue to search for an antiviral agent against enveloped viruses. The promise of chemotherapy for poxviruses (summarized in Fig. 1) and complications from vaccination against smallpox with vaccinia virus seem quite bleak because of the severe side effects.2 In addition, the looming threat of an influenza pandemic and lack of reliable treatment leave the world vulnerable to the upcoming emergence of new virulent strains. The spread of the SARS‐causing virus was nipped in the bud by the end of 2004, but that does not mean we can be complacent and ready to dispense a repertoire of antiserum treatments. Antiviral drugs would be the best ammunition to bolster the preparedness against this agent. For centuries, humans have looked at finding natural solutions to health problems. Recently, plants have been a focus of several investigators around the world in search of antiviral agents.3, 4, 5 Based on interesting anecdotal evidence suggesting properties protective against viral infections, a potentially promising plant extract was developed. Reliable scientific evidence was needed to indicate that the extract was truly worth considering as a potential treatment for viral infections and doing more research was justified. With a well‐established infrastructure and the methodology to cultivate and to titer viruses accurately6 to evaluate antiviral effects, it was possible to show that indeed a small volume of the plant extract termed Secomet‐V was able to inactivate approximately 1 million virus particles of the attenuated recombinant vaccinia virus vGK57 in 1 minute consistently and reproducibly. The recombinant attenuated virus vGK5 was chosen because it grows as well as the wild‐type Western Reserve strain of vaccinia virus in cell culture. The use of the highly attenuated vGK5 strain is also relatively safe, because of the lack of a neurovirulence factor8 attributed to its inability to replicate in the brain and cause complications.9 Anecdotal evidence in humans had indicated that the plant extract had beneficial effects against common colds, influenza virus, and HIV. Because Secomet‐V is registered as herbal medicine, it was provided by the Secomet company only to patients with no access to antiretroviral (ARV) drugs and under the care of a certified physician. The viral loads of all patients decreased significantly and to nearly minimal levels among the compliant patients, indicating that the extract does indeed have a positive effect on viremia. The levels do not decrease as rapidly as with ARV therapy or highly active antiretroviral therapy (HAART); however, there were no significant side effects with Secomet‐V, and the disease symptoms were reduced considerably soon after treatment. It is clear that the amount of active ingredient in the plant extract needs to be identified and increased. Besides increasing the efficacy of the active ingredient by identifying, purifying, and characterizing it, the hope is to reduce any other component that may cause toxicity. It has become evident that changing the process of plant extraction (and avoiding the addition of charcoal), as well as using ultrafiltration with a 3‐kDa cutoff filter, greatly reduces both the long‐term and short‐term toxicity. Secomet‐V is a broad‐spectrum agent that has the potential to be a safe and highly efficacious antiviral therapy. Identifying the active ingredient would characterize the extract to the fullest extent possible and have a major impact on the development of a new synthetic antiviral originating from a natural source. Another advantage of identifying the bioactive ingredient would be an understanding of the mechanism of action of Secomet‐V. Currently, all the evidences indicate that the mechanism of action is through rendering the virus noninfectious and subsequently blocking viral attachment.
Figure 1.
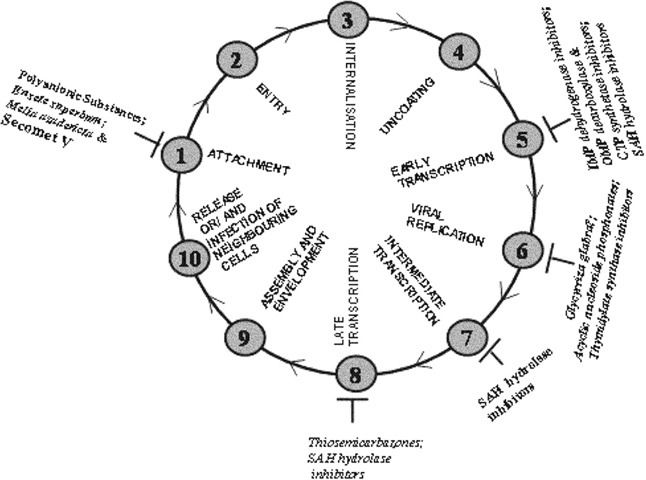
Life cycle of poxviruses and sites of inhibition by natural antivirals.
