Abstract
Background
We investigated the characteristics and clinical outcomes of respiratory syncytial virus (RSV)‐related pediatric intensive care unit (PICU) hospitalization and assessed the palivizumab (PZ) prophylaxis eligibility according to different guidelines from Korea, EU, and USA.
Methods
In this multicenter study, children <18 years of age hospitalized in six PICU from different hospitals due to severe RSV infection between September 2008 and March 2013 were included. A retrospective chart review was performed.
Results
A total of 92 patients were identified. The median length of PICU stay was 6 days (range, 1–154 days) and median PICU care cost was USD2,741 (range, USD556–98 243). Of 62 patients who were <2 years old at the beginning of the RSV season, 33 (53.2%) were high‐risk patients for severe RSV infection. Hemodynamically significant congenital heart disease (22.6%) was the most common risk factor, followed by chronic lung disease (11.3%), neuromuscular disease or congenital abnormality of the airway (NMD/CAA) (11.3%), and prematurity (8.1%). The percentage of patients eligible for PZ prophylaxis ranged from 38.7% to 48.4% based on the guidelines, but only two (2.2%) received PZ ≤30 days prior to PICU admission. The median duration of mechanical ventilation was longer in children with NDM/CAA than in those without risk factors (26 days; range, 24–139 days vs 6 days, range, 2–68 days, P = 0.033). RSV‐attributable mortality was 5.4%.
Conclusions
Children <2 years old with already well‐known high risks represent a significant proportion of RSV‐related PICU admissions. Increasing of the compliance for PZ prophylaxis practice among physicians is needed. Further studies are needed to investigate the burden of RSV infection in patients hospitalized in PICU, including children with NMD/CAA.
Keywords: eligibility, guideline, neuromuscular disorder or congenital abnormality of the airway, pediatric intensive care unit, respiratory syncytial virus
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection (LRTI) in infants and young children and causes a significant socioeconomic burden.1, 2, 3, 4 Prematurity, chronic lung disease (CLD) or hemodynamically significant congenital heart disease (HS‐CHD) are well‐known high‐risk factors for severe RSV infection, and these high‐risk children comprise approximately 10% of RSV‐related hospitalizations and 20–30% of pediatric intensive care unit (PICU) admissions.5 Palivizumab (PZ), a humanized monoclonal antibody against the RSV F protein, was licensed for the prevention of RSV‐related hospitalization and/or PICU admission in these high‐risk children.6, 7
Admission to a PICU is expensive in terms of medical resources, personnel, and treatment, yet there is a need for updated information on patient risk factors and PZ usage in PICU hospitalization. Despite the effectiveness of PZ prophylaxis, the eligibility for PZ varies widely between countries, with guidelines updated based on new evidence but also based on the changing health‐care policies and economic status of each country.6, 7, 8 In the American Academy of Pediatrics (AAP) guidelines, one new risk factor for PZ prophylaxis since 2009 is neuromuscular disorder and/or congenital abnormality of the airway (NMD/CAA), which is not included in the South Korean or EU guidelines.9, 10, 11, 12, 13, 14 In addition, the eligibility for PZ prophylaxis in premature infants also differs in these countries, with different age restrictions used to define prematurity (gestational age at birth or chronological age at the beginning of RSV season).11, 13, 15, 16, 17
In this study, we investigated the characteristics of children admitted to PICU due to RSV infection. We analyzed clinical outcomes, medical costs for PICU care and the eligibility for PZ prophylaxis according to different guidelines.
Methods
Patient selection
Children aged <18 years who were hospitalized in six PICU with RSV infection between 1 September 2008 and 31 March 2013 were included. All of these PICU are in tertiary care hospitals in South Korea: Samsung Medical Center (SMC) in Seoul; Asan Medical Center in Seoul; Korea University Ansan Hospital in Ansan; Gachon University Gill Hospital in Incheon; Pusan National University Children's Hospital in Yangsan; and Ewha Woman's University in Seoul. Diagnosis of RSV infection was confined to RSV detection ≤48 h after PICU admission on rapid RSV antigen test, multiplex respiratory virus real‐time polymerase chain reaction (PCR), and/or viral culture from a respiratory specimen such as a nasopharyngeal or endotracheal aspirate. Patients with underlying hematology–oncology disease and with RSV infection detected >48 h after PICU admission were excluded. The study was approved by the institutional review boards of Samsung Medical Center (IRB no. 2015‐01‐120) and all other participating hospitals. Requirement for informed consent and assent were waived. All patient‐identifiable data were deleted or modified prior to analysis.
Data collection and analysis
We retrospectively reviewed medical records including age, gender, underlying disease, RSV subtype, method of RSV detection, presence of co‐infection, and severity of RSV infection. LRTI was defined as clinical tachypnea with hypoxia (SpO2 < 93%) requiring oxygen supplementation and chest radiology confirmation of pneumonic infiltration or hyperinflation. Upper respiratory tract infection (URTI) was defined as respiratory symptoms without hypoxia and normal chest radiology. We analyzed the clinical outcomes of RSV‐related PICU hospitalizations according to risk factors. Clinical outcome parameters were length of PICU stay, prevalence and duration of mechanical ventilator support, rate of tracheostomy, and 30 day mortality. In patients who received a tracheostomy, we assumed that the date of discontinuation of ventilator support was the date of transfer to a general ward. High risk for severe RSV infection was defined based on previous studies or guidelines as the presence of one of the following risk factors:8, 18 prematurity (gestational age <35 weeks), CLD, HS‐CHD, CAA, NMD, Down syndrome, or profound immunocompromisation. We also analyzed the medical cost of PICU hospitalization at SMC.
