Abstract
The species Rhus chinensis Mill. (Anacardiaceae) is an important representative of the genus Rhus, which contains over 250 individual species found in temperate and tropical regions worldwide. Rhus chinensis has long been used by folk medicine practitioners in Asia. Leaves, roots, stem, bark, fruit and particularly the galls on Rhus chinensis leaves, Galla chinensis, are recognized to have preventative and therapeutic effects on different ailments (such as diarrhea, dysentery, rectal and intestinal cancer, diabetes mellitus, sepsis, oral diseases and inflammation). However, it is critical to separate evidence from anecdote. Fortunately, recent scientific research has revealed that Rhus chinensis compounds possess strong antiviral, antibacterial, anticancer, hepatoprotective, antidiarrheal and antioxidant activities. Moreover, compounds isolated from the stem of Rhus chinensis significantly suppressed HIV‐1 activity in vitro. Compounds from this plant were also found to inhibit enamel demineralization in vitro and enhance remineralization of dental enamel with fluoride. This review highlights claims from traditional and tribal medicinal lore and makes a contemporary summary of phytochemical, biological and pharmacological findings on this plant material. It aims to show that the pharmaceutical potential of this plant deserves closer attention. Copyright © 2010 John Wiley & Sons, Ltd.
Keywords: Rhus chinensis, Galla chinensis, traditional medicine, pharmacology, antiviral, anticaries, triterpene, gallotannins
INTRODUCTION
Rhus chinensis belongs to the genus Rhus and the Family Anacardiaceae (Miller et al., 2001). Commonly called sumac, Rhus consists of approximately 250 individual species of flowering plants, with six species found (four endemics) in China. Like most sumacs, Rhus chinensis is a dioecious shrub that can reach 8 m in height. It bears odd pinnately compound leaves and creamy‐white flowers. The fruits (drupes) are orange or red in color at maturity and contain one seed (Barkley, 1937; Miller et al., 2001; Tianlu and Barfod, 2008). The species grows in areas with marginal agricultural capacity, and is widely distributed in temperate, subtropical, and tropical regions, including China, Japan, Malaysia, Taiwan and India (Rayne and Mazza, 2007; Ren et al., 2008).
The species Rhus chinensis has two distinct varieties, Rhus chinensis var. chinensis (syn. Rhus semialata; Rhus semialata var. osbeckii; Rhus osbeckii) and Rhus chinensis var. roxburghii (Syn. Rhus semialata var. roxburghii; Rhus javanica Linnaeus var. roxburghii; Rhus roxburghii) (Tianlu and Barfod, 2008; GRIN; TROPICOS).
Galla chinensis or Galla rhois is the term used to describe the gall caused by the Chinese aphid, Schlechtendalia chinensis (Bell), on the leaves of Rhus chinensis (Lee et al., 1997). This gall is widely used as a separate drug. Other species in this genus also produce galls that are considered to have an inferior quality.
A plethora of traditional medicine references claim curative power for Rhus chinensis, despite its widespread use, many of these claims of efficacy were not supported by scientific evidence, whether for traditional use validation or for drug development endeavors.
Fortunately, recent scientific research on Rhus chinensis has revealed promising health benefits, including anticancer, antiviral, antimicrobial, antidiarrheal and antiinflammatory properties (Yang et al., 2005; Gu et al., 2007; Ahn et al., 1998; Chen et al., 2009; Kim et al., 2005). In recent years, the Chinese herbal medicine Galla chinensis has been discussed widely as a new alternative for carious disease (Chu et al., 2007).
So far, no comprehensive review has been compiled from the literature encompassing the efficacy of this plant. Widespread claims of the medicinal effectiveness of various Rhus chinensis tree preparations motivated us to bridge the information gap in this area.
TRADITIONAL MEDICINAL USE
Among Rhus species, Rhus chinensis and its gall, Galla chinensis, have a long history of use by indigenous peoples for medicinal care and others. Numerous curative properties are ascribed to different parts of this tree, namely root, bark, stem, leaf, fruit, flowers, seed and gall (Table 1). The leaves and the root are used as depuratives, stimulating blood circulation. Its decoction is used in the treatment of hemoptysis, inflammations, laryngitis, snakebite, stomachache and traumatic fractures (Duke and Ayensu, 1985; Kao, 1988; Ouyang et al., 2008). The ripe fruits of this plant have long been used in Asia to treat dysentery and diarrhea, as well as other gastrointestinal disorders (Kala, 2005; Pradhan and Badola, 2008; Bose et al., 2008). The fruit produces a sour juice when boiled with water. This juice, when diluted with water or/and mixed with raw eggs, treats diarrhea and dysentery (Pradhan and Badola, 2008). It is used for the treatment of colic (Chopra et al., 1986) and also as a food preservative (Pradhan and Badola, 2008). The seed is used in the treatment of cough, dysentery, fever, jaundice, malaria and rheumatism (Duke and Ayensu, 1985; Abbasi et al., 2009).
Table 1.
Summary of traditional medicinal uses of Rhus chinensis
| Plant parts | Medicinal use | References |
|---|---|---|
| Leaves | Depurative, can stimulate blood circulation, hemoptysis, inflammations, laryngitis, stomachache , traumatic fractures, spermatorrhea, snake bite, antitussive, diarrhea | Duke and Ayensu, 1985; Kao, 1988; Ouyang et al., 2008; Xiao, 1989 |
| Fruits | Colic, diarrhea, dysentery, jaundice and hepatitis | Chopra et al., 1986;Tangpu and Yadav, 2004; Kala, 2005; Pradhan and Badola, 2008; Abbasi et al., 2009 |
| Seeds | Coughs, dysentery, fever, jaundice, hepatitis, malaria and rheumatism | Duke and Ayensu, 1985; Abbasi et al., 2009 |
| Root | Diarrhea, spermatorrhea, malaria, antitussives, treatments of anasarca, jaundice and snake bite | Kao, 1988; Xiao, 1989; Ouyang et al., 2008; Duke and Ayensu, 1985; Abbasi et al., 2009 |
| Galls | Diarrhea, diabetes mellitus, antiseptic, antiphlogistic, astringent, haemostatic, persistent cough with blood, spontaneous sweating, urorrhoea, bloody sputum, burns, hemorrhoids, oral diseases, fever, malaria, inflammation, toxicosis, sore, skin infections, rectal and intestinal cancer | Duke and Ayensu, 1985; Zhu, 1998; Hupkens et al., 1995; Tian et al., 2009a; Ho et al., 2002; Yeung, 1985; Gao et al., 2000; Tian et al., 2009; Kee and Walter, 1999 |
The gall of Rhus chinensis has long been considered to possess natural medicinal properties with numerous benefits (Zhang et al., 2009). Galla chinensis is used internally for its astringent properties to treat disease such as diarrhea and hemorrhage (Duke and Ayensu, 1985). It is a frequent ingredient in polyherbal prescriptions for diabetes mellitus (Duke and Ayensu, 1985). It has hemostatic effects, often used to promote clotting following traumatic injuries and to treat burns (Yeung, 1985). It is also used to treat rectal and intestinal cancer, prolapse of the rectum, seminal enuresis and hemorrhoids (Yeung, 1985; Gao et al., 2000). In addition to its antiphlogistic and antiseptic uses for treating diseases such as persistent cough, Galla chinensis also has antiinflammatory properties (Tian et al., 2009). It is also used to counteract ulcers in the mouth and to treat fever and malaria (Duke and Ayensu, 1985; Gao et al., 2000).