METHODOLOGY
Antiviral Activity Assay
The attenuated recombinant vaccinia virus vGK5 was produced in BSC‐1 cells or chicken eggs in required quantities and titered in the presence or absence of the plant extract or the fractions from high‐pressure liquid chromatography (HPLC) columns as described earlier.6 Essentially, approximately 1 million virus particles were mixed with 10–20 μl of Secomet‐V.
Assay of HIV
Two different cell lines, CCRF CEM and CEM 174, were used and infected with a subtype B HIV virus (R482). Plant extracts were diluted with phosphate‐buffered saline. For the experiment, 12‐well plates were used and in each plate the following was added: 1 ml RPMI‐1640 medium (with Pen and Strep), 100 μl infected CCRF CEM (424.4 pg/ml), or 100 μl infected CEM 174 (20.3 pg/ml) and varying amounts of plant extract. For negative controls, the uninfected cell lines were set up in parallel with the infected cell lines. The positive controls were the infected cell lines on the days that the supernatant was collected. To look at the direct effects of the bioactive molecules on HIV infectivity, a pure virus stock of a million virus particles with varying amounts of the purified bioactive molecules was treated and compared with untreated samples. The p24 assay of surviving virus with and without treatment was also compared. Additional anti‐HIV assays were performed directly with Secomet‐V with direct contact with HIV at Virologic, Inc. (South San Francisco, CA).
Short‐Term Toxicity Assay
Varying amounts of African green monkey kidney cell lines (BSC‐1 cells) in a monolayer on 96‐well confluent plates were treated with varying amounts of the purified fractions and then stained with crystal violet to determine cell cytoxicity as judged by the integrity of the monolayer.
RESULTS AND DISCUSSION
Chemical Properties of Secomet‐V
Secomet‐V varies in color from almost colorless, when filtered with a 500‐Da cutoff filter, to dark brown when unfiltered. The bioactive agent therefore is smaller than or close to 500 Da in size. Autoclaving does not eliminate the activity, suggesting that the bioactive agent is heat stable up to at least 121°C. The pH of the undiluted extract is approximately 2.0. The virus comes in contact with the extract at a buffered pH of the medium (RPMI‐1640) of approximately 3.5. The dry weight varies from approximately 3 mg/ml to 40 mg/ml based on the ultrafiltration cutoff. The arsenic (undetectable) and iron (<10 ppm) content was found to be well below toxic levels, within limits prescribed in the National Formulary, USA (analyzed by using Perkin‐Elmer Atomic Absorption Spectrophotometer at the forensic chemistry lab, Western Cape Police Department, Cape Town, South Africa). Subjects have not complained about its taste, suggesting that it has at least a tolerable taste.
