ABSTRACT
Introduction
Acute bronchiolitis is a major cause of acute respiratory distress in infants. The soluble receptor for advanced glycation end‐products (sRAGE) is a biomarker of pulmonary damage processes, with a diagnostic and a prognostic value in acute respiratory distress syndrome (ARDS). The RAGE pathway is also implicated in the pathogenesis of other respiratory diseases like asthma, but the value of sRAGE levels in acute bronchiolitis remains under‐investigated.
Material and methods
A prospective, observational, and analytical study was conducted at Clermont‐Ferrand University Hospital. The main objective was to evaluate the correlation between serum sRAGE and clinical severity of bronchiolitis in hospitalized infants aged <1 year. We analyzed correlations between serum sRAGE and Wainwright score, short‐term morbidity attributable to bronchiolitis, causal viruses and risk for recurrent wheezing at 1 year.
Results
The study included 93 infants. sRAGE levels were significantly lower in acute bronchiolitis patients (mean 1101 pg/mL) than in controls (2203 pg/mL, P < 0.001) but did not correlate with clinical severity. No correlation was found between serum sRAGE and severity score, respiratory viruses, and recurrent wheezing at 1 year. Serum sRAGE levels were negatively correlated with age (r = −0.45, P < 0.001).
Conclusion
Serum sRAGE levels are decreased in acute bronchiolitis but not correlated with disease severity. sRAGE levels should be age‐adjusted in infants. Serum sRAGE levels measured in the setting of acute bronchiolitis were not predictive of recurrent wheezing.
Keywords: blood and urine biomarkers, infants, lower respiratory tract infection, recurrent wheezing, soluble receptor for advanced glycation end‐products
1. INTRODUCTION
Acute bronchiolitis is a common epidemic viral infection in infants responsible for numerous consultations in pediatric emergency wards in the autumn and winter seasons. In the first two years of life, relapses are frequent, at between 23% and 60%.1 Recurrent wheezing is diagnosed after the third episode of wheezing, and requires adapted therapeutics and follow‐up. The causal viruses of acute bronchiolitis are mainly respiratory syncytial virus (RSV) and rhinoviruses, but metapneumoviruses, parainfluenza, and influenza viruses also contribute.2, 3
The receptor for advanced glycation end‐products (RAGE) is a member of the immunoglobulin superfamily that is constitutively expressed in many tissues. RAGE is a multi‐ligand membrane receptor (mRAGE) that recognizes damage‐associated molecular patterns (DAMPS). Its most studied ligands are advanced glycation end‐products (AGEs), high mobility group box 1 protein (HMGB1), proteins from the S100 family and beta‐amyloid peptides.4 Binding with one of its ligands activates the transcription of pro‐inflammatory nuclear factor NF‐κB and leads to inflammatory processes.
RAGE levels in lung tissue are particularly high, suggesting an important role of RAGE in pulmonary physiology. RAGE can be found in the alveolar and bronchial epithelial cells. It is mainly located on the basolateral membrane of alveolar type 1 (AT1) cells, for which RAGE is now considered as a marker.5, 6, 7, 8 There is growing evidence of a pivotal role for RAGE in lung diseases such as asthma, allergic airway inflammation, acute respiratory distress syndrome (ARDS) or bronchopulmonary dysplasia.9
Soluble RAGE (sRAGE) represents the total pool of soluble forms of RAGE that can be measured in the extracellular compartment (eg, in biological fluids). sRAGE lacks the transmembrane domain by alternative splicing mechanisms or through proteolytic cleavage,10 but includes the extracellular domain of mRAGE. Thus, sRAGE acts as a decoy to prevent the activation of mRAGE by ligands and its pro‐inflammatory action10 (Figure 1).
Figure 1.