RSV seasonality and introduction of PZ in South Korea
In South Korea, the RSV season spans autumn through early spring (XX–XX) and is monitored by Korea Influenza and Respiratory Surveillance System (KINRESS) at the Centers for Disease Control and Prevention of South Korea.18 Typically, PZ prophylaxis could be initiated on 1 September and is given monthly until 31 March 2ith the limitation of a maximum number of five doses. PZ was introduced first in 2005 and has been covered by the National Healthcare Insurance Service in South Korea (K‐NHIS) for infants and young children with CLD since January 2006, for infants with HS‐CHD since 2009, and for infants born premature (gestational age <32 weeks) since 2012. Infants born at a gestational age 32–36 weeks with additional circumstances and children aged 12–24 months with HS‐CHD have been covered since September 2016, but these indications are not included in this study.
Guideline eligibility for PZ prophylaxis
We compared eligibility for PZ prophylaxis for patients <2 years of age between the guidelines of three different countries/regions: South Korea's K‐NHIS “Coverage Indications for Palivizumab” guidelines, updated 27 December 2012; the European Medicines Agency (EMA) guidelines, “Synagis: European Public Assessment Report–Product Information,” updated 18 December 2013; and the US AAP guidelines, “Modified Recommendations for Use of Palivizumab for Prevention of RSV infection in 2014”.12, 13 Detailed PZ prophylaxis eligibility criteria are listed in Table 1.
Table 1.
Indications for PZ prophylaxis according to guidelines
| Risk factor as an indication for PZ prophylaxis | 2012 K‐NHIS (South Korea) | 2013 EMA (EU) | 2014 AAP (USA) |
|---|---|---|---|
| Premature | |||
| <29 weeks’ GA | 6 months | 6 months | 12 months |
| 29–31 weeks’ GA | 6 months | 6 months | NI |
| 32–35 weeks’ GA | NI | 6 months | NI |
| CLD | 24 months | 24 months | 24 months |
| HS‐CHD | 12 months | 24 months | 12 months |
| NMD | NI | NI | 12 months† |
| CAA | NI | NI | 12 months† |
| Profoundly immunocompromised | NI | NI | 24 months† |
| Other risk factors | NI | NI | NI |
†Children with these risk factors may be considered for prophylaxis. AAP, American Academy of Pediatrics; CAA, congenital abnormality of airway; CLD, chronic lung disease; EMA, European Medicines Agency; GA, gestational age; HS‐CHD, hemodynamically significant congenital heart disease; K‐NHIS, National Healthcare Insurance Service in South Korea; NI, not indicated; NMD, neuromuscular disease; PZ, palivizumab.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Statistical analysis
Depending on the purpose of the analysis, the patients were classified according to different age criteria. We used age at RSV infection for the analysis of clinical outcomes. In addition, age at the beginning of the RSV season (<2 years) was used for the analysis of PZ prophylaxis. Continuous variables and categorical variables are summarized as median (range) and frequency (percentage), respectively. Clinical outcomes according to risk factors were compared using Mann–Whitney test or Kruskal–Wallis test for continuous variables, and Fisher's exact test or chi‐squared test for univariable analysis. Multiple linear regression analysis and multiple logistic regression analysis were performed to adjust for age at diagnosis, gender, and RSV infection clinical disease status for multivariable analysis. Non‐normal continuous outcome was log‐transformed and Dunnett's test was applied for multiple comparisons of each risk group with non‐risk group in multiple linear regression. P < 0.05 was considered statistically significant. Statistical analysis was carried out using SAS version 9.4 (SAS Institute, Cary, NC, USA) and GraphPad Prism version 6.04 (GraphPad Software, La Jolla, CA, USA).
Results
Patient characteristics
A total of 98 pediatric patients were admitted between September 2008 and March 2013 to the six PICU with RSV detection ≤48 h after admission. Of these admissions, 92 (93.8%) were identified as RSV‐related PICU admissions. Six were excluded for other reasons (Fig. 1). These six PICU admissions were not due to RSV infection itself but were due to severe or progressing status of underlying disease. Two patients with encephalitis were admitted to PICU with decreased mental status, not for respiratory tract infection itself. Three patients with congenital heart disease were admitted to PICU for planned open heart surgery, and RSV infection was found on routine active surveillance or evaluation. Median age at PICU admission was 6.6 months (range, 0.5–210.4 months; IQR, 2.1–23.9 months; Table 2). Sixty‐nine of 92 patients (75.0%) were <2 years of age at the time of RSV infection, and almost half (48.9%) were <6 months of age. Fifty‐five of 92 patients (59.8%) were male, and 59 of 92 (64.1%) had underlying disease. Six of 92 patients (6.5%) had received PZ prior to RSV infection, and only two (2.2%) received PZ ≤30 days prior to PICU admission. Eighty‐two patients (89.1%) presented with LRTI, and 10 (10.9%) presented with URTI. The median length of PICU stay was 6 days (range, 1–154 days; IQR, 4–12 days). Half of the patients required mechanical ventilation, with a median duration of mechanical ventilation of 7.5 days (range, 1–139 days; IQR, 5–19 days). Three patients (3.3%) required tracheostomy. The overall 30 day mortality was 7.6% (n= 7), and in five cases (5.4%) death was related to severe RSV infection (Table 2). All five patients who died had a high risk of severe RSV infection. Two had HS‐CHD, two had CLD, and one was a premature infant with a gestational age <29 weeks.