Antiviral Activity of Secomet‐V
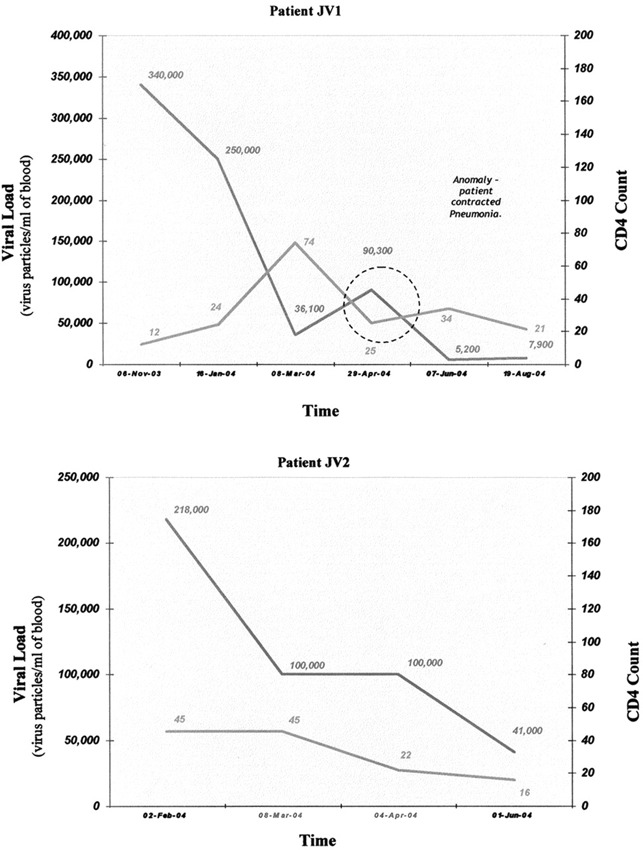
Secomet‐V, an extract of an African plant also found elsewhere in Asia, has been found to have potent antiviral activity against a poxvirus (vaccinia virus), rendering about 1 million particles noninfectious in 1 min with a 50th of a milliliter in in vitro assays (Fig. 2). Independent reports of anti‐HIV testing done by Virologic, Inc. by mixing the virus with the extract have shown that the HIV particles are neutralized by Secomet‐V. The same extract failed to cause any reduction in virus titers if the cells were first infected and then directly followed by addition of the plant extract, indicating that Secomet‐V neutralized viral infectivity but did not inhibit any of the post‐entry steps in the life cycle of the virus. HIV‐infected cells treated with plant extract showed no significant effect on the viral levels (Table 1). Therefore, apparently there is not any effect on the life cycle of HIV once it has entered cells. This was also observed with vGK5 infection. If Secomet‐V is added after the virus is allowed to attach and internalize for 2 h, there is no significant effect on the virus replication and plaque formation, once again confirming the hypothesis that one or more of the small molecules in the plant extract is, by some as yet unknown mechanism, rendering the virus noninfectious. This suggests that once the virus enters the cells, the extract is no longer effective. This key finding indicates that it is most likely Secomet‐V acting as a broad‐spectrum inhibitor of viral entry. Because of this novel mechanism of inhibition, it would not have the same problems of resistance that are common with ARVs, as well as the problems of viral evasion due to changes in a surface glycoprotein amino acid sequence. To determine whether Secomet‐V was able to reduce viremia, four HIV patients infected with HIV‐1 subtype C and who had full‐blown AIDS were given the plant extract. These patients had no access to ARVs, and their CD4 counts had decreased quite significantly prior to treatment with Secomet‐V. Viral loads were found to have been significantly reduced within a few months as observed (3, 3B). The SARS corona virus was also found to have been rendered inactive by Secomet‐V (data not shown) when tested along with other agents as a coded panel. The West Nile virus was similarly tested, but Secomet‐V had no effect on the West Nile virus at concentrations at which other viruses were inactivated. The dry weight of 1 ml of the crude plant extract is approximately 22 mg and that of the ultrafiltered (with a 5‐kDa cutoff) plant extract is 3 mg. There was no difference in the effectiveness of the plant extract in rendering vaccinia virus noninfectious whether it was autoclaved or not, suggesting that the bioactive agent is most likely but not necessarily a heat‐stable compound and not a small peptide. A preliminary HPLC separation of the ultrafiltered plant extract on a C18 column showed 3 major peaks, 3 minor peaks, and approximately 12 quite tiny peaks. Previously, it has been shown that the trifollium species has flavanoids and salicylic acid.
Figure 2.
