Abstract
Respiratory infections cause considerable morbidity during infancy. The impact of innate immunity mechanisms, such as mannose‐binding lectin (MBL), on respiratory symptoms remains unclear. The aims of this study were to investigate whether cord blood MBL levels are associated with respiratory symptoms during infancy and to determine the relative contribution of MBL when compared with known risk factors. This is a prospective birth cohort study including 185 healthy term infants. MBL was measured in cord blood and categorized into tertiles. Frequency and severity of respiratory symptoms were assessed weekly until age one. Association with MBL levels was analysed using multivariable random effects Poisson regression. We observed a trend towards an increased incidence rate of severe respiratory symptoms in infants in the low MBL tertile when compared with infants in the middle MBL tertile [incidence rate ratio (IRR) = 1.59; 95% confidence interval (CI): 0.95–2.66; p = 0.076]. Surprisingly, infants in the high MBL tertile suffered significantly more from severe and total respiratory symptoms than infants in the middle MBL tertile (IRR = 1.97; 95% CI: 1.20–3.25; p = 0.008). This association was pronounced in infants of parents with asthma (IRR = 3.64; 95% CI: 1.47–9.02; p = 0.005). The relative risk associated with high MBL was similar to the risk associated with well‐known risk factors such as maternal smoking or childcare. In conclusion the association between low MBL levels and increased susceptibility to common respiratory infections during infancy was weaker than that previously reported. Instead, high cord blood MBL levels may represent a so far unrecognized risk factor for respiratory morbidity in infants of asthmatic parents.
Keywords: asthma, infant, innate immunity, mannose‐binding lectin, respiratory infection
Respiratory infections during infancy represent a major cause of childhood morbidity and mortality (1). In addition, cough and wheeze result in a relevant financial burden on the healthcare system, accounting for a large proportion of medical consultations and unscheduled hospitalizations (2). Several risk factors for respiratory infections have been identified, such as parental smoking, male sex, atopy, prematurity and day‐care attendance (3, 4). Large cohort studies have shown that respiratory infections during early life can have both a protective and detrimental effect on long‐term respiratory outcomes, such as asthma (5, 6). Infant immunity depends in part on innate defence mechanisms as the protection through transplacentally acquired maternal antibodies gradually fades away while the adaptive immune defence is just about to mature (7, 8). Therefore, gene polymorphisms within the innate immune system might particularly affect individual susceptibility to infection in infants.
Mannose‐binding lectins (MBLs), an important component of innate immunity, are acute‐phase plasma collections that recognize repetitive sugar arrays present on many pathogens but not on mammalian cells (8, 9). Binding of MBL to various micro‐organisms activates complement by the lectin‐pathway, which finally leads to opsonization and lysis of pathogens and recruitment of inflammatory cells (10). Due to polymorphisms within the MBL2 gene and the associated promotor region, MBL serum concentrations show a wide variability (11, 12, 13). MBL deficiency is common with approximately 30% of the white population having reduced and 5–10% having very low MBL concentrations (10). MBL does not cross the placental barrier and the infant’s cord blood MBL concentration correlates well with the individual MBL level later in life (14, 15). MBL has repeatedly been postulated to play an important role in modulating susceptibility to respiratory tract infections during infancy (8, 10, 16), although data are partially conflicting (14, 17, 18, 19, 20). In a prospective population‐based cohort study, MBL deficient children experienced significantly more acute respiratory tract infections (21). On the contrary, several recent papers have shown associations between high MBL levels and asthma (14, 22, 23, 24). Interpretation of the available reports on MBL and respiratory symptoms in infants is hampered, since most studies did not adjust for known risk factors for respiratory disease such as atopy and environmental factors (19).
The aim of the current study was to investigate prospectively whether MBL levels measured in cord blood of healthy newborns are associated with respiratory symptoms during the first year of life and to determine the relative contribution of MBL in comparison to known risk factors. A secondary aim was to investigate whether MBL deficient infants are prone to infections with a specific respiratory virus.
Methods
Study subjects
This prospective birth cohort study comprised healthy term neonates in the region of Berne, Switzerland, born between January 1999 and December 2005. Exclusion criteria for the study were pre‐term delivery (<37 wk), major birth defects, respiratory distress after birth, other significant perinatal disease or a later diagnosis of airway malformation or specific chronic respiratory disease (3, 25). The Ethics Committee of the Region of Berne approved the study and written consent from all parents was obtained at enrolment.