Figure 1.
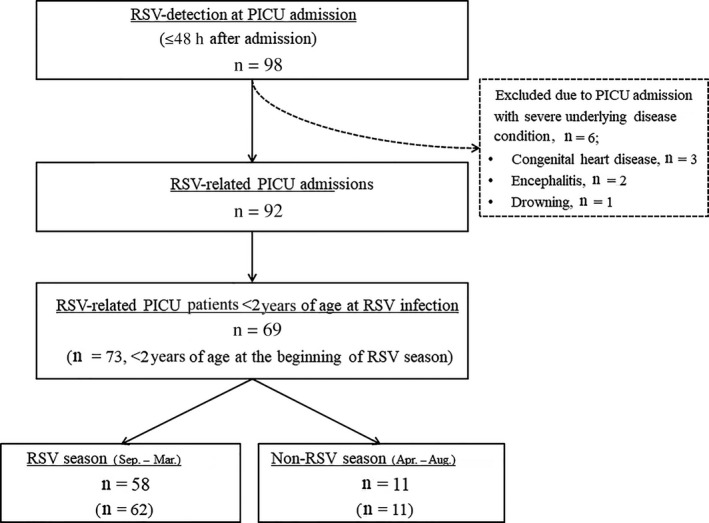
Flow of case selection. Of the two excluded cases of encephalitis, one was Listeria meningoencephalitis, and the other was of unknown origin in a 40‐month‐old boy. In the latter case, the patient had no respiratory symptom except fever, and follow‐up test was negative. PICU, pediatric intensive care unit; RSV, respiratory syncytial virus.
Table 2.
Patient characteristics and demographics (n = 92)
| Characteristics | n (%) or median (range) |
|---|---|
| Male | 55 (59.8) |
| Age at diagnosis (months) | 6.6 (0.5–210.4) |
| <6 months | 45 (48.9) |
| 6–11 months | 14 (15.2) |
| 12–23 months | 10 (10.9) |
| 24–59 months | 12 (13.0) |
| ≥60 months | 11 (12.0) |
| Presence of underlying disease† | 59 (64.1) |
| Diagnostic tool for RSV detection | |
| PCR | 77 (83.7) |
| Culture | 15 (16.3) |
| RSV subtype | |
| A | 49 (53.3) |
| B | 21 (22.8) |
| Unknown | 22 (23.9) |
| Co‐viral infection‡ | 16 (17.4) |
| PZ prophylaxis prior to RSV season (at least 1 dose) | 6 (6.5) |
| Status of RSV infection | |
| LRTI | 82 (89.1) |
| URTI | 10 (10.9) |
| Length of PICU stay (days) | 6 (1–154) |
| Ventilator support | |
| Requirement | 46 (50) |
| Duration (days) | 7.5 (1–139) |
| Tracheostomy | 3 (3.3) |
| Overall 30 day mortality | 7 (7.6)§ |
| RSV‐attributable 30 day mortality | 5 (5.4) |
†Prematurity, n = 7; chronic lung disease including bronchopulmonary dysplasia, interstitial lung disease and chronic aspiration, n = 11; congenital heart disease, n = 20; neuromuscular disorder due to mitochondrial disease and spinal muscular atrophy type 1, n = 2; airway malformation including tracheoesophageal fistula, lung sequestration, tracheomalacia and tracheal stenosis, n = 7; chromosomal anomaly including Down syndrome, n = 3; primary immunodeficiency, n = 1; acute kidney injury, n = 1; asthma, n = 1; arteriovenous malformation, n = 1; unknown severe developmental delay, n = 1; mediastinal lymphangiomatosis, n = 1; nephrotic syndrome, n = 1; renal tubular acidosis type 3, n = 1; and megacystic microcolon syndrome, n = 1. ‡Influenza A, n = 5; rhinovirus infection, n = 4; adenovirus infection, n = 4; coronavirus infection, n = 1; both parainfluenza and rhinovirus infection, n = 1; both adenovirus and rhinovirus infection, n = 1. §Two died from bacterial sepsis. LRTI, lower respiratory tract infection; PCR, polymerase chain reaction; PICU, pediatric intensive care unit; PZ, palivizumab; RSV, respiratory syncytial virus; URTI, upper respiratory tract infection.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Seasonal distribution of RSV‐related PICU hospitalization
Monthly distribution of RSV‐related PICU admissions follows the seasonal pattern of RSV circulation in South Korea: increasing in September, peaking between October and December, and ending in April (Fig. 2a). During the RSV season from September to March, 80 of 92 RSV‐related PICU hospitalizations (87.0%) occurred. Of these 80 patients, 58 (72.5%) were <2 years of age at the time of RSV infection, and 62 (77.5%) were <2 years of age at the beginning of the RSV season (Fig. 1). Forty‐nine of 92 patients (53.3%) had at least one risk factor for severe RSV infection regardless of age (Fig. 2b).
Figure 2.
