Abstract
Background
The objective of our study was to understand the epidemiological and clinical features of respiratory adenoviral infections among children at a single institution over the course of several years.
Methods
From January 2005 to April 2009, 1836 children (≤15 years old) who had been admitted to Korea University Ansan Hospital were tested for acute respiratory infection. The patients who were positive for an adenovirus infection were enrolled in this study, and their medical records were retrospectively reviewed.
Results
Adenoviruses were isolated from 310 patients. The male to female ratio was 1.6:1 and mean age was 32 ± 24 months. Children under 5 years of age had the highest prevalence. In 2007, adenovirus infections occurred endemically throughout the year. The clinical diagnoses were primarily upper respiratory tract infections (45.4%), lower respiratory tract infections (48.1%), and neurologic disease (5.2%). Associated symptoms, signs and laboratory findings included fever (91.9%), cough (83.9%), pharyngeal injection (62.3%), rale (32.6%) and elevated C‐reactive protein (93.9%). The most common radiologic findings were perihilar and peribronchial infiltrates (42.6%). Co‐infections were observed in 29 cases. The mean durations of hospitalization and fever were 6.2 ± 6.5 and 4.8 ± 3.1 days, respectively. The lengths of hospitalization were similar for patients admitted for upper respiratory tract infections with severe morbidity and those admitted for lower respiratory tract infections. No children in the study died.
Conclusion
Our study demonstrates that respiratory adenovirus infections are an important cause of hospitalization in young children, and contribute to a significant morbidity.
Keywords: adenovirus, children, hospitalization, morbidity, respiratory infection
Adenovirus is a major cause of acute respiratory illness in children worldwide.1, 2 Adenovirus‐related deaths in children are rare, but adenovirus‐attributable hospitalizations in young children and high‐risk populations are quite high.3, 4 A previous study found a high burden of hospital admission among children with adenoviral infection.5 The spectrum of adenoviral infection in children ranges from subclinical illness to complicated disease involving multiple organs, with pneumonia being one of the most common presentations. Although most infections are self‐limited, adenovirus can be associated with severe conditions in both immunocompromised and immunocompetent individuals.6, 7
Sporadic serotype analysis and some case reports have been performed in Korea since 1995.8, 9 However, little information on the epidemiology and clinical characteristics of respiratory adenoviral infection, especially in hospitalized children, has been published. The study was performed to more fully characterize the epidemiological pattern, clinical features and complications associated with hospitalization for adenoviral infection in Korean children.
Methods
Nasal aspirate specimens were collected from patients presenting with acute respiratory symptoms at Ansan Hospital, which is affiliated with Korea University and serves the communities of Ansan city, a city neighboring Seoul. Nasal aspirates were routinely tested to identify adenovirus, parainfluenza, respiratory syncytial virus (RSV) and influenza A/B by viral culture using three standard cell lines (HEp‐2, MDCK and LLC‐MK2).
We retrospectively analyzed data from hospitalized children 15 years of age or younger who had a laboratory‐confirmed adenovirus infection between January 2005 and April 2009. Study approvals were obtained from the Institutional Review Boards of Korea University Ansan Hospital. Clinical, laboratory and radiological data were extracted from electronic medical records. Clinical information regarding diagnosis, management during hospitalization, intensive care unit (ICU) stay, the need for mechanical ventilator assistance and any underlying conditions were obtained from the hospital records.
The statistical analysis was performed using spss version 12.0 (spss, Chicago, IL, USA). Data were presented as numbers (percentage), mean ± SD or median (range) as appropriate. Comparisons between groups were carried out using the Mann–Whitney U‐test. A P‐value of < 0.05 was considered to be significant in the analysis.