Study design
Subjects were recruited antenatally and cord blood was taken at birth for analysis of MBL levels. Health status of the infants during the first year of life was assessed by weekly phone calls of research nurses to the mothers with focus on respiratory symptoms and any changes in environmental factors such as nutrition, environmental tobacco smoke exposure, childcare or immunization (3, 25). Respiratory symptoms (cough and wheeze) were assessed using a standardized scoring system that stages symptoms into four levels according to the severity, with a high sensitivity for lower respiratory tract symptoms (Table 1) (26). Upper respiratory tract symptoms without cough (e.g. rhinitis, pharyngitis, middle ear infections) were not considered.
Table 1.
Symptom score used in weekly phone calls
| Symptom score | Day‐time symptoms (cough, wheeze or breathing difficulties) | Night‐time symptoms (cough, wheeze or breathing difficulties) |
|---|---|---|
| 0 | None | None |
| 1 | Slight; no treatment given | Slight; sleep not disturbed |
| 2 | Required treatment but no outside help | Sleep disturbed once; no help required |
| 3 | Severe; required help from GP | Sleep disturbed more than once or child needed help |
| 4 | Very severe; admitted to hospital | Sleep very disturbed or GP called |
Adapted with permission from the BMJ publishing group.
Main outcome measures were ‘weeks with respiratory symptoms’, defined as total number of weeks a child had any respiratory symptom, independent of type or severity; ‘weeks with severe respiratory symptoms’, defined as a symptom score of ≥3, e.g. repeated sleep disturbances during night or general practitioner consultation; and ‘audible wheeze’, defined as wheezing sound audible to the parents, as described in detail previously (3). We used a standardized questionnaire to assess sociodemographic conditions and pre‐ and post‐natal exposure to putative risk factors for respiratory disease (including maternal or paternal asthma, parental atopic disease, number of siblings and pre‐ or post‐natal environmental tobacco smoke exposure). Maternal atopy was ascertained by skin prick testing to eight common allergens (Allergomed AG, Therwil, Switzerland). For maternal smoking during pregnancy, validity of reported exposure was verified by comparisons with cotinine levels in the first urine of the newborn (gas–liquid chromatography, ITS, Lausanne, Switzerland).
MBL measurements
Cord blood was taken by venipuncture from the umbilical cord attached to the placenta, immediately following separation from the infant. Cord blood was then centrifuged for 6 min at 3000 g, the resulting serum immediately frozen in sterile tubes at −80°C. MBL concentration was measured in serum using a commercially available ELISA kit recognizing functional oligomers (MBL oligomer ELISA kit by Antibodyshop, Gentofte, Denmark).
Microbiological sampling and analysis
At the first acute respiratory infection (defined as more than 2 days with cough or wheeze, together with fever >38°C, acute rhinitis, otitis media or pharyngitis), the study nurse collected a nasal swab at the parents’ home. The samples were immediately stored at −80°C in phosphate‐buffered saline containing 40 U/ml RNase‐Inhibitor (Roche, Basel, Switzerland). The specimens were later thawed and analysed by separate qualitative Taqman® real‐time PCR assays targeting 16 different respiratory viruses that commonly infect humans, namely rhinovirus, human coronavirus (HCoV) ‐OC43, ‐E229, ‐NL63 and ‐HKU1, parainfluenza virus (PIV) ‐1, ‐2 and ‐3, respiratory syncytial virus A and B, human metapneumovirus, human bocavirus, influenza virus A and B, adenovirus and enterovirus, as described previously (27).
Statistical methods
The outcome for analysis was for each child the number of weeks with respiratory symptoms in the first year, which followed a Poisson distribution. Due to the distribution of our data, we fitted more conservative random‐effects Poisson regression models with child‐specific intercepts. Continuous variables were centred at their mean and categorical variables were entered as indicator variables. Results are expressed as incidence rate ratios (IRRs) with 95% confidence intervals (CIs).