PHYTOCHEMISTRY
Phytochemical studies on Rhus species have been reported earlier and resulted in the characterization of several compound groups such as flavonoids (Taniguchi et al., 2000; Lee et al., 2005; Lin et al., 2008), triterpenoids (Kuo, 1991; Parveen, 1991; Lee et al., 2005), phenolics (Parveen and Khan, 1988; Lee et al., 2005; Ouyang et al., 2008), tannins (Takechi et al., 1985) and aromatic alkanes (Kuo et al., 1991; Lee et al., 2005; Ouyang et al., 2008).
The galls on Rhus chinensis leaves are rich in gallotannin (50–70%), a type of hydrolysable tannin (Kee and Walter, 1999; Xiao et al., 2000). Gallotannins from Galla chinensis consist of a central glucose core, which is surrounded by several gallic acid units, and further gallic acid units can be attached through depside bonding of additional galloyl residues. Structures containing 1 to 14 galloyl residues result from such processes, yielding tri‐, tetra‐, penta‐, hepta‐ and nonagalloylglucose, and others (Xiang et al., 2007; Tian et al., 2009b). Pentagalloylglucose [1], 3‐galloyl‐gallic acid and 4‐galloyl‐gallic acid isomers isolated from Galla chinensis are reported to be the primary bioactive gallotannins, possessing numerous medicinal activities and health benefits (An et al., 2005; Sakai et al., 1990; Bhimani et al., 1993; Feldman et al., 2001; Choi et al., 2002). Rhus chinensis is rich in well known phenolic compounds, gallic acid [2] and methyl gallate [3] (Ahn et al., 1998, 2005; Bae et al., 1998; Choi et al., 2009). According to Buziashvili et al. (1973) Galla chinensis is composed of nearly 20% gallic acid and 7% methyl gallate.
A new benzofuranone, 5‐hydroxy‐3‐(propan‐2‐ylidene)‐7‐(3,7,11,15‐tetramethylhexade‐ca‐ 2,6,10,11‐tetraenyl)‐2(3H)‐benzofuranone [4], together with 16 known bioactive compounds, including 5‐hydroxy‐7‐(3,7,11,15‐tetramethylhexadeca‐ 2,6,10,11‐tetraenyl)‐ 2(3H)‐benzofuranone [5], 3‐oxo‐6β‐hydroxyolean‐12‐en‐28‐oic acid [6], 3‐oxo‐6β‐hydroxyolean‐18‐en‐28‐oic acid [7] moronic acid [8], betulonic acid [9], gallicin [10], dihydroxytoluene [11] and dimethylcaffic acid [12], have been isolated from the root stem of Rhus chinensis (Gu et al., 2007; Wang et al., 2008).
Phenol glycosides and lariciresinol‐based ligan glycosides compounds have been shown to be present in the butanol extract of Rhus chinensis root (Ouyang et al., 2007, 2008). 6‐Pentadecylsalicylic acid, an antithrombotic compound [13] (Kuo, 1991) and fisetin (3,7,3‐,4‐tetrahydroxyflavone) [14] (Lee et al., 2005) an antiinflammatory, have also been isolated from the stem of Rhus chinensis. The leaves of this plant are rich in essential oils, with palmitic acid, phytol and n‐heptacosane as the major components (Zhu et al., 2007).
BIOLOGICAL AND PHARMACOLOGICAL PROPERTIES
Antibacterial activity
The high level of gallotannins along with phenolic compounds, gallic acid and methyl gallate, known antimicrobial agents make Galla chinensis very useful in bacterial control (Wu‐Yuan et al., 1988; Ahn et al., 1998; Kang et al., 2008; Tian et al., 2009a, 2009b). Extracts from Galla chinensis inhibited several bacteria such as Bacillus subtilis, B. cereus, Escherichia coli, Enterobacter cloacae, Helicobacter pylori, Klebsiella oxytoca, Lactobacillus casei, L. acidophilus, L. salivarius, Salmonella derby, S. minesota, S. typhimurium, S. enteritidis, Shigella dysenteriae, Staphylococcus aureus, Streptococcus mutans, S. sobrinus, Ureaplasma urealyticum, with the minimal inhibitory concentration (MIC) in the range 0.5–8 mg/mL (Wu‐Yuan et al., 1988, Bae et al., 1998; Choi II et al., 2002; Kang et al., 2008; Zhu et al., 2008; Choi et al., 2009; Tian et al., 2009a).
Tian et al. (2009b) reported that different gallotannins from Galla chinensis separated according to the number of galloylglucose had significant antibacterial activities on Bacillus cereus and Salmonella typhimurium. Structure activity relationship studies indicated that antibacterial activity was positively correlated with the numbers of galloyl groups and generally, gallotannins with higher molecular weights had strong antibacterial activities (Tian et al., 2009a, 2009b).
A methanol extract of Galla chinensis was shown to have significant growth‐inhibitory activity towards harmful intestinal bacteria (Ahn et al., 1994, 1998). Activity‐directed fractionation of the methanol extract of Galla chinensis has led to the isolation of gallic acid and its derivative methyl gallate as the major components involved in the observed antimicrobial activity.
It was also reported that methyl gallate and gallic acid from Galla chinensis had inhibitory effects on periodontopathic bacteria (MIC = 1 mg/mL) and significantly reduced the in vitro biofilm formation of S. mutans (methyl gallate, 1 mg/mL gallic acid, 4 mg/mL, p < 0.05) (Kang et al., 2008).
Antiviral activities
Anti‐HIV activity.
In a recent study, different fractions of Rhus chinensis showed potent anti‐HIV‐1 activity (Wang et al., 2006). Subsequent anti‐HIV guided fractionation of Rhus chinensis led to the isolation of 17 compounds with potent anti‐HIV‐1 activity (Gu et al., 2007; Wang et al., 2008). Among those compounds, a new class of benzofuranone‐type compounds 5‐hydroxy‐3‐(propan‐2‐ylidene)‐7‐(3,7,11,15‐tetramethylhexadeca‐2,6,10,11‐tetraenyl)‐2(3H)‐benzofuranone [4] and 5‐hydroxy‐7‐(3,7,11,15‐tetramethylhexadeca‐ 2,6,10,11‐tetraenyl)‐2(3H)‐benzofuranone [5] were found significantly to suppress HIV‐1 replication (Gu et al., 2007). Compound [4] possessed significant anti‐HIV‐1 activity with a therapeutic index (TI) of 42.40, whereas compound [5] showed moderate anti‐HIV‐1 activity with a TI of 3.28 (Gu et al., 2007). Furthermore, the action mechanisms of the two benzofuranone‐type compounds were investigated by Wang et al. (2008). These authors found that both compounds [4] and [5] inhibited HIV‐1 replication in chronically infected H9 cells and may target late‐stages of the HIV‐1 life cycle.
Betulonic acid [9] an analogue of betulinic acid, a well known anti‐HIV‐1 agent (Kashiwada et al., 1996; Soler et al., 1996) exhibited moderate anti‐HIV‐1 activity with a TI value of 5.27–8.94 µm (Gu et al., 2007; Wang et al., 2008).
3‐Oxo‐6β‐hydroxyolean‐12‐en‐28‐oic acid [6], 3‐oxo‐6β‐hydroxyolean‐18‐en‐28‐oic acid [7] and moronic acid [8] are oleanolic acid‐related triterpenes previously reported to have potential anti‐HIV‐1 activity (Pengsuparp et al., 1994; Soler et al., 1996; Kashiwada et al., 1998). These compounds showed weak anti‐HIV activity with TI values of 4.14, 4.74 and 8.22, respectively (Gu et al., 2007; Wang et al., 2008). Mengoni et al. (2002) described the anti‐HIV and the mechanism of action for oleanolic acid, both of which suggested that oleanolic acid inhibits HIV‐1 protease activity in vitro.