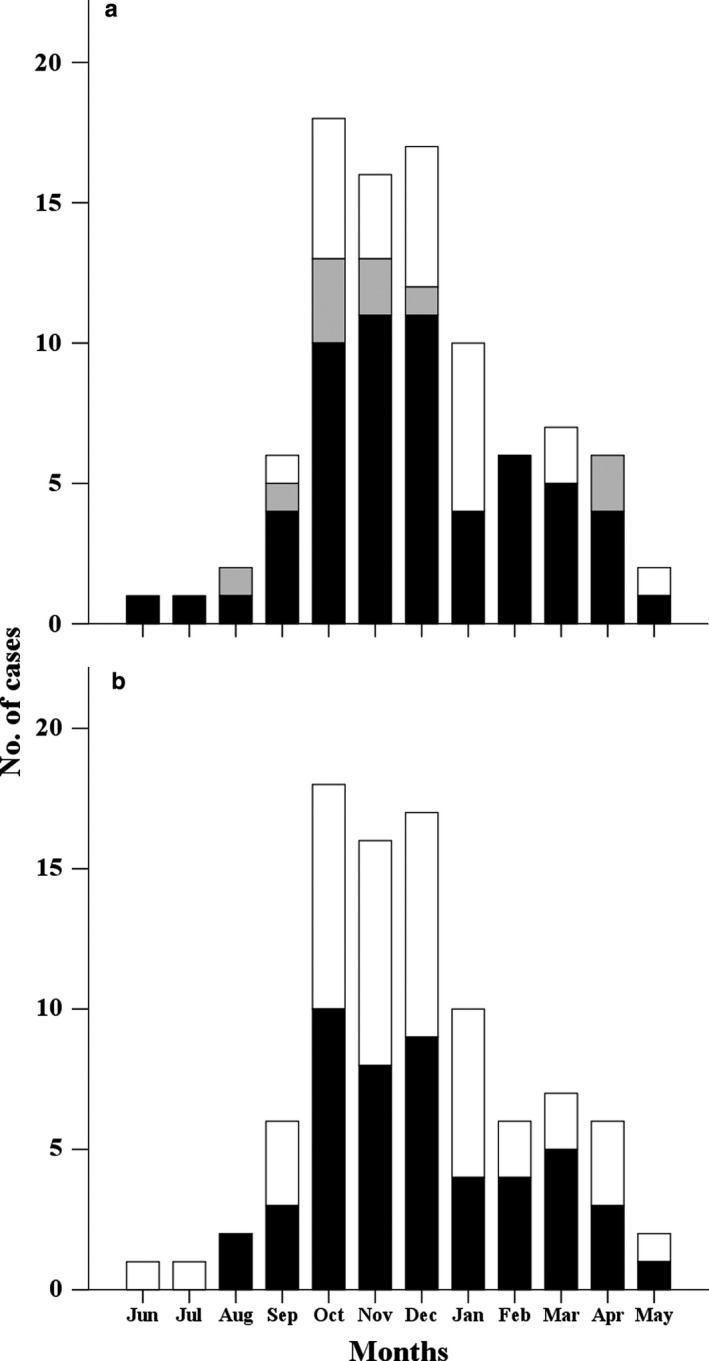
Seasonal distribution of respiratory syncytial virus (RSV)‐related pediatric intensive care unit (PICU) admissions according to (a) age at the time of RSV detection (■, <12 months;  , 12–24 months; □, >24 months); and (b) presence of risk factors (■, risk; □, no risk). (a) Sixty‐nine of 92 cases (75.0%) occurred in children <2 years of age at the time of RSV detection; 59 of 92 (64.1%) occurred in children <1 year of age. (b) Forty‐nine of 92 patients (53.3%) had at least one of risk factor for severe RSV infection. Risk factors for severe RSV infection include prematurity (gestational age <35 weeks), chronic lung disease, hemodynamically significant congenital heart disease, congenital abnormality of the airway or neuromuscular disorder, Down syndrome or profound immunocompromisation.
, 12–24 months; □, >24 months); and (b) presence of risk factors (■, risk; □, no risk). (a) Sixty‐nine of 92 cases (75.0%) occurred in children <2 years of age at the time of RSV detection; 59 of 92 (64.1%) occurred in children <1 year of age. (b) Forty‐nine of 92 patients (53.3%) had at least one of risk factor for severe RSV infection. Risk factors for severe RSV infection include prematurity (gestational age <35 weeks), chronic lung disease, hemodynamically significant congenital heart disease, congenital abnormality of the airway or neuromuscular disorder, Down syndrome or profound immunocompromisation.
High‐risk children in the PICU and clinical outcomes according to risk factor
There were 62 RSV‐related PICU admissions in children <2 years of age at the beginning of the RSV season. HS‐CHD (22.6%) was the most common risk factor, followed by CLD (11.3%), CAA (9.7%), prematurity (8.1%), and NMD (1.6%). Six patients (9.7%) had an underlying disease that did not increase the risk of severe RSV infection; 23 patients (37.1%) had no underlying disease.
There were 69 RSV‐related PICU admissions in children <2 years of age at the time of RSV infection. Of these, the prevalence of mechanical ventilation was significantly higher in the high‐risk group than in the no‐risk group on multivariable analysis (64.9% vs 31.3%, P = 0.006). There was no significant difference, however, in other outcome parameters, such as length of PICU stay, duration of mechanical ventilation, requirement for tracheostomy, and RSV‐related 30 day mortality. The NMD/CAA group had a longer median duration of mechanical ventilation compared with the no‐risk group (26 days; range, 24–139 days vs 6 days, range, 2–68 days, P = 0.033; Fig. S1; Table 3).
Table 3.