To minimize the effect of outliers and to examine a possible non‐linear relationship, cord blood MBL levels were categorized into tertiles (low, middle and high tertile) and entered via separate indicator variables into the model. The analysis was conducted in two steps. First, the association between cord blood MBL levels and weeks with respiratory symptoms was examined in univariable random‐effects Poisson regression models. We then obtained adjusted results by including other exposures associated with respiratory symptoms during infancy such as gender, birth weight, number of older siblings, nursery care, maternal smoking during pregnancy, parental smoking, maternal and parental (e.g. maternal and/or paternal) asthma and maternal allergic rhinitis (3). We included interaction terms to model effect modification by parental asthma and atopy and used likelihood ratio tests to assess the statistical significance of effect modification. Analysis of effect modification was pre‐planned for parental asthma and post hoc for parental atopy analysis.
Several sensitivity analyses were performed. First, we used additional outcome measures [total symptom score, average symptom score and number of infectious episodes as described previously (3)]. Second, we categorized cord blood MBL levels into quintiles and repeated the analysis with an additional subgroup [with MBL levels below 100 ng/ml (18, 20)] in order to examine the effect of very low MBL levels. All these analyses produced very similar results to the main analysis. All analyses were performed using Stata®, version 8.2 for Windows (STATA Corporation, College Station, TX, USA).
Results
The study enrolled 228 infants with data from 185 (81%) used for this analysis. This birth cohort has been previously described elsewhere in detail (3, 25). Reasons for exclusion were lack of cord blood (n = 33) or dropout from follow‐up (n = 10). Mothers of 172 (93%) children reported one or more weeks with wheeze or cough, with a median (range) of 4 (0–23) wk. Severe symptoms occurred in 81 (44%) infants, at a median (range) of 0 (0–6) wk and wheeze was present in 41 (22%) infants, with median (range) of 0 (0–10) wk. Four infants were hospitalized for respiratory reasons. The distribution of cord blood MBL levels is given in Fig. 1. Anthropometric data and presence of known risk factors for respiratory disease during infancy are given in Table 2 according to cord blood MBL levels in the low, middle and high tertile. One child without a history of pre‐natal smoke exposure was regarded as being exposed to pre‐natal tobacco smoke because elevated urine cotinine levels (93 ng/ml) suggested significant pre‐natal nicotine exposure.
Figure 1.
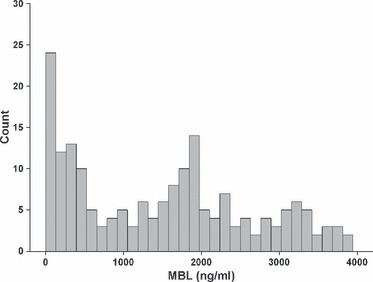
Distribution of cord blood MBL levels in ng/ml among the 185 healthy term infants of our cohort.
Table 2.
Anthropometric data and distribution of known risk factors for respiratory symptoms during the first year of life among the three MBL tertiles of the 185 study children
| Low MBL tertile (<600 ng/ml) | Middle MBL tertile (600–1931 ng/ml) | High MBL tertile (>1931 ng/ml) | |
|---|---|---|---|
| Number of subjects | 62 | 62 | 61 |
| Gender (male/female) | 37/25 | 38/24 | 32/29 |
| Gestational age, wk | 39.9 (38.9–40.6) | 40.1 (39.1–41.0) | 40.1 (39.6–40.7) |
| Birth weight, kg | 3410 (3160–3710) | 3210 (3040–3590) | 3430 (3100–3660) |
| Older siblings (none/one/two or more) | 26/21/15 | 36/17/9 | 32/19/10 |
| Parental* asthma | 12 (19) | 16 (26) | 13 (21) |
| Maternal asthma | 9 (15) | 8 (13) | 5 (8) |
| Paternal asthma | 5 (8) | 8 (13) | 8 (13) |
| Parental smoking* | 28 (45) | 13 (21) | 13 (21) |
| Maternal smoking during pregnancy | 11 (18) | 8 (13) | 5 (8) |
| Paternal smoking | 24 (39) | 10 (16) | 12 (20) |
| Day‐care attendance | 12 (20) | 16 (26) | 14 (23) |
Data are given as median (interquartile range) or number (percentage) of infants.
*Parental asthma is defined as presence of maternal and/or paternal asthma; parental smoking is defined accordingly.
Infants with MBL in the low tertile showed a trend towards an increased risk for respiratory symptoms compared with infants with MBL levels in the middle tertile (Table 3; left column). In the multivariable analysis, the strength of this association was decreased. Sensitivity analysis of a subgroup of 21 infants (11%) with very low MBL levels (below 100 ng/ml) resulted in a similar non‐significant trend: e.g. the IRR in the multivariable analysis was 1.25 (95% CI: 0.83–1.86; p = 0.283) for respiratory symptoms, 1.81 (0.94–3.47; p = 0.075) for severe symptoms and 1.78 (0.55–5.78; p = 0.335) for wheeze in relation to infants with MBL levels in the middle tertile.