Gallicins [10], gallic acid derivate‐type compounds, have been reported to inhibit HIV‐1 integrase (Kim et al., 1998). The work of Wang et al. (2008) confirmed the result in cell lines with a therapeutic index of 5.11. Dihydroxytoluene [11] had the same extent of anti‐HIV‐1 activity with a TI of 5.34. Wang and coworkers also showed that dimethylcaffic acid [12], caffeic acid phenylethyl ester derivate, has potent anti‐HIV‐1 activity with a TI value of 19.07.
These values are relatively low compared with the control AZT (TI > 471883) but the resistance and the adverse side effects to available conventional anti‐HIV drugs beg the need of identification and development of additional small‐molecule inhibitors that can be used in combination with currently available antiviral agents.
Anti herpes simplex virus activity.
In vivo studies performed in mice have shown that the hot‐water extract of Rhus chinensis had prophylactic and therapeutic efficacy against herpes simplex virus (HSV) type 1 (HSV‐1) (Kurokawa et al., 1993, 1995a, 1995b, 1997). This extract was also effective against acyclovir‐resistant HSV‐1 and HSV type 2 (HSV‐2) infections in mice (Kurokawa et al., 1995b) and improved the therapeutic efficacy of acyclovir in mice infected with HSV‐1 (Kurokawa et al., 1995a).
Subsequently, Nakano et al. (1998) also investigated the efficacy of Rhus chinensis extract in vivo, using a guinea‐pig primarily infected intravaginally with HSV‐2. Prophylactic oral administration of Rhus chinensis at the dose corresponding to human use significantly reduced the incidence and severity of spontaneous skin lesions compared with latently infected guinea‐pigs administered water. When recurrent HSV‐2 infection was induced by ultraviolet irradiation 3 months after primary infection, prophylaxis with Rhus chinensis was also significantly effective in reducing the severity of ultraviolet‐induced skin lesions.
Two terpene compounds, moronic acid [8] and betulonic acid [9], were separated from Rhus chinensis and their subsequent anti‐HSV activities were assessed in vitro and in vivo (Kurokawa et al., 1998). The effective concentrations of moronic acid and betulonic acid for 50% plaque reduction for HSV‐1 were consecutively, 3.9 and 2.6 µg/mL. The therapeutic index of moronic acid (10.3–16.3) was larger than that of betulonic acid (6.2). Oral administration of moronic acid thrice a day to mice infected cutaneously with HSV‐1, significantly retarded the development of skin lesions and/or prolonged the mean survival of infected mice without toxicity compared with the control. Moronic acid exerted stronger anti‐HSV‐1 activity in the brain of HSV‐1‐infected mice than in the skin, similar to the hot‐water extract of Rhus chinensis (Kurokawa et al., 1995a, 1998).
Anti‐HCV and anti‐SARS‐CoV activities.
Screening a library of traditional medicines, Duan et al. (2004) found that the EtOAc extract fraction from Galla chinensis was efficient in inhibiting the NS3 protease activity of hepatitis carcinoma virus (HCV). 1,2,6‐Tri‐O‐galloyl‐β‐d‐glucose, 1,2,3,6‐tetra‐O‐galloyl‐β‐d‐glucose and pentagalloylglucose [1] were identified as the active compounds. Tri‐, tetra‐ and pentagalloylglucose inhibited HCV NS3 protease with IC50 of 1.89, 0.75 and 1.60 µm, respectively (Duan et al., 2004).
Likewise, tetra‐O‐galloyl‐β‐d‐glucose isolated from Galla chinensis exhibited prominent inhibition against severe acute respiratory syndrome coronavirus (SARS‐CoV) with a 50% effective concentration of 4.5 µm (Yi et al., 2004).
Anticariogenic activity
Liu et al. (2003) found that crude aqueous extract of Galla chinensis has the ability to inhibit enamel demineralization in vitro. In another study, Chu et al. (2007) evaluated the effects of compounds from Galla chinensis on the remineralization of initial enamel carious lesions using an in vitro pH cycling model. The group demonstrated the potential of three different fractions of Galla chinensis to affect net rehardening of artificial carious lesions under dynamic pH‐cyclic conditions. Furthermore, Zou et al. (2008), using the same protocol, demonstrated the potential of Galla chinensis extract to inhibit the demineralization of initial enamel carious lesions.
The chemical compounds of Galla chinensis showed effects and combined effects with fluoride on enhancing remineralization of dental enamel (Cheng et al., 2008).
At this point, the active compound of Galla chinensis involved in remineralization or demineralization is still unknown. Chu et al. (2007) isolated gallic acid [2] and methyl gallate [3], both of which showed poor activity compared with the crude extract. This result was confirmed by Cheng et al. (2008) testing the combined effects of Galla chinensis extract or gallic acid with fluoride on remineralization of artificial early enamel caries. They found that both the crude extract of Galla chinensis and gallic acid had synergistic effects with fluoride on remineralization, but with apparent differing mechanisms. Thus, it seemed that gallic acid was not the only possible active constituent of Galla chinensis to enhance remineralization. Zou et al. (2008) similarly attempted to determine which of the constituent chemical fractions of Galla chinensis conferred a potential anticaries benefit by comparing the effects of four different fractions of Galla chinensis on demineralization of a bovine enamel model. The crude extract was the most active one, prone to some losses of other active compounds during the separation process.
Antioxidant activity
Cai et al. (2004) screened 112 Chinese medicinal plants for antioxidant activity; the results showed that the aqueous extract of Galla chinensis contained the highest antioxidant concentration of 17674 µmol TEAC/100 g.
More recently, two similar studies have investigated the antioxidant activity of gallotannins in four different systems, namely 1,1‐diphenyl‐2‐picrylhydrazyl (DPPH) radical scavenging, ferric reducing antioxidant power, β‐carotene linoleic acid system and hydroxyl radical scavenging assays. Tian et al. (2009a) tested the antioxidant activity of gallotannins with different polarities and found that all of the consecutive extracts of Galla chinensis possessed remarkable antioxidant activity. For example, DPPH radical scavenging activity, EC50 were in the sequence ethyl acetate (1.22 µg/mL) > ether (1.44 µg/mL) > ethanol (1.55 µg/mL) > water (2.11 µg/mL). Generally, all fractions showed better capacity to scavenge free radicals than the controls, BHT and TROLOX. The same results trend was observed with ferric reducing activity. Antioxidant activity increased when the polarity of extracts decreased, suggesting that extracts with weaker polarities contained higher molecular weight tannins, and thus had stronger antioxidant effects. Aware of this finding, (Tian et al. 2009b) isolated different gallotannins, containing 1–10 galloylglucoses (GG), from Galla chinensis and investigated their antioxidant activities in the above systems. Generally, gallotannins of high degrees of galloylation (5–10 GGs) had stronger antioxidant activities than those of low degrees of galloylation (1–4 GGs).The same conclusion was drawn in earlier work by Yokozawa et al. (1998).
Similarly, methyl gallate and gallic acid have been shown through in vivo and in vitro studies to have antioxidant and radical scavenging activity (Chen and Zhang, 2003; Whang et al., 2005; Madsen and Bertelsen, 1995; Peyrat‐Maillard et al., 2000).
It has been demonstrated that pentagalloylglucose possesses antioxidant activity and protects rat neuronal cells from oxidative damage (Choi et al., 2002; Feldman et al., 2001; Oh et al., 2001; Pan et al., 1999). Piao et al. (2009) showed that pentagalloylglucose exerts antiapoptotic activity through antioxidant properties.