Results
Patient characteristics
From January 2005 to April 2009, a total of 1836 specimens were collected from patients admitted with respiratory viral infection. Of these samples, 310 episodes were confirmed as adenoviral infections, an overall proportion of 16.9% (310/1836). Ages ranged from 1 month to 11.3 years, and the mean age was 32 ± 24 months. The patients in this study were grouped by age as 0–<1, 1–<2, 2–<3, 3–<4, 4–<5, and ≥5 years. About 90% of all the adenovirus‐related cases occurred primarily in children aged less than 5 years, and the peak incidence was in the 1–<2‐year group (23.5%) (Fig. 1). When the <1‐year‐old group was divided into two subgroups, the subgroup younger than 6 months represented only 8.4% of the total cases, while the 6–<12 month subgroup was 14.2%. The ratio of boys to girls was 1.6:1. Twenty‐six patients (8.4%) had one or more underlying conditions, including asthma, chronic neurologic disease and/or prematurity.
Figure 1.
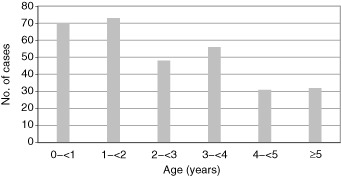
Age distribution of adenovirus infection.
Temporal distribution
The annual detection (detection rate, numbers of positive/numbers of specimen) of adenovirus was as follows: 68 cases (8%) in 2005, 55 cases in 2006 (3.8%), 142 cases (9.4%) in 2007, 34 cases (2.3%) in 2008 and 11 cases (2.1%) in 2009. Almost half (142/310, 45.8%) of the cases were detected in 2007 and showed distinct outbreaks compared with the other periods. The monthly distribution of infection caused by adenovirus is shown in Figure 2. The climatic seasons in this region of Korea are distinguished as follows: spring (March–May), summer with a rainy season (June–August), autumn (September–November) and winter (December–February). Adenovirus was more prevalent in the winter of 2005–2006, as opposed to higher rates of infection during the spring and summer of 2007. The season with the highest prevalence varied by year, but adenovirus infection occurred endemically throughout the year, except in 2007.
Figure 2.
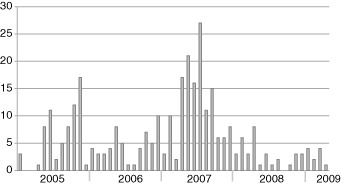
Trend of adenovirus isolation from January 2005 to April 2009. ( ) No. of cases.
) No. of cases.
Initial diagnosis
Diagnoses at admission were based on clinical, laboratory, and radiographic information. Adenovirus was associated with a wide variety of diagnoses, ranging from upper respiratory tract infections (URTI) to severe pneumonia and encephalitis (Table 1). The most frequent presentation was lower respiratory tract infections (LRTI) (149/310, 48.1%), followed by URTI (141/310, 45.4%). Among the LRTI, the admitting diagnosis was pneumonia for 107 patients (107/310, 34.5%), acute bronchiolitis in 20 (20/310, 6.5%), and acute bronchitis in 22 (22/310, 6.1%). URTI included pharyngitis (92/310, 29.6%), otitis media (26/310, 8.4%), sinusitis (13/310, 4.2%) and croup (10/310, 3.2%). Twenty patients (6.5%) presented with other diagnoses, including febrile convulsions, neonatal sepsis, gastroenteritis and encephalitis.
Table 1.
Primary diagnosis in adenoviral infection
| Diagnosis | No. of cases | % |
|---|---|---|
| URTI | 141 | 45.4 |
| LRTI | 149 | 48.1 |
| Tracheobronchitis | 22 | 7.1 |
| Bronchiolitis | 20 | 6.5 |
| Pneumonia | 107 | 34.5 |
| Neurologic disease | 16 | 5.2 |
| Sepsis | 3 | 1 |
| GI disease | 1 | 0.3 |
| Total | 310 | 100 |
GI, gastrointestinal; LRTI, lower respiratory tract infection; URTI, upper respiratory tract infection.