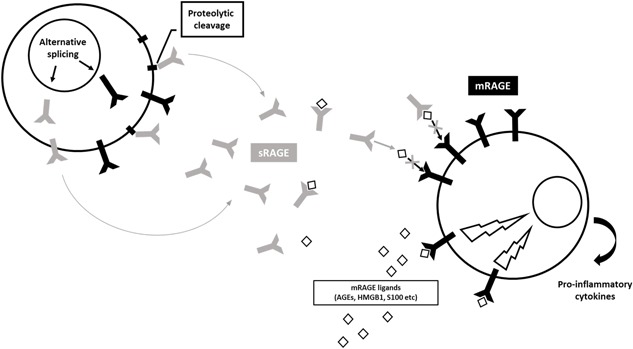
sRAGE arises by alternative splicing mechanisms or through proteolytic cleavage. sRAGE includes the extracellular domain of mRAGE. In the extracellular compartment, sRAGE acts as a decoy by capturing mRAGE receptor ligands and blocking its pro‐inflammatory signal
Several human studies argue that sRAGE is a marker of pulmonary damage processes. In both adults and children, plasma sRAGE is associated with severity and prognosis of ARDS.11, 12, 13 sRAGE measurements may also be valuable for assessing the severity of asthma in children.14, 15 However, the value of measuring sRAGE during acute bronchiolitis has been poorly investigated to date.16 We designed this study to investigate whether serum sRAGE could be a marker of severity in infants with acute bronchiolitis and a predictor of risk for the development of recurrent wheezing.
2. MATERIALS AND METHODS
This prospective observational single‐center study was carried in units at Clermont‐Ferrand University Hospital Department of Pediatrics (general hospitalization units, pediatric intensive care and step‐down units) from December 2015 to March 2018. The research protocol for this study was first approved by our institutional review board (Comité de Protection des Personnes Sud Est VI, France, approval number AU1224).
The main objective was to evaluate the correlation between serum sRAGE at hospital admission and clinical severity of acute bronchiolitis in hospitalized infants under 1‐year‐old. Secondary objectives were to study correlations between serum sRAGE and urinary sRAGE and associations between sRAGE levels and types of causal virus, immediate attributable morbidity and mortality, and risk for recurrent wheezing at 1 year.
2.1. Population
2.1.1. Inclusion criteria
All infants aged <1 year admitted to CHU Clermont‐Ferrand for acute bronchiolitis in two epidemic seasons, that is, 1 November 2015‐18 March 2016 and 21 November 2016‐1 March 2017, were eligible as cases. Bronchiolitis was diagnosed by the presence of a recent history of upper respiratory tract infection followed by onset of respiratory distress with cough, tachypnea, retraction and auscultatory findings of diffuse crackles or wheezes, in accordance with international recommendations on the diagnosis and management of acute bronchiolitis.17 Admission criteria were based on French national guidelines.18 Pediatric intensive care unit (PICU) was needed in case of signs of exhaustion such as listlessness or decreased respiratory effort, recurrent apnea, or failure to maintain adequate oxygen saturation despite oxygen supplementation.19 Infants born before 34 weeks of amenorrhea or infants with cystic fibrosis, bronchopulmonary dysplasia, suspected primary ciliary dyskinesia, congenital heart disease, immune deficiency, or acute renal failure were excluded.
Infants aged <1 year attending an anesthesia consultation ahead of planned surgical intervention were enrolled as control group. Health status was evaluated by a complete medical check‐up, with a clinical questionnaire. Children with a previous history of pulmonary disease (history of wheezing, cardiopulmonary disease, chronic pulmonary disease or prematurity) or with an infectious process were excluded.
Cases and controls were enrolled after providing the parents oral and written information and collecting their written consent.
2.1.2. Clinical data
Clinical severity was assessed at inclusion using the Wainwright clinical score and was based on respiratory effort, oxygen saturation, and respiratory rate. This score defines three stages of severity: non‐severe bronchiolitis, moderate, or severe.20 Other data collected included personal medical history (perinatal and atopic history), immediate complications related to acute bronchiolitis (bacterial superinfection, pneumothorax, atelectasis) and use of oral or inhaled corticosteroids. Immediate morbidity and mortality were assessed using data collected during hospitalization: length of hospital stay, use and durations of enteral nutrition or parenteral rehydration, need for oxygen therapy or respiratory support (continuous positive airway pressure [CPAP] or bi‐level positive airway pressure [BiPAP]) or invasive mechanical ventilation, incidence of superinfection as diagnosed biologically or radiologically, radiographic features of ventilation disorder or radiologically‐documented gas effusion, bronchospasm, use of inhaled beta‐agonists or corticosteroids, and death.
Families were phone‐surveyed at 30 days and 1 year after inclusion to collect data on recurrence of wheezing (bronchiolitis, asthma, wheezing without consultation) and use of respiratory treatments (oral corticosteroids, respiratory physiotherapy, hospital admissions for respiratory reasons). The diagnosis of recurrent wheezing, if not already established by another pediatrician, was based on occurrence of a third bronchiolitis episode or prescription of a long‐term treatment for respiratory symptoms.