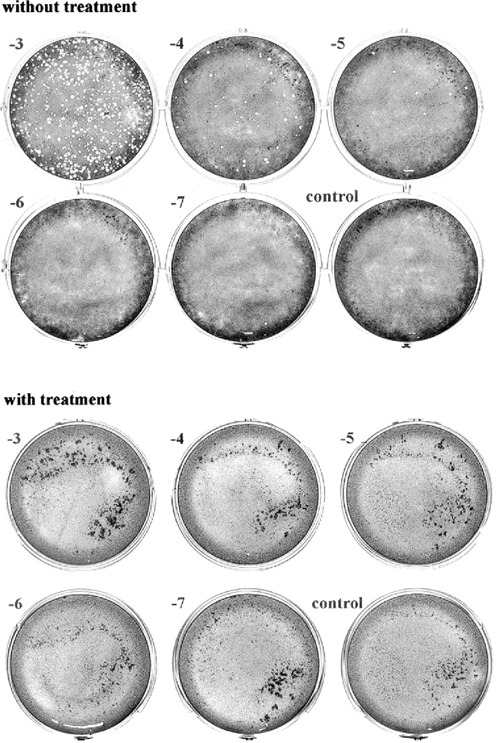
Effect of Secomet‐V on viral infectivity (as seen from inhibition of plaque formation).
Table 1.
Anti‐HIV activity
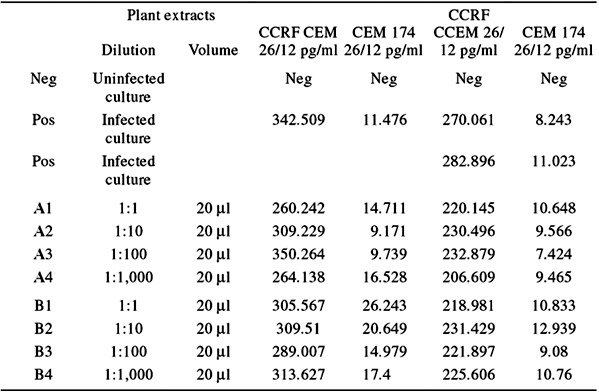
Figure 3.
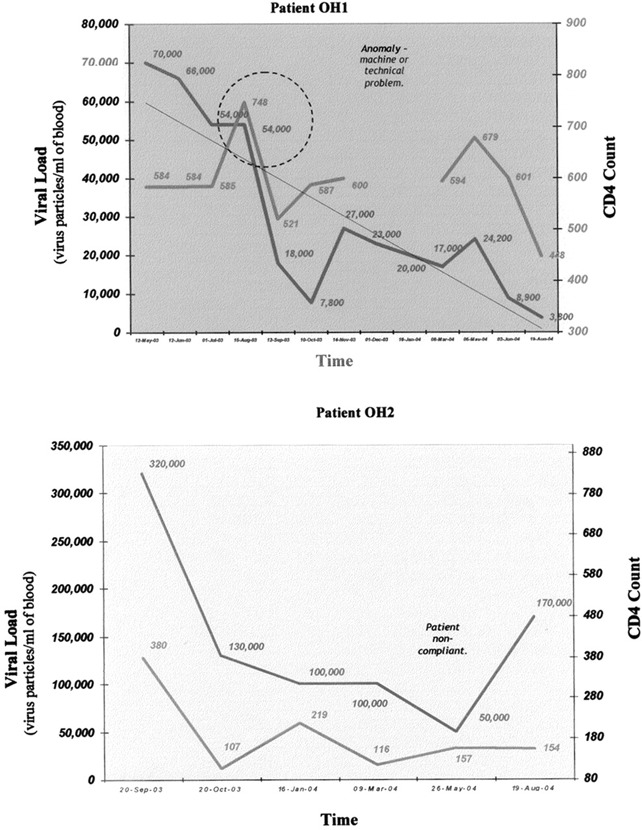
Viral load and CD4 profile of HIV‐infected patients over time after treatment with Secomet‐V.
Figure 3B.
No caption available.