Clinical symptoms and signs
Over 90% of adenoviral infections were accompanied by fever, which was the most frequent clinical finding. The mean duration of fever was 4.8 ± 3.1 days (range 0–15 days). A high fever (≥39°C) was described in one‐third of all patients, and prolonged fever (≥10 days) was reported in 6.8% of cases. Other common features were cough (83.9%), rhinorrhea (63.2%) and sputum (61.3%). Respiratory distress was seen in 19 cases (6.1%). Gastrointestinal symptoms were also commonly found. Sixty‐five (21%) patients presented with diarrhea, anorexia was seen in 63 (20.3%), and vomiting was found in 10 cases (3.2%). Other clinical symptoms included seizure (8.4%), headache (4.5%) and skin rash (2%). Crackles on auscultation were heard in 32.6% (101/310) of patients. Wheezing was noted in 37 (12%) patients, and retraction of the chest wall was seen in 14 (4.5%). Other abnormal findings on physical examination were pharyngeal injection (62.3%), redness of the tympanic membrane (12.6%) and conjunctival injection (8.7%). In addition, cervical lymphadenopathy was detected in eight (2.6%) patients, and hepatosplenomegaly was found in two (0.6%).
Laboratory and radiographic findings
Leukocytosis was detected in 69 (22.3%) patients, and leukopenia was seen in four (1.3%) (Table 2).10 Elevated C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were the most common laboratory findings (93.9%). Liver enzyme, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were elevated in about 10% of the patients.
Table 2.
Laboratory findings in adenoviral infection
| No. of cases | % | ||
|---|---|---|---|
| WBC (/μL) | Leukopenia | 4 | 1.3 |
| Normal range† | 263 | 84.8 | |
| Leukocytosis | 43 | 13.9 | |
| ESR (mm/hr) | Normal range (<9) | 19 | 6.1 |
| Elevated (≥9) | 291 | 93.9 | |
| CRP (mg/dl) | Normal range (<0.3) | 19 | 6.1 |
| Elevated (≥0.3) | 291 | 93.9 | |
| AST (IU/L) | Normal range (≤55) | 269 | 86.7 |
| Elevated (>55) | 41 | 13.3 | |
| ALT (IU/L) | Normal range (≤45) | 285 | 91.9 |
| Elevated (>45) | 25 | 8.1 |
†Normal range obtained from the age‐matched references.10 ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive peptide; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
Co‐infections with other pathogens were identified in 29 cases. The most common cause was Mycoplasma pneumoniae (M. pneumoniae) (12/29). Influenza A (2/29) and B (4/29), parainfluenza virus (2/29), RSV (2/29) and rotavirus (7/29) were also concomitantly identified with adenovirus infection. Five cases of rotavirus infection were identified as hospital‐acquired infection, while other bacterial isolates were not detected at the time of admission.
Chest radiography was performed in all patients at admission, and abnormal findings were found in 42.6% (132/310); 104 (35.5%) had perihilar or peribronchial infiltrates, 23 (7.4%) had lobar infiltration or consolidation, three (1%) had hyperinflation and two (0.6%) had pleural effusion.
Treatment and outcomes
The mean duration of hospital stay was 6.2 ± 6.5 days (range 3–98 days). The majority of patients (93.9%, 291/310) received antibiotics. Two or more antimicrobial agents were used in 34.2% of patients. The most commonly used agents were ampicillin/sulbactam (80.8%), third‐generation cephalosporin (24.7%), aminoglycoside (16.2%) and macrolide (8.6%). Oxygen supplementation was provided to 11 patients (3.6%). Five patients (1.6%) were admitted or transferred to the ICU, and three of them received mechanical ventilation. The characteristics of ICU patients are detailed in Table 3. Two of the ICU patients were admitted in 2005, while the remaining three patients were admitted in 2007. All were male, and had no underlying conditions, except one who was born prematurely. The first case was diagnosed with severe pneumonia, which progressed to ischemic‐hypoxic encephalopathy. The second case was pneumonia complicated with bronchiolitis obliterans. The third case was a 23‐month‐old premature infant with bronchopulmonary dysplasia who required ventilator care for 24 days. The fourth case was pneumonia with a long‐lasting fever (15 days) that was improved without complication. The fifth case was admitted for fever and seizure, and adenovirus from CSF was identified. No deaths were reported.
Table 3.