Table 3.
Association between cord blood MBL levels and number of weeks with respiratory symptoms during the first year of life
| Outcome measure | Univariable association | Adjusted association* | ||||
|---|---|---|---|---|---|---|
| IRR† | 95% CI | p‐Value | IRR† | 95% CI | p‐Value | |
| Weeks with any respiratory symptoms‡ | ||||||
| Low MBL§ | 1.27 | 0.94–1.73 | 0.125 | 1.21 | 0.90–1.62 | 0.218 |
| High MBL | 1.27 | 0.93–1.73 | 0.132 | 1.35 | 1.02–1.79 | 0.036 |
| Weeks with severe respiratory symptoms¶ | ||||||
| Low MBL§ | 1.61 | 0.93–2.76 | 0.086 | 1.57 | 0.93–2.64 | 0.092 |
| High MBL | 1.72 | 1.01–2.96 | 0.047 | 1.93 | 1.17–3.20 | 0.010 |
| Wk with wheeze** | ||||||
| Low MBL§ | 3.17 | 1.22–8.19 | 0.017 | 1.78 | 0.71–4.45 | 0.216 |
| High MBL | 2.80 | 1.07–7.30 | 0.036 | 2.03 | 0.83–4.97 | 0.120 |
Cord blood MBL levels in the low/high tertile of the cohort distribution are compared with MBL levels in the middle tertile.
*Adjusted for the following additional risk factors of respiratory symptoms during infancy: sex, gestational weight, maternal smoking during pregnancy and parental smoking, maternal and parental asthma, number of older siblings, day‐care attendance and maternal atopic disease.
†Incidence rate ratio of having weeks with the respective respiratory symptoms in first year of life compared to middle MBL levels.
‡Defined as respiratory symptoms independent of severity.
§Comparable results were obtained for 21 infants with very‐low MBL cord blood levels (<100 ng/ml); see ‘Results’ section for details.
¶Defined as respiratory symptoms with repeated sleep disturbances during night or GP consultation during day‐time.
**Defined as audible wheeze to the parents.
Infants with MBL levels in the high tertile had a significantly increased risk for respiratory symptoms compared with infants in the middle tertile (Table 3). The association was present for the number of weeks with ‘any respiratory symptoms’ but was more pronounced for the number of weeks with severe symptoms and with wheeze. The strength of association between cord blood MBL levels in the high tertile and respiratory symptoms was essentially unchanged in the multivariable analyses (Table 3) and was comparable to that of well‐known risk factors for respiratory symptoms in infancy, such as having two or more older siblings, smoking during pregnancy, childcare attendance and maternal asthma (see Fig. 2).
Figure 2.

Association between several risk factors and severe respiratory symptoms during infancy. The incidence rate ratio (number) and 95% CI for weeks with severe respiratory symptoms during the first year of life are given for the different risk factors, adjusted for all risk factors listed in the methods section.
An increased susceptibility to respiratory infections in MBL deficient children has been topic of considerable debate (19). Only sparse data, however, are available on the association between high MBL levels and respiratory symptoms, mainly based on studies about asthma in older children and adults (14, 22, 23, 24). We therefore further analysed the role of high cord blood MBL levels using stratification for parental asthma. We found that in infants of parents without asthma, MBL levels in the high tertile were not associated with an increased risk of respiratory symptoms (IRR = 1.12; 95% CI: 0.81–1.53; p = 0.49 for total respiratory symptoms; IRR = 1.57; 95% CI: 0.88–2.82; p = 0.13 for severe respiratory symptoms). In contrast, in infants of asthmatic parents (mother and/or father), MBL levels in the high tertile were significantly associated with an increased risk (IRR = 2.29; 95% CI: 1.30–4.03; p = 0.004 for total respiratory symptoms; IRR = 3.64; 95% CI: 1.47–9.02; p = 0.005 for severe respiratory symptoms). Thus, the association between cord blood MBL levels in the high tertile and subsequent respiratory symptoms was significantly modified by the presence of parental asthma (p value for interaction 0.013 for severe respiratory symptoms, Fig. 3). Similar findings were obtained for stratification by maternal or paternal asthmatic disease. No effect modification was found for paternal atopy as assessed by questionnaire or maternal atopy as assessed by skin prick test for six common respiratory allergens.