Anticancer activity
Yang et al. (2005) were the first to report the anticancer activity of Rhus chinensis extract on carcinogenic Cdc25 phosphatases. Several molecules found in Rhus chinensis such as pentagalloylglucose and gallic acid have been shown to have anticancer activity (Bhimani et al., 1993; Madsen and Bertelsen, 1995; Chung et al., 1998; Hu et al., 2008; Kuo et al., 2009). Pentagalloylglucose has been shown to exhibit in vivo anticancer effects against prostate cancer (Hu et al., 2008; Kuo et al., 2009), lung cancer (Huh et al., 2005) and sarcoma (Miyamoto et al., 1987), and in vitro inhibitory effects on the growth and/or invasion of breast cancer, leukemia, melanoma and liver cancer (Zhang et al., 2009). Pentagalloylglucose can exert anticancer activity via the inhibition of angiogenesis (Lee et al., 2004; Huh et al., 2005) and invasion of melanoma cells in metastasis (Ho et al., 2002). In vitro studies showed that pentagalloylglucose significantly inhibited the proliferation and tube formation of bFGF‐treated human umbilical vein endothelial cells (HUVEC) with an IC50 of 8 µm (Huh et al., 2005). The result is similar to the in vitro antiangiogenic activity of pentagalloylglucose in VEGF‐treated HUVECs (Lee et al., 2004). Daily injection of 4 and 20 mg/kg of pentagalloylglucose significantly inhibited the growth of the highly angiogenesis‐dependent Lewis Lung Cancer allograft by 57% and 91%, respectively (Huh et al., 2005). Similarly, pentagalloylglucose inhibited the invasion of highly metastatic mouse melanoma B16F10 cells in vitro in a dose‐ and time‐dependent manner, with IC50 of 15 µm (Ho et al., 2002).
Some other investigations have also demonstrated that derivatives of galloylglucose inhibit not only cancer cell growth (Pan et al., 1999; Hu et al., 2008) but also the invasion of HT1080 human fibrosarcoma cells (Ata et al., 1996).
Hepatoprotective activity
Several studies of natural hepatoprotective agents have revealed that the extract of Galla chinensis showed promising hepatoprotective activity (Oh et al., 2002; Tian et al., 2005). Based on an activity‐guided separation scheme An et al. (2005) purified pentagalloylglucose [1] and an equilibrium mixture of 3‐galloyl‐gallic acid and 4‐galloyl‐gallic acid isomer from the methanol extract of Galla chinensis and validated their hepatoprotective activity. Pentagalloylglucose [1] and the mixture compounds were found to have marked protective effects on tacrine‐induced cytotoxicity in human liver‐derived Hep G2 cells with EC50 values of 70.39 ± 5.4 and 29.51 ± 0.7 µm, respectively, and also inhibited nitrofurantoin‐induced cytotoxicity in Hep G2 cells at 150.9 ± 6.4 and 23.81 ± 0.5 µm respectively.
Furthermore, pentagalloylglucose treatment was able to reduce both hepatocyte necrosis induced by tert‐butyl hydroperoxide (4 and 20 µm) and apoptosis induced by glycochenodeoxycholic acid (3.125 to 50 µm) in primary rat hepatocytes (Park et al., 2008).
Antidiabetic activity
Diabetes mellitus is a chronic metabolic disorder characterized by high blood glucose level due to agents such as α‐glucosidase enzyme, which boosts the digestion of carbohydrate to monosaccharides in the process of intestinal absorption.
Therefore, Shim et al. (2003) tested the inhibitory effect of an aqueous extract from the gall of Rhus chinensis on α‐glucosidase activity in in vitro and in vivo models. Galla chinensis inhibited Bacillus α‐glucosidase activity with an IC50 of 0.9 µg/mL. Its inhibition on α‐glucosidase was determined to be noncompetitive and reversible when the enzyme–substrate mixture was simultaneously treated with Galla chinensis. Galla chinensis significantly suppressed the increase of blood glucose level in rats after oral administration of sucrose. These results suggest that Galla chinensis might exert antidiabetic effects by suppressing carbohydrate absorption from the intestine and thereby reducing the postprandial increase in the blood glucose.
Likewise, tannic acid, a mixture of gallotannins containing pentagalloylglucose, was found to have a hypoglycemic effect in patients with type 2 diabetes (Gin et al., 1999). Aware of this result, Li and coworkers hypothesized that pentagalloylglucose could have antidiabetic activity. Using synthetic pentagalloylglucose in vitro and in an animal assay, it was demonstrated that pentagalloylglucose effectively reduced blood glucose and insulin levels in vitro and in animal models (Li et al., 2005). Unlike most antidiabetic drugs, pentagalloylglucose may reduce blood glucose without increasing adiposity.
Antidiarrheal activity
The methanol extract of the dried ripe fruit of Rhus chinensis was tested in experimental models of castor oil‐induced diarrhea in Swiss albino mice (Tangpu and Yadav, 2004; Bose et al., 2008). At graded doses, the extract showed remarkable antidiarrheal activity evidenced by an 80.70% reduction in the rate of defecation of control animals at a dose of 600 mg/kg body weight. The extract also reduced intestinal fluid secretion induced by MgSO4 and gastrointestinal motility after charcoal meal administration in albino mice (Tangpu and Yadav, 2004).
In the same way, Galla chinensis extracts were found to be effective against enterotoxigenic Escherichia coli‐induced diarrhea that produces a heat‐labile enterotoxin (LT), which binds to the ganglioside GM1 on the surface of intestinal epithelial cells (Holmgren and Svennerholm, 1992) leading to a massive loss of fluids and ions from cells (Chen et al., 2009). Using the patent mouse gut assay in vivo study, Chen et al. (2006) found that Galla chinensis extract exhibited an anti‐LT‐induced diarrheal effect, with an IC50 value of 4.7 ± 1.3 mg/mL. Competitive GM1‐ELISA assay showed that Galla chinensis suppressed (IC50 = 0.17 ± 0.02 mg/mL) LT‐induced fluid accumulation by blocking the binding of LTB to GM1. Thin layer chromatography suggests that the most active fraction that inhibited the binding of LTB to GM1 was composed of mainly phenolics, especially gallic acid which significantly blocked the binding of LTB to GM1, with an IC50 value of 10.9 ± 0.3 mm, and suppressed the LT‐induced fluid accumulation in a dose‐dependent manner, with an IC50 value of 25.4 ± 11.6 mm.
Antiinflammatory and antithrombin activities
The work of Kim et al. (2005) showed that Galla chinensis had antiinflammatory activity in in vivo and in vitro models. Galla chinensis could control all of the inflammatory mediators, such as histamine, heparin, lipid‐derived mediators and various cytokines in the model of immediate‐type allergic reaction in a dose‐dependent manner through different mechanisms. Latter activity‐guided fractionation and purification of the EtOAc fractions of the Galla chinensis indicated that the main antiallergic component in Galla chinensis was gallic acid.
Fisetin a flavonoid found in the root of Rhus chinensis (Lin et al., 2008) was also found to down‐regulate inflammatory reactions in stimulated human mast cells (Park et al., 2007).
Similarly, pentagalloylglucose has been shown through in vivo and in vitro studies to exercise a strong antiinflammatory effect (Oh et al., 2004; Lee et al., 2007, 2003; Kang et al., 2005).
6‐Pentadecylsalicylic acid has been isolated from air‐dried stems of Rhus chinensis by bioassay‐directed fractionation of the n‐hexane extract of the stem (Kuo, 1991). This compound showed antithrombotic activity at 50 µg/mL using the amidolytic method (Kuo, 1991). It also prolonged clotting time in a dose‐dependent manner in the clotting assay of thrombin–fibrinogen interaction.1
Figure 1.