2.2. Collection of blood samples
At admission to hospital, blood samples (2.5 mL) was sampled by venipuncture into VACUETTE® tubes with lithium heparin, and urine (2 mL) was sampled into VACUETTE® Z tubes without additive by collecting pockets or compresses in the layer. Blood and urine samples were immediately centrifuged at 2500 rpm for 15 min, and the supernatants were kept frozen at −80°C until analysis. sRAGE levels were measured in duplicate using purpose‐designed commercially‐available ELISA kits (RAGE Quantikine, R&D Systems, Minneapolis, MN) with a manufacturer‐stated limit of detection of 78 pg/mL. The personnel responsible for performing sRAGE assays had no knowledge of the clinical setting.
Nasopharyngeal swab samples were collected by aspiration of nasal secretion. Viral analysis was carried out using enzymatic immunoassay chromatography for RSV antigen detection. Molecular biology methods included reverse transcription‐polymerase chain reaction (RT‐PCR) for RSV and rhinovirus in infants admitted to general pediatric and short‐stay units, and a multiplex PCR panel (adenovirus, coronavirus, metapneumovirus, enterovirus/rhinovirus, influenza A and B, parainfluenza virus, RSV, Bordetella pertussis, Chlamydia pneumoniae, Mycoplasma pneumoniae) in infants admitted to the intensive care unit. Rhinovirus strains were genotyped. Chest X‐ray was performed only when respiratory complications and/or recurrent wheezing were suspected.
2.3. Statistical analysis
All statistical analyses were performed using Stata 13 (StataCorp, College Station, TX) with a P‐value of <0.05 (two‐sided) considered statistically significant. Data were expressed as frequencies and percentages for qualitative variables and as means and standard deviations or medians and interquartile intervals for quantitative variables.
The case‐control analysis was performed using the χ 2 test (or Fisher's exact test if applicable) for qualitative variables and Student's t tests (or Mann and Whitney test if a data Shapiro‐Wilk test confirmed normality) for quantitative variables.
Severity analysis (non‐severe/moderate/severe) was carried out using quantitative analyses for quantitative data (or a Kruskal‐Wallis test) followed by a trend test, and by means of the χ 2 test (or Fisher's exact test if applicable) for qualitative data followed by a trend test.
sRAGE values were analyzed using the Mann and Whitney test (two groups) or Kruskal‐Wallis (three or more groups) test, taking into account non‐normally‐distributed data for qualitative criteria and using Spearman's correlation coefficient for quantitative criteria.
sRAGE values were then analyzed with a linear regression model correcting for clinical relevance and criteria found to be significant in univariate analysis.
3. RESULTS
3.1. Population
Ninety‐three cases were included. They were mainly young infants, with a median age of 1.8 (0.9‐3.5) months. Most had a first (n = 83, 89%) mild (n = 67, 72%) episode of acute bronchiolitis, 20 (22%) cases had a moderate episode, and 6 (7%) presented a severe acute bronchiolitis according to Wainwright score.
Viral analysis was performed in 90 infants and was negative in nine of them. Both epidemic seasons were dominated by RSV (n = 69, 74%) and rhinoviruses (n = 21, 23%). Eighteen (19%) viral co‐infections were identified, of which 14 (15%) were due to RSV and rhinovirus.
Median hospital stay was 4 (3‐6) days and was significantly associated with Wainwright score, with a median stay of 3 (2‐5) days for non‐severe bronchiolitis, 7.5 (5.5‐10) days for moderate bronchiolitis, and 8 (7‐10) days for severe bronchiolitis (P < 0.001). Twenty‐five infants were admitted to the intensive care unit, including eight cases admitted for apnea (Table 1). None developed ARDS.
Table 1.