Figure 3.

Association between cord blood MBL levels and severe respiratory symptoms according to parental asthma. The incidence rate ratio (number) and 95% CI for weeks with severe respiratory symptoms during the first year of life are given stratified for parental (e.g. maternal and/or paternal) asthmatic disease, adjusted for all risk factors listed in the methods section.
Nasal swabs for virological analysis were available from 93 infants at the time of the first acute respiratory tract infection, and one or more viruses were identified in 71 (75%) cases. No association between MBL levels and viral aetiology or age at onset of the first acute respiratory tract infection was found (Fig. 4).
Figure 4.
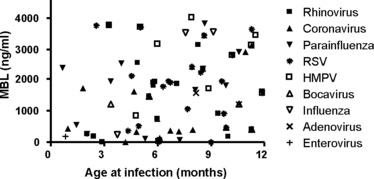
Respiratory viruses isolated using nasal swab PCR at the time of the first acute respiratory tract infection are shown in relation to the infants MBL cord blood concentration and to the age at onset.
Discussion
We assessed prospectively the association between cord blood MBL levels and subsequent respiratory symptoms in healthy term infants. We found that, when accounting for known risk factors for respiratory disease, low MBL levels were only weakly associated with respiratory symptoms in the first year of life. In contrast, infants with high MBL levels experienced significantly more respiratory symptoms. The association between high MBL levels and respiratory symptoms was pronounced in infants of parents with asthma.
The concentrations of MBL measured in cord blood in our cohort were in good agreement with published results (14, 18, 28) and showed a tri‐modal distribution, reflecting the polymorphisms resulting in low, middle and high MBL serum levels. Notably, the cut‐off distinguishing the low and middle MBL tertiles in our cohort (600 ng/ml) nearly coincides with the cut‐off proposed by receiver–operating curve analyses (700 ng/ml) to predict MBL deficiency (20, 28). Therefore, it can be assumed that the low MBL level group largely represented the 30% of the population with MBL deficient genotypes.
Although infants with low MBL levels experienced more weeks with respiratory symptoms in relation to children with middle MBL, this association did not reach statistical significance and was even weakened after adjustment for known risk factors. Sensitivity analysis focussing on infants with very low MBL levels [<100 ng/ml (18, 20)] confirmed these results. Currently available evidence concerning the role of MBL deficiency on respiratory infections in children is inconclusive (19) and arises mainly from hospital‐based or case control studies (16, 18, 20). Koch et al. (21) have published a prospective population‐based cohort study in Greenlandic children below two 2‐yr of age and reported a twofold‐increased risk of acute respiratory infections for MBL‐deficient children. A recent well‐designed large prospective German study (17) on MBL genotypes in 749 children could not confirm this finding. This study followed children until the age of 10 yr and interviewed the parents at 1, 3, 6 and 12 months during the first year of life.
In vitro binding of MBL has been shown for influenza A virus (29) and coronavirus (30). No obvious pattern between low MBL levels and the type of virus isolated from nasal swabs or age at the first respiratory tract infection was observed in our cohort. Since MBL‐mediated opsonization and direct lysis of pathogens have primarily been demonstrated for bacteria (9, 10), it may be argued that MBL deficiency plays a minor role in modulating susceptibility towards common respiratory infections (31) during infancy, which are mainly of viral origin. Furthermore, redundancy within innate immunity such as antibody‐mediated activation of the classical pathway of complement may compensate for MBL deficiency (32). Since our study addressed lower respiratory tract symptoms in a healthy population with a low incidence of hospitalizations, we cannot comment on the role of MBL deficiency in predominantly bacterial infections such as pneumonia or otitis media (11). MBL‐mediated immunity might indeed be more relevant in the context of invasive bacterial infections (33, 34).