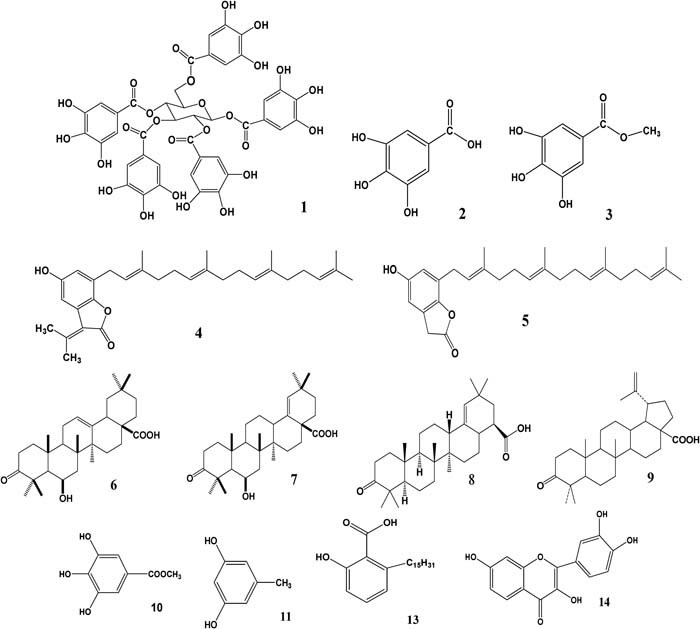
Structures of selected phytochemicals from Rhus chinensis and Galla chinensis: pentagalloylglucose [1], gallic acid [2], methyl gallate [3], 5‐hydroxy‐3‐(propan‐2‐ylidene)‐7‐(3,7,11,15‐tetramethylhexade‐ca‐2,6,10,11‐tetraenyl)‐2(3H)‐benzofuranone [4], 5‐hydroxy‐7‐(3,7,11,15‐tetramethylhexadeca‐2,6,10,11‐tetraenyl)‐2(3H)‐benzofuranone [5], 3‐oxo‐6β‐hydroxyolean‐12‐en‐28‐oic acid [6], 3‐oxo‐6β‐hydroxyolean‐18‐en‐28‐oic acid [7], moronic acid [8],betulonic acid [9], gallicin [10], dihydroxytoluene [11], dimethylcaffic acid [12], fisetin [13], 6‐pentadecylsalicylic acid [14].
CONCLUSIONS
Rhus chinensis species have long been recognized by folk medicine practitioners as having value and have been revealed to have great medicinal potential, much of which was completely unknown to Western scientists. Over the past few decades, the research efforts on Rhus chinensis extracts indicate that the extracts have promising potential as antiviral, anticarie, antidiarrheal, anticancer, antidiabetic and hepatoprotective agents, among others. Although the work reviewed here substantiated most of the traditional claims on its health effectiveness, more research is required for validation of the uses of this plant.
The available information on the different bioactive contents in samples of various parts of this medicinal plant is very limited, both qualitatively and quantitatively. The gall on Rhus chinensis leaves has received much scientific attention because of its high gallotannin content and subsequent health potential. However, other parts, such as fruits, leaves, and seeds, can also be investigated based on traditional uses and the findings in other Rhus species.
So far, Galla chinensis is the only medicine proven to remineralize a hard tissue like enamel. This is a unique potential for this plant, but the active constituent is still unknown.
The mechanistic activity of Rhus chinensis material as prophylactic, therapeutic, anti‐HSV, anti‐HIV and anti‐diarrheal medicine needs to be further examined. On the other hand, efforts should also be made to survey other sumac species to determine if these properties are generalized across the Rhus genus.
The safety of Rhus chinensis still needs to be rigorously established, since cases of toxicity from intake of gallotannins found in Rhus chinensis have been reported in the literature. Different gallotannins such as tri‐, tetra‐, hexa‐, hepta‐, octa‐, nona‐ and decagalloylglucose can reduce blood pressure and blood urea nitrogen, as reported in animal studies in the literature (Feldman et al., 1999; Hofmann et al., 2006; Nishizawa et al., 1983). Nonetheless, tannins diminish protein digestibility when present in high levels in diets with low protein content and also inhibit human salivary α‐amylase, thereby causing potential negative effects on starch digestion and food taste. This needs to be taken in account when testing the efficacy of Rhus chinensis compounds in human beings by clinical trials for drug use validation.
Acknowledgements
Support was provided by the Chinese government research funds of 111 project‐B07029, PCSIRT0627 and 2008BAD91B04‐2.
REFERENCES
- Abbasi AM, Khan MA, Ahmad M et al. 2009. Medicinal plants used for the treatment of jaundice and hepatitis based on socio‐economic documentation. Afr J Biotechnol 8: 1643–1650. [Google Scholar]
- Ahn YJ, Kwon JH, Chae SH, Park JH, Yoo JY. 1994. Growth‐inhibitory responses of human intestinal bacteria to extracts of Oriental medicinal plants. Microbiol Ecol Health Dis 7: 257–261 [Google Scholar]
- Ahn YJ, Lee CO, Kweon JH, Ahn JW, Park JH. 1998. Growth‐inhibitory effects of Galla Rhois‐derived tannins on intestinal bacteria. J Appl Microbiol 84: 439–443. [DOI] [PubMed] [Google Scholar]
- Ahn YJ, Lee HS, Oh HS, Kim HT, Lee YH. 2005. Antifungal activity and mode of action of Galla Rhois‐derived phenolics against phytopathogenic fungi. Pestic Biochem Physiol 81: 105–112. [Google Scholar]
- An RB, OH H, Kim YC. 2005. Phenolic Constituents of Galla Rhois with hepatoprotective effects on tacrine‐ and nitrofurantoin‐induced cytotoxicity in Hep G2 cells. Biol Pharm Bull 28: 2155–2157. [DOI] [PubMed] [Google Scholar]
- Ata N, Oku T, Hattori M et al. 1996. Inhibition by galloylglucose (GG6‐10) of tumor invasion through extracellular matrix and gelatinase‐mediated degradation of type IV collagens by metastatic tumor cells. Oncol Res 8: 503–511. [PubMed] [Google Scholar]
- Bae EA, Han MJ, Kim NJ, Kim DH. 1998. Anti‐Helicobacter pylori activity of herbal medicines. Biol Pharm Bull 21: 990–992. [DOI] [PubMed] [Google Scholar]
- Barkley FA. 1937. A monographic study of Rhus and its immediate allies in North and Central America, including the West Indies. Ann Mo Bot Gard 24: 265–499. [Google Scholar]
- Bhimani RS, Troll W, Grunberger D, Frenkel K. 1993. Inhibition of oxidative stress in HeLa cells by chemopreventive agents. Cancer Res 53: 4528–4533. [PubMed] [Google Scholar]
- Bose SK, Dewanjee S, Gupta AS, Samanta KC, Kundu M, Mandal SC. 2008. In vivo evaluation of antidiarroeal activity of Rhus semilata fruit extract in rat. Afr J Tradit Complement Altern Med 5: 97–102. [PMC free article] [PubMed] [Google Scholar]
- Buziashvili IS, Komissarenko NF, Kovalev IP, Gordienko VG, Kolesnikov DG. 1973. The structure of gallotanins. Chem Nat Comp 9: 752–755. [Google Scholar]
- Cai Y, Luo Q, Sun M, Corke H. 2004. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74: 2157–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Zhang L. 2003.The antioxidant (‐)‐epigallocatechin‐3‐gallate inhibits rat hepatic stellate cell proliferation in vitro by blocking the tyrosine phosphorylation and reducing the gene expression of platelet‐derived growth factor‐α receptor. J Biol Chem 278: 23381–23389. [DOI] [PubMed] [Google Scholar]
- Chen JC, Ho TY, Chang YS, Wu SL, Hsiang CY. 2006. Anti‐diarrheal effect of Galla Chinensis on the Escherichia coli heat‐labile enterotoxin and ganglioside interaction. J Ethnopharmacol 103: 385–391. [DOI] [PubMed] [Google Scholar]
- Chen JC, Ho TY, Chang YS, Wu SL, Li CC, Hsiang CY. 2009. Identification of Escherichia coli enterotoxin inhibitors from traditional medicinal herbs by in silico, in vitro, and in vivo analyses. J Ethnopharmacol 121: 372–378. [DOI] [PubMed] [Google Scholar]