Data on health care utilization and immediate complications in infants with acute bronchiolitis enrolled in the study
| n | % | Median duration (days) | IQR | |
|---|---|---|---|---|
| Hospital stay | ||||
| Short‐stay hospitalization unit | 35 | 38 | 3 | 2‐4 |
| General pediatric wards | 33 | 35 | 4 | 3‐5 |
| Intensive care unit | 25 | 27 | 8 | 7‐10 |
| Therapeutics | ||||
| Oxygen therapy | 51 | 55 | 3 | 2‐6 |
| Non‐invasive mechanical ventilation | 18 | 19 | 3 | 2‐4 |
| Enteral nutrition | 71 | 76 | 3 | 2‐5 |
| Intravenous rehydration | 42 | 45 | 2 | 1‐2 |
| Immediate complications | ||||
| Apnea | 8 | 9 | ||
| Bacterial superinfection | 16 | 17 | ||
| Atelectasis | 5 | 5 | ||
| Pneumothorax | 0 | 0 | ||
| Acute respiratory distress syndrome | 0 | 0 | ||
| Death | 0 | 0 |
Fifty‐five percent of cases had a first‐degree family history of atopic disease and 27% a family history of asthma. Fifteen percent of cases had a personal history of atopic diseases: 11% had eczema, 1% had cow's milk protein allergy, and 4% had recurrent wheezing at study inclusion (three cases were enrolled at their third episode, and one case included was enrolled during his fourth episode). Bronchial crackles were found in 42% of children. Eight percent were treated with beta‐agonists and 5% with inhaled corticosteroids.
One year after inclusion, 85 (91%) parents of cases responded to our phone survey: 37 (44%) infants developed recurrent wheezing.
Thirty‐two controls were included, with a median age of 6.3 (2.7‐9.3) months (P = 0.012, when age‐compared against cases). Most were boys (72%), but the gender difference between case and control populations was not statistically significant (P = 0.06). There was no difference between case and control groups on birth term (P = 0.45) and weight (P = 0.79).
3.2. sRAGE measurements
3.2.1. Mean sRAGE levels
Mean serum sRAGE was 1103 (SD: 654) pg/mL in infants with acute bronchiolitis. Mean serum sRAGE correlated negatively with age (r = −0.45, P < 0.001), with the highest rates found among younger infants (Table 2). This correlation was confirmed in multivariate analysis (P < 0.001). Mean serum sRAGE was 2203 (SD: 905) pg/mL in controls, with no statistically significant correlation with age (r = −0.32, P = 0.07, Table 2). Serum sRAGE levels were significantly higher in controls than in infants with acute bronchiolitis (P < 0.001), even after multivariate adjustment including age as a covariate (P < 0.001, Figure 2). The multivariate analysis showed a lowered attrition rate of 1382 points in cases, and each additional month of life lowered the attrition rate by 86.6 points. sRAGE levels did not differ according to causal virus. sRAGE was undetected in all urine samples.
Table 2.
Mean serum levels of soluble receptor for advanced glycation end‐products (sRAGE, in pg/mL), stratified by age
| Cases | Controls | |||||
|---|---|---|---|---|---|---|
| n | Mean serum sRAGE | SD | n | Mean serum sRAGE | SD | |
| <1 month | 26 | 1501 | 763 | 0 | ||
| 1‐2 months | 22 | 1248 | 719 | 6 | 2550 | 1171 |
| 2‐4 months | 29 | 838 | 379 | 7 | 2514 | 660 |
| >4 months | 16 | 737 | 270 | 19 | 1978 | 868 |
Figure 2.
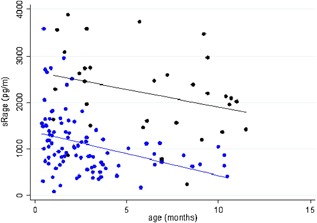
Serum levels of soluble receptor for advanced glycation end‐products (sRAGE, in pg/mL) in cases (blue) and in the control group (black), according to their age (p < 0.001)
3.2.2. Association with severity of acute bronchiolitis
There was no significant correlation between serum sRAGE and clinical severity of acute bronchiolitis at hospital admission, according to the Wainwright score (Figure 3). Mean serum sRAGE levels were 1063 (SD: 658), 1183 (SD: 596), and 1286 (SD: 837) pg/mL in infants with non‐severe, moderate, and severe acute bronchiolitis, respectively (P = 0.24).
Figure 3.