Infants with high cord blood MBL levels suffered more often from respiratory symptoms, which represents a novel finding. Subset analysis revealed that high MBL levels were particularly relevant if at least one of the parents suffered from asthma. Notably, infants of parents with asthma did not overall have higher MBL levels than infants of non‐asthmatic parents, which would be expected if the rise in MBL was caused primarily by a pro‐inflammatory in utero environment. Several authors have reported elevated MBL levels in patients with asthma (14, 22, 23, 24, 35), whereas studies based solely on genotyping MBL deficiency found no association with asthma (17, 36, 37). Recently, a novel intronic single nucleotide polymorphism within the MBL2 gene, associated with high MBL levels, peripheral blood eosinophilia and asthma severity, has been identified (23). Since most studies (14, 22, 23, 24, 35) measured MBL at a time when asthma had already been diagnosed, chronic inflammation could have caused the rise in MBL. In contrast, our results demonstrate that high cord blood MBL levels precede respiratory symptoms in infants of asthmatic parents. Thus, MBL may act as a disease‐modifier: when an asthmatic genetic background is present, high MBL levels may be either causative or represent a side‐phenomenon of an inflammatory process leading to increased respiratory symptoms. Considering that MBL activates the complement system, representing one of the most potent inflammatory cascades (38), it seems plausible that high MBL levels favour complement‐mediated inflammation (13, 39). No effect modification was found for atopy. This is in line with several studies who have failed to show associations between MBL and atopy (17, 37).
This study has several methodological strengths. Contrary to previous studies (16, 18, 20, 21), we assessed well‐known risk factors for respiratory disease and could therefore obtain carefully adjusted estimates of the impact of MBL cord blood levels. Weekly standardized phone calls to the parents allowed us to prospectively assess respiratory morbidity during infancy in detail. Although we studied unselected healthy infants, participants were likely to be biased towards a predominantly white middle class origin, with a high proportion of asthmatic parents and a low proportion of smoking parents as discussed in detail previously (3). This might have affected the incidence of respiratory symptoms, but is unlikely to have influenced the association between symptoms and MBL.
Our study design with the exclusion of premature infants and infants with neonatal disease makes a relevant bias in measuring MBL levels due to acute‐phase reactions unlikely. The present study was based on MBL serum concentrations and not MBL genotype. Since the effect of MBL is related to circulating functional oligomers (9, 10), this approach seems justified. In addition, MBL genotypes correlate closely with phenotypes (11, 12, 20, 36), and recent publications even categorize genotypes as low‐, medium‐, or high‐producing, thus by phenotype (13, 17, 28).
In conclusion, in this prospective birth cohort study, low cord blood MBL levels were only weakly associated with respiratory symptoms during infancy. In otherwise healthy term infants, environmental and genetic factors other than MBL deficiency may be more relevant for determining susceptibility to common respiratory infections (31), which are mainly of viral origin. In contrast, high cord blood MBL levels were significantly associated with increased respiratory morbidity during the first year of life, especially in offspring of asthmatic parents. The strength of this association was comparable to known risk factors such as maternal smoking during pregnancy or day‐care attendance. Innate immunity mechanisms thus need to be considered as important determinants for pulmonary disease. In addition to the principal function of recognizing microbial pathogens, MBL may modify how an individual reacts to an infectious or allergen challenge (24). Contrary to surfactant proteins A and D, which possess a high structural similarity to MBL (8), the biological role of MBL in bronchoalveolar fluids has received little attention (40). Future studies should therefore address the presence of MBL on airway surfaces and its implications in the pathogenesis of pulmonary diseases such as asthma.
Acknowledgment
The authors thank the study nurses Christine Becher and Monika Graf for performing the weekly phone calls to the parents, Laurent Kaiser, MD, und Christelle Deffernez, MD, for performing the virological analyses, Margrith Otth, laboratory technician, for measuring MBL levels, and the families for participating in this study.
Supported by Swiss National Science Foundation grant 3200‐B0‐112099 to U. Frey and P. Latzin and by Swiss National Science Foundation PROSPER grants 3233‐069348 and 3200‐069349 to C. Kuehni (38).
Authors Luregn Jan Schlapbach and Philipp Latzin contributed equally.