- Cheng L, Li JY, Hao YQ, Zhou XD. 2008. Effect of compounds of Galla chinensis and their combined effects with fluoride on remineralization of initial enamel lesion in vitro . J Dent 36: 369–373. [DOI] [PubMed] [Google Scholar]
- Choi BM, Kim HJ, Oh GS et al. 2002. 1,2,3,4,6‐Penta‐O‐galloyl‐beta‐D‐glucose protects rat neuronal cells (Neuro 2A) from hydrogen peroxide mediated cell death via the induction of heme oxygenase‐1. Neurosci Lett 328: 185–189. [DOI] [PubMed] [Google Scholar]
- Choi II, Chang HS, Young MY, Joo CM. 2002. Antimicrobial activity of medicinal herbs against Staphylococcus aureus and Salmonella gallinarum . San'oeb misaengmul haghoeji 30: 177–183. [Google Scholar]
- Choi JG, Kang OH, Lee YS et al. 2009. Antibacterial Activity of Methyl Gallate Isolated from Galla rhois or Carvacrol Combined with Nalidixic Acid against Nalidixic Acid Resistant Bacteria. Molecules 14: 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra RN, Nayar SL, Chopra IC. 1986. Glossary of Indian Medicinal Plants (Including the Supplement). Council of Scientific and Industrial Research: New Delhi. [Google Scholar]
- Chu JP, Li JY, Hao YQ, Zhou XD. 2007. Effect of compounds of Galla chinensis on remineralisation of initial enamel carious lesions in vitro . J Dent 35: 383–387. [DOI] [PubMed] [Google Scholar]
- Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. 1998. Tannins and human health: A review. Crit Rev Food Sci Nutr 38: 421–464. [DOI] [PubMed] [Google Scholar]
- Duan D, Li Z, Luo H, Zhang W, Chen L, Xu X. 2004. Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorgan Med Chem Lett 14: 6041–6044. [DOI] [PubMed] [Google Scholar]
- Duke JA, Ayensu ES. 1985. Medicinal Plants of China. Reference Publications, Inc., Algonac, Michigan. [Google Scholar]
- Feldman KS, Sahasrabudhe KS, Lawlor MD, Wilson SL, Lang CH, Scheuchenzuber WJ. 2001. In vitro and in vivo inhibition of LPS‐stimulated tumor necrosis factor‐a secretion by the gallotannin β‐D‐pentagalloylglucose. Bioorg Med Chem Lett 11: 1813–1815. [DOI] [PubMed] [Google Scholar]
- Feldman KS, Sahasrabudhe K, Smith RS, Scheuchenzuber WJ. 1999. Immunostimulation by plant polyphenols: a relationship between tumor necrosis factor‐alpha production and tannin structure. Bioorg Med Chem Lett 9: 985–990. [DOI] [PubMed] [Google Scholar]
- Gao XM, Xu ZM, Li ZW. 2000. Traditional Chinese Medicines. People's Health Publishing House: Beijing, 263–266. [Google Scholar]
- Gin H, Rigalleau V, Caubet O, Masquelier J, Aubertin J. 1999. Effects of red wine, tannic acid, or ethanol on glucose tolerance in non‐insulin‐dependent diabetic patients and on starch digestibility in vitro . Metabolism 48: 1179–1183. [DOI] [PubMed] [Google Scholar]
- GRIN : Germplasm Resources Information Network – (GRIN). USDA, ARS, National Genetic Resources Program. 12 November 2009. Online [database] http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?31679
- Gu Q, Wang RR, Zhang XM et al. 2007. A new benzofuranone and anti‐HIV constituents from the stems of Rhus chinensis . Planta Med 73: 279–282. [DOI] [PubMed] [Google Scholar]
- Ho LL, Chen WJ, Lin‐Shiau SY, Lin JK. 2002. Penta‐O‐galloyl‐β‐D‐glucose inhibits the invasion of mouse melanoma by suppressing metalloproteinase‐9 through down‐regulation of activator protein‐1. Eur J Pharmacol 453: 149–158. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Glabasnia A, Schwarz B, Wisman KN, Gangwer KA, Hagerman AE. 2006. Protein binding and astringent taste of a polymeric procyanidin, 1,2,3,4,6‐penta‐O‐galloyl‐beta‐D‐glucopyranose, castalagin, and grandinin. J Agric Food Chem 54: 9503–9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J, Svennerholm AM. 1992. Bacterial enteric infections and vaccine development. Gastroenterol Clin North Am 21: 283–302. [PubMed] [Google Scholar]
- Hu H, Lee HJ, Jiang C et al. 2008. Penta‐1,2,3,4,6‐O‐galloyl‐beta‐D‐glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo . Mol Cancer Ther 7: 2681–2691. [DOI] [PubMed] [Google Scholar]
- Huh JE, Lee EO, Kim MS et al. 2005. Penta‐O‐galloyl‐beta‐D‐glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: roles of cyclooxygenase‐2 and mitogen‐activated protein kinase pathways. Carcinogenesis 26: 1436–1445. [DOI] [PubMed] [Google Scholar]
- Hupkens P, Boxma H, Dokter J. 1995. Tannic acid as a topical agent in burns: historical considerations and implications for new developments. Burns 21: 57–61. [DOI] [PubMed] [Google Scholar]
- Kala CP. 2005. Ethnomedicinal botany of the Apatani in the Eastern Himalayan region of India. J Ethnobiol Ethnomed 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DG, Moon MK, Choi DH, Lee JK, Kwon TO, Lee HS. 2005. Vasodilatory and anti‐inflammatory effects of the 1,2,3,4,6‐penta‐O‐galloyl‐beta‐D‐glucose via a nitric oxide‐cGMP pathway. Eur J Pharmacol 524: 111–119. [DOI] [PubMed] [Google Scholar]
- Kang MS, Oh JS, Kang IC, Homg SJ, Choi CH. 2008. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J Microbiol 46: 744–750. [DOI] [PubMed] [Google Scholar]
- Kao MT. 1988. Popular Herbal Remedies of Taiwan (2); SMC Publishing Inc.: Taipei, Taiwan, 91. [Google Scholar]
- Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. 1996. Betulinic acid and dihydrobetulinic acid derivatives as potent anti‐HIV agents. J Med Chem 39: 1016–1017. [DOI] [PubMed] [Google Scholar]
- Kashiwada Y, Wang HK, Nagao T et al. 1998. Anti‐AIDS agents. 30. Anti‐HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J Nat Prod 61: 1090–1095. [DOI] [PubMed] [Google Scholar]
- Kee CH, Walter MW. 1999. The Pharmacology of Chinese Herbs, 2nd edn. CRC Press LLC, Boca Raton, FL; 239. [Google Scholar]
- Kim HJ, Woo ER, Shin CG, Park H. 1998. A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity against human immunodeficiency virus‐1 (HIV‐1) integrase. J Nat Prod 61: 145–148. [DOI] [PubMed] [Google Scholar]
- Kim SH, Park HH, Lee S et al. 2005. The anti‐anaphylactic effect of the gall of Rhus javanica is mediated through inhibition of histamine release and inflammatory cytokine secretion. Int Immunopharmacol 5: 1820–1829. [DOI] [PubMed] [Google Scholar]
- Kuo CM, Teng LG, Lee TH et al. 1991. 6‐Pentadecylsalicylic acid: an antithrombin component isolated from the stem of Rhus semialata var roxburghii . Planta Med 57: 247–249. [DOI] [PubMed] [Google Scholar]