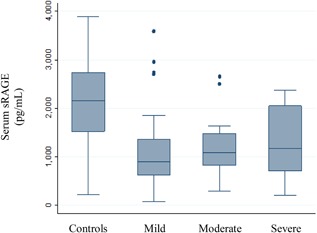
Serum levels of soluble receptor for advanced glycation end‐products (sRAGE, in pg/mL) in healthy infants and infants with acute bronchiolitis, stratified by clinical severity as defined by the Wainwright score
Serum sRAGE did not correlate with immediate morbidity, total hospital stay (r = 0.09, P = 0.41), type of hospital unit (P = 0.21), need for respiratory support (P = 0.74) or its duration (r = 0.20, P = 0.16). Patients with complications such as bacterial superinfection (P = 0.35) or atelectasis (P = 0.31) had similar sRAGE levels to those who did not.
3.2.3. Serum sRAGE and risk for recurrent wheezing
The presence of a family history of atopic diseases had no impact on sRAGE levels (asthma, P = 0.65; food allergy, P = 0.24; allergic rhinitis, P = 0.54; eczema, P = 0.41). Serum sRAGE was not associated with bronchial crackles (P = 0.39) nor with use of inhaled beta‐agonists and corticosteroids during hospitalization (P = 0.74).
sRAGE levels did not correlate with recurrent wheezing at one year: mean serum sRAGE was 1021 (SD: 589) pg/mL during the acute episode in patients who did not develop recurrent wheezing and 1227 (SD: 707) pg/mL in those who developed recurrent wheezing at 1 year (P = 0.11).
4. DISCUSSION
The main objective of this study was to investigate the association between serum sRAGE and severity of acute bronchiolitis in hospitalized infants. The study enrolled 93 infants (all aged under 1‐year‐old), making it the largest cohort of patients with acute bronchiolitis published to date. No correlation was found between serum sRAGE and severity of bronchiolitis as evaluated by a validated international clinical severity score at inclusion. Note that no ARDS was reported in our cohort.
sRAGE levels seem more likely to be linked to the presence or absence of the viral episode. Serum sRAGE levels were significantly lower in infants with acute bronchiolitis than in controls regardless of the nature of the causal virus. The lack of correlation with the severity of acute bronchiolitis is surprising. The presence of low rates in an acute context leads us to imagine lower rates among the most severe cases. However, the results do not show any correlation with the severity of acute bronchiolitis as evaluated by the Wainwright score, which could also be surprising. Additionally, there was no correlation between sRAGE rates and severity as evaluated by morbidity data such as total hospital stay, type of hospital unit, the need for respiratory support, or respiratory support duration. These morbidity data are probably even more reliable than the Wainwright score since evaluated once at inclusion of cases. The low number of severe cases could be a bias in our study, and this seems true according to the distribution of our population established on the Wainwright score. However, when we observe the severity on hospitalization data, 25 cases were admitted to the intensive care unit, representing the real most severe cases of acute bronchiolitis.
To explain low serum sRAGE levels during acute bronchiolitis, two physiopathological hypotheses can be made. It has been supposed that high sRAGE levels could exert beneficial anti‐inflammatory effects by blocking the pro‐inflammatory axis of RAGE.6, 21 If sRAGE is considered as a “blocker” of the inflammatory cascade, it could be “consumed” by its binding with pro‐inflammatory RAGE ligands in order to control the inflammation generated by viral aggression. It could partly explain the lower sRAGE levels in the bronchiolitis group than in the control group here. On the other hand, it may be hypothesized that constitutive lower sRAGE levels predispose for acute bronchiolitis and severe episodes. But the results of this first observational study must be confirmed by larger and more multicentric populations before concluding a physiopathological model. A cohort study from birth, with measured sRAGE kinetics could arbitrate between a predisposing role or a post‐event consequence of lower sRAGE levels in acute bronchiolitis.
Regardless, the relationship between sRAGE and the severity of acute bronchiolitis cannot be interpreted without consideration of age. Indeed, a singular feature of sRAGE levels in infancy is broad variability with age due to pulmonary maturation and alveolarization processes that last up to 12‐24 months.6, 22 sRAGE levels decrease progressively in the bronchoalveolar fluid (BALF) and serum from 30 weeks of amenorrhea to birth and again from birth to adulthood.23, 24 Our findings confirm this and are well described in Figure 2. Unfortunately, we did not obtain data in control infants under one month old, so our study may be underpowered to detect significant negative correlation between serum sRAGE and age in controls. This major variation factor of sRAGE levels could explain the discrepancies in the study results of García‐Salido et al.16 Their study showed a pattern opposite from ours, that is, higher serum sRAGE levels in severe acute bronchiolitis than in controls. This different pattern seems likely due to control‐group factors, as García‐Salido et al controls were older than the target population for acute bronchiolitis with an average age of 8 years (14 months to 18 years), which could skew comparisons between groups as physiological sRAGE levels are higher during the first months of life.23 Our control sample seems more precise, even though we had no controls aged under one month. To our knowledge, our study is the first to include healthy controls under 1 year of age, but there is no data available on sRAGE levels in this target population with which to compare our results.25
In fact, age is a double factor of bias in our study, since acute bronchiolitis is more severe in the youngest cases. An adjustment with age is thus necessary, and our results confirmed lower rates in the context of acute bronchiolitis even after this adjustment for age. The occurrence of acute bronchiolitis lowered sRAGE levels by 1382 points, and each additional month of life lowered sRAGE levels by 86.6 points.