Clinical profiles of ICU admitted patients
| Case no. | Sex | Age (months) | Diagnosis | Hospitalization (days) | Fever (days) | Underlying condition | Date detected (month/year) |
|---|---|---|---|---|---|---|---|
| 1 | M | 68 | Pneumonia, HIE | 98 | 5 | None | Aug/2007 |
| 2 | M | 17 | Pneumonia, BO | 23 | 11 | None | July/2007 |
| 3 | M | 23 | Acute bronchiolitis | 47 | 15 | Prematurity, BPD | June/2005 |
| 4 | M | 13 | Pneumonia | 19 | 15 | None | July/2007 |
| 5 | M | 3 | Encephalitis | 7 | 2 | None | Jan/2005 |
BO, bronchiolitis obliterans; BPD, bronchopulmonary dysplasia; HIE, hypoxic‐ischemic encephalopathy; ICU, intensive care unit.
Duration of hospital stay and fever were used as parameters for determining disease severity. Duration of hospital stay was significantly longer in children with LRTI (P < 0.001) and co‐infection (P = 0.001). However, the length of hospitalization was not related to age, sex or pre‐existing condition. On the other hand, prolonged fever was associated with underlying disease (P = 0.026) and asthma (P = 0.016). Age, sex, diagnosis and co‐infection, including M. pneumoniae, were not related to the duration of fever.
Discussion
This study revealed that adenoviral infection accounted for a relatively high proportion (16.9%) of all respiratory virus infections in hospitalized children compared to the results of previous reports.11, 12 Other pathogens included RSV (48%), influenza (19%), and parainfluenza (16.1%). In our study, 81.3% of the adenoviral infections occurred in young children between 6 months and 5 years of age. Similar demographic features have been reported in other studies.13, 14 According to Pereira's report, children aged 5 years had higher antibody positivity against adenovirus, and most infections happened in children younger than 5 years.15 Infants less than 6 months of age are known to have a neutralizing antibody by maternal transmission, which appears to be protective during the first 6 months of life. The frequency of infection was low in infants under 6 months of age; however, a severe case, such as encephalitis, as reported in this study, may occur. The male to female ratio was 1.6:1. The tendency for male predominance has been described in the literature concerning adenovirus infection, as well as in studies of other respiratory viruses.16, 17, 18
We observed that more cases of adenoviral infectious disease occurred in 2007. Adenovirus was the most common in the spring and summer of 2007, but occurred throughout the year in other years examined in this study. It seems that adenovirus shows a seasonal variation, with sporadic epidemics. In Korea, small outbreaks of adenovirus infection were reported in the summer of 1995 and in the spring of 1996.19 In a temperate climate, such as Korea, adenovirus infections are known to occur in the spring, early summer and winter.20 Our data showed similar findings, illustrating a slight tendency toward variations in the seasons in which adenovirus was detected more frequently.
In one study,21 adenoviral diseases in children were characteristically accompanied by a high and persistent (mean 5.4 days) fever. Over one‐third of the children in our study showed a high fever despite the supportive treatment, and a prolonged fever (≥10 days) was found in 6.8% of cases. Larrañaga et al.22 reported that 70% of hospitalized children with adenoviral infection had pneumonia, while our results revealed a significant proportion of patients (45.4%) with URTI. The prolonged fever associated with adenovirus infection highlights the primary cause for hospital treatment in patients with URTI. Also, many patients appeared to have gastrointestinal symptoms with hepatic involvement, as determined by a mild to high elevation of liver enzymes. Adenovirus was detected in the CSF of one infant who was diagnosed with encephalitis. According to our study, the clinical manifestation of adenovirus infection varied, with multiple organ involvement. Several studies have demonstrated that adenovirus is unique among the common respiratory viruses in that it can involve other organs, resulting in conjunctivitis, gastroenteritis, acute hemorrhagic cystitis and meningoencephalitis.23, 24
Although the white blood cell count was within the normal range in most patients (76.4%), elevated CRP and ESR levels were shown in 93.9% of all the patients. Elevated CRP and ESR levels are also generally thought to be related to bacterial infection, as they are inflammatory markers. Others have reported that adenoviral infection typically results in elevated ESR and CRP levels, unlike what is seen in other viral diseases.25, 26 Therefore, it is not surprising that many patients were initially treated with antibiotics. The clinical use of antigen detection tests is expected to make earlier diagnosis of adenoviral infection, and to reduce unnecessary treatment of antibiotics.