References
- 1. Williams BG, Gouws E, Boschi‐Pinto C, Bryce J, Dye C. Estimates of world‐wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2002: 2: 25–32. [DOI] [PubMed] [Google Scholar]
- 2. Stevens CA, Turner D, Kuehni CE, Couriel JM, Silverman M. The economic impact of preschool asthma and wheeze. Eur Respir J 2003: 21: 1000–6. [DOI] [PubMed] [Google Scholar]
- 3. Latzin P, Frey U, Roiha HL, et al. Prospectively assessed incidence, severity, and determinants of respiratory symptoms in the first year of life. Pediatr Pulmonol 2007: 42: 41–50. [DOI] [PubMed] [Google Scholar]
- 4. Kamper‐Jorgensen M, Wohlfahrt J, Simonsen J, Gronbaek M, Benn CS. Population‐based study of the impact of childcare attendance on hospitalizations for acute respiratory infections. Pediatrics 2006: 118: 1439–46. [DOI] [PubMed] [Google Scholar]
- 5. Nafstad P, Magnus P, Jaakkola JJ. Early respiratory infections and childhood asthma. Pediatrics 2000: 106: E38. [DOI] [PubMed] [Google Scholar]
- 6. Illi S, Von Mutius E, Lau S, et al. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ 2001: 322: 390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hargreaves DC, Medzhitov R. Innate sensors of microbial infection. J Clin Immunol 2005: 25: 503–10. [DOI] [PubMed] [Google Scholar]
- 8. Turner MW. Mannose‐binding lectin: the pluripotent molecule of the innate immune system. Immunol Today 1996: 17: 532–40. [DOI] [PubMed] [Google Scholar]
- 9. Neth O, Jack DL, Dodds AW, Holzel H, Klein NJ, Turner MW. Mannose‐binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect Immun 2000: 68: 688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eisen DP, Minchinton RM. Impact of mannose‐binding lectin on susceptibility to infectious diseases. Clin Infect Dis 2003: 37: 1496–505. [DOI] [PubMed] [Google Scholar]
- 11. Wiertsema SP, Herpers BL, Veenhoven RH, et al. Functional polymorphisms in the mannan‐binding lectin 2 gene: effect on MBL levels and otitis media. J Allergy Clin Immunol 2006: 117: 1344–50. [DOI] [PubMed] [Google Scholar]
- 12. Minchinton RM, Dean MM, Clark TR, Heatley S, Mullighan CG. Analysis of the relationship between mannose‐binding lectin (MBL) genotype, MBL levels and function in an Australian blood donor population. Scand J Immunol 2002: 56: 630–41. [DOI] [PubMed] [Google Scholar]
- 13. Biezeveld MH, Geissler J, Weverling GJ, et al. Polymorphisms in the mannose‐binding lectin gene as determinants of age‐defined risk of coronary artery lesions in Kawasaki disease. Arthritis Rheum 2006: 54: 369–76. [DOI] [PubMed] [Google Scholar]
- 14. Thorarinsdottir HK, Ludviksson BR, Vikingsdottir T, et al. Childhood levels of immunoglobulins and mannan‐binding lectin in relation to infections and allergy. Scand J Immunol 2005: 61: 466–74. [DOI] [PubMed] [Google Scholar]
- 15. Aittoniemi J, Miettinen A, Laippala P, et al. Age‐dependent variation in the serum concentration of mannan‐binding protein. Acta Paediatr 1996: 85: 906–9. [DOI] [PubMed] [Google Scholar]
- 16. Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ 1997: 314: 1229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muller S, Keil T, Gruber C, et al. MBL2 variants in relation to common childhood infections and atopy‐related phenotypes in a large German birth cohort. Pediatr Allergy Immunol 2007: 18: 665–70. [DOI] [PubMed] [Google Scholar]
- 18. Kielgast S, Thiel S, Henriksen TB, Bjerke T, Olsen J, Jensenius JC. Umbilical cord mannan‐binding lectin and infections in early childhood. Scand J Immunol 2003: 57: 167–72. [DOI] [PubMed] [Google Scholar]
- 19. Ruskamp JM, Hoekstra MO, Rovers MM, Schilder AG, Sanders EA. Mannose‐binding lectin and upper respiratory tract infections in children and adolescents: a review. Arch Otolaryngol Head Neck Surg 2006: 132: 482–6. [DOI] [PubMed] [Google Scholar]
- 20. Cedzynski M, Szemraj J, Swierzko AS, et al. Mannan‐binding lectin insufficiency in children with recurrent infections of the respiratory system. Clin Exp Immunol 2004: 136: 304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koch A, Melbye M, Sorensen P, et al. Acute respiratory tract infections and mannose‐binding lectin insufficiency during early childhood. JAMA 2001: 285: 1316–21. [DOI] [PubMed] [Google Scholar]