sRAGE levels did not correlate with recurrent wheezing at 1 year following the episode of acute bronchiolitis in our cohort. Several studies have found lower serum sRAGE levels in children with asthma than in healthy children, especially when asthma is uncontrolled, and a recent study found that serum sRAGE increased after inhaled corticosteroids.14, 15 Taken together, these data strongly suggest that sRAGE levels are lowered during acute (as in viral infection) and chronic (as in asthma) bronchial inflammation.
This potential deficit of sRAGE during inflammatory states would argue for therapy applications. In mouse studies, modulating RAGE by inhibiting the elimination of its expression protein and using anti‐RAGE monoclonal antibodies or exogenous recombinant sRAGE improved several features of lung injury in models of ARDS or of RSV infection.26, 27 As RSV is the main causal virus in acute bronchiolitis, especially the most severe forms,2 and given that neurogenic inflammation caused by RSV induces long‐term bronchial hyper‐responsiveness, sRAGE administration could be a promising candidate therapeutic target. Future studies should aim to test this idea.
This large‐cohort study found that serum sRAGE levels were lower in infants with acute bronchiolitis than without acute bronchiolitis, whatever their ages. Finally, our study does not show any difference in sRAGE levels according to acute bronchiolitis severity, even after adjusting for age. In our study, age was a major confounding factor for acute bronchiolitis severity (the more severe are the youngest) and sRAGE physiological levels (higher rates are found in the youngest). After age adjustment, sRAGE rates seem more related to the presence or absence of acute bronchiolitis rather than to its severity. In the long term, sRAGE levels did not correlated with risk for recurrent wheezing at 1 year.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
The authors thank Pr Cécile Henquel and her team at the Clermont‐Ferrand University Hospital Department of Medical Virology; Dr Isabelle Petit and Alexandra Usclade at the Clermont‐Ferrand University Hospital Department of Clinical Research for Children (CRECHE) for their valuable assistance. This study was supported by a hospital clinical research program (2015‐AO1587‐42) grant from Clermont‐Ferrand University Hospital.
Egron C, Roszyk L, Rochette E, et al. Serum soluble receptor for advanced glycation end‐products during acute bronchiolitis in infant: Prospective study in 93 cases. Pediatr Pulmonol. 2018;53:1429–1435. 10.1002/ppul.24141
REFERENCES
- 1.Haute Autorité de Santé. Pertinence du recours à l'hospitalisation pour bronchiolite (GHM 04M18). 2012.