Short‐Term Toxicity
Besides testing for efficacy, preliminary studies were completed to determine the short‐term and long‐term toxicity of the extract. In a simple assay in which varying amounts of the extract were added to 3 million African green monkey kidney cells (BSC‐1 cells), it was found that the 10 μl of the extract rendered half of the million virus particles inactive, and when 100 μl of the plant extract was added, the monolayer became detached and up to 50 μl of the extract was tolerated with no cytotoxicity present. Therefore, at 10% of the concentration of the toxic dose, 50% of the virus was rendered inactive. When the extract was filtered through a 3‐kDa filter, the toxicity diminished, but the antiviral activity remained intact. Similar studies have been conducted on potential long‐term effects of the crude extract (Ballardin et al., this volume) that could be carcinogenic. Secomet‐V was tested for mutagenicity using the Ames gene mutation test in Salmonella and the chromosome damage (clastogenic) micronucleus test in human lymphocytes. The crude extract presents weak clastogenic activity but powerful mutagenic activity in the Ames test with the addition of exogenous metabolic activation. The purification of the extract without the addition of charcoal results in a drastic reduction of the extract's mutagenicity, which has been virtually reduced by means of further ultrafiltration (cutoff <3,000 Da). If ultrafiltration almost eliminates the short‐term and long‐term toxic molecules, then pure bioactive molecules are likely to be free of all toxicity associated with the crude extract. These findings open new possibilities of HIV therapy because Secomet‐V, devoid of mutagenic activity, should reduce the possibility of the induction of resistant viral strains by previous ARV drugs.
ACKNOWLEDGMENTS
We thank Abubakar Jacobs and Andre Baard, Forensic Chemistry Laboratory, Cape Town Police Department, for their help in analyzing the samples for arsenic and iron. G.J.K. is currently Senior International Wellcome Trust Fellow for biomedical sciences in South Africa. A.P.K. and Y.T.G. are recipients of the Poliomyelitis Research Foundation (PRF) of South Africa and Senior Entrance Fellowship and International Students' Fellowship at the University of Cape Town, South Africa. This work was not supported by funding from Secomet pvt. Ltd. and is an independent study that was in no way influenced by Secomet.
REFERENCES
- 1. Kotwal, G.J. 2004. HIV treatment and eradication in South Africa. J. R. Soc. Med. 97: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Clercq, E. 2001. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 2: 382–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacob, J.R. et al. 2004. Korean medicinal plant extracts exhibit antiviral potency against viral hepatitis. J. Alt. Compl. Med. 10: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 4. Notka, F. et al. 2004. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo . Antiviral Res. 64: 93–102. [DOI] [PubMed] [Google Scholar]
- 5. Neurath, A.R. et al. 2004. Punica granatum (Pomegranate) juice provides an HIV‐entry inhibitor and candidate topical microbicide. BMC Infect. Dis. 4: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kotwal, G.J. & M.‐R. Abrahams. 2004. Growing poxviruses and determining virus titer. In Vaccinia Virus and Poxvirology, Methods and Protocols, S.N. Isaacs, Ed.:101‐112. Vol. 269. Humana Press. Totowa, NJ. [DOI] [PubMed]
- 7. Jonczy, E.A. , J. Daly & G.J. Kotwal. 2000. A novel approach using an attenuated recombinant vaccinia virus to test the antiviral effects of handsoaps. Antiviral Res. 45: 149–153. [DOI] [PubMed] [Google Scholar]
- 8. Kotwal, G.J. , A.W. Huegin & B. Moss. 1989. Mapping and insertional mutagenesis of a vaccinia virus gene encoding a 13,800 Da secretory protein. Virology 171: 579–587. [DOI] [PubMed] [Google Scholar]
- 9. Billings, B. , S.A. Smith, Z. Zhang, et al. 2004. Lack of N1L gene expression results in significant decrease in vaccinia virus replication in mice brain. Ann. N.Y. Acad. Sci. 1030: 297–302. [DOI] [PubMed] [Google Scholar]