- 22. Uguz A, Berber Z, Coskun M, Halide Akbas S, Yegin O. Mannose‐binding lectin levels in children with asthma. Pediatr Allergy Immunol 2005: 16: 231–5. [DOI] [PubMed] [Google Scholar]
- 23. Kaur S, Gupta VK, Shah A, Thiel S, Sarma PU, Madan T. Elevated levels of mannan‐binding lectin [corrected] (MBL) and eosinophilia in patients of bronchial asthma with allergic rhinitis and allergic bronchopulmonary aspergillosis associate with a novel intronic polymorphism in MBL. Clin Exp Immunol 2006: 143: 414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Staley KG, Stover C, Strippoli MP, Spycher BD, Silverman M, Kuehni CE. Mannan‐binding lectin in young children with asthma differs by level of severity. J Allergy Clin Immunol 2007: 119: 503–5. [DOI] [PubMed] [Google Scholar]
- 25. Latzin P, Kuehni CE, Baldwin DN, Roiha HL, Casaulta C, Frey U. Elevated exhaled nitric oxide in newborns of atopic mothers precedes respiratory symptoms. Am J Respir Crit Care Med 2006: 174: 1292–8. [DOI] [PubMed] [Google Scholar]
- 26. Silverman M, Wang M, Hunter G, Taub N. Episodic viral wheeze in preschool children: effect of topical nasal corticosteroid prophylaxis. Thorax 2003: 58: 431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Regamey N, Frey U, Deffernez C, Latzin P, Kaiser L. Isolation of human bocavirus from Swiss infants with respiratory infections. Pediatr Infect Dis J 2007: 26: 177–9. [DOI] [PubMed] [Google Scholar]
- 28. Frakking FN, Brouwer N, Zweers D, et al. High prevalence of mannose‐binding lectin (MBL) deficiency in premature neonates. Clin Exp Immunol 2006: 145: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thielens NM, Tacnet‐Delorme P, Arlaud GJ. Interaction of C1q and mannan‐binding lectin with viruses. Immunobiology 2002: 205: 563–74. [DOI] [PubMed] [Google Scholar]
- 30. Ip WK, Chan KH, Law HK, et al. Mannose‐binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis 2005: 191: 1697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kristensen IA, Thiel S, Steffensen R, Madhi S, Sorour G, Olsen J. Mannan‐binding lectin and RSV lower respiratory tract infection leading to hospitalization in children: a case‐control study from Soweto, South Africa. Scand J Immunol 2004: 60: 184–8. [DOI] [PubMed] [Google Scholar]
- 32. Roos A, Garred P, Wildenberg ME, et al. Antibody‐mediated activation of the classical pathway of complement may compensate for mannose‐binding lectin deficiency. Eur J Immunol 2004: 34: 2589–98. [DOI] [PubMed] [Google Scholar]
- 33. Frakking FN, Brouwer N, Van Eijkelenburg NK, et al. Low mannose‐binding lectin (MBL) levels in neonates with pneumonia and sepsis. Clin Exp Immunol 2007. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klein NJ. Mannose‐binding lectin: do we need it? Mol Immunol 2005: 42: 919–24. [DOI] [PubMed] [Google Scholar]
- 35. Kaur S, Gupta VK, Shah A, Thiel S, Sarma PU, Madan T. Plasma mannan‐binding lectin levels and activity are increased in allergic patients. J Allergy Clin Immunol 2005: 116: 1381–3. [DOI] [PubMed] [Google Scholar]
- 36. Leung TF, Tang NL, Sung YM, et al. Genetic association study between mbl2 and asthma phenotypes in Chinese children. Pediatr Allergy Immunol 2006: 17: 501–7. [DOI] [PubMed] [Google Scholar]
- 37. Aittoniemi J, Soranummi H, Rovio AT, et al. Mannose‐binding lectin 2 (MBL2) gene polymorphism in asthma and atopy among adults. Clin Exp Immunol 2005: 142: 120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol 2005: 23: 821–52. [DOI] [PubMed] [Google Scholar]
- 39. Walsh MC, Bourcier T, Takahashi K, et al. Mannose‐binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol 2005: 175: 541–6. [DOI] [PubMed] [Google Scholar]
- 40. Gomi K, Tokue Y, Kobayashi T, et al. Mannose‐binding lectin gene polymorphism is a modulating factor in repeated respiratory infections. Chest 2004: 126: 95–9. [DOI] [PubMed] [Google Scholar]