The relation to co‐infections has been reported by some authors. Korppi et al. reported that mixed infection was common (55%) in children with adenovirus infection, and bacterial co‐infection was demonstrated in 45% of patients.27 Another study noted that co‐infection with measles was a risk factor for mortality in the acute stage of adenovirus respiratory infections.28 In our study, the most common cause of co‐infection was M. pneumoniae. We found no significant difference in disease severity between adenovirus‐infected patients with co‐infection and those without. When more than one pathogen is identified with adenovirus, it is difficult to determine whether it is true co‐infection because adenovirus can persist in the respiratory or gastrointestinal tract after active infection. Further study will be necessary to determine whether co‐infection can affect the disease course and prognosis.
There were several limitations to our study. First, the serotypes of adenovirus were not determined. Therefore, we were unable to make more refined observations regarding differences in age distributions, clinical characteristics and determinants of severity based on the serotypes. Second, nearly all the children received antibiotic treatment, and the judicious use of antibiotics and resistant development is becoming increasingly important in Korea. Finally, we did not test for human bocavirus, coronavirus, metapneumovirus, or aerobic bacteria; therefore, some co‐infections may have been overlooked.
In conclusion, our study contributes to critical epidemiological baseline on respiratory adenoviral infection in Korean children, and highlights the importance of adenovirus as a major cause of hospitalization. Adenovirus was more frequently detected in young children and was associated with significant morbidity. Prolonged fever was associated with URTI and necessity of hospitalization, as well as LRTI. Large‐scale investigation through a multicenter approach is required to determine the optimal monitoring and treatment strategies and to achieve a better understanding of the clinical course of adenoviral infection in children.
Acknowledgments
We thank the doctors and laboratory staff of Korea University Ansan Hospital for their participation in the retrospective adenoviral infection study. We also thank our collaborators at Myung Moon Pediatrics for their support.
References
- 1. Carballal G, Videla CM, Espinosa MA et al. Multicentered study of viral acute lower respiratory infections in children from four cities of Argentina, 1993–1994. J. Med. Virol. 2001; 64: 167–174. [DOI] [PubMed] [Google Scholar]
- 2. Choi EH, Kim HS, Park KH, Lee HJ. Genetic heterogeneity of the hexon gene of adenovirus type 3 over a 9‐year period in Korea. J. Med. Virol. 2006; 78: 379–383. [DOI] [PubMed] [Google Scholar]
- 3. Wenman WM, Pagtakhan RD, Reed MH, Chernick V, Albritton W. Adenovirus bronchiolitis in Manitoba: epidemiologic, clinical, and radiologic features. Chest 1982; 81: 605–609. [DOI] [PubMed] [Google Scholar]
- 4. Dagan R, Schwartz RH, Insel RA, Menegus MA. Severe diffuse adenovirus 7a pneumonia in a child with combined immunodeficiency: possible therapeutic effect of human immune serum globulin containing specific neutralizing antibody. Pediatr. Infect. Dis. 1984; 3: 246–251. [DOI] [PubMed] [Google Scholar]
- 5. Kim YK, Nyambat B, Hong YS, Lee CG, Lee JW, Kilgore PE. Burden of viral respiratory disease hospitalizations among children in a community of Seoul, Republic of Korea, 1995–2005. Scand. J. Infect. Dis. 2008; 40: 946–953. [DOI] [PubMed] [Google Scholar]
- 6. Pichler MN, Reichenbach J, Schmidt H, Herrmann G, Zielen S. Severe adenovirus bronchiolitis in children. Acta Paediatr. 2000; 89: 1387–1389. [DOI] [PubMed] [Google Scholar]
- 7. Walls T, Shankar AG, Shingadia D. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect. Dis. 2003; 3: 79–86. [DOI] [PubMed] [Google Scholar]
- 8. Kim JH, Lee SI, Lee MH et al. Ten cases of severe adenoviral pneumonia in the spring 1995. Korean J Pediatr 1996; 39: 1247–1253. [Google Scholar]