Clinical outcome vs presence of risk (n = 69
| No risk | All risk† | Risk subgroup | P‐value | |||||
|---|---|---|---|---|---|---|---|---|
| Clinical outcome |
(n = 32) Median (range) or n (%) |
(n = 37) Median (range) or n (%) |
Prematurity (n = 6) Median (range) or n (%) |
CLD (n = 7) Median (range) or n (%) |
HS‐CHD (n = 16) Median (range) or n (%) |
NMD/CAA (n = 7) Median (range) or n (%) |
All risk vs non‐risk | Risk subgroup vs non‐risk |
| PICU length of stay (days) | 6 (1–68) | 7 (1–154) | 6 (4–12) | 5 (1–53) | 10.5 (1–37) | 7 (3–154) | 0.07 | 0.053 |
| Mechanical ventilation | ||||||||
| Requirement | 10 (31.3) | 24 (64.9) | 5 (83.3) | 4 (57.1) | 11 (68.8) | 3 (42.9) | 0.006 | 0.087 |
| Duration (days) | 6 (2–68) | 11.5 (1–139) | 5 (3–11) | 27 (1–53) | 12 (1–24) | 26 (24–139) | 0.16 | 0.031‡ |
| Tracheostomy | 0 (0) | 2 (8.3) | 0 (0) | 1 (25.0) | 0 (0) | 1 (33.3) | NA | NA |
| RSV‐related 30 day mortality | 0 (0) | 4 (10.8) | 1 (16.7) | 1 (14.3) | 2 (12.5) | 0 (0) | NA | NA |
†All risk groups includes prematurity, CLD, HS‐CHD, and NMD/CAA. Two cases of Down syndrome and one case of profoundly immunocompromised condition and were excluded in the analysis. ‡Significant difference in clinical outcome of ventilation duration (P = 0.031). In the subsequent subgroup analysis, the NMD/CAA group had a significantly prolonged duration of mechanical ventilation compared with the no‐risk group (NMD/CAA vs non‐risk, P = 0.033; Fig. S1). Multiple linear regression and multiple logistic regression analyses were performed to adjust for age at diagnosis, gender, and clinical disease status of RSV infection. Non‐normal continuous outcome was log‐transformed and Dunnett's test was applied for multiple comparisons of each risk group with non‐risk group in multiple linear regression. CAA, congenital abnormality of the airway; CLD, chronic lung disease; HS‐CHD, hemodynamically significant congenital heart disease; NA, not applicable; NMD, neuromuscular disorder; PICU, pediatric intensive care unit.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Eligibility for PZ prophylaxis
We estimated eligibility for PZ prophylaxis according to the three different guidelines. Twenty‐four of the 62 high‐risk children (38.7%) were eligible for PZ prophylaxis based on the 2013 K‐NHIS guidelines, 26 (41.9%) based on the 2015 EMA guidelines, and 30 (48.4%) based on the 2014 AAP guidelines (Table 4). The 2013 K‐NHIS guidelines appeared more restrictive than the 2014 AAP guidelines with regard to PZ eligibility, but the difference was not significant (38.7% vs 48.4%, P = 0.07; Fig. 3).
Table 4.
Risk factors for severe RSV infection and eligibility for PZ prophylaxis (n = 62)
| Risk factor |
<2 years of age at the beginning of RSV season n (%) |
2013 K‐NHIS (South Korea) n (%) |
2015 EMA (EU) n (%) |
2014 AAP (USA) n (%) |
|---|---|---|---|---|
| PM (weeks’ GA) | 5 (8.1) | 3 (12.5) | 5 (19.2) | 2 (6.7) |
| <29 | 2 (3.2) | 2 (8.3) | 2 (7.7) | 2 (6.7) |
| 29–31 | 1 (1.6) | 1 (4.2) | 1 (3.8) | 0 (0.0) |
| 32–35 | 2 (3.2) | 0 (0.0) | 2 (7.7) | 0 (0.0) |
| CLD | 7 (11.3) | 7 (29.2) | 7 (26.9) | 7 (23.3) |
| PM related | 6 (9.7) | 6 (25.0) | 6 (23.1) | 6 (20.0) |
| Non‐PM related | 1 (1.6) | 1 (4.2) | 1 (3.8) | 1 (3.3) |
| HS‐CHD† | 14 (22.6) | 14 (58.3) | 14 (53.8) | 14 (46.7) |
| Other | 9 (14.5) | 0 (0.0) | 0 (0.0) | 7 (23.3) |
| NMD‡ | 1 (1.6) | 0 (0.0) | 0 (0.0) | 1 (3.2) |
| CAA | 6 (9.7) | 0 (0.0) | 0 (0.0) | 6 (20.0) |
| Profoundly immunocompromised | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Down syndrome§ | 2 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Underlying disease without risk factor¶ | 4 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No underlying disease (no risk factor) | 23 (37.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 62 (100.0) | 24 (38.7) | 26 (41.9) | 30 (48.4) |
†One patient had two risk factors: pulmonary hypertension and congenital mitral regurgitation with laryngomalacia. ‡One patient had two risk factors: mitochondrial encephalomyopathy, lactic acidosis, and stroke‐like episodes (MELAS) with hypertrophic cardiomyopathy. §Without congenital heart disease. ¶Prematurity at 36 weeks and 0 days of gestation, n = 1; simple ventricular septal defect, n = 1; nephrotic syndrome, n = 1; and renal tubular acidosis type 3, n = 1. AAP, American Academy of Pediatrics; CAA, congenital abnormalities of the airway; CLD, chronic lung disease; EMA, European Medicines Agency; GA, gestational age; HS‐CHD, hemodynamically significant congenital heart disease; K‐NHIS, National Healthcare Insurance Service in South Korea; NMD, neuromuscular disorders; PM, prematurity; PZ, palivizumab; RSV, respiratory syncytial virus.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 3.