- Kuo PT, Lin TP, Liu LC et al. 2009. Penta‐O‐galloyl‐beta‐D‐glucose suppresses prostate cancer bone metastasis by transcriptionally repressing EGF‐induced MMP‐9 expression. J Agric Food Chem 57: 3331–3339. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Hozumi T, Basnet P et al. 1998. Purification and characterization of eugeniin as an anti‐herpes virus compound from Geum japonicum and Syzygium aromaticum. J Pharmacol Exp Ther 284: 728–735. [PubMed] [Google Scholar]
- Kurokawa M, Nagasaka K, Hirabayashi T et al. 1995a. Efficacy of traditional herb medicines in combination with acyclovir against herpes simplex virus type 1 infection in vitro and in vivo. Antivir Res 27: 19–37. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Nakano M, Ohyama H, Hozumi T, Namba T, Shiraki K. 1997. Prophylactic efficacy of traditional herbal medicines against recurrent herpes simplex virus type 1 infection from latently infected ganglia in mice. J Dermatol Sci 14: 76–84. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Ochiai H, Nagasaka K et al. 1993. Antiviral traditional medicines against herpes simplex virus (HSV‐1), poliovirus, and measles virus in vitro and their therapeutic efficacies for HSV‐1 infection in mice. Antivir Res 22: 175–188. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato H, Ohyama H et al. 1995b. Effects of traditional herb medicines against herpes simplex virus (HSV) type 2 and acyclovir‐resistant HSV type1 in vitro and in vivo. J Tradit Med 12: 187–194. [Google Scholar]
- Lee SM, Lee DW, Park JD, Kim JI. 1997. Study on formation and development of gall in Rhus javanica . Korean J Appl Entomol 36: 83. [Google Scholar]
- Lee SH, Park HH, Kim JE et al. 2007. Allose gallates suppress expression of pro‐inflammatory cytokines through attenuation of NF‐kappaB in human mast cells. Planta Med 73: 769–773. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee HM, Ji ST et al. 2004. 1, 2, 3, 4, 6‐Penta‐O‐galloyl‐beta‐D‐glucose blocks endothelial cell growth and tube formation through inhibition of VEGF binding to VEGF receptor. Cancer Lett 208: 89–94. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lee IS, Mar W. 2003. Inhibition of inducible nitric oxide synthase and cyclooxygenase‐2 activity by 1,2,3,4,6‐penta‐Ogalloyl‐beta‐D‐glucose in murine macrophage cells. Arch Pharmacal Res 26: 832–839. [DOI] [PubMed] [Google Scholar]
- Lee TH, Chiou JL, Lee CK, Kuo YH. 2005. Separation and determination of chemical constituents in the roots of Rhus javanica L. var. roxburghiana. J Chin Chem Soc 52: 833–841. [Google Scholar]
- Li Y, Kim J, Li J et al. 2005. Natural anti‐diabetic compound 1,2,3,4,6‐penta‐O‐galloyl‐D‐glucopyranose binds to insulin receptor and activates insulin‐mediated glucose transport signaling pathway. Biochem Biophys Res Commun 336: 430–437. [DOI] [PubMed] [Google Scholar]
- Lin CN, Chen HL, Yen MH. 2008. Flavonoids with DNA strand‐scission activity from Rhus javanica var. roxburghiana. Fitoterapia 79: 32–36. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Liu TJ, Li JY, Zhou XD, Zhang J. 2003. The effect of Galla chinensis on the demineralization of enamel. Sichuan Da Xue Xue Bao Yi Xue Ban (Chinese) 34: 507–509. [PubMed] [Google Scholar]
- Madsen HL, Bertelsen G. 1995. Spices as antioxidants. Trends Food Sci Technol 6: 271–277. [Google Scholar]
- Mengoni F, Lichtner M, Battinelli L et al. 2002. In vitro anti‐HIV activity of oleanolic acid on infected human mononuclear cells. Planta Med 68: 106–115. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Young DA, Wen J. 2001. Phylogeny and biogeography of Rhus (Anacardiaceae) based on ITS sequences data. Int J Plant Sci 162: 1401–1407. [Google Scholar]
- Miyamoto K, Kishi N, Koshiura R, Yoshida T, Hatano T, Okuda T. 1987. Relationship between the structures and the antitumor activities of tannins. Chem Pharm Bull 35: 814–822. [DOI] [PubMed] [Google Scholar]
- Nakano M, Kurokawa M, Hozumi T et al. 1998. Suppression of recurrent genital herpes simplex virus type 2 infection by Rhus javanica in guinea pigs. Antivir Res 39: 25–33. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Yamagishi T, Nonaka G, Nishioka I, Nagasawa T, Oura H. 1983. Tannins and related compounds. XII. Isolation and characterization of galloylglucoses from Paeoniae Radix and their effects on urea‐nitrogen concentration in rat serum. Chem Pharm Bull 31: 2593–2600. [DOI] [PubMed] [Google Scholar]
- Oh GS, Pae HO, Choi BM et al. 2004. Penta‐O‐galloyl‐β–D‐glucose inhibits phorbol myristate acetate‐induced interleukin‐8 [correction of intereukin‐8] gene expression in human monocytic U937 cells through its inactivation of nuclear factor‐kappaB. Int Immunopharmacol 4: 377–386. [DOI] [PubMed] [Google Scholar]
- Oh GS, Pae HO, Oh H et al. 2001. In vitro anti‐proliferative effect of 1,2,3,4,6‐penta‐O‐galloyl‐β‐D‐glucose on human hepatocellular carcinoma cell line, SK‐HEP‐1 cells. Cancer Lett 174: 17–24. [DOI] [PubMed] [Google Scholar]
- Oh H, Kim JS, Song EK et al. 2002. Sesquiterpenes with hepatoprotective activity from Cnidium monnieri on tacrine‐induced cytotoxicity in Hep G2 cells. Planta Med 68: 748–749. [DOI] [PubMed] [Google Scholar]
- Ouyang MA, Chang CI, Wein YS, Kuo YH. 2007. Four new lariciresinol‐based lignan glycosides from the roots of Rhus javanica var. roxburghiana. Helv Chim Acta 90: 1099–1106. [Google Scholar]
- Ouyang MA, Chang CI, Wein YS, Kuo YH. 2008. New phenol glycosides from the roots of Rhus javanica var. roxburghiana. J Chin Chem Soc 55: 223–227. [Google Scholar]
- Pan MH, Lin JH, Lin‐Shiau SY, Lin JK. 1999. Induction of apoptosis by penta‐O‐galloyl‐beta‐D‐glucose through activation of caspase‐3 in human leukemia HL‐60 cells. Eur J Pharmacol 381: 171–183. [DOI] [PubMed] [Google Scholar]
- Park HH, Lee S, Oh J et al. 2007. Anti‐inflammatory activity of fisetin in human mast cells (HMC‐1). Pharmacol Res 55: 31–37. [DOI] [PubMed] [Google Scholar]
- Park EJ, Zhao YZ, An RB, Kim, YC , Sohn DH. 2008. 1,2,3,4,6‐penta‐O‐galloyl‐β‐D‐glucose from Galla Rhois protects primary rat hepatocytes from necrosis and apoptosis. Planta Med 74: 1380–1383. [DOI] [PubMed] [Google Scholar]
- Parveen N, Khan NUD. 1988. Phenolic constituents from leaves of Rhus semialata . J Indian Chem Soc 65: 737–738. [Google Scholar]