- 2. Marguet C, Lubrano M, Gueudin M, et al. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS ONE. 2009; 4:e4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feuillet F, Lina B, Rosa‐Calatrava M, Boivin G. Ten years of human metapneumovirus research. J Clin Virol. 2012; 53:97–105. [DOI] [PubMed] [Google Scholar]
- 4. Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005; 83:876–886. [DOI] [PubMed] [Google Scholar]
- 5. Shirasawa M, Fujiwara N, Hirabayashi S, et al. Receptor for advanced glycation end‐products is a marker of type I lung alveolar cells. Genes Cells. 2004; 9:165–174. [DOI] [PubMed] [Google Scholar]
- 6. Buckley ST, Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol. 2010; 2010:917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen L, Wang T, Guo L, et al. Overexpression of RAGE Contributes to Cigarette Smoke‐Induced Nitric Oxide Generation in COPD. Lung. 2014; 192:267–275. [DOI] [PubMed] [Google Scholar]
- 8. Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol. 2006; 19:1437–1445. [DOI] [PubMed] [Google Scholar]
- 9. Oczypok EA, Perkins TN, Oury TD. All the “RAGE” in lung disease: the receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev. 2017; 23:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pilzweger C, Holdenrieder S. Circulating HMGB1 and RAGE as clinical biomarkers in malignant and autoimmune diseases. Diagnostics (Basel, Switzerland). 2015; 5:219–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jabaudon M, Futier E, Roszyk L, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011; 39:480–488. [DOI] [PubMed] [Google Scholar]
- 12. Yehya N, Thomas NJ, Meyer NJ, Christie JD, Berg RA, Margulies SS. Circulating markers of endothelial and alveolar epithelial dysfunction are associated with mortality in pediatric acute respiratory distress syndrome. Intensive Care Med. 2016; 42:1137–1145. [DOI] [PubMed] [Google Scholar]
- 13. Mrozek S, Jabaudon M, Jaber S, et al. Elevated plasma levels of sRAGE are associated with nonfocal CT‐Based lung imaging in patients with ARDS: a prospective multicenter study. Chest. 2016; 150:998–1007. [DOI] [PubMed] [Google Scholar]
- 14. El‐Seify MYH, Fouda EM, Nabih ES. Serum level of soluble receptor for advanced glycation end products in asthmatic children and its correlation to severity and pulmonary functions. Clin Lab. 2014; 60:957–962. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Wu R, Tian Y, Bao T, Tian Z. Fraction of exhaled nitric oxide and soluble receptors for advanced glycation end products are negatively correlated in children with recurrent wheezing. Asian Pac J Allergy Immunol. 2017; 35:33–37. [DOI] [PubMed] [Google Scholar]
- 16. García‐Salido A, Oñoro G, Melen GJ, et al. Serum sRAGE as a potential biomarker for pediatric bronchiolitis: a pilot study. Lung. 2015; 193:19–23. [DOI] [PubMed] [Google Scholar]
- 17. American academy of pediatrics subcommittee on diagnosis and management of bronchiolitis. diagnosis and management of bronchiolitis. Pediatrics. 2006; 118:1774–1793. [DOI] [PubMed] [Google Scholar]
- 18.Agence Nationale d'Accréditation et d'Evaluation en Santé. Conférence de consensus − Prise en charge de la bronchiolite du nourrisson. 2000.
- 19.National Collaborating Centre for Women's and Children's Health (UK). Bronchiolitis: Diagnosis and Management of Bronchiolitis in Children. London: National Institute for Health and Care Excellence (UK); 2015. [PubMed]
- 20. Wainwright C, Altamirano L, Cheney M, et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. N Eng J Med. 2003; 349:27–35. [DOI] [PubMed] [Google Scholar]
- 21. Bellon G. [Acute bronchiolitis in the infant. Definition]. Archives De Pédiatrie: Organe Officiel De La Sociéte Française De Pédiatrie. 2001; 8:25S–30S. [DOI] [PubMed] [Google Scholar]
- 22. Delacourt C, Jarreau P‐H, Bourbon J. [Normal and abnormal alveolar development]. Rev Mal Respir. 2003; 20:373–383. [PubMed] [Google Scholar]
- 23. Buschmann K, Tschada R, Metzger M‐S, et al. RAGE controls leukocyte adhesion in preterm and term infants. BMC Immunol. 2014; 15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yerkovich ST, Chang AB, Carroll ML, Petsky HL, Scrivener G, Upham JW. Soluble receptor for advanced glycation end products (sRAGE) is present at high concentrations in the lungs of children and varies with age and the pattern of lung inflammation. Respirology (Carlton, Vic.). 2012; 17:841–846. [DOI] [PubMed] [Google Scholar]
- 25. García‐Salido A, Melen G, Gómez‐Piña V, et al. Circulating soluble RAGE and cell surface RAGE on peripheral blood mononuclear cells in healthy children. J Pediatr Endocrinol Metab. 2018; 31:649–654. [DOI] [PubMed] [Google Scholar]
- 26. Miller AL, Sims GP, Brewah YA, et al. Opposing roles of membrane and soluble forms of the receptor for advanced glycation end products in primary respiratory syncytial virus infection. J Infect Dis. 2012; 205:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blondonnet R, Audard J, Belville C, et al. RAGE inhibition reduces acute lung injury in mice. Sci Rep. 2017; 7:7208. [DOI] [PMC free article] [PubMed] [Google Scholar]