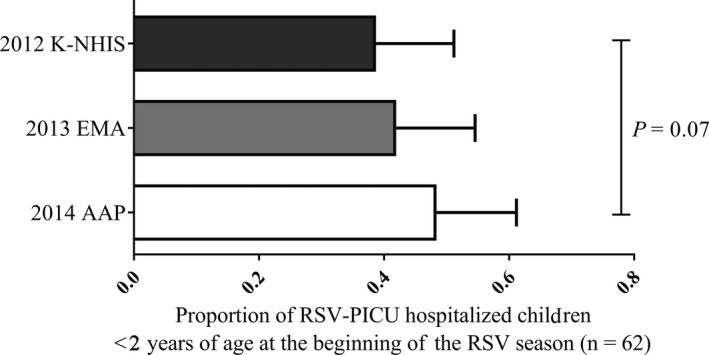
Palivizumab prophylaxis eligibility. 2013 K‐NHIS guidelines vs 2014 AAP guidelines, 38.7% vs 48.4% (P = 0.07, Mann–Whitney test). AAP, American Academy of Pediatrics; EMA, European Medicines Agency; K‐NHIS, National Healthcare Insurance Service in South Korea.
Economic burden according to risk factor
Medical cost was analyzed in 37 patients who were hospitalized at SMC. For the 28 children <2 years old at the time of RSV infection, the median cost of health care in the high‐risk group appeared to be higher than that of the no‐risk group, although the difference was not significant ($2,284; range, $705–$98 243 vs $1,899, range, $556–$30 449; P = 0.38). With regard to patients who received ventilator support, although patients with risk appeared to have higher median medical costs than patients with no risk, this was not statistically significant ($15 346; range, $2,575–$91 546 vs $4,634, range, $2,312–$30 449; P = 0.47; Table 5).
Table 5.
Economic cost according to risk factor (n = 28)
| Cost (USD)‡ | ||||||
|---|---|---|---|---|---|---|
| Characteristics | All (n = 28) | Mechanical ventilation (n = 13) | ||||
| Median | Range | P‐value† | Median | Range | P‐value† | |
| No risk | 1,898.8 | 556.1–30 448.9 | 4,634.3 | 2,311.9–30 448.9 | ||
| Risk | 2,284.0 | 705.4–98 243.1 | 0.38 | 15 346.3 | 2,575.5–91 545.9 | 0.47 |
| Subgroup analysis | 0.74 | 0.65 | ||||
| CLD | 1,865.9 | 824.6–38 340.4 | 20 457.9 | 2,575.5–38 340.4 | ||
| HS‐CHD | 3,729.9 | 705.4–35 040.7 | 9,836.1 | 4,071.0–32 198.8 | ||
| NMD/CAA | 9,928.8 | 1,125.1–98 243.1 | 20 226.3 | 10 466.2–91 545.9 | ||
†Mann–Whitney test using exact method. ‡Exchange rate on 15 March 2016: 1 USD = 1212.5 KRW. CAA, congenital abnormality of the airway; CLD, chronic lung disease; HS‐CHD, hemodynamically significant congenital heart disease; NMD, neuromuscular disorder.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Discussion
This study shows that high‐risk children account for a high proportion of RSV‐related PICU admissions. In clinical outcome analysis, NMD/CAA was associated with a prolonged duration of mechanical ventilation. Eligibility for PZ prophylaxis varied according to the guidelines of different countries.
The proportion of high‐risk patients in PICU was 53.3%, which was higher than that in other studies, at 20–30%.5, 19 Berger et al. reported that 23% of RSV‐related intermediate care unit (IMU) or ICU admissions for children <3 years of age in Switzerland were high risk, with at least one risk factor such as prematurity (gestational age <32 weeks), CLD, or HS‐CHD.19 In a retrospective study of 181 children admitted to the PICU with RSV infection in Canada from 2003 to 2009, Butt et al. found that 29.8% had an underlying disease.5 These studies, however, did not include NMD/CAA as an underlying disease; and NMD/CAA was present in 11.3% of the present RSV PICU cases.
The prevalence of HS‐CHD (22.6%) and of NMA/CAA (11.3%) were high in the present study compared with previous studies, while that of prematurity (gestational age <35 weeks, 8.1%) and of CLD (11.3%) were relatively low.5, 6, 19, 20, 21, 22 These observations might be due to differences in PZ prophylaxis according to risk factors in different countries.23, 24, 25 In a retrospective study of RSV‐related re‐admissions of preterm infants (gestational age <34 weeks) in South Korea, Lee et al. reported that 47.5% of 1,140 preterm infants received PZ prophylaxis, including 89.3% of those with CLD.24 The high PZ prophylaxis rates for CLD and premature infants might have reduced the RSV‐related PICU admissions for these groups in the present study. Infants with NMD/CAA are not eligible for PZ prophylaxis in South Korea, and no studies have reported PZ prophylaxis rates for children with HS‐CHD. A recent study of 466 infants with HS‐CHD in South Korea reported that 39% did not receive PZ prophylaxis on time.25 In addition, the present study period includes the 2008–2009 RSV season, during which HS‐CHD patients were not eligible for PZ prophylaxis. As a result, PZ prophylaxis for many children with HS‐CHD might have been delayed. Furthermore, as shown in the present study, even according to restrictive 2013 K‐NHIS guidelines, 24 patients (38.7%) were already eligible for PZ prophylaxis and should have received it. Only six patients (6.5%), however, had received PZ at least once before PICU admission due to RSV infection. Therefore, it is also important to promote adherence to PZ prophylaxis for high‐risk children among physicians in daily clinical practice.