- Parveen N, Singh MP, Khan NU et al. 1991. Semialatic acid, a triterpene from Rhus semialata. Phytochemistry 30: 2415–2416. [Google Scholar]
- Pengsuparp T, Cai LN, Fong HHS et al. 1994. Pentacyclic triterpenes derived from Maprouena Africana are potent inhibitors of HIV‐1 reverse transcriptase. J Nat Prod 57: 415–522. [DOI] [PubMed] [Google Scholar]
- Peyrat‐Maillard MN, Bonnely S, Berset C. 2000. Determination of the antioxidant activity of phenolic compounds by coulometric detection. Talanta 51: 709–716. [DOI] [PubMed] [Google Scholar]
- Piao MJ, Kang KA, Zhang R et al. 2009. Antioxidant properties of 1,2,3,4,6‐penta‐O‐galloyl‐β‐D‐glucose from Elaeocarpus sylvestris var. ellipticus. Food Chem 115: 412–418. [Google Scholar]
- Pradhan BK, Badola HK. 2008. Ethnomedicinal plant use by Lepcha tribe of Dzongu valley, bordering Khangchendzonga Biosphere Reserve, in North Sikkim, India. J Ethnobiol Ethnomed 4: 22. Online at http://www.ethnobiomed.com/content/4/1/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayne S, Mazza G. 2007. Biological activities of extracts from Sumac (Rhus spp.): A review. Plant Foods Human Nutr 62: 165–175. [DOI] [PubMed] [Google Scholar]
- Ren Z, Zhu B, Wang D, Ma E, Su D, Zhong Y. 2008. Comparative population structure of Chinese sumac aphid Schlechtendalia chinensis and its primary host‐plant Rhus chinensis . Genetica 132: 103–112. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Nagase H, Ose Y et al. 1990. Inhibitory action of peony root extract on the mutagenicity of benzo[a]pyrene. Mutat Res 244: 129–134. [DOI] [PubMed] [Google Scholar]
- Shim YJ, Doo HK, Ahn SY et al. 2003. Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha‐glucosidase activity and postprandial blood glucose. J Ethnopharmacol 85: 283–287. [DOI] [PubMed] [Google Scholar]
- Soler F, Poujade C, Evers M et al. 1996. Betulinic acid derivatives: A new class of specific inhibitors of human immunodeficiency virus type 1 entry. J Med Chem 39: 1069–1083. [DOI] [PubMed] [Google Scholar]
- Takechi M, Tanaka Y, Takehara M, Nonaka GI, Nishioka I. 1985. Structure and antileherpetic activity among the tannins. Phytochemistry 24: 2245–2250. [Google Scholar]
- Tangpu V, Yadav AK. 2004. Antidiarrhoeal activity of Rhus javanica ripen fruit extract in albino mice. Fitoterapia 75: 39–44. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Yazaki K, Ryoko YU et al. 2000. Galloylglucoses and riccionidin A in Rhus javanica adventitious root cultures. Phytochemistry 53: 357–364. [DOI] [PubMed] [Google Scholar]
- Tian F, Li B, Ji B et al. 2009a. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food Chem 113: 173–179. [Google Scholar]
- Tian F, Li B, Ji B, Zhang G, Luo Y. 2009b. Identification and structure–activity relationship of gallotannins separated from Galla chinensis . LWT – Food Sci Technol 42: 1289–1295. [Google Scholar]
- Tian YH, Kim HC, Cui JM, Kim YC. 2005. Hepatoprotective constituents of Cudrania tricuspidata . Arch Pharm Res 28: 44–51. [DOI] [PubMed] [Google Scholar]
- Tianlu M, Barfod A. 2008. Rhus Linnaeus, Sp. Pl. 1: 265. 1753. Flora of China 11: 345–348. [Google Scholar]
- Tropicos.org.Missouri Botanical Garden . 18 Nov 2009. Online [database] http://www.tropicos.org/Name/1300863
- Wang RR, Gu Q, Wang YH et al. 2006. Anti‐HIV‐1 activities of extracts from the medicinal plant Rhus chinensis . J Ethnopharmacol 105: 269–273. [DOI] [PubMed] [Google Scholar]
- Wang RR, Gu Q, Wang YH et al. 2008. Anti‐HIV‐1 activities of compounds isolated from the medicinal plant Rhus chinensis . J Ethnopharmacol 117: 249–256. [DOI] [PubMed] [Google Scholar]
- Whang WK, Park HS, Ham I et al. 2005. Methyl gallate and chemicals structurally related to methyl gallate protect human umbilical vein endothelial cells from oxidative stress. Exp Mol Med 37: 343–352. [DOI] [PubMed] [Google Scholar]
- Wu‐Yuan CD, Chen CY, Wu RT. 1988. Gallotannins inhibit growth, water‐insoluble glucan synthesis, and aggregation of Mutans Streptococci. J Dental Res 67: 51–55. [DOI] [PubMed] [Google Scholar]
- Xiang P, Lin YM, Lin P, Xiang C, Yang ZW, Lu ZM. 2007. Effect of cationization reagents on the matrix‐assisted laser desorption/ionization time of flight mass spectrum of Chinese gallotannins. J Appl Polymer Sci 105: 859–864. [Google Scholar]
- Xiao PG. 1989. Chinese Medicinal Herb Iconograph I, Taiwan Business & Affairs Publishing House: Taipei, 105. [Google Scholar]
- Xiao CH, Yang SS, Hong XK. 2000. Chemistry of Traditional Chinese Medicine. Shanghai Science and Technology Publishing House: Shanghai. [Google Scholar]
- Yang H, Zheng S, Meijer L et al. 2005. Screening the active constituents of Chinese medicinal herbs as potent inhibitors of Cdc25 tyrosine phosphatase, an activator of the mitosis‐inducing p34cdc2 kinase. J Zhejiang Univ Sci 6B: 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung HC. 1985. Handbook of Chinese Herbs and Formulas. Institute of Chinese Medicine: Los Angeles. [Google Scholar]
- Yi L, Li Z, Yuan K et al. 2004. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol 78: 11334–11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokozawa T, Cui PC, Dong E, Tanaka T, Nonaka GI, Nishioka I. 1998. Study on the inhibitory effect of tannins and flavonoids against the 1,1‐diphenyl‐2‐picrylhydrazyl radical. Biochem Pharmacol 56: 213–222. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li L, Kim SH, Hagerman AE, Lü J. 2009. Anti‐cancer, anti‐diabetic and other pharmacologic and biological activities of penta‐galloyl‐glucose. Pharm Res 26: 2066–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Ren ZM, Nan P, Jiang MX, Zhao JY, Zhong Y. 2007. Chemical variation in leaf essential oils of Rhus chinensis from eight locations in Southern and Eastern China. Chem Nat Comp 43: 741–743. [Google Scholar]
- Zhu C, Dong C, Kong Y, Liu L, Wu Q, Yao Y. 2008. Microdilution inhibition test of Chinese herbs to assess their effect against clinical strains of Ureaplasma urealyticum in vitro . J Nanjing Med Univ 23: 143–145. [Google Scholar]
- Zhu YP. 1998. Chinese Materia Medica. Harwood Academic Publishers: Amsterdam, 659–660. [Google Scholar]
- Zou L, Zhang L, Li J et al. 2008. Effect of Galla chinensis extract and chemical fractions on demineralization of bovine enamel in vitro . J Dent 36: 999–1004. [DOI] [PubMed] [Google Scholar]