- 9. Lee J, Choi EH, Lee HJ. Comprehensive serotyping and epidemiology of human adenovirus isolated from the respiratory tract of Korean children over 17 consecutive years (1991–2007). J. Med. Virol. 2010; 82: 624–631. [DOI] [PubMed] [Google Scholar]
- 10. Orkin SH, Nathan DG. Nathan and Oski's Hematology of Infancy and Childhood, 7th edn. Saunders/Elsevier, Philadelphia, PA, 2009. [Google Scholar]
- 11. Choi EH, Lee HJ, Kim SJ et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin. Infect. Dis. 2006; 43: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubin BA. Clinical picture and epidemiology of adenovirus infections (a review). Acta Microbiol. Hung. 1993; 40: 303–323. [PubMed] [Google Scholar]
- 13. Peled N, Nakar C, Huberman H et al. Adenovirus infection in hospitalized immunocompetent children. Clin Pediatr (Phila) 2004; 43: 223–229. [DOI] [PubMed] [Google Scholar]
- 14. Mandelboim M, Dror P, Azar R, Bromberg M, Mendelson E. Adenovirus infections in hospitalized patients in Israel: epidemiology and molecular characterization. J. Clin. Microbiol. 2011; 49: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pereira MS. Adenovirus infections. Postgrad. Med. J. 1973; 49: 798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gray GC, McCarthy T, Lebeck MG et al. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004–2006. Clin. Infect. Dis. 2007; 45: 1120–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quach C, Piché‐Walker L, Platt R, Moore D. Risk factors associated with severe influenza infections in childhood: implication for vaccine strategy. Pediatrics 2003; 112: e197–201. [DOI] [PubMed] [Google Scholar]
- 18. Schuurhof A, Bont L, Siezen CL et al. Interleukin‐9 polymorphism in infants with respiratory syncytial virus infection: an opposite effect in boys and girls. Pediatr. Pulmonol. 2010; 45: 608–613. [DOI] [PubMed] [Google Scholar]
- 19. Ahn KM, Chung SH, Chung EH et al. Clinical characteristics of acute viral lower respiratory tract infections in hospitalized children in Seoul, 1996–1998. J. Korean Med. Sci. 1999; 14: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chany C. [Adenovirus infections in children]. Arch. Gesamte Virusforsch. 1963; 13: 294–301. [PubMed] [Google Scholar]
- 21. Ruuskanen O, Meurman O, Sarkkinen H. Adenoviral diseases in children: a study of 105 hospital cases. Pediatrics 1985; 76: 79–83. [PubMed] [Google Scholar]
- 22. Larrañaga C, Kajon A, Villagra E, Avendaño LF. Adenovirus surveillance on children hospitalized for acute lower respiratory infections in Chile (1988–1996). J. Med. Virol. 2000; 60: 342–346. [PubMed] [Google Scholar]
- 23. Hong JY, Lee HJ, Piedra PA et al. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: epidemiology, clinical features, and prognosis. Clin. Infect. Dis. 2001; 32: 1423–1429. [DOI] [PubMed] [Google Scholar]
- 24. Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin. Infect. Dis. 1998; 27: 1194–1200. [DOI] [PubMed] [Google Scholar]
- 25. Barone SR, Pontrelli LR, Krilov LR. The differentiation of classic Kawasaki disease, atypical Kawasaki disease, and acute adenoviral infection: use of clinical features and a rapid direct fluorescent antigen test. Arch. Pediatr. Adolesc. Med. 2000; 154: 453–456. [DOI] [PubMed] [Google Scholar]
- 26. Appenzeller C, Ammann RA, Duppenthaler A, Gorgievski‐Hrisoho M, Aebi C. Serum C‐reactive protein in children with adenovirus infection. Swiss Med. Wkly 2002; 132: 345–350. [DOI] [PubMed] [Google Scholar]
- 27. Korppi M, Leinonen M, Mäkelä PH, Launiala K. Mixed infection is common in children with respiratory adenovirus infection. Acta Paediatr. Scand. 1991; 80: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murtagh P, Giubergia V, Viale D, Bauer G, Pena HG. Lower respiratory infections by adenovirus in children. Clinical features and risk factors for bronchiolitis obliterans and mortality. Pediatr. Pulmonol. 2009; 44: 450–456. [DOI] [PubMed] [Google Scholar]