Currently, PZ prophylaxis guidelines vary greatly between countries. The eligibility for PZ prophylaxis for the present patients ranged from 39% to 48% based on guidelines from South Korea, EU, and the USA. The main differences were inclusion or exclusion of children with NMD/CAA and differences in the age restrictions used to define prematurity and HS‐CHD.12, 13 The age restrictions used to identify high‐risk children are based on many studies on prophylactic effects and cost–benefit analyses.11, 13, 14 In contrast, children with NMD/CAA or profound immunocompromisation might not be included in the current guidelines due to insufficient data on the effectiveness of PZ prophylaxis. Therefore, large‐scale investigations to prove the effectiveness of PZ prophylaxis for these high‐risk groups are needed. In addition, although there is no doubt that Down syndrome is a risk factor for severe RSV infection, it remains controversial as to whether PZ prophylaxis is efficacious, including the cost–benefit aspects.26, 27, 28 Therefore, further studies are needed.
Notably, although the number of patients was small (n = 3), the median duration of mechanical ventilation was significantly longer in children with NMD/CAA (n = 3) than in those with no risk (26 days; range, 24–139 days vs 6 days, range, 2–68 days; P = 0.033; Table 4). Children with NMD/CAA were also more likely to receive tracheostomy than children in the no‐risk group, with borderline significance (40%, 2/5 vs 0%, 0/45; P = 0.053). Defects in respiratory defense mechanisms such as impaired ability to clear secretions and/or insufficient coughing due to weakness of the respiratory muscles are barriers to weaning children off mechanical ventilation. Although there were insufficient patients with NMD/CAA to show a cost difference, prolonged ventilator support means longer PICU care, which requires additional medical resources, thereby generating a larger socioeconomic burden (Table 5).
Given that this retrospective study was focused on tertiary‐care hospitals, it is likely that more children with severe RSV infection or other severe underlying disease were included than in studies that include intermediate‐care hospitals. In addition, despite the multicenter study design, the number of cases was insufficient for subgroup analysis due to the rarity of some diseases.
Nevertheless, this study provides needed data on the proportion of high‐risk children with severe RSV infection in PICU in the PZ era. We wanted to express the clinical outcomes of PICU admissions due to severe RSV infection including high mortality rate of 5%, as well as the economic burden of PICU admission due to RSV. We also assessed the eligibility for PZ prophylaxis based on different guidelines. Finally, we observed that children with NMD/CAA represent a significant portion of PICU admissions in South Korea, where these children are not eligible for PZ prophylaxis. Moreover, children with NMD/CAA appear to require a prolonged duration of mechanical ventilation than those with no risk. Therefore, it is worth noting that the children with NMD/CAA might have comprised a larger portion of the RSV‐related PICU admissions, if the PZ prophylaxis had been appropriately given to children with already well‐known high risks according to the K‐NHIS guidelines.
In conclusion, in South Korea, high‐risk children represented a higher proportion of RSV‐related PICU admissions than previously reported. Further studies are needed to investigate the burden of RSV infection in PICU hospitalizations, including that associated with children with NMD/CAA.
Disclosure
Y‐J.K. received a grant from Medimmune for RSV research and had received a grant from Celltrion for the research of RSV‐specific monoclonal antibody. Y‐J.K. has a contract with Janssen for RSV research. The other authors declare no conflict of interest.
Author contributions
J‐M.K. designed the study, carried out data collection and analysis, and drafted the manuscript. Y‐J.K. conceptualized and designed the study and critically reviewed and revised the manuscript. J.L., Y‐K.K., H.K.C., S.E.P., and K‐H.K. coordinated and supervised data collection at their own hospitals and critically reviewed the manuscript. M‐J.K. and S.K. performed analyses, interpreted data, and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supporting information
Fig. S1 Duration of mechanical ventilation according to risk factor. In subgroup analysis, children with NMD/CAA had a longer duration of mechanical ventilation than children with no risk factor (median, 26 days [range, 24‐139] vs. 6 days [range, 2‐68], P = .033).
References
- 1. Hall CB. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 2001; 344 (25): 1917–28. [DOI] [PubMed] [Google Scholar]
- 2. Stockman LJ, Curns AT, Anderson LJ, Fischer‐Langley G. Respiratory syncytial virus‐associated hospitalizations among infants and young children in the United States, 1997‐2006. Pediatr. Infect. Dis. J. 2012; 31: 5–9. [DOI] [PubMed] [Google Scholar]
- 3. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990‐2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 385 (9963): 117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heikkinen T, Ojala E, Waris M. Clinical and socioeconomic burden of respiratory syncytial virus infection in children. J. Infect. Dis. 2017; 215: 17–23. [DOI] [PubMed] [Google Scholar]
- 5. Butt ML, Symington A, Janes M, Elliott L, Steele S, Paes BA. The impact of prophylaxis on paediatric intensive care unit admissions for RSV infection: A retrospective, single‐centre study. Eur. J. Pediatr. 2011; 170: 907–13. [DOI] [PubMed] [Google Scholar]
- 6. Null D, Bimle C, Weisman L et al Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high‐risk infants. Pediatrics 1998; 102: 531–7. [PubMed] [Google Scholar]
- 7. Feltes TF, Cabalka AK, Meissner HC et al Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J. Pediatr. 2003; 143: 532–40. [DOI] [PubMed] [Google Scholar]
- 8. Andabaka T, Nickerson JW, Rojas‐Reyes MX, Rueda JD, Bacic VV, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst. Rev. 2013;4(4):CD006602. [DOI] [PubMed] [Google Scholar]
- 9. Prevention of respiratory syncytial virus infections: Indications for the use of palivizumab and update on the use of RSV‐IGIV. American Academy of Pediatrics Committee on Infectious Diseases and Committee of Fetus and Newborn. Pediatrics 1998; 102: 1211–6. [DOI] [PubMed] [Google Scholar]
- 10. Meissner HC, Long SS, American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn . Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 2003; 112: 1447–52. [DOI] [PubMed] [Google Scholar]
- 11. Committee on Infectious Diseases . From the American Academy of Pediatrics: Policy statements – Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics 2009; 124: 1694–701. [DOI] [PubMed] [Google Scholar]
- 12. European Medicines Agency . EPAR summary for the public “Synagis; palivizumab”. 2013. [Cited 7 June 2019.] Available from https://www.ema.europa.eu/en/medicines/human/EPAR/synagis
- 13. Macht M, Mull AC, McVaney KE et al Comparison of droperidol and haloperidol for use by paramedics: Assessment of safety and effectiveness. Prehosp. Emerg. Care 2014; 18: 375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schachtner T, Stein M, Sefrin A, Babel N, Reinke P. Inflammatory activation and recovering BKV‐specific immunity correlate with self‐limited BKV replication after renal transplantation. Transpl. Int. 2014; 27: 290–301. [DOI] [PubMed] [Google Scholar]
- 15. Simoes EA, Carbonell‐Estrany X, Guilbert T et al Clinical endpoints for respiratory syncytial virus prophylaxis trials in infants and children in high‐income and middle‐income countries. Pediatr. Infect. Dis. J. 2015; 34: 1086–92. [DOI] [PubMed] [Google Scholar]
- 16. Hasegawa K, Mansbach JM, Piedra PA et al Eligibility for palivizumab prophylaxis in a cohort of children with severe bronchiolitis. Pediatr. Int. 2015; 57: 1031–4. [DOI] [PubMed] [Google Scholar]
- 17. Yogev R, Krilov LR, Fergie JE, Weiner LB. Re‐evaluating the new committee on infectious diseases recommendations for palivizumab use in premature infants. Pediatr. Infect. Dis. J. 2015; 34: 958–60. [DOI] [PubMed] [Google Scholar]
- 18. Kim SY, Lee KE, Kang SY, Choi EH, Lee HJ. Evaluation of timeliness of palivizumab immunoprophylaxis based on the epidemic period of respiratory syncytial virus: 22 year experience in a single center. Pediatr. Infect. Vaccine 2015; 22: 172–7. [Google Scholar]
- 19. Berger TM, Aebi C, Duppenthaler A, Stocker M, Swiss Pediatric Surveillance Unit . Prospective population‐based study of RSV‐related intermediate care and intensive care unit admissions in Switzerland over a 4‐year period (2001‐2005). Infection 2009;37:109–16. [DOI] [PubMed] [Google Scholar]
- 20. Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J. Pediatr. 2003; 143 (5 Suppl): S112–7. [DOI] [PubMed] [Google Scholar]
- 21. Chi H, Chang IS, Tsai FY et al Epidemiological study of hospitalization associated with respiratory syncytial virus infection in Taiwanese children between 2004 and 2007. J. Formos. Med. Assoc. 2011; 110: 388–96. [DOI] [PubMed] [Google Scholar]
- 22. Paes B, Mitchell I, Li A, Harimoto T, Lanctot KL. Respiratory‐related hospitalizations following prophylaxis in the Canadian registry for palivizumab (2005‐2012) compared to other international registries. Clin. Dev. Immunol. 2013; 2013: 917068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han YM, Seo HJ, Choi SH et al Effect of prophylactic palivizumab on admission due to respiratory syncytial virus infection in former very low birth weight infants with bronchopulmonary dysplasia. J. Korean Med. Sci. 2015; 30: 924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee JH, Kim CS, Chang YS, Choi JH, Committee on Data Collection and Statistical Analysis of the Korean Society of Neonatology . Respiratory syncytial virus related readmission in preterm infants less than 34 weeks’ gestation following discharge from a neonatal intensive care unit in Korea. J. Korean Med. Sci. 2015; 30 (Suppl 1): S104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim AY, Jung SY, Choi JY et al Retrospective multicenter study of respiratory syncytial virus prophylaxis in Korean children with congenital heart diseases. Korean Circ. J. 2016; 46: 719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kashiwagi T, Okada Y, Nomoto K. Palivizumab prophylaxis against respiratory syncytial virus infection in children with immunocompromised conditions or Down syndrome: A multicenter post‐marketing surveillance in Japan. Paediatr. Drugs 2018; 20: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanchez‐Luna M, Medrano C, Lirio J, RISK‐21 Study Group. Down syndrome as risk factor for respiratory syncytial virus hospitalization: A prospective multicenter epidemiological study. Influenza Other Respir. Viruses 2017; 11: 157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yi H, Lanctot KL, Bont L et al Respiratory syncytial virus prophylaxis in Down syndrome: A prospective cohort study. Pediatrics 2014; 133: 1031–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Duration of mechanical ventilation according to risk factor. In subgroup analysis, children with NMD/CAA had a longer duration of mechanical ventilation than children with no risk factor (median, 26 days [range, 24‐139] vs. 6 days [range, 2‐68], P = .033).


